Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rational design and synthesis of atomically precise nanocluster-based nanocomposites: a step towards environmental catalysis
Alok
Kumar
,
Glory
James†
,
Ravari Kandy
Aparna†
and
Sukhendu
Mandal
 *
*
School of Chemistry, Indian Institute of Science Education and Research Thiruvananthapuram, Thiruvananthapuram, Kerala 695551, India. E-mail: sukhendu@iisertvm.ac.in
First published on 7th January 2025
Abstract
Atomically precise metal nanoclusters (NCs) and metal–organic frameworks (MOFs) possess distinct properties that can present challenges in certain applications. However, integrating these materials to create new composite functional materials has gained significant interest due to their unique characteristics through a range of applications, particularly in catalysis. Considering MOFs as hosts and NCs as guests, several synergistic effects have been observed in composites, particularly in environmental catalytic reactions. However, the precise role of encapsulated NCs within the MOF pore structure is still in its infancy. Besides, stabilizing NCs, whether through intact ligands or without ligands via the MOF host, presents challenges that are currently being investigated. This feature article reviews recent advancements in the synthesis of NC@MOF composites, focusing on cutting-edge strategies for selecting MOFs and the roles of NC ligands, as well as characterization and catalytic applications.
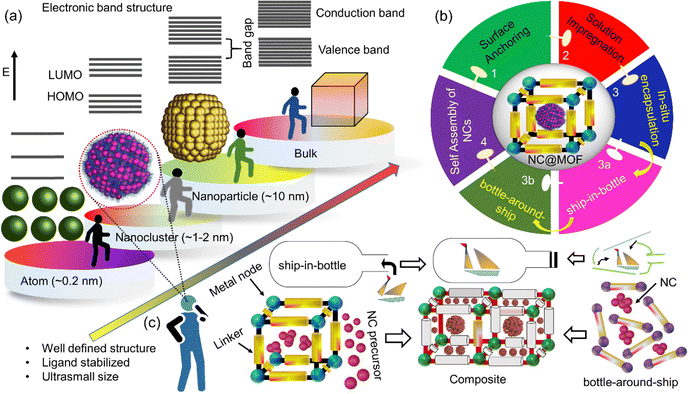
1. Introduction
The swift progression of globalization and industrialization has highlighted the critical importance of sustainable environmental development.1,2 Over the last few decades, a notable advancement has been made in creating innovative functional materials tailored for various applications.3,4 Atomically precise metal nanoclusters (NCs), a class of nanomaterials measuring less than 2 nm, are composed of a limited number of metal atoms, ranging from a few to several hundred, and are stabilized by ligands (Scheme 1a). These nanoclusters exhibit distinct energy levels that allow them to display unique properties akin to those of molecules, including optical, magnetic, biosensing, and catalytic characteristics.5–8 In contrast to traditional nanoparticles, the ligands attached to NCs offer significant potential for manipulating their properties to meet specific application requirements. The well-defined crystalline structures of these nanoclusters can be analyzed using X-ray crystallography, providing a crucial framework for tackling fundamental challenges and enhancing our understanding at the atomic and molecular levels, particularly in the context of environmental catalysis.9–16 In the realm of classical nanocatalysis, nanoparticles (NPs) such as TiO2, WO3, CeO2, and SiO2, which are primarily composed of metal oxides, are commonly utilized.17–21 Nanoparticles (NPs) typically exhibit a polydisperse nature, and structural constraint complicates a clear correlation between the catalytic active site and the underlying mechanisms.22,23 In recent years, NCs, have driven special attention owing to present a promising avenue for advancing fundamental value-added catalysis.8,24 NCs as catalysts stand out due to their plentiful unsaturated active sites, high surface area, ultra-small size, atomic-level precision, and exceptional molecular purity.6,25 However, challenges remain, particularly in addressing aggregation issues and identifying active sites hindered by steric effects from surface-bound ligands. To bridge the critical gaps in catalysis, precise ligand engineering and meticulous control over cluster atom arrangements are essential. Numerous comprehensive reviews have been documented for ligand design and synthetic methodologies for various NCs10 including silver (Ag),26 gold (Au),7,27,28 silver/gold (Ag/Au) alloys,29–31 and copper (Cu).32 It should be emphasized that coinage metals (e.g., Au, Ag), exhibit greater stability than other metal clusters (e.g., Cu, Ni). This stability is attributed to the ease of synthetic process, well-established purification methods, ultra-small size (<2 nm), remarkable photo/chemical stability, and minimal toxicity. The characteristics of NCs are largely dictated by the inorganic core selected, which defines the structure of the clusters, while the ligand shell plays a crucial role in ensuring solubility and functionality within the surrounding medium.9 Furthermore, coinage metals are known for their strong catalytic performance. However, the enhancement of properties such as efficiency, stability, selectivity, and the fine-tuning of ligand engineering remains in its early stages, prompting the exploration of diverse strategies to improve these characteristics. Researchers have engaged in efforts to anchor NCs to various support materials, which encompass porous carbon, mesoporous silica, porous polymers, and NPs.33–37 These support structures are generally inert concerning NC interactions or may restrict the engineering of ligands on the NCs for targeted catalytic processes. Additionally, the tendency of agglomeration on the host surface can compromise the active sites, making it crucial to select support materials that can maintain structural integrity while providing catalytic sites. Achieving a balance in both materials is desirable yet challenging. Recently, a relatively young family of porous materials known as metal–organic frameworks (MOFs) has garnered significant attention from researchers globally.38–41 The MOFs are constructed by metal ions or clusters, which make coordinative bonds with organic linkers, resulting in three-dimensional interconnected networks. Despite their potential, MOFs face certain challenges that limit their application in catalysis. Key issues include the (a) random distribution of active sites, (b) stability concerns in various environments, and (c) low thermal and mechanical resilience due to the relatively weak bonds between metal nodes and linkers.42,43 Nevertheless, MOFs present a unique opportunity to adjust their composition and pore structure through careful selection of precursors, synthetic methods, and post-synthetic modifications.44 To overcome these challenges, two primary approaches have been examined: enhancing the MOF backbone through functionalization and integrating MOFs with a range of functional materials.44–47 The composite of MOF with other functional materials, including metal NPs, quantum dots, silica, and polyoxometalates, has been thoroughly explored.47,48 The resulting composites reveal unique chemical and physical characteristics attributed to their synergistic effects. However, it is essential to recognize the nature of the interface significantly influences the assembly process, including aspects like growth, orientation, and nucleation, which are vital for the effective development of these composites and the realization of their synergistic benefits. Among these innovative strategies, the encapsulation of NC within MOFs has sparked considerable interest in the catalytic field, leading to the development of a new class of finely tuned functional materials. Various methodologies have been employed for the synthesis of composites, specifically NC@MOF, as illustrated in Scheme 1b. The deliberate design and fabrication of advanced composite catalysts, such as NC@MOF, have demonstrated superior performance compared to their bulk counterpart.46,49,50 However, the post-immobilization of NC and MOF has revealed a leaching phenomenon during reactions, attributed to insufficient host–guest interactions.42,49 The selection of ligand engineering and the modification of pore structures through suitable linkers and modulators present significant challenges. These factors are critical in enhancing the stability and efficacy of the composite materials, ultimately influencing their catalytic performance. Addressing these complexities is essential for advancing the development of more effective composite catalysts in various applications. Our research group engaged in the synthesis of NP/MOF composites and exotic functional materials for catalytic applications.51–54 Despite the advancements in this field, questions remain, particularly concerning how the ligands of NC engage with the MOF. Is there a ligand present in the MOF or NC that plays a role in the encapsulation of NC? Moreover, what is the potential stability of both the MOF pore and the NC once encapsulated, given the well-defined structure? With this aim, in this feature article, we begin with the state-of-the-art synthesis strategy for nanocluster–MOF composite material followed by the interaction of NC with the MOF through linker and pore matrices. Various factors like kinetic, choice of NC ligands, MOF linkers, and their pore matrices along with recent developments in manipulating the core structure were discussed. In addition, the composite characterization technique has been highlighted. The discussion further emphasizes the enhanced activity of NC@MOF nanocomposites and the underlying reasons for this improvement in catalysis.2. Synthesis of NCs@MOF
Typically, it is understood that the pure MOF framework itself collapses during the activation process (e.g., solvent removal/exchange) as shown in Fig. 1b. This phenomenon could be observed in PXRD peak broadening. These findings suggest that MOFs featuring longer, flexible linkers are more susceptible to structural collapse compared to those with shorter and rigid linkers.55,56 Therefore, the post-incorporation of guest molecules faces the challenges of maintaining the structural integrity upon encapsulation. Besides the understanding of two components, it is essential to distinguish how the guests (NCs) interact with the framework, whether through size or energy characteristics. Numerous opportunities exist for modifying the pore matrices of MOFs, and effective fabrication strategies could be achieved through careful consideration: (a) judicial choice of a linker (Fig. 1c), (b) metal node engineering, and (c) suitable templating or defect engineering (Fig. 1a). Different strategies have been adopted over the years for the synthesis of NC/MOF composites system. These methods can generally be classified into three categories: (A) post-synthetic modification, (B) in situ encapsulation, and (C) self-assembly of nanocomposites. A comprehensive discussion of these techniques, along with their pros and cons, are provided in the subsequent sections. | ||
| Fig. 1 (a) Depicted is UiO-6657 with metal nodes, linker (top), and purple polyhedral (below). The void space of the pore is emphasized in a yellow sphere. (b) A repressive model for MOF activation by solvent exchange and evacuation. (c) Representative ligands for MOF framework construction, which have scope to tune the pore size, functional interactions, and rigid structure. 5-Hydroxyisophthalic acid (L1), benzene-1,3,5-tricarboxylic acid (L2), 5-cyanoisophthalic acid (L3), 5-nitroisophthalic acid (L4), 2-aminoterephthalic acid (L5), terephthalic acid (L6), pyridine-3,5-dicarboxylic acid (L7), and 2-methyl imidazole (L8). | ||
2.1. Post-synthetic modification
Firstly, both components are synthesized prior to their integration. The first step involves the preparation of the MOF, which is subsequently subjected to post-synthetic modification through treatment with a pre-synthesized nanocluster. The MOF can be altered in two distinct ways: the NCs may either anchor to the surface of the MOF (termed surface anchoring) or diffuse into the MOF's pores, referred to as solution impregnation. These techniques face difficulties in establishing the composite system, primarily due to the absence of confinement and stabilization effects. | ||
| Fig. 2 (a) Schematic illustration of the synthetic route for the formation of the APNC/ZIF-8 (300 °C) composite system. Reproduced with permission from ref. 58. (b) Illustration of the charge transfer between Au25(SG)18 and metal–oxo/nitric clusters in MOFs and the reaction scheme of 4-NP reduction. Reproduced with permission from ref. 61. | ||
Luo et al. developed a nanocomposite (Au25/ZIF-8), in which the coordination between the terminal carboxylic protecting group of the Au25(GSH)18 nanocluster and the Zn2+ ions located at the nodes of MOF serves to stabilize the composite structure.62 This anchoring of the NC on the surface of the MOF provided unrestricted access to all the reactant molecules for the reduction of 4-nitrophenol in less than 12 minutes as compared to its counterpart Au25@ZIF-8, which took several hours for the catalytic process due to the diffusion selectivity provided by the ZIF-8 matrices. Zhu's group reported another work that uses the post-immobilization method for a nanocomposite system ZIF-8@Au25@ZIF-67.63 The pre-synthesized MOF (ZIF-8) and Au25(L-Cys)18 were used for composite formation via surface anchoring technique. The interaction of the terminal –COOH group on the ZIF-8 played a crucial role in the formation of the Au25/ZIF-8 composite. Subsequently, varying quantities of ZIF-67 precursors, specifically Co(NO3)2 and the linker 2-MeIm, were incorporated to achieve ZIF-67 coatings of differing thicknesses, resulting in the formation of the sandwich composite (ZIF-8@Au25@ZIF-67). The authors then followed a similar procedure to synthesize the ZIF-8@Au25@ZIF-8. To elucidate the interaction between metal nodes and NCs within a composite system, Shi et al. performed a theoretical study for the charge transfer dynamics involving Au25(GSH)18 NCs and the metal nodes of different MOFs, namely MIL-125 (Ti), MIL-101 (Cr), and ZIF-8 (Zn).61 It was observed that the charge on the Au NC significantly varied depending on the interaction with the metal node. Among these three composite systems, the XPS analysis of the composite system Au25(GSH)18/MIL-125 (Ti) showed a binding energy of 84.1 eV, similar to that of unsupported Au25(GSH)18. This result confirms that the oxidation state of the Au NC is metallic Au0. The Au25(GSH)18/MIL-101 (Cr) showed a positive shift in the binding energy, while Au25(GSH)18/ZIF-8 (Zn) revealed lower binding energy, unveiling Au in MIL-101 has a partial positive charge and the Au in ZIF-8 possess a partial negative charge. The reduction of 4-NP was used as a model reaction to evaluate the effect of charge on the catalytic activity of the Au NCs. Au25(GSH)18/MIL-125 and unsupported Au25(GSH)18 showed higher catalytic activity compared to the Au NCs immobilized on MIL-101 and ZIF-8. The outcome was attributed to the charge state of Auδ− in the Au25(GSH)18/ZIF-8 framework, which led to the repulsion of hydride (H−) species. In contrast, Auδ+ in the Au25(GSH)18/MIL-101 framework weakened the reductive capacity of the hydride (H−) species due to a strong affinity between them (Fig. 2b). Thus, only metallic Au0 demonstrated the highest activity, further highlighting the role of the MOF microenvironment in regulating the catalytic performance of encapsulated NCs.
 | ||
| Fig. 3 Schematic representation of “ideal” Au133(SR)52 organization in the periphery of gradient bMOF-102/106. Reproduced with permission from ref. 64. | ||
2.2. In situ encapsulation
In this method, the NC is integrated within the pore structure of the MOF through an in situ approach. There is in situ growth of the NC to form the resultant NC@MOF composite system. The encapsulation of the cluster utilizing this method could be achieved through two distinct approaches. The first approach involves a “bottle around a ship” strategy, where the MOF is constructed around pre-synthesized NC. While second approach is referred to as the “ship in a bottle” strategy, in which the NCs are synthesized within a pre-existing MOF (Scheme 1c). | ||
| Fig. 4 (a) Schematic representation of the process of Au NCs synthesis in MOFs and the example of Au11@ZIF-8. Reproduced with permission from ref. 66. (b) Schematic illustration of the synthesis of UiO-68-NHC, Au-NC@UiO-68-NHC, and UiO-68-NH2/Au mixture. Reproduced with permission from ref. 67. | ||
Horcajada and co-workers synthesized the AgNC@MIL-125-NH2 using this approach. The pre-synthesized amine-functionalized MIL-125 MOF was utilized, into which the silver precursor was integrated through an impregnation technique, subsequently followed by photoreduction to form the nanocomposite within the MOF.68 The optimizations of irradiation time, stepwise addition of reagents, stirring conditions, and the ratio of MOF to Ag were ascertained to achieve ultrasmall. The NC was uniformly distributed within the MOF, facilitated by the amine functional group of the MOF. Szilágyi et al. showed how the functional group (mono- or bi-functional) of a linker in the MOF contributes to the formation of the nanocomposite. The authors synthesized the nanocomposites Pd@UiO-66-NH2, Pd@UiO-66-(NH2)2, Pd@UiO-66-CH3, and Pd@UiO-66-(CH3)2.69 The findings indicated that the mono-functionalized Pd@UiO-66-NH2 outperformed the bi-functionalized Pd@UiO-66-(NH2)2 in terms of encapsulating Pd NCs. This advantage is due to the strong bond formation between the Pd and the bi-functionalized MOF, which requires harsh reduction conditions for the production of Pd NCs.
In addition, the previous research indicated that an increase in the Lewis base character of a functional group enhances the encapsulation of the nanocluster, as observed with –NH2 and –Br groups. Conversely, a higher Lewis acidity in functional groups diminishes the likelihood of Pd encapsulation, as seen with –H, –Cl, –OH, and –CH3. Notably, the Pd NC exhibited agglomeration in the presence of the CH3 functionalized MOF, attributed to insufficient stabilizing interactions between the CH3 group and the Pd precursor.
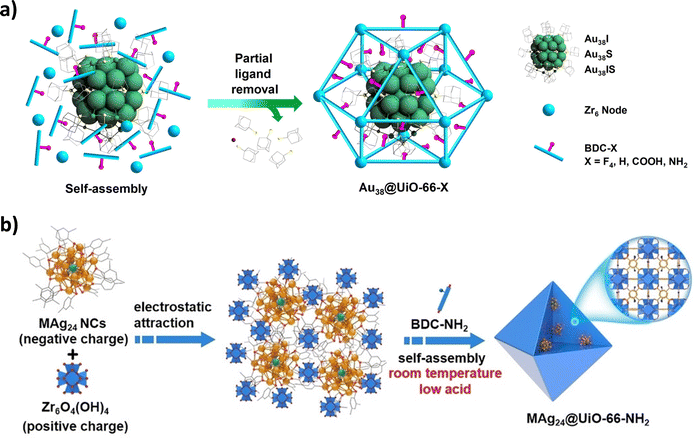 | ||
| Fig. 5 (a) Schematic illustration of the synthetic route to partial-ligand-removed Au38@UiO-66-X composites based on the self-assembly of Au38 NCs and UiO-66-X MOF precursors. Reproduced with permission from ref. 70. (b) Schematic illustration showing the synthetic route to MAg24@UiO-66-NH2 by encapsulating MAg24 clusters into UiO-66-NH2via electrostatic attraction and self-assembly at room temperature under low-acidity conditions. Reproduced with permission from ref. 71. | ||
Fischer et al. immobilized [NBu4]2[{Pt3(CO)6}3] NC into ZIF-8 MOF. The encapsulated cluster exhibited sensitivity to oxidation, leading to its transformation into the [NBu4]2[{Pt3(CO)6}4]@ZIF-8 nanocomposite upon exposure to air.72 The carbonyl groups were stripped off from the cluster by treating the composite at 200 °C for 4 h. Further, the FTIR data confirmed the total loss of CO ligands from the composite system. Further analysis revealed that the platinum clusters were evenly distributed within the MOF without any aggregation. This uniformity is attributed to the confinement effect provided by the MOF. Fischer and coworkers also encapsulated bimetallic Pt(M) (M = Co, Ni, Fe, Sn) clusters in ZIF-8 MOF using the bottle-around-ship approach.73 Following this, the decarbonylation of the CO ligand that protected the surface of the NC, led to yield of a ligand-free, NC encapsulated within the MOF.
The analysis of cluster size revealed that only the homometallic Pt9 and the bimetallic Co8Pt4 clusters were successfully encapsulated within the MOF. In contrast, the remaining clusters were located at the edges of the MOF, as their larger sizes and other limiting factors prevented them from being encapsulated inside the MOF's pores. Copper NCs have recently become more significant than gold and silver NCs because of their cost-effectiveness and availability, enabling their large-scale application. Serre et al. encapsulated copper NC in zirconium-based MOFs UiO-66-NH2 and MOF-801 using the bottle-around-a-ship strategy.74 The composite utilized for CO2 photoreduction. The mechanistic studies indicated that the stabilized CuI at the interface of the encapsulated copper NC and the MOF acted as the active site for catalysis due to a synergistic effect. Steunou et al. synthesized Au25SG18 NC and encapsulated it into an iron(III) carboxylate MOF MIL-100(Fe).75 The as-synthesized NC was mixed with the MOF precursors Fe(NO3)3 and linker trimesic acid to obtain the hybrid material Au25@MIL(Fe) at room temperature. Different amounts of NC were taken to prepare Au25(X)@MIL-100 (X = 3 to 13 wt%). Among composite systems, the highest loading of NC (e.g., Au25(13)@MIL) was investigated for the anti-inflammatory properties of the material.
Jiang et al. studied the interaction between the NC and the metal node of the MOF. The composite system was formed based on the coordinated self-assembly and electrostatic interactions, which yielded Au25@M-MOF-74 (M = Zn, Ni, Co, Mg).76 The microenvironment of Au25(Cys)18 could be modulated by different metal nodes (Fig. 6). The coordination interactions between the metal–oxo chain in M-MOF-74 and the free carboxyl groups from the surface-bound ligand Cys on Au25(Cys)18 effectively reduced the free vibration of the surface ligands on the Au25 NC. This modification enhanced the accessibility of the Au sites and resulted in variations in the fluorescence properties of these materials. It was noted that stronger interactions contributed to an increased electron density and core expansion within Au25(Cys)18 NC, thereby facilitating catalysis in the following order: Au25@Ni-MOF-74 > Au25@Co-MOF-74 > Au25@Zn-MOF-74 > Au25@Mg-MOF-74.
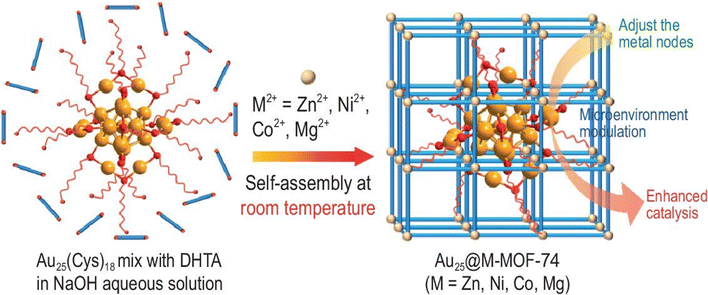 | ||
| Fig. 6 Schematic illustration showing the synthetic route to Au25@M-MOF-74 (M = Zn, Ni, Co, Mg) for enhanced catalysis through microenvironment modulation around Au25(Cys)18 by adjusting the metal nodes on MOF pore walls. Reproduced with permission from ref. 76. | ||
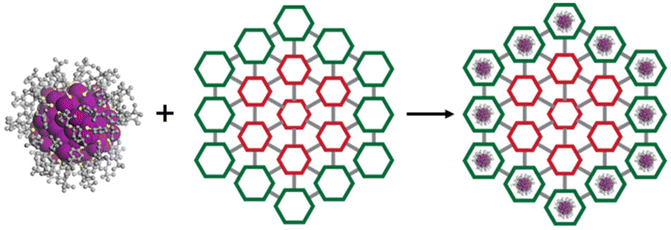
2.3. Self-assembly of nanocluster
This technique involves the NC as a metallic node, which is interconnected via a ligand present on the surface of the NC. This ligand acts as a bridging agent between two nanocluster units, facilitating their combination into a one-dimensional (1D), two-dimensional (2D), or three-dimensional (3D) framework that forms a cluster-based network similar to that of the MOF system. These materials demonstrated a well-structured and porous characteristic. A prominent example of cluster-based MOFs is the silver chalcogenolate, which utilizes Ag–N interactions to connect the network components. Zang et al. demonstrated the inter-nanocluster connection of Ag12 NCs (Ag12(StBu)6(CF3COO)6(CH3CN)6) to form a MOF based on Ag12 NC, referred to as Ag12bpy. This was achieved by replacing the coordinated CH3CN ligands in Ag12 NC with the linear bidentate linker 4,4′-bipyridine (bpy), resulting in an Ag12(StBu)8(CF3COO)4(bpy)4, as illustrated in Fig. 7. In the framework of Ag12bpy, each Ag12 node is linked to four adjacent nodes via eight μ2-bpy bridges and an open channel with a rectangle cross-section measuring 11.78 × 6.47 Å2.77 Several other Ag NCs, including Ag4,78 Ag8,79 Ag10,80 Ag14,81–84 Ag15,81 Ag18,85 and Ag27,86 have also been synthesized into 2D or 3D cluster-based MOFs using N-containing linkers with different configurations and functionalities. By linking the clusters together, there is a notable increase in the stability of the cluster along with an enhancement in its catalytic capabilities. The ability to adjust the surface ligands of NC enables the customization of linkers, resulting in different cluster assemblies. So far, research has predominantly concentrated on N-based linkers, while some assemblies using S/O-based linkers are also being developed. Dong et al. synthesized a series of Ag NC-based MOFs using a triangular, thiourea-based linker, yielding structures TNS-Ag8 and TOS-Ag4. In the TNS-Ag8 framework, the Ag8 cluster functions as a 6-connected node, creating a channel with a cross-sectional area of 9.0 × 9.0 Å2. Similarly, the TOS-Ag4 network features a channel of 10.0 × 10.0 Å2, formed by 3-connected Ag4 nanoclusters.83 | ||
| Fig. 7 Schematic illustration of the ligand-exchange strategy (method 1) used to obtain Ag12bpy crystals and one-pot synthesis (method 2) under identical conditions. Reproduced with permission from ref. 77. | ||
2.4. Synthetic challenges and opportunity
Upon reviewing the different synthesis techniques for NC@MOF composite, it is evident that the in situ encapsulation method is more advantageous than the post-synthetic modification approach. This advantage is largely attributed to the critical role of surface anchoring deriving forces such as a covalent bonding—between the MOF and the NC, van der Waals forces, π–π, coordination, and electrostatic interactions. These interactions are essential for maintaining stability, especially in challenging reaction environments, where there is an increased likelihood of NC aggregation or separation from the MOF due to insufficient interaction strength in the absence of a confinement effect. While the effectiveness of post-synthetic modification is influenced by the stability of the pre-synthesized MOF and the subsequent composite formation. A vital step in this process is the activation of the MOF, while the possibility of NC leaching exists, attributed to weak interactions and size discrepancies within the pore structure. The “bottle-around-ship” method (in situ) effectively reduces leaching, ensures a consistent distribution, and optimizes the stabilization of the embedded clusters. Besides, the “ship-in-a-bottle approach”, presents significant challenges. To achieve precise NC within MOF, it is essential to implement a rigorously controlled kinetic process during the reduction phase, followed by a size-focusing step or a purification procedure. This approach raises concerns during the in situ formation of clusters within the MOF, as impurities might be trapped in the framework, which could negatively affect the catalytic performance of the composite system. In addition, there is a limitation on the size of NC that can be integrated into MOF, as the growth of the NC is influenced by the pore size of the MOF due to in situ formation. The self-assembly of NCs into a cluster-based framework demonstrates a significant advantage in structural manipulation and the tailoring of NC properties. This technique not only promotes enhanced stabilization but also enables the fusion of functionalities between NCs and MOFs. However, a notable limitation is that the bridging linkers available for the construction of cluster-based MOFs are predominantly restricted to nitrogen-donor ligands. Although the research has been conducted on sulfur and oxygen-based linkers. The materials synthesized from these alternatives are limited. Thus, there is a compelling need for the introduction of novel bridging linkers or new bonding modes of cluster-based MOFs. The importance of including the NC in the MOF is noted for its role in manipulating the microenvironment. This could be achieved by changing the NC ligand functional group or MOF pore engineering. For example, the ligands on the NC entrapped inside the MOF could be stripped off under thermal conditions to yield a surface-clean NC. This exposes the active sites, reducing steric hindrance from the ligands, and allowing easier access for incoming reactant molecules, which enhances catalytic activity. Additionally, the MOF's confinement effect prevents NC aggregation, even after ligand removal, ensuring stability and maintaining catalytic efficiency. The functional group present on the organic linker can further modulate the stability and catalytic activity of the entrapped NC. Jiang et al. reported a nanocomposite system, in which they regulated the microenvironment around the Au25 nanocluster using the confinement effect of the MOF.87 Utilizing electrostatic attraction and coordination interactions, the pre-synthesized Au25(Capt)18 was successfully encapsulated in the pores of UiO-66-X (X = –NH2, –OH, –H, –NO2) as illustrated in Fig. 8a. Following this, the composite system underwent a thermal treatment at 275 °C to remove the surface-protecting ligands from the NC, yielding a surface-clean Au25 NC within the MOF structure. The surface-clean Au25 NC, confined within the MOF structure, demonstrates significantly enhanced activity and stability compared to pristine Au25 nanoclusters in the oxidative esterification of furfural. Zhao et al. recently used a rigid γ-cyclodextrin MOF (γ-CD-MOF) with sufficiently large cavities to host the ligands of NCs (Au40(Adm)22) via host–guest chemistry. Once anchored onto the γ-CD-MOF surface, each NC (Au40(Adm)22) is enclosed by the γ-CD molecules, thereby stabilizing the ligands. This stabilization restricts ligand movement, allowing reactants to interact readily with the exposed metal surface of the Au40 NCs through the gaps created by the rigidified ligands (Fig. 8b). Therefore, in a horseradish peroxidase-mimicking reaction, Au40/γ-CD-MOF demonstrated significantly enhanced catalytic activity compared to unmodified Au40 NCs. This approach is versatile and can also be applied to other NCs with distinct ligands, such as Au38(PET)24 and Au44(2,4-DMBT)26.88 | ||
| Fig. 8 (a) Schematic illustration showing the synthetic route to Au25(Capt)18@UiO-66-X to surface clean Au25@UiO-66-X for enhanced catalysis through microenvironment modulation around Au25 by adjusting the functional group on linker of MOF pore walls. Reproduced with permission from ref. 87. (b) Schematic illustration showing the encapsulation of Au40(S-Adm)22 by γ-CD-MOF to form Au40/γ-CD-MOF for phase transfer and enhanced catalysis in horseradish peroxidase reaction. Reproduced with permission from ref. 88. | ||
2.5. Structural integrity MOFs and NCs
It is of considerable interest to the researcher to maintain the well-defined architecture and chemical functionality of MOF during composite (e.g., NC@MOF) formation with NC guests. The unique characteristics of MOF matrices serve to prevent the aggregation of NC during heterogeneous catalysis, granting them notable features like the molecular sieving effect.89,90 However, the effectiveness of surface anchoring is diminished due to the weak nature of host–guest interactions (coordinative, electrostatic, etc.). Moreover, it becomes increasingly challenging to regulate NC aggregation in harsh environments, particularly at elevated temperatures. Unlike surface anchoring, pore-confined growth can effectively limit agglomeration and leaching. Additionally, since the NCs are physically situated within the MOF pores, aggregation can be mitigated even at elevated temperatures. However, this approach may face challenges in achieving the desired atomic precision of NCs due to the limited pore size of the MOF. Maintaining atomic precision during the reduction phase, along with subsequent size-focusing, kinetic control, and separation processes, presents difficulties. Therefore, the in situ encapsulation offers a potential solution to these challenges. This approach allows for the encapsulation of NC of various shapes and sizes within MOF. The MOF serves as a protective shell, preventing the migration and aggregation of the NC. Furthermore, the structural integrity of the MOF's pores is maintained, as it depends on the surface of the NC to facilitate interactions with external analytes for reactions. This method incorporates strategies such as coordination-assisted self-assembly and electrostatic attraction. For instance, Au25(SG)18 is encapsulated into ZIF-8, and the coordinating interaction between Zn2+ and the –COOH functional group of the SG ligand boosts the self-assembly of ZIF-8.62 Further examples can be found in the electrostatic technique, where the ionic NC (e.g., [Au12Ag32(SR)30]4−) is mixed with a Zn2+ solution containing a linker (e.g., 2-methylimidazole) to enable controlled encapsulation within ZIF-8 scaffolds.59 It is crucial to emphasize that the negatively charged NC and the positively charged metal node (e.g., Zn2+) were the fundamental driving forces for the composite formation. Therefore, with a strategic selection of NC and MOF, electrostatic attraction-assisted encapsulation offers greater versatility, accommodating a diverse range of NC shapes and sizes (e.g., Ag44, Ag12Cu28) within the frameworks (e.g., ZIF-8, ZIF-67, etc.). Notably, this method is particularly advantageous when conducted at room temperature, allowing for the utilization of temperature-sensitive MOFs. By employing this strategy, it is possible to successfully encapsulate NCs while maintaining the intrinsic properties and structural integrity of both the MOFs and the NCs.3. Composite characterization methods
The composites are analyzed using a range of advanced, cutting-edge analytical and spectroscopic techniques to gain comprehensive insights into their structure and properties. A deep understanding of the material is essential and envisioned for developing new methods for potential applications. In-depth characterization of composites not only facilitates a better understanding of their properties and potential uses but also enhances the optimization of synthesis techniques, which is fundamental to the progress of this field of study. The characterization of NC@MOF composite often considered to be challenging, as they are complex inorganic–organic hybrid materials. To obtain a comprehensive view of the structure and properties of the composites, information on their crystallinity, porosity, composition, etc., are collected using multiple spectroscopic and microscopic tools. The ultra-small size of the NCs renders their analysis within the composite particularly challenging, leading to the strategic utilization of novel analytical methods to effectively tackle this problem. Inductively coupled plasma (ICP) spectroscopy plays a crucial role in determining the composition of the composite. By utilizing ICP, the metal contents are measured with high accuracy, facilitating a quantitative confirmation of the incorporation of NCs.70,87 Powder XRD is one of the preliminary techniques that is used to characterize the NC@MOF composite. It gives information regarding the crystallinity and the phase purity of the material. There are instances in the synthesis of the composite; the crystallinity of the MOF could be compromised, like the agglomeration of NCs and problems in the MOF formation because of the presence of NCs in the bottle-around-the-ship approach, etc. A good match between the PXRD of the intact MOF and the composite is always an indication of the formation of a stable composite. The shift in peak position and variation in intensity observed in the composite, resulting from the interaction between the NC and MOF, also offer valuable insights. Zhu et al. incorporated Au25(L-Cys)18 and Au25(PET)18 in UiO-66-NH2 MOF, and they observed that the PXRD patterns of the MOF remained unchanged after the incorporation of the Au NCs, indicating the intact crystallinity of the MOF (Fig. 9a).60 Also, because of the ultrasmall size of the NC, there were no diffraction peaks associated with Au NCs. UV-vis spectroscopic analysis is a very powerful technique for the characterization of NC@MOF composites. It could reveal very crucial information, like the stability of the cluster in the composite and the interaction between NC and MOF, which is pivotal in understanding these hybrid materials. Wu et al. used UV-vis/NIR spectroscopic studies in their work to confirm the structural integrity of the NC after incorporation in MOF, where they constructed UiO-66 MOF around Au38 NCs in a bottom-up manner.70 Wang et al. also conducted UV-vis spectroscopic studies on their MAg24@UiO-66-NH2 (M = Ag, Pd, Pt, and Au) composite and observed the characteristic peaks of the synthesized cluster (Fig. 9b and c).71 The Fourier transform-infrared (FT-IR) spectroscopy is essential for characterization, as it offers valuable information regarding the interactions between NCs and MOFs. FT-IR spectra of the material could provide insights into the functional groups present in the composite, bonding interactions within the composite, and the incorporation of NCs. Yun et al. used FT-IR to investigate the –COO– functional group interaction of NC (Au25) ligand with Zn2+ or Co2+ ions in the ZIF-8, ZIF-67 MOFs, and ZIF-8@Au25@ZIF-67 composites, respectively.63 A new peak emerged at 520 cm−1, which was attributed to the Zn–O or Co–O stretching vibration, corresponding to the Au-Cys-Co or Au-Cys-Zn linkage. The porosity of the MOF is an interesting feature to study before and after the NC incorporation with MOF. This could provide information for the type of composite, comparative loading of NCs, etc. Typically, the surface area decreases upon the encapsulation of NC due to the pore's occupancy. In addition, it could also help in differentiating the surface-bound NC (e.g., NC/MOF) or encapsulated within the pore matrices of MOF. Wang et al. found that the surface area of the Au25@UiO-66 (853 m2 g−1) was comparatively lower than surface anchored NC (Au25/UiO-66, 1174 m2 g−1) (Fig. 9d).87 The findings indicated that the pore space of UiO-66 is occupied by Au25(Capt)18, resulting in the formation of Au25(Capt)18@UiO-66. Conversely, Au25(Capt)18 may remain on the surface of the MOF in the case of Au25(Capt)18/UiO-66. X-ray photoelectron spectroscopy (XPS) is an essential technique for investigating the electronic structure, elemental composition, and chemical state of NC@MOF composites. It primarily helps to identify the oxidation states of the elements within the composite. Additionally, XPS can provide insights into the interactions between NC and MOF. Su et al. employed XPS analysis to investigate the oxidation state of Au in Au25@ZIF-8 composite (Fig. 10a and c).62 Au25(SG)18-ZIF-8 nanocomposite shows a minor negative shift in the Au 4f7/2 peak (83.7 eV) compared to Au25(SG)18 (84.1 eV).91 This result indicates that the formation of negatively charged Au25 clusters occurs after the encapsulation of Au25(SG)18 within ZIF-8, presumably due to interactions with ZIF-8. In situ XPS experiments are also emerging tool in the field of composite materials characterization. Kratzl et al. used in situ XPS analysis to study the formation of Pt12±xNC@ZIF-8 composites by the thermal decarbonylation of [NBu4]2[{Pt3(CO)6}4]@ZIF-8 at 200 °C for 3 h.72 The binding energy peak of Pt 4f7/2 was found to be 71.1 eV, which is close to the reported value for decarbonylated Pt NCs. Signals for N 1s and Zn 2p match with the ZIF-8 MOF. X-ray absorption spectroscopy (XAS) is employed for studying the local atomic structure of the material and its coordination environment. XAS also provides the interaction between the NC and the MOF. Fischer et al. used XAS to study their system, where carbonyl-protected NCs (Co8Pt4) were incorporated in ZIF-8 (Fig. 10d).92 From XANES analysis, they could observe that the Co got oxidized further upon the incorporation into the MOF. This could be attributed to the interaction between MOF-matrices, partial removal of CO ligands, and damage caused by X-ray beams. EXAFS studies showed that dCo–Co decreased by 0.1 Å in the encapsulated Co8Pt4 as the cluster is located within the micropores of the framework. Microscopic analyses are crucial for investigating NC@MOF composite materials, as they offer insights into the size distribution of NCs. The TEM images analysis, such as high-angle annular dark field scanning (HAADF-STEM) and bright-field (BF-TEM) is curial for investigation. The TEM-energy dispersive X-ray spectroscopy (EDS), TEM-electron energy loss spectroscopy (EELS) along with elemental mapping are excellent for elemental analysis of the composite material. Jiang et al. used the HAADF-TEM to visualize and compare Ag25@UiO-66-NH2 and Ag25/UiO-66-NH2 (Fig. 10e and h).71 The careful analysis of the images revealed that the Ag25 NCs were incorporated into the pores of the MOF (Ag25@UiO-66-NH2) while other (Ag25/UiO-66-NH2) distributed over the surfaces. TEM-EDS analysis indicated the presence of uniformly distributed NCs all over the MOF in the case of Ag25@UiO-66-NH2. Whereas the Ag NCs were found to be occupying the edges of the MOF in Ag25/UiO-66-NH2. | ||
| Fig. 9 (a) XRD patterns of UiO-66-NH2-Au25(L-Cys)18, UiO-66-NH2/Au25(PET)18, and UiO-66-NH2 (simulated and experimental). Reproduced with permission from ref. 93 (b) UV-vis spectra of MAg24 (M = Ag, Pd, Pt, and Au) in CH2Cl2 solvent. (c) UV-vis spectra of MAg24 NCs loaded on or encapsulated into UiO-66-NH2 by subtracting the spectrum of UiO-66-NH2 from Ag25/UiO-66-NH2 and MAg24@UiO-66-NH2. Reproduced with permission from ref. 71. (d) N2 sorption isotherms of UiO-66, Au25(Capt)18@UiO-66, and Au25(Capt)18@UiO-66 at 77 K. Reproduced with permission from ref. 87. | ||
 | ||
| Fig. 10 XPS spectra of (a) Au25(SG)18, (b) 1000Au25(SG)18/ZIF-8 and (c) 1000Au25(SG)18@ZIF-8 from Au 4f and Zn 3p. Reproduced with permission from ref. 62. (d) Co Kα HERFD XANES of Co8Pt4@ZIF-8 and Pt27Co1/C. The XANES of Co(II)-oxide and Co-foil are also shown for comparison. Reproduced with permission from ref. 92. (e) and (f) HAADF-STEM images and the corresponding EDS mapping of Ag for Ag25@UiO-66-NH2 and (g) and (h) Ag25/UiO-66-NH2. Reproduced with permission from ref. 71. | ||
4. Catalytic performance of the nanocomposite
Since MOFs have been shown unprecedented properties and fine-tuning of liker and metal nodes provide the interactive host for the NC guest. The mutual support of MOF/NC not only retains the stability; however, approaches enhance catalytic efficiency for a variety of reactions.50,94 In addition, ultrasmall size, high surface area, known crystal structure, and active site warrant a comprehensive understanding of the mechanism. Here, we summarise the key factors that affect the reactivity efficiency and site selectivity for (a) photocatalysis and (b) value-added small molecule transformation.4.1. NC@MOF assisted photocatalysis
There are a plethora of reports and comprehensive reviews available for the immobilization of nanoparticles with the MOF and employed for photocatalytic application.47,48,93,95,96 However, NC@MOF composite has shown distinctive properties and selectivity for environmental photocatalysis (e.g., CO2 reduction). Fei et al. have reported ultrafine gold (Au) NC (Au-NC@UiO-68-NHC) composite for photochemical CO2 reduction.67 The Au NCs were stabilized by N-heterocyclic carbenes (NHCs) functionalized UiO-68-NHC pore matrices. The covalent bonding bridge between MOF-NHC-Au helps in modulating the reaction with precise control over the reaction mixtures. The composite afforded the yield of 57.6 μmol g−1 h−1 for CO2 to CO formation, which is four times higher than the UiO-68-NH2/Au.In addition, the comparative studies reveal that the Au-NC@UiO-68-NHC have a higher rate of CO production than MOF and UiO-68-NH2/Au mixture (Fig. 11a). The prepared catalyst was stable for up to 3 cycles and retains a reduction efficiency of up to 90 to 95% (Fig. 11b). The energy of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of Au-NCs were estimated to be 1.71 V and 0.43 V, suggesting the transfer of excited state electrons from Au-NCs to UiO-68 via Au-NHC bridges (Fig. 11c). Furthermore, the EPR signals is an evidence of the generation of Zr3+ species upon irradiation where Au-NCs facilitate the electron transfer (Fig. 11d). Authors proposed that the catalytic active site is found to be Zr3+ for the CO2 to CO conversion. Serre et al. reported Cu NCs@MOFs core–shell composite for CO2 photoreduction74 The photochemical CO2 reduction was performed under UV irradiation (385 nm), which yielded HCOOH (64.9%, 94 μmol h−1 g−1) as a major product along with CO (22.5%, 32 μmol h−1 g−1) as a minor (Fig. 12a and b). It was found that Cu NCs@UiO-66-NH2 showed better performance with an enhanced rate 128 (μmol h−1 g−1) for formic acid production (Fig. 12c). This is due to the more CO2 uptake driven by the NH2 functional group and strong host–guest interaction (Fig. 12d). Other examples, Xiong et al. have reported the encapsulation (in situ) of Au25(p-MBA)18 (p-MBA = 4-mercaptobenzoic acid) nanocluster into Cu3(BTC)2 (BTC = benzene-1,3,5-tricarboxylate) MOF.97
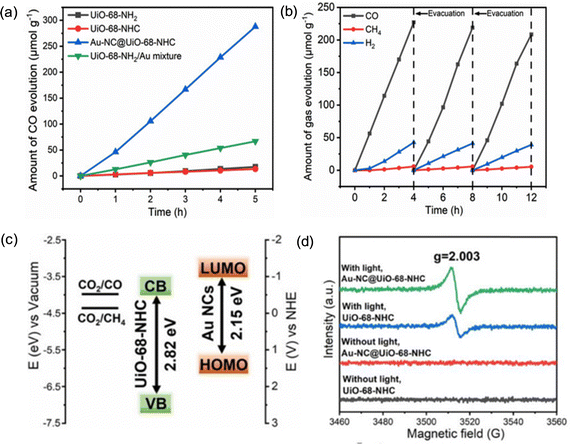 | ||
| Fig. 11 (a) Time courses of CO evolution by photocatalytic CO2 reduction using UiO-68-NHC, Au-NC@UiO-68-NHC, UiO-68-NH2, and Au/UiO-66-NH2 as photocatalysts upon AM 1.5G irradiation. (b) Time courses of photocatalytic CO2 reduction on Au-NC@UiO-68-NHC under AM 1.5G irradiation for 12 h, with evacuation every 4 h. (c) Band alignment of Au NCs and UiO-68-NHC. (d) ESR spectra of UiO-68-NHC and Au-NC@UiO-68-NHC with light and/or without light. Reproduced with permission from ref. 67. | ||
 | ||
| Fig. 12 (a) 13C NMR spectra of the photocatalysis products under Ar and 13CO2 gas feeding. (b) Production of the photocatalyzed CO2 with Cu NCs@MOF-801 and Cu NCs@UiO-66-NH2 compared to the corresponding blank experiments. (c) Comparative rates of HCOOH formation with Cu/MOFs, and core–shell CuNCs@MOFs. (d) PXRD patterns of Cu NCs@MOF-801 and Cu NCs@UiO-66-NH2 before and after photocatalysis. Reproduced with permission from ref. 74. | ||
The composite (Au25@Cu-BTC) has shown excellent activity for CO2 photoreduction to CO (Fig. 13a). The structure of p-MBA and BTC is anticipated to be similar, where the carboxyl group interacts with the Cu node, facilitating the transfer of electrons from the Au NC to the Cu site. The current density of the composite is 4 times higher than Au25/Cu-BTC and 8 times for bare Cu3(BTC)2 suggesting the excellent photogenerated charge separation (Fig. 13c). The authors observed that the strong interaction of Au NCs and Cu site promoting charge transfer and well-matched size shows the confinement effect.
 | ||
| Fig. 13 (a) Average production rates of H2, CO, CH4, and C2H4 in photoreduction CO2 by Au25@Cu-BTC in the first 2 h under visible-light (λ > 420 nm) irradiation, in comparison with those by Cu3(BTC)2, Au25(p-MBA)18, Au25/Cu-BTC and other control experiments under the same conditions. (b) GC-MS analysis of 13CO (m/z = 29) produced over Au25@Cu-BTC in light-driven reduction of 13CO2. (c) CO2 adsorption behavior for Au25@Cu-BTC and bare Cu3(BTC)2. (d) Transient photocurrent density. Reproduced with permission from ref. 97. | ||
In addition, Au helps in charge transfer while MOF is for CO2 uptake over the surface (Fig. 13b). Thus the composite performance has increased in comparison to Au NCs and MOF. Horcajada et al. have synthesized AgNC@MIL-125-NH2 composite and applied for hydrogenation reaction for p-nitroaniline (4-NA) to p-phenylenediamine (PPD).68 In addition, the composite was also investigated for the degradation of methylene blue (MB-dye) and sulfamethazine (SMT-antibiotic). They have achieved 100% transformation for p-nitroaniline and 92% and 96% for MB and SMT. This effect was attributed to the enhanced stability of Ag NC upon encapsulation, enabling the charge transfer process on the heterogeneous surface.
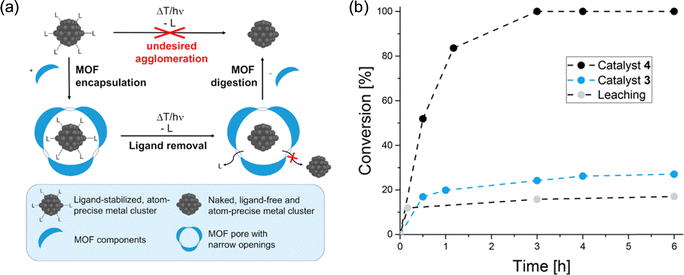 | ||
| Fig. 14 (a) General concept for the synthesis of ligand-free, atom-precise metal clusters and ultrasmall metal nanoparticles, based on the encapsulation of ligated metal clusters into size-matching pores of metal–organic frameworks chosen as removable protective matrix (b) catalytic hydrogenation of 1-hexene with [NBu4]2[{Pt3(CO)6}4]@ZIF-8 (3) and Pt12±x@ZIF-8 (4) as catalysts. While with 4, complete conversion is reached after 3 h, the ligand-stabilized clusters in 3 show a significantly slower conversion toward a steady state. When the catalyst 4 is separated after 10 min in a hot filtration experiment, only minimal further conversion is observed, presumably due to Pt species in the solution. Reproduced with permission from ref. 72. | ||
It is understood that the judicial choice of metal NCs and MOF hosts is important to bring selectivity and enhance catalytic activity. Notably, controlling ligand removal of NCs, which exceedingly helps for the encapsulation inside MOF's pore matrices leads to chemically and thermally stable and robust composite. Yun et al. reported sandwich composites ZIF-8@Au25@ZIF-67[tkn] and ZIF-8@Au25@ZIF-8[tkn] [tkn = thickness of shell] and investigated for the nitrophenol reduction and terminal alkyne carboxylation with CO2.63
ZIF-8@Au25@ZIF-67 has shown excellent yield (99%) for the carboxylation of the terminal alkyne using CO2. While bare NCs and MOF (ZIF-8, ZIF-8@ZIF-67) were less efficient and afforded only 10.8%, 27.9%, and 52.3% yields, respectively (Fig. 15a). The findings and observation revealed that the shell thickness and stable NC play an important role in providing the catalytic active site and assisting the substrate interaction for reaction (Fig. 15b). Besides the catalyst was stable up to more than three cycles. The mechanistic investigation suggests that the intermediate bond between an alkyne and an Au center is taking place (Fig. 15c). These studies unveil that encapsulation of NCs in the MOF matrices with the precise tuning of NCs's ligands stabilizing with MOF may give the desired catalytic active site for the selective products. Huang et al. reported palladium nanocluster and UiO-66-NH2 composite (Pd@UiO-66-NH2) and employed for the tandem oxidation reaction.98 Authors reported 99.9% acetal selective for conversion of benzyl alcohol in a single pot tandem reaction of benzyl alcohol and ethylene glycol. The Pd loading significantly modulates the conversion efficiency (1.0, 2.0, and 4.0 wt%). Higher Pd loading resulted in a high yield. The result suggests that palladium is an active site for catalytic conversion and is stable for up to more than 5 cycles in Pd@UiO-66-NH2. While Pd@UiO-66 shows the leaching. Overall, this affects the performance and diminishes the efficiency. The UiO-66-NH2 was recognized as an effective to enhance the stability of Pd NC.
 | ||
| Fig. 15 (a) Catalytic activity of various catalysts for the carboxylation of phenylacetylene. Reaction conditions: catalyst (1.12 × 10−4 mmol of Au25), alkyne (0.5 mmol), Cs2CO3 (0.24 mmol), CO2 (1.0 bar), 50 °C, 12 h. (b) Broken line diagram of catalytic performance of ZIF-8@Au25@ZIF-67 with various shell thicknesses. (c) Proposed catalytic mechanism of the reaction between terminal alkynes and CO2 over ZIF-8@Au25@ZIF-67[tkn]. Reproduced with permission from ref. 63. | ||
5. Conclusions and outlook
In this feature article, we reviewed the recent development of NCs@MOF composite and catalysis with a particular focus on the choice of MOF and ligand of NCs. MOF has shown unprecedented properties and characteristics for gas absorption and catalysis due to its porous nature and polymeric framework. There has been tremendous progress achieved in making the composite of MOF with other support materials e.g., nanoparticles. However, finding suitable composite materials for catalysis is still being investigated due to functional heterogeneous surfaces and multiple sites for reagent interaction, which complicates analysis. NCs are known to be ligand-stabilized and have well-defined structures, which significantly aid the advantage of establishing host–guest interaction and reactant. In addition, the synergy of NC and MOF composite can significantly influence the electronic structure of NC and heterogeneous surface contributing to boosting the catalytic efficiency. The progress achieved in this field underscores the importance of a new class of functional material and fine-tuning intrinsic properties and mutability. Furthermore, NCs unique properties provide the avenue for the rational design of new composite materials. Various synthetic techniques have been developed for NC@MOF composite preparation. We have outlined and classified these techniques into three main types: (a) post-synthetic modification (b) in situ encapsulation, and self-assembly of nanocluster. Furthermore, they are categorized into two subdivisions, which include surface anchoring, solution impregnation, the “ship-in-a-bottle”, and the “bottle-around-a-ship” method. Post-synthetic modification can show the leaching while dispersing in the solution phase. Besides the stability of NCs over the surface is a notable concern. The interaction between NCs’ ligand and MOF linker is difficult to analyze due to random distribution over the surface. In situ encapsulation is a popular technique substantiating the NCs embedded into the pore matrices. Among those methods, the bottle-around-a-ship is promising due to the scope for the NCs ligand and MOF linker modification. Due to its ultrasmall size (NCs), and low loading, difficult to identify through PXRD. However, the microscopic technique like HRTEM is frequently being used. Here, we highlighted the future perspective and scope for NC@MOF composite catalysis (Table 1).| Method of preparation | Composite | Application | Ref. |
|---|---|---|---|
| Surface anchoring | Au1Ag39/ZIF-8, Ag40/ZIF-8, Au12Ag32/ZIF-8, Ag46Au24/ZIF-8, Au4Cu4/ZIF-8, Pd3Cl/ZIF-8 | Phenylacetylene carboxylation, nitrophenol reduction, and para-substituted benzylamine oxidative coupling | 58 |
| Au25(L-Cys)18/UiO-66-NH2, Au25(PET)18/UiO-66-NH2 | Photocatalytic hydrogen evolution reaction | 60 | |
| Au25/ZIF-8 | Reduction of 4-nitrophenol | 62 | |
| Au25(GSH)18/MIL-125 (Ti), Au25(GSH)18/MIL-101 (Cr), Au25(GSH)18/ZIF-8 | Reduction of 4-nitrophenol | 61 | |
| ZIF-8@Au25@ZIF-67, ZIF-8@Au25@ZIF-8 | Reduction of 4-nitrophenol and carboxylation of terminal alkyne | 63 | |
| Solution impregnation | Au133(SR)52@bMOF-102/106 | — | 64 |
| Au NC@ZIF-8, Au NC@ZIF-90 | CO oxidation | 65 | |
| Ship in a bottle | Au11:PPh3@ZIF-8, Au13Ag12:PPh3@MIL-100 | Oxidation of benzyl alcohol | 66 |
| Au NC@UiO-68-NHC | Photocatalytic CO2 reduction | 67 | |
| AgNC@MIL-125-NH2 | Water purification, hydrogenation of 4-nitroaniline | 68 | |
| Pd@UiO-66-NH2, Pd@UiO-66-(NH2)2, Pd@UiO-66-CH3, Pd@UiO-66-(CH3)2 | — | 69 | |
| Bottle around a ship | Au12Ag32(SR)30@ZIF-8, Ag44(SR)30@ZIF-8, Ag12Cu28(SR)30@ZIF-8 | CO2 conversion | 59 |
| Au25(Capt)18@UiO-66-X | Oxidative esterification of furfural | 87 | |
| Au38I(S-Adm)19@UiO-66-X, Au38S(S-Adm)20@UiO-66-X, Au38IS(S-Adm)19@UiO-66-X | Reduction of 4-nitrophenol | 70 | |
| Ag25@UiO-66-NH2, AuAg24@UiO-66-NH2, PtAg24@UiO-66-NH2, PdAg24@UiO-66-NH2 | Photocatalytic hydrogen production | 70 | |
| Ptn@ZIF-8 (n ≈ 12) | Catalytic hydrogenation of 1-hexene | 72 | |
| Co8Pt4@ZIF-8 | — | 73 | |
| Cu NC@UiO-66-NH2, Cu NC@MOF-801 | Photocatalytic CO2 reduction | 74 | |
| Au25@Zn-MOF-74, Au25@Ni-MOF-74, Au25@Co-MOF-74, Au25@Mg-MOF-74 | Catalytic reduction of 2-nitro benzonitrile | 76 |
1. There are still limitations to the encapsulation of NCs into MOFs due to the disruption of NC's guest particles for the growth of MOF. Besides, it is limited to the type and number of MOFs e.g., ZIF-8, ZIF-67, UiO-66, UiO-66-NH2, MOF-108, MIL-101, etc. Controlling the both NC structure and MOF and recognizing the active site is still a challenge, however, scope to modulate. By carefully modifying the pore structures, components, and functional groups of the MOF supports, it is possible to substantially enhance and optimize the catalytic activity and selectivity of the immobilized composite.
2. Surface ligands of NCs often pose challenges to catalysis, yet they can also play a role in facilitating the activation of reactants and enabling the regulation of activity and selectivity. The complex role of ligands presents an intriguing subject for future exploration. The aspect of selectivity control is particularly significant and merits systematic examination, given that achieving elevated selectivity is a key aim in catalytic processes.
3. Recent research has uncovered instances where the support may actively engage in specific reactions. Analyzing the interactions between ligand-protected NCs and the support (MOFs) is significantly more difficult than assessing bare clusters on supports. Therefore, overcoming the associated challenges through experimental investigation of interfacial processes with computational support will be crucial for future advancements in this area.
4. The catalytic process occurring on NC catalysts will be examined at the atomic level and in real-time. We anticipate that the integration of NC with MOF give rise to a new class of functional composite and uncover the fundamental atomic origins of structure–activity relationships. Ultimately, this advancement will enable chemists to tailor active sites for targeted reactions.
Data availability
The data used for this feature article will be available on request for academic use.Conflicts of interest
There are no conflicts to declare.Acknowledgements
AK thanks Anusandhan National Research Foundation (ANRF), New Delhi for a National Post-Doctoral Fellowship (PDF/2023/002916). GJ thanks the MoE, India for Prime Minister Research Fellowship (PMRF).References
- K. N. Ganesh, D. Zhang, S. J. Miller, K. Rossen, P. J. Chirik, M. C. Kozlowski, J. B. Zimmerman, B. W. Brooks, P. E. Savage, D. T. Allen and A. M. Voutchkova-Kostal, Environ. Sci. Technol. Lett., 2021, 8, 487–491 CrossRef CAS.
- J. R. Mihelcic, R. O. Barra, B. W. Brooks, M. L. Diamond, M. J. Eckelman, J. MacDonald Gibson, S. Guidotti, A. Ikeda-Araki, M. Kumar, Y. Maiga, J. McConville, S. L. Miller, V. Pizarro, F. Rosario-Ortiz, S. Wang and J. B. Zimmerman, Environ. Sci. Technol. Lett., 2023, 10, 961–962 Search PubMed.
- K.-G. Liu, Z. Sharifzadeh, F. Rouhani, M. Ghorbanloo and A. Morsali, Coord. Chem. Rev., 2021, 436, 213827 CrossRef CAS.
- X. F. Lu, Y. Fang, D. Luan and X. W. D. Lou, Nano Lett., 2021, 21, 1555–1565 CrossRef CAS PubMed.
- M. A. Abbas, M. Jeon and J. H. Bang, J. Phys. Chem. C, 2022, 126, 16928–16942 CrossRef CAS.
- Y. Du, H. Sheng, D. Astruc and M. Zhu, Chem. Rev., 2020, 120, 526–622 CrossRef CAS PubMed.
- Q. Yan, Z. Yuan, Y. Wu, C. Zhou, Y. Dai, X. Wan, D. Yang, X. Liu, N. Xue, Y. Zhu and Y. Yang, Precis. Chem., 2023, 1, 468–479 CrossRef CAS.
- D. Yang, J. Wang, Q. Wang, Z. Yuan, Y. Dai, C. Zhou, X. Wan, Q. Zhang and Y. Yang, ACS Nano, 2022, 16, 15681–15704 CrossRef CAS PubMed.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- A. Baghdasaryan and T. Bürgi, Nanoscale, 2021, 13, 6283–6340 RSC.
- X. Sun, P. Wang, X. Yan, H. Guo, L. Wang, Q. Xu, B. Yan, S. Li, J. He, G. Chen, H. Shen and N. Zheng, iScience, 2023, 26, 107850 CrossRef CAS PubMed.
- X. Cai, Y. Sun, J. Xu and Y. Zhu, Chem. – Eur. J., 2021, 27, 11539–11547 CrossRef CAS PubMed.
- N. Yan, L. Liao, J. Yuan, Y.-J. Lin, L.-H. Weng, J. Yang and Z. Wu, Chem. Mater., 2016, 28, 8240–8247 CrossRef CAS.
- H. Liang, Q. Chen, Q.-L. Mo, Y. Wu and F.-X. Xiao, J. Mater. Chem. A, 2023, 11, 9401–9426 RSC.
- M. H. Naveen, R. Khan and J. H. Bang, Chem. Mater., 2021, 33, 7595–7612 CrossRef CAS.
- N. Baig, I. Kammakakam and W. Falath, Mater. Adv., 2021, 2, 1821–1871 RSC.
- A. Kumar and R. Ananthakrishnan, ACS Appl. Energy Mater., 2022, 5, 2706–2719 CrossRef CAS.
- H. Chen, L. Zou, E. Hossain, Y. Li, S. Liu, Y. Pu and X. Mao, Biomater. Sci., 2024, 12, 4283–4300 RSC.
- P. Viswanathan, G. Sivakumar, A. Gowrisankar, R. Tolani, S. Manivannan, K. Kim and R. Ramaraj, J. Environ. Chem. Eng., 2023, 11, 111431 CrossRef CAS.
- J. Yang, Y. Peng, S. Li, J. Mu, Z. Huang, J. Ma, Z. Shi and Q. Jia, Coord. Chem. Rev., 2022, 456, 214391 CrossRef CAS.
- M. A. Mahmoud, B. Garlyyev and M. A. El-Sayed, J. Phys. Chem. C, 2013, 117, 21886–21893 CrossRef CAS.
- S. B. Beil, S. Bonnet, C. Casadevall, R. J. Detz, F. Eisenreich, S. D. Glover, C. Kerzig, L. Næsborg, S. Pullen, G. Storch, N. Wei and C. Zeymer, JACS Au, 2024, 4, 2746–2766 CrossRef CAS PubMed.
- G. Deng, H. Yun, M. S. Bootharaju, F. Sun, K. Lee, X. Liu, S. Yoo, Q. Tang, Y. J. Hwang and T. Hyeon, J. Am. Chem. Soc., 2023, 145, 27407–27414 CrossRef CAS PubMed.
- T. Kawawaki, T. Okada, D. Hirayama and Y. Negishi, Green Chem., 2024, 26, 122–163 RSC.
- Y.-P. Xie, Y.-L. Shen, G.-X. Duan, J. Han, L.-P. Zhang and X. Lu, Mater. Chem. Front., 2020, 4, 2205–2222 RSC.
- Y. Wang and T. Bürgi, Nanoscale Adv., 2021, 3, 2710–2727 RSC.
- Q. Zhu, X. Huang, Y. Zeng, K. Sun, L. Zhou, Y. Liu, L. Luo, S. Tian and X. Sun, Nanoscale Adv., 2021, 3, 6330–6341 RSC.
- S. Masuda, K. Sakamoto and T. Tsukuda, Nanoscale, 2024, 16, 4514–4528 RSC.
- A. Ma, J. Wang, Y. Wang, Y. Zuo, Y. Ren, X. Ma and S. Wang, Polyoxometalate, 2024, 3, 9140054 CrossRef.
- Y. Zhang, J. Zhang, Z. Li, Z. Qin, S. Sharma and G. Li, Chem. Commun., 2023, 6, 24 CrossRef CAS PubMed.
- X. Liu and D. Astruc, Coord. Chem. Rev., 2018, 359, 112–126 CrossRef CAS.
- M. A. Habeeb Muhammed and T. Pradeep, Small, 2011, 7, 204–208 CrossRef CAS PubMed.
- R. Tian, S. Zhang, M. Li, Y. Zhou, B. Lu, D. Yan, M. Wei, D. G. Evans and X. Duan, Adv. Funct. Mater., 2015, 25, 5006–5015 CrossRef CAS.
- S. Zhao, R. Jin, H. Abroshan, C. Zeng, H. Zhang, S. D. House, E. Gottlieb, H. J. Kim, J. C. Yang and R. Jin, J. Am. Chem. Soc., 2017, 139, 1077–1080 CrossRef CAS PubMed.
- H. Huang, Z. He, X. Lin, W. Ruan, Y. Liu and Z. Yang, Appl. Catal., A, 2015, 490, 65–70 CrossRef CAS.
- Z. Bian, N. Dewangan, Z. Wang, S. Pati, S. Xi, A. Borgna, H. Kus and S. Kawi, ACS Appl. Nano Mater., 2021, 4, 1112–1125 CrossRef CAS.
- Z. Hong-Cai, L. R. Jeffrey and Y. M. Omar, Chem. Rev., 2012, 112, 673–674 CrossRef PubMed.
- B. P. Biswal, A. Bhaskar, R. Banerjee and U. K. Kharul, Nanoscale, 2015, 7, 7291–7298 RSC.
- R. Khajavian, M. Mirzaei and H. Alizadeh, Dalton Trans., 2020, 49, 13936–13947 RSC.
- D. Narváez-Celada and A. S. Varela, J. Mater. Chem. A, 2022, 10, 5899–5917 RSC.
- C. H. Hendon, A. J. Rieth, M. D. Korzyński and M. Dincă, ACS Cent. Sci., 2017, 3, 554–563 CrossRef CAS PubMed.
- D. Li, H.-Q. Xu, L. Jiao and H.-L. Jiang, Energy Chem., 2019, 1, 100005 CrossRef.
- S. Mandal, S. Natarajan, P. Mani and A. Pankajakshan, Adv. Funct. Mater., 2021, 31, 2006291 CrossRef CAS.
- T. Islamoglu, S. Goswami, Z. Li, A. J. Howarth, O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2017, 50, 805–813 CrossRef CAS PubMed.
- Y. Wen, J. Zhang, Q. Xu, X.-T. Wu and Q.-L. Zhu, Coord. Chem. Rev., 2018, 376, 248–276 CrossRef CAS.
- L. Chen and Q. Xu, Matter, 2019, 1, 57–89 CrossRef.
- M. Mukoyoshi and H. Kitagawa, Chem. Commun., 2022, 58, 10757–10767 RSC.
- Y.-M. Li, J. Hu and M. Zhu, Coord. Chem. Rev., 2023, 495, 215364 CrossRef CAS.
- L. Luo and R. Jin, iScience, 2021, 24, 103206 CrossRef CAS PubMed.
- R. K. Aparna, S. Mukherjee, S. S. Rose and S. Mandal, Inorg. Chem., 2022, 61, 16441–16447 CrossRef CAS PubMed.
- R. K. Aparna, V. Surendran, D. Roy, B. Pathak, M. M. Shaijumon and S. Mandal, ACS Appl. Energy Mater., 2023, 6, 4072–4078 CrossRef CAS.
- S. Muhamed, R. K. Aparna, A. Karmakar, S. Kundu and S. Mandal, Inorg. Chem., 2023, 62, 7195–7202 CrossRef CAS PubMed.
- R. K. Aparna, A. Karmakar, R. T. Arsha, S. Kundu and S. Mandal, Chem. Commun., 2023, 59, 10444–10447 RSC.
- J. An, O. K. Farha, J. T. Hupp, E. Pohl, J. I. Yeh and N. L. Rosi, Nat. Commun., 2012, 3, 604 CrossRef PubMed.
- A. Dutta, A. G. Wong-Foy and A. J. Matzger, Chem. Sci., 2014, 5, 3729–3734 RSC.
- S. Øien, D. Wragg, H. Reinsch, S. Svelle, S. Bordiga, C. Lamberti and K. P. Lillerud, Cryst. Growth Des., 2014, 14, 5370–5372 CrossRef.
- Y. Yun, Y. Fang, W. Fu, W. Du, Y. Zhu, H. Sheng, D. Astruc and M. Zhu, Small, 2022, 18, 2107459 CrossRef CAS PubMed.
- L. Sun, Y. Yun, H. Sheng, Y. Du, Y. Ding, P. Wu, P. Li and M. Zhu, J. Mater. Chem. A, 2018, 6, 15371–15376 RSC.
- A. Yao, Y. Du, M. Han, Y. Wang, J. Hu, Q. Zhu, H. Sheng and M. Zhu, Nano Res., 2023, 16, 1527–1532 CrossRef CAS.
- K. Chu, Y. Luo, D. Wu, Z. Su, J. Shi, J. Z. Zhang and C.-Y. Su, J. Phys. Chem. Lett., 2021, 12, 8003–8008 CrossRef CAS PubMed.
- Y. Luo, S. Fan, W. Yu, Z. Wu, D. A. Cullen, C. Liang, J. Shi and C. Su, Adv. Mater., 2018, 30, 1704576 CrossRef PubMed.
- Y. Yun, H. Sheng, K. Bao, L. Xu, Y. Zhang, D. Astruc and M. Zhu, J. Am. Chem. Soc., 2020, 142, 4126–4130 CrossRef CAS PubMed.
- C. Liu, C. Zeng, T.-Y. Luo, A. D. Merg, R. Jin and N. L. Rosi, J. Am. Chem. Soc., 2016, 138, 12045–12048 CrossRef CAS PubMed.
- L. Dou, S. Wu, D.-L. Chen, S. He, F.-F. Wang and W. Zhu, J. Phys. Chem. C, 2018, 122, 8901–8909 CrossRef CAS.
- L. Liu, Y. Song, H. Chong, S. Yang, J. Xiang, S. Jin, X. Kang, J. Zhang, H. Yu and M. Zhu, Nanoscale, 2016, 8, 1407–1412 RSC.
- Y. Jiang, Y. Yu, X. Zhang, M. Weinert, X. Song, J. Ai, L. Han and H. Fei, Angew. Chem., Int. Ed., 2021, 60, 17388–17393 CrossRef CAS PubMed.
- A. Arenas-Vivo, S. Rojas, I. Ocaña, A. Torres, M. Liras, F. Salles, D. Arenas-Esteban, S. Bals, D. Ávila and P. Horcajada, J. Mater. Chem. A, 2021, 9, 15704–15713 RSC.
- J. King, Z. Lin, F. Zanca, H. Luo, L. Zhang, P. Cullen, M. Danaie, M. Hirscher, S. Meloni, A. M. Elena and P. Á. Szilágyi, Phys. Chem. Chem. Phys., 2024, 26, 25021–25028 Search PubMed.
- Q. You, H. Wang, Y. Zhao, W. Fan, W. Gu, H.-L. Jiang and Z. Wu, J. Am. Chem. Soc., 2024, 146, 9026–9035 CrossRef CAS PubMed.
- H. Wang, X. Zhang, W. Zhang, M. Zhou and H.-L. Jiang, Angew. Chem., Int. Ed., 2024, 63, e202401443 CrossRef CAS PubMed.
- K. Kratzl, T. Kratky, S. Günther, O. Tomanec, R. Zbořil, J. Michalička, J. M. Macak, M. Cokoja and R. A. Fischer, J. Am. Chem. Soc., 2019, 141, 13962–13969 CrossRef CAS PubMed.
- K. L. Kollmannsberger, Poonam, C. Cesari, R. Khare, T. Kratky, M. Boniface, O. Tomanec, J. Michalička, E. Mosconi, A. Gagliardi, S. Günther, W. Kaiser, T. Lunkenbein, S. Zacchini, J. Warnan and R. A. Fischer, Chem. Mater., 2023, 35, 5475–5486 CrossRef CAS.
- S. Dai, T. Kajiwara, M. Ikeda, I. Romero-Muñiz, G. Patriarche, A. E. Platero-Prats, A. Vimont, M. Daturi, A. Tissot, Q. Xu and C. Serre, Angew. Chem., Int. Ed., 2022, 61, e202211848 CrossRef CAS PubMed.
- H. Zhao, S. Becharef, E. Dumas, F. Carn, G. Patriarche, S. Mura, F. Gazeau, C. Serre and N. Steunou, Nanoscale, 2024, 16, 12037–12049 RSC.
- H. Wang, X. Liu, Y. Zhao, Z. Sun, Y. Lin, T. Yao and H.-L. Jiang, Natl. Sci. Rev., 2024, 11, nwae252 CrossRef PubMed.
- R.-W. Huang, Y.-S. Wei, X.-Y. Dong, X.-H. Wu, C.-X. Du, S.-Q. Zang and T. C. W. Mak, Nat. Chem., 2017, 9, 689–697 CrossRef CAS PubMed.
- Z. Chang, X. Jing, C. He, X. Liu and C. Duan, ACS Catal., 2018, 8, 1384–1391 CrossRef CAS.
- X.-H. Wu, P. Luo, Z. Wei, Y.-Y. Li, R.-W. Huang, X.-Y. Dong, K. Li, S.-Q. Zang and B. Z. Tang, Adv. Sci., 2019, 6, 1801304 CrossRef PubMed.
- X.-Y. Dong, H.-L. Huang, J.-Y. Wang, H.-Y. Li and S.-Q. Zang, Chem. Mater., 2018, 30, 2160–2167 CrossRef CAS.
- C. Wang, Y.-J. Wang, C.-L. He, Q.-Y. Wang and S.-Q. Zang, JACS Au, 2021, 1, 2202–2207 CrossRef CAS PubMed.
- Z.-Y. Wang, M.-Q. Wang, Y.-L. Li, P. Luo, T.-T. Jia, R.-W. Huang, S.-Q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2018, 140, 1069–1076 CrossRef CAS PubMed.
- M. J. Alhilaly, R.-W. Huang, R. Naphade, B. Alamer, M. N. Hedhili, A.-H. Emwas, P. Maity, J. Yin, A. Shkurenko, O. F. Mohammed, M. Eddaoudi and O. M. Bakr, J. Am. Chem. Soc., 2019, 141, 9585–9592 CrossRef CAS PubMed.
- G. Deng, B. K. Teo and N. Zheng, J. Am. Chem. Soc., 2021, 143, 10214–10220 CrossRef CAS PubMed.
- X.-H. Ma, J.-Y. Wang, J.-J. Guo, Z.-Y. Wang and S.-Q. Zang, Chin. J. Chem., 2019, 37, 1120–1124 CrossRef CAS.
- M. Zhao, S. Huang, Q. Fu, W. Li, R. Guo, Q. Yao, F. Wang, P. Cui, C.-H. Tung and D. Sun, Angew. Chem., Int. Ed., 2020, 59, 20031–20036 CrossRef CAS PubMed.
- H. Wang, X. Liu, W. Yang, G. Mao, Z. Meng, Z. Wu and H.-L. Jiang, J. Am. Chem. Soc., 2022, 144, 22008–22017 CrossRef CAS PubMed.
- Y. Zhao, S. Zhuang, L. Liao, C. Wang, N. Xia, Z. Gan, W. Gu, J. Li, H. Deng and Z. Wu, J. Am. Chem. Soc., 2020, 142, 973–977 CrossRef CAS PubMed.
- W. Zhang, G. Lu, C. Cui, Y. Liu, S. Li, W. Yan, C. Xing, Y. R. Chi, Y. Yang and F. Huo, Adv. Mater., 2014, 26, 4056–4060 CrossRef CAS PubMed.
- A. Kirchon, L. Feng, H. F. Drake, E. A. Joseph and H.-C. Zhou, Chem. Soc. Rev., 2018, 47, 8611–8638 RSC.
- Y. Lu, Y. Jiang, X. Gao and W. Chen, Chem. Commun., 2014, 50, 8464–8467 RSC.
- P. M. Schneider, K. L. Kollmannsberger, C. Cesari, R. Khare, M. Boniface, B. Roldán Cuenya, T. Lunkenbein, M. Elsner, S. Zacchini, A. S. Bandarenka, J. Warnan and R. A. Fischer, ChemElectroChem, 2024, 11, e202300476 CrossRef CAS.
- Q. Yang, Q. Xu and H.-L. Jiang, Chem. Soc. Rev., 2017, 46, 4774–4808 RSC.
- S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li and H.-C. Zhou, Adv. Mater., 2018, 30, 1704303 CrossRef PubMed.
- B. Li, J.-G. Ma and P. Cheng, Small, 2019, 15, 1804849 CrossRef PubMed.
- J. Yu, C. Mu, B. Yan, X. Qin, C. Shen, H. Xue and H. Pang, Mater. Horiz., 2017, 4, 557–569 RSC.
- J. Zhang, X. Cui, Y. Zhou, T. Kong, Y. Wang, X. Wei and Y. Xiong, Chem. Commun., 2023, 59, 2299–2302 RSC.
- X. Li, Z. Guo, C. Xiao, T. W. Goh, D. Tesfagaber and W. Huang, ACS Catal., 2014, 4, 3490–3497 CrossRef CAS.
Footnote |
| † Both GJ and RKA made equal contribution. |
| This journal is © The Royal Society of Chemistry 2025 |