Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Functional flexible adsorbents and their potential utility
Kyriaki
Koupepidou†
 ,
Aizhamal
Subanbekova†
,
Aizhamal
Subanbekova†
 and
Michael J.
Zaworotko
and
Michael J.
Zaworotko
 *
*
Bernal Institute, Department of Chemical Sciences, University of Limerick, Limerick V94T9PX, Republic of Ireland. E-mail: xtal@ul.ie
First published on 20th January 2025
Abstract
Physisorbents are poised to address global challenges such as CO2 capture, mitigation of water scarcity and energy-efficient commodity gas storage and separation. Rigid physisorbents, i.e. those adsorbents that retain their structures upon gas or vapour exposure, are well studied in this context. Conversely, cooperatively flexible physisorbents undergo long-range structural transformations stimulated by guest exposure. Discovered serendipitously, flexible adsorbents have generally been regarded as scientific curiosities, which has contributed to misconceptions about their potential utility. Recently, increased scientific interest and insight into the properties of flexible adsorbents has afforded materials whose performance suggests that flexible adsorbents can compete with rigid adsorbents for both storage and separation applications. With respect to gas storage, adsorbents that undergo guest-induced phase transformations between low and high porosity phases in the right pressure range can offer improved working capacity and heat management, as exemplified by studies on adsorbed natural gas storage. For gas and vapour separations, the very nature of flexible adsorbents means that they can undergo induced fit mechanisms of guest binding, i.e. the adsorbent can adapt to a specific adsorbate. Such flexible adsorbents have set several new benchmarks for certain hydrocarbon separations in terms of selectivity and separation performance. This Feature Article reviews progress made by us and others towards the crystal engineering (design and control) of flexible adsorbents and addresses several of the myths that have emerged since their initial discovery, particularly with respect to those performance parameters of relevance to natural gas storage, water harvesting and hydrocarbon gas/vapour separation.
Introduction
The International Union of Pure and Applied Chemistry (IUPAC) defines an adsorbent as a condensed phase at the surface of which adsorption may occur.1 Adsorbents serve important everyday applications in separation and purification such as gas separation and recovery,2,3 water decontamination4,5 and air purification,6,7 and they are of topical interest for water harvesting8 and gas storage applications.9,10 Adsorbents can be conveniently classified by three criteria that influence their functional properties, (i) mechanism of adsorption, (ii) degree of porosity and (iii) composition:(i) Physisorption or chemisorption. Physisorbents rely on physical adsorption (physisorption),11i.e. adsorbent–adsorbate interactions result from weak noncovalent forces. Chemisorbents exploit covalent bond breakage and formation (chemisorption).12
(ii) Nonporous or porous. Porous adsorbents such as zeolites,13 most metal–organic frameworks (MOFs)14 and some hydrogen bonded organic frameworks (HOFs)15 or halogen bonded organic frameworks (XOFs)16 exhibit internal porosity,17 whereas nonporous adsorbents such as silica and carbon black do not.
(iii) Network or molecular solid. Network solids such as diamond and MOFs are sustained by covalent bonds. Molecular solids such as organic inclusion compounds18 are sustained by noncovalent forces.19
Physisorbents are of topical interest for their potential utility since they generally require less energy for desorption of the adsorbate than chemisorbents.20 Porous adsorbents tend to be preferred as they can have an internal surface area in addition to external surface area, meaning that their uptakes are typically higher than nonporous adsorbents.21 There should be no inherent prejudice as to whether an adsorbent is a molecular or network solid, but their nature can profoundly impact mechanism and performance.
Most porous physisorbents are considered to be “rigid” adsorbents, i.e. they retain their structures during gas or vapour sorption cycles. Both molecular and network solids that behave as rigid adsorbents were studied decades ago by Barrer, as exemplified by Dianin's compound22 and zeolites.23 Zeolites can be naturally occurring24 or synthetic,25 with 256 zeolite framework topologies archived in the International Zeolite Association (IZA) database.26 MOFs and coordination networks (CNs)27–29 such as hybrid ultramicroporous materials (HUMs)30 have emerged in the past three decades and differ from zeolites as they are highly modular in terms of their chemical composition. This makes them amenable to design using principles such as crystal engineering,31–33 so that a particular topology can have hundreds or even thousands of related or isoreticular34 members of the same family. The first reports of permanent porosity in MOFs were made in 1997 by the groups of Kitagawa and Mori, Co2(4,4′-bpy)3(NO3)4,35 and Cu(II) terephthalate, Cu(bdc),36 respectively, and in 1998 by Yaghi's group, Zn(bdc).37 The gas sorption isotherms of these adsorbents are concave to the pressure axis, which is classified by IUPAC as a type I isotherm38 and corresponds to loading of a rigid pore (rp, Fig. 1a). The slope of the curve is related to the strength and uniformity of adsorbent–adsorbate interactions. Many high surface area MOFs exhibit weak adsorbent–adsorbate interactions resulting in nearly linear adsorption profiles, whereas HUMs can offer steeper uptakes at low pressure due to strong adsorbent–adsorbate interactions (Fig. 1a).39
Flexible adsorbents, i.e. adsorbents that undergo structural transformation(s) upon guest exposure, were to our knowledge first reported by Barrer for a Werner complex, a nonporous molecular solid, in 1969.40 Although the crystal structures corresponding to the guest-induced phase transformations were not then reported, this work laid the groundwork for what would later be called flexible adsorbents. Prediction of flexible adsorbents in the then emerging field of MOFs was made in 1998 by Kitagawa,41,42 validated three years later when ELM-11(Cu) was reported by Kaneko.43 It was noted that the observed isotherm types, which typically involve one or more steps, “cannot be classified into the representative six types recommended by IUPAC”.43 Today, a relatively small but growing subset of MOFs (perhaps ≤ 1%) are known to exhibit flexibility.44,45 We herein define a flexible adsorbent as an adsorbent that undergoes a guest-induced structural transformation during gas, vapour or liquid sorption, resulting in changes to its pore size, shape and/or chemistry, as usually evidenced by in situ structural characterisation. Structural characterisation is typically necessary to validate that phase transformations have indeed occurred during gas or vapour uptake. Whereas a stepped adsorption isotherm can be an indicator of flexibility, it is not definitive, as stepped isotherms can also occur in rigid adsorbents for vapours and liquids.46 Although flexibility can manifest in both global and local dynamics,47 locally dynamic adsorbents exhibit only localised structural changes without long-range transformations in response to guest exposure. In contrast, globally dynamic or “cooperatively flexible” adsorbents, herein referred to as flexible adsorbents, undergo discontinuous phase changes leading to stepped isotherms, and are the focus of this Feature Article.
Flexible adsorbents tend to exhibit distinctive isotherm “steps” (Fig. 1b–f).48 Although some of the isotherm types defined by IUPAC are similar in shape, they are associated with monolayer or multilayer adsorption in which the surface layer of the adsorbent is equally accessible to the adsorbate.49 However, this is not the case in flexible adsorbents as the initial phase has less (or no) accessible surface, whereas the subsequent guest-loaded phase does. Therefore, additional isotherm types must be defined to reflect sorption in flexible adsorbents. Isotherm step(s) are associated with structural transformation(s), typically (but not always) from a less porous (narrow pore; np) or nonporous (closed pore; cp) activated phase to a more porous (large pore; lp) loaded phase. The nature of the initial phase impacts the profile of the isotherm before the step: np phases result in type F-I (gradual) or F-II (sudden) isotherms (np → lp; Fig. 1b and e); cp phases afford type F-III (gradual) or F-IV (sudden) isotherms (cp → lp; Fig. 1c and f). Sharp isotherm steps corresponding to cp → lp transformations can be classified as “switching”,50 and can involve one (cp → lp; type F-IVs, where “s” stands for single-step isotherm; Fig. 1c) or multiple (cp → np → lp; type F-IVm, where “m” stands for multistep isotherm; Fig. 1d) steps depending on the adsorbent and adsorbate. Hysteresis, where the adsorption and desorption curves do not match is typically a result of kinetic barriers and metastable phases.41,51 The pressure threshold at which a step occurs during adsorption and desorption can be termed the gate-opening (Pgo) and gate-closing (Pgc) pressures, respectively.
Insight into the underlying mechanisms behind different isotherm profiles is typically gained through in situ structural characterisation, with computational modelling being a powerful complementary technique to provide further insight. The increasing availability and utilisation of in situ X-ray diffraction (XRD) and advances in computational modelling have provided insight into the design and properties of flexible adsorbents and enable us to better assess their potential utility. The sorption behaviour of flexible adsorbents suggests possible advantages: (i) enhanced working capacity; (ii) intrinsic thermal management; (iii) improved molecular recognition and selectivity. Working capacity, i.e. the uptake difference between deliverable (high) and operable (low) pressures, can be higher in flexible adsorbents than rigid ones. This is because rigid adsorbents tend to retain gas at the deliverable pressure, therefore limiting the uptake difference between deliverable and operable pressures (Fig. 1g). A flexible adsorbent with a type F-IVs isotherm (Fig. 1c) retains no gas at deliverable pressure, if Pgc is above the deliverable pressure. That flexible adsorbents undergo an endothermic phase transformation induced by gas pressure can partly offset exothermic heat release during adsorption. Finally, as phase transformations are guest-induced, flexible adsorbents can exhibit molecular recognition (selectivity) if a phase transformation is induced by only one component of a mixture.
With increasing scientific studies concerning flexible adsorbents, several misconceptions or “myths” have emerged, all of which seem intuitive but would mitigate against utility: lack of design principles to identify parent (first generation, Gen 1) adsorbents; hydrolytic or mechanical instability, a feature of many MOFs; slow kinetics vs. rigid adsorbents since structural transformation(s) are required; hysteresis; low gas uptake because phase changes are not extreme enough; lack of selectivity resulting from co-adsorption in lp phases. Although many reviews have previously highlighted the potential utility of flexible adsorbents,47,52–55 none of them have directly addressed these misconceptions. This Feature Article provides the state-of-the-art in the context of these perceived weaknesses by addressing design and control principles from a crystal engineering perspective, before addressing each misconception through examples of flexible adsorbents studied in our group. We intend for this review to emphasise that functional flexible adsorbents are now candidates for utility in natural gas storage, water harvesting and hydrocarbon gas/vapour separation.
Chronology of key discoveries in flexible adsorbents for energy applications
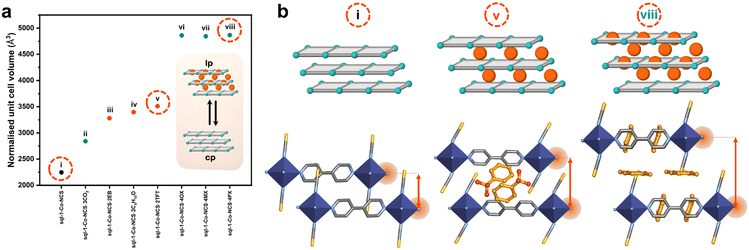
Since Barrer introduced the first flexible adsorbent, [Ni(4-MePy)4(NCS)2],40 both network and molecular solids have been studied (Fig. 2). Kitagawa's prediction of “third-generation” flexible porous coordination polymers, PCPs,42 was validated by Kaneko et al. when they reported a two-dimensional (2D) framework, Cu(bpy)2(BF4)2, in 2001 (now commonly known as ELM-11(Cu)).43 Three-dimensional (3D) flexible MOFs were reported in the following year, including Cu2(bdc)2(bpy) by Seki,56,57Cu2(pzdc)2(dpyg) by Kitagawa,58 and MIL-53(Cr) by Férey and Serre.59 Also in 2002, flexibility was reported for the nonporous organic molecular solid p-tert-butylcalix[4]arene by Atwood and Barbour.60 However, practical applications of flexible adsorbents remained understudied until 2009, when MIL-53(Cr) was investigated for CO2/CH4 separation.61 Long's report concerning Co(bdp) for CH4 storage in 2015 enabled flexible adsorbents to gain momentum for energy applications.62 In 2017, MIL-53(Al) demonstrated the separation of hydrogen isotopes, with selectivity for D2 over H2.63 In 2018, our group reported high working capacity for CH4 storage by X-dia-1-Ni48 and one year later selective C8 separation was reported for sql-1-Co-NCS.64 Our study of induced fit binding of C2H2 by sql-SIFSIX-bpe-Zn in 2021 set a new benchmark for C2H2 binding and C2H2/CO2 selectivity.65 The extensively studied DUT-8(Ni) introduced by Kaskel's group was found to exhibit D2/H2 separation in 2022 through selective cp → lp transformation for D2.66X-dia-2-Cd was found in 2023 to be the first flexible adsorbent meeting criteria for atmospheric water harvesting,67 while a molecular solid, TPBD, exhibited benchmark p-xylene binding.68 Most recently, cobalt doping of X-dia-1-Ni afforded X-dia-1-Ni0.89Co0.11, which exhibited inverse C2H6/C2H4 selectivity.69 Interestingly, these reports do not fully address composition/property relationships. The next section therefore addresses if flexibility can be expected: | ||
| Fig. 2 Chronology of the “discovery” (dark cyan) and “structure/function” (light cyan) phases of the development of flexible adsorbents, for which representative isotherms corresponding to phase transformations were reported. Compound names are colour coded for molecular (green) solids, 2D network (blue) and 3D network (red) solids. Crystal structures are generated from .cif files archived in the Cambridge Structural Database (ref. 45). CCDC numbers: 1179557 ([Ni(4-MePy)4(NCS)2]), 974376 (ELM-11(Cu)), 797261 (MIL-53(Cr)), 863312 (Cu2(bdc)2(bpy)), 168595 (Cu2(pzdc)2(dpyg)), 1242903 (p-tert-butylcalix[4]arene), 1058444 (Co(bdp)), 1818655 (sql-1-Co-NCS), 2048415 (sql-SIFSIX-bpe-Zn), 2020029 (DUT-8(Ni)) 2240524 (X-dia-2-Cd), 2243659 (TPBD), 1426847 (X-dia-1-Ni). Atom colours: C, grey; N, blue; O, red; S, yellow; F, neon green; B, pink; Cu, orange; Cr, burnt orange; Co, indigo; Ni, green; Zn, purple; Cd, gold; Al, dusty pink. Hydrogen atoms are omitted for clarity. | ||
Myth 1: “Flexible adsorbents are curiosities that are difficult to design or control.”
Once a Gen 1 adsorbent has been identified, the inherent modularity of CNs and most molecular compounds allows for crystal engineering of families of second generation (Gen 2) flexible adsorbents through systematic substitution of components. CNs based on molecular building blocks (MBBs)31 can provide platforms based on the most commonly observed and accessible topologies:70,71 square lattice (sql), primitive cubic (pcu) and diamondoid (dia). Indeed, sql-1-Co-NCS belongs to the same sql family as ELM-11(Cu), X-dia-2-Cd has dia topology like X-dia-1-Ni, and DUT-8(Ni) has pcu topology as does Cu2(bdc)2(bpy). Network solids based on rod building blocks (RBBs)72 include MIL-53(Cr) and Co(bdp). The primary issue is, therefore, not systematic development of Gen 2 materials, but rather discovery of the prototypal Gen 1 member. In this context, mechanisms of flexibility can provide guidance.
Design and control of flexible adsorbents
Flexible adsorbents undergo structural changes upon both guest removal and introduction. Upon guest removal, this flexibility originates from transitions to more thermodynamically favourable states via flexibility mechanisms, which promote densification and frequently facilitate the formation of exothermic noncovalent interactions within the adsorbent.47 The subsequent flexibility exhibited upon guest introduction is facilitated through compensatory host–guest interactions. Understanding these mechanisms of flexibility is a prerequisite for control and fine-tuning of flexible adsorbents. While CNs and Werner complexes can be readily fine-tuned by metal and (equatorial and axial) ligand substitution, organic molecular solids can only be tuned by covalent bond modification. The high degree of modularity of CNs and Werner complexes affords several variables from a crystal engineering perspective,73 making them the primary focus of this section.Flexibility in molecular solids
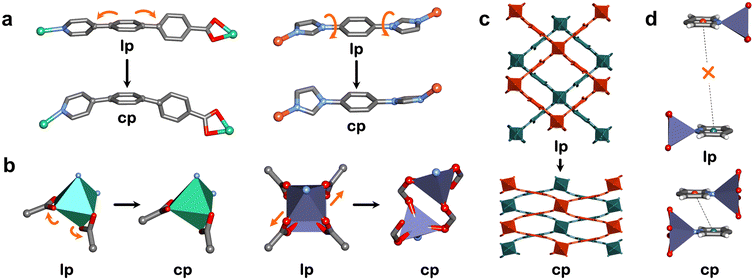
In molecular solids, crystal packing can change to accommodate guest molecules within pockets formed by adjacent molecular units. Early examples of organic and metal-organic molecular solids where both the guest-loaded and guest-free phases were characterized are p-tert-butylcalix[4]arene and [Ni(4-MePy)4(NCS)2].40,60,74 Both exist as nonporous guest-free solids that undergo structural transformation through rearrangement of noncovalent bonds: for p-tert-butylcalix[4]arene, ab/cd packing in the cp phase changes to ab/ab packing to accommodate two aromatic molecules; for [Ni(4-MePy)4(NCS)2], translation between adjacent complexes creates a cavity to fit one benzene molecule. Werner complexes can be readily modified through ligand or metal substitution. For example, Barrer also reported gas sorption for the Co analogue of [Ni(4-MePy)4(NCS)2]. Further, [Co(4-EtPy)4(NCS)2] was found to exhibit phase transformations when exposed to benzene, toluene and C8 isomers (where 4-MePy = 4-methylpyridine and 4-EtPy = 4-ethylpyridine).40 Pyridine (Py) ligands were investigated in the series [Cu(Py)4X2] (X = PF6, BF4, CF3SO3, CH3SO3), with [Cu(Py)4(PF6)2] and [Cu(Py)4(BF4)2] exhibiting inflected isotherms for acetonitrile (298 K) and methanol (283 K).75 The phase transformations for [Cu(Py)4(PF6)2] are consistent with Barrer's earlier studies (Fig. 3).In order to find more examples of molecular solids that can act as adsorbents, our approach is to first identify host–guest complexes in “as-synthesised” crystal structures, including hydrates and solvates. Werner complexes have been known to form Werner clathrates since the late 1950s,76 and several have since been found to exhibit reversible guest-induced structural transformations. Identification of host–guest complexes can aid the design of flexible adsorbents by studying what happens after the removal of guests. For example, we recently revisited the Werner complex [Ni(4-MePy)4(NCS)2], which was reported to form clathrates with aromatic molecules in 1957,77 while its nonporous phase was reported in 1952.78 By identifying these phases we found that [Ni(4-MePy)4(NCS)2] can serve as a flexible adsorbent for p-xylene and that it shows a rarely observed shape-memory effect.79 Solvate or hydrate formation is therefore one indicator of a potential flexible adsorbent. Organic molecular solids that are promiscuous with respect to conformational polymorphism is another indicator, an example of which is discussed in the separation section.68
Flexibility in network solids
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ph, and –N
N–Ph, and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ph(CH3)2) resulted in a new platform of flexible adsorbents with sql topology, Zn(5-Ria)(bphy) (ia = isophthalic acid and bphy = 1,2-bis(pyridin-4-yl)hydrazine).87
N–Ph(CH3)2) resulted in a new platform of flexible adsorbents with sql topology, Zn(5-Ria)(bphy) (ia = isophthalic acid and bphy = 1,2-bis(pyridin-4-yl)hydrazine).87
Based on the above, we can categorise our research efforts into three categories of linker ligands that offer potential for flexibility: (i) linkers that contort (X ligands); (ii) linkers that isomerise (e.g. syn/anti); (iii) linkers with pendant groups. However, these results do not preclude the use of rigid ligands in flexible adsorbents, as there are other features that can promote flexibility. Indeed, rigid ligands such as 4,4′-bipyridine, which propagate linear linkages between coordination sites, cannot induce flexibility on their own. Nevertheless, they are frequently encountered in flexible adsorbents as there are other mechanisms that can induce flexibility.
Our approach has focused on MBBs that distort or isomerise. The simplest MBBs comprise a single metal moiety where O–M–O, N–M–O and N–M–N angles can vary (O = oxygen, N = nitrogen, M = metal). This was observed in ZIF-7 (ZIF = zeolitic imidazolate framework), where flexibility primarily originates from the flapping motion of the rigid benzimidazolate linker ligand around the tetrahedral MBBs.90 In our studies, octahedral distortion was seen in X-dia-1-Ni (Fig. 4b).48 In other cases, breakage of one of the chelating M–O bonds of a carboxylate moiety can result in change of geometry, e.g. octahedral to tetrahedral, as seen in X-dia-4-Co and X-dia-5-Co.83 Whereas bond angle deformations in monometallic MBBs are typically subtle, metal cluster MBBs can undergo more extreme deformations. For example, isomerisation of the paddle-wheel MBB in X-pcu-5-Zn by changing the coordination mode of the O-donor linker from bidentate to monodentate resulted in phase transformation to a new cp polymorph (Fig. 4b).91 This isomerisation resulted in the geometry changing from square pyramidal to tetrahedral, which is more expected for Zn than Co, Ni or Cu due its d10 electronic configuration. Such a situation is exemplified by DUT-8(Ni) and DUT-8(Co), which retained square pyramidal geometry after phase transformation, whereas DUT-8(Zn) underwent isomerisation.92,93 More recently, we reported a new MBB that enables deformations similar to RBBs by mimicking a butterfly waving its wings (“butterfly” motions). We employed this MBB in a double-walled dia network (X-dia-6-Ni)94 as well as a double diamondoid (ddi) network (X-ddi-1-Ni).82
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) exhibited the highest Pgo.101 Substitutions of C atoms in phenyl rings with N atoms, e.g.X-dia-4-Co and X-dia-5-Co;83X-ddi-1-Ni and X-ddi-2-Ni;82SIFSIX-23-Cu and SIFSIX-23-CuN,102 typically result in lower Pgo, and in one case a shape-memory effect was observed.102
N–) exhibited the highest Pgo.101 Substitutions of C atoms in phenyl rings with N atoms, e.g.X-dia-4-Co and X-dia-5-Co;83X-ddi-1-Ni and X-ddi-2-Ni;82SIFSIX-23-Cu and SIFSIX-23-CuN,102 typically result in lower Pgo, and in one case a shape-memory effect was observed.102
We have also employed metal substitution as a strategy to tune Pgo. In a series of transiently porous networks, X-dmp-1-M (M = Co, Zn, Cd), we found that metal substitution affected the geometries during phase transformations: distorted square pyramidal (in lp) to tetrahedral geometry (in cp) for Co, and retention of tetrahedral and octahedral geometries for Zn and Cd, respectively.103 Out of the three analogues studied, Co achieved the lowest Pgo values and Cd the highest (for CO2 at 195 K and CH4 at 298 K). Even in platforms where MBB distortion is not the main mechanism of flexibility, e.g.sql-1-M-NCS (M = Fe, Co, Ni), we have observed that, besides layer expansion, the geometry of pores can change in shape from a rhombus to a square upon CO2 sorption, accompanied by coordination sphere distortion.104 While all three members exhibited a clear isotherm step, metal substitution affected its position: Fe had the highest Pgo, while Ni showed the lowest, indicating that octahedral distortion was more facile for Ni.
Truth 1: “Certain classes of flexible adsorbents are inherently modular and can have their sorption performance parameters controlled by fine-tuning of composition, while design of Gen 1 flexible adsorbents is now possible from first principles.”
Over the past two decades, flexible adsorbents have progressed from serendipity to systematic design, leading to understanding of structure–function relationships. During the early 2000s, the identification of key components enabling flexibility, such as ligands with contortional flexibility or the ability to isomerise, alongside RBBs and MBBs capable of “butterfly” motions, marked significant developments. These findings allowed for the classification of parameters that can induce flexibility, equipping crystal engineers with the ability to design families of flexible adsorbents from first principles. As a result, the 2010s saw the development of new families of flexible adsorbents where the focus was their properties, particularly in relevance to natural gas storage, water harvesting, and most recently, hydrocarbon separations. In the 2020s, now that we have identified features of flexible adsorbents that can result in promising properties, we have insight for design of the next generation (Gen 3). Concurrently, we can recognise and then address limitations that may hinder performance. One of the key areas requiring further exploration is our inability to accurately predict Pgo for a given combination of flexible adsorbent components. However, once a flexible adsorbent platform is established, Pgo can be systematically tuned to suit a specific application. This tunability is an inherent feature of most flexible adsorbents, and could make them highly adaptable for a broad spectrum of industrial applications as explained below.
Flexible adsorbents for energy applications
Natural gas storage
Burning of fossil fuels to produce energy generates over 35 billion tons of carbon dioxide (CO2) annually and CO2 is the most persistent greenhouse gas.105,106 Whereas there is a trend for fossil fuels to produce a smaller share of global energy production in relative terms, increasing global energy production means that in absolute terms the use of fossil fuels continues to grow along with CO2 emissions.107 Methane (CH4), the main component of natural gas (NG), is an attractive intermediate fuel that could facilitate a move towards energy sustainability because it is both more abundant and cleaner as an energy source compared to petroleum and coal. Specifically, CH4 produces 40% less CO2 emissions than conventional fossil fuels upon burning.108 CH4 is also attracting attention as a vehicular fuel, not only because of its low carbon emissions, but also thanks to its high octane number (107).109,110The primary challenge for the use of gaseous fuels, such as CH4, for transportation lies in their low volumetric density under ambient conditions of temperature and pressure. In particular, at room temperature and 1 atm, CH4 has a density of 0.668 kg m−3, about 1000 times lower than petroleum (820–870 kg m−3).111 In order to increase storage density, compressed NG (CNG), liquefied NG (LNG) and adsorbed NG (ANG) are available but there are drawbacks to each. In order to increase the storage density of NG to 430–470 kg m−3, LNG requires low temperature (−161.5 °C), while CNG means compression at high pressure (200–250 bar) to achieve storage densities of 160–200 kg m−3.109 Both CNG and LNG are therefore hindered by cost, energy footprint, space and safety issues. In principle, ANG, which exploits adsorbents to store and deliver CH4 at ambient temperatures and lower pressures than CNG (for delivery at 5–35 or 5–65 bar), is advantageous. The CH4 storage targets set by the US Department of Energy (DOE) involve gravimetric values of 700 cm3 (STP) g−1 or 0.5 g g−1, or a volumetric target of 350 cm3 (STP) cm−3.112 When omitting the incorporated calculated packing loss of 25%, the volumetric target is 263 cm3 (STP) cm−3. The problem is that no adsorbent has yet achieved this performance, and a modelling study indicated that that rigid physisorbents are unlikely to exceed volumetric capacity of 200 cm3 (STP) cm−3.113
With respect to the study of CNs in the context of CH4 storage, in 1997 Kitagawa's group provided the first report of high-pressure CH4 sorption involving a rigid adsorbent, Co2(4,4-bipyridine)3(NO3)4,35 and followed this up with a study on CuSiF6(4,4′-bipyridine)2.114 Subsequent work tended to focus upon rigid adsorbents with high gravimetric surface areas, including the archetypal MOF HKUST-1(Cu).115 The study of flexible adsorbents for NG storage did not occur until 2009 with the study of CH4 sorption by MIL-53(Fe)116 and gained strong momentum in 2015 with Long's report on the performance of Co(bdp) and Fe(bdp) for CH4 storage.62 In terms of performance, deliverable capacity, also referred to as working capacity, is a key parameter. High performing adsorbents should minimise gas uptake at deliverable pressure (5 bar), while offering relatively high uptake at the single or dual compressor operable pressures of 35 and 65 bar, respectively. Rigid adsorbents tend not to be ideally suited for CH4 storage as their type I isotherms result in retention of gas at low pressure. Flexible adsorbents exhibiting type F-IV isotherms can have isotherm profiles that reduce or eliminate this parasitic CH4 uptake at 5 bar. Unfortunately, with few exceptions,48,62 transformations from nonporous to porous phases that enable type F-IV isotherm profiles tend to not be extreme enough to afford volumetric working capacities close to 200 cm3 cm−3, or exhibit phase switching within the needed pressure range. Additionally, to meet the goal of working capacity, the desorption profile needs to be stepped with minimal hysteresis. This section will thus address flexible adsorbents in relation to the following myth:
Myth 2: “The nature of flexible adsorbents means that working capacity is unlikely to exceed 200 cm3cm−3.”
Our pursuit of CH4 storage with flexible adsorbents began in 2018 with X-dia-1-Ni.48 The X ligand serves two purposes: enabling flexibility, as previously discussed, and large pores in the lp phase to enhance potential CH4 uptake. Indeed, we found that X-dia-1-Ni exhibited a type F-IV isotherm through a cp → lp transformation (Fig. 6a and 7a), with CH4 uptake of 222 cm3 g−1 (189 cm3 cm−3) at 65 bar.48 However, the working capacity between 5–65 bar was only 149 cm3 cm−3 (110 cm3 cm−3 for 5–35 bar) due to the hysteresis in the desorption branch of the isotherm, resulting in retention of more than 50 cm3 cm−3 at 5 bar. To regulate this, we prepared mixed crystals through Co substitution, affording a series of mixed crystals of X-dia-1-Ni.117 The best performing adsorbent, X-dia-1-Ni0.89Co0.11, exhibited an uptake of 221 cm3 g−1 at 65 bar, while also achieving a higher Pgc than the parent X-dia-1-Ni. As a result, the amount of gas retained at 5 bar was significantly reduced, yielding working capacities of 159 cm3 g−1 (5–35 bar) and 202 cm3 g−1 (5–65 bar). Although this was a significant improvement, the previously studied Co(bdp) achieves higher working capacities of 155 cm3 cm−3 for 5–35 bar and 197 cm3 cm−3 for 5–65 bar through a cp → lp transformation (Fig. 6a).62 The dearth of adsorbents that exhibit extreme cp → lp phase transformations in the relevant pressure range prompted us to explore a flexible adsorbent that exhibits np → lp transformation, X-dia-6-Ni (Fig. 6b).94 As anticipated, CH4 sorption revealed a type F-II isotherm with an uptake of 235 cm3 g−1 (200 cm3 cm−3) at 65 bar (Fig. 7b). While the achieved working capacity (5–65 bar) was only 166 cm3 cm−3, this study serves as a proof-of-concept for the potential utility of flexible adsorbents with np → lp transformations, which remain understudied. Likewise, a MIL-53 variant, MIL-53(Al)-OH, exhibited a working capacity of 156 cm3 g−1 from 5–65 bar (np → lp).118
 | ||
| Fig. 7 Experimental CH4 adsorption and desorption isotherms at 298 K for (a) X-dia-1-Ni and (b) X-dia-6-Ni. Adsorption = full circles, desorption = empty circles. Dashed vertical lines show the range of 5–65 bar. Isotherms were generated from raw data collected in our laboratory.48,94 | ||
Overall, there are now examples of flexible adsorbents that outperform rigid adsorbents in terms of CH4 working capacity. This is because, whereas rigid adsorbents such as Ni-MOF-74, HKUST-1(Cu), and UTSA-76a(Cu) have higher uptakes at 35 bar than flexible adsorbents, they retain at least a third of their uptakes at 5 bar, reducing their 5–35 bar working capacities to ca. 150 cm3 cm−3.115,119 Nevertheless, flexible adsorbents do not yet achieve the DOE targets (197 cm3 cm−3 for Co(bdp)vs. 263 cm3 cm−3 target). The potential upper limits for CH4 working capacities in rigid adsorbents were projected almost a decade ago, with the conclusion that rigid adsorbents are unlikely to meet the targets.113 Nevertheless, recent studies suggest that flexible adsorbents offer potential.120 The current limitation is their inability to achieve the fully open lp phase when exposed to CH4. For example, Co(bdp) exhibits uptake of 672 cm3 g−1 for N2 at 77 K after five gate-opening steps, but only achieves 256 cm3 g−1 for CH4 at 298 K after one gate-opening step.121 This limitation might be addressed by reduction of Pgo through crystal engineering, as detailed above.
In summary, although flexible adsorbents are not yet ready to address ANG, rigid adsorbents are inherently disadvantaged due to their isotherm profiles. Moving forward, screening of flexible adsorbents that can sustain Pgo to high porosity lp phases is crucial to meet applicable working capacities. This section suggests the following:
Truth 2: “Current flexible adsorbents do not address DOE targets; however, it is unlikely that we have reached the potential upper limit of their working capacity.”
Water harvesting
Water and water vapour are ubiquitous and perhaps the most important resources on earth. Water scarcity, from both a quantity and quality perspective, represents an urgent global challenge that is exacerbated by climate change.122,123 Harvesting water vapour from air (atmospheric water harvesting, AWH) is an attractive way to tackle water scarcity as the atmosphere contains 12.9 × 1015 tons of water124 that is inherently replenishable. This is perhaps the most urgent of all global challenges, as water is required to sustain life and agriculture. In addition, water is relevant in other applications including indoor humidity control (dehumidification),125,126 food preservation and heating/cooling.127–129Whereas AWH technologies such as fog water collection and dehumidification/condensation are feasible, they are ineffective under dry (low relative humidity, RH) conditions where water is scarce. Traditional desiccants such as zeolites, silica, metal chlorides and metal oxides come with drawbacks: zeolites require high regeneration temperatures due to their hydrophilicity;130 silica has generally low uptake due to low surface area;131 metal chlorides and oxides require chemical reactions and are prone to deliquescence.132 There is therefore an urgent need for the development of improved and energy-efficient AWH technologies.
Regeneration optimised adsorbents (ROSs), i.e. adsorbents that offer optimal performance (fast kinetics, low regeneration energy, recyclability, high selectivity, low hysteresis), have emerged in the past decade.133 In the context of AWH, water adsorbents have been developed to target two primary applications: (i) AWH at low humidity (5–35% RH); (ii) dehumidification of high humidity air to 40–60% RH. The prototypal high surface area rigid adsorbent HKUST-1(Cu) and a prototypal flexible adsorbent MIL-53(Al) were studied with respect to their water vapour sorption properties in 2002 and 2010, respectively.134,135 Whereas HKUST-1(Cu) would be unsuitable for AWH at low RH, MIL-53(Cr) exhibited a stepped isotherm (type F-IV) at low RH, suggesting potential AWH utility for flexible MOFs. Subsequently, a series of rigid MOFs exemplified by Al-fumarate,136CAU-10,137,138MOF-303139 and ROS-037140,141 were found to exhibit stepped isotherms at relatively low RH from pore filling and have attracted attention for further development as ROSs. The perception that flexible adsorbents would be unable to be classified as ROSs has perhaps discouraged their study in the context of AWH. Therefore, this section addresses the following myth:
Myth 3: “Flexible adsorbents will be unsuitable as ROSs for water harvesting as they are likely to suffer from poor recyclability (mechanical stress), slow kinetics and high hysteresis.”
To explore water harvesting ROSs, we focused on identifying potentially flexible adsorbents that crystallise in lp phases with 1D channels filled with water. In 2022, we reported a 1D flexible adsorbent, Cu(HQS)(TMBP), synthesised as a hydrated lp phase, which underwent a facile lp → cp transformation upon activation (Fig. 8a).142 Water sorption studies revealed a stepped isotherm, with an inflection point at 10% RH, which is relevant for AWH at low humidity, achieving an uptake of 150 mg g−1 at 90% RH. The kinetics of loading were also investigated, which revealed comparable kinetics to leading rigid adsorbents such as MOF-303(Al) and CAU-10-H, indicating that concomitant phase transformation did not slow kinetics. A more extensive range of flexible adsorbents were exchanged in water and, in cases where the lp phases could form 1D channels filled with water, water sorption properties were investigated. This led us to the discovery of sql-(azpy)(pdia)-Ni (Fig. 8a), which exhibited a stepped isotherm with a step at 50% RH, identifying it as suitable for indoor humidity control (Fig. 9a).96 However, the final uptake was lower than Cu(HQS)(TMBP) (115 mg g−1). Most recently, we reported a new flexible adsorbent through similar screening, X-dia-2-Cd (Fig. 8b), which undergoes a np → lp transformation upon water uptake and exhibits a step at 18% RH.67 This study prompted us to address the misconceptions regarding flexible adsorbents in the context of water harvesting. We found that X-dia-2-Cd meets the main criteria for potential utility: a stepped isotherm with a step below 30% RH; negligible hysteresis (<5 RH%); mild regeneration temperature (333 K); hydrolytic stability during temperature-humidity swing cycles for 100+ cycles. The saturation uptake of X-dia-2-Cd reached 130 mg g−1 at 95% RH (Fig. 9b). Cycling studies on these flexible desiccants demonstrated that they are stable to repeated adsorption–desorption stress: X-dia-2-Cd (128 cycles);67 [Cu(HQS)(TMBP)] (>100 cycles);142sql-(azpy)(pdia)-Ni (100 cycles).96
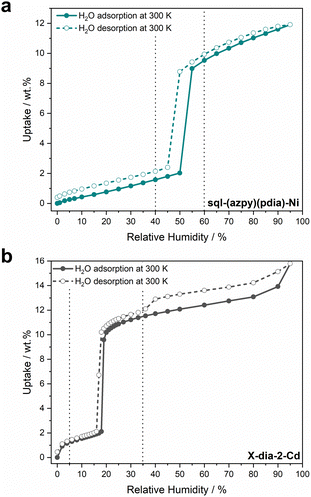 | ||
| Fig. 9 Experimental water adsorption and desorption isotherms at 300 K for (a) sql-(azpy)(pdia)-Ni and (b) X-dia-2-Cd. Adsorption = full circles, desorption = empty circles. Dashed vertical lines show the range of 40–60% RH in (a) and 5–35% RH in (b). Isotherms were generated from raw data collected in our laboratory.67,96 | ||
Compared to rigid adsorbents, the flexible adsorbents studied in our lab are not leading in terms of uptake. For example, the rigid adsorbents MOF-808(Zr)-Br and MOF-303(Al) also have steps below 30% RH but with much higher uptakes: 700 mg g−1 and 447 mg g−1, respectively.139,143 However, kinetics of adsorption/desorption are also key performance indicators.144 Recently, our group provided a method for projecting productivity, i.e. litres of water per kg of adsorbent per day (L kg−1 day−1), through heatmaps based on non-equilibrium cycling.133 In this context, some rigid adsorbents with high uptake were found to exhibit relatively slow adsorption kinetics, and, consequently, lower productivities than denser adsorbents with lower gravimetric uptake but faster kinetics. For example, compared to MOF-303(Al) (447 mg g−1), the rigid adsorbent ROS-039(Zn) achieved about half of the uptake (248 mg g−1), but exhibited faster kinetics. That loading was found to be controlled by surface diffusion to the adsorbent bed means that adsorbents with a step at lower % RH will adsorb at a faster rate. Through non-equilibrium cycling, productivities of 1.3 and 7.3 L kg−1 d−1 were found for MOF-303(Al) and ROS-039(Zn), respectively. Given that kinetics of water sorption, as well as non-equilibrium cycling, has rarely been studied in flexible adsorbents, they cannot yet be benchmarked as AWH adsorbents.
In summary, whereas rigid adsorbents can achieve higher water vapour uptake than flexible variants, uptake is only part of the picture. Considering that the study of flexible adsorbents as desiccants has only recently attracted attention, it is too early to assert if flexible adsorbents can be suited for AWH. This section supports the following assertion:
Truth 3: “Flexible adsorbents remain understudied with respect to AWH applications and it is too early to judge how well they compete with rigid adsorbents in terms of performance.”
Separations
Purification of speciality and commodity chemicals is an energy-intensive step in their manufacture because conventional processes that involve distillation, solvent extraction or catalytic conversion are inherently energy intensive and wasteful in terms of by-products.145 Adsorbents, especially physisorbents, offer an opportunity to greatly reduce the energy and environmental footprint of chemical purification processes. This is because separation by physisorbents is based on the molecular properties of the gas mixture components, rendering adsorptive separation up to 10 times more energy-efficient than traditional methods.109However, adsorptive separation is not as easy as it sounds. This is because most porous physisorbents tend not to have high enough selectivity to address removal of trace impurities. This is because “breaking up is hard to do”,146 especially for (i) molecules with similar physicochemical properties and (ii) trace impurities for which high selectivity is a necessity. For example, polymer-grade ethylene (C2H4) contains acetylene (C2H2) as a trace impurity (ca. 1%) that must be reduced to levels below 5 ppm.147 Similarly, as-produced propylene (C3H6) contains trace (ca. 1%) propyne (C3H4), which must be reduced below 5 ppm prior to catalytic polymerisation to polypropylene.148 Therefore, a suitable physisorbent must not only be able to distinguish between components with similar physicochemical properties, they must be effective at trace concentrations of the impurity. The challenge of separations is apparent from the kinetic diameters of common chemical commodities that must be purified: CO2, 3.3 Å; N2, 3.6 Å; CH4, 3.8 Å; C2H2, 3.3 Å; C2H4, 4.2 Å; C2H6, 4.4 Å; C3H8, 4.3 Å; C3H6, 4.7 Å; C3H4, 4.8 Å; p-xylene, 5.8 Å; ethylbenzene, 5.8 Å; o-xylene, 6.8 Å; m-xylene, 6.8 Å.149
Rigid physisorbents with ultra-high selectivity have emerged over the past decade and resulted in dramatic improvements in performance for several commodity separation processes: CO2/N2; CO2/CH4; C2H2/CO2; C2H2/C2H4; C2H6/C2H4; C3H4/C3H6; C8 isomers.150–152 In particular, the 2010s saw HUMs that could effect “gas separations at will”.153 HUMs, comprised of inorganic and organic linkers, have outperformed previous benchmark adsorbents, sometimes by one or more orders of magnitude in terms of selectivity. For example, the first adsorbent reported to selectively adsorb C2H2 from a C2H2/CO2 mixture was reported by Kitagawa's group in 2005, namely Cu2(pzdc)2(pyz).154SIFSIX-3-Zn reported in 2013 by our group39 improved selectivity for CO2/N2 by one order of magnitude compared to previous benchmarks such as UTSA-16(Co),155 and Mg-MOF-74 (Mg/DOBDC or CPO-27-Mg).156,157 HUMs are now the benchmarks for a variety of hydrocarbon separations, and generally function though the presence of a high density of bespoke identical binding sites.30 In effect, these rp adsorbents have the right pore size, shape and chemistry for a specific separation.158 The binding site interactions can be described as being analogous to the “lock and key” mechanism from biochemistry,159,160i.e. the adsorbate binds to the adsorbent in a manner similar to tight substrate binding by enzymes, where ultra-high selectivity is also needed (Fig. 10a).
 | ||
| Fig. 10 (a) Lock and key binding for a rigid adsorbent and (b) induced fit binding for a flexible adsorbent. | ||
Nevertheless, there are limitations to rigid adsorbents. First, real world gas mixtures are non-binary, and so performance can be affected by the other components, especially water vapour.161–163 Second, higher density adsorbents tend to have relatively low gravimetric uptakes and so a trade-off tends to exist between uptake, selectivity and binding affinity.164 In principle, flexible adsorbents can address this trade-off and combine high uptake with high selectivity, especially if they can engage in induced fit binding of adsorbates (Fig. 10b), i.e. where the adsorbent adapts to the shape and size of the adsorbate in order to facilitate optimal binding.165,166 This section addresses recent progress concerning the potential utility of flexible adsorbents in separations by addressing the following myth:
Myth 4: “Flexible adsorbents will have poor selectivity once they transform to alpphase.”
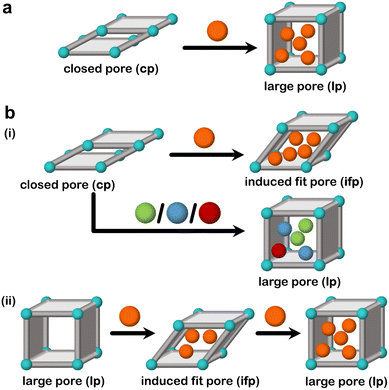
To date, only a few flexible adsorbents are known to exhibit induced fit behaviour.59,65,68,167 Induced fit binding is a feature of some flexible adsorbents and differs from the more commonly observed guest-induced cp → lp or np → lp transformations (Fig. 11a). Generally, we can classify induced fit adsorbents into two categories:
(a) Category I: adsorbents that undergo a bespoke structural transformation for one guest out of a mixture, leading to an induced fit phase that exists only for that particular guest;
(b) Category II: adsorbents that undergo a bespoke structural transformation for one guest out of a mixture, leading to an induced fit phase that would not correspond to the energy minimum in the absence of that guest.
Category I adsorbents are exemplified by those that undergo induced fit mechanisms towards a particular guest leading to an induced fit pore (ifp), but undergo phase transformation to the as-synthesised lp phase for other guests under the same conditions (Fig. 11b(i)). In category II adsorbents, the guest enforces a shrinkage on the adsorbent, which is not the energetically favoured phase in the absence of the guest (Fig. 11b(ii)). This leads to a lp → np → lp transformation, and, when this is true for only one particular guest, then the np can be viewed as an induced fit phase (ifp), classifying the transformation as lp → ifp → lp. Examples of such adsorbents will be discussed below.
C2H2/CO2 and C2H6/C2H4 separation
Separation of C2H2 and CO2 is particularly challenging due to their similar molecular size (ca. 3.3 Å) and boiling points (189 and 195 K respectively). Therefore, the design of adsorbents for this purpose should focus on host–guest interactions for preferential binding rather than size exclusion. Our contributions initially focused on the development of HUMs that can serve as rigid adsorbents with high C2H2/CO2 selectivity at 100 kPa and 298 K: NKMOF-1-Ni (23.9),168DICRO-4-Ni-i (13.9),169SIFSIX-21-Cu (10.0)164 and NbOFFIVE-3-Cu (9.5).164 Recently, we used a flexible ligand to develop a flexible adsorbent that combines the enhanced electrostatics of HUMs with the flexibility of conventional flexible adsorbents.65 We found that sql-SIFSIX-bpe-Zn exhibited induced fit binding for C2H2, the np phase slightly contracting to fit guest molecules into an ifp phase (Fig. 12a). Upon full loading, the network transformed to the lp phase. Exposure to CO2 under the same conditions resulted in np → lp transformation as is typical for flexible adsorbents. The mechanism of binding was supported by contortion of the linker, as well as host–guest interactions (hydrogen bonding, Fig. 12a). In terms of separation, the induced fit binding resulted in record-high heat of adsorption (Qst) for C2H2 of 67.5 kJ mol−1, while C2H2/CO2 selectivity reached 8.4, enabling sql-SIFSIX-bpe-Zn to separate an equimolar binary mixture.In order to produce polymer grade C2H4, C2H6 impurities must also be removed. Most adsorbents reported to date are C2H4/C2H6 selective due to the higher quadrupole moment of C2H4 and the presence of π electrons. However, preferential adsorption of C2H4 implies the need for additional desorption steps to obtain C2H4 as an effluent. A potential solution to this problem is the design of adsorbents which are “inverse selective” for C2H6. This concept was introduced in 2010 by a seminal study on the flexible adsorbent ZIF-7, which demonstrated “inverse selectivity” for a C2H6/C2H4 mixture.170 The benchmark inverse selective adsorbents have C2H6/C2H4 selectivities of 2–4 at 298 K and 100 kPa: Fe2(O2)(dobdc) (4.4);171Cu(Qc)2 (3.4);172NPU-3 (3.2);173ZJU-120 (2.7; at 296 K);174MAF-49 (2.7),175,176ZIF-7 (1.5).170,175,176 Recently, we discovered that a flexible adsorbent, the mixed crystal X-dia-1-Ni0.89Co0.11, offers proof-of-principle for flexible adsorbents in this context.69 Gas sorption revealed gate-opening towards C2H6 at 273 K, but no gate-opening towards C2H4 under the same conditions, corresponding to a cp → lp transformation only for C2H6. Selective gate-opening towards C2H6 resulted in corresponding C2H6/C2H4 selectivity of 5.5, which was also supported by dynamic column breakthrough experiments. Although the study was not performed at ambient (298 K) temperature, it reiterates that flexible adsorbents can exhibit benchmark molecular recognition properties, in some cases leading to inverse selectivity.
C8 isomer separations
Purification of the C8 isomers, o-xylene (OX), m-xylene (MX), p-xylene (PX), and ethylbenzene (EB), is necessary since they are typically produced as mixtures. Separation of C8 isomers in our group has generally been investigated through Werner complexes or CNs. Among these are flexible adsorbents that offer selective capture of OX, exemplified by the Werner complex SAMM-3-Cu-OTf (SOX/PX = 23.1)177 and the CN sql-1-Co-NCS (SOX/PX = 9.6).64 Although the current benchmark is another Werner complex, SAMM-3-Ni-NCS, otherwise known as [Ni(NCS)2(ppp)4] (SOX/PX = 40.5),178 its uptake is lower than SAMM-3-Cu-OTf (420 mg g−1) and sql-1-Co-NCS (852 mg g−1). Last year, we unexpectedly found that a nonporous organic molecular solid can exhibit induced fit binding towards PX. Specifically, the host–guest complex of TPBD could only be formed with PX through a cp → ifp phase transformation, while OX, MX, and EB, were excluded (Fig. 12b).68 The induced fit binding enabled high selectivity over OX (SPX/OX = 76.1) which compares to the leading rigid, MAF-89 (SPX/OX = 221),179 and flexible, Mn-dhbq (SPX/OX = 76.9) adsorbents.180 Even though MAF-89 leads for SPX/OX, it was outperformed by TPBD for selectivity towards MX (SPX/MX = 22.1 for TPBD). The induced fit binding mechanism also enabled relatively high gravimetric uptake by TPBD (160 mg g−1) and recyclable separation of C8 isomers.The sum of this work suggests that flexible adsorbents not only have the potential for highly efficient hydrocarbon separation, but also that their ability to selectively bind guests through induced fit mechanisms could make flexible adsorbents uniquely suited for trace separations. Therefore, myth 4 can be addressed as follows:
Truth 4: “Just as in biochemistry, the induced fit mechanism in flexible adsorbents can result in highly selective binding in a manner that cannot be achieved by lock-and-key binding.”
Conclusions and perspectives
While rigid adsorbents have been studied and extensively optimised over the past 25 years, flexible adsorbents have only recently been regarded as serious candidates for utility. This contribution highlights how flexible adsorbents not only compete with traditional rigid adsorbents, but can surpass them if the right adsorbent is selected for the right application. This is because their inherent flexibility is accompanied by properties that cannot be exhibited by rigid adsorbents, such as enhanced working capacity, heat management and potential for induced fit binding. Although several of the concerns about flexible adsorbents persist, some flexible adsorbents are hydrolytically stable, recyclable, and exhibit benchmark selectivity towards specific guests. While some myths are still in play, e.g. the potential upper limit of working capacity, others can now be debunked. Notably, design and control principles for flexible adsorbents are now in hand and their properties can make them well-suited as ROSs and/or induced fit binding.That insights into the mechanisms of flexibility were gained from the discovery phase (Phase 1) has enabled deliberate design or screening of other Gen 1 flexible adsorbents. Although Gen 1 adsorbents are now widely available, their properties, particularly whether Pgo will occur in the desirable pressure range, remain difficult to predict. However, that tuning of Pgo has nowadays become controllable, underscores that significant progress has been achieved over the last decade. We are therefore perhaps now approaching the end of the structure/function phase (Phase 2) which deals with the predictability and reproducibility of properties. Further translation of the properties of some identified candidates is now required to broaden the scope of flexible adsorbents. Herein we see two key areas that require more work: (i) non-equilibrium cycling, which could potentially optimise adsorption kinetics and enhance productivity, has not been thoroughly investigated in flexible adsorbents; (ii) the potential of induced fit binding in flexible adsorbents for separations, which has been introduced only in the last five years, requires further insight given that adsorbents that can adapt to a specific adsorbate could offer superior performance compared to traditional rigid adsorbents.
We assert that we are now at the beginning of the “utility phase” but there remain unresolved challenges. The next major step for flexible adsorbents is not only to make them better, but also cheaper and greener, as these are prerequisites for large-scale applications that might address global challenges. In terms of formulation of flexible adsorbents for practical applications, two areas require attention. First, the effect of particle size, which directly impacts not just adsorbent kinetics, but in some cases whether flexibility can occur in the first place. Second, current methods for screening adsorbents are time-consuming, being reliant on serial sorption experiments or computational simulations that can take weeks or months. It is therefore essential to develop faster, more efficient screening methods to accelerate the discovery and optimisation of flexible adsorbents especially as many existing structures deposited in the CSD may exhibit flexibility and remain to be investigated.
To conclude, a few promising flexible adsorbents with benchmark properties have now been identified, indicating that others with equal or even better performance remain to be discovered. The potential utility of flexible adsorbents could extend well beyond initial interest in ANG, including into societally important areas such as AWH and hydrocarbon separations. We therefore feel that it is time to challenge the misconceptions that surround flexible adsorbents and encourage a paradigm shift in terms of both scientific study and technological utility.
Data availability
There are no data to support this Feature Article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Research Ireland (SFI Awards 16/IA/4624 and 12/RC/2275_P2), the Irish Research Council (IRCLA/2019/167) and the European Research Council (ADG 885695). The authors thank Dr. Xia Li, Dr. Shao-Min Wang, and Prof. Qing-Yuan Yang for sharing the isotherm data for sql-(azpy)(pdia)-Ni, X-dia-6-Ni and X-dia-1-Ni.References
- adsorbent, International Union of Pure and Applied Chemistry (IUPAC), 3.0.1 edn, 2019 DOI:10.1351/goldbook.A00153.
- A. I. Osman, M. Hefny, M. Abdel Maksoud, A. M. Elgarahy and D. W. Rooney, Environ. Chem. Lett., 2021, 19, 797–849 CrossRef CAS.
- K. Gaj, Clean Technol. Environ. Policy, 2017, 19, 2181–2189 CrossRef CAS.
- Q. Sun, B. Aguila, Y. Song and S. Ma, Acc. Chem. Res., 2020, 53, 812–821 CrossRef CAS PubMed.
- A. M. A. Pintor, V. J. P. Vilar, C. M. S. Botelho and R. A. R. Boaventura, Chem. Eng. J., 2016, 297, 229–255 CrossRef CAS.
- E. S. Sanz-Pérez, C. R. Murdock, S. A. Didas and C. W. Jones, Chem. Rev., 2016, 116, 11840–11876 CrossRef PubMed.
- X. Han, S. Yang and M. Schröder, Nat. Rev. Chem., 2019, 3, 108–118 Search PubMed.
- Y. Tu, R. Wang, Y. Zhang and J. Wang, Joule, 2018, 2, 1452–1475 Search PubMed.
- Y. Lin, C. Kong, Q. Zhang and L. Chen, Adv. Energy Mater., 2017, 7, 1601296 CrossRef.
- J. Yang, A. Sudik, C. Wolverton and D. J. Siegel, Chem. Soc. Rev., 2010, 39, 656–675 RSC.
- physisorption, International Union of Pure and Applied Chemistry (IUPAC), 3.0.1 edn, 2019 DOI:10.1351/goldbook.P04667.
- chemisorption, International Union of Pure and Applied Chemistry (IUPAC), 3.0.1 edn, 2019 DOI:10.1351/goldbook.C01048.
- P. Jacobs, E. M. Flanigen, J. Jansen and H. van Bekkum, Introduction to zeolite science and practice, Elsevier, 2001 Search PubMed.
- S. Kaskel, The Chemistry of metal-organic frameworks, 2 volume set: synthesis, characterization, and applications, John Wiley & Sons, 2016 Search PubMed.
- R.-B. Lin, Y. He, P. Li, H. Wang, W. Zhou and B. Chen, Chem. Soc. Rev., 2019, 48, 1362–1389 RSC.
- M. P. Moghadasnia, B. J. Eckstein, H. R. Martin, J. U. Paredes and C. M. McGuirk, Cryst. Growth Des., 2024, 24, 2304–2321 CrossRef CAS.
- porosity, International Union of Pure and Applied Chemistry (IUPAC), 3.0.1 edn, 2019 DOI:10.1351/goldbook.P04762.
- J. Davies, W. Kemula, H. Powell and N. Smith, J. Inclusion Phenom., 1983, 1, 3–44 Search PubMed.
- T. L. Brown, H. E. LeMay, B. E. Bursten and L. S. Brunauer, Chemistry: the central science, Prentice Hall, Englewood Cliffs, NJ, 1997 Search PubMed.
- M. Khraisheh, S. Mukherjee, A. Kumar, F. Al Momani, G. Walker and M. J. Zaworotko, J. Environ. Manage., 2020, 255, 109874 CrossRef CAS PubMed.
- K. Unger, Angew. Chem., Int. Ed. Engl., 1972, 11, 267–278 CrossRef CAS.
- R. M. Barrer and V. H. Shanson, J. Chem. Soc., Chem. Commun., 1976, 333–334 Search PubMed.
- R. M. Barrer and D. W. Riley, J. Chem. Soc., 1948, 133–143 RSC.
- A. F. Cronstedt, J. L. Schlenker and G. H. Kühl, 1993.
- H. d S. Claire-Deville, Comptes Rendus, 1862, 54, 324–327 Search PubMed.
- Database of Zeolite Structures, International Zeolite Association, https://www.iza-structure.org/databases/, (accessed 12/08/2024).
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- S. Kitagawa, R. Kitaura and S. I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- S. R. Batten, N. R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O’Keeffe, M. P. Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715–1724 Search PubMed.
- S. Mukherjee and M. J. Zaworotko, Trends Chem., 2020, 2, 506–518 Search PubMed.
- B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629–1658 CrossRef CAS PubMed.
- B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1990, 112, 1546–1554 CrossRef CAS.
- B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1989, 111, 5962–5964 CrossRef CAS.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS PubMed.
- M. Kondo, T. Yoshitomi, H. Matsuzaka, S. Kitagawa and K. Seki, Angew. Chem., Int. Ed. Engl., 1997, 36, 1725–1727 CrossRef CAS.
- W. Mori, F. Inoue, K. Yoshida, H. Nakayama, S. Takamizawa and M. Kishita, Chem. Lett., 1997, 1219–1220 Search PubMed.
- H. Li, M. Eddaoudi, T. L. Groy and O. M. Yaghi, J. Am. Chem. Soc., 1998, 120, 8571–8572 CrossRef CAS.
- K. S. Sing, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80–84 CrossRef CAS PubMed.
- S. A. Allison and R. M. Barrer, J. Chem. Soc. A, 1969, 1717–1723 RSC.
- S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695–704 CrossRef CAS PubMed.
- S. Kitagawa and M. Kondo, Bull. Chem. Soc. Jpn., 1998, 71, 1739–1753 CrossRef CAS.
- D. Li and K. Kaneko, Chem. Phys. Lett., 2001, 335, 50–56 CrossRef CAS.
- S.-Q. Wang, S. Mukherjee and M. J. Zaworotko, Faraday Discuss., 2021, 231, 9–50 RSC.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B, 2016, 72, 171–179 CrossRef CAS PubMed.
- N. Hanikel, X. Pei, S. Chheda, H. Lyu, W. Jeong, J. Sauer, L. Gagliardi and O. M. Yaghi, Science, 2021, 374, 454–459 CrossRef CAS PubMed.
- S. Krause, N. Hosono and S. Kitagawa, Angew. Chem., Int. Ed., 2020, 59, 15325–15341 CrossRef CAS PubMed.
- Q.-Y. Yang, P. Lama, S. Sen, M. Lusi, K.-J. Chen, W.-Y. Gao, M. Shivanna, T. Pham, N. Hosono, S. Kusaka, J. J. Perry IV, S. Ma, B. Space, L. J. Barbour, S. Kitagawa and M. J. Zaworotko, Angew. Chem., Int. Ed., 2018, 57, 5684–5689 CrossRef CAS PubMed.
- K. S. W. Sing, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- N. Klein, C. Herzog, M. Sabo, I. Senkovska, J. Getzschmann, S. Paasch, M. R. Lohe, E. Brunner and S. Kaskel, Phys. Chem. Chem. Phys., 2010, 12, 11778–11784 RSC.
- S. Watanabe, H. Sugiyama, H. Adachi, H. Tanaka and M. T. Miyahara, J. Chem. Phys., 2009, 130, 164707 CrossRef PubMed.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Chem. Soc. Rev., 2014, 43, 6062–6096 RSC.
- J. H. Lee, S. Jeoung, Y. G. Chung and H. R. Moon, Coord. Chem. Rev., 2019, 389, 161–188 CrossRef CAS.
- C. R. Murdock, B. C. Hughes, Z. Lu and D. M. Jenkins, Coord. Chem. Rev., 2014, 258–259, 119–136 CrossRef CAS.
- Y. Li, Y. Wang, W. Fan and D. Sun, Dalton Trans., 2022, 51, 4608–4618 RSC.
- K. Seki, Phys. Chem. Chem. Phys., 2002, 4, 1968–1971 RSC.
- Y. Sakata, S. Furukawa, M. Kondo, K. Hirai, N. Horike, Y. Takashima, H. Uehara, N. Louvain, M. Meilikhov, T. Tsuruoka, S. Isoda, W. Kosaka, O. Sakata and S. Kitagawa, Science, 2013, 339, 193–196 CrossRef CAS PubMed.
- R. Kitaura, K. Fujimoto, S.-I. Noro, M. Kondo and S. Kitagawa, Angew. Chem., Int. Ed., 2002, 41, 133 CrossRef CAS PubMed.
- C. Serre, F. Millange, C. Thouvenot, M. Noguès, G. Marsolier, D. Louër and G. Férey, J. Am. Chem. Soc., 2002, 124, 13519–13526 CrossRef CAS PubMed.
- J. L. Atwood, L. J. Barbour, A. Jerga and B. L. Schottel, Science, 2002, 298, 1000–1002 CrossRef CAS PubMed.
- L. Hamon, P. L. Llewellyn, T. Devic, A. Ghoufi, G. Clet, V. Guillerm, G. D. Pirngruber, G. Maurin, C. Serre, G. Driver, W. van Beek, E. Jolimaître, A. Vimont, M. Daturi and G. Férey, J. Am. Chem. Soc., 2009, 131, 17490–17499 CrossRef CAS PubMed.
- J. A. Mason, J. Oktawiec, M. K. Taylor, M. R. Hudson, J. Rodriguez, J. E. Bachman, M. I. Gonzalez, A. Cervellino, A. Guagliardi, C. M. Brown, P. L. Llewellyn, N. Masciocchi and J. R. Long, Nature, 2015, 527, 357–361 CrossRef CAS PubMed.
- J. Y. Kim, L. Zhang, R. Balderas-Xicohténcatl, J. Park, M. Hirscher, H. R. Moon and H. Oh, J. Am. Chem. Soc., 2017, 139, 17743–17746 Search PubMed.
- S.-Q. Wang, S. Mukherjee, E. Patyk-Kaźmierczak, S. Darwish, A. Bajpai, Q.-Y. Yang and M. J. Zaworotko, Angew. Chem., Int. Ed., 2019, 58, 6630–6634 Search PubMed.
- M. Shivanna, K.-i Otake, B.-Q. Song, L. M. van Wyk, Q.-Y. Yang, N. Kumar, W. K. Feldmann, T. Pham, S. Suepaul, B. Space, L. J. Barbour, S. Kitagawa and M. J. Zaworotko, Angew. Chem., Int. Ed., 2021, 60, 20383–20390 CrossRef CAS PubMed.
- L. Bondorf, J. L. Fiorio, V. Bon, L. Zhang, M. Maliuta, S. Ehrling, I. Senkovska, J. D. Evans, J.-O. Joswig, S. Kaskel, T. Heine and M. Hirscher, Sci. Adv., 2022, 8, eabn7035 CrossRef CAS PubMed.
- A. Subanbekova, V. I. Nikolayenko, A. A. Bezrukov, D. Sensharma, N. Kumar, D. J. O'Hearn, V. Bon, S.-Q. Wang, K. Koupepidou, S. Darwish, S. Kaskel and M. J. Zaworotko, J. Mater. Chem. A, 2023, 11, 9691–9699 RSC.
- M. Rahmani, C. R. M. O. Matos, S.-Q. Wang, A. A. Bezrukov, A. C. Eaby, D. Sensharma, Y. H. Andaloussi, M. Vandichel and M. J. Zaworotko, J. Am. Chem. Soc., 2023, 145, 27316–27324 CrossRef CAS PubMed.
- S.-M. Wang, M. Shivanna, S.-T. Zheng, T. Pham, K. A. Forrest, Q.-Y. Yang, Q. Guan, B. Space, S. Kitagawa and M. J. Zaworotko, J. Am. Chem. Soc., 2024, 146, 4153–4161 Search PubMed.
- L. T. Glasby, J. L. Cordiner, J. C. Cole and P. Z. Moghadam, Chem. Mater., 2024, 36, 9013–9030 CrossRef CAS PubMed.
- E. V. Alexandrov, V. A. Blatov, A. V. Kochetkov and D. M. Proserpio, CrystEngComm, 2011, 13, 3947–3958 Search PubMed.
- A. Schoedel, M. Li, D. Li, M. O’Keeffe and O. M. Yaghi, Chem. Rev., 2016, 116, 12466–12535 Search PubMed.
- D. J. O'Hearn, A. Bajpai and M. J. Zaworotko, Small, 2021, 17, 2006351 CrossRef PubMed.
- D. V. Soldatov, G. D. Enright and J. A. Ripmeester, Cryst. Growth Des., 2004, 4, 1185–1194 Search PubMed.
- S.-i Noro, K. Fukuhara, K. Sugimoto, Y. Hijikata, K. Kubo and T. Nakamura, Dalton Trans., 2013, 42, 11100–11110 RSC.
- H. M. Powell and J. H. Rayner, Nature, 1949, 163, 566–567 CrossRef CAS.
- W. D. Schaeffer, W. S. Dorsey, D. A. Skinner and C. G. Christian, J. Am. Chem. Soc., 1957, 79, 5870–5876 CrossRef CAS.
- A. V. Logan and D. W. Carle, J. Am. Chem. Soc., 1952, 74, 5224–5225 CrossRef CAS.
- S.-Q. Wang, S. Darwish, C. R. M. O. Matos, Z. M. Wong, A. Nalaparaju, Y. Luo, J. Zhu, X. Zhang, Z. Xu and M. J. Zaworotko, Inorg. Chem. Front., 2024, 11, 3254–3262 RSC.
- A. Subanbekova, A. A. Bezrukov, V. Bon, V. I. Nikolayenko, K. Koupepidou, D. Sensharma, S. Javan Nikkhah, S.-Q. Wang, S. Kaskel, M. Vandichel and M. J. Zaworotko, ACS Appl. Mater. Interfaces, 2024, 16, 24132–24140 CAS.
- B.-Q. Song, Q.-Y. Yang, S.-Q. Wang, M. Vandichel, A. Kumar, C. Crowley, N. Kumar, C.-H. Deng, V. GasconPerez, M. Lusi, H. Wu, W. Zhou and M. J. Zaworotko, J. Am. Chem. Soc., 2020, 142, 6896–6901 Search PubMed.
- K. Koupepidou, V. I. Nikolayenko, D. Sensharma, A. A. Bezrukov, M. Shivanna, D. C. Castell, S.-Q. Wang, N. Kumar, K.-I. Otake, S. Kitagawa and M. J. Zaworotko, Chem. Mater., 2023, 35, 3660–3670 Search PubMed.
- K. Koupepidou, V. I. Nikolayenko, D. Sensharma, A. A. Bezrukov, M. Vandichel, S. J. Nikkhah, D. C. Castell, K. A. Oyekan, N. Kumar, A. Subanbekova, W. G. Vandenberghe, K. Tan, L. J. Barbour and M. J. Zaworotko, J. Am. Chem. Soc., 2023, 145, 10197–10207 CrossRef CAS PubMed.
- Y. H. Andaloussi, D. Sensharma, A. A. Bezrukov, D. C. Castell, T. He, S. Darwish and M. J. Zaworotko, Cryst. Growth Des., 2024, 24, 2573–2579 Search PubMed.
- K. Koupepidou, A. A. Bezrukov, D. C. Castell, D. Sensharma, S. Mukherjee and M. J. Zaworotko, Chem. Commun., 2023, 59, 13867–13870 RSC.
- V. I. Nikolayenko, D. C. Castell, D. Sensharma, M. Shivanna, L. Loots, K. A. Forrest, C. J. Solanilla-Salinas, K.-I. Otake, S. Kitagawa, L. J. Barbour, B. Space and M. J. Zaworotko, Nat. Chem., 2023, 15, 542–549 CrossRef CAS PubMed.
- X. Li, D. Sensharma, K. Koupepidou, X.-J. Kong and M. J. Zaworotko, ACS Mater. Lett., 2023, 5, 2567–2575 CrossRef CAS PubMed.
- F. Salles, G. Maurin, C. Serre, P. L. Llewellyn, C. Knöfel, H. J. Choi, Y. Filinchuk, L. Oliviero, A. Vimont, J. R. Long and G. Férey, J. Am. Chem. Soc., 2010, 132, 13782–13788 CrossRef CAS PubMed.
- K. Barthelet, J. Marrot, D. Riou and G. Férey, Angew. Chem., Int. Ed., 2002, 41, 281–284 CrossRef CAS PubMed.
- R. A. Klein, S. Shulda, P. A. Parilla, P. Le Magueres, R. K. Richardson, W. Morris, C. M. Brown and C. M. McGuirk, Chem. Sci., 2021, 12, 15620–15631 RSC.
- A.-X. Zhu, Q.-Y. Yang, A. Kumar, C. Crowley, S. Mukherjee, K.-J. Chen, S.-Q. Wang, D. O′Nolan, M. Shivanna and M. J. Zaworotko, J. Am. Chem. Soc., 2018, 140, 15572–15576 CrossRef CAS PubMed.
- S. Ehrling, I. Senkovska, V. Bon, J. D. Evans, P. Petkov, Y. Krupskaya, V. Kataev, T. Wulf, A. Krylov, A. Vtyurin, S. Krylova, S. Adichtchev, E. Slyusareva, M. S. Weiss, B. Büchner, T. Heine and S. Kaskel, J. Mater. Chem. A, 2019, 7, 21459–21475 RSC.
- L. Abylgazina, I. Senkovska, S. Ehrling, V. Bon, P. St. Petkov, J. D. Evans, S. Krylova, A. Krylov and S. Kaskel, CrystEngComm, 2021, 23, 538–549 Search PubMed.
- X. Li, D. Sensharma, L. Loots, S. Geng, S. J. Nikkhah, E. Lin, V. Bon, W. Liu, Z. Wang, T. He, S. Mukherjee, M. Vandichel, S. Kaskel, L. J. Barbour, Z. Zhang and M. J. Zaworotko, J. Am. Chem. Soc., 2024, 146, 18387–18395 Search PubMed.
- S.-Q. Wang, V. Bon, S. Darwish, S.-M. Wang, Q.-Y. Yang, Z. Xu, S. Kaskel and M. J. Zaworotko, ACS Mater. Lett., 2024, 6, 666–673 Search PubMed.
- X. Li, D. Sensharma, V. I. Nikolayenko, S. Darwish, A. A. Bezrukov, N. Kumar, W. Liu, X.-J. Kong, Z. Zhang and M. J. Zaworotko, Chem. Mater., 2023, 35, 783–791 CrossRef CAS PubMed.
- A. Halder and C. M. McGuirk, Cryst. Growth Des., 2024, 24, 1200–1213 CrossRef CAS.
- A. Halder, R. A. Klein, S. Shulda, G. A. McCarver, P. A. Parilla, H. Furukawa, C. M. Brown and C. M. McGuirk, J. Am. Chem. Soc., 2023, 145, 8033–8042 CrossRef CAS PubMed.
- C. M. McGuirk, T. Runčevski, J. Oktawiec, A. Turkiewicz, M. K. Taylor and J. R. Long, J. Am. Chem. Soc., 2018, 140, 15924–15933 CrossRef CAS PubMed.
- M. K. Taylor, T. E. Runčevski, J. Oktawiec, M. I. Gonzalez, R. L. Siegelman, J. A. Mason, J. Ye, C. M. Brown and J. R. Long, J. Am. Chem. Soc., 2016, 138, 15019–15026 Search PubMed.
- A.-X. Zhu, Q.-Y. Yang, S. Mukherjee, A. Kumar, C.-H. Deng, A. A. Bezrukov, M. Shivanna and M. J. Zaworotko, Angew. Chem., Int. Ed., 2019, 58, 18212–18217 CrossRef CAS PubMed.
- B.-Q. Song, M. Shivanna, M.-Y. Gao, S.-Q. Wang, C.-H. Deng, Q.-Y. Yang, S. J. Nikkhah, M. Vandichel, S. Kitagawa and M. J. Zaworotko, Angew. Chem., Int. Ed., 2023, 62, e202309985 CrossRef CAS PubMed.
- V. I. Nikolayenko, D. C. Castell, D. Sensharma, M. Shivanna, L. Loots, K.-I. Otake, S. Kitagawa, L. J. Barbour and M. J. Zaworotko, J. Mater. Chem. A, 2023, 11, 16019–16026 RSC.
- S.-Q. Wang, S. Darwish, D. Sensharma and M. J. Zaworotko, Adv. Mater., 2022, 3, 1240–1247 RSC.
- Global Carbon Budget, https://globalcarbonbudget.org/, (accessed 18/06/2024).
- World Bank Group, https://data.worldbank.org/, (accessed 18/06/2024).
- H. R. A. P. Rosado, Fossil fuels, https://ourworldindata.org/fossil-fuels) Search PubMed.
- A. Schoedel, Z. Ji and O. M. Yaghi, Nat. Energy, 2016, 1, 16034 Search PubMed.
- H. Li, L. Li, R.-B. Lin, W. Zhou, Z. Zhang, S. Xiang and B. Chen, EnergyChem, 2019, 1, 100006 Search PubMed.
- J. Kubesh, S. R. King and W. E. Liss, Effect of gas composition on octane number of natural gas fuels, Report 0148-7191, SAE Technical Paper, 1992.
- NIST Chemistry WebBook, https://webbook.nist.gov/cgi/cbook.cgi?ID=C74828&Mask=2, (accessed 18/06/2024) Search PubMed.
- U.S. Department of Energy, https://www.energy.gov/, (accessed 18/06/2024).
- C. M. Simon, J. Kim, D. A. Gomez-Gualdron, J. S. Camp, Y. G. Chung, R. L. Martin, R. Mercado, M. W. Deem, D. Gunter, M. Haranczyk, D. S. Sholl, R. Q. Snurr and B. Smit, Energy Environ. Sci., 2015, 8, 1190–1199 RSC.
- S. i Noro, S. Kitagawa, M. Kondo and K. Seki, Angew. Chem., Int. Ed., 2000, 39, 2081–2084 CrossRef PubMed.
- Y. Peng, V. Krungleviciute, I. Eryazici, J. T. Hupp, O. K. Farha and T. Yildirim, J. Am. Chem. Soc., 2013, 135, 11887–11894 CrossRef CAS PubMed.
- P. L. Llewellyn, P. Horcajada, G. Maurin, T. Devic, N. Rosenbach, S. Bourrelly, C. Serre, D. Vincent, S. Loera-Serna, Y. Filinchuk and G. Férey, J. Am. Chem. Soc., 2009, 131, 13002–13008 Search PubMed.
- S.-M. Wang, M. Shivanna, P. Lama, Q.-Y. Yang, L. J. Barbour and M. J. Zaworotko, ChemSusChem, 2023, 16, e202300069 CrossRef CAS PubMed.
- T. Kundu, B. B. Shah, L. Bolinois and D. Zhao, Chem. Mater., 2019, 31, 2842–2847 Search PubMed.
- B. Li, H.-M. Wen, H. Wang, H. Wu, M. Tyagi, T. Yildirim, W. Zhou and B. Chen, J. Am. Chem. Soc., 2014, 136, 6207–6210 Search PubMed.
- Y. Lim, B. Kim and J. Kim, Chem. Mater., 2024, 36, 5465–5473 Search PubMed.
- M. K. Taylor, T. Runčevski, J. Oktawiec, M. I. Gonzalez, R. L. Siegelman, J. A. Mason, J. Ye, C. M. Brown and J. R. Long, J. Am. Chem. Soc., 2016, 138, 15019–15026 CrossRef CAS PubMed.
- M. M. Mekonnen and A. Y. Hoekstra, Sci. Adv., 2016, 2, e1500323 Search PubMed.
- M. Wang, B. L. Bodirsky, R. Rijneveld, F. Beier, M. P. Bak, M. Batool, B. Droppers, A. Popp, M. T. H. van Vliet and M. Strokal, Nat. Commun., 2024, 15, 880 CrossRef CAS PubMed.
- P. H. Gleick., Encyclopedia of climate, weather, 1996, pp. 817–823 Search PubMed.
- Y. Zhang, L. Wu, X. Wang, J. Yu and B. Ding, Nat. Commun., 2020, 11, 3302 CrossRef CAS PubMed.
- B. Li, L. Hua, Y. Tu and R. Wang, Joule, 2019, 3, 1427–1436 CrossRef CAS.
- K. H. Cho, D. D. Borges, U. H. Lee, J. S. Lee, J. W. Yoon, S. J. Cho, J. Park, W. Lombardo, D. Moon, A. Sapienza, G. Maurin and J.-S. Chang, Nat. Commun., 2020, 11, 5112 CrossRef CAS PubMed.
- S. Wang, J. S. Lee, M. Wahiduzzaman, J. Park, M. Muschi, C. Martineau-Corcos, A. Tissot, K. H. Cho, J. Marrot, W. Shepard, G. Maurin, J.-S. Chang and C. Serre, Nat. Energy, 2018, 3, 985–993 CrossRef CAS.
- M. F. de Lange, K. J. F. M. Verouden, T. J. H. Vlugt, J. Gascon and F. Kapteijn, Chem. Rev., 2015, 115, 12205–12250 CrossRef CAS PubMed.
- A. Karmakar, V. Prabakaran, D. Zhao and K. J. Chua, Appl. Energy, 2020, 269, 115070 Search PubMed.
- E.-P. Ng and S. Mintova, Microporous Mesoporous Mater., 2008, 114, 1–26 Search PubMed.
- X. Zhang and L. Qiu, Energy Convers. Manage., 2007, 48, 320–326 CrossRef CAS.
- A. A. Bezrukov, D. J. O’Hearn, V. Gascón-Pérez, S. Darwish, A. Kumar, S. Sanda, N. Kumar, K. Francis and M. J. Zaworotko, Cell Rep. Phys. Sci., 2023, 4, 101252 Search PubMed.
- Q. Min Wang, D. Shen, M. Bülow, M. Ling Lau, S. Deng, F. R. Fitch, N. O. Lemcoff and J. Semanscin, Microporous Mesoporous Mater., 2002, 55, 217–230 Search PubMed.
- S. Bourrelly, B. Moulin, A. Rivera, G. Maurin, S. Devautour-Vinot, C. Serre, T. Devic, P. Horcajada, A. Vimont, G. Clet, M. Daturi, J.-C. Lavalley, S. Loera-Serna, R. Denoyel, P. L. Llewellyn and G. Férey, J. Am. Chem. Soc., 2010, 132, 9488–9498 CrossRef CAS PubMed.
- F. Jeremias, D. Fröhlich, C. Janiak and S. K. Henninger, RSC Adv., 2014, 4, 24073–24082 Search PubMed.
- D. Fröhlich, E. Pantatosaki, P. D. Kolokathis, K. Markey, H. Reinsch, M. Baumgartner, M. A. van der Veen, D. E. De Vos, N. Stock, G. K. Papadopoulos, S. K. Henninger and C. Janiak, J. Mater. Chem. A, 2016, 4, 11859–11869 Search PubMed.
- A. Cadiau, J. S. Lee, D. Damasceno Borges, P. Fabry, T. Devic, M. T. Wharmby, C. Martineau, D. Foucher, F. Taulelle, C.-H. Jun, Y. K. Hwang, N. Stock, M. F. De Lange, F. Kapteijn, J. Gascon, G. Maurin, J.-S. Chang and C. Serre, Adv. Mater., 2015, 27, 4775–4780 Search PubMed.
- N. Hanikel, M. S. Prévot, F. Fathieh, E. A. Kapustin, H. Lyu, H. Wang, N. J. Diercks, T. G. Glover and O. M. Yaghi, ACS Cent. Sci., 2019, 5, 1699–1706 CrossRef CAS PubMed.
- M. J. Zaworotko, V. Gascón-Pérez, A. A. Bezrukov, D. J. O'Hearn and S.-Q. Wang, US20200030737A1, 2020.
- B. Rather and M. J. Zaworotko, Chem. Commun., 2003, 830–831, 10.1039/B301219K.
- M. Shivanna, A. A. Bezrukov, V. Gascón-Pérez, K.-I. Otake, S. Sanda, D. J. O’Hearn, Q.-Y. Yang, S. Kitagawa and M. J. Zaworotko, ACS Appl. Mater. Interfaces, 2022, 14, 39560–39566 CrossRef CAS PubMed.
- Z. Lu, J. Duan, L. Du, Q. Liu, N. M. Schweitzer and J. T. Hupp, J. Mater. Chem. A, 2022, 10, 6442–6447 RSC.
- A. A. Bezrukov, D. J. O’Hearn, V. Gascón-Pérez, C. R. M. O. Matos, K. Koupepidou, S. Darwish, S. Sanda, N. Kumar, X. Li, M. Shivanna and M. J. Zaworotko, Chem, 2024, 10, 1458–1470 CAS.
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef PubMed.
- S. Mukherjee, Y. He, D. Franz, S.-Q. Wang, W.-R. Xian, A. A. Bezrukov, B. Space, Z. Xu, J. He and M. J. Zaworotko, Chem. – Eur. J., 2020, 26, 4881 CrossRef.
- T.-L. Hu, H. Wang, B. Li, R. Krishna, H. Wu, W. Zhou, Y. Zhao, Y. Han, X. Wang, W. Zhu, Z. Yao, S. Xiang and B. Chen, Nat. Commun., 2015, 6, 7328 Search PubMed.
- Q. Wang, J. Hu, L. Yang, Z. Zhang, T. Ke, X. Cui and H. Xing, Nat. Commun., 2022, 13, 2955 Search PubMed.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- J.-X. Wang, C.-C. Liang, X.-W. Gu, H.-M. Wen, C. Jiang, B. Li, G. Qian and B. Chen, J. Mater. Chem. A, 2022, 10, 17878–17916 RSC.
- X. Zhao, Y. Wang, D.-S. Li, X. Bu and P. Feng, Adv. Mater., 2018, 30, 1705189 Search PubMed.
- T. Wang, E. Lin, Y.-L. Peng, Y. Chen, P. Cheng and Z. Zhang, Coord. Chem. Rev., 2020, 423, 213485 Search PubMed.
- B. Li and B. Chen, Chem, 2016, 1, 669–671 Search PubMed.
- R. Matsuda, R. Kitaura, S. Kitagawa, Y. Kubota, R. V. Belosludov, T. C. Kobayashi, H. Sakamoto, T. Chiba, M. Takata, Y. Kawazoe and Y. Mita, Nature, 2005, 436, 238–241 Search PubMed.
- S. Xiang, Y. He, Z. Zhang, H. Wu, W. Zhou, R. Krishna and B. Chen, Nat. Commun., 2012, 3, 954 CrossRef PubMed.
- D. Britt, H. Furukawa, B. Wang, T. G. Glover and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 20637–20640 CrossRef CAS PubMed.
- S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870–10871 CrossRef CAS PubMed.
- D. O’Nolan, A. Kumar, K.-J. Chen, S. Mukherjee, D. G. Madden and M. J. Zaworotko, ACS Appl. Nano Mater., 2018, 1, 6000–6004 Search PubMed.
- R. U. Lemieux and U. Spohr, Adv. Carbohydr. Chem. Biochem., 1994, 50, 9–13 Search PubMed.
- N. Punekar, Enzymes: catalysis, kinetics and mechanisms, Springer, 2018 Search PubMed.
- P. G. Boyd, A. Chidambaram, E. García-Díez, C. P. Ireland, T. D. Daff, R. Bounds, A. Gładysiak, P. Schouwink, S. M. Moosavi, M. M. Maroto-Valer, J. A. Reimer, J. A. R. Navarro, T. K. Woo, S. Garcia, K. C. Stylianou and B. Smit, Nature, 2019, 576, 253–256 Search PubMed.
- T. Li, X. Jia, H. Chen, Z. Chang, L. Li, Y. Wang and J. Li, ACS Appl. Mater. Interfaces, 2022, 14, 15830–15839 CrossRef CAS PubMed.
- H. Zeng, X.-J. Xie, M. Xie, Y.-L. Huang, D. Luo, T. Wang, Y. Zhao, W. Lu and D. Li, J. Am. Chem. Soc., 2019, 141, 20390–20396 CrossRef CAS PubMed.
- N. Kumar, S. Mukherjee, N. C. Harvey-Reid, A. A. Bezrukov, K. Tan, V. Martins, M. Vandichel, T. Pham, L. M. van Wyk, K. Oyekan, A. Kumar, K. A. Forrest, K. M. Patil, L. J. Barbour, B. Space, Y. Huang, P. E. Kruger and M. J. Zaworotko, Chem, 2021, 7, 3085–3098 CAS.
- I. Wong, S. S. Patel and K. A. Johnson, Biochemistry, 1991, 30, 526–537 CrossRef CAS PubMed.
- K. A. Johnson, J. Biol. Chem., 2008, 283, 26297–26301 CrossRef CAS PubMed.
- H. Zeng, M. Xie, Y.-L. Huang, Y. Zhao, X.-J. Xie, J.-P. Bai, M.-Y. Wan, R. Krishna, W. Lu and D. Li, Angew. Chem., Int. Ed., 2019, 58, 8515–8519 CrossRef CAS PubMed.
- Y.-L. Peng, T. Pham, P. Li, T. Wang, Y. Chen, K.-J. Chen, K. A. Forrest, B. Space, P. Cheng, M. J. Zaworotko and Z. Zhang, Angew. Chem., Int. Ed., 2018, 57, 10971–10975 CrossRef CAS PubMed.
- H. S. Scott, M. Shivanna, A. Bajpai, D. G. Madden, K.-J. Chen, T. Pham, K. A. Forrest, A. Hogan, B. Space, J. J. Perry Iv and M. J. Zaworotko, ACS Appl. Mater. Interfaces, 2017, 9, 33395–33400 Search PubMed.
- C. Gücüyener, J. van den Bergh, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2010, 132, 17704–17706 CrossRef PubMed.
- L. Li, R.-B. Lin, R. Krishna, H. Li, S. Xiang, H. Wu, J. Li, W. Zhou and B. Chen, Science, 2018, 362, 443–446 Search PubMed.
- R.-B. Lin, H. Wu, L. Li, X.-L. Tang, Z. Li, J. Gao, H. Cui, W. Zhou and B. Chen, J. Am. Chem. Soc., 2018, 140, 12940–12946 Search PubMed.
- B. Zhu, J.-W. Cao, S. Mukherjee, T. Pham, T. Zhang, T. Wang, X. Jiang, K. A. Forrest, M. J. Zaworotko and K.-J. Chen, J. Am. Chem. Soc., 2021, 143, 1485–1492 Search PubMed.
- J. Pei, J.-X. Wang, K. Shao, Y. Yang, Y. Cui, H. Wu, W. Zhou, B. Li and G. Qian, J. Mater. Chem. A, 2020, 8, 3613–3620 Search PubMed.
- S. Mukherjee, D. Sensharma, K.-J. Chen and M. J. Zaworotko, Chem. Commun., 2020, 56, 10419–10441 RSC.
- P.-Q. Liao, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Nat. Commun., 2015, 6, 8697 CrossRef PubMed.
- A. M. Kałuża, S. Mukherjee, S.-Q. Wang, D. J. O’Hearn and M. J. Zaworotko, Chem. Commun., 2020, 56, 1940–1943 Search PubMed.
- M. Lusi and L. J. Barbour, Angew. Chem., Int. Ed., 2012, 51, 3928–3931 CrossRef CAS PubMed.
- Z.-M. Ye, X.-F. Zhang, D.-X. Liu, Y.-T. Xu, C. Wang, K. Zheng, D.-D. Zhou, C.-T. He and J.-P. Zhang, Sci. China Chem., 2022, 65, 1552–1558 CrossRef CAS.
- L. Li, L. Guo, D. H. Olson, S. Xian, Z. Zhang, Q. Yang, K. Wu, Y. Yang, Z. Bao, Q. Ren and J. Li, Science, 2022, 377, 335–339 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |