Open Access Article
Open Access ArticleAnion exchange polymer modified electrodes for detection of Δ9-tetrahydrocannabinol (Δ9-THC): a potential electrochemical sensor for point-of-care and roadside testing†
Artemis
Oddy
a,
Steven
Holdcroft
b and
Sandra
Hernandez-Aldave
*a
aDepartment of Chemistry, University of Huddersfield, Queensgate, Huddersfield HD1 3DH, UK. E-mail: s.hernandezaldave@hud.ac.uk
bDepartment of Chemistry, Simon Fraser University, Burnaby, Metropolitan Vancouver, British Columbia, V5A 1S6, Canada
First published on 24th December 2024
Abstract
The effect of a polybenzimidazolium anion exchange polymer on improving sensitivity and selectivity toward the electrochemical detection of Δ9-tetrahydrocannabinol (Δ9-THC) has been investigated. Herein we report a rapid, inexpensive and stable approach to detecting 10–1000 ng mL−1 of Δ9-THC in buffered solutions and in human saliva.
Cannabis, commonly known as marijuana, is increasing in use worldwide, with over 192 million users,1 who use it not only for recreational but also for medicinal purposes. In 2023, 7.6% of the UK population, ∼2.5 million people, reported using cannabis in the last 12 months.2 Over one-third of people aged 16 to 59 years who used cannabis in the last year consumed the drug more than once a month, with 8.3% using it in their everyday life. The frequent use of cannabis has increased to over 8% since 2020 for those aged 30 to 34 years.
Several countries worldwide have legalised the use of cannabis for recreational purposes; however, its use is restricted in public parks, schools, and hospitals. Driving under the influence of cannabis is a major concern. A recent meta-analysis has found that cannabis increased impairment for lane control and reaction time, as well as decreased compensatory speed.3 In the UK, among the deceased drivers between 2014 and 2021 in road accidents, cannabis was the most common illegal drug leading to road fatality.4
Δ9-Tetrahydrocannabinol (Δ9-THC) is the psychoactive component in cannabis; because of its high lipophilicity it can easily cross over the blood–brain barrier and interact with the central nervous system (CNS) cannabinoid receptors. This leads to several psychological effects such as euphoria, relaxation, decreased concentration, somnolence, temporal distortions, and hallucinations.5,6
Therefore, multiple jurisdictions have attempted to set per se Δ9-THC limits in blood and saliva. In Canada, a roadside threshold of 5 ng mL−1 when analysed in blood and 25 ng mL−1 when measured in saliva has been adopted.3 In England and Wales, a per se threshold of 2 ng mL−1 in blood has been established.7 In the US the per se limits or cutoffs vary among different states but range from THC blood levels of 1 to 5 ng mL−1 Δ9-THC.8 On-site testing for THC levels has proved problematic, and hence there is a need for rapid roadside detection of Δ9-THC that can be used to accurately screen drivers.
Δ9-THC can be tested in urine, blood, breath, and saliva.9 Urine tests, a commonly employed method for drug testing, can only detect Δ9-THC metabolites, which are only traceable in urine several hours after the intake of cannabis, far past the window of intoxication and impairment.10 Blood testing is not necessarily a good approach for Δ9-THC detection probably due to its high log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and enterohepatic recirculation: Δ9-THC usually accumulates in the tissues for long periods of time and it can be detected in the blood up to 30 days post-consumption.6 Δ9-THC is retained in the breath for short periods of time, no longer than 3 h, making it difficult to determine if the driver is still under its influence. Oral fluid appears to be preferable for Δ9-THC roadside monitoring, being quick and less invasive. Preliminary evidence exists that indicates that the concentration of Δ9-THC in saliva can be correlated with impairment.10
P and enterohepatic recirculation: Δ9-THC usually accumulates in the tissues for long periods of time and it can be detected in the blood up to 30 days post-consumption.6 Δ9-THC is retained in the breath for short periods of time, no longer than 3 h, making it difficult to determine if the driver is still under its influence. Oral fluid appears to be preferable for Δ9-THC roadside monitoring, being quick and less invasive. Preliminary evidence exists that indicates that the concentration of Δ9-THC in saliva can be correlated with impairment.10
The Δ9-THC concentration in saliva has been found to be affected by the method of consumption. Via smoking, it is reported to take 0.17 h to detect THC's maximum concentration. The Δ9-THC level in the saliva of frequent smokers is on average 9 μM (2.83 μg mL−1) and 3 μM (944 ng mL−1) for occasional users. For Δ9-THC consumed orally, it takes 0.33–0.41 h to detect the maximum salivary concentration presenting concentrations of 944 nM (297 ng mL−1) in frequent users and 642 nM (202 ng mL−1) in occasional users.11 Hence, it is necessary to develop a detection method able to comply with current jurisdictions and capable of detecting a range of salivary Δ9-THC concentrations from 25 to 1000 ng mL−1.
In recent years, multiple techniques for detecting Δ9-THC in saliva have been researched including the use of liquid chromatography with UV-vis or mass spectrometry detection, immunoassays, and surface enhanced Raman scattering.5,12 Although these techniques can detect low levels of Δ9-THC, they rely on relatively costly, non-portable, or non-scalable instrumentation. For example, the current on-market roadside test device, Dräger DrugTest 5000, employs an immunoassay approach to detect Δ9-THC with a detection limit of 5 ng mL−1 but at high cost (∼5500 USD). Another device is the Druglizer LE5 Drug Testing System, based on a competitive lateral flow immunoassay with a Δ9-THC cut-off of 15 ng mL−1, provides qualitative test result costs in excess of 3195 USD plus cartridges. Other commercial roadside test devices are based on colour test strips, but do not provide quantitative information (Rapid STAT®, DrugWipe® S), which is a key requirement in jurisdictions where there is a Δ9-THC threshold.
Electrochemical sensors, on the other hand, offer the possibility of low cost, rapid, high selectivity and sensitivity, and low sample volume. Electrochemical sensors are simple and portable devices for the detection of Δ9-THC in biological fluids such as saliva.5,12
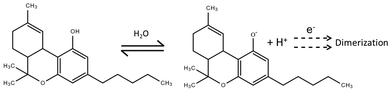
Δ9-THC is detectable electrochemically due to the direct oxidation of the phenol moiety. The hydroxy group is deprotonated at high pH, leading to the formation of a phenoxide anion that can be oxidised under low potential values. The oxidation of the Δ9-THC has been proven to be favoured at carbon-based surfaces. However, the instability of phenoxy radical produced leads to subsequent chemical reactions (Fig. 1).13,14
Several electrochemical approaches for Δ9-THC detection have been published. However, some of the designs required lengthy electrodes or sample pre-treatments, using modified electrodes which have a short shelf-life (Table S1, ESI†).
In this context, the presented work explores the potential of a low-cost anion conductive polymer as a modification for screen printed carbon electrodes (SPCE). Anion exchange polymers, widely used as alkaline anion exchange membranes in fuel cells and electrolysers, are positively charged, and are anticipated to result in the pre-concentration of Δ9-THC and facilitate its stabilisation by hindering inherent dimerization reactions. Pre-concentration of Δ9-THC is expected to enhance the signal to noise ratio, improving the sensitivity of the sensor. Anion exchange polymers can also be designed to possess hydrophobic domains leading to hydrophobic interactions with Δ9-THC, promoting its stabilisation and hindering the formation of by-products.
Lastly, anion exchange polymers repel positively charged interferences present in saliva, such as nicotine.
In this work, we demonstrate that anion exchange polymer, HMT-PMBI, meets all of the above and can be used to fabricate modified screen printed electrodes (SPEs)s, which can electrochemically detect Δ9-THC in concentrations as low as 10 ng mL−1 in buffered solutions. Moreover, preliminary studies performed on untreated saliva are presented, wherein Δ9-THC is detected, opening the possibility in the near future, of rapidly identifying and quantifying Δ9-THC in the saliva samples of human subjects.
In order to achieve sensing detection, several experimental conditions were optimised. In the initial studies the goal was to voltammetrically evaluate screen printed electrodes prepared by the drop casting HMT-PMBI polymer solutions. Two different electrodes were investigated: Gii-Sense (pure 3D graphene foam sensing electrodes) and Metrohm C11L, details of which are presented in the ESI.† All the electrochemical cells employed were based on screen printed electrodes presenting a carbon working electrode, carbon counter electrode and Ag/AgCl reference electrode. For both Gii-Sense and Metrohm electrodes the presence of the anionic polymer film increases the anodic current density of Δ9-THC oxidation (Fig. S1, ESI†). In the presence of 10 μg mL−1 Δ9-THC, a typical unmodified Gii-Sense SPCE presents a peak current density of 5.6 μA cm−2 and unmodified Metrohm SPE, 14.6 μA cm−2. These results were unexpected as it was thought that the hydrophobic Δ9-THC molecule would form hydrophobic interactions with the graphene foam leading to higher preconcentration and higher sensitivity. However, it is worth noting that the 3D foam structure of the Gii-Sense electrode presents higher capacitive currents, increases the background noise, possibly reducing sensitivity. The modification of SPEs with a thin polymer film leads to an increase in anodic current density, consistent with the hypothesis of preconcentration by the positively charged HMT-PMBI solid polymer electrolyte with the negatively charged Δ9-THC analyte. Furthermore, Δ9-THC also possesses a hydrophobic character which is complementary with the highly hydrophobic segments of the polymer (Fig. 1 and 2).15 These segmented electrostatic and hydrophobic interactions are deemed responsible for the preconcentration of Δ9-THC and an increase in sensitivity of its detection. The polymer modified Metrohm electrodes exhibited a 4-fold increase in the anodic peak current (63.4 μA cm−2), and the modified Gii-Sense electrodes yielded a 13× response (71.2 μA cm−2) compared to the pristine electrodes. The larger increase of the latter is believed to be due to its foam-like structure, presenting a higher electrode effective area and increased porosity, thus, facilitating the integration of the polymer within the foam during the drop-cast method and easing the diffusion of the electroactive species in comparison with packet carbon working electrodes.
Both electrodes were examined under conditions of low Δ9-THC concentrations (50 ng mL−1) which are more akin to roadside saliva Δ9-THC concentrations (Fig. S2, ESI†). For 50 ng mL−1 Δ9-THC concentrations, the polymer modified Metrohm electrodes presented a clearly visible oxidation peak, whereas modified Gii-Sense electrodes did not. No reproducible anodic peak was achievable with polymer modified Gii-Sense electrodes with Δ9-THC concentrations below 500 ng mL−1. These results are consistent with a large capacitive current masking the low Δ9-current density of THC oxidation.
Differential pulse voltammetry (DVP) (E step: 0.1 V, E pulse: 0.2 V, T pulse: 0.02 s, scan rate: 0.01 V s−1) using Metrohm C11L electrodes provided greater consistency and smaller background, capacitive signals, resulting in enhanced Δ9-THC detection at low Δ9-THC concentrations.9 Modified Gii-Sense electrodes on the other hand showed complementary sensitivity in the high concentration regime.
Taking into consideration the pKa of Δ9-THC phenol (10.6), the effect of solution pH on electrochemical detection of Δ9-THC at polymer modified electrodes was examined using Metrohm electrodes (Fig. S3, ESI†). The electrochemical response to Δ9-THC was found to increase with increasing pH, which is deemed to be due to increasing deprotonation, rendering an anion which may diffuse into the cationic polymer film. Moreover, at higher pH values the Δ9-THC oxidation signal shifts to more negative potentials. This effect was previously observed by Williams et al.,9 where a shift in the reaction quotient was suggested. The use of solutions of pH > 11 were avoided as the high concentration of OH− competes against Δ9-THC anions for polycation sites in the film, lowering the concentration of electrostatically bound Δ9-THC. In contrast, the anodic current decreases below pH 5.0–6.0 due to protonation of Δ9-THC to form a cation, which is repelled by the polycationic film.12,16,17 Therefore, the pH was optimized to 10 employing TRIS buffer.
The quantity of HMT-PMBI deposited on electrodes was found to have a direct impact on the anodic oxidation of Δ9-THC (Fig. S4, ESI†). The anodic current corresponding to Δ9-THC oxidation increased with increasing concentrations of drop cast HMT-PMBI solutions, up to 2% (w/v). However, the oxidative peak decreased when higher concentration polymer solutions were deposited, dropping to virtually no observable current for 10% (w/v) solutions, presumably due to high ionic resistance and long diffusion lengths imposed by the film. Consequently, 2% (w/v) of polymer was considered to be the optimal concentration for further study.
The rate of Δ9-THC uptake on the modified electrodes was investigated by immersing modified electrodes in a pH 10, TRIS buffered solution containing 1000 ng mL−1 Δ9-THC (Fig. S5, ESI†). It was found that the electrochemical response of Δ9-THC increases with time, reaching a maximum after 45 minutes. 40 minutes was chosen as the pre-concentration period.
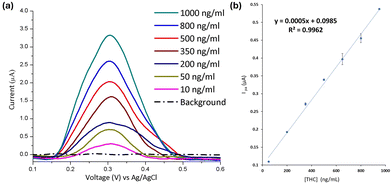
With all the above parameters investigated, a Δ9-THC calibration curve was constructed (Fig. 3). Fig. 3a presents DVP curves of polymer modified electrodes recorded in buffered solutions of increasing Δ9-THC concentration, and Fig. 3b shows the plot of the anodic peak current vs. Δ9-THC concentration. A linear regressive response for Δ9-THC was found (Ipa (μA) = 0.0005X (ng mL−1) + 0.0985 (R2 = 0.996)). Δ9-THC was detected at concentrations as low as 10 ng mL−1. The calculated limit of detection (LOD) was 5.28 ng mL−1 obtained from the relation LOD = 3.3Sb/S.18 Consequently, the calculated limit of quantification (LOQ), was 16 ng mL−1 (10 SD blank per slope).
Reproducibility of the response to 1000 ng mL−1 Δ9-THC was examined by preparing 11 different modified electrodes (Fig. S6, ESI†). The mean of the anodic peaks was 0.614 μA [std. dev., 0.0587, 9.5% RSD, and 0.614 ± 0.0347 (± 6.65%) margin of error at 95% confidence interval] (Fig. S6, ESI†). Moreover, the electrodes exhibited notable stability over time, when stored in a dry room and room temperature environment; no visible differences in their Δ9-THC detection capabilities were detected after several weeks of storage.
A variety of components are expected to be found in human saliva during a roadside testing. These include ascorbic acid (AA) or caffeine (CF).12 The impact of these analytes on pristine and modified electrodes was therefore investigated (Fig. 4) by mixing 1 μg mL−1 of Δ9-THC with increasing concentrations of AA and CF (concentrations ranging from 10 μg mL−1 to 100 μg mL−1) in TRIS buffer solutions (pH 10). The electrochemical responses of bare electrodes are significantly affected (up to 400% increase in the anodic current) by the presence of the AA and/or CF, indicating their clear interference.
In contrast, the presence of AA and CF did not influence the electrochemical response of Δ9-THC even for 100-fold mass ratios of AA and/or CF.
Selectivity is deemed to be due to the hydrophobic nature of the polymer structure repelling the relatively hydrophilic ascorbic acid and promoting the preconcentration of the hydrophobic Δ9-THC.
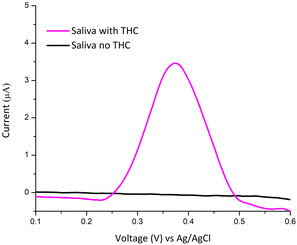
Preliminary studies of modified electrodes were performed on human saliva (Fig. 5) spiked with Δ9-THC. 5 μg mL−1 of Δ9-THC was added to 1 mL of saliva and diluted to a 9 mL solution. Dilution of the saliva is recommended based on two conditions: (i) typical human saliva possesses kinematic viscosities of 1.40 mm2 s−1 whereas that of water is 1 mm2 s−1; high viscosities lower the absorption rate of electroactive species on the surface of the carbon working electrode. (ii) While saliva has neutral pH, detection is optimised at pH 10. Voltammetry on saliva alone did not reveal any anodic peak in the voltage range of interest; whereas the spiked saliva yielded a peak current of 0.39 μA.
In summary, a simple, affordable, portable, and stable electrochemical method is presented for the detection of low concentrations of Δ9-THC (10–1000 ng mL−1). An anion exchange polymer coated electrode is demonstrated to have sensitivity towards electrochemical oxidation of Δ9-THC, increasing the current density by 400%. The polycationic polymer films provides selectivity against interferents such as caffeine and ascorbic acid. These findings may prove important in the future design of low-cost, portable, roadside systems for Δ9-THC detection.
A.O. – investigation, validation, methodology, data curation; S.H.A. – funding acquisition, conceptualization, resources, formal analysis, project administration, writing – original draft, supervision; S.H. – investigation, synthesis of HMT-PMBI, writing – review and editing. All authors have read and agreed to the published version of the manuscript.
This work was financially supported by the Royal Society of Chemistry through an RSC Research Fund grant (R21-8000280413).
Data availability
The data supporting this article have been included as part of the ESI.† All of the differential pulse voltammetry graphs produced in this research have been presented in the main article or in the ESI.† The calibration data obtained are also presented in Fig. 3. Raw data for the experiments not involving human participants are available by contacting the corresponding author. Data collected from human participants, described in Fig. 5, are not available for confidentiality reasons.Conflicts of interest
There are no conflicts to declare.Notes and references
- N. S. Miller, R. Ipeku and T. Oberbarnscheidt, Int. J. Environ. Res. Public Health, 2020, 17, 1578 CrossRef PubMed.
- Office for National Statistics (ONS), Drug misuse in England and Wales: year ending March 2023, https://www.ons.gov.uk/peoplepopulationandcommunity/crimeandjustice/articles/drugmisuseinenglandandwales/latest, 2024.
- P. Di Ciano, B. Brands, A. Fares, M. Wright, G. Stoduto, P. Byrne, M. McGrath, O. S. M. Hasan, B. Le Foll and C. M. Wickens, Cannabis Cannabinoid Res., 2023, 8, 408–413 CrossRef PubMed.
- Department for Transport UK, Drugs in reported road fatalities in Great Britain, data to 2021: summary, https://www.gov.uk/government/statistics/developing-drug-driving-statistics/drugs-in-reported-road-fatalities-in-great-britain-data-to-2021-summary, 2024.
- B. Zanfrognini, A. Monari, G. Foca, A. Ulrici, L. Pigani and C. Zanardi, Microchem. J., 2022, 183, 108108 CrossRef CAS.
- V. Ramzy and R. Priefer, Talanta, 2021, 222, 121528 CrossRef CAS PubMed.
- Department for Transport UK, Changes to drug driving law, https://www.gov.uk/government/collections/drug-driving, 2024.
- T. R. Arkell, T. R. Spindle, R. C. Kevin, R. Vandrey and I. S. McGregor, Traffic Inj. Prev., 2021, 22, 102–107 CrossRef PubMed.
- K. J. Williams, C. J. White, A. P. Hunt and M. E. Meyerhoff, Electroanalysis, 2023, 35, e202200451 CrossRef CAS.
- J.-R. Lee, J. Choi, T. O. Shultz and S. X. Wang, Anal. Chem., 2016, 88, 7457–7461 CrossRef CAS PubMed.
- M. J. Swortwood, M. N. Newmeyer, M. Andersson, O. A. Abulseoud, K. B. Scheidweiler and M. A. Huestis, Drug Test. Anal., 2017, 9, 905–915 CrossRef CAS PubMed.
- T. Pholsiri, W. Khamcharoen, S. Vimolmangkang, W. Siangproh and O. Chailapakul, Sens. Actuators, B, 2023, 383, 133571 CrossRef CAS.
- K. Amini, A. Sepehrifard, A. Valinasabpouri, J. Safruk, D. Angelone and T. de Campos Lourenco, J. Cannabis Res., 2022, 4, 12 CrossRef PubMed.
- M. Renaud-Young, R. M. Mayall, V. Salehi, M. Goledzinowski, F. J. E. Comeau, J. L. MacCallum and V. I. Birss, Electrochim. Acta, 2019, 307, 351–359 CrossRef CAS.
- A. G. Wright, J. Fan, B. Britton, T. Weissbach, H.-F. Lee, E. A. Kitching, T. J. Peckham and S. Holdcroft, Energy Environ. Sci., 2016, 9, 2130–2142 RSC.
- S. Hernandez-Aldave, A. Tarat and P. Bertoncello, Chemosensors, 2021, 9, 325 CrossRef CAS.
- M. Rees, A. G. Wright, S. Holdcroft and P. Bertoncello, Sensors, 2020, 20, 443 CrossRef PubMed.
- European Medicines Agency, Note for Guidance on Validation of Analytical Procedures: Text and Methodology (CPMP/ICH/381/95), EMEA 2006, Canary Wharf, London, 1994.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and addition information. See DOI: https://doi.org/10.1039/d4cc05254d |
| This journal is © The Royal Society of Chemistry 2025 |