Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Supramolecular structure@MXenes for photocatalytic applications – a review
Pankaj
Verma
 a,
Jan
H. van Maarseveen
a,
Jan
H. van Maarseveen
 b and
N. Raveendran
Shiju
b and
N. Raveendran
Shiju
 *a
*a
aCatalysis Engineering Group, Van’t Hoff Institute for Molecular Sciences, University of Amsterdam, Science Park 904, 1098 XH Amsterdam, The Netherlands. E-mail: n.r.shiju@uva.nl
bSynthetic Organic Chemistry Group, Van’t Hoff Institute for Molecular Sciences, University of Amsterdam, Science Park 904, 1098 XH Amsterdam, The Netherlands
First published on 13th March 2025
Abstract
Recently, supramolecules have emerged as innovative and eco-friendly options for photocatalytic applications due to their tunable porous structures and photophysical properties. However, their low thermal stability and chemical stability pose a significant challenge. To address this, combining supramolecules with more stable materials like MXenes, which have a low Fermi energy level, is a useful strategy, in which they can form heterostructures that enhance stability and improve photocatalytic activity. The synthesis process, whether through in situ or post-synthesis modifications, plays a crucial role in controlling the formation of both covalent and non-covalent interactions, as well as the morphology of the heterostructures. These interactions and the resulting morphology significantly influence the recombination and separation of charge carriers (electron–hole pairs), ultimately affecting the stability and recyclability of the heterostructures in photocatalytic applications. In this review, we discuss the importance of supramolecule/MXene heterostructures, detailing their synthesis and morphology, as well as the mechanisms involved in various applications.
1. Introduction
In the face of pressing global challenges such as fossil fuel depletion, rising energy demands, and environmental degradation, photocatalysis emerges as a promising solution.1 This method is particularly eco-friendly because it involves the harvesting of solar energy, which is a renewable energy source, to drive chemical reactions without generating byproducts. This approach utilizes a semiconductor, as a photocatalyst, to absorb solar energy and generate charge carriers (electron–hole pairs)2 (Fig. 1). These charge carriers are responsible for initiating various chemical transformations, such as hydrogen generation via water splitting, pollutant degradation, CO2 reduction etc. The breakthrough work of Fujishima and Honda in 1972 set the foundation for photocatalysis research.3 This work demonstrated the potential of titanium dioxide (TiO2) photocatalysts for hydrogen generation via photocatalytic water splitting. Their discovery opened new avenues for researchers to produce clean hydrogen fuels from renewable sources, sparking widespread interest in developing new photocatalytic materials for various applications. Despite these advancements, the photocatalytic performance of TiO2 is limited by many factors such as (i) a relatively wide band gap (∼3.2 eV) which limits its ability for light absorption and (ii) rapid recombination of photogenerated charge carriers (electron–hole pairs), which are mainly responsible for the efficiency of photocatalytic reactions.4Thus, these limitations have driven researchers to explore a wide range of other semiconductor materials with an objective of enhancing photocatalytic activity by improving the range of light absorption and minimizing charge recombination.5 Some notable semiconductors include oxides such as SnO2 and ZnO, as well as a variety of chalcogenide materials, such as CdSe, CdS etc.6 In addition to these advanced semiconductors, researchers have also focused on next-generation materials with advanced architectures designed to enhance charge separation and improve stability, such as one-dimensional (1-D) (e.g., carbon nanotubes) and two-dimensional (2-D) (e.g., carbon nitrides and layered materials) materials.7
Recently, attention has shifted towards heterostructures, combining two or more photocatalytically active materials, such as Z-scheme systems, core–shell structures or p–n junctions. In Z-scheme systems, two photocatalysts are combined in a manner that mimics the natural photosynthesis process, enhancing charge separation and extending the range of light absorption.8 In core–shell heterojunctions, the core material is surrounded by a shell of another photocatalytic material. This design not only enhances the charge transfer but also shields the core material from degradation, extending the lifetime of photocatalysts.9 These heterostructures not only address the limitations of individual semiconductors but also significantly enhance photocatalytic performance. The heterostructures of supramolecules with MXenes are potential candidates in this direction.
Supramolecules, commonly known as “beyond molecules,” encompass a wide range of structures formed through various non-covalent interactions, including hydrogen bonding, π–π interactions, van der Waals forces, and more.10 Metal–organic frameworks (MOFs)11 and covalent organic frameworks (COFs)12 represent a distinct category of “Supramolecular Host–Guest Assemblies.” In these systems, the framework serves as the host, providing space for guest molecules, such as ions or solvent molecules, to reside through weak or non-covalent interactions. In photocatalytic reactions, supramolecular materials, especially MOFs13 and COFs,14 have emerged as innovative, eco-friendly options and have many advantages, such as (i) high surface area and tunable pore size, providing more active sites for photocatalytic activity, (ii) high porosity enhancing reactant adsorption and diffusion,15 (iii) tunable energy levels by selecting appropriate metal centers (in MOFs) or organic linkers (in both MOFs and COFs), enabling them to absorb in a long range, (iv) conductive linkers and π-conjugated pathways in COFs enhancing charge transport, while MOFs with appropriate metal centers acting as electron acceptors or donors,16 and (v) possibility of post-synthetic modifications or functionalization in both MOFs and COFs.17 However, these supramolecules often suffer from low thermal/chemical stability and rapid recombination of photogenerated electrons and holes, resulting in low photocatalytic activity with very low recyclability. Thus, combining them with more stable materials like MXenes can yield heterostructures with enhanced stability18,19 and improved photocatalytic activity.20
MXenes are a class of two-dimensional (2-D) materials, known for their excellent metallic electrical conductivity, hydrophilicity similar to graphene oxide, and robust mechanical properties.21 MXenes are typically derived from MAX phases (e.g., Ti2AlC, Ti3AlC2, and Ti4AlC3) through selective etching of aluminum, creating interlayer spaces suitable for intercalation and the formation of sandwich-like structures.22 The presence of terminal functional groups (e.g., –OH, –O, and –F) on MXene surfaces enhances their versatility, enabling the formation of hybrids with other materials such as MOFs and COFs. This makes MXenes ideal candidates for various applications due to their tunable properties.23,24 Our group has shown the effectiveness of MXenes and MAX phases in several applications.25–27 Although extensive research has been conducted on supramolecule/MXene composites for applications such as supercapacitors, batteries, and electro materials, there is a notable lack of comprehensive reviews focusing on their photocatalytic activity.28 This review seeks to address this gap by summarizing the current state of research on supramolecule/MXene composites for photocatalysis (Table 1) and providing a framework for future investigations into these materials for eco-friendly applications.
| S. N. | Photocatalyst | Preparation method | Structure | BET surface area (m2 g−1) | Light source/range | Other conditions | Photocatalytic activity | Result | Recyclability | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | TU series (Ti3C2/UiO-66-NH2 | One-pot hydrothermal method | Irregular cubic-spherical morphology of UiO-66-NH2 was decorated with Ti3C2 nanosheets | 984 | Simulated solar irradiation | Sacrificial reagent (0.1 M Na2S and 0.1 M Na2SO3) | Hydrogen production | 204 μmol h−1 g−1 | 3 runs | 29 |
| 2. | Ti3C2/TiO2/UiO-66-NH2 | Electrostatic adsorption via one-step hydrothermal method | Uniform attachment of UiO-66-NH2 on the surface of TCA with more exposed reactive sites | 987.5 | Simulated solar irradiation (350 < λ < 780 nm) | Sacrificial reagent 0.1 M Na2S and 0.1 M Na2SO3 | Hydrogen production | 1980 μmol h−1 g−1 | 3 runs | 30 |
| 3. | TCs/Cu-PMOF | One-step solvothermal process | Both TCs and Cu-PMOF had 2D lamellar structures while 2D sheets’ structure was observed in the image of the TCs/Cu-PMOF heterostructure | 20.3 | 300 W Xe lamp (340–780 nm) | Sacrificial reagent triethanolamine | Hydrogen production | 10.15 mmol g−1 | 5 runs | 31 |
| 4. | TT/CuTMOF nanocomposite | Cu2+ is introduced into Ti3C2@TiO2 (TT) to provide sites for the in situ assembly of the Cu-TCPP MOF | TT/CuTMOF showed thin sheet-like structures with close interfacial contact | — | Simulated solar irradiation (340 < λ < 780 nm) | Sacrificial reagent triethanolamine (TEOA) | Hydrogen production | 19.06 mmol g−1 | 5 runs | 32 |
| 5. | Ti3C2@MIL-NH2 | In situ hydrothermal growth method | Irregular nanoparticles of MOFs were in situ grown on the accordion like layered MXene structure | 69.2 | Simulated solar irradiation | Sacrificial reagent 20 mL CH3OH with 0.3 mL TEOA | Hydrogen production | 4383.1 mmol g−1 | 4 runs | 33 |
| 6. | Ti-MOF/QDs/ZIS | Electrostatic attraction method | ZnIn2S4 nanosheets covering the surface of the Ti-MOF crystals and 2D Ti3C2 MXene lamellae stacks | 179.8 | λ > 420 nm | Sacrificial reagent 0.35 M Na2S and 0.25 M Na2SO3) | Hydrogen production | 2931.9 μmol g−1 h−1 | 4 runs | 34 |
| 7. | ATNT | In situ growth of β-ketoenamine-linked COFs onto NH2−Ti3C2Tx MXenes via covalent connection | COF sticks with a sheet-like microstructure are uniformly attached on NH2–Ti3C2Tx | 683.6 | λ > 420 nm | Sacrificial reagent L-ascorbic acid | Hydrogen production | 14.20 mmol g−1 | 6 runs | 35 |
| 8. | MXene/MOF (MXOF) | In situ synthesis of MOFs in the presence of MXene nanosheets | MOF particles with spherical shapes on the MXene nanosheet surface | 37.69 | Visible-light irradiation (mercury vapor (250 W)) | Time required (60 min) | Photodegradation of DR31 (azo dye) and MB (thiazine dye) | 62% of MB and 35% of DR31 dyes | 4 runs | 36 |
| 9. | Sn–Bi–MOF/Ti3C2 | In situ solvothermal method | Sn–Bi–MOF nanoparticles were inserted into the layered structure of Ti3C2 | 145.8 | 500 W xenon lamp | Time required (90 min) | Photocatalytic degradation of tetracycline (TC) | 96.2% degradation efficiency of TC | 4 runs | 37 |
| 10. | MIL-88A(Fe)/MXene (D-MIL-88A(Fe), W-MIL-88A(Fe), S-MIL-88A(Fe)) | Hydrothermal in situ growth | Many small fine grain particles of MIL-88A(Fe) on the smooth Ti3C2 MXene surface. | 12.4 | Xenon lamp | Time required (10 min) | Degradation of common pollutants in wastewater | 70% degradation of sulfamethoxazole by DA-M100 | 3 runs | 38 |
| 11. | NH2-MIL-125(Ti)(TiO2)/Ti3C2 | One-step solvothermal strategy | MT0 – plate-like nanosheet morphology, MT0.1 – rod-like nanoparticles, MT1 – enlarged particles with the surface uniformly coated by TiO2 nanosheets, MT5 – enlarged particles with the surface uniformly coated by TiO2 nanoparticles | 329 | λ > 420 nm | Time required (60 min) | Photocatalytic degradation of tetracycline hydrochloride (TC-HCl) | 82.80% degradation of tetracycline over MT5 | 4 runs | 39 |
| 12. | TP-COFs/Ti3C2Tx (TCM) | In situ growth of COFs over MXenes | TC nanofibers (about 500–1500 nm in length) attach in an orderly fashion on the layered NH2−Ti3C2Tx, forming a lawn-like TCM composite | — | 300 W xe lamp and equipped with an ultraviolet cutoff filter (λ > 420 nm) | Electron donor (15% w/v TEOA) | CO2 reduction | Formate yield – 11.9 & 2.7 mmol L−1 (with & without an electron mediator, respectively) | — | 40 |
| 13. | ZT-450 | ZIF-67 was in situ grown on Ti3C2Tx MXene | Spreading of polyhedral ZIF-67 on the Ti3C2Tx MXene surface | 62.6 | Xe lamp (420–780) | — | CO2 reduction | Yields – CO (62.7 μmol g−1), CH4 (6.7 μmol g−1) and H2 (7.3 μmol g−1) | 4 runs | 41 |
| 14. | pink/TC/SBM | A simple electrostatic self-assembly method | 2D structure of pine and SBM rapped around TC and all three are connected with electrostatic connections | — | 300 W he lamp (simulated sunlight) | Electron donor (20 ml TEOA) | CO2 reduction | Yields – CO (36.33 μmol g−1 h−1) | 4 runs | 42 |
| 15. | Co–Co LDH/TNS | In situ MOF derived solvothermal method | LDH nanosheets staggered and standing on a TNS substrate to form a nanoarray structure | — | 5 W LED lamp (400–1000 nm) | Electron donor (1 ml TEOA) | CO2 reduction | Yields – CO (1.25 × 104 μmol g−1 h−1) | 5 runs | 43 |
| 16. | Ti3C2/TpPa-1/Cu2O | In situ growth of a TpPa-COF on MXene through the Schiff base reaction, followed by NP anchoring | Cu2O spherical nanoparticles grew on the accordion like structure of Ti3C2/TpPa-1 | — | — | Method – plate colony counting method | Antibacterial activity | High antibacterial properties against P. aeruginosa and S. aureus, with antibacterial rates of 99.62% and 98.90%, respectively | 3 runs | 44 |
| 17. | Ti3C2/TpPa-1/Ag | Covalently connected Ti3C2/TpPa-1 through the Schiff base reaction, anchored by Ag NPs | Ag nanoparticles were highly dispersed on the surface of Ti3C2/TpPa-1 | — | — | Method – plate colony counting method | Antibacterial activity | Antibacterial activity against P. aeruginosa and S. aureus, with antibacterial rates of 99.60% and 99.78%, respectively | 3 runs | 45 |
| 18. | Cu-TCPP/Ti3C2 | In situ self-assembly method | Sheet-like structure | — | — | Method – spread plate method | Antibacterial activity | Antibacterial activity against S. aureus, with antibacterial rates of 99.73% | — | 46 |
2. MXenes and their optical properties
MXenes, which are carbides and nitrides of transition metals, are emerging as a new class of useful two-dimensional (2D) materials. They are renowned for their outstanding electronic conductivity and highly active metal sites with hydrophilic surfaces.47 In 1964, Jeitschko et al.48 reported the TMC phase (Zr2TlC, Zr2PbC, Hf2TlC, and Hf2PbC), which is now known as the MAX phase. Although these compounds contained thallium (Tl) in those days, note that the soluble thallium salts are highly toxic and hence it is better to avoid their use. The general empirical formula for the MAX phase is Mn+1AXn, where M denotes a transition metal, such as titanium (Ti), scandium (Sc), niobium (Nb), molybdenum (Mo), tantalum (Ta), or vanadium (V); A represents a group 13 (boron family) or group 14 (carbon family) element; and X is carbon (C) and/or nitrogen (N). In the MAX phase, the M–A bond, which is a chemically active metallic bond, can be easily broken using a selective chemical etching method, resulting in MXenes.49 In the simplest way, MXenes can be divided into three parts: (1) an intramolecular region composed of alternative arrangement of titanium (Ti) and carbon (C) via the formation of an ionic bond. This is the main skeleton structure. (2) The interlayer region formed by non-covalent interactions, such as hydrogen bonding or van der Waals forces, between the layered structures; and (3) the surface terminated functional groups.50,51 Depending on the etching agents used, MXenes have different terminal functional groups (Tx), such as –O, –F, –OH, etc. A schematic representation of possible MXene structures (M2XTx, M3X2Tx, M4X3Tx, and M5X4Tx) is shown in Fig. 2. Similar to their corresponding MAX phases, MXenes have a hexagonal close-packed structure, in which the octahedral voids are occupied by X atoms.21,52 | ||
| Fig. 2 Representation of the structure and compositions of MXenes. Reprinted with permission from ref. 21; Copyright 2021 Wiley-VCH GmbH. | ||
To date, various methods have been documented for the preparation of MXenes from their corresponding MAX phases. Synthesizing high-quality MXenes is challenging, and the process is influenced by many factors, such as reaction conditions, the type of etching agent, the type of MAX precursor, and others. The most popular synthesis method is etching, which utilizes various chemicals and bio-materials such as hydrofluoric acid, alkalis, Lewis acidic salts, and algae extracts, as well as electrochemical processes and photolithography.53 The selection of the etching method used primarily depends on the type of A-group element, the transition metals, and the bonding between them.54 According to the literature, acid etching is beneficial for MAX phases containing Al and Si as A-group elements, whereas MAX phases containing Al, Si, Zn, and Ga as A-group elements can be easily converted into MXene nanosheets using molten salt etching methods. Additionally, the exfoliation of nanosheets also depends on the strength of bonding between transition metals and A-group elements.55 In the periodic table, the reactivity of transition metals decreases from left to right. Therefore, more stringent conditions are necessary for MAX phases containing transition metals such as Cr, Mo, and W, in contrast to those with Ti, Zr, and Hf.56
The optical properties mainly depend on the structural and electronic properties of materials. In MXenes, the presence of terminal functional groups and chemical composition significantly affect the optical properties. Lashgari et al.57 demonstrated through DFT calculations that without terminal functional groups (Tx), pristine MXenes exhibit metallic character due to the overlapping of the conduction band (CB) and valence bands (VB) at the Fermi level. During the course of synthesis, the outermost metallic layer in MXenes is terminated by terminal functional groups. Further DFT studies predicted that the optical properties are mainly dependent on the type as well as the orientation of terminal functional groups, irrespective of the composition of MXenes. Berdiyorov58 employed DFT calculations to reveal the role of terminal functional groups (such as −F, −O, and −OH) in the electronic structure of Ti3C2T2 MXenes. In the visible range, fluoride (–F) and hydroxy (–OH) groups exhibited lower absorption and reflectivity compared to bare MXenes. In contrast, the surface with an –O terminal functional group showed enhanced absorption and reflectivity. In the ultra-violet (UV) range, surface functionalization showed a positive impact on improving the anti-ultraviolet properties of MXenes.
3. MOFs and their electronic properties
MOFs, a subset of porous frameworks, are widely recognized as inorganic–organic hybrid materials. They are prepared from inorganic nodes and organic linkers.59 The inorganic nodes are metal centers (mainly transition or lanthanide metals) or metal oxide clusters, known as secondary building units (SBUs), whereas organic linkers consist of functional groups or anions60 such as carboxylates, phosphonates, sulfonates etc. The geometry of MOFs mainly depends on the coordination number and geometry of the central metal ion, and also the nature of functional groups, such as monodentate, bidentate or multidentate (Fig. 3(a)).61 In principle, a bridging linker reacts with a metal ion that has more than one vacant or labile site, resulting in the formation of frameworks with different topologies. | ||
| Fig. 3 (a) Basic topological diagrams for 1D, 2D and 3D MOFs (reprinted with permission from ref. 61. Copyright 2012 American Chemical Society). (b) Comparison of calculated and experimental band gaps in functionalized ligands (reprinted from open access under Creative Commons CC BY 3.0 from ref. 62). (c) Band energy structure of UiO-66(Ce)-X (reprinted with permission from ref. 63) Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (d) Optical band gap energy of a Zn-based MOF (reprinted with permission from ref. 64). Copyright 2012 American Chemical Society. | ||
In MOFs, the electronic properties (band gap shifting) can be achieved by (i) exchanging ligands with more conjugated ligand systems,65 (ii) generating vacancies and defects in the system,66–69 (iii) altering the shape of MOFs at the nanoscale70,71 and (iv) functionalization of organic linkers (such as –NH2, –NO2, –OH, –Me etc.) leading to a band shift by donating a 2p electron to the aromatic linker.72 For example, MIL-125, synthesized from the 4-benzenedicarboxylate (bdc) linker, has a band gap in the UV region (3.6 eV, 345 nm)73 whereas MIL-125-NH2, synthesized from a mono-aminated bdc-NH2 linker, has a band gap in the visible region (2.6 eV, 475 nm).74 Treger et al.62 performed a DFT study on the effect of electron-donating and/or electron-withdrawing functional groups on the optical properties of MOFs by introducing functional groups into the ligand (terephthalic acid) of UiO-66 MOFs (2.96 eV). These ligands contain amine (–NH2) or dimethylamine (–NMe2) as an electron donating group and a nitro (–NO2) or dicyanovinyl (DCV) group as an electron withdrawing group. Generally, the introduction of functional groups in UiO-66 MOFs decreases the band gap but the extent of the effect is based on the electron donating/withdrawing nature of the group. The electron-donating functional group lowers the band gap more significantly compared to electron-withdrawing groups (Fig. 3(b)).62 In the case of the –NH2 group, a new band gap state is generated over the valence band maximum (VBM) due to the presence of free electron pairs of nitrogen atoms (UiO-66-NH2, 2.75 eV). In contrast, introduction of –NO2 groups showed only a small decrease in the band gap due to strongly bound electrons which formed a localized state (UiO-66-NO2, 2.93 eV).
The functionalization of ligands in MOFs not only decreases the band gap but also alters the alignment of the VB and CB.70 For example, –NH2 functionalization increases the reduction potential of UiO-66(Ce)-NH2 by shifting the CB band (LUMO) to more negative values, whereas –NO2 groups shift the VB (HOMO) position to more positive values relative to the non-functionalized UiO-66(Ce) with respect to the NHE (Fig. 3(c)).63,70 For photocatalytic overall water splitting (OWS), the HOMO (VB edge) and LUMO (CB edge) must align with the thermodynamic potentials of the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). The energy difference (band gap) between the HOMO (VB edge, approximately −0.41 V vs. NHE) and LUMO (CB edge, approximately 0.82 V vs. NHE) should fall within the range of 1.8–2.4 eV.75 For example, utilizing terephthalate-based mixed ligands with −NH2 substituents (HOMO approximately −0.94 V vs. NHE, LUMO approximately 1.15 V vs. NHE, band gap 2.09 eV) and/or −Br substituents (HOMO approximately −0.75 V vs. NHE, LUMO approximately 1.93 V vs. NHE, band gap 2.68 eV) offers a promising approach to synthesize functionalized UiO-66(Ce) with the desired band gap energy and band alignment.63
Additionally, the incorporation of a halogen atom into an aromatic system can also reduce the band gap. The combined effect of two factors—the electron-donating effect (+C effect, conjugative effect) and the electron-withdrawing effect (−I effect, inductive effect)—contributes to an increase in the HOMO energy level.76 Thus, among all halogens, iodine is the best candidate for reducing the band gap and increasing the valence band maximum (VBM) (Table 2). In addition to the above, the size and type of metal centres and/or SBUs also affect the electronic properties of MOFs (Fig. 3(d)).64 These modifications not only change the electronic properties of MOFs but also open new avenues for exploring MOFs as new semiconductors.77
4. COFs and their optical properties
COFs, another subclass of porous crystalline materials, are formed by covalent bonds between two or more organic building blocks. These building blocks range from small organic molecules to macromolecules such as polymers78 (Fig. 4). The high stability of COFs mainly depends on the strength of covalent bonds formed between the building blocks such as C–N, C–C, and C–O bonds. For the first time in 2005, Yaghi et al.79 introduced COFs, formed by the condensation reaction of organic building blocks. In contrast to MOFs, COFs are formed solely through covalent bonds between light elements such as hydrogen, boron, carbon, nitrogen, and oxygen, without the involvement of heavy metal atoms. MOF frameworks are created through co-ordination bonds formed between metal centers and organic ligands, whereas COFs are characterized by formation of covalent bonds, providing them high stability.80 Generally, COFs have low densities with exceptional stability under harsh conditions, such as acidic, basic, oxidative, and reductive conditions. | ||
| Fig. 4 Basic topological diagrams of COFs. Reprinted with permission from ref. 78. Copyright 2020 American Chemical Society. | ||
The optical properties of COFs mainly depend upon the type of framework, topology, chemical composition, presence of functional groups, specific surface area and porosity. The presence of chromophores such as aromatic rings or extended conjugated systems enhances the photophysical properties of COFs in visible or UV-visible ranges. The type of covalent bonding formed during synthesis, such as imine linkages, triazine-based linkages, and β-ketoenamine linkages, and the presence of functional groups, such as –NO2, –NH2etc., can also alter the electron density and photophysical properties (Table 3). In a critical review, Wang et al.81 clearly explained that the structure of frameworks, from the 0-dimensional structure to the 3-dimensional structure, and the intact weak bonding such as H-bonding, π–π interactions etc. between layers, directly influence the separation and transfer of electron–hole pairs through the frameworks. Li et al.82 demonstrated that the presence of donor–acceptor (D–A) groups and increased conjugated systems that participated in COFs results in a narrower optical band gap. Pachfule et al.83 synthesized β-ketoenamine based COFs functionalized with phenyl, acetylene and diacetylene, named TP-DPT, TP-EDDA and TP-BDDA COFs respectively, for photocatalytic hydrogen generation via water splitting. A comparison of their optical properties revealed a notable reduction in the band gap by the introduction of a diacetylene group. Specifically, TP-BDDA exhibited a small band gap (2.31 eV), in comparison to TP-EDDA (2.34 eV) and TP-DTP (2.42 eV) (Fig. 5(a)). Chen et al.84 investigated the impact of proton tautomerism (Fig. 5(b)) on the optical properties of COFs, named COF-OH-n (where n = 0–3 denotes the number of –OH groups), formed by β-ketoenamine linkages. COF-OH-0, with a band gap of 2.68 eV, exhibited the narrowest absorption spectrum due to the absence of –OH groups. In contrast, COF-OH-1, COF-OH-2 and COF-OH-3 displayed progressively broader absorption spectra with band gaps of 1.90, 2.02 and 2.28 eV, respectively (Fig. 5(c)).
 | ||
| Fig. 5 (a) UV-Vis DRS of COFs showing the calculation of the band gaps as well as the optical images of COF powders (reprinted with permission from ref. 83). Copyright 2017 American Chemical Society; (b) color change due to proton tautomerism; (c) band position and bandgap of COFs (b & c reprinted with permission from ref. 84. Copyright 2022 Royal Society of Chemistry). | ||
5. Synthesis methods
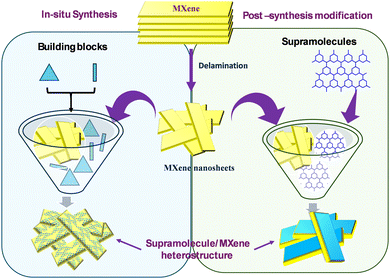
Supramolecules, MOFs and COFs, are significant branches of porous polymeric materials with tunable structures, either in terms of porosity or functionality of frameworks. MOFs are synthesized from metal centers coordinated with multi-functional ligand systems whereas COFs are organic polymers formed through the creation of covalent bonds between organic moieties.88 The presence of organic ligands with functional groups makes these materials ideal precursors for the conversion to other carbon based materials.89In air and water environments, the metastable metal atoms present on the surface of MXenes can transform into stable metal oxides through a spontaneous reaction with oxygen-containing groups.90,91 To enhance the stability and photophysical properties, MXenes are often used to form composites with supramolecules and their derivatives.92 Thus, for the synthesis of MXene based composites with supramolecules, many strategies have been employed which can be classified into two categories an in situ method and post-synthetic modification (Fig. 6).
5.1 In situ method
In this method, composite formation occurs in a single step, enabling the components to bond through the available active sites. A large number of interactions are formed between the components during the growth of the framework. Typically, the process of supramolecule/MXene heterostructure formation involves mixing MXenes with the building blocks of supramolecular frameworks under controlled synthesis conditions93 (Fig. 6). In the case of MOFs, the building blocks are deprotonated ligands and metal ions. Qu et al.52 synthesized Ni-MOF/Ti3C2Tx hybrids by adding MXenes to the solution containing nickel salt followed by the addition of terephthalic acid as a ligand, with stirring at room temperature. Similarly, Wen et al.94 synthesized a bimetallic CoNi-ZIF-67/Ti3C2Tx composite by adding MXenes to the solution containing Co2+ and Ni2+ ions, followed by the addition of 2-methylimidazole and hexadecyl trimethyl ammonium bromide (CTAB), with continuous stirring at room temperature.The process of COF/MXene hybrid formation is similar to that of MOF/MXene hybrids, with the only difference being the use of metal ions in MOFs whereas only organic molecules are used for the formation of COF/MXene. Geng et al.95 reported in situ synthesis of a series of COF@MXene heterostructures through the formation of covalent bonds between the terminal C–O groups of anthraquinone (AQ)-COFs and amino units of modified MXene nanosheets. Similarly Zhao et al.96 and Gong et al.97 reported a solid-phase micro-extraction coating method and an electrostatic self-assembly method for the preparation of COF/MXene heterostructures.
5.2 Post-synthesis modification
This method involves the integration of synthesized supramolecules with MXenes through various approaches, such as mechanical mixing, self-assembly and electrochemical methods, depending on the desired properties of composites and their application. Direct or mechanical mixing and sonication are easy and effective approaches for the preparation of hybrid materials. Non-covalent interactions formed between the components help simultaneously increase the properties of hybrid materials. Wei et al.98 prepared MXene/COF-LZU1 composite films through an ultrasound treatment by the addition of COF particles into the MXene solution. In the direct mixing method, both components, pre-synthesized supramolecules and MXenes, are mixed in a solution to form supramolecule/MXene composites. For example, Wang et al.99 fabricated a novel MIL-100(Fe)/Ti3C2 microporous photocatalyst by directly mixing a MOF solution with MXenes for improved nitrogen fixation ability.In a self-assembly synthesis method, electrostatic interactions, π–π stacking, hydrogen bonding and other interactions are mainly responsible for forming an ordered structure. Liu et al.100 synthesized three dimensional NiCo-MOF/Ti3C2 nanosheets through self-assembly. During the self-assembly process, hydrogen bonds were formed between 2D MXene and NiCo-MOF nanosheets. Similarly, Sun et al.101 fabricated a new Co-ZIF-9/MXene photocatalyst through the electrostatic self-assembly method for hydrogen generation.
6. Advantages of hybrid structures over individual components
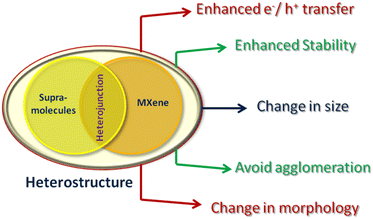
In photocatalytic reactions, pure supramolecules have many limitations like-low stability, rapid charge carriers’ recombination and fast agglomeration after a few cycles, which restrict their photocatalytic application on a large-scale. The compounding of supramolecules with MXenes not only alleviates the aforementioned limitations but also enhances the activity of supramolecules through synergy. In this review, we discuss the newly generated functionalities arising from the combination of MXenes with supramolecules in detail (Fig. 7).6.1 Improved stability
In some MOFs, poor stability limits their large-scale application, which could be enhanced by rational integration with other materials to produce a synergic effect. Liu et al.100 improved the stability of a bimetallic (Ni–Co) MOF by the formation of hydrogen bonds with Ti3C2 MXenes in three-dimensional inter-connected hierarchical porous composites, named Ti3C2/NiCo-MOF. Xioan et al.102 prevented the oxidation of MXenes by incorporating lignin into Ti3C2Tx/NiCo-MOF heterostructures, where the hydroxyl group of lignin formed bonds with the carboxylic group of MOFs and the terminal (Tx) group of MXenes. Wang et al.103 prevented restacking of MXene nanosheets by the formation of electrostatic attraction between two-dimensional Cu-HHTP MOFs and MXenes. Zhao et al.104 improved the cyclic stability of MXenes through electrostatic integration with ZIF derived layered double hydroxide nanosheets, where the surfaces of metal oxides were coated with MXenes to prevent the structural alteration of the electrode. In another study, Wei et al.105 improved the stability by forming a hollow core–shell structure in which binary-metal oxide ZnCo2O4 formed the core, coated by the Ti3C2 layer.6.2 Change in morphology
The combination of MXenes with MOFs also modifies the morphology of MOFs by creating new interactions with functionality of ligands as well as the bonded central metal center. Ruan et al.106 successfully modulated the rough sheet morphology into a typical dendrite structure by adding MXene suspension (Fig. 8(a)). These structures consisted a thick truck and delicate branches, which were wrapped in MXene nanosheets (Fig. 8(b)–(d)). These unique nano-trees served as electro-active sites where the wrapped conductive MXenes facilitated charge transfer. Zhang et al.107 also modulated the freely grown 2D nanosheet structure (Ni-MOF) into a layered microstrip. The functionality of composite materials was further enhanced by introducing TiO2 between MXenes and MOFs. Similarly, Wu et al.39 induced morphological changes in the nanosheet structure of the NH2-MIL-125(Ti) MOF by adding different concentrations of MXenes (Fig. 8(e)–(h)). With 0.1 wt% MXene, nanosheet structures were transformed to rod-shaped nanoparticles (Fig. 7(f)). At higher concentrations [MT1 (1%) and MT5 (5%)], TiO2 nanosheets and nanoparticles were uniformly dispersed on the surface (Fig. 8(g) and (h)), respectively. This demonstrated the modulating effect of Ti3C2 during crystallization. | ||
| Fig. 8 (a) Synthesis scheme for the CoNi2S4/MXene/NF hybrid; (b)–(d) SEM images of growth of the CoNi2S4/MXene/NF hybrid with time (reprinted with permission from ref. 106. Copyright 2021 Elsevier); and (e)–(h) different morphologies of the NH2-MIL-125(Ti)/MXene hybrid with different contents of MXenes. Reprinted with permission from ref. 39. Copyright 2020 Elsevier. | ||
6.3 Controlled agglomeration
An important advantage in forming composite materials is to decrease the chance of agglomeration. In composite materials of supramolecules with MXenes, the interactions between the free functional group of supramolecules and the group present on the surface of MXenes must be considered. This interaction not only reduces the agglomeration but also simultaneously improves the activity and recyclability of the composite materials. Yang et al.108 controlled the agglomeration of MXenes by uniformly anchoring nanosheets of CoSe2/Ni3Se4 on both sides. This anchoring prevented the restacking of MXene sheets and the synergistic effect of MXene@CoSe2/Ni3Se simultaneously improved the performance, enhanced charge transfer dynamics and increased recyclability.6.4 Improved electron/ion transport
Supramolecules are advanced materials for photocatalytic activity due to their designed tunable structures, high specific surface areas and porosity. These features not only increase the availability of active sites but also create spaces for the adsorption of catalytic materials. However, the main drawback is the quick recombination of charge carriers. In a supramolecule/MXene heterostructure, the interface formed at the junction facilitates charge transfer between the supramolecules and MXenes, while the low Fermi level of MXenes acts as a driving force for electron transfer from the supramolecules to MXenes109,110 (Fig. 9). Additionally, the presence of terminal functional groups on MXenes (Tx) and MOFs formed strong interactions during the self-assembly process, which also reduce the transfer pathway of charge carriers. Cheng et al.111 demonstrated that a 2D/2D UNiMOF/Ti3C2 hybrid exhibited four times higher photocatalytic activity compared to a bare ultrathin nickel metal organic framework, UniMOF. This is attributed to the smooth transfer of electrons from MOFs to the Fermi level of MXenes and the reduced recombination of charge carriers (Fig. 9(a)). Similarly, Sun et al.101 reported high hydrogen production (3538.5 μmol g−1 h−1) via a photocatalytic reaction over a noble metal free 2D/2D Co-ZIF-9/Ti3C2 heterostructure, which was 9.6 times higher than that of Co-ZIF-9 alone due to the efficient transfer and separation of photogenerated electron–hole pairs (Fig. 9(b)). | ||
| Fig. 9 (a) Band alignment in the UNiMOF/Ti3C2 hybrid (reprinted with permission from ref. 111. Copyright 2021 Elsevier) and (b) band alignment in the Co-ZIF-9/Ti3C2 heterostructure (reprinted with permission from ref. 101. Copyright 2022 Wiley-VCH GmbH). | ||
7. Applications
7.1 Hydrogen generation
In recent years, the depletion of fossil fuels underscored the importance of sustainable fuels with minimal carbon footprints. The demand for clean hydrogen sourced from low-carbon outlets is projected to increase substantially, with estimates indicating a potential annual demand of 200 million metric tonnes by 2030, a substantial rise from the 71 million tonnes recorded in 2019.112 Among the various technologies (fossil fuel reforming, electrolysis, plasma reforming, and biomass) available for hydrogen production, photocatalytic water splitting stands out as an environmentally friendly approach to replace fossil fuels. In photocatalytic hydrogen generation, water is split into oxygen and hydrogen through the effect of solar light113 (Fig. 10). The kinetic behaviour of this procedure can be influenced by several factors, such as alterations in pH during the reactions, the concentration of the immediate substrate, and the light absorption capacity of the suspended particles.114 Tian et al.29 used MXenes as non-metallic co-catalysts in combination with UiO-66-NH2 MOFs as ideal candidates for photocatalytic hydrogen generation activity. MXene nanosheets, prepared by the intercalation method, were formed in intimate face to face contact with the UiO-66-NH2 porous MOF prepared by a hydrothermal method (Fig. 11(a)). The synthesized samples were labeled as TU-X where X represents the dilution concentration of the original MXene concentration by 5, 10, 15 and 20 times. Both the MOF and TU10 exhibited a typical type I hysteresis loop in their nitrogen adsorption and desorption profile, indicating their microporous nature. Further BJH analysis showed that TU10 was more porous than the pristine MOF itself, supporting the fact that MXenes acted as substrates for the growth of UiO-66-NH2.115 A comparative photocatalytic study showed that TU10 had the highest photocatalytic hydrogen generation activity, approximately 8-fold greater than that of the bare MOF (Fig. 11(b)). In the TU series, the Schottky junction formed at the interfaces not only suppressed the recombination of electron–hole pairs but also promoted the spatial separation and transfer of charge carriers (Fig. 11(c)). The photocatalytic activity was also further enhanced by the electron-donating ability of MXenes due to the accumulation of photo-induced electrons at the interface. Additionally, DFT calculations supported that O-terminated MXenes showed high photocatalytic activity due to their most positive Fermi level and the lowest Gibbs free energy (|ΔGH*| = 0.08 ≈ 0).29 | ||
| Fig. 10 Pictorial representation of photocatalytic hydrogen production over supramolecule/MXene heterostructures. | ||
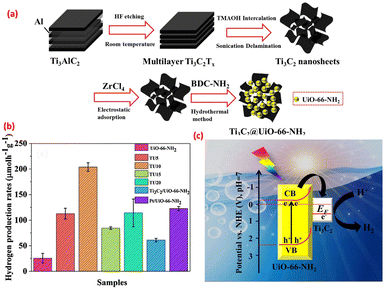 | ||
| Fig. 11 (a) Synthesis scheme of the TU series; (b) H2-generation rates under photocatalytic conditions of samples; and (c) proposed mechanism. Reprinted with permission from ref. 29 Copyright 2019 Elsevier. | ||
Zhu et al.31 fabricated a nano-hybrid with matched dimensions (2D on 2D) by combining two-dimensional porphyrin-based metal–organic frameworks (Cu-PMOFs) on two-dimensional MXene/TiO2 nanosheets using a solvothermal reaction at a temperature 120 °C. This ternary photocatalyst demonstrated significantly enhanced photocatalytic hydrogen generation activity, nearly 29-fold that of bare TiO2. The VB of Cu-PMOFs was more negative than that of TiO2, facilitating the transfer of holes from TiO2 to Cu-PMOFs which was very weak and consumed by the TEOA sacrificial reagent. Simultaneously, the electrons present in Cu-PMOFs were transferred at the interface of Cu-PMOFs and TiO2. In the composite, TiO2 also acted as a semiconductor and the photogenerated electron also present in the VB of TiO2. Thus, the VB electron of TiO2 along with the electrons present at interfaces flowed to a lower Fermi level of MXenes and reacted with water to produce hydrogen. Thus, the synergistic effect between Cu-PMOFs and Ti3C2/TiO2 enhanced the activity in the visible range, suppressed the recombination of charge carriers and also improved the recyclability. Similarly, Chen et al.32 also fabricated a ternary photocatalyst by compounding Cu-TMOFs with Cu2+ anchored to the partially oxidized MXene (TT) as the nucleation sites. The morphology of the TT/Cu-TMOF composite consisted of thin, sheet-like structures with tight interfacial contact. The photocatalytic activity of the nanocomposite (TT/Cu-TMOF) was about 55-fold higher than that of pure TT and also demonstrated high cycle stability.
Due to their layered structure, MXenes can be used as substrates for the growth of supramolecules, which not only facilitates the in situ formation of connections but also increases the uniform porosity of supramolecules. Li et al.33 developed a Ti-based MOF (MIL-NH2) by in situ growth onto layered Ti3C2 MXenes. Here, MXenes served as both a substrate and a source of Ti for MOF formation. In the composite, the –NH2 group in the MIL-NH2 MOF coordinated with the Ti atom in Ti3C2, accelerating the electron transfer through interfacial contact and simultaneously enhancing the separation efficiency. As a result, Ti3C2@MIL-NH2 (4383 μmol h−1 g−1) exhibited higher hydrogen generation performance than bare materials. Similarly, Liu et al.34 synthesized a novel ternary MXene quantum dot based photocatalyst, NH2-MIL-125(Ti)/MXene quantum dot/ZnIn2S4 (Ti-MOF/QD/ZIS), to evaluate hydrogen generation activity (2932 μmol g−1 h−1) under visible light irradiation. MXene quantum dots acted as a bridge between the semiconducting components for the Z-scheme mechanism and facilitated the transfer of electrons from MOFs to ZnIn2S4.
Similar to MOFs, the integration of COFs with MXenes not only improves the photocatalytic activity but also significantly enhances the stability of composite materials compared to bare materials. Wang et al.35 synthesized COFs through an acid-catalyzed Schiff base reaction with variation in the ratio of β-ketoenamine to imine as shown in Fig. 12(a). During integration with amine functionalized MXenes, covalent connections were formed between β-ketoenamine-linked COFs and the –NH2 group of MXenes. The increased number of hydroxy groups (–OH) which participated in the formation of β-ketoenamine linkages directly affects the HOMO energy. In photocatalytic activity, an in situ synthesized COF/MXene hybrid (ATNT) showed a higher hydrogen generation yield in comparison to physical mixing samples. The enhancement in activity was due to the formation of heterojunctions in ATNT hybrids which provided short pathways for the transfer of photogenerated electrons through the synergistic effect of photoactive COFs and amine functionalized conductive MXenes. Most importantly, the hybrid with optimized content of MXene (ATNT-4) showed higher photocatalytic activity as an increase in content of amine functionalized MXenes blocked the active site and also shielded open areas for light absorption (Fig. 12(b)). Recyclability experiments demonstrated that the COF hybrid had high recyclability (more than 6 cycles) (Fig. 12(c)) and good stability.
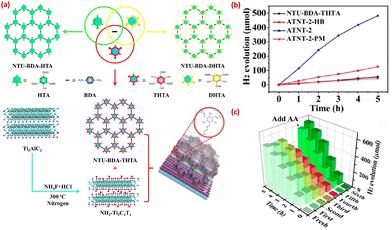 | ||
| Fig. 12 (a) Synthesis of the COF/MXene–NH2 hybrid; (b) photocatalytic activity of synthesized hybrids; and (c) recyclability. Reprinted with permission from ref. 35. Copyright 2020 American Chemical Society. | ||
In addition to the above binary examples (supramolecule/MXene), Tian et al.30 used annealed MXenes (Ti3C2Tx) for the synthesis of a ternary photocatalyst for hydrogen production. During the annealing process, MXenes retained their original layered structure with the formation of TiO2, which combined with a water-stable porous UiO-66-NH2 MOF to form a layered porous ternary catalyst (Ti3C2/TiO2/UiO-66-NH2). Detailed structural analysis with FE-SEM and TEM showed that all the three components of the ternary catalyst (MOF, titanium dioxide and Ti3C2) were arranged in such a manner that they formed three types of contact interfaces: MXene/TiO2/MOF, MXene/TiO2, and MXene/MOF. The N2 adsorption–desorption isotherms showed that the ternary catalyst (988 m2 g−1) had a slightly higher specific surface area with a microporous structure compared to the pure MOF (977 m2 g−1). In a porous ternary catalyst, three interfaces were formed at the contact of the MOF, TiO2 and MXene. These three interfaces initiated three pathways for photocatalytic hydrogen generation (1980 mmol h−1 g−1) in the ternary catalyst which was higher than that of pristine UiO-66-NH2 under simulated sunlight irradiation. In pathway-I, the photogenerated electron was directly transferred to the Fermi level of TiO2 from the MOF, suppressing the recombination of charge carriers. In pathway-II, the photocatalytic generated electron present in the conduction band of TiO2 is directly transferred to Ti3C2 for further photocatalytic activity. In pathway-III, the photogenerated electron in the conduction band of the MOF was directly transferred to TiO2 which was then indirectly transferred to Ti3C2 for further activity. Thus, the synergistic effect of all three components, formed through three contact interfaces, was mainly responsible for the enhanced photocatalytic activity and for successfully suppressing the recombination of charge carriers. This was also confirmed by the low PL intensity of the ternary composite in comparison to other bare components. This work established a foundation for MOF-based ternary photocatalysts for further studies in the field of photocatalytic hydrogen generation activity.
7.2 Pollutant degradation
Statistical estimations indicate that industries using organic dyes for manufacturing and applications in textile, pharmaceuticals, leather, pulp and paper, personal care products and food processing produce over 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of organic dyes globally each year. Approximately, 10–15% of these dyes are released as waste effluents.116 In comparison to other methods such as ozonation,117,118 adsorption119,120 and filtration121,122 degradation of pollutants through the photocatalytic method is one of the preferred approaches. This method is simple, energy-efficient, low-cost and stable, with a minimal production of secondary pollutants. In photocatalytic degradation, semiconductor materials absorb light with enough energy to transfer electrons from the valence band (VB) to conduction band (CB), leading to the generation of oxidative holes in the valence band (VB). Highly reactive species are produced from the reaction of reductive electron and oxidative holes with dissolved oxygen/H2O/H2O2 which can directly degrade the large molecular structure of pollutants to small molecules (monomers, CO2etc.) (Fig. 13). Additionally, the role of active species in the degradation process can also be investigated by scavenger tests. In these tests, scavengers, such as potassium iodide (KI), p-benzoquinone (p-BQ), and isopropyl alcohol (IPA), are added to the reaction mixture to capture the holes (h+), superoxide ions (˙O2−) and hydroxyl radical (˙OH), respectively. A detailed review of the photocatalytic activity of supramolecular/MXene composites for pollutant degradation is summarized in Table 1.
000 tons of organic dyes globally each year. Approximately, 10–15% of these dyes are released as waste effluents.116 In comparison to other methods such as ozonation,117,118 adsorption119,120 and filtration121,122 degradation of pollutants through the photocatalytic method is one of the preferred approaches. This method is simple, energy-efficient, low-cost and stable, with a minimal production of secondary pollutants. In photocatalytic degradation, semiconductor materials absorb light with enough energy to transfer electrons from the valence band (VB) to conduction band (CB), leading to the generation of oxidative holes in the valence band (VB). Highly reactive species are produced from the reaction of reductive electron and oxidative holes with dissolved oxygen/H2O/H2O2 which can directly degrade the large molecular structure of pollutants to small molecules (monomers, CO2etc.) (Fig. 13). Additionally, the role of active species in the degradation process can also be investigated by scavenger tests. In these tests, scavengers, such as potassium iodide (KI), p-benzoquinone (p-BQ), and isopropyl alcohol (IPA), are added to the reaction mixture to capture the holes (h+), superoxide ions (˙O2−) and hydroxyl radical (˙OH), respectively. A detailed review of the photocatalytic activity of supramolecular/MXene composites for pollutant degradation is summarized in Table 1.
 | ||
| Fig. 13 Pictorial representation of photocatalytic degradation of organic pollutants over supramolecule/MXene heterostructures. | ||
In supramolecules, the fast recombination of photocatalytically generated charge carriers can be controlled by the compounding of supramolecules with MXenes which can abstract the electron to the low Fermi level. This synergistic effect of composite materials can significantly enhance the efficiency of pollutant degradation. Far et al.36 developed a highly efficient MXOF photocatalyst by decorating Ti3C2Tx MXene nanosheets with a highly porous ZIF-8 MOF under solvothermal conditions. In the MXOF photocatalyst, spherical ZIF-8 nanoparticles (<100 nm) were uniformly distributed on the MXene nanosheets. The MXOF, with a specific surface area of 37.7 m2 g−1, was mesoporous in nature whereas the bare MOF was microporous with a specific surface area of 851 m2 g−1. In this heterostructure, the MXOF benefited from a tunable band gap of 4.99 eV which was intermediate between the bare MXene (5.18 eV) and MOF (4.79 eV). This tunable band gap not only enhanced the charge transfer but also reduced the recombination rate of photogenerated charge carriers through the transfer of electrons between the band of MOFs and MXene. The synergistic effect of both components resulted in impressive photodegradation efficiency, achieving 62% degradation of methylene blue (MB) and 35% degradation of Direct Red 31 (DR31) dyes, along with excellent recyclability. Additionally, scavenger tests were also performed which showed that the photodegradation efficiency of the DR31 dye with and without p-BQ was 35%, whereas photodegradation of MB was 62% and 48% with and without p-BQ respectively. These results indicated that ˙O2− did not play a major role in the photodegradation of DR31.
Similarly, Cao et al.37 also reported a MOF–MXene photocatalyst that showed 96% degradation of the tetracycline (TC) pollutant after 90 min of photocatalysis, with a mineralization rate of 45.5%. In the solvothermally synthesized bimetallic/MXene (Sn–Bi–MOF/Ti3C2) heterostructure, bulk MXene (Ti3C2) had a multilayered structure with a layer spacing ranging from 75 to 240 nm while bimetallic Sn–Bi–MOF nanoparticles had small particles (nearly 60 nm size) with rough surface and slight agglomeration. The degree of agglomeration of the bimetallic MOF was effectively low in MXOF composites. MXenes, bimetallic Sn–Bi–MOF, and MXOF catalysts were all mesoporous and have type IV isotherms with H3-type hysteresis loops. However, the MXOF had a higher specific surface area, pore volume, and pore diameter than the bare MXene and bimetallic MOF, providing more active sites for the photocatalytic degradation reaction. The photocatalytic mechanism of Sn–Bi–MOF/Ti3C2 showed that the Schottky junction formed at the surface of MOFs and MXenes accelerated the transfer of photogenerated electrons to MXenes and enhanced charge separation and led to free radical generation. In the degradation process, ˙O2 and h+ are the main active substances. The recyclability of the Sn–Bi–MOF/Ti3C2 composite was good even after four cycles of the photocatalytic experiment, which was further confirmed by PXRD patterns of samples before and after the reaction.
In photocatalysis, the enhanced activity of composite materials compared to bare materials is mainly due to the formation of a heterojunction at the surface, which promotes the transfer of charge carriers through heterojunction barriers and decreases the rate of recombination. However, in some cases, the controlled morphology of materials acts as a supporting factor to enhance the activity, as reported by Tan et al.38 The morphology of the photocatalyst was controlled by the H2O–DMF solvent ratio during the synthesis process. Different morphologies of the MIL-88A(Fe) MOF, from elongated spindle shape to short spindle shape, were obtained by decreasing the H2O ratio in the H2O/DMF mixture, named S-MIL-88A(Fe) (in pure water), W-MIL-88A(Fe) (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF 1
DMF 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and D-MIL-88A(Fe) (pure DMF) (Fig. 14(a)). These highly stable MIL-88A(Fe) MOFs with modulated active crystal planes were used to form composites with MXenes, creating a type-I heterojunction. This series of composites was named XA-MY where X denotes the morphology such as SA-M100, WA-M100, and DA-M100 and Y denotes the different MXene loadings (DA-M50, DA-M100, and DA-M150). Due to a large elongated structure, S-MIL-88A(Fe) was highly agglomerated on the surface of MXenes in comparison to W-MIL-88A(Fe) and D-MIL-88A(Fe). The DA-M100 composite exhibited the most effective activity, achieving approximately 70% degradation of sulfamethoxazole within 10 minutes of light exposure and reaching 93% efficiency after 60 minutes compared to other samples (Fig. 14(b) and (c)). The rate constant for the degradation of sulfamethoxazole of DA-M100 was 1.03 and 2.37 time higher than that of WA-M100 and SA-M100, respectively. The excess amount of MXenes also masked the active sites of MOFs, resulting in reduction in the catalytic performance of the photocatalyst. Additionally, universality tests demonstrated that DA-M100 acted as a dual photo catalyst, showing strong oxidation efficiency for common dyes and efficient reduction for heavy metal ions in sewage. The removal capabilities for Cr(VI), Cu(II), and Ni(II) were 95.88%, 79.77%, and 66.67%, respectively, after 1 hour of irradiation, indicating good environmental adaptability. A detailed study showed that at the interface, the composite exhibited a type-I heterojunction which was mainly responsible for the transport and separation of photogenerated charge carriers and enhanced photocatalytic activity. Additionally, this work presented a new approach for designing and synthesizing heterostructures with dual reduction and oxidation capabilities.
1) and D-MIL-88A(Fe) (pure DMF) (Fig. 14(a)). These highly stable MIL-88A(Fe) MOFs with modulated active crystal planes were used to form composites with MXenes, creating a type-I heterojunction. This series of composites was named XA-MY where X denotes the morphology such as SA-M100, WA-M100, and DA-M100 and Y denotes the different MXene loadings (DA-M50, DA-M100, and DA-M150). Due to a large elongated structure, S-MIL-88A(Fe) was highly agglomerated on the surface of MXenes in comparison to W-MIL-88A(Fe) and D-MIL-88A(Fe). The DA-M100 composite exhibited the most effective activity, achieving approximately 70% degradation of sulfamethoxazole within 10 minutes of light exposure and reaching 93% efficiency after 60 minutes compared to other samples (Fig. 14(b) and (c)). The rate constant for the degradation of sulfamethoxazole of DA-M100 was 1.03 and 2.37 time higher than that of WA-M100 and SA-M100, respectively. The excess amount of MXenes also masked the active sites of MOFs, resulting in reduction in the catalytic performance of the photocatalyst. Additionally, universality tests demonstrated that DA-M100 acted as a dual photo catalyst, showing strong oxidation efficiency for common dyes and efficient reduction for heavy metal ions in sewage. The removal capabilities for Cr(VI), Cu(II), and Ni(II) were 95.88%, 79.77%, and 66.67%, respectively, after 1 hour of irradiation, indicating good environmental adaptability. A detailed study showed that at the interface, the composite exhibited a type-I heterojunction which was mainly responsible for the transport and separation of photogenerated charge carriers and enhanced photocatalytic activity. Additionally, this work presented a new approach for designing and synthesizing heterostructures with dual reduction and oxidation capabilities.
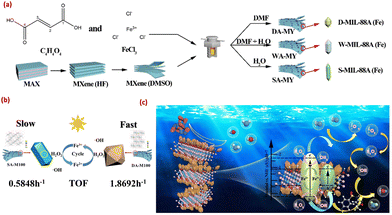 | ||
| Fig. 14 (a) Synthesis scheme of XA-MY; (b) TOF diagram; and (c) proposed mechanism. Reprinted with permission from ref. 38; Copyright 2024 Elsevier. | ||
Similarly, Wu et al.39 also used Ti3C2–MXenes as modulators to affect the growth of the MIL-125-NH2 MOF during the synthesis of nanohybrid structures. As shown in Fig. 15(a), the morphology of the composite changed with the amount of MXene from 0 to 5 mL. MT0 (0 mL MXene with MIL-125-NH2 MOF) exhibited plate-type nanosheets morphology which changed to rod type morphology (MT0.1) with the addition of 0.1 mL MXene. As the amount of MXene increased, the rod-type structure was converted to enlarged particle morphology with a uniform distribution of TiO2 nanosheets and nanoparticles on the surface of NH2-MIL-125(Ti) in MT1 and MT5 respectively, along with a partial loss of the organic ligand (Fig. 15(a)). Due to the presence of the –NH2 group in the ligand, MT0 showed the characteristics adsorption band at 480 nm,123 which was further extended to 570 nm with enhanced absorption intensity in MT5 due to a high amount of black-colored MXene. This enhancement in absorption intensity was mainly responsible for the generation of charge carriers and high photocatalytic activity. The optimized photocatalytic degradation efficiency of MT5 nanohybrids towards higher tetracycline hydrochloride (TC-HCl) was 82.80% in 60 min which was 11.5 times higher that of pristine MT0 (MIL-125-NH2) under visible light irradiation (λ > 420 nm) (Fig. 15(b)). The order of rate constant is – MT5 (0.034 min−1) > MT1 (0.005 min−1) > MT0.1 (0.003 min−1) > MT0 (0.001 min−1) (Fig. 15(c)). This highest photocatalysis activity of MT5 was mainly due to the presence of a dual heterojunction which not only increased the charge carrier density but also remarkably enhanced the interfacial charge separation as well as transfer. Additionally, the recyclability of MT5 in photocatalytic TC-HCl degradation was approximately 81.00% after four consecutive recycles. This work provided insight into the modification of semiconductor morphology in the presence of a modulator and demonstrated the importance of dual heterojunctions in the hybrid material.
 | ||
| Fig. 15 (a) Synthesis scheme of Ti3C2/MOF heterostructures; (b) photocatalytic degradation of TC-HCl (λ > 420 nm) of all as-synthesized samples; and (c) pseudo first-order kinetic fitting curves. Reprinted with permission from ref. 39. Copyright 2020 Elsevier. | ||
7.3 CO2 reduction
Carbon dioxide (CO2) is a greenhouse gas which contributes to nearly 76% of global warming. Thus, the conversion of CO2 to value-added products by photocatalysis could effectively address the critical issues of climate changes and energy shortages. Research on efficient photocatalysts for CO2 reduction is still in its early stage. The regeneration of photocatalytic nicotinamide adenine dinucleotide (NADH) conjugated with enzymatic CO2 reduction is crucial for global sustainable development. Wei et al.40 synthesized a rationally designed lawn-like TP-COF/Ti3C2Tx (TCM) photocatalyst by the in situ and post synthesized loading of COFs on NH2 functionalized Ti3C2Tx-MXenes for NADH regeneration. The synthesized hybrid samples were labeled as TCM-y%, where y% denotes the MXene percentage ranging from 5% to 20% resulting in TCM-5%, TCM-10%, TCM-15%, and TCM-20%, respectively. As shown in Fig. 16(a), in the presence of an electron mediator, the order of photocatalysts for the formate yield was TCM-15% (11.9 mmol L−1) > TCM-10% (8.0 mmol L−1) > TCM-20% (6.3 mmol L−1) > TCM-5% (5.9 mmol L−1) > TC (5.4 mmol L−1), demonstrating the crucial role of NADH in the FDH-catalyzed CO2 reduction reaction. Similarly, in the absence of an electron mediator, the order of photocatalysts for the formate yield was TCM-15% (2.7 mmol L−1) > TCM-10% (2.1 mmol L−1) > TCM-20% (1.6 mmol L−1) > TCM-5% (1.4 mmol L−1) > TC (1.2 mmol L−1) as shown in Fig. 16(b). Thus, the NADH regeneration yield achieved by TCM-15% was nearly 95% and 46% with and without an electron mediator ([Cp*Rh-(bpy)(H2O)]2+) respectively, whereas TP-COFs achieved only 42% with an electron mediator (Fig. 16(c) and (d)). Also, the TOF value for NADH regeneration was about 4.05 and 4.90 times higher than those of pure TP-COFs, with and without an electron mediator, respectively. This work established a strong foundation for efficiently achieving NADH regeneration without the need for an electron mediator. | ||
| Fig. 16 Formate yields (a) with and (b) without an electron mediator. (c) Comparison of production rates and (d) possible mechanism. Reprinted with permission from ref. 40. Copyright 2024 American Chemical Society. | ||
Li et al.41in situ grew a ZIF-66 MOF (zeolitic imidazolate framework-67) on the surface of MXenes using a self-assembly method. The addition of Co salts followed by 2-MIM in MXene solution allowed the MOFs to grow on the MXene surface. The ZIF-67/Ti3C2Tx MXene photocatalyst is denoted as ZT-a, where a (= 90, 180, 270, 360, 450, 540, and 630 mg) represents the specific weight of MXenes. The polyhedral structure of ZIF-67 was distributed on the layered surface of MXenes. The Schottky heterojunction formed at the junction of ZIF-67 and MXenes in ZT-450 showed the smooth transfer of electrons from ZIF-67 to Ti3C2Tx, indicating the highest rate of separation and longest lifetime of photogenerated charge carriers. Among the other synthesized composites, ZT-450 showed the highest activity towards photocatalytic CO2 reduction yielding carbon mono-oxide (CO), methane (CH4), and hydrogen (H2) at 62.7, 6.7, and 7.3 μmol g−1, respectively. The production of CO over ZT-450 was 16 and 4.8 higher than that of bare ZIF-67 and Ti3C2Tx, respectively, due to higher adsorption ability of CO2 in ZT-450 (127.90 μmol g−1). In situ DRIFTS measurements revealed the presence of intermediates such as *COOH, *CHO, *OCH3, etc. during the photocatalytic CO reduction reaction (CO2RR) on the photocatalyst. In the heterostructure, the excited electrons of ZIF-67 were transferred to the Fermi level of Ti3C2Tx facilitating the CO2 reduction reaction. Meanwhile, the holes in the valence band of ZIF-67 drove the oxidation reactions to generate O2. This localized separation of charge carriers in heterostructures was mainly responsible for the enhancement of photocatalytic efficiency.
According to theoretical calculations, the photocatalytic activity of MXenes can be improved through the formation of a Schottky junction by coupling with a n-type semiconductor that has a smaller work function than that of MXenes. Song et al.42 synthesized a ternary heterostructure with a Z-scheme heterojunction by combining two semiconductors with highly conductive MXenes. In a ternary photocatalyst, porous flakes of g-carbon nitride were loaded with the clusters of the Sn–Bi–MOF that wrapped around the layered structure of MXenes. Under simulated sunlight, the reduction of CO2 to CO over the ternary g-CN/TC/SBM composite was 36.33 μmol g−1 h−1, which was 4.36-fold that of g-carbon nitride and 3.5-fold that of SBM. The quantum apparent efficiency (AQY) was 3.2% at 420 nm. In ternary composites, three interfaces formed at the junctions of surfaces which were responsible for the transfer of charge carriers in the Z-scheme. In the presence of light, the photo-generated electrons moved to the low Fermi level of MXenes and formed a barrier at the surface, controlling the reflow of electrons from MXenes to g-carbon nitride. Simultaneously, these electrons accelerated to the Sn–Bi–MOF due to the Schottky barrier formed with MXenes. Thus, this Z-scheme Schottky barrier not only separated the charge carrier but also controlled the recombination of electrons and holes which was mainly responsible for the increased production of CO. Chen et al.43 also demonstrated the significance of three dimensional hybrid structures, Co–Co LDH/TNS composites, based on MXenes as a promising candidate for photoreduction of CO2 to value added products. In a 3D hierarchical nanoarray, Co acted as active species and was mainly responsible for the significant enhancement of the CO2-to-CO conversion rate (1.25 × 104 μmol h−1 g−1) and excellent stability (Fig. 17).
 | ||
| Fig. 17 (a) Synthesis scheme of Co–Co LDH/TNS nanosheets; (b) release of CO and H2 with time intervals; and (c) proposed mechanism. Reprinted with permission from ref. 43. Copyright 2020 Elsevier. | ||
7.4 Antibacterial activity
In marine engineering, marine fouling is an emerging problem that negatively impacts the environment and human health. Thus, it is crucial for researchers to develop efficient, highly active and environment friendly antifouling agents that can function under normal environmental conditions. One emerging concept is the use of an antifouling agent that can be activated under sunlight. However, traditional semiconductors such as TiO2,124 ZnO,125 CuO,126 MoS2127etc. suffer from the major disadvantage of fast recombination of charge carriers. Thus, Liu et al.44in situ grew a TpPa-COF semiconductor on the surface of highly conductive MXenes through the Schiff base reaction, which simultaneously anchored the metal oxide nanoparticles (Cu2O NPs) through covalent bonding to obtain a highly efficient photocatalytic bactericide (Fig. 18(a)). In the ternary photocatalyst, Ti3C2/TpPa-1/Cu2O and TpPa-COF acted as semiconductors. Heterojunctions formed between TpPa-1 and Cu2O facilitated the migration of photogenerated charge carriers. Due to their excellent conductivity and surface plasmon resonance effect, MXenes promoted the transport of charge carriers and acted as co-catalysts (Fig. 18(b)). The high porosity of TpPa-1 with large pore size/volume reduced the lattice density and enhanced the contact of active site with medium, along with improving the storage of active oxygen species. This synergistic effect of all three components in the ternary photocatalyst was mainly responsible for high antibacterial properties against P. aeruginosa and S. aureus, with antibacterial rates of 99.62% and 98.90% which were 33% and 50% higher in comparison to the bare TpPa-1-COF. This combination of the ternary catalyst not only improved the active transport of charge carriers but also reduced the chance of photocorrosion. | ||
| Fig. 18 (a) Synthesis scheme of Ti3C2/TpPa-1/Cu2O; (b) proposed mechanism, reprinted with permission from ref. 45. Copyright 2022 Elsevier; (c) synthesis scheme of Ti3C2/TpPa-1/Ag; and (d) proposed antibacterial mechanism. Reprinted with permission from ref. 46. Copyright 2023 Elsevier. | ||
Similar to Cu2O which released the Cu2+ during the sterilization process, Ag nanoparticles also have importance in sterilization as they release Ag+ which enhance the activity in the visible region but also have large work function to accelerate the migration of electrons. Wang et al.45 utilized this advantage and developed a Ag NP-based ternary photocatalyst to achieve a highly active photocatalytic antifouling material. Similar to the method mentioned above, Ti3C2/TpPa-1/Ag was obtained by in situ growth of a COF on the 2D surface of MXenes through the Schiff base reaction, followed by covalent anchoring of Ag nanoparticles (Fig. 18(c)). The surface plasmon resonance (SPR) phenomenon of both MXenes and Ag NPs was mainly responsible for the transfer of electrons from the TpPa-COF to the Fermi level of MXenes and Ag NPs through a Schottky barrier (Fig. 18(d)). Additionally, the ternary composite controlled the release of Ag+ ions, enhancing antibacterial efficacy. The higher electron transport and lower electron–hole recombination activities of the Ti3C2/TpPa-1/Ag ternary system contributed to its efficiency. Due to the synergistic effect of all the three components, the ternary composite (Ti3C2/TpPa-1/Ag) demonstrated a superior antibacterial activity against S. aureus and P. aeruginosa, with antibacterial rates reaching 99.60% and 99.78%, respectively.
MOFs consisting of suitable bioactive metal ions and functionalized organic ligands also showed excellent antibacterial activity. Li et al.46 grew the porphyrin based porous 2D MOF (Cu-TCPP) nanosheets in situ on the layered Ti3C2–MXene to form a Cu-TCPP/Ti3C2 composite for bacteria-killing efficiency under photocatalytic conditions. The conjugation of MOFs with MXenes formed a Schottky barrier and a space charge layer at the interface due to the difference in work functions. Under light irradiation, photocatalytically generated electrons were transferred to the MXenes through Schottky barriers, enhancing the separation of charge carriers by inhibiting the backflow of electrons from MXenes. As a result, more ROS were produced and over 99% of S. aureus were killed over the MOF/Ti3C2 composite. This work provided deeper insights into designing MOF-based composites with high photophysical properties through in situ interfacial engineering strategies for photocatalytic antibacterial activity.
8. Challenges and future perspectives
Regardless of promising and remarkable improvement of supramolecules/MXene heterostructures in few years, several challenges still need to be overcome to fully explore their potential and make sure their transition from low scale laboratory research to large scale real-world applications. This section will focus on these challenges, their potential solutions and future perspectives in this rapidly growing research field.Despite the many reported works on the synthesis of hybrid materials, the synthesis of supramolecules/MXenes is not an easy task. MXenes have the tendency to form staking layered structures which are connected through weak bonding. Sometime, this behavior restricts the synthesis of heterostructures with supramolecules. Thus, a controlled synthesis process is required for the uniform distribution and interface formation between supramolecules and MXenes. Low stability of supramolecules is still a challenge which can be controlled by uniform layering of supramolecules over MXenes. This layering can be controlled by the interaction formed between supramolecules and MXenes. Otherwise, supramolecular frameworks may collapse and consequently decrease the performance and recyclability. Thus, rational design of heterostructures and their accurate characterization are still crucial challenges because of the complex design of heterostructures and overlapping of properties of all components. These challenges can be solved by the development of advanced characterization techniques that can give comprehensive data about the structure, involved interactions, morphology and properties.
The ultimate objective of the research is the commercialization of prepared materials in desired applications, which is another significant challenge. This commercialization is only possible if the material can be produced cost-effectively under safety guidelines and its stability under the given environmental conditions can be ensured. Secondly, because of environmental concerns, the method should be green, that is, without producing hazardous by-products. In the view of next-generation materials, supramolecule/MXene heterostructures have high potential to be used in multiple applications from environmental application to green fuel generation. For the scale-up, more work needs to be done in the field of advance methods and instruments. Furthermore, integration of advanced computational studies, machine learning and artificial intelligence tools with research will help to predict the details of controlled reaction conditions for the synthesis of desired structures and the behavior of designed materials towards novel applications. Thus, the combined efforts of chemical, material and environmental scientists and also engineers will help in the commercialization of supramolecule/MXene heterostructures.
9. Conclusions
In this comprehensive review, a supramolecule/MXene heterostructure is highlighted as a potential candidate for advancing the photocatalytic activity, with applications ranging from green fuel production (hydrogen generation) to addressing environmental problems (such as CO2 conversion, pollutant degradation, and more). By exploring the unique properties of supramolecules, photocatalytically active tunable porous structures, in combination with highly stable conductive structures of MXenes, these heterostructures can exhibit an enhanced photocatalytic activity simultaneously addressing the inherit stability limitations of supramolecules. The morphology of these heterostructures significantly impacts charge carrier dynamics, stability and recyclability which can be precisely controlled by the modification of synthesis methods, including in situ and post-synthesis adjustments. Future research should focus on the development of new synthesis techniques that facilitate new bonding between composite materials and explore their performance in other applications. Overall, the integration of supramolecules with MXenes opens up new opportunities for the development of sustainable materials for photocatalytic applications, contributing to the solution of energy and environmental problems through green technology.Author contributions
P. Verma: writing – original draft, J. V. Marseveen: writing – review & editing, N. R. Shiju: funding acquisition, supervision, writing – review & editing.Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
P. Verma gratefully acknowledges Research Priority Area (RPA) Sustainable Chemistry of the University of Amsterdam for a postdoctoral fellowship.Notes and references
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38(1), 253 RSC.
- K. Wenderich and G. Mul, Chem. Rev., 2016, 116(23), 14587 CrossRef CAS PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238(5358), 37 CrossRef CAS PubMed.
- S. A. Rawool, M. R. Pai, A. M. Banerjee, A. Arya, R. S. Ningthoujam, R. Tewari, R. Rao, B. Chalke, P. Ayyub, A. K. Tripathi and S. R. Bharadwaj, Appl. Catal., B, 2018, 221, 443 CrossRef CAS.
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43(22), 7520 RSC.
- S. Zhong, Y. Xi, S. Wu, Q. Liu, L. Zhao and S. Bai, J. Mater. Chem. A, 2020, 8(30), 14863 RSC.
- D. Vaya and P. K. Surolia, Environ. Technol. Innovation, 2020, 20, 101128 CrossRef CAS.
- L. Wang, C. Bie and J. Yu, Trends Chem., 2022, 4(11), 973 CrossRef CAS.
- S. Liu, N. Zhang and Y. J. Xu, Part. Part. Syst. Charact., 2014, 31(5), 540 CrossRef CAS.
- B. M. McVey and P. J. Cragg, Supramolecules, Supramolecular Chemistry in Corrosion and Biofouling Protection, CRC Press, 2021, pp. 3–16 Search PubMed.
- T. R. Cook, Y. R. Zheng and P. J. Stang, Chem. Rev., 2013, 113(1), 734 CrossRef CAS PubMed.
- X. Wu, Z. Liu, H. Guo, Y. L. Hong, B. Xu, K. Zhang, Y. Nishiyama, W. Jiang, S. Horike, S. Kitagawa and G. Zhang, ACS Appl. Mater. Interfaces, 2021, 13(31), 37172 CrossRef CAS PubMed.
- P. Verma, U. P. Singh and R. J. Butcher, CrystEngComm, 2019, 21(36), 5470 RSC.
- J. Yang, Z. Chen, L. Zhang and Q. Zhang, ACS Nano, 2024, 18(33), 21804 CrossRef CAS PubMed.
- P. Verma, U. P. Singh, T. Verma, R. J. Butcher and P. Mohanty, J. Coord. Chem., 2024, 77(12), 1566 CrossRef CAS.
- G. Yuan, L. Tan, P. Wang, Y. Wang, C. Wang, H. Yan and Y. Y. Wang, Cryst. Growth Des., 2021, 22(1), 893 CrossRef.
- P. Verma, R. V. Singh, A. M. Banerjee and M. R. Pai, Int. J. Hydrogen Energy, 2025, 107, 569 CrossRef CAS.
- W. Liang, M. Liu, X. Shi, B. Chen, L. Shao, J. Xu and Z. Sun, Batteries Supercaps, 2024, 7(2), e202300462 CrossRef CAS.
- X. Shi, W. Liang, G. Liu, B. Chen, L. Shao, Y. Wu, Z. Sun and F. García, Chem. Eng. J., 2023, 462, 142271 CrossRef CAS.
- L. Shi, C. Wu, Y. Wang, Y. Dou, D. Yuan, H. Li, H. Huang, Y. Zhang, L. D. Gates, X. Sun and T. Ma, Adv. Funct. Mater., 2022, 32(30), 2202571 CrossRef CAS.
- M. Naguib, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2021, 33(39), 2103393 CrossRef CAS PubMed.
- T. K. Slot, V. Natu, E. V. Ramos-Fernandez, A. Sepulveda-Escribano, M. Barsoum, G. Rothenberg and N. R. Shiju, 2D Mater., 2021, 8(3), 035003 CrossRef CAS.
- X. Ma, J. Kang, Y. Wu, C. Pang, S. Li, J. Li, Y. Xiong, J. Luo, M. Wang and Z. Xu, Chem. Eng. J., 2023, 469, 143888 CrossRef CAS.
- T. Ramachandran, F. Hamed, Y. A. Kumar, R. K. Raji and H. H. Hegazy, J. Energy Storage, 2023, 73, 109299 CrossRef.
- A. E. Mathew, S. Jose, A. M. Babu and A. Varghese, Mater. Today Chem., 2024, 36, 101927 CrossRef CAS.
- T. K. Slot, F. Yue, H. Xu, E. V. Ramos-Fernandez, A. Sepúlveda-Escribano, Z. Sofer, G. Rothenberg and N. R. Shiju, 2D Mater., 2020, 8(1), 015001 CrossRef.
- M. Ronda-Lloret, V. S. Marakatti, W. G. Sloof, J. J. Delgado, A. Sepúlveda-Escribano, E. V. Ramos-Fernandez, G. Rothenberg and N. R. Shiju, ChemSusChem, 2020, 13, 6401 CrossRef CAS PubMed.
- W. Ng, E. S. Gnanakumar, E. Batyrev, H. F. Greer, W. Zhou, R. Sakidja, G. Rothenberg, M. Barsoum and N. R. Shiju, Angew. Chem., Int. Ed., 2018, 57, 1485 CrossRef CAS PubMed.
- P. Tian, X. He, L. Zhao, W. Li, W. Fang, H. Chen, F. Zhang, Z. Huang and H. Wang, Sol. Energy, 2019, 188, 750 CrossRef CAS.
- P. Tian, X. He, L. Zhao, W. Li, W. Fang, H. Chen, F. zhang, Z. Huang and H. Wang, Int. J. Hydrogen Energy, 2019, 44(2), 788 CrossRef CAS.
- H. Zhu, Y. Chen, T. Zhang, L. Qin, S. Z. Kang and X. Li, J. Solid State Chem., 2023, 327, 124298 CrossRef CAS.
- Y. Chen, H. Zhu, S. Z. Kang, T. Zhang, L. Qin and X. Li, J. Alloys Compd., 2023, 961, 170929 CrossRef CAS.
- Y. Li, Y. Liu, Z. Wang, P. Wang, Z. Zheng, H. Cheng, Y. Dai and B. Huang, Chem. Eng. J., 2021, 411, 128446 CrossRef CAS.
- S. Liu, X. Jiang, G. I. Waterhouse, Z. M. Zhang and L. M. Yu, Sep. Purif. Technol., 2022, 294, 121094 CrossRef CAS.
- H. Wang, C. Qian, J. Liu, Y. Zeng, D. Wang, W. Zhou, L. Gu, H. Wu, G. Liu and Y. Zhao, J. Am. Chem. Soc., 2020, 142(10), 4862 CrossRef CAS PubMed.
- H. S. Far, M. Najafi, M. Hasanzadeh and R. Rahimi, Inorg. Chem. Commun., 2023, 152, 110680 CrossRef.
- Y. Cao, L. Yue, Z. Li, Y. Han, J. Lian, H. Qin and S. He, Appl. Surf. Sci., 2023, 609, 155191 CrossRef CAS.
- Q. Tan, Z. Yu, Q. Xiang, N. He, R. Long and J. Wang, Process Saf. Environ. Prot., 2023, 179, 405–420 CrossRef CAS.
- Y. Wu, X. Li, Q. Yang, D. Wang, F. Yao, J. Cao, Z. Chen, X. Huang and X. Li, Chem. Eng. J., 2020, 390, 124519 CrossRef CAS.
- P. Wei, J. Dong, X. Gao, L. Chang, Z. Huang, H. Zheng, S. M.-Y. Lee, W.-Y. Lou and C. Peng, ACS Sustainable Chem. Eng., 2024, 12(18), 6881 CrossRef CAS.
- Z. Li, J. Xiong, H. Song, S. Liu, Y. Huang, Y. Huang, G. I. N. Waterhouse, Z. Wang, Y. Mao, Z. Liang and X. Luo, Sep. Purif. Technol., 2024, 341, 126817 CrossRef CAS.
- Z. Song, S. Song, W. Zhang, H. Han, K. Wei, D. Liu, Q. Wang, C. Ma and S. Feng, Fuel, 2024, 366, 131154 CrossRef CAS.
- W. Chen, B. Han, Y. Xie, S. Liang, H. Deng and Z. Lin, Chem. Eng. J., 2020, 391, 123519 CrossRef CAS.
- C. Liu, W. Wang, M. Zhang, C. Zhang, C. Ma, L. Cao, D. Kong, H. Feng, W. Li and S. Chen, Chem. Eng. J., 2022, 430, 132663 CrossRef CAS.
- W. Wang, C. Liu, M. Zhang, C. Zhang, L. Cao, C. Zhang, T. Liu, D. Kong, W. Li and S. Chen, J. Colloid Interface Sci., 2022, 608, 735–748 CrossRef CAS PubMed.
- J. Li, Z. Yang, C. Wang, S. Wu, Y. Zheng, Z. Cui, H. Jiang, Z. Li, S. Zhu, L. Feng and X. Liu, Appl. Catal., B, 2023, 339, 123163 CrossRef CAS.
- M. Ronda-Lloret, T. K. Slot, N. P. van Leest, B. de Bruin, W. G. Sloof, E. Batyrev, A. Sepúlveda-Escribano, E. V. Ramos-Fernandez, G. Rothenberg and N. R. Shiju, ChemCatChem, 2022, 14(18), e202200446 CrossRef CAS.
- W. Jeitschko, H. T. Nowotny and F. Benesovsky, J. Less-Common Met., 1964, 7(2), 133 CrossRef CAS.
- T. K. Slot, P. Oulego, Z. Sofer, Y. Bai, G. Rothenberg and N. R. Shiju, ChemCatChem, 2021, 13(15), 3470 CrossRef CAS.
- J. A. Kumar, P. Prakash, T. Krithiga, D. J. Amarnath, J. Premkumar, N. Rajamohan, Y. Vasseghian, P. Saravanan and M. Rajasimman, Chemosphere, 2022, 286, 131607 CrossRef CAS PubMed.
- K. Chaturvedi, V. Hada, S. Paul, B. Sarma, D. Malvi, M. Dhangar, H. Bajpai, A. Singhwane, A. K. Srivastava and S. Verma, Top. Curr. Chem., 2023, 381(2), 11 CrossRef CAS PubMed.
- Y. Qu, C. Shi, H. Cao and Y. Wang, Mater. Lett., 2020, 280, 128526 CrossRef CAS.
- I. Amin, H. V. D. Brekel, K. Nemani, E. Batyrev, A. de Vooys, H. van der Weijde, B. Anasori and N. R. Shiju, ACS Appl. Mater. Interfaces, 2022, 14(38), 43749–43758 CrossRef CAS PubMed.
- M. Benchakar, L. Loupias, C. Garnero, T. Bilyk, C. Morais, C. Canaff, N. Guignard, S. Morisset, H. Pazniak, S. Hurand, P. Chatier, J. Pacaud, V. Mauchamp, M. W. Barsoum, A. Habrioux and S. Celerier, Appl. Surf. Sci., 2020, 530, 147209 CrossRef CAS.
- D. Dolz, A. Morales-García, F. Viñes and F. Illas, Nanomaterials, 2012, 11(1), 127 CrossRef PubMed.
- C. Zhang and M. Naguib, Transition Metal Carbides and Nitrides (MXenes) Handbook: Synthesis, Processing, Properties and Applications, John Wiley & Sons, 2024 Search PubMed.
- H. Lashgari, M. R. Abolhassani, A. Boochani, S. M. Elahi and J. Khodadadi, Solid State Commun., 2014, 195, 61 CrossRef CAS.
- G. R. Berdiyorov, AIP Adv., 2016, 6(5), 055105 CrossRef.
- P. Verma, U. P. Singh, R. J. Butcher, S. Banerjee and P. Roy, J. Mol. Struct., 2022, 1249, 131590 CrossRef CAS.
- U. P. Singh, P. Verma and R. J. Butcher, J. Mol. Struct., 2021, 1224, 129161 CrossRef CAS.
- M. O’Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112(2), 675 CrossRef PubMed.
- M. Treger, A. Hannebauer, A. Schaate, J. L. Budde, P. Behrens and A. M. Schneider, Phys. Chem. Chem. Phys., 2023, 25(8), 6333–6341 RSC.
- X. Qiu, Y. Zhu, X. Zhang, Y. Zhang, L. T. Menisa, C. Xia, S. Liu and Z. Tang, Solar RRL, 2020, 4(8), 1900449 CrossRef CAS.
- C. K. Lin, D. Zhao, W. Y. Gao, Z. Yang, J. Ye, T. Xu, Q. Ge, S. Ma and D.-J. Liu, Inorg. Chem., 2012, 51(16), 9039 CrossRef CAS PubMed.
- J. Gascon, M. D. Hernández-Alonso, A. R. Almeida, G. P. van Klink, F. Kapteijn and G. Mul, ChemSusChem, 2008, 1(12), 981 CrossRef CAS PubMed.
- X. Ma, L. Wang, Q. Zhang and H.-L. Jiang, Angew. Chem., 2019, 131, 12303 CrossRef.
- Z. Qian, R. Zhang, H. Hu, Y. Xiao, H. Li, X. Sun and T. Ma, Sol. RRL, 2023, 7, 2300547 CrossRef CAS.
- A. Jamma, B. Jaksani, C. S. Vennapoosa, S. Gonuguntla, S. Sk, M. Ahmadipour, M. Abraham B, I. Mondal and U. Pal, Mater. Adv., 2024, 5(7), 2785 RSC.
- S. Sk, A. Jamma, D. S. Gavali, V. Bhasin, R. Ghosh, K. Sudarshan, R. Thapa and U. Pal, ACS Appl. Mater. Interfaces, 2023, 15, 55822 CrossRef CAS PubMed.
- S. Navalón, A. Dhakshinamoorthy, M. Álvaro, B. Ferrer and H. García, Chem. Rev., 2022, 123(1), 445–490 CrossRef PubMed.
- X. Tan, J. Zhang, J. Shi, X. Cheng, D. Tan, B. Zhang, L. Liu, F. Zhang, B. Han and L. Zheng, Sustainable Energy Fuels, 2020, 4(6), 2823–2830 RSC.
- A. Maleki and R. Taheri-Ledari, Physicochemical Aspects of Metal-Organic Frameworks: A New Class of Coordinative Materials, Springer Nature, 2023 Search PubMed.
- M. Dan-Hardi, C. Serre, T. Frot, L. Rozes, G. Maurin, C. Sanchez and G. Férey, J. Am. Chem. Soc., 2009, 131(31), 10857–10859 CrossRef CAS PubMed.
- T. Davide, F. Marc, S. Clément, S. Capucine, R. Laurence, M. D. Caroline and W. Aron, J. Am. Chem. Soc., 2013, 135, 10942 CrossRef PubMed.
- M. Sohail, S. Rauf, M. Irfan, A. Hayat, M. M. Alghamdi, A. A. El-Zahhar, D. L. Ghernaout, Y. Al-Hadeethi and W. Lv, Nanoscale Adv., 2024, 6(5), 1286–1330 RSC.
- H. Q. Pham, T. Mai, N. N. Pham-Tran, Y. Kawazoe, H. Mizuseki and D. Nguyen-Manh, J. Phys. Chem. C, 2014, 118(9), 4567–4577 CrossRef CAS.
- J. Patel, G. Bury and Y. Pushkar, Small, 2024, 2310106 CrossRef CAS PubMed.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Chem. Rev., 2020, 120(16), 8814 CrossRef CAS PubMed.
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O’Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310(5751), 1166 CrossRef CAS.
- S. Patial, V. Soni, A. Kumar, P. Raizada, T. Ahamad, X. M. Pham, Q. V. Le, V.-H. Nguyen, S. Thakur and P. Singh, Environ. Res., 2023, 218, 114982 CrossRef CAS.
- H. Wang, H. Wang, Z. Wang, L. Tang, G. Zeng, P. Xu, M. Chem, T. Xiong, C. Zhou, X. Li, D. Huang, Y. Zhu, Z. Wang and J. Tang, Chem. Soc. Rev., 2020, 49(12), 4135–4165 RSC.
- M. Li, C. Gong, J. Du, D. Ding, D. Du, D. Wang, J. Jiang, T. Li, C. Zhang, Y.-F. Yang, Y. She and J. Jia, ACS Mater. Lett., 2023, 5(3), 694 CrossRef CAS.
- P. Pachfule, A. Acharjya, J. Roeser, T. Langenhahn, M. Schwarze, R. Schomäcker, A. Thomas and J. Schmidt, J. Am. Chem. Soc., 2018, 140(4), 1423 CrossRef CAS PubMed.
- Y. Chen, X. Luo, J. Zhang, L. Hu, T. Xu, W. Li, L. Chen, M. Shen, S.-B. Ren, D.-M. Han, G.-H. Ning and D. Li, J. Mater. Chem. A, 2022, 10(46), 24620 RSC.
- L. Yin, Y. Zhao, Y. Xing, H. Tan, Z. Lang, W. Ho, Y. Wang and Y. Li, Chem. Eng. J., 2021, 419, 129984 CrossRef CAS.
- X. Pei, P. He, K. Yu, Y. Li, Y. Tang and L. Ma, Adv. Funct. Mater., 2024, 2410827 CrossRef CAS.
- W. Wang, D. Huang, W. Zheng, X. Zhao, K. He, H. Pang and Y. Xiang, Chem. Mater., 2023, 35(17), 7154 CrossRef CAS.
- R. Freund, O. Zaremba, G. Arnauts, R. Ameloot, G. Skorupskii, M. Dincă, A. Bavykina, J. Gascon, A. Ejsmont, J. Goscianska, M. Kalmutzki, U. Lachelt, E. Ploetz, C. S. Diercks and S. Wuttke, Angew. Chem., Int. Ed., 2021, 60(45), 23975 CrossRef CAS PubMed.
- F. Ahmadijokani, A. Ghaffarkhah, H. Molavi, S. Dutta, Y. Lu, S. Wuttke, M. Kamkar, O. J. Rojas and M. Arjmand, Adv. Funct. Mater., 2023, 2305527 Search PubMed.
- N. H. Solangi, R. R. Karri, S. A. Mazari, N. M. Mubarak, A. S. Jatoi, G. Malafaia and A. K. Azad, Coord. Chem. Rev., 2023, 477, 214965 CrossRef CAS.
- Q. Zhong, Y. Li and G. Zhang, Chem. Eng. J., 2021, 409, 128099 CrossRef CAS.
- S. Kashif, S. Akram, M. Murtaza, A. Amjad, S. S. A. Shah and A. Waseem, Diamond Relat. Mater., 2023, 136, 110023 CrossRef CAS.
- K. Nabeela, R. Deka, Z. Abbas, P. Kumar, M. Saraf and S. M. Mobin, Cryst. Growth Des., 2023, 23(5), 3057 CrossRef CAS.
- Y. Wen, Z. Wei, C. Ma, X. Xing, Z. Li and D. Luo, Nanomaterials, 2019, 9(5), 775 CrossRef CAS PubMed.
- Q. Geng, H. Wang, Y. Wu, L. P. Lv, S. Chen, W. Sun and Y. Wang, ChemElectroChem, 2022, 9(16), e202200340 CrossRef CAS.
- Y. Zhao, K. Hu, C. Yang, X. Liu, L. Li, Z. Li, P. Wang, Z. Zhang and S. Zhang, Anal. Chim. Acta, 2023, 1237, 340581 CrossRef CAS PubMed.
- X. Gong, G. Zhang, H. Dong, H. Wang, J. Nie and G. Ma, J. Membr. Sci., 2022, 657, 120667 CrossRef CAS.
- C. Wei, Y. Wang, Y. Zhang, L. Tan, Y. Qian, Y. Tao, S. Xiong and J. Feng, Nano Res., 2021, 14, 3576 CrossRef CAS.
- H. Wang, R. Zhao, J. Qin, H. Hu, X. Fan, X. Cao and D. Wang, ACS Appl. Mater. Interfaces, 2019, 11(47), 44249 CrossRef CAS PubMed.
- Y. Liu, Y. He, E. Vargun, T. Plachy, P. Saha and Q. Cheng, Nanomaterials, 2020, 10(4), 695 CrossRef CAS PubMed.
- Y. Sun, M. Xie, H. Feng and H. Liu, ChemPlusChem, 2022, 87(4), e202100553 CrossRef CAS PubMed.
- T. Xiao, J. Jin, Y. Zhang, W. Xi, R. Wang, Y. Gong, B. He and H. Wang, Electrochim. Acta, 2022, 427, 140851 CrossRef CAS.
- Y. Wang, J. Song and W. Y. Wong, Angew. Chem., 2023, 135(8), e202218343 CrossRef.
- X. Zhao, H. Xu, Z. Hui, Y. Sun, C. Yu, J. Xue, R. Zhou, L. Wang, H. Dai, Y. Zhao, J. Yang, J. Zhou, Q. Chen and W. Huang, Small, 2019, 15(47), 1904255 CrossRef CAS PubMed.
- A. Wei, L. Wang and Z. Li, J. Alloys Compd., 2022, 899, 163369 CrossRef CAS.
- C. Ruan, D. Zhu, J. Qi, Q. Meng, F. Wei, Y. Ren, Y. Sui and H. Zhang, Surf. Interfaces, 2021, 25, 101274 CrossRef CAS.
- X. Zhang, S. Yang, W. Lu, D. Lei, Y. Tian, M. Guo, P. Mi, N. Qu and Y. Zhao, J. Colloid Interface Sci., 2021, 592, 95 CrossRef CAS PubMed.
- Y. Yang, X. Huang, C. Sheng, Y. Pan, Y. Huang and X. Wang, J. Alloys Compd., 2022, 920, 165908 CrossRef CAS.
- B. P. Mishra, L. Biswal, S. Das, L. Acharya and K. Parida, Langmuir, 2023, 39(3), 957 CrossRef CAS PubMed.
- H. Gu, H. Zhang, X. Wang, Q. Li, S. Chang, Y. Huang, L. Gao, Y. Cui, R. Liu and W.-L. Dai, Appl. Catal., B, 2023, 328, 122537 CrossRef CAS.
- L. Cheng, Y. Tang, M. Xie, Y. Sun and H. Liu, J. Alloys Compd., 2021, 864, 158913 CrossRef CAS.
- S. G. Nnabuife, J. Ugbeh-Johnson, N. E. Okeke and C. Ogbonnaya, Carbon Capture Sci. Technol., 2022, 3, 100042 CrossRef CAS.
- M. M. Ayyub, M. Chhetri, U. Gupta, A. Roy and C. N. R. Rao, Chem. – Eur. J., 2018, 24(69), 18455 CrossRef CAS PubMed.
- T. Ohta, Int. J. Hydrogen Energy, 1988, 13(6), 333–339 CrossRef CAS.
- L. Zhao, B. Dong, S. Li, L. Zhou, L. Lai, Z. Wang, S. Zhao, M. Han, K. Gao, M. Lu, X. Xie, B. Chen, Z. Liu, X. Wang, H. Zhang, H. Li, J. Liu, H. Zhang, X. Huang and W. Huang, ACS Nano, 2017, 11(6), 5800 CrossRef CAS PubMed.
- D. Chen, Y. Cheng, N. Zhou, P. Chen, Y. Wang, K. Li, S. Huo, P. Cheng, P. Peng, R. Zhang, L. Wang, H. Liu, Y. Liu and R. Ruan, J. Cleaner Prod., 2020, 268, 121725 CrossRef CAS.
- N. M. Mahmoodi, J. Mol. Catal. A: Chem., 2013, 366, 254 CrossRef CAS.
- E. Issaka, J. N. O. Amu-Darko, S. Yakubu, F. O. Fapohunda, N. Ali and M. Bilal, Chemosphere, 2022, 289, 133208 CrossRef CAS PubMed.
- O. Tavakoli, V. Goodarzi, M. R. Saeb, N. M. Mahmoodi and R. Borja, J. Hazard. Mater., 2017, 334, 256 CrossRef CAS PubMed.
- H. Assad, I. Fatma, A. Kumar, S. Kaya, D. V. N. Vo, A. Al-Gheethi and A. Sharma, Chemosphere, 2022, 298, 134221 CrossRef CAS PubMed.
- N. Nasrollahi, S. Aber, V. Vatanpour and N. M. Mahmoodi, Composites, Part B, 2018, 154, 388 CrossRef CAS.
- W. Zhou, P. Wu, L. Zhang, D. Zhu, X. Zhao and Y. Cai, J. Hazard. Mater., 2022, 421, 126721 CrossRef CAS PubMed.
- X. Li, Y. Pi, Q. Hou, H. Yu, Z. Li, Y. Li and J. Xiao, Chem. Commun., 2018, 54(15), 1917 RSC.
- E. Erdural, U. Bolukbasi and G. Karakas, J. Photochem. Photobiol., A, 2014, 283, 29 CrossRef.
- O. Bechambi, M. Chalbi, W. Najjar and S. Sayadi, Appl. Surf. Sci., 2015, 347, 414 CrossRef CAS.
- K. Dulta, G. Koşarsoy Ağçeli, P. Chauhan, R. Jasrotia, P. K. Chauhan and J. O. Ighalo, Sustainable Environ. Res., 2022, 32, 1 CrossRef.
- M. Zhang, K. Wang, S. Zeng, Y. Xu, W. Nie, P. Chen and Y. Zhou, Chem. Eng. J., 2021, 411, 128517 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |