Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent development of azahelicenes showing circularly polarized luminescence
Chihiro
Maeda
 * and
Tadashi
Ema
* and
Tadashi
Ema
 *
*
Division of Applied Chemistry, Graduate School of Natural Science and Technology, Okayama University, Tsushima, Okayama 700-8530, Japan. E-mail: cmaeda@okayama-u.ac.jp; ema@cc.okayama-u.ac.jp
First published on 25th February 2025
Abstract
Recently, a variety of circularly polarized luminescence (CPL) dyes have been developed as next-generation chiroptical materials. Helicenes, ortho-fused aromatics, have been recognized as some of the most promising CPL dyes. Although typical carbohelicenes show CPL, weak fluorescence is often emitted in the blue region. In contrast, heteroatom-embedded helicenes (heterohelicenes) can show intense fluorescence and CPL in the visible region because heteroatoms alter the electronic states of helicene frameworks. Among various heterohelicenes, nitrogen-embedded helicenes (azahelicenes) have unique features such as facile functionalization and sensitive responses to acid/base or metal ions. Furthermore, polycyclic aromatic hydrocarbons (PAHs) containing azaborine units have been recognized as excellent luminescent materials, and the helical derivatives, B,N-embedded helicenes, have been rapidly growing recently. In this feature article, we review and summarize the synthesis and chiroptical properties of azahelicenes, which are classified into imine-type and amine-type azahelicenes and B,N-embedded helicenes. CPL switching systems of azahelicenes are also reviewed.
1. Introduction
Circularly polarized luminescence (CPL) is associated with the difference in intensities of the left-handed (IL) and right-handed circularly polarized light (IR), and CPL-active dyes have been actively studied for their potential application in 3D displays, security paints, information storage, and optical communications.1 CPL is generally evaluated using the luminescence dissymmetry factor, glum, defined as 2(IL − IR)/(IL + IR). A variety of chiral organic molecules and metal complexes with high glum values have been developed, although it has often been difficult to achieve high values of both fluorescence quantum yield (ΦF) and glum. Recently, CPL brightness (BCPL) defined as ε × ΦF × glum/2 has also been used to quantify the whole chiroptical performance including the molar extinction coefficient (ε) and ΦF along with glum.1fHelicenes are polycyclic aromatic hydrocarbons (PAHs) consisting of ortho-fused aromatic rings showing chiroptical properties derived from inherent helical chirality and have been recognized as some of the most promising CPL dyes.2 Although general carbohelicenes exhibit CPL, their fluorescence is often weak and emitted in the blue region. In addition, CPL and circular dichroism (CD) are more sensitive to substituents than to helical sense.3 On the other hand, the incorporation of heteroatoms into helicene frameworks can modulate the electronic states, and such heteroatom-embedded helicenes (heterohelicenes) show intense fluorescence and CPL in the visible to near infrared (NIR) region. Among the heterohelicenes, nitrogen-embedded helicenes (azahelicenes) have attracted considerable attention due to easy functionalization and derivatization and possible mutual interactions such as hydrogen bonding and metal coordination.
Azahelicenes can be classified into imine-type and amine-type (Fig. 1). The former often contains a pyridine ring, while the latter usually contains a pyrrole or carbazole ring. Imine-type nitrogen allows protonation to generate cationic species or coordination with metal ions, which enables a pH- or coordination-driven chiroptical switching. Amine-type nitrogen allows various functionalizations such as alkylation, arylation, borylation, and metal complexation, and such azahelicenes function as a chiral electron-donating unit.
Azaborines contain both nitrogen and boron atoms in an aromatic six-membered ring in place of two carbon atoms and have unique electronic states, aromaticity, and reactivities.4 Recently, PAHs containing azaborine unit(s) have been recognized as excellent luminescent materials such as thermally activated delayed fluorescence (TADF) emitters and organic light-emitting diodes (OLEDs).4c–e Very recently, helicenes with azaborine unit(s) have been actively studied for their potential application as chiral TADF materials and in CP-OLEDs.
Here, we summarize recent advances in CPL-active azahelicenes, classifying them into imine-type and amine-type azahelicenes and B,N-embedded helicenes containing azaborine units (Fig. 1). The key synthetic schemes to obtain azahelicenes and examples of CPL switching are highlighted.
2. Synthesis and CPL properties of azahelicenes
Although the distortion of helicenes sometimes hinders the synthesis, extensive and intensive efforts have been devoted to the development of synthetic methods for a variety of helicenes including long helicenes, multiple helicenes, and cationic helicenes. In this section, the synthesis of CPL-active azahelicenes is summarized. Some examples of the enantioselective synthesis are also highlighted.2.1. Azahelicenes with imine-type nitrogen
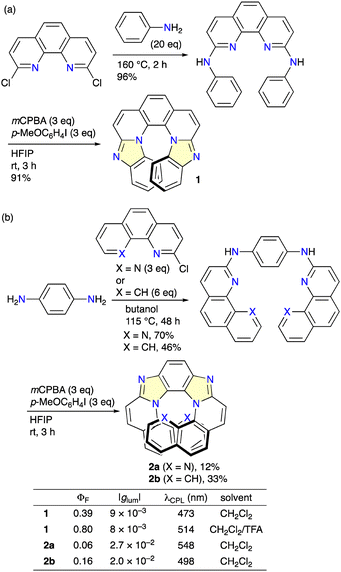
Otani, Shibata, and co-workers developed the facile two-step synthesis of tetraaza[7]helicene 1 from 2,9-dichloro-1,10-phenanthroline via the double amination reaction followed by hypervalent iodine-mediated intramolecular coupling (Scheme 1a).5a Racemate 1 was resolved by means of HPLC with a CHIRALCEL OD stationary phase into each enantiomer showing fluorescence and CPL with ΦF and |glum| values of 0.39 and 9 × 10−3, respectively. Interestingly, the ΦF value increased to 0.80 upon protonation with TFA. The same group also synthesized polyaza[9]helicenes 2a and 2bvia the reaction of p-phenylenediamine with 2-chloro-1,10-phenanthroline or 2-chlorobenzoquinoline followed by intramolecular coupling (Scheme 1b).5b2a and 2b showed even higher |glum| values of 2.7 × 10−2 and 2.0 × 10−2, respectively. The redshifted CPL suggests the structural change or aggregation in the excited state, which might contribute to large |glum| values.Mori, Miura, and co-workers reported the two-step synthesis of tetraaza[7]helicene 3 from indolocarbazole via direct arylation with 2-bromo-6-phenylpyridine, followed by dehydrogenative intramolecular coupling (Scheme 2).6 Racemic compound 3 was resolved by means of HPLC using a YMC CHIRAL ART amylose-SA stationary phase into each enantiomer showing CPL with a |glum| value of 4.4 × 10−3 at 429 nm, which increased to 5.8 × 10−3 at 509 nm upon protonation.
Gu and co-workers reported a ring-expansion strategy for the synthesis of α-aryl aza[7]helicene 4via the acid-mediated reaction of helical dinaphthofluoren-9-ols with NaN3 (Scheme 3).7 The reaction mechanism was proposed as follows. Carbocation Int-1 is generated via the protonation of the tertiary alcohol. Azido fluorene Int-2 was formed via SN1 type substitution. Subsequently, 1,2-migration/ring expansion occurs with the release of N2 to deliver N-heterocyclic cation species Int-3. Finally, aromatization/deprotonation of Int-3 produces azahelicene 4. Because the tertiary alcohols could be readily prepared by the reactions of helical ketones with Grignard reagents or organolithiums, a variety of azahelicenes were obtained. The azahelicenes showed CPL at around 490 nm with |glum| values of 2.2–4.5 × 10−3.
Lacour and co-workers have investigated the synthesis and chiroptical properties of cationic azahelicenes 5, which are of particular interest due to the chemical and configurational stabilities, easy preparation, and chiroptical properties in up to the far-red region (Fig. 2).8 Functionalization of the 6-position produced various cationic azahelicenes with tunable chiroptical properties, and CPL was recorded in the red region with a |glum| value of ca. 1 × 10−3.8a The authors also synthesized oligoacene-incorporated azahelicene 6 showing CPL at 640 nm both in acetonitrile solution and in the PMMA film with |glum| values of 1.4 × 10−3 and 8 × 10−4, respectively.8c
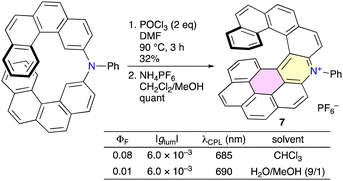
Olivier, Ferrand, and co-workers synthesized phenanthrene-fused cationic aza[7]helicene 7 (Scheme 4).9 When bis(benzo[c]phenanthren-2-yl)phenylamine was treated under Vilsmeier–Haack conditions, intramolecular cycloaromatization and simultaneous quaternarization occurred to form the dibenzoacridinium derivative, and the subsequent intramolecular dehydrogenative C–C coupling between one of the phenanthrene units and the neighboring pyridinium ring took place leading to the formation of 7. 7 was soluble both in organic and aqueous media (H2O/MeOH = 9/1) and showed CPL with |glum| values of 6 × 10−3 in both media after the optical resolution by means of HPLC on a CHIRALPAK IA stationary phase.
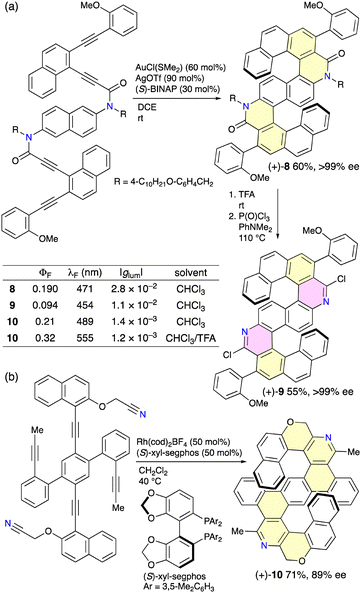
The development of efficient methods for the enantioselective synthesis of heterohelicenes is important and challenging. Tanaka and co-workers achieved the enantioselective synthesis of S-shaped double azahelicenes (Scheme 5).10 The gold-catalyzed sequential intramolecular hydroarylation of alkynes in the presence of AgOTf and (S)- or (R)-BINAP produced S-shaped double azahelicene 8 in 60% yield with an enantiomeric excess (ee) of >99% (Scheme 5a).10a Removal of the 4-alkoxybenzyl groups on the nitrogen atoms followed by chlorination afforded double azahelicene 9 possessing two pyridine units without racemization. 8 and 9 showed intense CPL with |glum| values of 2.8 × 10−2 and 1.1 × 10−2, respectively. The authors also reported the rhodium-catalyzed intramolecular [2+2+2] cycloaddition of cyanodiynes in the presence of (S)-xyl-segphos giving S-shaped double azahelicene-like molecule 10 in 71% yield with 89% ee (Scheme 5b).10b10 showed fluorescence at 489 nm with a ΦF value of 0.21, which increased to 0.32 at 555 nm upon protonation with TFA. 10 is also CPL-active with |glum| values of 1.4 × 10−3 in the neutral state and 1.2 × 10−3 in the cationic state.
Zhong, Luo, Zhu, and co-workers achieved the palladium-catalyzed modular synthesis of pyridohelicenes through double cyclization reactions, and a variety of pyrido[6]helicenes and furan-containing pyrido[7]helicenes were obtained by the enantioselective reactions of diisocyanides with aryl iodides.11 A representative example is shown in Scheme 6. (P)-/(M)-Enantiomers of 11 exhibited mirror-image CD spectra with absorption dissymmetry factors (gabs) of +3.9/–5.0 × 10−3 at 340 nm, and they also exhibited mirror-image CPL with a |glum| value of 6.25 × 10−4 at 430 nm. The origin of enantioselectivity was studied by DFT calculations on the second pyridine ring formation. A pivalate ion-assisted concerted metalation–deprotonation (CMD) takes place in the enantioselectivity-determining step. The formation of the major enantiomer is 3.4 kcal mol−1 more favorable than that of the minor enantiomer. This calculated energy difference corresponds to 98% ee, which agrees with the experimental result (98% ee). Clearly, this energy difference in the C–H bond activation is the key to the highly enantioselective reaction.
2.2. Azahelicenes with amine-type nitrogen
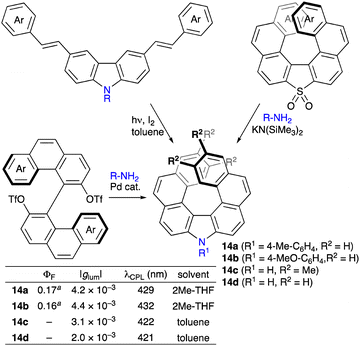
Carbazole is known as a planar tricyclic aromatic showing highly emissive and electron-conducting abilities and chemical stabilities. From another viewpoint, carbazole can be regarded as an aza[3]helicene, and π-extension at the 3,4- and 5,6-positions leads to aza[n]helicenes showing chiroptical properties. Hiroto, Shinokubo, and co-workers developed the synthesis of aza[5]helicene 12via the oxidative coupling of a 2-aminoanthracene derivative with DDQ in the presence of EtOH (Scheme 7a).12a Thanks to the bulky triisopropylsilyl groups, optically active compound 12 was resolved by means of HPLC using a CHIRALPAK IA stationary phase and showed fluorescence and CPL with a ΦF value of 0.36 and a |glum| value of 3 × 10−3. The same group also synthesized figure-eight aza[5]helicene dimer 13 showing more intense CPL with a ΦF value of 0.58 and a |glum| value of 8.5 × 10−3, and the BCPL reached 152 M−1 cm−1 (Scheme 7b).12bThe photocyclization of 3,6-bis(styryl)carbazoles giving aza[7]helicenes is an efficient and practical method because the substrates are readily prepared via the Mizoroki–Heck reaction of 3,6-dihalogenated carbazoles (Scheme 8).13 On the other hand, similar aza[7]helicenes were also synthesized via the double amination reaction14 or SNAr reaction of thia[7]helicene S,S-dioxides.15 Nakano and co-workers investigated the chiroptical properties of 14a and 14b, and |gabs| and |glum| values of 4 × 10−3 were recorded.15 Wang, Wang, and co-workers found that tetramethyl-substituted aza[7]helicene 14c showed an enhanced racemization barrier and chiroptical performance with a |glum| value of 3.1 × 10−3 as compared to unsubstituted aza[7]helicene 14d (2.0 × 10−3).14c
Maeda, Ema, and co-workers developed the facile synthesis of aza[7]helicene 15via the intramolecular oxidative aromatic coupling of 3,6-bis(1,1′-biphenyl-2-yl)carbazole (Scheme 9a).16a Attachment of benzoxazole or benzothiazole to 15, followed by the boron complexation produced chiral BODIPY analogues 16a and 16b showing CPL with glum values of 8.7 × 10−4 and 7.0 × 10−4, respectively. Furthermore, (R)-BINOL was attached to the boron atom via the Et2AlCl-mediated reaction developed by the same group.17 The chiroptical properties of 17a and 17b were enhanced by the attachment of axial chirality. Interestingly, the (R,P)-diastereomers showed somewhat higher glum values (−1.5 × 10−3/−1.2 × 10−3 for 17a/17b) than the (R,M)-diastereomers (+1.2 × 10−3/+8.8 × 10−4 for 17a/17b), suggesting the enhancement effect of chirality by the (P)-azahelicene and (R)-binaphthyl group. They also investigated the oxidative aromatic coupling of several 3,6-bis(2-arylphenyl)carbazoles, and the corresponding aza[7]helicenes 18a–c and hetero[9]helicenes 18d–f were synthesized (Scheme 9b).16b Interestingly, triple helicene 18g was obtained from the 3,6-bis(2-naphthylphenyl)carbazole via double rearrangement. This rearrangement was controlled by the N-substituents of the carbazoles; Similar rearrangement products were obtained from the N-ethyl and N-4-tert-butylphenyl carbazoles, while the aza[9]helicene was obtained from the N-benzoyl carbazole.16c These azahelicenes showed CPL with |glum| values in the range of 2.9 × 10−4–3.5 × 10−3.
 | ||
| Scheme 9 (a) Synthesis of aza[7]helicene 15 and derivatized boron complexes 16 and 17. (b) Synthesis of aza[7]helicenes and aza[9]helicenes 18. | ||
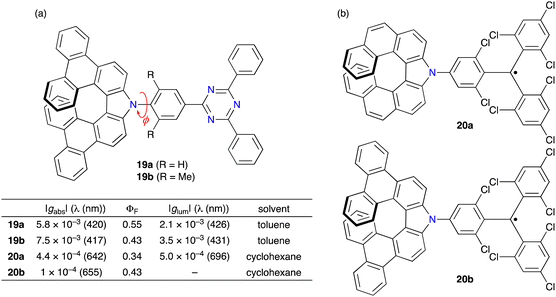
Because of the synthetic ease and potential applications as a chiral electron-donating unit, azahelicene 15 was also used by other groups.18–21 Wang and co-workers synthesized donor–acceptor (D–A) type CPL materials 19 composed of azahelicene 15 and triphenyltriazine (Fig. 3a).18 DFT calculations of 19a suggested that the magnitude of the transition dipole moment |μ| and magnetic dipole moment |m| was highly dependent on the dihedral angle (ϕ) of the D–A moiety and that the minimum |μ| value was calculated at a ϕ value of 105°. Because the μ and m were aligned in parallel at any ϕ, a maximum glum was expected at 105°. Based on the calculations, 19b with two methyl groups was also prepared since the ϕ value of the calculated structure of 19b was 92°, close to the ideal value of 105°. 19a and 19b showed similar CPL responses at 390–500 nm, while 19b showed a higher |glum| value of 3.5 × 10−3 than 19a (2.1 × 10−3). This work provides a new insight to boost the |glum| value of D–A type CPL materials by modulating the dihedral angle (ϕ).
Ravat, Kuehne, and co-workers synthesized aza[7]helicenes 20 functionalized with triphenylmethyl radicals via the Buchwald–Hartwig amination of iodinated-tris(2,4,6-trichlorophenyl)methyl radical with 14d or 15 (Fig. 3b).19 These radicals were stable enough to be isolated and resolved into the enantiomers. 20a and 20b displayed broad absorption bands at around 650 nm originating from the transition of the highest occupied molecular orbital (HOMO) to singly occupied molecular orbital (SOMO) and fluorescence at around 700 nm with ΦF values of ca. 0.4. Optically active 20a showed CPL with a |glum| value of 5.0 × 10−4, while 20b was CPL-inactive under the measurement conditions.
Zhang, Chen, and co-workers synthesized double aza[7]helicene 21via the KMnO4-mediated N–N coupling of the brominated aza[7]helicene, followed by the Yamamoto coupling (Scheme 10).2021 showed a relatively weak absorption band at 560 nm probably due to the existence of the central N2C4 core with antiaromatic character. Optical resolution of 21 was performed by means of HPLC using a CHIRALPAK IA-3 stationary phase to furnish (P,P)-21 and (M,M)-21 showing mirror-imaged CD along with the achiral compound (P,M)-21. The optically active compound (P,P)-21 showed red CPL with a ΦF value of 0.86, a |glum| value of 2.2 × 10−4, and a BCPL value of 13.2 M−1 cm−1. 21 also showed solid-state fluorescence with a ΦF value of 0.10, and CPL was recorded in the film state with a |glum| value of 2.0 × 10−4. Furthermore, cyclic voltammetry (CV) measurements of 21 revealed two reversible one-electron oxidation waves at +0.30 and +0.73 V (vs. Fc+/Fc in CH2Cl2). Hence, chemical oxidation of 21 was also conducted using NOSbF6, which generated radical cation 21˙+ and dication 212+ in two steps. Nucleus-independent chemical shift (NICS) values of the central N2C4 core were calculated to be +9.25 and −10.68 for 21 and 212+, respectively, indicating switching from antiaromaticity to aromaticity character. This aromaticity and antiaromaticity switching was further supported by the anisotropy of the induced current density (ACID) calculations and harmonic oscillator model of aromaticity (HOMA) values.
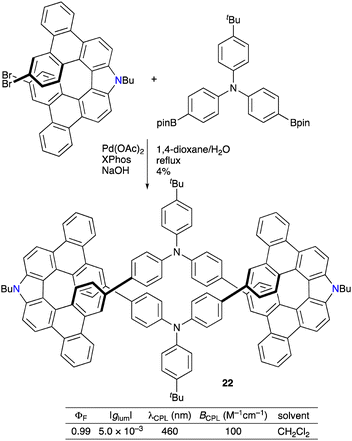
Li, Jin, Chen, and co-workers synthesized triphenylamine-bridged aza[7]helicene dimer 22via the Suzuki–Miyaura coupling of racemic dibromoaza[7]helicene and triphenylamine 4,4′-diboronic acid pinacol ester (Scheme 11).21 Interestingly, meso compound (P,M)-22 was not detected. Optical resolution of 22 was performed by means of HPLC on a CHIRALPAK IG-3 stationary phase to furnish (P,P)-22 and (M,M)-22 showing mirror-imaged CD with a |gabs| value of 2.5 × 10−3 at 435 nm and CPL with a |glum| value of 5.0 × 10−3 at 460 nm. The ΦF value was nearly quantitative (0.99), and the BCPL value reached 100 M−1 cm−1. Furthermore, the chemical oxidation of electron-rich chiral macrocycle 22 with NOSbF6 generated a tetraradical cation species as confirmed by the UV/vis-NIR absorption and ESR spectroscopy.
Casado, Liu, and co-workers prepared hexa-peri-hexabenzocoronene (HBC)-fused aza[7]helicene 23 (Fig. 4).2223 adopts a twist-helix geometry, while the energy barrier for racemization dramatically increases to 143 kcal mol−1, which is much higher than the value of typical aza[7]helicenes such as 15 (ca. 30 kcal mol−1). 23 exhibited excellent chiroptical properties with a |gabs| value of 1.0 × 10−2, a |glum| value of 7.0 × 10−3, and a BCPL value of 95.2 M−1 cm−1.
Expanded helicenes, which are defined as helicene analogues containing linearly fused aromatic rings in the helical structures, are promising candidates for molecular springs due to their large helical diameter. However, the optical resolution has rarely been achieved because of the low racemization barrier. In addition, it is also difficult to achieve high glum values due to the flexible structures in the excited state. Yang, Wu, and co-workers reported the synthesis of expanded azahelicenes 24a–e consisting of up to 43 fused rings via the Suzuki–Miyaura coupling of 3,6-dibromo-2,7-bis(2-methoxyvinyl)carbazole and anthracene 1,8-diboronic ester and the subsequent Bi(OTf)3-catalyzed cyclization (Scheme 12).23a24a–e were obtained in 81% total yields in two steps and easily separated by means of gel permeation chromatography (GPC). 24b–d were characterized by X-ray diffraction analysis and resolved by means of HPLC on a CHIRALPAK IG stationary phase into each enantiomer. They showed CPL with |glum| and BCPL values of up to 2.1 × 10−2 and 76 M−1 cm−1, respectively, which are superior to the all-carbon counterparts.23b
It is quite challenging to synthesize longer helicenes. Tanaka and co-workers reported the synthesis of benzannulated aza[n]helicenes 25a–f (n = 9–19) via the one-shot oxidative fusion reaction of ortho-phenylene-bridged oligopyrroles with PIFA (Scheme 13a).24a The structures of all the aza[n]helicenes were characterized by X-ray diffraction analysis, which revealed the triple-layer helixes of the aza[17]helicene and aza[19]helicene for the first time. 25a–d were butylated with an excess amount of butyl iodide, and the optical resolution of the N-butylated azahelicenes was determined to give two fractions showing mirror-imaged CD and CPL spectra with |glum| values of 1.7–5.7 × 10−3 in THF. The same group also reported the two-step synthesis of benzannulated double aza[9]helicenes via the Suzuki–Miyaura coupling of 1,2,4,5-tetrabromobenzene and 5-{2-(indol-2-yl)phenyl}pyrrole 2-boronic ester and the subsequent oxidative fusion reaction using PIFA.24b Optical resolution of N-alkylated double aza[9]helicenes 26-Et and 26-Bu was achieved, and the optically active double helicenes showed fluorescence and CPL with ΦF and |glum| values of 0.35 and 1 × 10−3, respectively (Scheme 13b).
 | ||
| Scheme 13 (a) Synthesis of benzannulated aza[n]helicenes 25 up to n = 19. (b) Benzannulated double aza[9]helicenes 26. | ||
Hu and co-workers reported the two-step synthesis of double aza[7]helicene 27via the Suzuki–Miyaura coupling of 4,4′′-di-tert-butyl-2,2′′,6,6′′-tetrabromo-p-terphenyl and 9-phenylcarbazole-2-boronic acid and the subsequent Scholl reaction using DDQ/TfOH (Scheme 14).25 Interestingly, the use of a larger amount of DDQ or the further reaction of 27 furnished aza-nanographene 28 with two octagons, which bound C60 and C70 with binding constants (Ka) of 9.5 × 103 and 3.7 × 104 M−1, respectively. DFT calculations and VT-NMR analysis indicated the fast racemization of 28 at rt, while 27 was resolved by means of HPLC on a CHIRALPAK IE stationary phase into each enantiomer showing fluorescence and CPL with ΦF and |glum| values of 0.45 and 2.4 × 10−3, respectively. The BCPL was calculated to be 173 M−1 cm−1, which was among the highest values for ever-reported helical molecules.
Ito, Jin, and co-workers demonstrated the crystalline-induced chirality transfer in conformationally flexible aza[5]helicene Au(I) complexes inducing CPL (Scheme 15).26 Chiral NHC Au(I) complexes (S,S)- and (R,R)-29 were obtained from (S,S)- or (R,R)-NHC Au(I) chloride and aza[5]helicene in 96% and 92%, respectively. (S,S)-29 exhibited dynamic chirality in the aza[5]helicene moiety because of the low flipping barrier, and CPL was inactive in solution. In sharp contrast, the chiral diphenyl moieties of the (S,S)-NHC ligand interacted with the aza[5]helicene unit of another complex in the crystal to induce (M)-chirality in the aza[5]helicene, and the crystal showed CPL at 390–470 nm with a |glum| value of 3 × 10−3.
 | ||
| Scheme 15 Crystalline-induced chirality transfer in the aza[5]helicene Au(I) complex (S,S)-29. R = 3,5-di-tert-butylbenzyl. | ||
As shown above, most of the optically active azahelicenes were resolved by means of chiral HPLC on a small scale. Therefore, the development of the efficient preparation of optically active helicenes has been desired for wide applications. Diastereomer methods are one of the promising strategies to separate (P)- and (M)-diastereomers of helicenes or their precursors by silica gel chromatography. Yorimitsu and co-workers reported the systematic asymmetric synthesis of dihetero[8]helicenes 30 from common intermediate 31, which was resolved into optically active species by the diastereomer method (Scheme 16).27 Treatment of (rac)-31 with (+)-10-camphorsulfonyl chloride gave diastereomers 31′, which were successfully separated by silica gel chromatography into (S,S)-31′ and (R,R)-31′. The sulfonyl groups could be removed with NaBH4. The optically active 31 was converted into bis-sulfone 32 and dithiol 33, and the subsequent cyclization reactions of 32 and 33 furnished dioxa[8]helicene 30a and dithia[8]helicene 30b, respectively. Dithia[8]helicene 30b was oxidized to the corresponding tetraoxide 30c, which was further converted into diaza[8]helicene 30d and spiro-shaped helicenes 30e and 30f. Importantly, the enantiopurities were mostly retained, and these dihetero[8]helicenes were obtained in enantiomerically pure forms (>97% ee). These [8]helicenes were CPL-active with the |glum| in the range of 7.6 × 10−4–9.5 × 10−3, among which diaza[8]helicene 30d recorded the highest |glum| value of 9.5 × 10−3 with a ΦF value of 0.13.
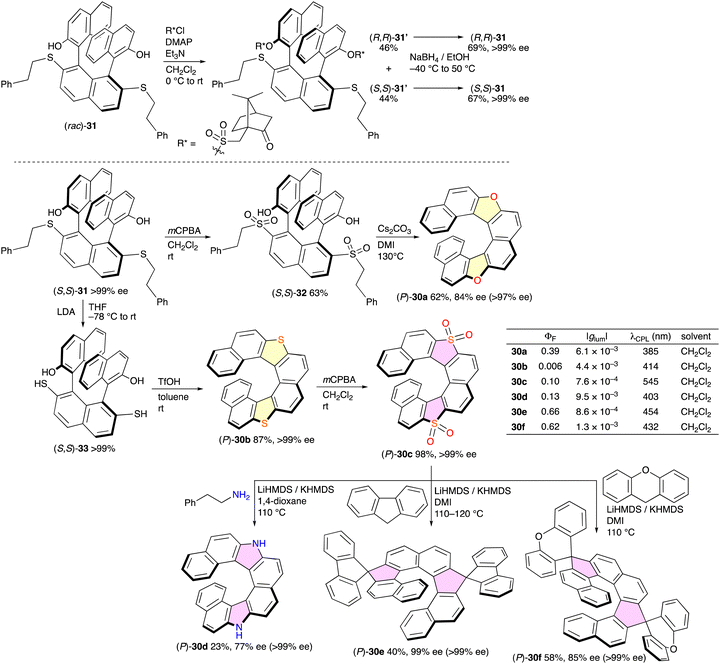 | ||
| Scheme 16 Synthetic routes to dihetero[8]helicenes 30a–f. Values of ee in parenthesis are those after recrystallization. | ||
Maeda, Ema, and co-workers prepared diastereomeric azahelicenes with (1R)-menthylcarbonate groups (Scheme 17).28 The azahelicenes and closed azahelicenes were selectively synthesized under different conditions of the Scholl reactions, and the (P)- and (M)-diastereomers were separated by silica gel chromatography. The optically active aza[7]helicenes were converted into the corresponding butadiyne-bridged dimers (P,P)- or (M,M)-34 and 35 showing CPL with BCPL values of 17–31 M−1 cm−1, which were higher than the corresponding monomers (2.3–6.7 M−1 cm−1). Furthermore, 34 and 35 were found to recognize a fluoride ion selectively with binding constants (Ka) of up to 2.0 × 105 M−1, which induced fluorescence and CPL in the red region.
 | ||
| Scheme 17 Synthesis of diastereomeric aza[7]helicenes and conversions into cyclic aza[7]helicene dimers 34 and 35. | ||
Chen, Zhou, and co-workers reported an organocatalytic central-to-helical chirality conversion strategy for the enantioselective synthesis of indolohelicenes.29 The best chiral phosphoric acid (CPA) was selected for the cycloaddition–elimination reactions between azonaphthalenes and enecarbamates to give indolohelicenoids in high yields and enantioselectivities. A representative example is shown in Scheme 18. The indolohelicenoid 36 was successfully oxidized with DDQ to give indolohelicene 37. (P)-/(M)-36 showed two CD signals at around 310 and 390 nm with maximum gabs of +3.0/−2.7 × 10−3 at 398 nm, while (P)-/(M)-37 showed three CD signals at 341, 391, and 415 nm with maximum gabs of +4.2/−2.8 × 10−3 at 343 nm. A clear mirror-image relationship was observed for the CPL spectra of (P)-/(M)-enantiomers with maximum glum of +9.8/−9.6 × 10−4 at 435 nm.
2.3. B,N-embedded helicenes
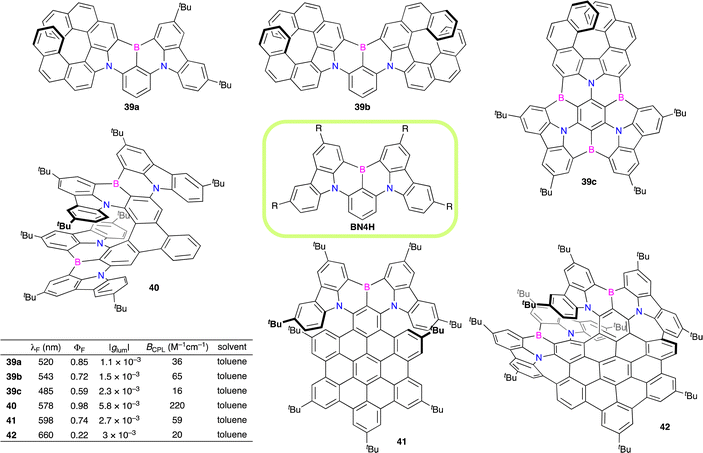
Zhang, Duan, and co-workers developed B,N-embedded double helicenes 38a and 38b as deep-red emitters for highly efficient OLEDs (Scheme 19).30a The double helicenes were readily synthesized in two steps from carbazoles and 1,4-dibromo-2,3,5,6-tetrafluorobenzene without using transition metal catalysts. The para-positioned B atoms and N atoms enhance the electronic coupling to allow for the formation of restricted π-bonds on the benzene core for delocalized excited states and a narrow energy gap. In addition, the mutually ortho-positioned B and N atoms also induce a multi-resonance effect on the peripheral skeleton for non-bonding orbitals, creating shallow potential energy surfaces to suppress the nonradiative transition. The HOMO and LUMO exhibit alternate distribution, which induces a small energy gap between S1 and T1. As a result, 38a and 38b showed efficient TADF emission at 662 nm and 692 nm with the ΦF reaching 100%. OLEDs were further constructed to record a maximum external quantum efficiency (EQE) of 28%. Wang and co-workers synthesized 38a–c to investigate the chiroptical properties.30b Racemates 38a–c were resolved by means of HPLC using a CHIRALPAK IE stationary phase into each enantiomer showing CPL with a |glum| value of 2 × 10−3. BCPL values of 38a–c were determined to be 29, 37, and 40 M−1 cm−1, respectively, representing the highest BCPL values in helicenes showing CPL in the red-NIR region.After the development of 38 with the multi-resonance effect, various CPL-active helicenes containing the B,N-embedded [4]helicene (BN4H) segment(s) have been investigated (Fig. 5).31 Ravat and co-workers developed B,N-embedded helicenes 39a and 39b comprising aza[7]helicene in place of the carbazole(s), which showed sharp fluorescence and CPL.31a39a and 39b showed ΦF values of 0.85 and 0.72, |glum| values of 1.1 × 10−3 and 1.5 × 10−3, and BCPL values of 36 and 65 M−1 cm−1, respectively. The fluorescence and CPL bands are narrow (full width at half maximum (FWHM) of 23–28 nm in toluene), which are essential for application to CP-OLEDs. The authors also synthesized 39c showing ultranarrow band fluorescence (FWHM of 17 nm in toluene) and CPL (FWHM of 18 nm in toluene) with a ΦF value of 0.59 and |glum| value of 2.3 × 10−3.31b
Li, Chen, and co-workers synthesized B,N-embedded [9]helicene 40, in which two BN4Hs were fused to triphenylene.31c40 showed bright photoluminescence with a ΦPL value of 0.98 and CPL with a |glum| value of 5.8 × 10−3. Furthermore, CP-OLED devices incorporating 40 as an emitter showed a maximum EQE of 35.5%, a small FWHM of 48 nm, and a high |gEL| value of 6.2 × 10−3. The Q-factor (EQE × |gEL|) of CP-OLEDs was determined to be 2.2 × 10−3, which was the highest among helicene analogues.
Furthermore, BN4H-fused HBCs such as 41 and 42 with TADF character were developed by Tan, Zhang, and co-workers and Liu and co-workers, respectively.31d,e41 and 42 showed CPL with |glum| values of 2.7 × 10−3 and 3 × 10−3, respectively. Interestingly, the treatment of 42 with TBAF generated a difluoride adduct with the tetracoordinate borons as confirmed by UV/vis and CD spectroscopy.
Yang and co-workers succeeded in the gram-scale synthesis of multiple helicenes 43 and 44 containing 1,2-azaborine units via the Suzuki–Miyaura coupling of 1,2,4,5-tetrabromobenzene and 3,6-di-tert-butylcarbazole-1-boronic acid pinacol ester and the subsequent boron insertion and the Scholl reaction (Scheme 20).3244 adopts a (P,P)- or (M,M)-configuration, and the (P,M)-configuration, less stable by 12.07 kcal mol−1, was not observed. Although the optical resolution of 43 was unsuccessful because of instability, racemate 44 was readily resolved by means of HPLC on a CHIRALPAK ID stationary phase into the enantiomers, which showed fluorescence with a ΦF value of 0.65 and CPL with a |glum| value of 1.0 × 10−3. Furthermore, 44 underwent the stepwise recognition of fluoride ions to generate monofluoride and difluoride adducts, which also showed CPL. The ΦF and |glum| values of the former were 0.99 and 6 × 10−4, respectively, while those of the latter were 0.90 and 7 × 10−4, respectively. Furthermore, the authors confirmed that the addition of BF3·OEt2 to the fluoride adducts regenerated the parent tricoordinate boron species 44.
Staubitz and co-workers synthesized [5] and [6]helicenes 45a and 45b comprising two 1,2-azaborine rings via Suzuki–Miyaura coupling and metal-catalyzed cyclization (Scheme 21).3345a had a relatively high racemization barrier (t1/2rac ≈ 80 min at 60 °C and ΔG‡ = 25.7 kcal mol−1), while racemization of 45b did not occur even at 200 °C for 14 h. Optical resolution of 45a and 45b was performed by means of HPLC on a CHIRALPAK IA stationary phase into enantiomers showing mirror-imaged CD and CPL with |gabs| and |glum| values of 9.9 × 10−3 and 4.2 × 10−3, respectively, for 45a and 1.1 × 10−2 and 1.3 × 10−2, respectively for 45b. The BCPL values of 45a and 45b were 10 and 59 M−1 cm−1, respectively, which were much higher than those of carbohelicenes despite the similar structural geometries.
Liu and co-workers synthesized benzo-extended heli(aminoborane)s 46a and 46b as a B,N-embedded [8]helicene and [10]helicene, respectively (Fig. 6).3446a and 46b had significant racemization barriers of 46.92 and 40.61 kcal mol−1, respectively and were resolved by means of HPLC on a CHIRALPAK IF stationary phase into the enantiomers. Optically active compounds 46a and 46b exhibited excellent chiroptical properties with the |gabs| values of 0.036 and 0.061, |glum| values of 0.024 and 0.048, and BCPL values of 294 and 292 M−1 cm−1, respectively.
3. Azahelicenes showing CPL switching
Chiral dyes with multiple interconvertible states can exhibit a chiroptical switch by external stimuli. Although CPL switching systems have been growing in the last decade,35 they are still limited as compared to CD switching systems, especially for helicene-based ones. Unlike carbohelicenes, azahelicenes are good candidates for CPL switching systems because the nitrogen-containing functional groups can respond to external stimuli such as protonation, metal coordination, and oxidation.Autschbach, Crassous, and co-workers reported pioneering works on the chiroptical switches of bis(pyridine)-containing aza[6]helicenes (Scheme 22).36 2-Pyridyl-aza[6]helicene 47 (Scheme 22a) was synthesized in two steps from 2,2′-bipyridine-6-carbaldehyde by the Wittig reaction, followed by a photocyclization reaction.36a The racemate was readily resolved by means of HPLC on a CHIRALPAK IC stationary phase into the enantiomers, which were converted into cycloplatinated derivative 47Pt. Both 47 and 47Pt underwent protonation with HBF4 to give 47·2H+ and 47Pt·H+, respectively, which returned with Na2CO3 or Et3N to the neutral forms. Optically active 47 and 47Pt showed CPL and circularly polarized phosphorescence (CPP) with |glum| values of 3 × 10−3 at around 426 nm and 1 × 10−3 at around 547 nm, respectively. The protonated forms were also CPL and CPP active, and the |glum| values of 3 × 10−3 at around 590 nm and 2 × 10−3 at around 555 nm were recorded, respectively. Thus, the acid/base triggered switching of CPL and CPP was achieved. The same group also reported CPL switching systems based on bis-aza[6]helicenes 48 and 49 upon Zn2+ binding or protonation (Scheme 22b and c).36b,c
 | ||
| Scheme 22 CPL switching systems based on bis(pyridine)-containing aza[6]helicenes (a) 47, (b) 48, and (c) 49. TPEN = N,N,N′,N′-tetrakis(2-pyridylmethyl)ethane-1,2-diamine. | ||
Pieters, Champagne, Audisio, and co-workers synthesized 24 kinds of azahelicenes such as 50 through 1,3-dipolar cycloaddition of sydnones with arynes (Scheme 23).37 Two enantiomers (+)-50 and (−)-50 were successfully separated by HPLC on a CHIRALPAK IC stationary phase. The pyridine-containing azahelicene 50 exhibited high proton affinity, which allowed a chiroptical switch. Whereas (+)-50 showed positive CPL at 430 nm with a glum of +1.1 × 10−3, (+)-50·H+ showed negative CPL at 585 nm with a glum of −1.2 × 10−3 upon protonation of (+)-50 with TFA. Deprotonation was successfully conducted with DBU, and this reversibility enabled at least three-time switches without CPL deterioration. Thus, the pH-triggered chiroptical switch with the distinct color change and sign-inversion was achieved upon protonation and deprotonation with acid and base, respectively.
 | ||
| Scheme 23 Synthesis of azahelicene 50 through 1,3-dipolar cycloaddition of sydnones with arynes and the pH-triggered chiroptical switch. | ||
Tanaka and co-workers have synthesized heteroatom-doped PAHs via the oxidative fusion reaction of ortho-phenylene-bridged cyclic pyrrole/thiophene pentamers. Interestingly, the oxidation of the pentapyrroles with DDQ/Sc(OTf)3 in toluene at reflux gave pentaaza[10]circulene 51, while that with PIFA in CH2Cl2 at −78 °C gave closed-aza[7]helicene 52a (Scheme 24).38a The authors later conducted the oxidation of the substrates containing two or three thiophenes in place of the pyrroles with DDQ/Sc(OTf)3 or FeCl3, which gave the corresponding closed-[7]helicenes 52b and 52c.38b Surprisingly, closed-[7]helicene dimers (52b)2 and (52c)2 were obtained when PIFA was used as an oxidant. Furthermore, the closed azahelicenes were found to be interconvertible between the monomers, 52b and 52c, and dimers, (52b)2 and (52c)2, via the chemical oxidation and photoreduction. Because only the monomers showed fluorescence and CPL with the |glum| values of 4 × 10−4 for 52b and 1.2 × 10−3 for 52c, the dimers functioned as light-driven turn-ON CPL emitters.
 | ||
| Scheme 24 Synthesis of pentaaza[10]circulene 51, closed-azahelicenes 52a–c, and dimers (52b)2 and (52c)2. | ||
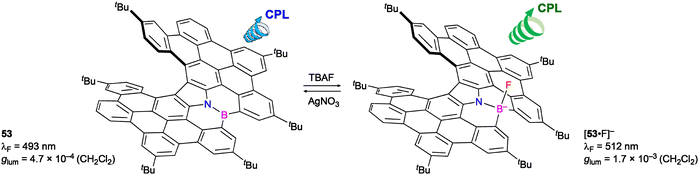
Maeda, Ema, and co-workers synthesized B,N-embedded π-extended helicene 53 (Scheme 25).39 The three-coordinate boron moiety recognized anions to form four-coordinate boron species such as 53·F− showing red-shifted and enhanced CPL. The four-coordinate boron species was converted back to 53 with Ag+, and the ion-triggered chiroptical switch was demonstrated.
4. Conclusions
In this feature article, we have summarized recently developed azahelicenes showing CPL. Representative examples are listed in Table 1. A variety of CPL-active azahelicenes have been synthesized by oxidative coupling, cyclization of alkynes or alkenes, photocyclization, and so on. Enantioselective synthesis has also been developed with high ee values reaching >99%. The azahelicenes with imine-type nitrogen undergo protonation or coordination with metal ions, and pH- or coordination-driven CPL switching systems have been reported. The azahelicenes with amine-type nitrogen allow facile functionalization such as alkylation, arylation, borylation, and metal complexation, which led to the development of various azahelicene derivatives showing CPL with tunable chiroptical properties. Furthermore, B,N-embedded helicenes have been rapidly growing quite recently because of its potential application as chiral TADF materials and in CP-OLEDs. Some derivatives show fluorescence and CPL in the red-NIR regions with quite high ΦF values, which leads to high BCPL values and high EQE in the OLEDs. The chiroptical performance has greatly advanced, while it is still difficult to achieve high values of both ΦF and glum in the NIR region partly because nonradiative decay often accelerates in the longer wavelength region to decrease ΦF and because simple π-extension increases the planarity and decreases glum. Nevertheless, such problems will be solved by combining π-extension, intra- and intermolecular electronic coupling, and molecular recognition systems in the near future. We believe that this feature article will be useful for the further development of new CPL-active helicenes in the future.| Aza[n]helicene | Φ F | |glum| (× 10−3) | B CPL (M−1 cm−1) | λ (nm)a | Solvent | Remark | Ref. | |
|---|---|---|---|---|---|---|---|---|
| a Maximum wavelength of fluorescence or CPL. b Data not available. | ||||||||
| 1 | n = 7 | 0.39 | 9 | 15 | 473 | CH2Cl2 | Φ F = 0.80 with TFA | 5a |
| 3 | n = 7 | 0.14 | 4.4 | 19 | 429 | CHCl3 | λ = 509 nm with TFA | 6 |
| 4d | n = 7 | 0.073 | 3.0 | 7.4 | 490 | CH2Cl2 | Solid-state fluorescence | 7 |
| 6 | n = 6 | 0.21 | 1.4 | 1.2 | 640 | CH3CN | Cationic aza[6]helicene | 8c |
| 7 | n = 7 | 0.08 | 6.0 | 14 | 685 | CHCl3 | Cationic aza[7]helicene | 9 |
| 8 | n = 6 | 0.19 | 28 | 67 | 471 | CHCl3 | Enantioselective synthesis | 10a |
| 11 | n = 6 | 0.059 | 0.63 | —b | 430 | CH2Cl2 | Enantioselective synthesis | 11 |
| 13 | n = 5 | 0.55 | 8.5 | 152 | 588 | CH2Cl2 | Figure-eight azahelicene dimer | 12b |
| 14a | n = 7 | 0.17 | 4.2 | —b | 429 | 2Me-THF | Various derivatives | 15 |
| 15 | n = 7 | 0.31 | 1.9 | 8.8 | 428 | CH2Cl2 | Various derivatives | 16a |
| 19b | n = 7 | 0.43 | 3.5 | 38 | 431 | toluene | D–A system | 18 |
| 20a | n = 7 | 0.34 | 0.5 | 4 | 696 | cyclohexane | Radical | 19 |
| 21 | n = 7 | 0.86 | 0.22 | —b | 583 | CH2Cl2 | Aromaticity switching | 20 |
| 22 | n = 7 | 0.99 | 5.0 | 100 | 460 | CH2Cl2 | Azahelicene dimer | 21 |
| 23 | n = 7 | 0.32 | 7.0 | 95 | 588 | CH2Cl2 | Large racemization barrier | 22 |
| 24d | — | 0.066 | 21 | 76 | 530 | CH2Cl2 | Expanded azahelicene | 23a |
| 25d-Bu | n = 15 | 0.07 | 5.7 | 13 | 519 | THF | Longest CPL-active helicene | 24a |
| 26-Bu | n = 9 | 0.35 | 1 | 19 | 522 | THF | Double helicene | 24b |
| 27 | n = 7 | 0.45 | 2.4 | 173 | 531 | CH2Cl2 | Double helicene | 25 |
| 29 | n = 5 | —b | 3 | —b | 410 | crystal | Crystallization-induced CPL | 26 |
| 30d | n = 8 | 0.13 | 9.5 | 34 | 403 | CH2Cl2 | Asymmetric synthesis | 27 |
| 35 | n = 7 | 0.54 | 1.3 | 31 | 483 | CH2Cl2 | F− sensor | 28 |
| 37 | n = 6 | 0.05 | 0.98 | 1 | 435 | CHCl3 | Enantioselective synthesis | 29 |
| 38a | n = 7 | 1 | 2 | 28.5 | 662 | CH2Cl2 | Φ F = 1 in the red-NIR region, EQE = 28%, TADF | 30 |
| 39c | n = 7 | 0.59 | 2.3 | 16 | 485 | toluene | FWHMCPL = 18 nm | 31b |
| 40 | n = 9 | 0.98 | 5.8 | 220 | 578 | toluene | CP-OLED (EQE = 35.5%, |gEL| = 6.2 × 10−3) | 31c |
| 41 | n = 6 | 0.74 | 2.7 | 59 | 598 | toluene | Double helicene | 31d |
| 42 | n = 9 | 0.22 | 3 | 20 | 660 | toluene | F− sensor | 31e |
| 44 | n = 5 | 0.65 | 1 | 11 | 522 | toluene | F− sensor | 32 |
| 45b | n = 6 | 0.17 | 13 | 59 | 432 | CH2Cl2 | High glum | 33 |
| 46b | n = 10 | 0.24 | 48 | 292 | 430 | CH2Cl2 | High glum | 34 |
| 47 | n = 6 | 0.084 | 3.4 | 4.9 | 426 | CH2Cl2 | CPL switching and CPP | 36a |
| 48 | n = 6 | 0.084 | 8.6 | 22 | 420 | CH2Cl2 | CPL switching and Zn2+ binding | 36b |
| 49 | n = 6 | 0.22 | 4.8 | 22 | 425 | CH2Cl2 | CPL switching and Zn2+ binding | 36c |
| 50 | n = 7 | 0.17 | 1.1 | 4 | 430 | CH2Cl2 | CPL switching | 37 |
| 52c | n = 7 | 0.03 | 1.2 | 0.8 | 422 | THF | CPL on/off | 38 |
| 53 | n = 7 | 0.48 | 0.47 | 5.8 | 493 | CH2Cl2 | CPL switching | 39 |
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research on azahelicenes in our group has been supported by JSPS KAKENHI Grant Number 21K05039 and 24K08395.Notes and references
- (a) H. Maeda and Y. Bando, Pure Appl. Chem., 2013, 85, 1967 Search PubMed; (b) E. M. Sánchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz and S. de la Moya, Chem. – Eur. J., 2015, 21, 13488 Search PubMed; (c) J. Kumar, T. Nakashima and T. Kawai, J. Phys. Chem. Lett., 2015, 6, 3445 CrossRef CAS PubMed; (d) E. Yashima, N. Ousaka, D. Taura, K. Shimomura, T. Ikai and K. Maeda, Chem. Rev., 2016, 116, 13752 Search PubMed; (e) H. Tanaka, Y. Inoue and T. Mori, ChemPhotoChem, 2018, 2, 386 CrossRef CAS; (f) L. Arrico, L. Di Bari and F. Zinna, Chem. – Eur. J., 2021, 27, 2920 Search PubMed; (g) K. Takaishi, C. Maeda and T. Ema, Chirality, 2023, 35, 92 CrossRef CAS PubMed; (h) C. Maeda, I. Yasutomo, K. Takaishi and T. Ema, J. Porphyrins Phthalocyanines, 2023, 27, 903 CrossRef CAS.
- (a) Y. Shen and C.-F. Chen, Chem. Rev., 2012, 112, 1463 CrossRef CAS PubMed; (b) M. Gingras, Chem. Soc. Rev., 2013, 42, 968 Search PubMed; (c) M. Gingras, G. Félix and R. Peresutti, Chem. Soc. Rev., 2013, 42, 1007 RSC; (d) H. Isla and J. Crassous, C. R. Chimie, 2016, 19, 39 CrossRef CAS; (e) W.-L. Zhao, M. Li, H.-Y. Lu and C.-F. Chen, Chem. Commun., 2019, 55, 13793 RSC; (f) K. Dhbaibi, L. Favereau and J. Crassous, Chem. Rev., 2019, 119, 8846 CrossRef CAS PubMed; (g) M. Jakubec and J. Storch, J. Org. Chem., 2020, 85, 13415 CrossRef CAS PubMed; (h) A. Tsurusaki and K. Kamikawa, Chem. Lett., 2021, 50, 1913 CrossRef CAS; (i) T. Mori, Chem. Rev., 2021, 121, 2373 CrossRef CAS PubMed; (j) P. Ravat, Chem. – Eur. J., 2021, 27, 3957 CrossRef CAS PubMed; (k) M. Cei, L. Di Bari and F. Zinna, Chirality, 2023, 35, 192 CrossRef CAS PubMed; (l) A. Nowak-Król, P. T. Geppert and K. R. Naveen, Chem. Sci., 2024, 15, 7408 RSC; (m) Q. Huang, Y.-P. Tang, C.-G. Zhang, Z. Wang and L. Dai, ACS Catal., 2024, 14, 16256 CrossRef CAS.
- (a) Y. Nakai, T. Mori and Y. Inoue, J. Phys. Chem. A, 2013, 117, 83 CrossRef CAS PubMed; (b) S. Abbate, G. Longhi, F. Lebon, E. Castiglioni, S. Superchi, L. Pisani, F. Fontana, F. Torricelli, T. Caronna, C. Villani, R. Sabia, M. Tommasini, A. Lucotti, D. Mendola, A. Mele and D. A. Lightner, J. Phys. Chem. C, 2014, 118, 1682 Search PubMed.
- (a) P. G. Campbell, A. J. V. Marwitz and S.-Y. Liu, Angew. Chem., Int. Ed., 2012, 51, 6074 Search PubMed; (b) Z. X. Giustra and S.-Y. Liu, J. Am. Chem. Soc., 2018, 140, 1184 CrossRef CAS PubMed; (c) X. Chen, D. Tan and D.-T. Yang, J. Mater. Chem. C, 2022, 10, 13499 Search PubMed; (d) C. Chen, Y. Zhang, X.-Y. Wang, J.-Y. Wang and J. Pei, Chem. Mater., 2023, 35, 10277 Search PubMed; (e) M. Mamada, M. Hayakawa, J. Ochi and T. Hatakeyama, Chem. Soc. Rev., 2024, 53, 1624 Search PubMed.
- (a) T. Otani, A. Tsuyuki, T. Iwachi, S. Someya, K. Tateno, H. Kawai, T. Saito, K. S. Kanyiva and T. Shibata, Angew. Chem., Int. Ed., 2017, 56, 3906 CrossRef CAS PubMed; (b) T. Otani, T. Sasayama, C. Iwashimizu, K. S. Kanyiva, H. Kawai and T. Shibata, Chem. Commun., 2020, 56, 4484 RSC.
- T. Taniguchi, Y. Nishii, T. Mori, K. Nakayama and M. Miura, Chem. – Eur. J., 2021, 27, 7356 CrossRef CAS PubMed.
- J. Feng, L. Wang, X. Xue, Z. Chao, B. Hong and Z. Gu, Org. Lett., 2021, 23, 8056 CrossRef CAS PubMed.
- (a) I. Hernández Delgado, S. Pascal, A. Wallabregue, R. Duwald, C. Besnard, L. Guénée, C. Nançoz, E. Vauthey, R. C. Tovar, J. L. Lunkley, G. Muller and J. Lacour, Chem. Sci., 2016, 7, 4685 RSC; (b) S. Pascal, C. Besnard, F. Zinna, L. Di Bari, B. Le Guennic, D. Jacquemin and J. Lacour, Org. Biomol. Chem., 2016, 14, 4590 RSC; (c) R. Duwald, J. Bosson, S. Pascal, S. Grass, F. Zinna, C. Besnard, L. Di Bari, D. Jacquemin and J. Lacour, Chem. Sci., 2020, 11, 1165 Search PubMed; (d) P. Moneva Lorente, A. Wallabregue, F. Zinna, C. Besnard, L. Di Bari and J. Lacour, Org. Biomol. Chem., 2020, 18, 7677 RSC.
- C. Olivier, N. Nagatomo, T. Mori, N. McClenaghan, G. Jonusauskas, B. Kauffmann, Y. Kuwahara, M. Takafuji, H. Ihara and Y. Ferrand, Org. Chem. Front., 2023, 10, 752 RSC.
- (a) K. Nakamura, S. Furumi, M. Takeuchi, T. Shibuya and K. Tanaka, J. Am. Chem. Soc., 2014, 136, 5555 Search PubMed; (b) K. Hanada, J. Nogami, K. Miyamoto, N. Hayase, Y. Nagashima, Y. Tanaka, A. Muranaka, M. Uchiyama and K. Tanaka, Chem. – Eur. J., 2021, 27, 9313 Search PubMed.
- T. Yu, Z.-Q. Li, J. Li, S. Cheng, J. Xu, J. Huang, Y.-W. Zhong, S. Luo and Q. Zhu, ACS Catal., 2022, 12, 13034 Search PubMed.
- (a) K. Goto, R. Yamaguchi, S. Hiroto, H. Ueno, T. Kawai and H. Shinokubo, Angew. Chem., Int. Ed., 2012, 51, 10333 Search PubMed; (b) A. Ushiyama, S. Hiroto, J. Yuasa, T. Kawai and H. Shinokubo, Org. Chem. Front., 2017, 4, 664 RSC.
- (a) G. M. Upadhyay, H. R. Talele, S. Sahoo and A. V. Bedekar, Tetrahedron Lett., 2014, 55, 5394 Search PubMed; (b) G. M. Upadhyay and A. V. Bedekar, Tetrahedron, 2015, 71, 5644 Search PubMed; (c) G. M. Upadhyay, H. R. Talele and A. V. Bedekar, J. Org. Chem., 2016, 81, 7751 Search PubMed; (d) C. Maeda, K. Akiyama and T. Ema, Org. Lett., 2023, 25, 3932 Search PubMed.
- (a) K. Nakano, Y. Hidehira, K. Takahashi, T. Hiyama and K. Nozaki, Angew. Chem., Int. Ed., 2005, 44, 7136 CrossRef CAS PubMed; (b) K. Uematsu, K. Noguchi and K. Nakano, Phys. Chem. Chem. Phys., 2018, 20, 3286 RSC; (c) X.-Y. Chen, J.-K. Li, C. Wang and X.-Y. Wang, Tetrahedron Lett., 2024, 139, 154981 Search PubMed.
- K. Uematsu, C. Hayasaka, K. Takase, K. Noguchi and K. Nakano, Molecules, 2022, 27, 606 Search PubMed.
- (a) C. Maeda, K. Nagahata, T. Shirakawa and T. Ema, Angew. Chem., Int. Ed., 2020, 59, 7813 Search PubMed; (b) C. Maeda, S. Nomoto, K. Akiyama, T. Tanaka and T. Ema, Chem. – Eur. J., 2021, 27, 15699 Search PubMed; (c) C. Maeda, S. Michishita and T. Ema, Chem. – Eur. J., 2025, 31, e202404325 CrossRef PubMed.
- (a) C. Maeda, K. Nagahata, K. Takaishi and T. Ema, Chem. Commun., 2019, 55, 3136 RSC; (b) C. Maeda, K. Suka, K. Nagahata, K. Takaishi and T. Ema, Chem. – Eur. J., 2020, 26, 4261 Search PubMed; (c) C. Maeda, S. Nomoto, K. Takaishi and T. Ema, Chem. – Eur. J., 2020, 26, 13016 Search PubMed.
- X.-Y. Chen, J.-K. Li, W.-L. Zhao, C.-Z. Du, M. Li, C.-F. Chen and X.-Y. Wang, J. Mater. Chem. C, 2023, 11, 893 RSC.
- M. Gross, F. Zhang, M. E. Arnold, P. Ravat and A. J. C. Kuehne, Adv. Opt. Mater., 2024, 12, 2301707 Search PubMed.
- C. Li, C. Zhang, P. Li, Y. Jia, J. Duan, M. Liu, N. Zhang and P. Chen, Angew. Chem., Int. Ed., 2023, 62, e202302019 CrossRef CAS PubMed.
- Y. Shi, C. Li, J. Di, Y. Xue, Y. Jia, J. Duan, X. Hu, Y. Tian, Y. Li, C. Sun, N. Zhang, Y. Xiong, T. Jin and P. Chen, Angew. Chem., Int. Ed., 2024, 63, e202402800 CrossRef CAS PubMed.
- S. Qiu, A. C. Valdivia, W. Zhuang, F.-F. Hung, C.-M. Che, J. Casado and J. Liu, J. Am. Chem. Soc., 2024, 146, 16161 Search PubMed.
- (a) G.-F. Huo, W.-T. Xu, Y. Han, J. Zhu, X. Hou, W. Fan, Y. Ni, S. Wu, H.-B. Yang and J. Wu, Angew. Chem., Int. Ed., 2024, 63, e202403149 Search PubMed; (b) G.-F. Huo, T. M. Fukunaga, X. Hou, Y. Han, W. Fan, S. Wu, H. Isobe and J. Wu, Angew. Chem., Int. Ed., 2023, 62, e202218090 Search PubMed.
- (a) Y. Matsuo, M. Gon, K. Tanaka, S. Seki and T. Tanaka, J. Am. Chem. Soc., 2024, 146, 17428 Search PubMed; (b) Y. Matsuo, M. Gon, K. Tanaka, S. Seki and T. Tanaka, Chem. – Asian J., 2024, 19, e202400134 Search PubMed.
- J. Liu, J. Hong, Z. Liao, J. Tan, H. Liu, E. Dmitrieva, L. Zhou, J. Ren, X.-Y. Cao, A. A. Popov, Y. Zou, A. Narita and Y. Hu, Angew. Chem., Int. Ed., 2024, 63, e202400172 CrossRef CAS PubMed.
- P. Jiang, A. S. Mikherdov, H. Ito and M. Jin, J. Am. Chem. Soc., 2024, 146, 12463 CrossRef CAS PubMed.
- T. Yanagi, T. Tanaka and H. Yorimitsu, Chem. Sci., 2021, 12, 2784 RSC.
- C. Maeda, I. Yasutomo and T. Ema, Angew. Chem., Int. Ed., 2024, 63, e202404149 CrossRef CAS PubMed.
- W.-L. Xu, R.-X. Zhang, H. Wang, J. Chen and L. Zhou, Angew. Chem., Int. Ed., 2024, 63, e202318021 CrossRef CAS PubMed.
- (a) Y. Zhang, D. Zhang, T. Huang, A. J. Gillett, Y. Liu, D. Hu, L. Cui, Z. Bin, G. Li, J. Wei and L. Duan, Angew. Chem., Int. Ed., 2021, 60, 20498 CrossRef CAS PubMed; (b) J.-K. Li, X.-Y. Chen, Y.-L. Guo, X.-C. Wang, A. C.-H. Sue, X.-Y. Cao and X.-Y. Wang, J. Am. Chem. Soc., 2021, 143, 17958 CrossRef CAS PubMed.
- (a) F. Zhang, F. Rauch, A. Swain, T. B. Marder and P. Ravat, Angew. Chem., Int. Ed., 2023, 62, e202218965 CrossRef CAS PubMed; (b) F. Zhang, V. Brancaccio, F. Saal, U. Deori, K. Radacki, H. Braunschweig, P. Rajamalli and P. Ravat, J. Am. Chem. Soc., 2024, 146, 29782 CrossRef CAS PubMed; (c) W.-C. Guo, W.-L. Zhao, K.-K. Tan, M. Li and C.-F. Chen, Angew. Chem., Int. Ed., 2024, 63, e202401835 CrossRef CAS PubMed; (d) Y.-Y. Ju, L.-E. Xie, J.-F. Xing, Q.-S. Deng, X.-W. Chen, L.-X. Huang, G.-H. Nie, Y.-Z. Tan and B. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202414383 CrossRef PubMed; (e) W. Zhuang, F.-F. Hung, C.-M. Che and J. Liu, Angew. Chem., Int. Ed., 2024, 63, e202406497 CAS.
- D. Tan, J. Dong, T. Ma, Q. Feng, S. Wang and D.-T. Yang, Angew. Chem., Int. Ed., 2023, 62, e202304711 CrossRef CAS PubMed.
- Y. Appiarius, S. Míguez-Lago, P. Puylaert, N. Wolf, S. Kumar, M. Molkenthin, D. Miguel, T. Neudecker, M. Juríček, A. G. Campaña and A. Staubitz, Chem. Sci., 2024, 15, 466 RSC.
- Y. Yu, C. Wang, F.-F. Hung, C. Chen, D. Pan, C.-M. Che and J. Liu, J. Am. Chem. Soc., 2024, 146, 22600 Search PubMed.
- (a) H. Maeda, Y. Bando, K. Shimomura, I. Yamada, M. Naito, H. Nobusawa, H. Tsumatori and T. Kawai, J. Am. Chem. Soc., 2011, 133, 9266 CrossRef CAS PubMed; (b) B. A. San Jose, J. Yan and K. Akagi, Angew. Chem., Int. Ed., 2014, 53, 10641 CrossRef CAS PubMed; (c) Y. Nagata, T. Nishikawa and M. Suginome, Chem. Commun., 2014, 50, 9951 RSC; (d) S. P. Morcillo, D. Miguel, L. Á. de Cienfuegos, J. Justicia, S. Abbate, E. Castiglioni, C. Bour, M. Ribagorda, D. J. Cárdenas, J. M. Paredes, L. Crovetto, D. Choquesillo-Lazarte, A. J. Mota, M. C. Carreño, G. Longhi and J. M. Cuerva, Chem. Sci., 2016, 7, 5663 RSC; (e) Y. Hashimoto, T. Nakashima, D. Shimizu and T. Kawai, Chem. Commun., 2016, 52, 5171 RSC; (f) S. Ito, K. Ikeda, S. Nakanishi, Y. Imai and M. Asami, Chem. Commun., 2017, 53, 6323 RSC; (g) K. Takaishi, M. Yasui and T. Ema, J. Am. Chem. Soc., 2018, 140, 5334 CrossRef CAS PubMed; (h) A. Homberg, E. Brun, F. Zinna, S. Pascal, M. Górecki, L. Monnier, C. Besnard, G. Pescitelli, L. Di Bari and J. Lacour, Chem. Sci., 2018, 9, 7043 Search PubMed; (i) S. Fa, T. Tomita, K. Wada, K. Yasuhara, S. Ohtani, K. Kato, M. Gon, K. Tanaka, T. Kakuta, T. Yamagishi and T. Ogoshi, Chem. Sci., 2022, 13, 5846 RSC; (j) Y. Wang, J. Gong, X. Wang, W.-J. Li, X.-Q. Wang, X. He, W. Wang and H.-B. Yang, Angew. Chem., Int. Ed., 2022, 61, e202210542 CrossRef CAS PubMed.
- (a) N. Saleh, B. Moore, M. Srebro, N. Vanthuyne, L. Toupet, J. A. G. Williams, C. Roussel, K. K. Deol, G. Muller, J. Autschbach and J. Crassous, Chem. – Eur. J., 2015, 21, 1673 CrossRef CAS PubMed; (b) H. Isla, M. Srebro-Hooper, M. Jean, N. Vanthuyne, T. Roisnel, J. L. Lunkley, G. Muller, J. A. G. Williams, J. Autschbach and J. Crassous, Chem. Commun., 2016, 52, 5932 RSC; (c) H. Isla, N. Saleh, J.-K. Ou-Yang, K. Dhbaibi, M. Jean, M. Dziurka, L. Favereau, N. Vanthuyne, L. Toupet, B. Jamoussi, M. Srebro-Hooper and J. Crassous, J. Org. Chem., 2019, 84, 5383 CrossRef CAS PubMed.
- E. Yen-Pon, F. Buttard, L. Frédéric, P. Thuéry, F. Taran, G. Pieters, P. A. Champagne and D. Audisio, JACS Au, 2021, 1, 807 CrossRef CAS PubMed.
- (a) Y. Matsuo, K. Kise, Y. Morimoto, A. Osuka and T. Tanaka, Angew. Chem., Int. Ed., 2022, 61, e202116789 CrossRef CAS PubMed; (b) Y. Matsuo, C. Maeda, Y. Tsutsui, T. Tanaka and S. Seki, Angew. Chem., Int. Ed., 2023, 62, e202314968 CrossRef CAS PubMed.
- C. Maeda, S. Michishita, I. Yasutomo and T. Ema, Angew. Chem., Int. Ed., 2025, 64, e202418546 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif)