 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heterogeneous copper-catalyzed Grignard reactions with allylic substrates†
Shobhan
Mondal
a,
Luca
Deiana
b,
Armando
Córdova
 *b,
Haibo
Wu
*b,
Haibo
Wu
 *a and
Jan-E.
Bäckvall
*a and
Jan-E.
Bäckvall
 *ab
*ab
aDepartment of Organic Chemistry, Arrhenius Laboratory, Stockholm University, Stockholm, SE-10691, Sweden. E-mail: haibo.wu@su.se; jeb@organ.su.se
bDepartment of Natural Sciences, Mid Sweden University, Holmgatan 10, Sundsvall, 85179, Sweden. E-mail: armando.cordova@miun.se
First published on 15th January 2025
Abstract
Herein, we present a highly efficient allylic substitution of carbonates with Grignard reagents using a reusable cellulose-supported nanocopper catalyst. This approach highlights the first instance of heterogeneous catalysis for the cross-coupling of allylic alcohol substrates with Grignard reagents. The method features high yields, excellent regioselectivity, and complete chirality transfer.
Transition-metal-catalyzed allylic substitution reactions are fundamental tools for construction of carbon–carbon bonds in organic synthesis.1–3 In particular, the enantiocontrolled version of these reactions are widely employed for the preparation of various chiral α-substituted alkenes.2,4 Significant progress over the years has led to the development of stereoselective palladium-catalyzed allylic substitutions with stabilized carbon nucleophiles, such as malonate carbanions.5,6 In contrast, copper catalysis2,7–9 enables the use of non-stabilized carbon nucleophiles, such as Grignard and organozinc reagents, offering a complementary approach to palladium catalysis. Traditionally, these Cu-catalyzed cross-coupling reactions have predominantly relied on homogeneous catalysis (or the use of stoichiometric cuprates), and the reactivity, regioselectivity (SN2 versus SN2′), and stereospecificity are influenced by several factors such as leaving group, copper source, reaction conditions, solvent and ligand (Scheme 1A).10–14
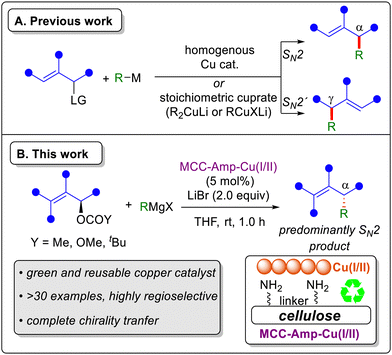 | ||
| Scheme 1 Copper-catalyzed cross-coupling of allylic substrates with Grignard reagents: (A) previous work. (B) This work. | ||
Growing concerns about the environment and climate change have driven chemists to develop more sustainable and environmentally benign methodologies.15 Compared with homogenous catalysis, heterogeneous catalysis presents a compelling alternative,16–18 offering advantages such as easier recovery, and reuse of metal catalysts as well as the production of cleaner products with lower heavy metal contamination.19 In our recent work, we developed a heterogeneous nanocopper catalyst supported on modified microcrystalline cellulose (MCC), which demonstrated excellent performance in Alder-ene cyclization20 and Crabbé type SN2′ propargylic substitution,21 both in terms of catalytic activity and sustainability. Expanding this sustainable system to a wider window of organic synthesis will be highly beneficial for a broader audience.
In the present study (Scheme 1B) we have developed a novel nanocopper-catalyzed Grignard reaction of allylic substrates leading to allylic substitution, where the previously reported heterogeneous nanocopper catalyst21 was successfully employed. We have demonstrated the practical advantages of the new heterogeneous catalytic method, including catalyst recovery and reuse, reduced heavy metal contamination, and improved environmental sustainability, offering a viable and greener alternative to traditional homogeneous methods.
The objective was to mimic the widely applied homogeneous copper-catalyzed allylic substitution reaction with Grignard reagents by the use of a heterogeneous nanocopper catalyst. Our initial investigations started by adapting the previously reported nanocopper catalytic system that was used with propargylic acetates.21 (E)-2-Nonenol derivatives were chosen as the model substrates together with n-butyl magnesium chloride. We were pleased to observe a complete conversion of 1a (LG = OAc), yielding 86% of 3a and 10% (E)-2-nonenol (Table 1, entry 1). Changing the leaving group of 1a to carbonate 1a′ (LG = OCO2Me) increased the yield to 98% without generating any significant side products (entry 2). Changing the additive to LiCl slightly decreased the yield (entry 3). Further control experiments (see ESI,† for details) indicate that LiBr plays a key role in enhancing the reaction, likely through the formation of more reactive Li-Grignard reagent complex (RMgX·LiBr).22 Performing the reaction at 0 °C resulted in a yield similar to that at room temperature (entry 4). Along with THF, diethyl ether also proved to be an efficient solvent for this allylic substitution reaction (entry 5). The use of the nanocopper catalyst was essential as its exclusion resulted in no product formation, with 65% of compound 1a′ remaining and with 35% of (E)-2-nonenol (entry 6) being formed. The presence of LiBr also proved important for reactivity as its omission reduced the yield to 54% along with 35% of 1a′ and 8% of alcohol 1-OH (entry 7). Employing a bulkier pivalate ester leaving group (1a′′) also proved to be highly efficient for this transformation (entry 8).
| Entry | LG | Conditions | Yield% (3a, 1, 1-OH) |
|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), LiBr (0.4 mmol) and THF (1.0 mL) were used under N2 atmosphere; yields were determined by 1H-NMR using 1,3,5-trimethoxybenzene as an internal standard. | |||
| 1 | OAc (1a) | Standard | 86, 0, 10 |
| 2 | OCO2Me (1a′) | Standard | 98, 0, 2 |
| 3 | OCO2Me (1a′) | LiCl instead of LiBr | 89, 7, 3 |
| 4 | OCO2Me (1a′) | At 0 °C | 95, 0, 3 |
| 5 | OCO2Me (1a′) | Et2O as solvent | 95, 0, 2 |
| 6 | OCO2Me (1a′) | No Cu catalyst | 0, 65, 35 |
| 7 | OCO2Me (1a′) | No LiBr additive | 54, 35, 8 |
| 8 | OPiv (1a′′) | Standard | 95, 0, 0 |
With the optimized reaction conditions in hand, the generality and scope of the protocol was explored (Scheme 2). Grignard reaction of allylic carbonate 1a afforded 3a with exclusive α-substitution in 88% yield. A similar yield (87%) of 3a was obtained when the reaction was carried on a gram-scale. Comparable high yields of 92% and 95% of shorter chain olefin products 3b and 3c, respectively, were obtained from the corresponding allylic carbonates (1b and 1c). An aliphatic secondary alcohol derivative (1d) was suitable for this protocol, providing the desired product 3d in 81% yield with minor amounts of γ-substituted product (α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ 10
γ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Extension of our methodology towards the cinnamyl system 1e produced styryl derivative 3e in 95% yield. A secondary cinnamyl carbonate 1f underwent the reaction smoothly, and gave the corresponding targeted olefin 3f in 95% yield. Then this reactivity was extended to the β-methyl substituted carbonate 1g, which afforded product 3g in 94% yield. The reaction was also extended to naturally abundant allylic alcohol analogs. The substituted product 3h, obtained from farnesol derivative 1h, was isolated in high yield. Likewise, phytol derived olefin 3i was obtained in 81% yield from the corresponding carbonate derivative 1i. Next, the carbonate derived from geraniol (1j) yielded the corresponding product 3j in 92% yield. The cis-2-nonenol derivative 1k was found to be amenable to the reaction conditions affording a mixture of internal and terminal (α
1). Extension of our methodology towards the cinnamyl system 1e produced styryl derivative 3e in 95% yield. A secondary cinnamyl carbonate 1f underwent the reaction smoothly, and gave the corresponding targeted olefin 3f in 95% yield. Then this reactivity was extended to the β-methyl substituted carbonate 1g, which afforded product 3g in 94% yield. The reaction was also extended to naturally abundant allylic alcohol analogs. The substituted product 3h, obtained from farnesol derivative 1h, was isolated in high yield. Likewise, phytol derived olefin 3i was obtained in 81% yield from the corresponding carbonate derivative 1i. Next, the carbonate derived from geraniol (1j) yielded the corresponding product 3j in 92% yield. The cis-2-nonenol derivative 1k was found to be amenable to the reaction conditions affording a mixture of internal and terminal (α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ 8
γ 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) olefins 3k. Interestingly, the cis configuration was maintained in the reaction. The protocol was applied to cyclic allylic derivatives as well. The six-membered olefin derivatives 3l–3n were obtained in high to excellent yield from 1l–1n respectively, while seven-membered olefin 3o was obtained in 93% yield from 1o. We applied this method to electron-rich cinnamyl derivatives 1p and 1q, constructing cinnamyl derivative 3p and 3q in 95% and 80% yield respectively. The reaction with the carbonate derived from 1-phenylprop-2-en-1-ol resulted in γ-substituted derivative 3e in 81% yield with a slight amount of α-substituted product (γ
1) olefins 3k. Interestingly, the cis configuration was maintained in the reaction. The protocol was applied to cyclic allylic derivatives as well. The six-membered olefin derivatives 3l–3n were obtained in high to excellent yield from 1l–1n respectively, while seven-membered olefin 3o was obtained in 93% yield from 1o. We applied this method to electron-rich cinnamyl derivatives 1p and 1q, constructing cinnamyl derivative 3p and 3q in 95% and 80% yield respectively. The reaction with the carbonate derived from 1-phenylprop-2-en-1-ol resulted in γ-substituted derivative 3e in 81% yield with a slight amount of α-substituted product (γ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α 15
α 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Subsequently, various Grignard reagents were examined under standard reaction conditions. Cyclic Grignard reagents like cyclopentyl (2b) and cyclohexyl (2c) were demonstrated to be efficient nucleophiles producing 3r and 3s, respectively in moderate to good yields. Also, aliphatic Grignard reagents (2d, 2e) proved to be equally effective as nucleophiles providing olefins 3t and 3u in high yields. Next, aromatic Grignard reagents with neutral and different ring substitution were employed for this substitution reactions. Excellent yields of 3v, 3w, 3x and 3y were attained when unsubstituted (2f), 4-chloro substituted (2g), 3-methoxy (2h), and 2-methyl (2i) substituted phenyl Grignard reagents, respectively, were utilized under these reaction conditions. The benzyl (2j) and 3-phenylpropyl Grignard reagent (2k) exhibited satisfactory efficiency, forming 3z and 3aa in 82% and 92% isolated yields respectively. Subsequently, the trimethyl silyl-based Grignard reagent (2l) demonstrated moderate reactivity, affording 3ab in 78% yield. However, employing the bulky tert-butyl magnesium chloride (2m) resulted in an excellent yield of 96%, nonetheless a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of α- and γ-substituted regioisomers was observed. In case of isopropyl Grignard reagent (2n), moderate yield of 3ad was observed with 20
1 mixture of α- and γ-substituted regioisomers was observed. In case of isopropyl Grignard reagent (2n), moderate yield of 3ad was observed with 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of regioisomers. In this instance, the cross-coupling reactions of a vinyl Grignard reagent (2o) with an allylic alcohol derivative (1a′), afforded 37% of non-conjugated diene derivative 3ae.
1 ratio of regioisomers. In this instance, the cross-coupling reactions of a vinyl Grignard reagent (2o) with an allylic alcohol derivative (1a′), afforded 37% of non-conjugated diene derivative 3ae.
Additionally, a cinnamyl carbonate derivative (1af) produced aryl substituted olefin 3af in 92% yield. A long alkyl chain Grignard reagent (2q) performed well with cinnamyl derivative (1p) under the developed protocol providing 3ag in high yield. Additionally, we applied our methodology to a previously reported drug precursor synthesis, successfully yielding desired compound 3ah in 81% yield.23
It is known that homogeneous copper catalysts offer transfer of chirality from enantioenriched allylic substrates,24 and we therefore wanted to mimic this property with the heterogeneous catalyst used here (Scheme 3). Enantiopure acetate derivative (1d′) of allylic alcohol was obtained via enzymatic kinetic resolution.25 Remarkably, under standard conditions, a complete chirality transfer was obtained for the corresponding allylic substitution product 3d along with a high yield (81%).
To further establish the sustainability of this protocol, we performed the model reactions with recycled catalyst several times using two different types of substrates (Scheme 4). In these cases, the catalyst recovered (washed with dry solvent) after each cycle via centrifugation was used directly for the next run. Both for carbonate (1a′) and pivalate (1a′′) derivatives of (E)-2-nonenol, the yields of 3a were found to be maintained at a high level for at least four cycles when butyl magnesium chloride was employed under the standard reaction conditions.
In conclusion, we have utilized a cellulose supported nanocopper-catalyst to mimic the reactivity of homogeneous copper catalysis in nucleophilic substitution of allylic alcohol derivatives with Grignard reagents. A wide range of allylic alcohol derivatives including natural products were well-tolerated, providing the corresponding α-substituted product in high yields. Additionally, a variety of commercially available Grignard reagents proved to be efficient for this reaction. The broader application includes the complete chirality transfer similar to that with homogeneous copper catalysis. The efficient nanocopper catalyst used here can be recovered and reused for several times providing a green alternative with low metal contamination. The present work has shown that the heterogeneous nanocopper catalyst can efficiently mimic the homogeneous copper catalytic system, and the extension to Grignard reactions with allylic substrates has further increased the utility of the MCC-Amp-Cu in organic synthesis.
The authors are grateful to the financial support from the Wallenberg Initiative Materials Science for Sustainability (WISE) funded by the Knut and Alice Wallenberg Foundation, Swedish Foundation for Strategic Environmental Research (Mistra: project Mistra SafeChem), (2018/11 and 2023/11), the Swedish Knowledge Foundation (Neopulp), and the Swedish Research Council (2022-03682, 2018-04425).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- Z. Lu and S. Ma, Angew. Chem., Int. Ed., 2008, 47, 258–297 CrossRef CAS.
- A. Alexakis, J.-E. Bäckvall, N. Krause, O. Pàmies and M. Diéguez, Chem. Rev., 2008, 108, 2796–2823 CrossRef CAS.
- N. A. Butt and W. Zhang, Chem. Soc. Rev., 2015, 44, 7929–7967 RSC.
- B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921–2944 CrossRef CAS.
- O. Pàmies, J. Margalef, S. Cañellas, J. James, E. Judge, P. J. Guiry, C. Moberg, J.-E. Bäckvall, A. Pfaltz, M. A. Pericàs and M. Diéguez, Chem. Rev., 2021, 121, 4373–4505 CrossRef.
- M. Majdecki, J. Jurczak and T. Bauer, ChemCatChem, 2015, 7, 799–807 CrossRef CAS.
- H. Yorimitsu and K. Oshima, Angew. Chem., Int. Ed., 2005, 44, 4435–4439 CrossRef CAS PubMed.
- N. Krause and A. Hoffmann-Röder, Modern Organocopper Chemistry, 2002, pp. 145–166 Search PubMed.
- S. Thapa, B. Shrestha, S. K. Gurung and R. Giri, Org. Biomol. Chem., 2015, 13, 4816–4827 RSC.
- J.-E. Bäckvall, M. Sellen and B. Grant, J. Am. Chem. Soc., 1990, 112, 6615–6621 CrossRef.
- J. Norinder and J.-E. Bäckvall, Chem. – Eur. J., 2007, 13, 4094–4102 CrossRef CAS PubMed.
- J.-E. Bäckvall, E. S. M. Persson and A. Bombrun, J. Org. Chem., 1994, 59, 4126–4130 CrossRef.
- E. S. M. Persson, M. van Klaveren, D. M. Grove, J.-E. Bäckvall and G. van Koten, Chem. – Eur. J., 1995, 1, 351–359 CrossRef CAS.
- S. Okamoto, S. Tominaga, N. Saino, K. Kase and K. Shimoda, J. Organomet. Chem., 2005, 690, 6001–6007 CrossRef CAS.
- P. Fantke, L. Huang, M. Overcash, E. Griffing and O. Jolliet, Green Chem., 2020, 22, 6008–6024 RSC.
- W.-J. Yoo, H. Ishitani, Y. Saito, B. Laroche and S. Kobayashi, J. Org. Chem., 2020, 85, 5132–5145 CrossRef CAS.
- K. Masuda, T. Ichitsuka, N. Koumura, K. Sato and S. Kobayashi, Tetrahedron, 2018, 74, 1705–1730 CrossRef CAS.
- S. Vásquez-Céspedes, R. C. Betori, M. A. Cismesia, J. K. Kirsch and Q. Yang, Org. Process Res. Dev., 2021, 25, 740–753 CrossRef.
- L. Järup, Br. Med. Bull., 2003, 68, 167–182 CrossRef PubMed.
- Z. Zheng, L. Deiana, D. Posevins, A. A. Rafi, K. Zhang, M. J. Johansson, C.-W. Tai, A. Córdova and J.-E. Bäckvall, ACS Catal., 2022, 12, 1791–1796 CrossRef CAS PubMed.
- H. Wu, Z. Zheng, K. Zhang, J. Kajanus, M. J. Johansson, A. Córdova and J.-E. Bäckvall, Angew. Chem., Int. Ed., 2023, 62, e202314512 CrossRef CAS.
- A. Krasovskiy and P. Knochel, Angew. Chem., Int. Ed., 2004, 43, 3333–3336 CrossRef CAS PubMed.
- L. K. Thalén, A. Sumic, K. Bogár, J. Norinder, A. K. Persson and J.-E. Bäckvall, J. Org. Chem., 2010, 75, 6842–6847 CrossRef.
- J. Norinder, K. Bogár, L. Kanupp and J.-E. Bäckvall, Org. Lett., 2007, 9, 5095–5098 CrossRef CAS.
- M.-B. Li, D. Posevins, K. P. J. Gustafson, C.-W. Tai, A. Shchukarev, Y. Qiu and J.-E. Bäckvall, Chem. – Eur. J., 2019, 25, 210–215 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc05366d |
| This journal is © The Royal Society of Chemistry 2025 |




