Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metal catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides: structural diversity at the dipole partner
Juan Carlos
Carretero
 ab,
Nuria
Rodríguez
ab,
Nuria
Rodríguez
 *ab and
Javier
Adrio
*ab and
Javier
Adrio
 *ab
*ab
aDepartamento de Química Orgánica, Facultad de Ciencias, Universidad Autónoma de Madrid, Cantoblanco, 28049, Madrid, Spain. E-mail: n.rodriguez@uam.es; Javier.adrio@uam.es
bInstitute for Advanced Research in Chemical Sciences (IAdChem) and Center for Innovation in Advanced Chemistry (ORFEO-CINQA), Spain
First published on 4th February 2025
Abstract
The 1,3-dipolar cycloaddition of azomethine ylides represents a versatile approach for synthesizing pyrrolidines, valuable structural motifs in synthetic and medicinal chemistry. However, most studies to date have relied predominantly on α-iminoesters as ylide precursors, thereby limiting the broader synthetic applications of this strategy. This feature article highlights alternative azomethine ylide precursors, beyond conventional α-iminoesters, which have facilitated the preparation of pyrrolidines with new subtitution patterns.
1 Introduction
Cycloaddition reactions are fundamental in organic chemistry, providing a primary method for the preparation of complex cyclic molecules with multiple stereocenters.1 In particular, 1,3-dipolar cycloadditions offer efficient stereocontrolled approaches for synthesizing different heterocyclic compounds.2 For instance, the use of azomethine ylides as dipole partners enables the effective synthesis of chiral polysubstituted pyrrolidines.3 Pyrrolidine is a privileged heterocycle in organic and medicinal chemistry, as many of its derivatives exhibit a wide range of bioactivities, including antibiotic, antibacterial, anticancer, and antihypertensive properties. Consequently, pyrrolidine derivatives provide an excellent opportunity for the potential discovery of new pharmaceutical agents.4 In addition, chiral pyrrolidines can be used as optimal scaffolds for ligand design in both transition metal-mediated and organocatalytic processes.5In recent decades, metal-catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides has emerged as one of the most widely employed methodologies for the enantioselective synthesis of pyrrolidines.6 A common procedure involves the in situ generation of the azomethine ylide from the corresponding α-iminoester in combination with a transition metal salt and a base. The high efficacy of this approach arises from the N,O-coordination of the α-iminoester to the metal forming a five-membered chelate ring. This coordination significantly enhances the acidity of the proton at the α position of the ester group and also fixes the W-conformation of the 1,3-dipole, which determines the usual 2,5-cis configuration of the final pyrrolidine. Furthermore, if a chiral ligand is used, the formation of a rigid five-membered chelate ring facilitates asymmetric induction, allowing for the preparation of enantioenriched pyrrolidines (Scheme 1).
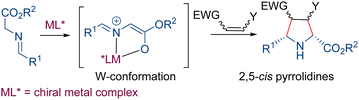 | ||
| Scheme 1 α-Iminoesters as dipole precursors in catalytic asymmetric 1,3-dipolar cycloaddition reactions. | ||
The first example of this kind of process was reported by Grigg and co-workers7 in 1991, using stoichiometric amounts of a cobalt salt and a chiral ligand derived from ephedrine, and the reaction proceeded with high yield and enantioselectivity. Inspired by this work, the first catalytic examples of this cycloaddition were reported by the groups of Zhang8 and Jorgensen9 in 2002, employing Zn- and Ag-based catalytic systems, respectively.
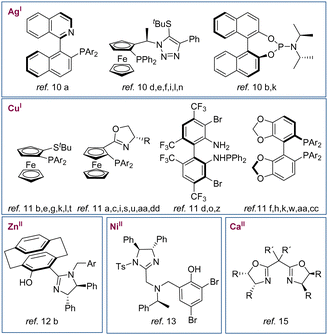
Since these early papers, the effort of many research groups has been focussed on the development of new catalyst systems. As a result, many Lewis acids have been described based on the combination of different metals (such as Ag,10 Cu,11 Zn,12 Ni,13 Au14 and Ca15) and a wide variety of ligands with central, axial or planar chirality (Fig. 1). In addition, efficient organocatalysts have also been developed.16
The use of these catalyst systems has facilitated the preparation of proline derivatives with high reactivity and impressive levels of diastereo- and enantioselectivity. Two diastereomers can form depending on the endo or exo approach of the 1,3-dipole to the dipolarophile. Similar to the Diels–Alder reaction, the endo approach is typically favored by secondary orbital interactions. However, these stabilizing interactions are generally much weaker in 1,3-dipolar cycloadditions, making diastereoselectivity challenging to predict in most cases. As a general trend, silver-catalyzed 1,3-dipolar cycloadditions tend to exhibit greater endo selectivity compared to copper-catalyzed reactions.
Azomethine ylides generated from iminoesters, due to their electron-rich nature, preferentially react with electron-deficient dipolarophiles. The excellent regioselectivity observed in these reactions is attributed to the dominant FMO interaction between the HOMO of the dipole and the LUMO of the dipolarophile.17 Recently, the structural scope of this asymmetric cycloaddition has been expanded through the incorporation of novel types of dipolarophiles, beyond the standard acrylates and related α,β-unsaturated esters.6 In contrast, the structural scope of the cycloaddition has been more limited with regard to the azomethine ylide precursor since most of the reported examples used α-iminoesters of non-enolizable aldehydes (mainly aryliminoesters) as ylide precursors. Accordingly, the variety of pyrrolidines that can be obtained using this strategy is limited to derivatives that have an ester group at C-2 and usually an aryl substitution at C-5 (Scheme 2).18
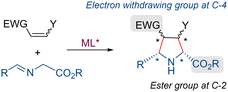 | ||
| Scheme 2 Structural pattern of pyrrolidines formed through 1,3-dipolar cycloadditions using α-iminoesters as azomethine ylide precursors. | ||
This structural pattern is not present in most of the pyrrolidine scaffolds contained in natural or biologically active products. Therefore, to enhance the synthetic applicability of the process, it is crucial to broaden the scope of the cycloaddition to include the use of other types of dipole precursors.
Our research group has been deeply involved in the study of azomethine ylide dipolar cycloadditions since 2005, focusing on developing new reaction partners. This Feature Article reviews literature examples that explored the use of azomethine ylide precursors beyond the classic α-iminoesters. Emphasis is placed on methodologies developed by our research group. Additionally, selected works by other groups, chosen for their relevance, will also be discussed.
2. Azomethine ylide precursors beyond α-iminoesters
2.1. α-Iminophosphonates
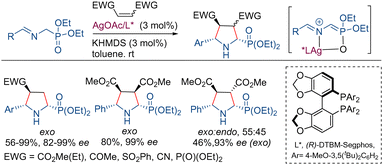
α-Aminophosphonic acids and their phosphonate esters are regarded as bioisosteres of α-amino acids and represent a significant class of compounds with diverse applications in medicinal chemistry.19 The use of Schiff bases of α-aminophosphonates as azomethine ylide precursors in 1,3-dipolar cycloadditions for the preparation of proline phosphonate derivatives has been hampered probably due to the lower acidity of their α-protons.20 In 2010, Kobayashi and co-workers described the first example of the use of iminophosphonates in catalytic asymmetric 1,3-dipolar cycloadditions. This process is based on the use of a chiral silver amide complex, prepared from AgOTf, KHMDS, and the ligand DTBM-Segphos.21 Under these conditions, the reaction proceeds with excellent yields and high levels of exo-diastereo- and enantioselectivities for a wide variety of dipolarophiles (α,β-unsaturated alkenes such as esters, amides, ketones, sulfones, and phosphonates, Scheme 3).2.2. α-Iminoamides
Despite their inherent interest, there are few examples in the literature where α-iminoamides have been used as azomethine ylide precursors.22 In 2012, our research group reported the first methodology enabling the use of α-iminoamides in the catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides.23 The catalytic systems formed by Cu(CH3CN)4PF6 as a metal and several ligands from the Segphos family showed outstanding efficiency, giving rise to the corresponding 2-amidopyrrolidines with high yields and excellent levels of diastereo- and enantioselectivities (Scheme 4). The methodology exhibited an unusual broad structural scope, being compatible with α-iminoamides of different electronic nature, as well as with both diactivated (fumarates and maleates) and monoactivated (such as acrylates, vinylsulfones, nitrostyrenes, and chalcones) dipolarophiles. Remarkably, most of the reactions take place using only 1 mol% of catalyst loading. The synthetic utility of the method was demonstrated through the synthesis of enantioenriched pyrrolizidines. The 1,3-dipolar cycloaddition between Weinreb derived iminoamide and phenyl vinyl sulfone afforded the corresponding pyrrolidine with high yield and excellent enantiomeric excess. Subsequent acylation, cyclization, and desulfonylation steps yielded the target pyrrolizidine (39% overall yield, Scheme 4).More recently, Garner and co-workers published a procedure based on the use of glycyl sultams as precursors of azomethine ylides.24 In this multicomponent reaction, the sultam group activates the α position of the Schiff base, making feasible the generation of azomethine ylides in situ from the corresponding aldehydes (Scheme 5). Using a [(CH3CN)4Cu]PF6/DTBM-Segphos complex as a catalytic system, in the absence of an external base, pyrrolidines are obtained with high yields and enantioselectivities. Interestingly, this method is compatible with the use of labile enolizable aldehydes (such as acetaldehyde and propionaldehyde).
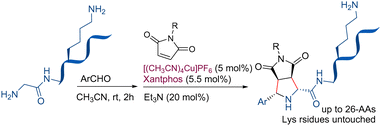
These studies have paved the way for using (3+2) cycloadditions of azomethine ylides in late-stage peptide modification, a crucial advancement for developing new applications of this important class of molecules. In 2024, Kanemoto and co-workers25 developed a diastereoselective methodology for the N-terminal-specific modification of peptides via 1,3-dipolar cycloaddition between glycine amide derived azomethine ylides and maleimides. The cycloaddition under copper catalysis afforded the exo adduct with excellent yield and diastereoselectivity. Oligopeptides containing up to 26 amino acids, including several lysine moieties, are suitable substrates affording exclusively the N-termini adducts (Scheme 6).
Cyclic peptides are highly valuable molecules due to their unique biological properties. Guéret Waldmann and co-workers26 described the incorporation of pyrrolidines as structural elements that define and constrain the conformation of macrocyclic hot loop-derived peptides. The cyclic peptides were synthesized on a solid support through macrocyclization via imine formation. The subsequent 1,3-dipolar cycloaddition in the presence of lithium bromide and Et3N efficiently afforded the corresponding adducts. The configuration of the pyrrolidine fragment determines the conformation of the peptide residue responsible for binding with the target protein (Scheme 7).
Recently, our research group has developed an efficient strategy for synthesizing unnatural cyclic peptides through Cu-catalyzed 1,3-dipolar cycloaddition of azomethylene ylides.27 This method enables the incorporation of pyrrolidine units with up to four new stereocenters into the cyclic peptide structure. Exceptional stereocontrol over the newly formed stereogenic centers within the pyrrolidine ring was achieved taking advantage of the stereocenters already present in the peptide chain. This cycloaddition approach is compatible with a broad range of natural amino acids in the peptide chain, accessing various macrocyclic ring sizes, and being compatible with different dipolarophiles (Scheme 8). Mechanistic studies suggest that copper coordination with both the dipole and dipolarophile is essential for establishing the preorganization necessary for the 1,3-dipolar reaction to proceed efficiently.
2.3. α-Iminonitriles
Considering that α-iminoacetonitriles have an α-hydrogen with an acidity comparable to that of α-iminoesters, they are expected to readily form azomethine ylides stabilized by the cyano group. The 1,3-dipolar cycloaddition of these ylides leads to 2-cyanopyrrolidines, structural motifs known for their significant biological activity. Additionally, 2-cyanopyrrolidines may serve as valuable synthetic intermediates, as the cyano group could act as a leaving group in further transformations, facilitating the synthesis of a diverse range of C-2 substituted pyrrolidines. Despite this potential, before 2010, only a few non-asymmetric examples of the use of α-iminonitriles in 1,3-dipolar cycloaddition reactions had been reported.28In 2010, our research group described a protocol for the catalytic asymmetric cycloaddition between α-iminonitriles and activated alkenes.29 In the presence of an AgOAc/Taniaphos complex, the reaction with methyl fumarate and N-methylmaleimide afforded the corresponding 2-cyanopyrrolidines with good endo diastereoselectivities and enantioselectivities (Scheme 9). X-ray diffraction studies and DFT theoretical calculations suggest that coordination between the metal complex and the α-iminonitrile occurs solely through the imine nitrogen, resulting in a 1,3-metalodipole with syn configuration. The approach of the dipolarophile takes place through the less hindered face, which explains the good diastereo- and enantioselectivity of the process. This methodology enabled the synthesis of pyrrolidines diversely substituted at the C-2 position by nucleophilic substitution of the cyano group.
Using the same catalytic system, Ramström and co-workers30 developed a dynamic systemic resolution protocol of α-iminonitriles using a transamination/catalytic asymmetric 1,3-dipolar cycloaddition sequence, in which the silver complex worked as both a catalyst and an external selector. The dynamic imine system was generated from the α-iminophenylacetonitrile mixed with 12 different aldehydes in the presence of Sc(OTf)3. This system was subsequently subjected to 1,3-dipolar cycloaddition conditions (AgOAc/Taniaphos complex, Et3N, in CH2Cl2) affording exclusively the 5-furyl-pyrrolidine products with good yield and diastereo- and enantioselectivity (Scheme 10). The formation of a bidentate metallacycle enhances the interaction with the catalyst and favours the dipolar cycloaddition. The authors argue that the less effective overlap between silver and sulfur accounts for the lower reactivity of the thiophenyl derivative.
 | ||
| Scheme 10 Dynamic systemic resolution of α-iminonitriles via catalytic asymmetric 1,3-dipolar cycloaddition. | ||
Later, Zhang and coworkers developed a new strategy for the preparation of racemic 5-unsubstituted pyrrolidines using a silver-catalyzed diastereoselective 1,3-dipolar cycloaddition of α-iminonitriles and a subsequent NaBH4-induced reductive decyanation reaction.31 The methodology was applied to the total synthesis of the pyrrolizidine natural product isoretronecanol (Scheme 11).
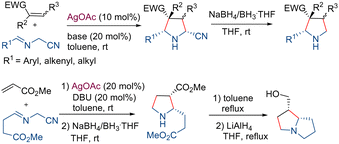 | ||
| Scheme 11 Diastereoselective synthesis of 5-unsubstituted pyrrolidines by 1,3-dipolar cycloaddition of α-iminonitriles. | ||
2.4. α-N-(Heteroaryl-methyl)imine derivatives
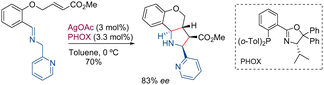
The first work in which imines with heteroaryl groups, instead of an ester group at position C-2, were used as precursors of azomethine ylides was reported by Grigg in 1983.32 Specifically, this involved the reaction between α-iminoheterocycles and N-phenylmaleimide carried out in the absence of a catalyst at elevated temperatures which gave rise to racemic pyrrolidines with yields ranging from 60% to 82%, although the diastereoselectivity was low. Seven years later, the same research group described the intramolecular version of this reaction.33In 2005, Pfaltz and co-workers34 described a catalytic asymmetric version of the intramolecular process. When AgOAc and the PHOX ligand were used as the catalytic system, the final tricyclic system was obtained with a good yield and an enantiomeric excess of 83% (Scheme 12).
In 2010, our research group studied the intermolecular cycloaddition reaction between α-iminopyridines and activated olefins.35 The coordination of the metal to both the imine and pyridine nitrogens facilitated the formation of a highly reactive metallodipole, which was crucial to achieve good stereochemical discrimination in the cycloaddition with the dipolarophile. Using CuI/BOX as the catalytic system, the reaction with N-phenylmaleimide exhibited almost complete exo-selectivity and excellent enantioselectivity (Scheme 13). Although the reaction with dipolarophiles with a trans configuration was less diastereoselective, it still maintained high enantioselectivity (72–96% ee).
 | ||
| Scheme 13 Intermolecular (3+2) cycloaddition between N-(2-pyridylmethyl)imines and different dipolarophiles. | ||
2.5. α-Silylimines
α-Silylimines represent a different and less commonly used type of azomethine ylide precursor, leading to 5-unsubstituted pyrrolidines, a substitution pattern that cannot be directly achieved through the typical process involving Schiff bases of amino acid esters.36 Despite the synthetic interest in these dipoles, the asymmetric catalytic version of this reaction has been barely studied.In 2012, our research group developed the first catalytic asymmetric cycloaddition using α-silyliminoesters as azomethine ylide precursors.37 The use of the CuI/(R)-DTBM-Segphos complex as a catalytic system enabled the formation of a wide variety of enantioenriched pyrrolidines with a quaternary centre at position C-2. Employing maleimide as a dipolarophile the corresponding adducts were obtained with good yields and enantioselectivities (81–98% ee). Interestingly, the reaction worked well from alkyl substituted α-silylimines. Excellent results were also obtained using sulfonylated dipolarophiles, which after desulfonylation facilitated the preparation of proline derivatives with a quaternary centre. The presence of a coordinating group (carboxylate) in the silylimine, which allows the formation of a five-membered metallodipole, is essential for the reaction to occur with good selectivity (Scheme 14).
 | ||
| Scheme 14 Catalytic asymmetric cycloaddition using α-silyliminoesters as azomethine ylide precursors. | ||
In 2016, Kumar and co-workers38 explored the application of this methodology for the preparation of complex quaternary-carbon rich scaffolds. To this end, they chose pyrones and benzopyrones with trisubstituted olefin moieties as dipolarophiles. The use of AgOAc/iPr-DuPhos as a catalyst system allowed accessing the expected bicyclic and tricyclic adducts with excellent yield and diastereo- and enantioselectivity. The resulting adducts served as starting materials for the synthesis of tetracyclic structures with three quaternary chiral centres. The authors again proposed a transition state in which the coordination of the AgI/DuPhos complex with the oxygen of the carbonyl and the nitrogen of the azomethine ylide provided the necessary chiral environment for achieving high asymmetric inductions (Scheme 15).
Considering the good results obtained by our research group using α-iminopyridines as azomethine ylide precursors,35 we envisaged that a pyridylsilylmine could form the 5-membered metallodipole necessary for achieving high reactivity and asymmetric induction. The reaction in the presence of CuI/(R)-Walphos provided the expected pyrrolidines with high yields and high levels of enantioselectivity (up to ≥99% ee) and diastereoselectivity (major formation of C-2/C-4 trans-substituted pyrrolidines).39 The use of water as an additive significantly improved the reactivity of the process, probably by accelerating the desilylation step in the formation of the metallodipole. It is worth noting that the method is compatible with different heteroaryl groups such as 2-quinolyl, 2-benzothiazolyl and 2-thiazolyl (Scheme 16).
The effectiveness of this methodology was demonstrated through the formal total synthesis of α-nicotine. The (3+2) cycloaddition between pyridylsilylimine and vinyl sulfone produced the corresponding adduct as a mixture of regioisomers, likely due to the participation of the two resonance structures of the dipole (Scheme 17). Subsequent protection of the nitrogen as Cbz and reductive sulfonyl elimination by treatment with Na(Hg) provided the α-nicotine precursor (87% ee).
2.6. α-Fluoromethylimines
Fluorinated pyrrolidines are of significant interest in medicinal chemistry as it is well known that substituting hydrogen atoms with fluorine in organic compounds can enhance their biological properties.40 The catalytic asymmetric 1,3-dipolar cycloaddition between azomethine ylides and activated fluorinated alkenes has emerged as one of the most convenient methodologies for preparing enantioenriched pyrrolidines with fluorine atoms in positions 3 or 4.41 However, the use of fluorinated azomethine ylide precursors in 1,3-dipolar cycloadditions has been rarely explored, and only some non-enantioselective examples have been reported.42In 2016, our research group reported a new trifluoromethylated azomethine ylide precursor with a coordinating group (either ester or pyridine) that promotes the formation of a bidentate metallodipole, providing the required activation of the imine for the reaction to proceed.43 A variety of 2-fluorinated pyrrolidines were obtained with good yields and high diastereoselectivities using simply AgOAc/PPh3 as a catalyst system. The highest enantioselectivities (86–92% ee) were achieved using the AgOAc/(R)-taniaphos catalytic system.
NBO analysis revealed that the charge is mainly localized at the α-position to the CF3 group, which is consistent with the regioselectivity experimentally observed (Scheme 18).
Simultaneously with the development of this work, Wang and co-workers reported the first example of organocatalytic 1,3-dipolar cycloaddition of N-(2,2,2-trifluoroethyl)-ketimines (obtained by the condensation of trifluoroethylamine and isatins) with enals.44 Using the Hayashi–Jorgensen catalyst and benzoic acid as the additive, the 1,3-dipolar cycloaddition gave the corresponding products bearing four contiguous stereogenic centres in excellent yields and diastereo- and enantioselectivities (Scheme 19). The chiral iminium intermediate, formed by the reaction of diphenylprolinol silyl ether with the 2-enals, induces Si-facial cycloaddition on the oxindole-derived azomethine ylide by shielding the Re-face with bulky aryl groups. Later, this methodology was extended to the use of diethyl 2-oxomalonate derived trifluoromethyl imines as azomethine ylide precursors.45
In 2015, the same research group reported the catalytic asymmetric cycloaddition of trifluoromethyl-containing azomethine ylides with β-nitroalkenes.46 Various spiro[pyrrolidin-3,2′-oxindoles] were synthesized with excellent yields and high diastereo- and enantioselectivities using a cinchona alkaloid-derived squaramide-catalyst. The bifunctional catalyst activated both substrates, the squaramide moiety coordinates via the nitro styrene while the tertiary nitrogen of the chinchona coordinates via the azomethine ylide, then the cycloaddition by the Re-face would take place (Scheme 20).
Since the publication of these works, numerous examples of the use of isatin derived imines in organocatalytic cycloadditions with different dipolarophiles47 such as arylfuran-2(3H)-ones,48 β-trifluoromethyl electron-deficient alkenes,49 aurones,50 2,3-dioxopyrrolidines,51 β-unsaturated pyrazolones,52 ethylene sulfonyl fluoride,53 β-γ-unsaturated α-keto esters,54 2-nitrobenzothiophene,55 isatylidenyl isoxazoles,56 methyleneindolinones,57 5-alkenyl thiazolones,58 and rhodamine derivatives59 and benzylidenemalononitriles,60 have been described.
In comparison with the organocatalytic procedures, the transition metal-catalyzed asymmetric 1,3-dipolar cycloaddition of N-2,2,2-trifluoroethylisatinketimines has been much less studied. Wang and co-workers have reported the use of chiral dinuclear Zn complexes as catalysts. First, they demonstrated that the cycloaddition between CF3-containing isatin derived azomethine ylides and methyleneindolinones afforded the corresponding spirocyclic adducts with two adjacent spiro quaternary stereocenters in high yield and enantio- and exo′-diastereoselectivity (3,4-trans-configuration). This stereochemical outcome indicates that the reaction takes place by a stepwise mechanism via an anti-selective Michael/Mannich sequence (Scheme 21).61 Later, they reported that under similar conditions aurones are also suitable dipolarophiles for this transformation.62
 | ||
| Scheme 21 Zn-catalyzed asymmetric (3+2) cycloaddition of trifluoromethylated azomethine ylides and methyleneindolinones. | ||
The same catalytic system was also effective in the dearomative cycloaddition of CF3-containing N-unprotected isatin-derived azomethine ylides with 2-nitrobenzofurans and 2-nitrobenzothiophenes. Pyrrolidinyl-spirooxindoles containing a 2,3-fused-dihydrobenzofuran (or dihydrobenzothiphene) moiety were obtained with excellent diastereoselectivity and moderate to high enantiomeric excess (Scheme 22).63
In 2022, Yuan, Zhao, Zhang and co-workers64 described the use of benzo[b]thiophene sulfones as dipolarophiles. Using a Cu(OTf)2/Phosferrox complex as a ligand, and Cs2CO3 as a base, different substituted pentacyclic spirooxindoles were obtained with excellent diastereo- and enantioselectivites. The umpolung of N-2,2,2-trifluoroethylisatin ketimines led to an unexpected regioisomer of the spirooxindole compounds (Scheme 23).
Shortly later,65 the same research group described the desymmetrization of N-arylmaleimides by copper catalyzed 1,3-dipolar cycloaddition with trifluoroethylisatin ketimines. Using a Cu(OTf)2/chiral ferrocenyl P,N-ligand complex as a catalyst, a series of octahydropyrrolo[3,4-c]pyrroles, with a chiral C–N axis, were obtained with excellent yields and high diastereo- and enantioselectivity (Scheme 24).
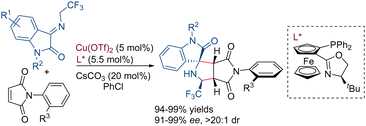 | ||
| Scheme 24 Desymmetrization of N-arylmaleimides by 1,3-dipolar cycloaddition with trifluoroethylistatin ketimines. | ||
2.7. HMF derived azomethine ylides
The use of renewable raw materials derived from biomass instead of those arising from petrochemicals for the preparation of fine chemicals is a key goal of modern organic synthesis. A remarkable example of these platform molecules is 5-hydroxymethylfurfural (HMF), which is obtained by the dehydration of 6-carbon sugars and offers great synthetic potential.66 However, the use of HMF as a starting material for the preparation of enantioenriched products by asymmetric catalysis has been scarcely studied.67 Recently, our research group has demonstrated that catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides is an efficient tool for the preparation of enantioenriched highly functionalized pyrrolidines from HMF.68 The cycloaddition between the HMF-iminoester (obtained by condensation with methyl glycinate) and N-methylmaleimide using a CuI/Fesulphos catalyst system exclusively afforded endo-pyrrolidine with excellent enantiocontrol (75% yield, 95% ee, Scheme 25).2.8. Synthesis of axially chiral arylpyrroles
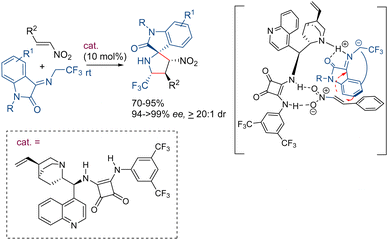
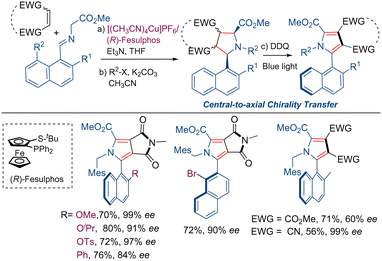
Axial chiral molecules are important structural motifs present in natural products and biologically active substances.69 Moreover, a great number of chiral ligands and organocatalysts are based on skeletons with axial chirality. In 2024, our research group has developed a new method for the synthesis of axially chiral arylpyrroles using the 1,3-dipolar cycloaddition reaction of azomethine ylides as the key step.70 The CuI/Fesulphos catalyzed (3+2)-cyclization using naphthyl derived iminoesters as azomethine ylide precursors takes place with good yield and high levels of diastereo- and enantioselectivity. The subsequent nitrogen alkylation and aromatization gives rise to the corresponding axial chiral pyrroles. The mild conditions used in the DDQ/blue light mediated aromatization process provide an effective central-to-axial chirality transfer affording the corresponding pyrroles with high atroposelectivity (Scheme 26).3. Conclusions
The catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides has witnessed significant progress over the past few decades, providing a reliable procedure for the enantioselective synthesis of polysubstituted pyrrolidines. During this time, numerous research groups have greatly expanded the range of both dipoles and dipolarophiles compatible with this reaction. This feature article highlights examples that use azomethine ylide precursors beyond the standard α-iminoesters, including α-iminophosphonates, α-iminoamides, α-iminonitriles, α-iminopyridines, α-silylimines and α-fluoromethylimines. These advancements have expanded the scope of pyrrolidine derivatives that can be synthesized through this methodology, opening up new avenues for the preparation of complex pyrrolidines. Despite these notable achievements, challenges remain, for example, while non-stabilized azomethine ylides have been extensively employed in natural product synthesis, their use in asymmetric catalysis remains underexplored. In this regard, a key aspect is the generation of active monodentate chiral chelates through coordination of a metal complex to the imine nitrogen. Addressing this gap could significantly enhance the synthetic applicability of this methodology.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank FEDER/Ministerio de Ciencia, Innovación y Universidades–Agencia Estatal de Investigación (Grants PGC2018-098660-B-I00 and PID2021-1248553NB-100) for financial support. We also thank the MINECO for the financial support for the RED2022-134331-T.Notes and references
- Methods and Applications of Cycloaddition Reactions in Organic Syntheses, ed., N. Nishiwaki, Wiley, Hoboken, 2014 Search PubMed.
- (a) M. Breugst and H.-U. Reissig, Angew. Chem., Int. Ed., 2020, 59, 12293–12307 CrossRef CAS PubMed; (b) T. Hashimoto and K. Maruoka, Chem. Rev., 2015, 115, 5366–5412 CrossRef CAS PubMed.
- (a) S. Dubey, A. Pal, S. Roy, S. Sasmal, A. Tamrakar, R. Jana and T. Das, New J. Chem., 2023, 47, 8997–9034 RSC; (b) S. S. Panda, M. N. Aziz, J. Stawinski and A. S. Girgis, Molecules, 2023, 28, 668–741 CrossRef CAS PubMed; (c) C. Najera and J. M. Sansano, Pure Appl. Chem., 2019, 91, 575–596 CrossRef CAS; (d) C. Nájera and J. M. Sansano, Curr. Org. Chem., 2003, 7, 1105–1150 CrossRef.
- More than 37 marketed drugs contain the pyrrolidine ring in its structure (a) S. Poyraz, H. A. Dondas, N. Y. Dondas and J. M. Sansano, Front. Pharmacol., 2023, 14, 1239658 CrossRef CAS PubMed; (b) G. L. Petri, M. V. Raimondi, V. Spanò, R. Holl, P. Barraja and A. Montalbano, Top. Curr. Chem., 2021, 379, 34 CrossRef PubMed; (c) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed.
- (a) G. D. Yadav, P. Chaudhary, B. Pani and S. Singh, Tetrahedron Lett., 2024, 134, 154835 CrossRef CAS; (b) T. Mukaiyama and M. Asami, Top. Curr. Chem., 1985, vol. 127, Springer, Berlin, Heidelberg Search PubMed.
- (a) S. V. Kumar and P. J. Guiry, Chem. – Eur. J., 2023, 29, e202300296 CrossRef CAS PubMed; (b) L. Wei, X. Chang and C.-J. Wang, Acc. Chem. Res., 2020, 53, 1084–1100 CrossRef CAS PubMed; (c) J. Adrio and J. C. Carretero, Chem. Commun., 2019, 55, 11979–11991 RSC; (d) I. Arrastia, A. Arrieta and F. P. Cossio, Eur. J. Org. Chem., 2018, 5889–5904 CrossRef CAS PubMed; (e) X. Fang and C.-J. Wang, Org. Biomol. Chem., 2018, 16, 2591–2601 RSC; (f) B. Bdiri, B.-J. Zhao and Z.-M. Zhou, Tetrahedron: Asymmetry, 2017, 28, 876–899 CrossRef CAS; (g) H. A. Döndas, M. G. Retamosa and J. M. Sansano, Synthesis, 2017, 2819–2851 CrossRef; (h) T. Hashimoto and K. Maruoka, Chem. Rev., 2015, 115, 5366–5412 CrossRef CAS PubMed; (i) J. Adrio and J. C. Carretero, Chem. Commun., 2014, 50, 12434–12446 RSC; (j) C. Nájera and J. M. Sansano, J. Organomet. Chem., 2014, 771, 78–92 Search PubMed; (k) R. Narayan, M. Potowski, Z. J. Jia, A. P. Antonchick and H. Waldmann, Acc. Chem. Res., 2014, 47, 1296–1310 CrossRef CAS PubMed; (l) Y. Xing and N.-X. Wang, Coord. Chem. Rev., 2012, 256, 938–952 CrossRef CAS; (m) J. Adrio and J. C. Carretero, Chem. Commun., 2011, 47, 6784–6794 RSC; (n) C. Nájera, J. M. Sansano and M. Yus, J. Braz. Chem. Soc., 2010, 21, 377–412 CrossRef; (o) C. Nájera and J. M. Sansano, Top. Heterocycl. Chem., 2008, 12, 117–145 Search PubMed; (p) L. M. Stanley and M. P. Sibi, Chem. Rev., 2008, 108, 2887–2902 CrossRef CAS PubMed; (q) H. Pellisier, Tetrahedron, 2007, 63, 3235–3285 CrossRef; (r) G. Pandey, P. Banerjee and S. R. Gadre, Chem. Rev., 2006, 106, 4484–4517 CrossRef CAS PubMed; (s) T. M. V. D. Pinho e Melo, Eur. J. Org. Chem., 2006, 2873–2888 CrossRef CAS; (t) M. Bonin, A. Chauveau and L. Micouin, Synlett, 2006, 2349–2363 CrossRef CAS; (u) S. Husinec and V. Savic, Tetrahedron: Asymmetry, 2005, 16, 2047–2061 CrossRef CAS; (v) I. Coldham and R. Hufton, Chem. Rev., 2005, 105, 2765–2810 CrossRef CAS PubMed; (w) C. Nájera and J. M. Sansano, Angew. Chem., Int. Ed., 2005, 44, 6272–6276 CrossRef PubMed.
- P. Allway and R. Grigg, Tetrahedron Lett., 1991, 32, 5817–5820 CrossRef CAS.
- J. M. Longmire, B. Wang and X. Zhang, J. Am. Chem. Soc., 2002, 124, 13400–13401 CrossRef CAS PubMed.
- A. S. Gothelf, K. V. Gothelf, R. G. Hazell and K. A. Jørgensen, Angew. Chem., Int. Ed., 2002, 41, 4236–4238 CrossRef CAS PubMed.
- (a) I. Ohno, K. Kanemoto, S. Furuya, Y. Suzuki and S. Fukuzawa, Org. Lett., 2024, 26, 1880–1885 CrossRef CAS PubMed; (b) J. Liu, R. Zhang, S. Mallick, S. Patil, C. Wientjens, J. Flegel, A. Krupp, C. Strohmann, C. Grassin, C. Merten, A. Pahl, M. Grigalunas and H. Waldmann, Chem. Sci., 2023, 14, 7936–7943 RSC; (c) E. García-Mingüens, M. Ferrándiz-Saperas, M. de Gracia Retamosa, C. Nájera, M. Yus and J. M. Sansano, Molecules, 2022, 27, 4579–4594 CrossRef PubMed; (d) S. Furuya, K. Kanemoto and S. Fukuzawa, Chem. – Asian J., 2022, 17, e202200239 CrossRef CAS PubMed; (e) Y. Suzuki, K. Kanemoto, A. Inoue, K. Imae and S. Fukuzawa, J. Org. Chem., 2021, 86, 14586–14596 CrossRef CAS PubMed; (f) O. Yildirim, M. Grigalunas, L. Brieger, C. Strohmann, A. P. Antonchick and H. Waldmann, Angew. Chem., Int. Ed., 2021, 60, 20012–20020 CrossRef CAS PubMed; (g) K.-Q. Zhang, Q.-F. Deng, J. Luo, C.-L. Gong, Z.-G. Chen, W. Zhong, S.-Q. Hu and H.-F. Wang, ACS Catal., 2021, 11, 5100–5107 CrossRef CAS; (h) H. Liu, H. Tao, H. Cong and C.-J. Wang, J. Org. Chem., 2016, 81, 3752–3760 CrossRef CAS PubMed; (i) K. Imae, T. Konno, K. Ogata and S. Fukuzawa, Org. Lett., 2012, 14, 4410–4413 CrossRef CAS PubMed; (j) I. Oura, K. Shimizu, K. Ogata and S. Fukuzawa, Org. Lett., 2010, 12, 1752–1755 CrossRef CAS PubMed; (k) K. Shimizu, K. Ogata and S.-I. Fukuzawa, Tetrahedron Lett., 2010, 51, 5068–5070 CrossRef CAS; (l) G. Liang, M. Tong and C.-J. Wang, Adv. Synth. Catal., 2009, 351, 3101–3106 CrossRef CAS; (m) C. Nájera, M. de Gracia Retamosa and J. M. Sansano, Angew. Chem., Int. Ed., 2008, 47, 6055–6058 CrossRef PubMed; (n) C. Chen, X. Li and S. L. Schreiber, J. Am. Chem. Soc., 2003, 125, 10174–10175 CrossRef CAS PubMed.
- (a) X. Wang, R. Gao and X. Li, J. Am. Chem. Soc., 2024, 146, 21069–21077 CrossRef CAS PubMed; (b) B. R. Wang, Y. B. Li, Q. Zhang, D. Gao, P. Tian, Q. Li and L. Yin, Nat. Commun., 2023, 14, 4688 CrossRef CAS PubMed; (c) X. Cheng, X. Chang, Y. Yang, Z. Zhang, J. Li, Y. Li, W. Zhao, L. W. Chung, H. Teng, X.-Q. Dong and C.-J. Wang, Sci. China: Chem., 2023, 66, 3193–3204 CrossRef CAS; (d) C. Cristóbal, D. Gaviña, I. Alonso, M. Ribagorda, J. C. Carretero, C. del Pozo and J. Adrio, Chem. Commun., 2022, 58, 7805–7808 RSC; (e) X. Chang, X. Cheng and C.-J. Wang, Chem. Sci., 2022, 13, 4041–4049 RSC; (f) H. Wang, J. Li, L. Peng, J. Song and C. Guo, Org. Lett., 2022, 24, 7828–7833 CrossRef CAS PubMed; (g) X. Xu, L. Bao, L. Ran, Z. Yang, D. Yan, C.-J. Wang and H. Teng, Chem. Sci., 2022, 13, 1398–1407 RSC; (h) M. Gill, A. Das and V. K. Singh, Org. Lett., 2022, 24, 5629–5634 CrossRef CAS PubMed; (i) X. Chang, Y. Yang, C. Shen, K.-S. Xue, Z.-F. Wang, H. Cong, H.-Y. Tao, L. W. Chung and C.-J. Wang, J. Am. Chem. Soc., 2021, 143, 3519–3535 CrossRef CAS PubMed; (j) S. N. Greszler, G. Zhao, M. Buchman, X. B. Searle, B. Liu and E. A. Voight, J. Org. Chem., 2020, 85, 7620–7632 CrossRef CAS PubMed; (k) A. Molina, S. Díaz-Tendero, J. Adrio and J. C. Carretero, Chem. Commun., 2020, 56, 5050–5053 RSC; (l) H. Deng, T.-T. Liu, Z.-D. Ding, W.-L. Yang, X. Luo and W.-P. Deng, Org. Chem. Front., 2020, 7, 3247–3252 RSC; (m) S. Furuya, S. Kanemoto and S. Fukuzawa, J. Org. Chem., 2020, 85, 8142–8148 CrossRef CAS PubMed; (n) T. Rigotti, J. Asenjo-Pascual, A. Martín-Somer, P. Milán Rois, M. Cordani, S. Díaz-Tendero, A. Somoza, A. Fraile and J. Alemán, Adv. Synth. Catal., 2020, 362, 1345–1355 CrossRef CAS; (o) X. Cheng, D. Yan, X. Dong and C. J. Wang, Asian J. Org. Chem., 2020, 9, 1567–1570 CrossRef CAS; (p) Z. Wang, D.-C. Wang, M.-S. Xie, G.-R. Qu and H.-M. Guo, Org. Lett., 2020, 22, 164–167 CrossRef CAS PubMed; (q) J. Corpas, A. Ponce, J. Adrio and J. C. Carretero, Org. Lett., 2018, 20, 3179–3182 CrossRef CAS PubMed; (r) B. Xu, Z. Zhang, B. Liu, S. Xu, L. Zhou and J. Zhang, Chem. Commun., 2017, 53, 8152–8155 RSC; (s) A. Pascual-Escudero, A. de Cózar, F. P. Cossío, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2016, 55, 15334–15338 CrossRef CAS PubMed; (t) H. Takayama, Z.-J. Jia, L. Kremer, J. O. Bauer, C. Strohmann, S. Ziegler, A. P. Antonchick and H. Waldmann, Angew. Chem., Int. Ed., 2013, 52, 12404–12408 CrossRef CAS PubMed; (u) Z. He, T. Liu, H. Tao and C. J. Wang, Org. Lett., 2012, 14, 6230–6233 CrossRef CAS PubMed; (v) A. P. Antonchick, C. Gerding-Reimers, M. Catarinella, M. Schürmann, H. Preut, S. Ziegler, D. Rauh and H. Waldmann, Nat. Chem., 2010, 2, 735–740 CrossRef CAS PubMed; (w) R. Robles-Machín, M. González-Esguevillas, J. Adrio and J. C. Carretero, J. Org. Chem., 2010, 75, 233–236 CrossRef PubMed; (x) S. Filippone, E. E. Maroto, A. Martín-Domenech, M. Suarez and N. Martín, Nat. Chem., 2009, 1, 578–582 CrossRef CAS PubMed; (y) A. López-Pérez, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2009, 48, 346–349 CrossRef; (z) A. López-Pérez, J. Adrio and J. C. Carretero, J. Am. Chem. Soc., 2008, 130, 10084–10085 CrossRef PubMed; (a a) C.-J. Wang, G. Liang, Z.-Y. Xue and F. Gao, J. Am. Chem. Soc., 2008, 130, 17250–17251 CrossRef CAS PubMed; (a b) X.-X. Yan, Q. Peng, Y. Zhang, K. Zhang, W. Hong, X.-L. Hou and Y.-D. Wu, Angew. Chem., Int. Ed., 2006, 45, 1979–1983 CrossRef CAS PubMed; (a c) S. Cabrera, R. Gómez Arrayás and J. C. Carretero, J. Am. Chem. Soc., 2005, 127, 16394–16395 CrossRef CAS PubMed; (a d) W. Gao, X. Zhang and M. Raghunath, Org. Lett., 2005, 7, 4241–4344 CrossRef CAS PubMed.
- (a) S. V. Kumar and P. J. Guiry, Angew. Chem., Int. Ed., 2022, 61, e202205516 CrossRef CAS PubMed; (b) O. Dogan, H. Koyuncu, P. Garner, A. Bulut, W. J. Youngs and M. Panzner, Org. Lett., 2006, 8, 4687–4690 CrossRef CAS PubMed.
- (a) A. Awata and T. Arai, Chem. – Eur. J., 2012, 12, 8278–8282 CrossRef PubMed; (b) T. Arai, N. Yokoyama, A. Mishiro and H. Sato, Angew. Chem., Int. Ed., 2010, 49, 7895–7898 CrossRef CAS PubMed.
- (a) M. Martín-Rodríguez, C. Nájera, J. M. Sansano, A. de Cózar and F. P. Cossío, Chem. – Eur. J., 2011, 17, 14224–14233 CrossRef PubMed; (b) M. Martín-Rodríguez, Carmen Nájera, J. M. Sansano and F.-L. Wu, Tetrahedron: Asymmetry, 2010, 21, 1184–1186 CrossRef.
- (a) T. Tsubogo, S. Saito, K. Seki, Y. Yamashita and S. Kobayashi, J. Am. Chem. Soc., 2008, 130, 13321–13332 CrossRef CAS PubMed; (b) S. Saito, T. Tsubogo and S. Kobayashi, J. Am. Chem. Soc., 2007, 129, 5364–5365 CrossRef CAS PubMed.
- (a) Y. A. Pronina, L. A. Teglyai, A. I. Ponyaev, M. L. Petrova, V. M. Boitsov and A. V. Stepakov, Russ. J. Gen. Chem., 2024, 94, 1–44 CrossRef CAS; (b) J. Przydacz, A. Bojanowski, A. Albrecht and Ł. Albrecht, Org. Biomol. Chem., 2021, 19, 3075–3086 RSC; (c) S. Reboredo, J. L. Vicario, D. Badía, L. Carrillo and E. Reyes, Adv. Synth. Catal., 2011, 353, 3307–3312 CrossRef CAS; (d) X.-H. Chen, Q. Wei, S.-W. Luo, H. Xiao and L.-Z. Gong, J. Am. Chem. Soc., 2009, 131, 13819–13825 CrossRef CAS PubMed; (e) J. L. Vicario, S. Reboredo, D. Badia and L. Carrillo, Angew. Chem., Int. Ed., 2007, 46, 5168–5170 CrossRef CAS PubMed.
- (a) R. Sustmann and H. Trill, Angew. Chem., Int. Ed. Engl., 1972, 11, 838–840 CrossRef CAS; (b) K. N. Houk, J. Sims, C. R. Watts and L. J. Luskus, J. Am. Chem. Soc., 1973, 95, 7301–7315 CrossRef CAS.
- Aliphatic imines are very challenging substrates since they are easily transformed into the corresponding enamines in the presence of a base. For catalytic asymmetric examples, see: (a) P. Garner, L. Weerasinghe, W. Youngs, B. Wright, D. Wilson and D. Jacobs, Org. Lett., 2012, 14, 1326–1329 CrossRef CAS PubMed; (b) Y. Yamashita, T. Imaizumi and S. Kobayashi, Angew. Chem., Int. Ed., 2011, 50, 4893–4896 CrossRef CAS PubMed; C. Alemparte, G. Blay and K. A. Jørgensen, Org. Lett., 2005, 7, 4569–4572 Search PubMed.
- H. A. Dondas, Y. Durust, R. Grigg, M. J. Slater and M. A. B. Sarker, Tetrahedron, 2005, 61, 10667–10682 CrossRef CAS.
- A. Dehnel and J. M. Kanabus-Kaminska, Can. J. Chem., 1988, 66, 310–318 CrossRef CAS.
- Y. Yamashita, X.-X. Guo, R. Takashita and S. Kobayashi, J. Am. Chem. Soc., 2010, 132, 3262–3263 CrossRef CAS PubMed.
- (a) T. O. Painter, K. Kaszas, J. Gross, J. T. Douglas, V. W. Day, M. J. Iadarola and C. Santini, Bioorg. Med. Chem. Lett., 2014, 24, 963–968 CrossRef CAS PubMed; (b) R. Grigg, P. McMeekin and V. Sridharan, Tetrahedron, 1995, 51, 13347–13356 CrossRef CAS; (c) O. Tsuge, S. Kanemasa and M. Yoshioka, J. Org. Chem., 1988, 53, 1384–1391 CrossRef CAS.
- M. González-Esguevillas, J. Adrio and J. C. Carretero, Chem. Commun., 2012, 48, 2149–2151 RSC.
- R. Joseph, C. Murray and P. Garner, Org. Lett., 2014, 16, 1550–1553 CrossRef CAS PubMed.
- H. Machida and K. Kanemoto, Angew. Chem., Int. Ed., 2024, 63, e202320012 CrossRef CAS PubMed.
- S. M. Guéret, S. Thavam, R. J. Carbajo, M. Potowski, N. Larsson, G. Dahl, A. Dellsén, T. N. Grossmann, A. T. Plowright, E. Valeur, M. Lemurell and H. Waldmann, J. Am. Chem. Soc., 2020, 142, 4904–4915 CrossRef PubMed.
- E. Gallent, I. Alonso, J. C. Carretero, N. Rodríguez and J. Adrio, Org. Lett., 2024, 26, 10394–10398 CrossRef CAS PubMed.
- (a) S. Kanemasa, H. Yamamoto, E. Wada, T. Sakurai and K. Urshido, Bull. Chem. Soc. Jpn., 1990, 63, 2857–2865 CrossRef CAS; (b) O. Tsuge, S. Kanemasa, K. Yorozu and K. Ueno, Bull. Chem. Soc. Jpn., 1987, 60, 3359–3366 CrossRef CAS; (c) O. Tsuge, K. Ueno, S. Kanemasa and K. Yorozu, Bull. Chem. Soc. Jpn., 1986, 59, 1809–1824 CrossRef CAS; (d) O. Tsuge, S. Kanemasa, K. Yorozu and K. Ueno, Chem. Lett., 1985, 1601–1604 CrossRef CAS.
- R. Robles-Machín, I. Alonso, J. Adrio and J. C. Carretero, Chem. – Eur. J., 2010, 16, 5286–5291 CrossRef PubMed.
- L. Hu and O. Ramström, Chem. Commun., 2014, 50, 3792–3794 RSC.
- (a) J. Li, H. Zhao and Y. Zhan, Synlett, 2015, 2745–2750 CAS; (b) J. Li, H. Zhao, X. Jiang, X. Wang, H. Hu, L. Yu and Y. Zhang, Angew. Chem., Int. Ed., 2015, 54, 6306–6310 CrossRef CAS PubMed.
- (a) V. Sridharan, H. Q. N. Gunaratne and R. Grigg, Tetrahedron Lett., 1983, 24, 4363–4366 CrossRef; (b) E. E. Elboray, R. Grigg, C. W. G. Fishwick, C. Kilner, M. A. B. Sarker, M. F. Aly and H. H. Abbas-Temirek, Tetrahedron, 2011, 67, 5700–5710 CrossRef CAS.
- R. Grigg, G. Donegan, H. Q. N. Gunaratne, D. A. Kennedy, J. F. Malone, V. Sridharan and S. Thianpatanagul, Tetrahedron, 1989, 45, 1723–1746 CrossRef CAS.
- R. Stohler, F. Wahl and A. Pfaltz, Synthesis, 2005, 1431–1436 CAS.
- S. Padilla, R. Tejero, J. Adrio and J. C. Carretero, Org. Lett., 2010, 12, 5608–5611 CrossRef CAS PubMed.
- (a) K. Achiwa and M. Sekiya, Chem. Pharm. Bull., 1987, 35, 2646–2655 CrossRef; (b) O. Tsuge, S. Kanemasa, T. Yamada and K. Matsuda, J. Org. Chem., 1987, 52, 2523–2530 CrossRef CAS; (c) M. Komatsu, H. Okada, T. Akaki, Y. Oderaotoshi and S. Minakata, Org. Lett., 2002, 4, 3505–3508 CrossRef CAS PubMed.
- J. Hernández-Toribio, S. Padilla, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2012, 51, 8854–8858 CrossRef PubMed.
- N. Kesava-Reddy, C. Golz, C. Strohmann and K. Kumar, Chem. – Eur. J., 2016, 22, 18373–18377 CrossRef CAS PubMed.
- A. Pascual-Escudero, M. González-Esguevillas, S. Padilla, J. Adrio and J. C. Carretero, Org. Lett., 2014, 16, 2228–2231 CrossRef CAS PubMed.
- (a) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC; (b) K. Muller Gouverneur, Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical aspects to Clinical Applications, Imperial College Press, London, UK, 2012 CrossRef; (c) J. Wangó, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef PubMed; (d) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315–8359 CrossRef CAS PubMed.
- (a) Q.-H. Li, Z.-Y. Xue, H.-Y. Tao and C.-J. Wang, Tetrahedron Lett., 2012, 53, 3650–3653 CrossRef CAS; (b) D. Yan, Q. Li and C.-J. Wang, Chin. J. Chem., 2012, 30, 2714–2720 CrossRef CAS; (c) I. McAlpine, M. Tran-Dube, F. Wang, S. Scales, J. Matthews, M. R. Collins, S. K. Nair, M. Nguyen, J. Bian, L. Martinez Alsina, J. Sun, J. Zhong, J. S. Warmus and B. T. O’Neill, J. Org. Chem., 2015, 80, 7266–7274 CrossRef CAS PubMed; (d) C. Cristóbal, D. Gaviña, I. Alonso, M. Ribagorda, J. C. Carretero, C. del Pozo and J. Adrio, Chem. Commun., 2022, 58, 7805–7808 RSC.
- (a) K. Burger, A. Meffert and S. J. Bauer, J. Fluor. Chem., 1977, 10, 57–62 CrossRef CAS; (b) K. Tanaka, S. Nagatani, M. Ohsuga and K. Mitsuhashi, Bull. Chem. Soc. Jpn., 1994, 67, 589–591 CrossRef CAS; F. Laduron and H. G. Viehe, Tetrahedron, 2002, 58, 3543–3551 Search PubMed; (c) X. Zhang, M. Liu, W. Zhang, M. Legris and W. Zhang, J. Fluor. Chem., 2017, 204, 18–22 CrossRef CAS.
- A. Ponce, I. Alonso, J. Adrio and J. C. Carretero, Chem. – Eur. J., 2016, 22, 4952–4959 CrossRef CAS PubMed.
- M. Ma, Y. Zhu, Q. Sun, X. Li, J. Su, L. Zhao, Y. Zhao, S. Qiu, W. Yan, K. Wang and R. Wang, Chem. Commun., 2015, 51, 8789–8792 RSC.
- (a) J. Su, Z. Ma, X. Li, L. Lin, Z. Shen, P. Yang, Y. Li, H. Wang, W. Yan, K. Wang and R. Wang, Adv. Synth. Catal., 2016, 358, 3777–3785 CrossRef CAS; (b) X.-H. Chen, Q. Wei, S.-W. Luo, H. Xiao and L.-Z. Gong, J. Org. Chem., 2017, 82, 3482–3490 CrossRef PubMed.
- Q. Sun, X. Li, J. Su, L. Zhao, M. Ma, Y. Zhu, Y. Zhao, R. Zhu, W. Yan, K. Wang and R. Wang, Adv. Synth. Catal., 2015, 357, 3187–3196 CrossRef CAS.
- For reviews, see: (a) H.-Z. Gui, Y. Wei and M. Shi, Chem. – Asian J., 2020, 15, 1225–1233 CrossRef CAS PubMed; (b) B. Borah, N. S. Veeranagaiah, S. Sharma, M. Patat, M. S. Prasad, R. Pallepogu and L. R. Chowhan, RSC Adv., 2023, 13, 7063–7075 RSC; (c) Y. Liu, L. Wang, D. Ma and Y. Song, Molecules, 2023, 28, 2990–3032 CrossRef CAS PubMed; (d) F. R. Miankooshki, M. Bayat, S. Nasri and N. H. Samet, Mol. Diversity, 2023, 27, 2365–2397 CrossRef CAS PubMed.
- Z.-H. Wang, Z.-J. Wu, D.-F. Yue, W.-F. Hu, X.-M. Zhang, X.-Y. Xu and W.-C. Yuan, Chem. Commun., 2016, 52, 11708–11711 RSC.
- (a) Y. You, W.-Y. Lu, Z.-H. Wang, Y.-Z. Chen, X.-Y. Xu, X.-M. Zhang and W.-C. Yuan, Org. Lett., 2018, 20, 4453–4457 CrossRef CAS PubMed; (b) W.-R. Zhu, Z.-W. Zhang, W.-H. Huang, N. Lin, Q. Chen, K.-B. Chen, B.-C. Wang, J. Weng and G. Lu, Synthesis, 2019, 1969–1979 CAS.
- B. Li, F. Gao, X. Feng, M. Sun, Y. Guo, D. Wen, Y. Deng, J. Huang, K. Wang and W. Yan, Org. Chem. Front., 2019, 6, 1567–1571 RSC.
- (a) X. Zhao, J. Xiong, J. An, J. Yu, L. Zhu, X. Feng and X. Jiang, Org. Chem. Front., 2019, 6, 1989–1995 RSC; (b) X. Liu, D. Lu, J. H. Wu, J. P. Tan, C. Jiang, G. Gao and T. Wang, Adv. Synth. Catal., 2020, 362, 1490–1495 CrossRef CAS.
- C. Wang, D. Wen, H. Chen, Y. Deng, X. Liu, X. Liu, L. Wang, F. Gao, Y. Guo, M. Sun, K. Wang and W. Yanm, Org. Biomol. Chem., 2019, 17, 5514–5519 RSC.
- J. Chen, D. Zhu, X. Zhang and M. Yan, J. Org. Chem., 2021, 86, 3041–3048 CrossRef CAS PubMed.
- Y. Xiong, X. X. Han, Y. Lu, H. J. Wang, M. Zhang and X. W. Liu, Tetrahedron, 2021, 87, 132112 CrossRef CAS.
- J. Q. Zhao, S. Zhou, L. Yang, H. Y. Du, Y. You, Z. H. Wang, M. Q. Zhou and W. C. Yuan, Org. Lett., 2021, 23, 8600–8605 CrossRef CAS PubMed.
- T.-H. Li and D.-M. Du, Org. Biomol. Chem., 2022, 20, 817–823 RSC.
- (a) W. J. Juang, Q. Chen, N. Lin, X. W. Long, W. G. Pan, Y. S. Xiong, J. Weng and G. Lu, Org. Chem. Front., 2017, 4, 472–482 RSC; (b) Y. Zhi, K. Zhao, C. von Essen, K. Rissanen and D. Enders, Synlett, 2017, 2876–2880 CAS.
- Y. He, Y. Liu, Y. Liu, X. X. Kou, Q. Z. Li, J. H. Li, H. Z. Jiang, H. J. Leng, C. Peng and J. L. Li, Adv. Synth. Catal., 2020, 362, 2052–2058 CrossRef CAS.
- Y. X. Song and D. M. Du, J. Org. Chem., 2018, 83, 9278–9290 CrossRef CAS PubMed.
- C. Duffy, W. E. Roe, A. M. Harkin, R. McNamee and P. C. Knipe, New J. Chem., 2021, 45, 22034–22038 RSC.
- Y. Yi, Y. Z. Hua, H. J. Lu, L. T. Liu and M. C. Wang, Org. Lett., 2020, 22, 2527–2531 CrossRef CAS PubMed.
- R. L. Wang, S. K. Jia, Y. J. Guo, Y. Yi, Y. Z. Hua, H. J. Lu and M. C. Wang, Org. Biomol. Chem., 2021, 19, 8492–8496 RSC.
- S. H. Chen, Y. H. Miao, G. J. Mei, Y. Z. Hua, S. K. Jia and M.-C. Wang, Chem. – Eur. J., 2022, 28, e202103688 CrossRef PubMed.
- W. C. Yuan, L. Yang, J. Q. Zhao, H. Y. Du, Z. H. Wang, Y. You, Y. P. Zhang, J. Liu, W. Zhang and M. Q. Zhou, Org. Lett., 2022, 24, 4603–4608 CrossRef CAS PubMed.
- Z. H. Wang, J. H. Liu, Y. P. Zhang, J. Q. Zhao, Y. You, M. Q. Zhou, W. Y. Han and W. C. Yuan, Org. Lett., 2022, 24, 4052–4057 CrossRef CAS PubMed.
- W. Fan, C. Verrier, Y. Queneau and F. Popowycz, Curr. Org. Synth., 2019, 16, 583–614 CrossRef CAS PubMed.
- (a) P.-F. Koh and T.-P. Loh, Green Chem., 2015, 17, 3746–3750 RSC; (b) Y.-L. Su, Z.-Y. Han, Y.-H. Li and L.-Z. Gong, ACS Catal., 2017, 7, 7917–7922 CrossRef CAS.
- C. Cristóbal, C. Corral, J. C. Carretero, M. Ribagorda and J. Adrio, Chem. Commun., 2023, 59, 4336–4639 RSC.
- M. C. Kozlowski, B. J. Morgan and E. C. Linton, Chem. Soc. Rev., 2009, 38, 3193–3207 RSC.
- I. Maclean, E. Gallent, O. Orozco, A. Molina, N. Rodríguez, J. Adrio and J. C. Carretero, Org. Lett., 2024, 26, 922–927 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |