Open Access Article
Open Access ArticleCombining broadband absorbing electrochromic materials for hybrid grey-to-colourless flexible devices†
Lukas
Niklaus‡
 a,
Rúben R.
Ferreira‡
a,
Rúben R.
Ferreira‡
 b,
Sven
Macher
b,
Sven
Macher
 a,
Antoine
Stopin
b,
Marco
Schott
a,
Antoine
Stopin
b,
Marco
Schott
 *a,
Laura
Maggini
*a,
Laura
Maggini
 *b and
Davide
Bonifazi
*b and
Davide
Bonifazi
 b
b
aFraunhofer Institute for Silicate Research ISC, Neunerplatz 2, D-97082 Würzburg, Germany. E-mail: marco.schott@isc.fraunhofer.de
bDepartment of Organic Chemistry, University of Vienna, Währinger Straße 38, A-1090 Vienna, Austria. E-mail: laura.maggini@univie.ac.at
First published on 6th March 2025
Abstract
This study presents a grey-to-colourless hybrid electrochromic device combining a novel red-to-colourless polymer with blue-to-colourless Prussian blue. The device achieves a neutral tint in both the dark and bleached states, with notable changes in visible light transmittance and fast response.
Electrochromic materials (ECMs) and devices (ECDs) exhibit a colour change upon the application of an electrical stimulus.1 This colour change is caused by variations in the oxidation state of ECMs, accompanied by the insertion or extraction of ions into the ECM layer to balance the charge. ECMs can be classified as inorganic2–5 (e.g., metal oxides), organic6–8 (e.g., conjugated polymers), or hybrid inorganic–organic9–11 (e.g., metallo-polymers). Among these, electrochromic conjugated polymers (ECPs) have attracted considerable interest due to their high optical contrast and colouration efficiency, fast response, and broad colour range12–17 achievable through structural design. As a result, ECP-based ECDs have found applications in diverse fields, including smart windows,8 colour-changing fabrics,7 and adaptive camouflage.18,19 Interest in neutral-tint ECDs has increased due to their potential application in light-dimmable systems, eyewear, automobiles, and architecture, where black/grey-to-colourless transitions with minimal colour distortion are desirable.20,21 Although donor–acceptor (DA) ECPs are commonly used to obtain these transitions,22–24 they suffer from residual colour in the bleached state.25 Additionally, their complex synthesis, challenges with large-scale production, and cost hinder practical application.
An alternative approach involves combining two or more ECMs with complementary colours, achieving neutral hues through subtractive colour mixing.26,27 The careful selection of complementary ECMs can optimise the final hue by broadening the light absorption across the visible spectrum, resulting in a perceived neutral colouration.12,28–30 For instance, Sotzing et al. demonstrated colour neutrality achieved by subtractive colour mixing of either blue PEDOT31 or purple PProDOT electropolymerised in situ in combination with a yellow organic dye in the electrolyte.32 To eliminate potential issues related to the in situ polymerisation of complex ECMs, an alternative could involve the controlled deposition of the complementary ECMs onto separate electrodes. In this arrangement, the secondary ECM can serve as both a hue regulator for the primary ECM and a charge-balancing layer,33 simplifying ECD assembly. Complementary ECMs with opposing redox behaviours can create the desired subtractive colour mixing effect, ensuring that the display alternates between coloured and colourless states when an electrical potential or current is applied.
In this work, we present a hybrid grey-to-colourless switching ECD that combines a novel organic ECP (Red1), which transitions from red to colourless, with Prussian blue (PB), an inorganic material known for its reversible blue-to-colourless transition (Fig. 1). While Red1 bleaches upon oxidation, PB bleaches upon the reduction of [Fe(III)CN6]3− to [Fe(II)CN6]4−.34,35 This pairing leverages broadband absorption in the visible range (400–800 nm), with complementary redox behaviour, making it highly effective for neutral-tint ECDs while maintaining scalability and assembly simplicity.
Achieving a fully red-to-colourless, fast-switching ECP is a challenging task, as the high bandgap needed to produce a red colour makes it difficult to shift the bipolaronic transition to the NIR after oxidation.36–39 Reynolds and coworkers tackled this task by developing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 copolymer of an electron-rich bulky acyclic dioxythiophene (3,4-di(2-ethylhexyloxy)thiophene; DEHOT), with smaller monomers (3,4-di(methoxy)thiophene; DMOT).36 The electron-donating oxygenated substituents on the thiophene ring lower the oxidation potential and enable a transmissive oxidised state by stabilising a closed-shell bipolaron structure.40
1 copolymer of an electron-rich bulky acyclic dioxythiophene (3,4-di(2-ethylhexyloxy)thiophene; DEHOT), with smaller monomers (3,4-di(methoxy)thiophene; DMOT).36 The electron-donating oxygenated substituents on the thiophene ring lower the oxidation potential and enable a transmissive oxidised state by stabilising a closed-shell bipolaron structure.40
The branched alkyl groups enhance solubility and introduce steric effects that reduce the conjugation length. Additionally, these groups create an “open” morphology that facilitates ion transport through the film.36,37 However, the 2-ethylhexyl chains alone would introduce excessive steric repulsion in the ECP, resulting in an orange colour and slow response. Smaller monomers are needed to lower the band gap further and achieve a red hue. Additionally, side-chain entanglement can reduce interchain spacing, impeding rapid ion transport.
To address these issues and facilitate scalable synthesis for industrial applications, we designed a dioxythiophene monomer, 3,4-di(2-methylbutyloxy)thiophene (DMBOT; 1), that can polymerise into a red-coloured ECP (Red1; Fig. 1a) exhibiting a rapid red-to-colourless transition. The branched 2-methylbutyl side chain provides sufficient steric bulkiness to reduce conjugation length for the red hue while maintaining a transparent oxidised state and an open morphology to support efficient ion intercalation and fast switching.
Monomer 1 was synthesised according to published procedures,41 before undergoing ferric chloride (FeCl3) catalysed oxidative polymerisation42 to produce Red1 (see ESI†). Initially, a Red1/Red1 ECD with an active area of 1 cm2 was prepared to characterise the ECP (Fig. 2). Spectroelectrochemical analysis revealed the desired broad transmittance minimum centred at 500 nm (Fig. 2a), responsible for the red colour. Upon applying different cell voltages (±1.5 V), the absorbance of the ECD shifted bathochromically into the NIR region, with a clear isosbestic point observed at 610 nm (Fig. S6, ESI†). This behaviour is characteristic of polythiophenes26,43 and is attributed to the increased conjugation along the polymer backbone due to the formation of bipolarons during oxidation. The visible light transmittance (VLT, τv) of the dark (37%) and bleached (61%) state corresponds to a total modulation (Δτv) of 24%. The colour of the ECD was assessed using the CIELAB system, as described in DIN 5033.44 This ECD presented a significant increase in the a* value from the bleached (a* = −2.2) to the dark (a* = 27.9) state, indicative of its red colour. Switching times for both bleaching and colouring processes were evaluated using chronoamperometry while recording the voltage-induced colour change in transmittance at 500 nm (Fig. 2b). The switching time is defined as the time required to achieve 90% of the total transmittance change, in a given timeframe. The determined values at ± 1.5 V were 1.8 s for colouring and 3.8 s for bleaching, highlighting how Red1 can achieve a high optical contrast with fast switching.
 | ||
| Fig. 2 (a) Spectroelectrochemical characterization and (b) switching time determination of the Red1/Red1 ECD at cell voltage ± 1.5 V. | ||
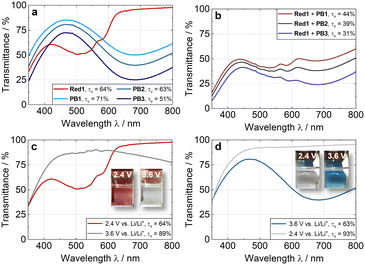
After evaluating the EC properties of Red1, we investigated its combination with PB counter electrodes presenting different thicknesses (PB1, PB2, and PB3) to achieve a neutral-tint ECD (Fig. 3a and b). PB thin-film electrodes were prepared on ITO-coated PET substrates (see ESI†), and their transmittance spectra were measured (Fig. 3a). The light blue PB films exhibited a broad intervalence charge transfer (IVCT) band centred around 700 nm, characteristic of mixed-valence compounds.45 By adding the absorbance of the PB films to that of Red1, the predicted transmittance of the resulting ECDs was calculated (Fig. 3b). Based on these calculations, we determined the L*a*b* values for the various electrode combinations (Table S2, ESI†). Among these, the ECD using PB2 as counter electrode is expected to achieve the lowest a* and b* colour coordinates, indicating a near-neutral tint. Based on these results, we evaluated the individual EC performance of the Red1 and PB2 electrodes in an electrochemical half-cell with Li as the counter electrode (CE) and reference electrode (RE), using 1 M lithium bis(trifluoromethane)sulfonimide (LiTFSI) in propylene carbonate (PC) as the electrolyte (Fig. 3c and d). For Red1, the broad transmittance band (λmax = 504 nm) increased upon oxidation at 3.6 V vs. Li/Li+ and reduced upon reduction at 2.4 V vs. Li/Li+ (cathodically colouring, Fig. 3c), while PB2 exhibited a colour change between 2.4 V and 3.6 V vs. Li/Li+ (anodically colouring), observable by the broad IVCT transition at around 685 nm after oxidation (Fig. 3d).
The τv value [dark/bleached in %] changed between 63/89 and 63/93 for Red1 and PB2, respectively (Table 1). Notably, both electrodes reached an almost completely colourless bleached state, with a*/b* values of −1.0/3.6 for Red1 and −0.9/−3.1 for PB2. Additionally, both electrodes were analysed using cyclic voltammetry (CV), charging/discharging, and cycle stability measurements over 1000 switching cycles. Red1 exhibited anodic and cathodic peak potentials, Epa and Epc, corresponding to the oxidation (bleaching) and reduction (colouring) processes at 3.36 V and 3.51 V vs. Li/Li+, respectively, at a scan rate of 10 mV s−1 (Fig. S8, ESI†). For PB2, the anodic and cathodic peak potentials appeared at 2.78 V and 3.04 V vs. Li/Li+ (Fig. S8, ESI†). No side reactions were observed within the applied potential window (2.4–3.6 V).
| EC electrodes | Potential/V vs. Li/Li+ | L* | a* | b* | τ v/% | Δτv/% | η/cm2 C−1 |
|---|---|---|---|---|---|---|---|
| Red1 | 2.4 | 83.0 | 17.7 | 4.8 | 63 | 26 | 359 |
| 3.6 | 95.3 | −1.0 | 3.6 | 89 | |||
| PB2 | 2.4 | 97.0 | −0.9 | 3.1 | 93 | 30 | 78 |
| 3.6 | 84.7 | −12.8 | −10.3 | 63 |
Charge densities determined by galvanostatic measurements (Fig. S9, ESI†) revealed a maximum charge density of 0.62 mC cm−2 for Red1 and 4.87 mC cm−2 for PB2 at a current density of 1 μA cm−2. Another key performance indicator for ECMs is the colouration efficiency (η), which is calculated using the equation: η = log(Tb/Td)/q, where Tb and Td are the transmittance values in the bleached and dark states, respectively, and q is the charge density. The η values for Red1 and PB2 were determined to be 359 cm2 C−1 (at 504 nm) and 78 cm2 C−1 (at 685 nm), respectively. The CV (Fig. S8, ESI†) and charge/discharge measurements of the Red1 electrodes confirmed the high reversibility of the bleaching and colouring processes (Fig. 4a). At a current density of 50 μA cm−2, the Coulombic efficiency (defined as the ratio of discharge to charge density) exceeded 92%. Cycling tests of the individual electrodes in inert conditions (see ESI†) revealed that after 1000 charge/discharge cycles between 2.4 V and 3.6 V vs. Li/Li+, the Red1 electrode retained over 84% of its initial charge (0.67 mC cm−2 → 0.56 mC cm−2). A similar evaluation of the PB2 electrode showed a Coulombic efficiency above 97% (Fig. 4b), with charge retention over 88% (4.69 mC cm−2 → 4.13 mC cm−2), indicating stable redox behaviour.
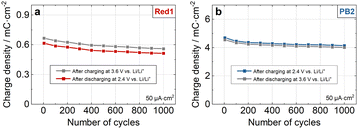 | ||
| Fig. 4 Charge/discharge measurements over 1000 switching cycles for (a) Red1 and (b) PB2, performed at 50 μA cm2. | ||
Finally, the Red1 and PB2 electrodes were assembled into a grey-to-colourless ECD (Red1/PB2) with an active area of 5 × 5 cm2. The device was fabricated using a sheet-to-sheet assembly process (see ESI†), using a proprietary LiTFSI-containing gel electrolyte.46 Spectroelectrochemical analysis of the device revealed that in its dark state, a grey colour can be achieved at a cell voltage of −2.0 V (Fig. 5). This grey hue results from the PB2 counter electrode absorbing in the 600–800 nm region, which perfectly complements the lower wavelength absorption (400–600 nm) of the cathodically colouring Red1 (Fig. 5a). The measured colour coordinates of the dark (a*/b* = −5.3/−4.7) and bleached state (a*/b* = −3.2/−0.5) closely match the calculated values and confirm the neutral colouration. The ECD could be reversibly switched to a colourless state at 2.0 V, with τv values of 29% in the dark state and 63% in the bleached state. The switching time of the ECD, determined from the τv-time profile shown in Fig. 5b, was around 11 s for bleaching and 9 s for colouring, highlighting the device's suitability for EC applications requiring fast response. The relevant optical properties and switching times are summarised in Table 2. Further characterization by CV (Fig. S10, ESI†), showed well-defined, reversible redox peaks at cell voltages of approximately 0.76 V (bleaching) and 0.55 V (colouring) at a scan rate of 10 mV s−1. Moreover, the scan-rate-dependent CVs (10–100 mV s−1) indicate that the oxidative and reductive peak currents are linearly proportional to the square root of the scan rate, i.e. the bleaching/colouring processes are diffusion-controlled.
 | ||
| Fig. 5 (a) Spectroelectrochemical characterization and (b) switching time determination of the Red1/PB2 ECD at cell voltage ± 2 V. | ||
| Full Cell | Cell voltage/V | L* | a* | b* | τ v/% | Δτv/% | Switching time/s |
|---|---|---|---|---|---|---|---|
| Red1/PB2 | 2.0 | 83.6 | −3.2 | −0.5 | 29 | 34 | 11 |
| −2.0 | 61.7 | −5.3 | −4.7 | 63 | 9 |
In summary, we developed a simple and scalable method for producing grey-to-colourless switching ECDs. A novel red ECP (Red1) was designed and straightforwardly synthesised, presenting a fast red-to-colourless transition and cycling resilience within flexible ECDs. By combining Red1 with PB for complementary switching, we achieved a hybrid grey-to-colourless switching ECD with a VLT modulation of 34% and switching times of approximately 10 s (active area 5 × 5 cm2). This approach shifts grey-to-colourless ECD development from synthetic challenges to an assembly-focused strategy, enhancing the potential of electrochromic technology for cost-effective applications.
This work was supported by the European Union's Horizon 2020 research and innovation program (grant no. 760973, DecoChrom). DB, LM and RF further acknowledge the University of Vienna for funding and Dong Min Lee and Dr Sayan Sarkar for the NMR, HRMS and GPC analysis of 1 and Red1 polymer. The authors would also like to thank Christine Müller for the preparation of the PB electrodes and hybrid ECDs.
Data availability
The data supporting this article have been included in the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- Electrochromic materials and devices, ed. D. R. Rosseinsky, P. M. S. Montk and R. J. Mortimer, Wiley-VCH, Weinheim, 2015 Search PubMed.
- G. A. Niklasson and C. G. Granqvist, J. Mater. Chem., 2007, 17, 127–156 RSC.
- L. Niklaus, M. Schott, U. Posset and G. A. Giffin, ChemElectroChem, 2020, 7, 3274–3283 CrossRef CAS.
- A. Paolella, C. Faure, V. Timoshevskii, S. Marras, G. Bertoni, A. Guerfi, A. Vijh, M. Armand and K. Zaghib, J. Mater. Chem. A, 2017, 5, 18919–18932 RSC.
- C. G. Granqvist, Thin Solid Films, 2014, 564, 1–38 Search PubMed.
- K. Madasamy, D. Velayutham, V. Suryanarayanan, M. Kathiresan and K.-C. Ho, J. Mater. Chem. C, 2019, 7, 4622–4637 Search PubMed.
- W. M. Kline, R. G. Lorenzini and G. A. Sotzing, Color. Technol., 2014, 130, 73–80 CAS.
- R. J. Mortimer, A. L. Dyer and J. R. Reynolds, Displays, 2006, 27, 2–18 CrossRef CAS.
- L. Niklaus, M. Schott, M. Mihelčič, I. Jerman, U. Posset and G. Sextl, Sol. Energy Mater. Sol. Cells, 2019, 200, 110002 CrossRef CAS.
- M. Schott, H. Lorrmann, W. Szczerba, M. Beck and D. G. Kurth, Sol. Energy Mater. Sol. Cells, 2014, 126, 68–73 CrossRef CAS.
- M. Schott, L. Niklaus, J. Clade and U. Posset, Sol. Energy Mater. Sol. Cells, 2019, 200, 110001 CrossRef CAS.
- R. H. Bulloch, J. A. Kerszulis, A. L. Dyer and J. R. Reynolds, ACS Appl. Mater. Interfaces, 2015, 7, 1406–1412 Search PubMed.
- J. A. Kerszulis, K. E. Johnson, M. Kuepfert, D. Khoshabo, A. L. Dyer and J. R. Reynolds, J. Mater. Chem. C, 2015, 3, 3211–3218 Search PubMed.
- P. M. Beaujuge, S. Ellinger and J. R. Reynolds, Adv. Mater., 2008, 20, 2772–2776 CrossRef CAS PubMed.
- A. Cihaner and F. Algı, Adv. Funct. Mater., 2008, 18, 3583–3589 CrossRef CAS.
- L. You, J. He and J. Mei, Polym. Chem., 2018, 9, 5262–5267 RSC.
- M. Schott, L. Niklaus, S. Janietz, C. Völkel, T. Egorov-Brening and T. Bilkay-Troni, Polymers, 2024, 16, 799 CrossRef CAS PubMed.
- H. Fu, L. Zhang, Y. Dong, C. Zhang and W. Li, Mater. Chem. Front., 2023, 7, 2337–2358 RSC.
- J. Wang, L. Zhang, W. Zhan, J. Dong, Y. Ma, J. Li, W. Li and C. Zhang, Chem. Eng. J., 2024, 483, 149078 CrossRef CAS.
- M. Sassi, M. M. Salamone, R. Ruffo, G. E. Patriarca, C. M. Mari, G. A. Pagani, U. Posset and L. Beverina, Adv. Funct. Mater., 2016, 26, 5240–5246 CrossRef CAS.
- V. Rai, R. S. Singh, D. J. Blackwood and D. Zhili, Adv. Eng. Mater., 2020, 22, 2000082 CrossRef CAS.
- G. Sonmez, C. K. F. Shen, Y. Rubin and F. Wudl, Angew. Chem., 2004, 116, 1524–1528 CrossRef.
- S. Ozdemir, M. Sendur, G. Oktem, Ö. Doğan and L. Toppare, J. Mater. Chem., 2012, 22, 4687 RSC.
- H. Yue, S. Ming, H. Du, J. Zhao and Y. Zhang, Eur. Polym. J., 2022, 175, 111395 CrossRef CAS.
- A. M. Österholm, L. Nhon, D. E. Shen, A. M. Dejneka, A. L. Tomlinson and J. R. Reynolds, Mater. Horiz., 2022, 9, 252–260 RSC.
- P. M. Beaujuge and J. R. Reynolds, Chem. Rev., 2010, 110, 268–320 CrossRef CAS PubMed.
- K.-R. Lee and G. A. Sotzing, Chem. Commun., 2013, 49, 5192–5194 RSC.
- P. Chandrasekhar, B. J. Zay, C. Cai, Y. Chai and D. Lawrence, J. Appl. Polym. Sci., 2014, 131, 41043 Search PubMed.
- R. H. Bulloch, J. A. Kerszulis, A. L. Dyer and J. R. Reynolds, ACS Appl. Mater. Interfaces, 2014, 6, 6623–6630 CrossRef CAS PubMed.
- X. Yu, M. Chang, W. Chen, D. Liang, X. Lu and G. Zhou, ACS Appl. Mater. Interfaces, 2020, 12, 39505–39514 CrossRef PubMed.
- Y. Zhu, M. T. Otley, A. Kumar, M. Li, X. Zhang, C. Asemota and G. A. Sotzing, Chem. Commun., 2014, 50, 8167–8170 RSC.
- M. Li, O. A. Yassin, M. L. Baczkowski, X. Zhang, R. Daniels, A. A. Deshmukh, Y. Zhu, M. T. Otley and G. A. Sotzing, Org. Electron., 2020, 84, 105748 CrossRef CAS.
- J. Jensen, M. Hösel, A. L. Dyer and F. C. Krebs, Adv. Funct. Mater., 2015, 25, 2073–2090 CrossRef CAS.
- M. Schott, L. Niklaus, C. Müller, B. Bozkaya and G. A. Giffin, Mater. Adv., 2021, 2, 4659–4666 RSC.
- P. Andersson Ersman, U. Boda, I. Petsagkourakis, J. Åhlin, U. Posset, M. Schott and R. Brooke, Adv. Eng. Mater., 2023, 25, 2201299 CrossRef CAS.
- A. L. Dyer, M. R. Craig, J. E. Babiarz, K. Kiyak and J. R. Reynolds, Macromolecules, 2010, 43, 4460–4467 Search PubMed.
- X. Chen, Z. Xu, S. Mi, J. Zheng and C. Xu, New J. Chem., 2015, 39, 5389–5394 RSC.
- G. Atakan and G. Gunbas, RSC Adv., 2016, 6, 25620–25623 RSC.
- Z. Xu, X. Chen, S. Mi, J. Zheng and C. Xu, Org. Electron., 2015, 26, 129–136 Search PubMed.
- C. Lin, T. Endo, M. Takase, M. Iyoda and T. Nishinaga, J. Am. Chem. Soc., 2011, 133, 11339–11350 CrossRef CAS PubMed.
- S. Rajappa and A. R. Deshmukh, in Comprehensive heterocyclic chemistry III, ed. A. R. Katritzky, Elsevier, Amsterdam, 2009, vol. 4, pp. 741–841 Search PubMed.
- M. A. Ochieng, J. F. Ponder and J. R. Reynolds, Polym. Chem., 2020, 11, 2173–2181 RSC.
- A. J. Heeger, R. Pethig, R. J. Gillespie and P. Day, Philos. Trans. R. Soc., A, 1985, 314, 17–35 CrossRef.
- Deutsches Institut für Normung, e.V. DIN 5033-1:2017-10, Colorimetry – Part 1: Basic terms of colorimetry, Beuth Verlag, Berlin, 2017 Search PubMed.
- T. Dumas, D. Guillaumont, P. Moisy, D. K. Shuh, T. Tyliszczak, P. L. Solari and C. D. Auwer, Chem. Commun., 2018, 54, 12206–12209 RSC.
- U. Posset, B. Herbig, G. Schottner, K. Zaghib, J.-F. Labrecque, A. Guerfi, M. Perrier, R. Ruffo, M. Salamone, C. Mari, L. Beverina and G. Pagani, EU Pat., 2570846A1, 2011 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc06804a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |