Open Access Article
Open Access ArticleSpontaneous adsorption of iridium chloride complex on oxychloride photocatalysts provides efficient and durable reaction site for water oxidation†
Hajime
Suzuki
 *a,
Kengo
Minamimoto
a,
Yusuke
Ishii
*a,
Kengo
Minamimoto
a,
Yusuke
Ishii
 a,
Yudai
Furuta
a,
Osamu
Tomita
a,
Yudai
Furuta
a,
Osamu
Tomita
 a,
Akinobu
Nakada
a,
Akinobu
Nakada
 ab,
Shunsuke
Nozawa
c and
Ryu
Abe
ab,
Shunsuke
Nozawa
c and
Ryu
Abe
 *a
*a
aDepartment of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto 615-8510, Japan
bPrecursory Research for Embryonic Science and Technology (PRESTO), Japan Science and Technology Agency (JST), 4-1-8 Honcho, Kawaguchi, Saitama 332-0012, Japan
cPhoton Factory (PF), Institute of Materials Structure Science (IMSS), High Energy Accelerator Research Organization (KEK), Tsukuba, Ibaraki 305-0801, Japan
First published on 23rd January 2025
Abstract
The visible-light-driven O2 evolution on oxychloride photocatalysts, such as Bi4NbO8Cl, was significantly enhanced by stirring in an aqueous solution containing IrCl63− in the dark. Various characterizations indicated that highly dispersed IrOxHyClz-like species spontaneously formed on the oxychloride surface, serving as effective and stable cocatalysts for enhancing O2 evolution.
Water splitting using semiconductor photocatalysts is a promising approach to clean hydrogen production.1–5 Till now, ultraviolet (UV)-light-driven water splitting with a quantum efficiency of almost 100% has been achieved using SrTiO3-based photocatalysts.6 Large-scale photocatalytic hydrogen production tests are also underway.7 However, since UV light accounts for only a small fraction of the solar spectrum, developing photocatalysts capable of harvesting visible light, which constitutes nearly half of sunlight, is necessary to achieve practically high efficiencies.
Mixed-anion compounds, such as oxynitrides and oxysulfides, have been developed to address this requirement.8 These photocatalyst materials have the valence band maximum (VBM) composed of their N-2p or S-3p orbitals hybridized with O-2p. The contribution of these orbitals to the VBM reduces the bandgap and enables visible light absorption. However, these materials often suffer from self-oxidative deactivation by photogenerated holes during water splitting. Recently, Sillén(-Aurivillius)-type layered oxyhalides, such as Bi4NbO8Cl, have emerged as promising photocatalysts for visible-light-driven water splitting (Fig. 1).9,10 These materials feature a unique valence band structure, in which elevated O-2p orbitals primarily contribute to the VBM. This structure enables photogenerated holes to be preferentially consumed by water oxidation, minimizing self-oxidation and providing remarkable stability as O2 evolution photocatalysts in Z-scheme water-splitting systems.11,12
 | ||
| Fig. 1 Crystal structures of Sillén(-Aurivillius)-type layered oxyhalide photocatalysts: Bi4MO8Cl (M = Nb, Ta), Bi2REO4Cl (RE = rare earth), and SrBi3O4Cl3. | ||
Previous efforts to enhance photocatalytic activity have included the exploration of related oxyhalide materials and synthetic methods such as flux-assisted synthesis.13,14 However, further exploration of their surface engineering is needed, particularly for loading effective water oxidation cocatalysts. Unlike the well-studied surfaces of metal oxides, layered oxyhalides possess more complex surfaces with volatile halogen species, requiring innovative surface modification strategies to improve both charge transfer and surface reactions.
While exploring effective cocatalysts and their loading methods to enhance O2 evolution on the representative oxychloride photocatalyst Bi4NbO8Cl, we discovered that highly dispersed IrOxHyClz species were spontaneously formed by simply stirring Bi4NbO8Cl particles in an aqueous Na3IrCl6 solution in the dark. This novel adsorption method significantly enhanced the O2 evolution rate under visible light irradiation and proved effective for various oxyhalide photocatalysts.
The oxyhalide photocatalyst Bi4NbO8Cl was synthesized using the previously reported flux method.13 X-ray diffraction (XRD) analysis, scanning electron microscopy (SEM), and diffuse reflectance spectroscopy confirmed the successful synthesis of single-phase, plate-like particles of Bi4NbO8Cl (Fig. S1, ESI†). Initially, various metal oxide species (RuO2, CoOx, and IrO2, each with 0.5 wt% metal content) were loaded onto Bi4NbO8Cl via a conventional impregnation (IMP) method. The samples were then calcinated at 450 °C in air, and their O2 evolution activities were evaluated. As summarized in Fig. S2 (ESI†), the loading of IrO2 provided the highest enhancement, increasing the O2 evolution rate approximately 11-fold compared to unmodified Bi4NbO8Cl. RuO2 and CoOx also increased the rate to some extent.13,15 Then, loading of IrO2 cocatalysts was carried out by employing various methods, including colloidal adsorption (COL), microwave-assisted (MW) method, and photodeposition (PD) method, all of which have reportedly been effective for loading IrO2 onto metal oxides and oxynitride photocatalysts.16–18 Notably, the PD method was proposed to provide IrO2 species through oxidation of IrIIICl63− precursor to IrIVO2 by photogenerated holes on the photocatalyst, accompanied by nitrate ion reduction (e.g., NO3− + 2H+ + 2e− → NO2− + H2O) by photoexcited electrons.18
Additionally, we introduced the simple adsorption (ADS) method as a control test for the PD method. In this approach, the photocatalyst particles were suspended in aqueous Na3IrCl6 solution (in the absence of NO3−) in the dark.
Fig. 2 shows the O2 evolution rates of various Bi4NbO8Cl samples (0.1 g) in an aqueous AgNO3 solution (8 mM, 100 mL) under visible light irradiation (λ > 400 nm) from a 300-W Xe-arc lamp. Among the tested methods, PD achieved the highest O2 evolution rate, outperforming the IMP method. The COL and MW methods showed slightly higher rates than the bare sample but considerably lower values than IMP. Notably, the newly introduced ADS method, in which Bi4NbO8Cl particles were stirred in an aqueous Na3IrCl6 solution under dark conditions, achieved a rate comparable to that of PD, the highest among all the methods. The O2 evolution rate of the ADS sample remained steady until the Ag+ ions in the solution were completely consumed (Fig. S3, ESI†). Attempts to apply the ADS method with other metal species (e.g., Ru and Co) did not significantly enhance the O2 evolution rates (Fig. S4, ESI†), indicating that the enhancement is unique to the Ir species. The ADS sample also exhibited higher activity than the bare sample, not only with the sacrificial electron acceptors (Ag+) but also with the reversible electron acceptors (Fe3+ and polyoxometalate [SiVVW11O40]5−) (Fig. S5, ESI†). These findings highlight the potential of such modified oxyhalide photocatalysts in Z-scheme water-splitting systems with redox mediators.
The Ir species loaded via ADS and other methods were characterized using various techniques. Inductively coupled plasma (ICP) spectroscopy measurements indicated that most of the introduced Ir species (0.5 wt% metal content) were successfully loaded onto the surface of Bi4NbO8Cl, regardless of the loading method (Fig. S6, ESI†). The driving force for the spontaneous adsorption of Ir species is likely related to the electrostatic attraction between the Bi4NbO8Cl particles and the IrCl63− precursor. The pH of the solution during the ADS method was measured to be around 5. At pH 5, the Bi4NbO8Cl particles are positively charged,19 suggesting that the electrostatic attraction between them possibly promotes the adsorption of Ir species. Fig. 3 and Fig. S7 (ESI†) show scanning transmission electron microscope (STEM) images of each Bi4NbO8Cl sample. For the ADS sample (Fig. 3), identifying the Ir species was almost impossible, suggesting highly dispersed, fine particles or clusters on the surface. Alternatively, Ir species are distinctly evident in the MW sample but are indistinguishable in the PD sample, as shown in Fig. S7 (ESI†). The IMP sample exhibited much smaller Ir species compared to those from the MW and COL methods. These observations imply that Ir species with high dispersion and small size are critical for enhancing O2 evolution on Bi4NbO8Cl photocatalysts.
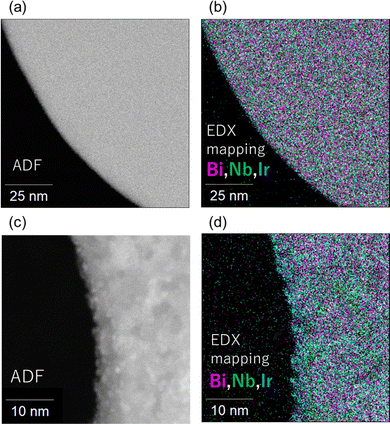 | ||
| Fig. 3 (a) and (c) STEM image and (b) and (d) EDX mapping of Bi4NbO8Cl loaded with Ir species via ADS (a) and (b) and MW (c) and (d) method. | ||
The X-ray absorption fine structure (XAFS) results are shown in Fig. 4 and Fig. S8 (ESI†). The X-ray absorption near-edge structure (XANES) spectra (Fig. S8, ESI†) revealed that the loaded Ir species predominantly existed in the +4 oxidation state across all loading methods. In the extended XAFS (EXAFS) spectra (Fig. 4), the Ir species loaded using the COL, MW, and IMP methods showed only Ir–O bonds in the first coordination shell, indicating that the Ir species existed as oxides or hydroxides, as previously reported. In contrast, the samples prepared via ADS and PD methods exhibited both Ir–O and Ir–Cl bonds, indicating the formation of IrOxHyClz species, which are effective cocatalysts for O2 evolution. The PD method was originally proposed to deposit IrO2 on a photocatalyst through the oxidation of IrCl63− by photogenerated holes, accompanied by the reduction of NO3− by photoexcited electrons under light irradiation.18 However, the present ADS method achieved comparably high activity than the PD method without light or electron acceptor. Additionally, the states of the Ir species loaded via ADS and PD were almost identical, as confirmed by STEM and XAFS measurements. Thus, for both methods, the IrOxHyClz species are probably loaded on the oxyhalide through the partial hydrolysis of IrCl63− in water.20,21 Notably, the EXAFS spectrum of the ADS-loaded sample post-photocatalytic O2 evolution still showed Ir–Cl bonds, indicating stability under the photocatalytic reaction conditions (Fig. S9, ESI†). These findings indicate that active Ir species were spontaneously formed from IrCl63− on the Bi4NbO8Cl surface during stirring in the dark, for both the PD and ADS methods.
 | ||
| Fig. 4 Fourier-transformed Ir L3-edge EXAFS spectra of Bi4NbO8Cl loaded with Ir species via various methods (COL, MW, IMP, PD, and ADS), along with those of reference samples. | ||
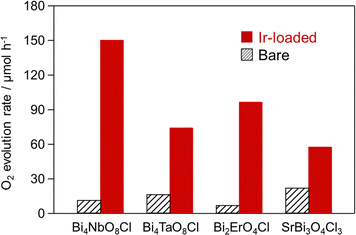
We applied this novel ADS method to other oxychloride photocatalysts, including Bi4TaO8Cl, Bi2ErO4Cl, and SrBi3O4Cl3 (Fig. 1 and Fig. S10, ESI†). Fig. 5 shows the O2 evolution rates of these oxyhalide photocatalysts with Ir species loaded using the ADS method. The time course of O2 evolution is shown in Fig. S11 (ESI†). Ir loading significantly improved the O2 evolution rates for both Sillén-type (Bi2ErO4Cl and SrBi3O4Cl3) and Sillén-Aurivillius-type (Bi4NbO8Cl and Bi4TaO8Cl) oxychlorides. In contrast, applying the ADS method to oxide-based (BiVO4 and WO3) and oxynitride-based (TaON) photocatalysts negligibly improved the O2 evolution (Fig. S12, ESI†). This distinction indicates that the IrCl63− complex possibly interacts with the surface of oxychlorides, facilitating the formation of highly active Ir species and/or enabling efficient charge transfer, thereby significantly enhancing O2 evolution rates. As shown in Fig. S13 (ESI†), using Na2IrCl6 significantly enhances O2 evolution similar to Na3IrCl6 on Bi4NbO8Cl, whereas Ir(acac)3 without Cl anions yields less pronounced improvement. These findings underscore the critical role of surface Cl interactions on oxychlorides. Further investigation is required to elucidate the mechanisms underlying this activity enhancement. Nonetheless, the novel ADS method is a mild, simple, and versatile method for loading active and stable Ir species, significantly enhancing the O2 evolution rates of various oxychloride photocatalysts.
In summary, this study demonstrated the potential of the novel ADS method as a simple, mild, and versatile approach for enhancing the visible-light-driven O2 evolution activity of oxyhalide photocatalysts by loading highly dispersed and active Ir species. This method effectively improved the performance of not only a representative oxyhalide Bi4NbO8Cl but also of various other oxyhalide photocatalysts through simple stirring in an aqueous Ir precursor solution. The success of the ADS method, particularly with oxyhalides rather than with conventional oxides and oxynitrides, suggests the importance of Cl-mediated interactions in facilitating effective cocatalyst deposition and/or charge transfer. These findings provide valuable insights for the development of tailored cocatalysts and their loading techniques to optimize the photocatalytic efficiency of mixed-anion photocatalysts.
This work was supported by JSPS KAKENHI (JP20H00398 and JP23H02061) through Grants-in-Aid for Scientific Research (A) and (B), respectively. This study was also supported by the JSPS Core-to-Core Program (JPJSCCA20200004), Kansai Research Foundation for Technology Promotion, and ENEOS Tonengeneral Research/Development Encouragement & Scholarship Foundation. Part of this study was supported by the Advanced Characterization Platform and AIST Nanocharacterization Facility (ANCF) Platform as a program of the “Nanotechnology Platform” (JPMXP1223KU0001). The XAFS experiments were performed with the approval of the Photon Factory Program Advisory Committee (Proposal No. 2024G638). The authors would like to acknowledge Dr Rie Haruki of the High Energy Accelerator Research Organization (KEK) for assistance with the XAFS measurements. We are also grateful to Mr Takaaki Toriyama of Kyushu University for his support with STEM analysis.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed
.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC
.
- R. Abe, Bull. Chem. Soc. Jpn., 2011, 84, 1000–1030 CrossRef CAS
.
- F. E. Osterloh, Chem. Soc. Rev., 2013, 42, 2294–2320 RSC
.
- S. S. Chen, T. Takata and K. Domen, Nat. Rev. Mater., 2017, 2, 17050 CrossRef CAS
.
- T. Takata, J. Jiang, Y. Sakata, M. Nakabayashi, N. Shibata, V. Nandal, K. Seki, T. Hisatomi and K. Domen, Nature, 2020, 581, 411–414 CrossRef CAS PubMed
.
- H. Nishiyama, T. Yamada, M. Nakabayashi, Y. Maehara, M. Yamaguchi, Y. Kuromiya, H. Tokudome, S. Akiyama, T. Watanabe, R. Narushima, S. Okunaka, N. Shibata, T. Takata, T. Hisatomi and K. Domen, Nature, 2021, 598, 304–307 CrossRef CAS PubMed
.
- A. Miyoshi and K. Maeda, Solar RRL, 2020, 5, 2000521 CrossRef
.
- H. Fujito, H. Kunioku, D. Kato, H. Suzuki, M. Higashi, H. Kageyama and R. Abe, J. Am. Chem. Soc., 2016, 138, 2082–2085 CrossRef CAS PubMed
.
- H. Suzuki, H. Kunioku, M. Higashi, O. Tomita, D. Kato, H. Kageyama and R. Abe, Chem. Mater., 2018, 30, 5862–5869 CrossRef CAS
.
- H. Kunioku, M. Higashi, O. Tomita, M. Yabuuchi, D. Kato, H. Fujito, H. Kageyama and R. Abe, J. Mater. Chem. A, 2018, 6, 3100–3107 RSC
.
- D. Kato, K. Hongo, R. Maezono, M. Higashi, H. Kunioku, M. Yabuuchi, H. Suzuki, H. Okajima, C. C. Zhong, K. Nakano, R. Abe and H. Kageyama, J. Am. Chem. Soc., 2017, 139, 18725–18731 CrossRef CAS PubMed
.
- K. Ogawa, A. Nakada, H. Suzuki, O. Tomita, M. Higashi, A. Saeki, H. Kageyama and R. Abe, ACS Appl. Mater. Interface, 2019, 11, 5642–5650 CrossRef CAS PubMed
.
- Y. Ishii, H. Suzuki, K. Ogawa, O. Tomita, A. Saeki and R. Abe, Sustainable Energy Fuels, 2022, 6, 3263–3270 RSC
.
- S. Chang, L. Shi, J. Yu, R. Wang, X. Xu and G. Liu, Appl. Catal., B, 2023, 328, 122541 CrossRef CAS
.
- K. Maeda and K. Domen, Angew. Chem., Int. Ed., 2012, 51, 9865–9869 CrossRef CAS PubMed
.
- K. Chen, J. Xiao, J. J. M. Vequizo, T. Hisatomi, Y. Ma, M. Nakabayashi, T. Takata, A. Yamakata, N. Shibata and K. Domen, J. Am. Chem. Soc., 2023, 145, 3839–3843 CrossRef CAS PubMed
.
- A. Iwase, H. Kato and A. Kudo, Chem. Lett., 2005, 34, 946–947 CrossRef CAS
.
- K. Ogawa, R. Sakamoto, C. Zhong, H. Suzuki, K. Kato, O. Tomita, K. Nakashima, A. Yamakata, T. Tachikawa, A. Saeki, H. Kageyama and R. Abe, Chem. Sci., 2022, 13, 3118–3128 RSC
.
- J. C. Chang and C. S. Garner, Inorg. Chem., 1965, 4, 209–215 CrossRef CAS
.
- K.-C. Tso, T.-Y. Chan, T.-C. Yu, Y.-J. Tao, C.-Y. Chu, S.-Y. Chen, J.-F. Lee, J. Ohta, P.-C. Chen and P.-W. Wu, Surf. Interfaces, 2024, 44, 103785 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: XRD pattern, diffuse reflectance spectrum, SEM image, photocatalytic activity, STEM image, XAFS spectrum. See DOI: https://doi.org/10.1039/d4cc06683a |
| This journal is © The Royal Society of Chemistry 2025 |