Open Access Article
Open Access ArticleChiral synthetic hosts for efficient enantioselective molecular recognition. Design principles and synthetic aspects
Hugo
Marchi Luciano
 a and
Agustí
Lledó
a and
Agustí
Lledó
 *ab
*ab
aInstitut de Química Computacional i Catàlisi (IQCC), Universitat de Girona, Maria Aurèlia Capmany 69, 17003 Girona, Spain. E-mail: agusti.lledo@udg.edu
bInstitute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain
First published on 24th December 2024
Abstract
Discrimination of enantiomeric substrate molecules is one of the fundamental properties of biological hosts. Replicating enantioselective molecular recognition with synthetic receptors is a topic of interest with implications in diverse applications such as bioinspired enantioselective catalysis, enantiomer separation, or sensing. In this review, five different systems reported in the literature are discussed, and their performance and versatility are analyzed. A recently reported host featuring a flexible scaffold challenges the long-established view that a high degree of preorganization in combination with strongly directional non-covalent interactions is required for efficient enantiodiscrimination. The review is complemented with an analysis of the synthetic effort required for each of the hosts presented.
Introduction
The capacity to discriminate efficiently between two enantiomeric guest molecules is a crucial property of biological receptors that underpins some of their most crucial functions. The emergence of differential biological and pharmacological responses in response to a chiral drug or metabolite and the production of a chiral substance through enzymatic catalysis, for instance, all rely on precise molecular recognition events within the binding pocket of the respective biological hosts. Replicating these phenomena with synthetic hosts has been a long-sought objective given the implications in essential applications such as sensing devices,1 chiral separation technologies,1 or enzyme-like enantioselective catalysis.2–6 While constructing a synthetic receptor containing stereogenic elements and/or featuring chiral point group symmetry may be relatively straightforward,7 the main challenge of this approach is efficiently translating the chirality to the binding space to exert differential molecular recognition onto opposite enantiomers of a given guest. The “three-point contact” theory developed in the 20th century stated that efficient molecular recognition of chiral compounds originates from at least three strongly directional interactions—hydrogen bonds, dipole–dipole interactions, and the like—between the guest and buried polar functional groups.8,9 Proteinogenic active sites combine hydrophobic regions with inwardly directed polar functional groups from the peptide sequence. However, this feature is difficult to integrate by design in synthetic hosts, because of the synthetic challenge associated with the functionalization of hindered concave surfaces. Hence, integrating multiple polar functional groups within or near the concavity of a preorganized host would provide, in principle, the highest chances of discriminating between two enantiomers. Preorganization is one of the canonical design principles of synthetic hosts, which has led to a prevalence of rigid host structures in the supramolecular chemistry literature. This bias is probably caused by the challenges associated with developing receptors with flexibility akin to that of enzymes. The design and synthesis of hosts with rigid building blocks and geometries are more straightforward, and the spectroscopic characterization is simplified by reduced fluxional behaviour. Notably, this preference for rigid structures contradicts one of the universal features of proteinogenic receptors—their intrinsic flexibility. While a synthetic host with rigidly oriented molecular recognition units may provide high discrimination between enantiomers of a given ideal guest, chances are that the scope of guests providing high enantioselectivity is reduced. For the field of enantioselective molecular recognition to evolve towards real applications, host design needs to move away from this restricted lock-and-key notion and embrace the induced fit and conformational selection phenomena that govern biological binding, allowing the design of hosts that can be adapted to different applications through systematic derivatization. Herein, we highlight and compare recent reports on enantioselective molecular recognition using chiral hosts of diverse architecture (H1–H6), including a recent example from our group. While a quantitative evaluation of the conformational mobility for each of these hosts is not available, a qualitative picture of the systems’ flexibility can be derived from the number of Sigma bonds with “free” rotation, which can be gauged against the enantioselectivity ratios obtained and the scope of the guests studied in each case. Finally, we provide a comparison of the synthetic effort required to access each of these receptors and their potential for diversification and further development.Discussion
In the following subsections, we will discuss the following hosts or families of hosts: triptycene-based cages (H1),10,11 naphthotubes (H2),12–14 binol-derived hosts (H3, H4),15–17 calix[5]arene based self-folding cavitands (H5)18,19 and Tröger's base derived hosts (H6).20 For each of these structures, the structurally non-equivalent sigma bonds acting as conformational hinges are highlighted (green arrows). The total number of rotatable bonds is indicated throughout the discussion to give an approximate idea of the degrees of freedom in each system. Fig. 1–6 highlight the main features of each of the systems discussed. Receptors marked in red squares have been tested in organic media, while blue squares indicate water-soluble receptors. The total number of guests tested for each system is indicated, as well as the range of association constants (Ka) measured. Selected guests for each system are detailed, and the degree of enantioselectivity in the molecular recognition process is indicated by the selectivity ratio , where Ka and
, where Ka and  are the absolute association constants of diastereomeric host–guest complexes, measured from the combination of opposite enantiomers of a given host with the same enantiomer of the guest, or vice versa.
are the absolute association constants of diastereomeric host–guest complexes, measured from the combination of opposite enantiomers of a given host with the same enantiomer of the guest, or vice versa.
 | ||
| Fig. 1 Triptycene derived hosts and selectivity factors (S) obtained in the binding of chiral quaternary ammonium salts. X = PF6− (acetone); X = I− (H2O). | ||
 | ||
| Fig. 2 Chiral naphthotubes reported in the literature. Privileged guests and the corresponding selectivity ratios are highlighted in each case. | ||
 | ||
| Fig. 3 Corral[4]binol host H3, and selected privileged guests for enantioselective molecular recognition. | ||
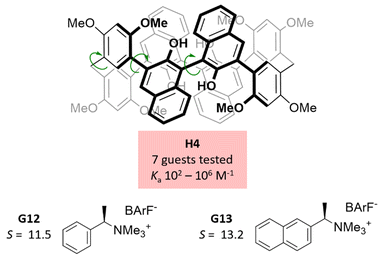 | ||
| Fig. 4 Binol-derived macrocycle H4 and selected privileged guests for enantioselective molecular recognition. | ||
 | ||
| Fig. 6 Structure of Tröger's base derived hosts H6a and H6b, and selected privileged guests providing exceptional enantioselectivity values. | ||
Triptycene-based hosts
In 2016, Chen and co-workers developed a new calixarene-like receptor based on triptycene building blocks, H1a, which displayed enantioselective molecular recognition of chiral quaternary ammonium salts in organic media (Fig. 1).11 Later on, the same group adapted this scaffold to obtain a water-soluble host H1b.10 While the water-soluble version required the separation by chiral HPLC of the key cyclization precursor in order to obtain enantiopure hosts, H1a was obtained directly in racemic form and later resolved into the separate enantiomers using a chiral auxiliary, providing more convenient access to the enantiopure host. H1 hosts provided remarkable enantioselectivity ratios towards chiral quaternary ammonium salts (up to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), although the scope of the guests tested in these studies is relatively narrow. The similarity and reduced range of guests tested in these studies suggest that H1a–b are very rigid and can only accommodate guests with good shape complementarity. The presence of 6 Sigma bonds at the methylene hinges may provide some breathing ability, but the structure is nevertheless restrained by the total rigidity of the triptycene scaffold. Remarkably, though, good enantioselectivity is maintained in water, which is typically perceived as more challenging since the magnitude of the non-covalent dipolar interactions is attenuated by the medium polarity. Given the fact that the molecular recognition in these examples is governed by non-directional interactions such as CH–π and cation–π interactions, the excellent levels of selectivity observed—both in organic media and water—would suggest that strong dipolar interactions are not a strict requirement for efficient enantioselective molecular recognition, at least in synthetic systems. For the study carried out in water, the enthalpic term is the main contributor to the observed selectivity (for G3: ΔΔH = −5.0 kJ mol−1, Δ(−TΔS) = −1.4 kJ mol−1),† corroborating that the poorly directional cation–π interaction between the trimethylammonium knob and the aromatic panels is driving the observed selectivity.
1), although the scope of the guests tested in these studies is relatively narrow. The similarity and reduced range of guests tested in these studies suggest that H1a–b are very rigid and can only accommodate guests with good shape complementarity. The presence of 6 Sigma bonds at the methylene hinges may provide some breathing ability, but the structure is nevertheless restrained by the total rigidity of the triptycene scaffold. Remarkably, though, good enantioselectivity is maintained in water, which is typically perceived as more challenging since the magnitude of the non-covalent dipolar interactions is attenuated by the medium polarity. Given the fact that the molecular recognition in these examples is governed by non-directional interactions such as CH–π and cation–π interactions, the excellent levels of selectivity observed—both in organic media and water—would suggest that strong dipolar interactions are not a strict requirement for efficient enantioselective molecular recognition, at least in synthetic systems. For the study carried out in water, the enthalpic term is the main contributor to the observed selectivity (for G3: ΔΔH = −5.0 kJ mol−1, Δ(−TΔS) = −1.4 kJ mol−1),† corroborating that the poorly directional cation–π interaction between the trimethylammonium knob and the aromatic panels is driving the observed selectivity.
Naphthotubes
Naphthotubes (H2) are an important family of receptors first introduced by Glass in 200421 and further developed by Jiang, among others (Fig. 2).22 They feature a cavity that combines hydrophobic regions—defined by flanking 2-naphthol panels—with hydrophilic groups in the linkages, and are very amenable to solubilization in water. The synthesis of H2 is based on a key cyclocondensation of rigid bis-naphthalene building blocks, resulting typically in the formation of two diastereomers, syn (H2a, H2b, H2d) and anti (H2c, H2e), each possessing molecular recognition properties. A variety of chiral H2 receptors have been developed over the last years, showing very promising results in the enantiodiscrimination of chiral guest molecules of different nature both in organic and aqueous solutions.12–14 The first example (H2a) was reported by Jiang and co-workers, and its synthesis required the separation of a key chiral intermediate by chiral preparative HPLC.14H2a was tested against a set of chiral heterocyclic guests (oxazoles, epoxides, ketals), and enthalpic contributions were found to be the main driving force for binding. This finding validated the proposed design with amide groups amid section of the host poised to establish directional dipolar interactions with bound guests. On the downside, H2a provided only moderate selectivity values, showcasing the challenges of integrating the “three-point contact” theory in synthetic systems. Later on, analogous receptors with amide (H2b–c)12 and thiourea (H2d–e)13 spacers, including nearby stereogenic centers, were prepared more conveniently using a chiral auxiliary approach. Remarkably, large association constants are also observed for these hosts in organic solvents (often producing competitive binding), reinforcing the crucial role of the amide/urea functionalities in the molecular recognition capabilities of H2. Most notably, very high enantioselectivities (up to S = 34.4) were obtained with this second generation of H2 hosts. The reduced symmetry of receptors H2b–e with respect to H2a—aC2v symmetrical structure—appears to be essential for this enhancement in S values. The breadth of different guests tested with H2 receptors—more than 60 compounds, including diketopiperazines, amino acids, small peptides, and biologically relevant molecules—is a testament to the versatility of this family of hosts. Unlike the previously discussed H1, which has only been modified at the periphery, H2 hosts can be diversified by varying the nature of the linker joining the bis-naphthalene building blocks. In combination with the syn and anti configurations, various hosts with different shapes and sizes can be accessed, providing a richer chemical space for molecular recognition. Like in the case of H1, the flexibility of H2 hosts also appears to be strongly influenced by the rigidity of the bis-naphthalene building blocks, although the congeners with larger spacers—H2d and H2e, containing up to 8 rotatable sigma bonds—may provide some increased adaptability in comparison to H1. In this context, it is worth noting that related structures devoid of the ketal rigidification unit of H2 have been shown to display rich conformational dynamics, but homochiral versions of such hosts have not been reported.23Binol-based receptors
Binol has been popularized over the last years as a building block for chiral macrocycles and hosts, probably because it is a readily available compound in enantiopure form.24 The parent structure of corral[4]binol (H3, Fig. 3) was first synthesized by Hasegawa, Imai, and co-workers using a 4-fold Yamamoto coupling as the key step, although these authors did not explore the molecular recognition properties of the host.25 Capitalizing on this seminal study, Cai and co-workers adapted the synthesis to obtain an analogous water-soluble derivative.16 Host H3 was initially studied in the enantioselective molecular recognition of a set of 4 chiral guests, providing selectivity ratios up to 18.7, as ascertained by competitive fluorometric titration experiments in water. In a follow-up study, an additional set of 16 different steroidal guests was tested against H3, providing selectivity values as high as 15.5. It is worth noting that, in analogy with H1, H3 does not feature functional groups that can establish strong and directional interactions with bound guests, which will engage instead in non-directional interactions with the aromatic panels of the hots. In addition, H3 was implemented in a sensory array that allowed the discrimination between steroids at μM concentrations, highlighting the remarkable capacity of H3 to discern subtle stereochemical differences among guests of similar shapes. Corral[4]binol features 8 rotatable biaryl linkages that may provide it with some adaptability, although this potential for induced fit behaviour was not studied in these works. Although a respectable number of guests were tested for H3, the range of guest sizes and shapes explored is limited in relation to the diverse library of guests tested against H2.Another remarkable binol-based host was recently reported by Li and co-workers (H4, Fig. 4).17 This receptor stands out for its ease of synthesis, and provided very good selectivity factors for selected trimethylammonium salts. Importantly, the phenol functional groups in H4 point inwards the confined space, and proved crucial for the molecular recognition events taking place therein. A similar analogue with outward oriented phenol groups was devoid of specific molecular recognition properties with quaternary ammonium salts. The dipolar interactions between the trimethylammonium knob of the guests and the phenol groups of H4 were hypothesized to be crucial for the observed enantioselectivity. For G12, for instance, molecular models showed that in the disfavoured diastereomeric host–guest pair, the dominating interactions with the phenol groups positioned the guest away from the cavity, preventing additional non-covalent contacts. Notably, receptor H4 features a total of 10 rotatable bonds that may endow it with some breathing ability or adaptability, although this was not discussed in detail in this study.
Calix[5]arene-based hosts
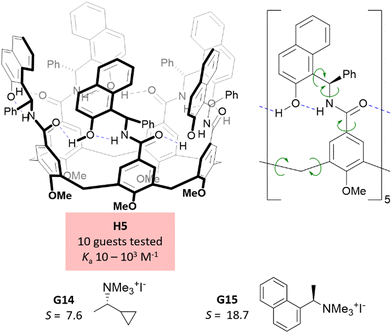
Our group reported in 2023 a chiral receptor based on calix[5]arene and a readily available chiral amine building block (H5, Fig. 4).18,19 This host is stabilized in the closed conformation through a network of hydrogen bonds involving secondary amide and phenol groups. This feature constitutes a major structural difference with respect to the previously discussed hosts (H1–H4), relying purely on covalent bridging to arrange the confined space. The enantiodiscrimination capacity of H5 was evaluated by testing a family of chiral quaternary ammonium salts, given the electron-rich nature of the host's inner surface. Moderate to good S rations were obtained, and the highest S value so far reported at that time for chiral quaternary ammonium guests was obtained for naphthalene derivative G15.10,11,26 Perhaps more interestingly, a wide range of guest sizes was covered in the set of 10 guests tested (solvent accessible volume of the cation: 153–254 Å3), and good enantioselectivity results were obtained at opposed ends of this range (G14, G15), showcasing the advantage of the flexible and adaptable structure of H5 in comparison to more rigid hosts. The excellent S values obtained indicate a good relay of stereochemical information from the stereogenic elements to the confined space, as opposed to other systems based on calix[5]arene, where this relay is inefficient.27 Indeed, the structure of H5 features 25 rotatable bonds that increase the available conformational space while preserving a sufficient degree of preorganization, as demonstrated by molecular dynamics simulations. The moderate association constants obtained for this system in chloroform (Ka = 10–103 M−1) could be seen as a trade-off of the increased flexibility. However, association constants for the much more rigid H1a in acetone are in a similar range, demonstrating that conformational flexibility is not necessarily deleterious for binding in synthetic host–guest systems. Unlike for other host structures discussed herein, a water-soluble version of H5 has not been reported so far.Tröger's base derived hosts
In 2024, a new set of receptors based on the Tröger's base scaffold (H6) was reported by Yang, Wu, and co-workers.20 Receptor H6a was soluble in organic solvents and provided a respectable selectivity (S = 4.4) in chloroform for the enantiomers of binol derivative G16. Interestingly, oxidation of the tertiary amine groups in H6a provided the N-oxide derivative H6b, which turned out to be water soluble without the requirement of additional solubilizing groups. This finding departs from previously discussed hosts that require the bespoke addition of ionisable functions to attain water solubility. Receptor H6b was tested in water against a series of chiral guests including axially chiral biaryl derivatives and biologically relevant molecules, providing in general very good selectivity factors (S > 13 for 4 guests). An exceptional and unprecedented selectivity (S = 41) was obtained for guest G16. At first glance, it would be tempting to rationalize that the basis of the enhanced selectivity obtained with H6a would derive simply from the fact that the highly polarized amine N-oxide groups can establish attractive and directional dipolar interactions with bound guests. However, ITC titration experiments carried out by the authors show that, while the enthalpy factor dominates the Gibbs free energy of binding in general, the observed enantiodiscrimination originates from entropic effects. For instance, for the H6b⊂G16 complex, ΔΔH = 11.1 kJ M−1 and Δ(−TΔS) = −20.3 kJ M−1.† These results showcase again the limitations of the “three-point contact” theory in the design of enantioselective molecular recognition processes in general. In terms of rigidity, H6 hosts are very similar to H1 inasmuch conformational mobility is limited by the rigid Tröger's base scaffold—only partial rotation about the Sigma bonds of the methylene hinges is available (6 rotatable bonds).Synthetic aspects
In the following paragraphs, we provide a comparative analysis of the synthetic effort required for each of the hosts analysed, highlighting the potential for diversification. Overall, this analysis will be helpful to gauge the potential of each of these systems to develop specific applications.Conclusions and outlook
The development of enantioselective molecular recognition processes with synthetic hosts has witnessed important improvements in the last decade. A number of structurally diverse hosts is now available providing excellent enantioselectivity values in the molecular recognition of chiral guests. Molecular recognition in water in particular has progressed significantly, proving that it is an addressable challenge despite the established belief that the high polarity of the medium would be counterproductive for selectivity arising from differences in dipole–dipole interactions, which were hypothesized to be the determinant factor in enantioselective discrimination. As it turns out, recent examples have proven that very good levels of selectivity can be obtained even in the absence of highly directional non covalent interactions, and that entropic effects can be responsible alone for the observed selectivity. Such effects have been shown to be important in aqueous solution, and the previously established paradigm that enantioselective molecular recognition in water was more challenging may be simply due to the limited number of chiral water-soluble receptors that had been so far synthesized. After all, hydrophobic effects typically dominate binding in biological receptors.The results obtained in our lab with a highly flexible receptor showcase that a high degree of preorganization—as established by the legacy supramolecular chemistry principles—is not strictly necessary to obtain good selectivity levels. Indeed, the flexibility in host H5 proved beneficial in accommodating guests of different sizes, providing a more versatile platform for enantioselective molecular recognition. On the other hand, rigid hosts like H1 or H6 have the potential to reach excellent selectivity levels with highly complementary guests, but have more limited prospects for expanding substrate scope. In any case, the flexibility of the host needs to be carefully balanced. For instance, induction of chirality on the highly conformationally mobile pillar[n]arenes has proved challenging so far.37 Future developments in this area may benefit from computational methods, allowing a quantitative analysis of the host's flexibility and its impact on the underlying molecular recognition phenomena. Discrete conformational analysis is a possible solution to rationalizing the host–guest behaviour of highly flexible hosts.38 The most promising approach for the rational design of molecular recognition phenomena in flexible synthetic hosts is to incorporate the tools that have found widespread used in biomolecular modelling to account specifically for host flexibility and disorder—such as molecular dynamics simulations.39
Ease of access and diversification to synthetic receptors is paramount for exploring and expanding their applications. In this sense, the condensation reactions required to forge the necessary macrocyclic scaffolds and their obtention in enantiopure form are the most critical points. In addition, potential for synthetic diversification is desirable to access confined spaces of different shapes and sizes, and to explore subtle substitution effects. In this regard, calix[5]arene derived host H5 stands out overall, combining an early cyclization step that can be scaled-up, a reasonably short synthetic sequence, and a versatile architecture that be diversified at late stages by incorporation of chiral small building blocks, overriding the typically more challenging resolution of the host structure itself.
The incorporation of proteinogenic α-amino acids or short peptides in a macrocyclic structure is also a synthetically appealing strategy.40 The development of peptide based cages was pioneered in the 90s by Still, and outstanding selectivity levels towards the recognition of α-amino acid and small peptide derivatives were reported.41–46 Some of these studies already recognized the importance of flexibility in receptor design, although a quantitative analysis was not provided. Implementation of modern molecular modelling techniques could foster a resurgence of the de novo design of peptide based hosts. Quantitative models could allow the exploitation of subtle and non directional host–guest interactions, expanding the breadth of suitable guests beyond peptidic structures, the binding of which is dominated by strong and directional dipolar interactions.
The results summarized herein provide a whole new body of knowledge for the rational design of chiral hosts and their applications, surpassing the “three-point contact” model that has been frequently invoked to rationalize the discrimination of enantiomers by molecular recognition processes in both biological and synthetic hosts. This field is now ripe for translating the exceptional enantioselectivity levels obtained in specific applications in sensing, enantiomer separation technologies, and, most importantly, enantioselective catalysis. For the latter, the excellent enantioselectivity levels recently obtained would translate into synthetically useful levels of enantiomeric excess if such S values can be replicated in the molecular recognition of diastereomeric transition states. It is anticipated that privileged receptors for this purpose will have to combine structures that are amenable to fine tuning and diversification (H5) with the presence of functionality that is inwardly directed to the confined space in order to trigger reactivity (H2).47
Data availability
This feature article is a review of works on enantioselective molecular recognition published in the 2016–2024 period. No new data of any kind—experimental or computational—has been used in the preparation of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from grants TED2021-130573B-I00, PID2023-146498NB-I00 and REQ2021_B_05 funded by MICIU/AEI/10.13039/501100011033 and by the European Union NextGenerationEU/PRTR. We thank AGAUR/Generalitat de Catalunya for funding (2021SGR623).Notes and references
- G. A. Hembury, V. V. Borovkov and Y. Inoue, Chem. Rev., 2008, 108, 1–73 CrossRef CAS PubMed.
- J. Chen, X. Wu, S. Huang, J. Yang, Y.-L. Lu, Z. Jiao and C.-Y. Su, ACS Catal., 2024, 14, 3733–3741 CrossRef CAS.
- D. Sokolova, G. Piccini and K. Tiefenbacher, Angew. Chem., Int. Ed., 2022, 61, e202203384 CrossRef CAS PubMed.
- S. M. Bierschenk, J. Y. Pan, N. S. Settineri, U. Warzok, R. G. Bergman, K. N. Raymond and F. D. Toste, J. Am. Chem. Soc., 2022, 144, 11425–11433 CrossRef CAS PubMed.
- C. Zhao, Q.-F. Sun, W. M. Hart-Cooper, A. G. DiPasquale, F. D. Toste, R. G. Bergman and K. N. Raymond, J. Am. Chem. Soc., 2013, 135, 18802–18805 CrossRef CAS PubMed.
- C. J. Brown, R. G. Bergman and K. N. Raymond, J. Am. Chem. Soc., 2009, 131, 17530–17531 CrossRef CAS PubMed.
- F. Begato, G. Licini and C. Zonta, Angew. Chem., Int. Ed., 2023, 62, e202311153 CrossRef CAS PubMed.
- V. A. Davankov, Chirality, 1997, 9, 99–102 CrossRef CAS.
- L. H. Easson and E. Stedman, Biochem. J., 1933, 27, 1257–1266 CrossRef CAS PubMed.
- X.-N. Han, P.-F. Li, Y. Han and C.-F. Chen, Angew. Chem., Int. Ed., 2022, 61, e202202527 CrossRef CAS PubMed.
- G.-W. Zhang, P.-F. Li, Z. Meng, H.-X. Wang, Y. Han and C.-F. Chen, Angew. Chem., Int. Ed., 2016, 55, 5304–5308 CrossRef CAS PubMed.
- X. Yang and W. Jiang, J. Am. Chem. Soc., 2024, 146, 3900–3909 CrossRef CAS PubMed.
- S.-M. Wang, Y.-F. Wang, L. Huang, L.-S. Zheng, H. Nian, Y.-T. Zheng, H. Yao, W. Jiang, X. Wang and L.-P. Yang, Nat. Commun., 2023, 14, 5645 CrossRef CAS PubMed.
- H. Chai, Z. Chen, S.-H. Wang, M. Quan, L.-P. Yang, H. Ke and W. Jiang, CCS Chem., 2020, 2, 440–452 CrossRef CAS.
- R. Fu, D.-Y. Li, J.-H. Tian, Y.-L. Lin, Q.-Y. Zhao, W.-L. Li, F.-Y. Chen, D.-S. Guo and K. Cai, Angew. Chem., Int. Ed., 2024, 63, e202406233 CrossRef CAS PubMed.
- R. Fu, Q.-Y. Zhao, H. Han, W.-L. Li, F.-Y. Chen, C. Tang, W. Zhang, S.-D. Guo, D.-Y. Li, W.-C. Geng, D.-S. Guo and K. Cai, Angew. Chem., Int. Ed., 2023, 62, e202315990 CrossRef CAS PubMed.
- G. Sun, X. Zhang, Z. Zheng, Z.-Y. Zhang, M. Dong, J. L. Sessler and C. Li, J. Am. Chem. Soc., 2024, 146, 26233–26242 CrossRef CAS PubMed.
- R. Álvarez-Yebra, R. López-Coll, N. Clos-Garrido, D. Lozano and A. Lledó, Isr. J. Chem., 2024, 64, e202300077 CrossRef.
- R. Álvarez-Yebra, R. López-Coll, P. Galán-Masferrer and A. Lledó, Org. Lett., 2023, 25, 3190–3194 CrossRef PubMed.
- X. Liang, T. Zhao, Y. Shen, L. Fang, L. Chen, D. Zhou, W. Wu and C. Yang, Angew. Chem., Int. Ed., 2024, e202416975 Search PubMed.
- B. J. Shorthill, C. T. Avetta and T. E. Glass, J. Am. Chem. Soc., 2004, 126, 12732–12733 CrossRef CAS PubMed.
- L.-P. Yang, X. Wang, H. Yao and W. Jiang, Acc. Chem. Res., 2020, 53, 198–208 CrossRef CAS PubMed.
- L.-P. Yang, L. Zhang, M. Quan, J. S. Ward, Y.-L. Ma, H. Zhou, K. Rissanen and W. Jiang, Nat. Commun., 2020, 11, 2740 CrossRef CAS PubMed.
- Y. Yu, Y. Hu, C. Ning, W. Shi, A. Yang, Y. Zhao, Z. Y. Cao, Y. Xu and P. Du, Angew. Chem., Int. Ed., 2024, 63, e202407034 CrossRef CAS PubMed.
- Y. Nojima, M. Hasegawa, N. Hara, Y. Imai and Y. Mazaki, Chem. Commun., 2019, 55, 2749–2752 RSC.
- S. J. Nemat, H. Jędrzejewska, A. Prescimone, A. Szumna and K. Tiefenbacher, Org. Lett., 2020, 22, 5506–5510 CrossRef CAS PubMed.
- T. Haino, H. Fukuoka, H. Iwamoto and Y. Fukazawa, Supramol. Chem., 2008, 20, 51–57 CrossRef CAS.
- R. Zimmer, L. Schefzig, A. Peritz, V. Dekaris and H.-U. Reissig, Synthesis, 2004, 1439–1445 CrossRef CAS.
- P. J. Cox, W. Wang and V. Snieckus, Tetrahedron Lett., 1992, 33, 2253–2256 CrossRef CAS.
- H.-F. Chow, C.-W. Wan and M.-K. Ng, J. Org. Chem., 1996, 61, 8712–8714 CrossRef CAS.
- D. Lozano, R. Álvarez-Yebra, R. López-Coll and A. Lledó, Chem. Sci., 2019, 10, 10351–10355 RSC.
- D. R. Stewart and C. D. Gutsche, Org. Prep. Proced. Int., 1993, 25, 137–139 CrossRef CAS.
- J. Garcia-Hartjes, S. Bernardi, C. A. G. M. Weijers, T. Wennekes, M. Gilbert, F. Sansone, A. Casnati and H. Zuilhof, Org. Biomol. Chem., 2013, 11, 4340–4349 RSC.
- Y. Dong, R. Li, J. Lu, X. Xu, X. Wang and Y. Hu, J. Org. Chem., 2005, 70, 8617–8620 CrossRef CAS PubMed.
- M. Betti, Org. Synth., 1929, 9, 60 CrossRef CAS.
- R. Álvarez-Yebra, A. Sors-Vendrell and A. Lledó, Chem. Commun., 2023, 59, 11556–11559 RSC.
- K. Diao, C. Ruan, R. Wang, S. Li, J. Jiang and L. Wang, Tetrahedron Lett., 2024, 137, 154941 CrossRef CAS.
- Z. Xu, W. Yang, H. Liu, S. Jiang and A. C. H. Sue, JACS Au, 2024, 4, 3475–3483 CrossRef CAS PubMed.
- M. Karplus and J. A. McCammon, Nat. Struct. Mol. Biol., 2002, 9, 646–652 CrossRef CAS PubMed.
- S. E. Gibson and C. Lecci, Angew. Chem., Int. Ed., 2006, 45, 1364–1377 CrossRef CAS PubMed.
- S. S. Yoon and W. C. Still, Angew. Chem., Int. Ed. Engl., 1995, 33, 2458–2460 CrossRef.
- M. R. Carrasco and W. C. Still, Chem. Biol., 1995, 2, 205–212 CrossRef CAS PubMed.
- M. F. Cristofaro and A. R. Chamberlin, J. Am. Chem. Soc., 1994, 116, 5089–5098 CrossRef CAS.
- A. Borchardt and W. C. Still, J. Am. Chem. Soc., 1994, 116, 7467–7468 CrossRef CAS.
- S. S. Yoon and W. C. Still, J. Am. Chem. Soc., 1993, 115, 823–824 CrossRef CAS.
- J. I. Hong, S. K. Namgoong, A. Bernardi and W. C. Still, J. Am. Chem. Soc., 1991, 113, 5111–5112 CrossRef CAS.
- F. R. Pinacho Crisóstomo, A. Lledó, S. R. Shenoy, T. Iwasawa and J. Rebek, Jr., J. Am. Chem. Soc., 2009, 131, 7402–7410 CrossRef.
Footnote |
| † Energy differences calculated by subtracting ΔH and −TΔS values of the less stable diastereomeric host–guest pair from those of the more stable one, where ΔH and ΔS are the thermodynamic parameters of to the host–guest association equilibria. |
| This journal is © The Royal Society of Chemistry 2025 |