Open Access Article
Open Access ArticleTailoring the oxidation of benzyl alcohol and its derivatives with (photo)electrocatalysis
Jingjing
Qiu
 *,
Tucker
Forbes
and
Timothy
Lin
*,
Tucker
Forbes
and
Timothy
Lin
Department of Chemistry and Biochemistry, San Francisco State University, San Francisco, California 94132, USA. E-mail: qiu@sfsu.edu
First published on 24th January 2025
Abstract
The electrochemical oxidation of alcohol molecules has gained significance as a key anode reaction, offering an alternative to the oxygen evolution reaction (OER) for hydrogen (H2) production and carbon dioxide (CO2) reduction. The (photo)electrochemical oxidation of benzyl alcohol and its derivatives serves as an important model system, not only because benzyl alcohol oxidation is a critical industrial process, but also because it offers valuable insights into electrocatalytic biomass conversion. Tailoring this reaction through electrochemical and photoelectrochemical methods using heterogeneous noble and transition metal electrocatalysts presents a green approach and the potential for uncovering new reaction mechanisms. This review article positions the electrochemical oxidation of benzyl alcohol as an alternative to the OER to produce H2, highlighting recent mechanistic studies involving noble and transition metal electrocatalysts. Furthermore, we discuss the electronic substituent effects on this reaction, which have been well-explored in organic oxidation pathways but remain underexplored in (photo)electrocatalytic contexts.
1. Introduction
Electrolysis is one approach to transform electrical energy into chemical energy by storing electrons within stable chemical bonds. This stored chemical energy can later be used as fuel or converted back into electricity when required. Water (H2O) electrolysis to produce hydrogen (H2) and oxygen (O2) gases is one well-established technology while the electrolysis of carbon dioxide (CO2) and nitrogen (N2) reduction holds potential for renewable energy storage.1 In the electrochemical reduction of H2O and CO2, the accompanying anodic reaction is typically the oxygen evolution reaction (OER) (eqn (1) and (2)). However, the high overpotential of the OER at the anode increases the overall voltage required for the above electrolysis processes.| acid: 2H2O → O2 + 4H+ + 4e− | (1) |
| base: 4OH− → O2 + 2H2O + 4e− | (2) |
Recently, the electrochemical oxidation of alcohols, such as benzyl alcohol and its derivates2 and glycerol,3 has gained significant research attention for its potential to replace the OER as the anodic process in the hydrogen evolution reaction (HER) and CO2 reduction. This alternative approach aims to lower the overall cell voltage of the electrolyzer compared to the traditional OER as the anodic reaction.4–6 In addition, higher-value products, such as aldehyde or acids, can be generated from the alternative anodic reaction of alcohol oxidation. Among the various alcohols in the alternative anodic reactions, the electrochemical oxidation of benzyl alcohol has attracted considerable research interest. It not only produces benzaldehyde or benzoic acid without strong oxidants, but also serves as an excellent model system for understanding the electrochemical oxidation of bio-derived molecules, with potential to develop electrified synthetic pathways for producing value-added products.2,7 The generic electrochemical oxidation of primary alcohol molecules to aldehyde and carboxylic acid in acidic and alkaline electrolytes are shown in eqn (3)–(6):
| acid: RCH2OH → RCHO + 2H+ + 2e− | (3) |
| RCHO + H2O → RCOOH +2H+ + 2e− | (4) |
| base: RCH2OH + 2OH− → RCHO + 2H2O + 2e− | (5) |
| RCHO + 2OH− → RCOOH + H2O + 2e− | (6) |
The electrochemical oxidation of small alcohols such as methanol and ethanol has been extensively studied for their use in alcohol fuel cells, which convert chemical energy into electrical energy.8 This anodic reaction in the fuel cells produces CO2 as the product (eqn (7) and (8)). The efficiency and selectivity of alcohol oxidation are influenced by the type of the alcohol and the electrode material. The electrode used, whether noble or transition metals, has its unique catalytic properties and can influence the energy consumption and durability of the alcohol fuel cells.8 Although the reactions of electrochemical oxidation of alcohols as an alternative reaction of the OER are different from the anodic reaction in the fuel cells, insights gained from these studies are crucial for advancing research into alternative anode reactions of alcohol oxidation.
| acid: CH3OH + H2O → CO2 + 6H+ + 6e− | (7) |
| base: CH3OH + 6OH− → CO2 + 5H2O + 6e− | (8) |
Since the electrochemical oxidation of alcohols, used as an alternative anode reaction, generally occurs in aqueous electrolytes under anodic bias, it should compete effectively with the OER with high faradaic efficiency (FE). Both noble metal electrode, such as gold (Au) and platinum (Pt), and first-row transition metals, such as nickel (Ni), cobalt (Co), have been explored for this purpose. While it was expected that the optimal electrode materials would be those with poor OER activity, it is noteworthy that several transition metal-based electrocatalysts (e.g., Ni- and Co-based oxides, hydroxides, and oxyhydroxides), which are known to be good OER catalysts, have demonstrated high FEs for the electrochemical oxidation of alcohols.7,9 Although the basic steps in the electrochemical oxidation of alcohols have been proposed, recent studies on the fundamental mechanisms, particularly for benzyl alcohol and its derivates, achieved new insights.2,7,9 In addition to new insights into the reaction mechanisms of alcohol oxidation, recent research has also focused on tailoring different interfaces in this reaction. For example, the liquid–liquid interface with water and organic solvent mixtures was explored to control the product selectivity.10 The interfacial properties related to the adsorption of reactant molecules at the solid–liquid interface are also variables that have received recent research attention.11 These studies all highlight the need for further investigation in this area from new perspectives.
In this review article, we will explore benzyl alcohol and its derivatives as a model system, highlighting recent mechanistic studies on their (photo)electrochemical oxidation. Given that different electronic substituents on benzyl alcohol are expected to exhibit distinct adsorption properties at the solid–liquid interface—potentially leading to varied reaction mechanisms and tuning the electrochemical oxidation—we will discuss the effects of these substituents on the reaction. The article begins with a brief overview of alternative anodic reactions to the OER, followed by a review of common chemical and catalytic approaches for benzyl alcohol oxidation. This foundation will lead to a discussion of electrochemical and photoelectrochemical routes for the oxidation of benzyl alcohol. Our focus will center on recent studies of electrochemical oxidation of benzyl alcohol and its derivatives on various metal electrodes under alkaline conditions. Additionally, we will examine mechanistic insights into the electrochemical oxidation of benzyl alcohol and its derivatives and explore photochemical and photoelectrochemical pathways for their oxidation.
2. Alternative anodic reaction of the OER
The application of water electrolysis for H2 production is primarily limited by its large cell voltage and low energy conversion efficiency associated with the OER.12 To address these limitations, various alternative anodic reactions have been explored to reduce the overpotential and increase the efficiency of H2 production.13 In addition to the HER process, these alternative anode reactions have also been applied in CO2 reduction14 and nitrate (NO3−) reduction.15 Some of these alternative anodic reactions include halide oxidation,16 amine oxidation,17 aldehyde oxidation6 and alcohol oxidation.14,18 Each of these alternative anodic reactions presents its own challenges and advantages, making the choice dependent on specific applications, economic factors, and environmental considerations. By utilizing these alternative reactions, it is possible to optimize the water-splitting process for higher efficiencies, reduced costs, and valuable by-products.2.1 Formaldehyde oxidation
Formate is widely used in various industrial applications. The formaldehyde (HCHO) oxidation reaction is one promising alternative anodic reaction, producing formate (HCOO−) and H2 as a by-product at a relatively low potential under alkaline conditions compared to the OER (Fig. 1). Coupling both formaldehyde oxidation with the HER would produce H2 on both the cathode and the anode, while producing value-added product such as formate. Recent research has shown that the formaldehyde oxidation has the capacity to reach industrially relevant current densities with the Cu3Ag7 anode and Ni3N/Ni cathode electrocatalysts.6 The Cu3Ag7 electrocatalyst is unique that the copper (Cu) and silver (Ag) work cooperatively to stabilize the H2C(OH)O− intermediate due to the separate d-bands of Cu and Ag which facilitates the C–H bond cleavage in the electrochemical oxidation of formaldehyde to formate.6 | ||
| Fig. 1 Schematics of (a) conventional electrocatalytic water electrolysis under alkaline conditions and (b) electrocatalytic water reduction coupled with formaldehyde oxidation under alkaline conditions. Figure adapted from ref. 6. Copyright 2023 The Author(s). | ||
2.2 Benzyl alcohol oxidation
Compared to other alternative oxidation reactions, electrochemical oxidation of alcohols shows massive promise with various candidates such as methanol, ethanol, benzyl alcohol, and glycerol which is a simple triol compound. For example, methanol has been shown to be able to produce formate as a value-added product, and the cell voltage is 1.427 V to achieve a current density of 10 mA cm−2 coupled with the HER.19 While glycerol oxidation could produce formate with a cell voltage of 1.36 V to achieve 10 mA cm−2 with the HER,20 its oxidation also presents the potential to produce dihydroxyacetone or glyceraldehyde at 0.05 and 0.09 V vs. RHE, respectively.21 The electrochemical oxidation of benzyl alcohol, on the other hand, produces benzaldehyde and/or benzoic acid using both first-row transition and noble metal electrocatalysts with the product selectivity tuned by the electrode materials and reaction conditions.18 As illustrated in Table 1, alcohol oxidation reactions typically occur at much lower potentials compared to the OER, which generally requires approximately 1.56–1.73 V vs. RHE to achieve a current density of 10 mA cm−2.22The electrochemical oxidation of benzyl alcohol reaction is particularly attractive for its ability to operate at high current densities (>250 mA cm−2) for over 24 hours coupled with H2 production,18 making it suitable for industrial applications. Moreover, the electrochemical approach removes the use of explosive oxidizers like potassium permanganate (KMnO4), offering a green alternative for the synthesis of benzaldehyde or benzoic acid. The electrochemical oxidation of alcohols can not only produce value-added products but also provide insights into electrochemical oxidation of more complex molecules containing hydroxyl (–OH) functional group(s).
Fundamentally, the electrochemical oxidation of benzyl alcohol not only provides a green alternative for the conventional synthesis of benzaldehyde and benzoic acid under moderate conditions but also serves as an important model system for understanding biomass conversion electrochemically. One key intermediate in the oxidative valorization of biomass is 5-hydroxymethylfurfural (HMF), which is derived from cellulosic biomass and can be oxidized to 2,5-furandicarboxylic acid (FDCA). FDCA is a potential alternative to terephthalic acid in polyethylene terephthalate (PET) plastics.23 The electrochemical oxidation of HMF to FDCA involves a more complex reaction of converting the hydroxymethyl and the aldehyde groups of HMF into carboxyl groups. Recent research has explored the electrochemical and photoelectrochemical oxidation of HMF with Ni-based electrocatalysts and achieved important understanding of the system.24,25 In addition to developing more stable and efficient catalysts that deliver satisfactory FDCA yields, effective separation and purification of the products are also crucial.26 The insights gained from the electrochemical oxidation of aldehyde and alcohol will help contribute to the understanding of the conversion of these biomass-derived intermediates.
As described above, the electrochemical oxidation of benzyl alcohol (Scheme 1) is a reaction of significant importance. The thermodynamic potentials of the electrochemical oxidation of benzyl alcohol and benzaldehyde27 (Scheme 1) are lower than that of the OER process. As a result, significantly higher current densities for the electrochemical oxidation of benzyl alcohol are typically observed at the same anode potential when compared to the OER.18 Key variables controlling the efficiency and selectivity of the electrochemical oxidation of benzyl alcohol include the metal electrocatalysts,28 the electrolyte10 and the operating conditions.18 A high yield of benzaldehyde, exceeding 90%, has been achieved using a Ni(OH)2 electrocatalyst with an organic–aqueous mixed electrolyte in the direct electrochemical oxidation of benzyl alcohol.10 From a fundamental study perspective, voltametric analyses are frequently applied as an analytical tool. The onset potential and peak current in the experimental cyclic voltametric data could provide information of the direct benzyl alcohol oxidation. In addition, the cyclic voltametric data is used to investigate the relationship between the redox properties of the electrocatalysts and benzyl oxidation.9 To achieve the electrosynthesis of benzyl alcohol oxidation, a flow-cell setup is commonly applied to evaluate its performance coupled with a chosen cathode reaction.10,18 As the reaction yield and selectivity vary with the dynamic changes of the electrocatalysts,9,18 there is growing research interest in understanding the reaction mechanisms of benzyl alcohol on metal/metal oxide-based electrocatalysts.7,9
 | ||
| Scheme 1 Electrochemical oxidation of benzyl alcohol in alkaline electrolytes which generates benzaldehyde and benzoic acid. The standard potential for the oxidation reaction shown corresponds to the anode potential that will lead to an isoergic equilibrium (ΔG° = 0) between the oxidized and reduced molecule under standard state conditions, when protons are reduced to H2 at the cathode.27 | ||
A deeper understanding of the reaction mechanism has enabled the development of various electrochemical strategies to optimize the reactions and expand the applicability to benzyl alcohol derivatives.18 Recent efforts have investigated the use of organic–water interfaces for the electrochemical oxidation of benzyl alcohol. This approach shows promise in addressing the challenge of low solubility of some benzyl alcohol derivatives.10 These mechanistic insights of benzyl alcohol will be discussed in greater details in Section 4.
3. Chemical oxidation of benzyl alcohol
The selective oxidation of benzyl alcohol to benzaldehyde or benzoic acid can be achieved through various chemical methods such as the use of stoichiometric oxidants or O2 as a milder oxidant coupled with different catalysts (Scheme 2). Benzyl alcohol could be selectively oxidized to benzaldehyde or fully oxidized to benzoic acid. The strength of the oxidants, catalysts, and reaction conditions (e.g. temperature and time) could be tuned to achieve the desired products.29 Each method has its own advantages, disadvantages, and specific applications based on the desired products, environmental impact, and industrial scalability.On an industrial scale, benzyl alcohol oxidation is commonly performed using expensive stoichiometric oxidants such as dichromate, chromic acid, and permanganate to produce benzoic acid. Alternatively, commercial hydrogen peroxide (H2O2) can be used as a more moderate oxidant to promote the reaction at relatively low temperatures, and benzyl alcohol could be selectively converted to benzaldehyde.30 The use of more environmentally friendly oxidants, such as molecular oxygen or air, can also produce high yields of benzaldehyde. However, these reactions generally require elevated temperatures and the presence of catalysts.29 This process, known as the aerobic oxidation, is considered a more environmentally friendly approach compared to using hazardous chemical oxidants like dichromate or permanganate. The challenge with aerobic oxidation lies in activating the oxygen double bond (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at low temperature, often necessitating the use metal-based catalysts, such as noble metals (e.g., Pd, Au), transition metal oxides or metal oxide supported metal catalysts. The electrochemical oxidation of benzyl alcohol relies on metal electrocatalysts, such as Au, Ni, or Co, to facilitate the process of proton and electron transfer. In both liquid-phase aerobic oxidation and electrochemical oxidation, the liquid–solid interfacial properties play a critical role in determining reaction efficiency and selectivity. Insights from liquid-phase aerobic oxidation of alcohols on solid catalysts are particularly valuable for understanding the interactions between alcohol and catalysts.
O) at low temperature, often necessitating the use metal-based catalysts, such as noble metals (e.g., Pd, Au), transition metal oxides or metal oxide supported metal catalysts. The electrochemical oxidation of benzyl alcohol relies on metal electrocatalysts, such as Au, Ni, or Co, to facilitate the process of proton and electron transfer. In both liquid-phase aerobic oxidation and electrochemical oxidation, the liquid–solid interfacial properties play a critical role in determining reaction efficiency and selectivity. Insights from liquid-phase aerobic oxidation of alcohols on solid catalysts are particularly valuable for understanding the interactions between alcohol and catalysts.
3.1 Aerobic oxidation of benzyl alcohol
In performing aerobic oxidation of benzyl alcohol in the liquid phase, several factors must be considered including catalysts, solvents, temperature, and pressure. These factors should also be considered from a holistic perspective. For example, the interaction between the catalysts and reactants is significantly influenced by the solvent used, as water-induced effects can enhance their interactions during alcohol oxidation.31 For aerobic oxidation of benzyl alcohol, it was determined that there are two primary reaction paths: (1) an alkoxy pathway leading to toluene, benzaldehyde, and benzyl ether, and (2) a carbonyloxyl pathway (“neutral carboxylate”) leading to benzoic acid, benzene, and benzyl benzoate.32 The catalyst system plays a key role in tailoring the reaction product selectivity, and Pd and Au are the most commonly used systems. For example, the metal oxide supported bimetallic system, PdFe/TiO2, demonstrated high selectivity in oxidizing benzyl alcohol into benzaldehyde outperforming other bimetallic combinations through the in situ production of H2O2 from molecular H2 and O2.32 Additionally, the polymer supported bimetallic AuPd/polyaniline catalyst was shown to enhance the selectivity of benzyl alcohol oxidation to benzaldehyde, achieving a maximum conversion rate of 98% at 100 °C, likely due to the modified valence d-band occupation of the bimetallic system.33 This bimetallic catalyst approach has been investigated in the electrochemical oxidation of formaldehyde, where the different d-bands of Cu and Ag contribute to stabilizing reaction intermediates.6 Beyond Pd and Au, the liquid-phase oxidation of benzyl alcohol can also be catalyzed by various first-row transition metal catalysts, such as MnOx clusters, which exhibit good activity and selectivity towards benzaldehyde formation.34To gain deeper insight into the reaction mechanism from a surface chemistry perspective, it is essential to consider the coverages of atomic hydrogen, atomic oxygen, and surface hydroxyls on the catalyst surface. A thorough understanding of how these species adsorb onto metal surfaces is critical for elucidating reaction pathways and designing more effective catalysts.
3.2 Surface chemistry of benzyl alcohol oxidation on noble metals
Although the aerobic oxidation of benzyl alcohol typically occurs in the liquid phase, studies conducted in the gas phase using single crystals and ultrahigh vacuum (UHV) techniques have significantly advanced our understanding of the fundamental reaction mechanisms. These studies effectively bridge surface science with catalytic processes, offering valuable insights into the reaction's underlying principles.35The surface chemistry of benzyl alcohol is very dynamic. The reaction of benzyl alcohol was shown to be highly dependent on its adsorption orientation, where the adsorption orientation can be manipulated by covering the metal catalyst surface with surface oxygen. For example, temperature-programmed desorption (TPD) and high-resolution electron energy loss spectroscopy (HREELS) experiments showed that surface oxygen has many uses, acting as a Brønsted base to abstract the hydroxyl hydrogen of the benzyl alcohol, as well as a nucleophile to oxidize aldehydes and carboxylates.36 On a clean Pd(111) surface, the aromatic ring of benzyl alcohol interacts strongly with the surface, orienting itself almost parallel to the metal surface. Similarly, at low coverages of surface oxygen, the benzyl alcohol proceeds through a flat-lying benzaldehyde adsorbate to produce CO and benzene. However, at high coverages, the benzyl alcohol is more upright producing deoxygenation products and toluene.36
Au, as a well-studied model catalyst, exhibits interesting selectivity with a strong size dependence and has gathered research interest for this property.37 After the surprising discovery of Au nanoparticles’ high activity as a low-temperature CO oxidation catalyst, extensive research into Au nanoparticles for oxidation reactions were initiated.38 Aerobic oxidation of alcohols catalyzed by Au has been widely explored, especially for organic synthesis involving carbonyl and carboxyl compounds, serving as a model for oxygen activation. Among various substrates, benzyl alcohol and glycerol have been the most thoroughly studied ones. Generally, the adsorption and catalytic properties of Au strongly depend on its surface properties.38 A study of the interaction between benzyl alcohol and a clean Au(111) surface revealed that the remarkable selectivity achieved in the Au-mediated oxidation of benzyl alcohol is associated with conditions where benzyl alcohol adsorption exceeds the surface atomic oxygen.35 Under such conditions, the kinetic barrier for aldehyde formation is sufficiently lower than that for benzoate, which ultimately leads to benzoic acid, to ensure a selective oxidation to benzaldehyde.35 The general reaction pathways of aldehyde from alcohol oxidation on a Au(111) surface with atomic oxygen are summarized in Fig. 2.39
 | ||
| Fig. 2 Schematics of aldehyde formation on O/Au(111) via two pathways: the β-H elimination (top pathway) of alkoxys or introduced externally (bottom pathway, blue). Adapted with permission from ref. 39. Copyright 2010 American Chemical Society. | ||
These surface studies emphasize the critical role of benzyl alcohol adsorption on the catalyst surface in determining product selectivity. However, in the presence of a liquid electrolyte and an electrochemical interface, adsorption processes behave substantially more complex.11 Gaining a deeper understanding of these dynamic adsorption processes in the electrochemical oxidation of benzyl alcohol is essential for interpreting the reaction mechanism, and needs further investigation.
3.3 Computational studies of metal-catalyzed alcohol oxidation
In addition to the experimental surface chemistry studies, the advances in surface science theory have significantly improved our understanding of the mechanisms governing alcohol oxidation, especially from the perspective of alcohol adsorption on metal surfaces.In the alcohol oxidation reactions, the catalyst must bind alcohol strongly enough to break the necessary chemical bonds, but weakly enough to release relevant intermediates or products. This is known as the Sabatier's principle, which often creates an inherent upper limit on catalyst activity. Meanwhile, reaction dynamics are affected by the structure of the d-band of the metal catalysts, an important descriptor in predicting the chemistry of metal catalysts.40 The d-band model links the energy of the d-band of the metal to the adsorption energies of adsorbates, directly affecting the energy barriers in catalytic reactions. This occurs because the position of the d-band center dictates the energy of electronic states involved in the adsorbate–surface bond.
Density functional theory (DFT) studies play an important role of predicting the most thermodynamically favored orientation of molecular adsorption. One DFT study with van der Waals interaction found that ethanol prefers to adopt a parallel orientation on the metal surfaces which enhances its attraction to the oxidation products and the metallic surfaces.41 In another study, the adsorption energies of benzyl alcohol and benzaldehyde were calculated on TiO2-supported PdZn catalyst surfaces to understand the high selectivity of the PdZn component.42 Since benzyl alcohol adopts a more stable configuration on Pd, it is identified as the primary metal component responsible for the observed high activity of the catalyst. Conversely, benzaldehyde is more stable on the Zn component of the bimetallic system. Therefore, Zn is proposed to play a role in enhancing reaction selectivity.42 In the aerobic oxidation of benzyl alcohol, computational comparisons of the oxygen adsorption on Pd and Au surfaces were also conducted. The calculations demonstrated that the AuPd alloy decreased the oxygen adsorption energies relative to pure Pd, which in turn enhanced the selectivity of the AuPd alloy in benzyl alcohol oxidation.43 These studies highlight the critical role of the metal catalyst surface in influencing the molecular orientation and bonding strength during alcohol oxidation, which directly impacts the subsequent reaction activity and selectivity. The addition of functional groups to benzyl alcohol derivatives creates a wider range of possible surface adsorption orientations and energetics. Both experimental and computational studies should account for how these changes in surface chemistry might influence reaction activity and selectivity.
In the liquid-phase reactions, the involvement of water molecules and organic solvent molecules adds another layer of complexity to the surface chemistry. While organic solvent-free oxidation of benzyl alcohol is a green approach, experimental studies have shown that small amount of organic solvent in water is one way to tune the adsorption behaviors of both benzyl alcohol and the product benzaldehyde.44 Computational modelling has proven invaluable in elucidating the influence of solvent effects on the energetics of alcohol and its oxidation intermediates, as well as identifying the rate-limiting steps in alcohol oxidation. For example, one study on methanol electrooxidation on Au reveals that incorporating both implicit solvent effects and explicit solvation can more stabilize the product formic acid compared to models in a vacuum.45 While both implicit and explicit solvent models identify the same potential-determining step and yield similar theoretical limiting potentials, the more advanced model suggests that aldehyde and formate formation may also play a competitive role in the potential-determining step, a possibility the simpler model overlooks.45 Advanced models are needed to enhance understanding of the interactions between larger benzyl alcohol molecules or their derivatives and metal surfaces in the presence of solvents, as well as their impact on the oxidation reaction.
3.4 Electronic substituent effects in benzyl alcohol oxidation
In the oxidation of benzyl alcohol, the electronic effects of substituents on the benzyl ring play a significant role in determining the rate and pathway of the reaction as they can change the local electron density of the molecule and surface interaction with the catalyst in heterogeneous catalysis.The catalytic performance in the oxidation of substituted benzyl alcohol depends on the electronic and steric effects of the substituents on the phenyl ring. Substituents that are electron-withdrawing or bulky lead to longer reaction times for the complete conversion of benzylic alcohols. For example, in the selective oxidation of substituted benzyl alcohol to the corresponding aldehydes using copper–manganese mixed oxide (CuMn2Ox) nanoparticles catalyst, substituted benzyl alcohol with electron withdrawing groups (e.g. –NO2) at the para-position of the phenyl ring exhibited slower conversion rates compared to unsubstituted benzyl alcohol while electron donating groups (e.g. –CH3 and –OCH3) accelerate the reaction.46 Similar to this observation, it was also shown that the Au-catalyzed oxidation of substituted benzyl alcohol was promoted by electron-donating groups like –OCH3 compared to electron-withdrawing groups.37 This is interesting as the opposite trend of the electronic substituent effects is often observed for benzyl alcohol oxidation with liquid oxidants. The results indicated that electron density on the phenyl ring induced by the substituents played an important role in the reactivity of the oxidation reaction.
Hammett developed the Hammett equation in physical organic chemistry, which relates the reaction rates or equilibrium constants of a chemical reaction to the electronic properties of substituents on an aromatic ring.47 The Hammett study provides a quantitative way to understand how substituents on aromatic rings influence reaction rates and equilibria, and has been applied for the heterogenous catalysis of benzyl alcohol oxidation on metal oxide supported Au catalysts.48 Hammett studies are typically performed by substituting the para position of a phenyl ring and measuring differences in the reaction rates. The electron-donating or electron-withdrawing ability of the substituent is quantified by its substituent constant.49 The Hammett studies were conducted on Au/metal-oxide catalyzed benzyl alcohol oxidation. Two substituents of benzyl alcohol, including the electron donating group –OCH3 and the electron withdrawing group –CF3, were studied.48 The reaction rate of the –OCH3 substituted benzyl alcohol oxidation is higher than that of the –CF3 substituted one.48 The reaction mechanism of benzyl alcohol and its derivative was proposed to proceed in three steps (Fig. 3) of alcohol adsorption, deprotonation of the hydroxyl group to form an alkoxide (BnO−), and the rate-determining hydride transfer to the Au nanoparticle.48 The early stage of the hydride transfer results in the development of a partial positive charge on the benzylic carbon, which is sensitive to the electron-donating or -withdrawing ability of the aryl substituent. It was found the substituents induce two competing effects, including affecting the alcohol affinities for the metal catalyst and the surface coverage of alkoxide. Benzyl alcohol with electron donating groups such as –OCH3 is electron-rich but is less acidic. The adsorption of electron-rich alcohol is more favored, which further affect the deprotonation equilibria and increase the number of active alkoxides.48
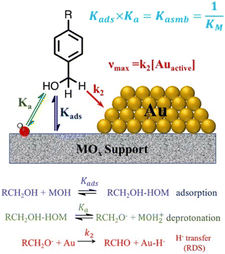 | ||
| Fig. 3 Schematic of the equilibria of alcohol binding, deprotonation and hydride transfer. Adapted with permission from ref. 48. Copyright 2020 American Chemical Society. | ||
In benzyl alcohol oxidation catalyzed by Au, where alcohol deprotonation is required prior to rate-determining hydride transfer, electronic substituents of the benzyl alcohol impact the reaction by impacting the surface coverage of the active intermediate.48 When studying the electronic substituents, all the surface processes need be accounted for.
4. Electrochemical oxidation of benzyl alcohol molecules and mechanistic studies
In theory, any successful chemical oxidation reaction should have an electrochemical equivalent, and the reverse should also hold true, assuming the specific chemical oxidant is identified.50 However, in practice, the two methods are not directly parallel but often serve as complementary approaches. Once the numerous variables involved in the electrochemical process are optimized, the method offers several advantages. These include simplified workup procedures, as no chemical oxidant or by-products need to be removed—often requiring only the removal of solvent and electrolytes. Additionally, electrochemical oxidation can be cost-effective, especially when the initial investment in equipment is excluded, and it frequently results in higher yields.50In the electrochemical oxidation of benzyl alcohol, the electrochemical potential of the electrode materials where the alcohol adsorbs and undergoes oxidation can be regulated. Because of this, a more complex interfacial environment is also presented as the surface charges and the electrochemical potentials control the reactant adsorption and reactivity. In the presence of a liquid electrolyte and an electrochemical interface, adsorption processes behave substantially differently than those in the gas phase. Understanding these adsorption processes becomes an important component for designing effective electrocatalysts.11
As an alternative anode reaction to the OER in the production of H2, the electrochemical oxidation of benzyl alcohol and its derivatives is typically performed in aqueous electrolytes with anodic bias. To outcompete effectively with the OER kinetically, it is reasonable to assume that the electrode materials should not be effective towards the OER. However, experimental studies have demonstrated that both noble metal and first-row transition metal catalysts—despite their varying effectiveness as OER catalysts—can efficiently facilitate the electrochemical oxidation of benzyl alcohol and its derivatives. The influence of the redox chemistry of the electrodes and electrolytes has attracted research attention to gain deeper mechanistic insights into electrochemical oxidation of benzyl alcohol.
4.1 Noble metal electrocatalyst for benzyl alcohol oxidation
As an electrocatalyst, Au enables the oxidation of alcohol molecules with a small overpotential under alkaline conditions via the on-surface formation of adsorbed hydroxide.51 While surface hydroxyl groups adsorbed on the Au metal surface are crucial for catalyzing the electrochemical oxidation of alcohols, further oxidation of the metal can lead to the formation of surface-poisoning oxides. Consequently, the oxidation activity of alcohols depends on the metal's resistance to forming these surface-poisoning oxides. This poisoning effect can be observed in the cyclic voltammograms (CVs) of alcohol oxidation on noble metal electrodes under alkaline conditions.The electrochemical oxidation of alcohol molecules on noble metal surfaces such as Au and Pt often shows interesting voltametric features. Double oxidation peaks are often observed for the electrochemical oxidation of alcohol molecules in the cyclic voltammetry (CV) in both the forward and reverse scans.52 This is closely associated with the redox features of the noble metals. The formation of the surface oxide on the noble metal electrode is considered to poison the active sites for alcohol oxidation and the reduction of the metal oxide surface re-actives the catalytic sites.53 The oxidation of the alcohol in the reverse scan in the CVs is often observed after the metal oxide is reduced to its metallic phase. Depending on the type of alcohol molecules and the noble metal electrodes, the relative magnitudes of the currents in the forward and reveres scans vary. While the reduction of some metal electrodes leads to higher current in the reverse scan, implying a more active surface, others show the opposite.54 To avoid this surface deactivation due to metal oxidation formation, a recent study showed a strategy of functionalizing the electrode surface with polymer, which is effective to enhance the electrochemical oxidation of methanol on Pt electrodes.53
As Au is effective for the electrochemical oxidation of alcohols under alkaline conditions, it is an inefficient electrocatalyst for the OER.55 A mechanistic investigation of the electrocatalytic role of Au in the OER under alkaline conditions would provide further insights into its catalytic role in the electrochemical oxidation of benzyl alcohol and guidelines of controlling reaction selectivity. It was found that hydroxide ion adsorption on a Au(111) single crystal electrode leads to oxide formation when hydroxide ion surface concentration exceeds one third of a monolayer. At negatively charged surfaces, adsorbed hydroxide ions creates a highly polar surface bond, but as the surface charge density becomes more positive, this polarity decreases significantly, with oxide formation occurring at higher charge densities.56 The chemical identify of the surface species of Au at different pH values was determined with spectroscopic studies, in which it reveals that the surface of Au forms the β-oxide layer at anodic potentials at high pH values (Fig. 4).57 Although this study primarily aimed to elucidate the reaction mechanism of the OER at different pH values, it also offers valuable insights into alcohol oxidation reactions, where the surface redox properties of Au are crucial.
 | ||
| Fig. 4 Proposed mechanism for electrocatalytic water oxidation on gold. In the high-pH region, the rate-limited step of oxygen evolution is deprotonation of Au(OH)3 involving a decoupled proton-transfer step. Adapted with permission from ref. 57. Copyright 2020 American Chemical Society. | ||
The electrochemical oxidation of benzyl alcohol to benzaldehyde on Au requires the removal of two protons and two electrons. As shown Fig. 5, one possible mechanism starts with the deprotonation of the hydroxyl group in alkaline electrolytes. Such dependence of the activity of Au as an electrocatalyst towards alcohol oxidation is highly dependent on the pH, necessitating a distinction between the roles of the base and the Au. A study correlating the electrochemical oxidation efficiency of alkyl alcohols with their pKa values revealed that more acidic alcohols exhibit lower onset potentials.55 It was suggested that the initial deprotonation at the hydroxyl group is catalyzed by the base, while the subsequent deprotonation at the –CH2 group is promoted by the Au surface.55 This proposed reaction mechanism agrees with the aerobic oxidation of benzyl alcohol catalyzed by Au nanoparticles.48
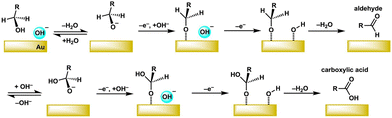 | ||
| Fig. 5 Proposed mechanism for electrocatalytic oxidation of benzyl alcohol on gold. Adapted with permission from ref. 51. Copyright 2021 the Authors. | ||
In addition to the view that base catalysis is the main driver behind the high oxidation activity of many alcohols, the importance of the surface-bound hydroxyl groups on the Au(111) model surface in alkaline electrolytes was highlighted in the computational study of electrochemical oxidation of glycerol, which has two primary alcohol groups and one secondary alcohol group.58 The reaction barriers on the OH-covered Au(111) surface were substantially lower than those on the pristine surface, which explains the higher activity of glycerol oxidation under alkaline conditions. At low OH coverage, computational studies confirmed the presence of dual active sites, where both OH-covered Au and pristine Au participate in catalyzing the protonation processes of glycerol.58
These studies demonstrate that the electrochemical oxidation of benzyl alcohols is highly sensitive to the redox chemistry of the Au surface under alkaline conditions.
4.2 Transition metal electrocatalysts for benzyl alcohol oxidation
Compared to noble metal Au, first-row transition metal-based based electrocatalysts, such as Ni, Co and Fe, are effective OER catalysts in alkaline electrolytes59–61 and have also been investigated in the electrochemical oxidation of alcohols especially benzyl alcohol.2,7,9 Interestingly, these catalysts are effective for both the OER and the electrochemical oxidation of benzyl alcohol. For example, porous Ni-based electrocatalysts with three-dimensional hierarchical structures have been used for the electrochemical oxidation of benzyl alcohol on the anode for H2 production. This low-cost electrocatalyst achieved almost unity faradaic efficiencies.2 Despite the fact that Ni and Co hydroxides are great OER catalysts, they were shown to electrochemically oxidize benzyl alcohols at a large current density of over 400 mA cm−2 without the interference of the OER.62 In addition, the product of the oxidation reaction was able to be separated with conventional crystallization technique.62 Similar to Ni and Co electrocatalysts, the Fe/Co (oxides) heterostructures have also been shown to be an excellent bifunctional OER and benzyl alcohol electrochemical oxidation catalyst.28 A current density of 10 mA cm−2 was achieved at a cell voltage of 1.42 V to simultaneously drive water splitting and the electrochemical oxidation of benzyl alcohol, which is lower than the 1.48 V required for water splitting alone.28 The faradaic efficiency of the HER at the cathode is approximately 99.4%, with the Co/Fe composite anode yielding benzaldehyde and benzoic acid as products from the electrochemical oxidation of benzyl alcohol.28Since first-row transition metals often act as dual electrocatalysts for both the OER and alcohol oxidation, it remains a question whether these two reactions share similar mechanisms when catalyzed by transition metal electrocatalysts. Unlike noble metals, where oxide formation can inhibit alcohol oxidation, the electrochemical oxidation of alcohols on first-row transition metal oxides often follows the concurrent oxidation of the transition metal electrode. Consequently, there is growing research interest in understanding how transition metals facilitate the electrochemical oxidation of benzyl alcohol and other alcohols. Early kinetic studies of the electrochemical oxidation of alcohols in alkaline conditions using oxide-covered Ni, Ag, Cu, and Co anodes proposed that hydrogen was abstracted from the alcohol by the oxide species rather than direct electron transfer to the anode.63
The Choi group revealed new reaction mechanism of nickel oxyhydroxide (NiOOH), an OER electrocatalyst that has been extensively investigated, in electrochemical oxidation of benzyl alcohol.9,23 Since the oxidation of benzyl alcohol is initiated following the oxidation of the Ni metal cations (Fig. 6(a)), their studies aim to answer the question of whether the oxidation of benzyl alcohol depends on the applied potentials. They developed a 3-step rate deconvolution procedure and found that there are two pathways for the electrochemical oxidation of benzyl alcohol on NiOOH electrode including a potential-dependent oxidation mechanism and an indirect oxidation mechanism (Fig. 6(b)). In addition, the new pathway found in this study implies hydride transfer from benzyl alcohol to Ni4+ sites in NiOOH in the electrochemical oxidation process.9 Since NiOOH oxidizes alcohols via two dehydrogenation mechanisms, hydrogen atom transfer and hydride transfer, it has also been explored in selectively oxidizing the secondary alcohol group of glycerol.64
 | ||
| Fig. 6 (a) Cyclic voltammograms of electrochemical oxidation of benzyl alcohol in alkaline electrolytes and (b) the deconvoluted reaction mechanism for 10 mM benzyl alcohol electrochemical oxidation. Adapted with permission from ref. 9. Copyright 2020 American Chemical Society. | ||
In a different study, the dependence of the catalytic activities of Ni–Fe thin film electrodes on the redox activities was evaluated for the electrochemical oxidation of benzyl alcohols as Fe doping can change the electrochemical potential (E0) of the Ni(OH)2/NiOOH redox species. A linear correlation between electrochemical oxidation of benzyl alcohol potentials and Ni redox peak positions (E0) was identified under various alkaline microenvironment conditions, confirming the observation that the formation of the conductive metal oxyhydroxide phase is necessary for benzyl alcohol oxidation.7
The reaction mechanism of the benzyl alcohol oxidation was primarily investigated computationally with DFT. The adsorption energy of benzyl alcohol was computed on the β-NiOOH surface. It was found the strongest adsorption of benzyl alcohol occurs on the oxygen vacancy, but it competes with the OH− ions from electrolyte to fill the vacancy. Because of this, two reaction mechanisms, which are redox mechanism and vacancy-driven mechanism, were both examined (Fig. 7).7 A key difference between these two mechanisms is the site of the initial deprotonation of benzyl alcohol. In the redox mechanism, the proton is first removed from the –CH2 group, whereas in the vacancy-driven mechanism, the proton is removed from the –OH group when benzyl alcohol adsorbs onto the oxygen vacancy.7 The vacancy-driven mechanism of NiOOH-catalyzed benzyl alcohol oxidation is analogous to the Au-catalyzed mechanism in terms of the first deprotonation step (Fig. 5).
 | ||
| Fig. 7 Benzyl alcohol oxidation mechanisms and their corresponding free energy pathways on the edge site of β-NiOOH (with or without Fe-doped). Schematic representation of reaction intermediates for the benzyl alcohol oxidation process via the (a) redox mechanism and (b) vacancy-driven mechanism. Adapted with permission from ref. 7. Copyright 2023 American Chemical Society. | ||
A recent study demonstrated experimentally and computationally that the oxygen vacancy of NiOOH affects the reaction mechanism of the electrochemical oxidation of methanol.65 The in situ surface-enhanced infrared absorption spectroscopy (SEIRAS) showed that formate and (bi)carbonate are formed after the onset of the methanol oxidation, with their distributions dependent on the applied potentials.65 These spectroscopic results were corroborated by DFT-computed reaction profiles over an oxygen vacancy, suggesting that the methanol oxidation mainly proceeds through the formate-involving pathway. In this pathway, methanol is initially reacted to produce formate as the major product, while increasing potential drives the further oxidation of formate to (bi)carbonate.65
4.3 Cooperative effect of noble and transition metal electrocatalysts for benzyl alcohol oxidation
With both Au and transition metal electrocatalysts being effective towards alcohol oxidation, a cooperative effect of Au nanoparticles supported on cobalt oxyhydroxide nanosheets (Au/CoOOH) was recently reported.18 The Au/CoOOH electrocatalyst was used as the anode to drive the electrochemical oxidation of benzyl alcohol coupled with the HER process, and a high current density of 340 mA cm−2 was achieved at a potential of 1.3 V vs. RHE in 1 M KOH with 0.1 M benzyl alcohol at room temperature.18 The reaction mechanism of the superior composite electrocatalyst was investigated with computational tools and the reaction pathway was proposed (Fig. 8). In a typical alcohol electrooxidation reaction over Au catalyst, the alcohol molecules tend to interact with Au to form adsorbed R-CH2O* species at a moderate potential after deprotonation of the hydroxyl group, which is then oxidized by the in situ formed electrophilic OH* species adsorbed on Au (Au(OH)ads) at higher potentials. This is followed by catalytic deprotonation of the beta-hydrogen (C–Hβ) of alcohol from the –CH2 group to give corresponding ketones and carboxylates.18 This is the same reaction mechanism of aerobic oxidation benzyl alcohol catalyzed by supported Au nanoparticles.49 Since Koper and co-workers reported that the presence of adsorbed CO on a Au(111) surface could strengthen OH adsorption on Au at low potentials, which promotes C–Hβ elimination and electrochemical oxidation of alcohols,66 the role of CoOOH in the Au/CoOOH system was proposed to provide reactive adsorbed OH species for the benzyl alcohol oxidation at the interface.18 | ||
| Fig. 8 (a) Traditional electrochemical oxidation of alcohol process over Au catalyst and its deactivation at high potential. (b) Alcohols enrichment and reactive adsorbed OH generation over the interface of Au/CoOOH. Adapted with permission from ref. 18. Copyright 2022, The Author(s). | ||
Similar to this observation, it was reported in another study that incorporation of Ni in AuNi alloys also facilitate the formation of Au(OH)ads species and thus improve the electrochemical oxidation of glycerol.67
Since Au and transition metals, like Ni and Co, operate within different potential ranges for their redox transformations, exploring the dynamic adsorption profiles of benzyl alcohol and its intermediate, benzaldehyde, would be valuable. Building on previous studies, the combination of Au and the oxygen vacancy on transition metal oxyhydroxides presents an intriguing system to explore. Additionally, studying the product selectivity when combining these two types of electrocatalysts needs further investigation. Metal doping in transition metal electrocatalysts and the effects of different conductive substrates have proven effective in modifying the OER process.60,61,68,69 Although the electrochemical oxidation of benzyl alcohol and the OER involve different reaction mechanisms, it would also be interesting to investigate how doping transition metal electrocatalysts to alter their redox properties and varying the conductive noble metal substrates impact these reactions.
4.4 Electronic substituent effects in electrochemical oxidation of benzyl alcohol
To electrochemically oxidize benzyl alcohol by removing electrons and protons, the next question centers on the nature of the highest occupied molecular orbital (HOMO), as this is the orbital that will most readily lose an electron.70 Since toluene oxidizes at 1.96 V vs. Ag/0.1 M AgNO3 and methanol oxidizes beyond 2.5 V vs. Ag/0.1 M AgNO3, it is anticipated that the oxidation potential of the π electrons should be lower than that of the oxygen electrons.70 In benzyl alcohol, the two functional groups should largely retain their individual identities and electrochemical properties, as there is no direct conjugation between them. It has been shown that the oxidation potentials of benzyl alcohol are sensitive to substitution on the phenyl ring. The half-wave potential for benzyl alcohol (2.0 V vs. Ag/0.1 M AgNO3) is higher than those of p-methylbenzyl alcohol (1.59 V vs. Ag/0.1 M AgNO3), and p-methoxybenzyl alcohol (1.25 V vs. Ag/0.1 M AgNO3).50 This implies that the electron donating groups make the electrochemical oxidation of benzyl alcohol more feasible.The electronic effects on the electrochemical oxidation of benzyl alcohol using a hierarchically porous Ni catalyst were investigated, revealing that benzyl alcohol, 4-nitrobenzyl alcohol and 4-methylbenzyl alcohols (molecular structures shown in Scheme 3) were all electrooxidized without significant influence from the electronic substituents.2 However, the onset potentials of these molecules were largely determined by the desirable oxidation potential of the Ni catalysts regardless of the differing intrinsic thermodynamics of the alcohol oxidation reactions and their functional groups.2 This observation is similar to an early study of electrochemical oxidation of a range of primary alcohols and secondary alcohols at a Ni anode in aqueous alkaline solution, in which most of the organic compounds oxidize at the same potential, following the oxidation of the Ni electrode.71
More recently, the plasmon-assisted electrochemical oxidation of 4-(hydroxymethyl)benzoic acid (4-HMBA) (Scheme 3), a derivative of benzyl alcohol, was demonstrated with nanostructured plasmonic Au electrodes under alkaline conditions.72 4-HMBA is one of the most essential intermediates in the synthetic process of Eprosartan, which is a new antihypertensive drug. 4-HMBA has been widely used in the field of medicine and chemical synthesis and can also be used in the synthesis of photocatalytic and photoelectric materials.73 In the study of the (photo)electrochemical oxidation of 4-HMBA, CV analysis (Fig. 9(a)) revealed that its rate of electrochemical oxidation is slower compared to that of benzyl alcohol.72 This agrees with the observation in aerobic oxidation of benzyl alcohol catalyzed by Au in which the electron withdrawing group on the para site of benzyl alcohol impedes the reaction kinetics.37 Furthermore, electrochemical quartz crystal microbalance (EC-QCM) studies revealed distinct mass changes associated with alcohol adsorption, oxidation and Au surface oxidation and reduction for both 4-HMBA and benzyl alcohol (Fig. 9(b)–(d)).72 The potential for the formation of poisoning AuOx with benzyl alcohol is more anodic than with 4-HMBA, as indicated by the mass increase in the EC-QCM measurements (Fig. 8(c) and (d)). This suggests that the –COOH electronic group alters the surface adsorption of 4-HMBA compared to benzyl alcohol, potentially contributing to the differences in their observed reactivities.
 | ||
| Fig. 9 Cyclic voltammograms of electrochemical oxidation of benzyl alcohol and 4-HMBA on a Au disk electrode (b)–(d) EC-QCM measurements in 0.1 M KOH electrolyte and. Reproduced with permission from the SI of ref. 72. Copyright 2022 AIP Publishing. | ||
The various experimental phenomena related to the electronic substituent effects in the electrochemical oxidation of benzyl alcohol may be influenced by the choice of electrode materials, experimental conditions, and the steric effects of the substituents. Future studies, both experimental and computational, are needed to provide further insights into this topic.
4.5 Operando/in situ studies of the electrochemical oxidation of alcohols
The mechanisms of electrochemical oxidation of benzyl alcohol remain a topic of debate. A fundamental understanding of the key electrocatalytic and interfacial reactions for different molecules is crucial for precise control of the reaction process, tailoring product selectivity, and designing targeted catalysts. Thus, the development of advanced in situ and operando characterization techniques is essential for real-time analysis of key intermediates and active catalytic species at various applied potentials. Additionally, DFT calculations serve as a powerful tool to explore preferred reaction pathways. Integrating current operando techniques with more advanced simulations will enhance our understanding of structural evolution and the reaction mechanisms.13For example, in situ Raman and in situ X-ray absorption spectroscopy (XAS) were applied to investigate the reaction mechanism of β-Ni(OH)2 in the OER and alcohol oxidation. In situ electrochemical Raman studies showed that the Ni3+–O bending and stretching modes were observed during the OER after the oxidation potential of Ni metal cations at 1.35 V vs. RHE while these modes were only observable at more anodic potentials above 1.60 V vs. RHE in alcohol oxidation (Fig. 10). This study proposed that for β-Ni(OH)2, the alcohol oxidation activity origin is a β-Ni(OH)O intermediate rather than Ni2+δOxHy or the OER intermediates.74
 | ||
| Fig. 10 Relationship between surface species, interfaces, and reactions based on in situ Raman spectroscopy. Adapted with permission from ref. 74. Copyright 2020, Elsevier. | ||
Another example of the operando studies is using Raman and FT-IR to reveal the reaction mechanisms of the electrochemical oxidation of phenol to benzoquinone.75 The operando spectroscopic studies showed that the Fe single atoms in nitrogen-doped hierarchically porous carbon supported FeRu help to avoid the excessive oxidation of the desired products.75
5. Photo-assisted oxidation of alcohols
In addition to chemical and electrochemical oxidation of benzyl alcohol, photochemical and photoelectrochemical oxidation introduce light as a new variable to control the reaction. Researchers have employed optically active catalysts or electrocatalysts, such as semiconductors, plasmonic metal nanoparticles, or a combination of both, to facilitate the oxidation of benzyl alcohol. Another approach involves integrating photovoltaics (PV) to drive the electrochemical reactions with light. The following discussion will focus on the first scenario.5.1 Photocatalytic oxidation of benzyl alcohol
Semiconductors are often used in photocatalytic oxidation reactions due to their innate ability to generate electron–hole pairs. Reactive oxygen species (ROS) such as the superoxide radical (˙O2−) and the hydroxyl radical (˙OH) can be produced from the photogenerated holes on semiconductors. These ROS act as replacements for liquid oxidants such as permanganate, chromate, and hydrogen peroxide.76 ROS play an important role in the selectivity of products, such as the selective conversion of benzyl alcohol to benzaldehyde. Titanium dioxide (TiO2) is one the most commonly studied semiconductor system,77,78 and metal nanoparticles such as Au are often coupled with the semiconductors to facilitate surface electron and hole separation.79More recently, plasmonic metal nanoparticles such as Au have been applied in supporting the oxidation of benzyl alcohols with visible light. For example, plasmonic Au nanoparticles supported on bismuth oxychloride (BiOCl) were used to photocatalyze the selective oxidation of benzyl alcohol with O2 under visible light.80 The reaction mechanism of this selective benzyl alcohol oxidation was proposed to be related to the energetic electron and holes generated by the plasmonic Au nanoparticles and the oxygen vacancies on BiOCl.
Oxygen vacancies on BiOCl were proposed to facilitate the trapping and transfer of plasmonic hot electrons to adsorbed O2, producing ˙O2− radicals, while plasmonic hot holes remaining on the Au surface mildly oxidize benzyl alcohol to corresponding carbon-centered radicals (Fig. 11).80 Compared to the O2 adsorption on the oxygen vacancies, it would be interesting to study the possible competing adsorption of benzyl alcohol on the oxygen vacancy, as it has been shown that the oxygen vacancy plays an important role for benzyl alcohol adsorption in electrochemical oxidation approach.7
 | ||
| Fig. 11 Proposed reaction mechanism of selective benzyl alcohol oxidation over Au–BiOCl. Adapted with permission from ref. 80. Copyright 2017 American Chemical Society. | ||
5.2 Photoelectrochemical oxidation of benzyl alcohol
In photoelectrochemical (PEC) oxidation of water, semiconductors are often paired with electrocatalysts. The semiconductors absorb light to generate electron–hole pairs, while the electrocatalysts act as hole collectors, facilitating water oxidation.81 The applied potential plays a crucial role in controlling surface band bending in the semiconductors, which in turn affects the surface charge separation process.Similar strategies have been employed in the photoelectrochemical oxidation of benzyl alcohol on various photoanode materials. For example, bismuth vanadate (BiVO4), a semiconductor that absorbs visible light, has been utilized for the efficient selective conversion of benzyl alcohol to benzaldehyde. While Co-based hydroxide has been combined with BiVO4 to drive PEC water oxidation, the active ˙OH species derived during water oxidation further promote the selective oxidation of benzyl alcohol.76 Studies with this photoanode have shown varying rates of aldehyde production for different substituted benzyl alcohols, attributed to differences in steric and electronic effects of the substitutions. Electron-donating groups on the benzyl ring were found to favor reactivity more than electron-withdrawing groups.76 Additionally, BiVO4 has been demonstrated as a photoanode for the selective oxidation of glycerol to glycolaldehyde,82 providing insights into electrochemical C–C bond cleavage and coupling.
In addition to semiconductors, plasmonic metal has also been demonstrated in assisting oxidation reactions of alcohols. Plasmon-assisted electrocatalysis represents a novel approach where plasmonic metal materials are used as electrodes and/or electrocatalysts to drive reactions by introducing visible and near-infrared light into the system.83 For instance, the plasmon-enhanced electrocatalytic oxidation of glucose has been demonstrated using Au nanoparticles and visible light. It is proposed that hot holes generated by Au nanoparticles under resonant light illumination facilitate the oxidation of hydroxide anions into ˙OH radicals, which then diffuse through the solution to accelerate glucose oxidation.84 More recently, plasmonic effects have been applied to the electrochemical oxidation of 4-HMBA. A nanostructured Au electrode serves both to absorb light and catalyze the oxidation reaction. 4-HMBA was electrochemically oxidized to 4-carboxybenzaldehyde and terephthalic acid, with the reaction kinetics significantly enhanced under visible light illumination.72 It is suggested that the nanostructured Au electrode increases the percentage of Au(OH)ads on the electrode surface through energetic holes generated by resonant light, thereby enhancing the dehydrogenation of the hydroxyl group to form benzyl alkoxide, which further leads to the oxidation of 4-HMBA.72 Although plasmon effects have been shown to enhance several electrocatalytic reactions, the precise role of photothermal effects versus energetic charge carriers in electrocatalysis remains debated,85,86 and requires further research to clarify the enhancement mechanisms.
6. Future work
To further tune the (photo)electrocatalytic oxidation of benzyl alcohol and its derivatives to desired products, it is essential to investigate not only the impact of the electrocatalyst or electrode, but also the broader interfacial environment. Since the adsorption of reactant molecules involves replacement processes, it is crucial to consider how factors such as solvent, electrolyte, and electronic substituents of benzyl alcohol affect the adsorption of reactants, intermediates, and products under electrochemical conditions, and how these factors influence subsequent reactivity.Given the diverse experimental phenomena observed regarding the electronic substituent effects in the electrochemical oxidation of benzyl alcohol, further research is needed to correlate the substituent effects with the reaction activity and selectivity. This study intersects several fields, including surface chemistry, electrocatalysis, and (physical) organic chemistry, requiring both experimental and computational approaches to elucidate the reaction mechanisms at the molecular level. Leveraging tools from physical organic chemistry, such as Hammett studies, which provide mechanistic insights into active site electronics, could be valuable for understanding the electrochemical oxidation of benzyl alcohol and its derivatives.
This highlights the need to assess how electronic changes affect the adsorption, dehydrogenation, and activation of benzyl alcohol at the liquid–solid interface under different electrochemical potentials through both experimental and computational studies. A deeper understanding of the kinetic coupling between liquid-phase and surface reactions is also crucial. Moreover, integrating light into the electrochemical oxidation of benzyl alcohol and its derivatives introduces new opportunities for exploring different reaction mechanisms, activity, and selectivity and new electrode systems.
7. Conclusions
In summary, the electrochemical oxidation and photoelectrochemical oxidation of benzyl alcohol and its derivatives are an important model system as an alternative anode reaction for the OER to produce H2 and understanding the biomass conversion. Given the various oxidation pathways, gaining deeper mechanistic insights is essential to understand the similarities and differences among these systems. This understanding will aid in selecting the most appropriate method and tailoring the reaction more precisely. Interdisciplinary research combining surface chemistry, organic chemistry, electrochemistry, and catalysis will enhance the potential for optimizing this important industrial reaction.Author contributions
Jingjing Qiu: conceptualization, recent progress analysis, writing and reviewing draft; Tucker Forbes: writing and editing text; Timothy Lin: writing and editing text.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this highlight article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Acknowledgement is made to the National Science Foundation (NSF) under grant no. 2102196.References
- Z. Yan, J. L. Hitt, J. A. Turner and T. E. Mallouk, Renewable Electricity Storage Using Electrolysis, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 12558–12563 CrossRef CAS PubMed
.
- B. You, X. Liu, X. Liu and Y. Sun, Efficient H2 Evolution Coupled with Oxidative Refining of Alcohols via A Hierarchically Porous Nickel Bifunctional Electrocatalyst, ACS Catal., 2017, 7, 4564–4570 CrossRef CAS
.
- Z. G. Schichtl, S. K. Conlin, H. Mehrabi, A. C. Nielander and R. H. Coridan, Characterizing Sustained Solar-to-Hydrogen Electrocatalysis at Low Cell Potentials Enabled by Crude Glycerol Oxidation, ACS Appl. Energy Mater., 2022, 5, 3863–3875 CrossRef CAS
.
- L. Quan, H. Jiang, G. Mei, Y. Sun and B. You, Bifunctional Electrocatalysts for Overall and Hybrid Water Splitting, Chem. Rev., 2024, 124, 3694–3812 CrossRef CAS PubMed
.
- B. You, G. Han and Y. Sun, Electrocatalytic and Photocatalytic Hydrogen Evolution Integrated with Organic Oxidation, Chem. Commun., 2018, 54, 5943–5955 RSC
.
- G. Li, G. Han, L. Wang, X. Cui, N. K. Moehring, P. R. Kidambi, D. Jiang and Y. Sun, Dual Hydrogen Production from Electrocatalytic Water Reduction Coupled with Formaldehyde Oxidation via a Copper-silver Electrocatalyst, Nat. Commun., 2023, 14, 525 CrossRef CAS PubMed
.
- L. Wei, M. D. Hossain, M. J. Boyd, J. Aviles-Acosta, M. E. Kreider, A. C. Nielander, M. B. Stevens, T. F. Jaramillo, M. Bajdich and C. Hahn, Insights into Active Sites and Mechanisms of Benzyl Alcohol Oxidation on Nickel–Iron Oxyhydroxide Electrodes, ACS Catal., 2023, 13, 4272–4282 CrossRef CAS
.
- A. Hamnett, Mechanism and Electrocatalysis in the Direct Methanol Fuel Cell, Catal. Today, 1997, 38, 445–457 CrossRef CAS
.
- M. T. Bender, Y. C. Lam, S. Hammes-Schiffer and K.-S. Choi, Unraveling Two Pathways for Electrochemical Alcohol and Aldehyde Oxidation on NiOOH, J. Am. Chem. Soc., 2020, 142, 21538–21547 CrossRef CAS PubMed
.
- R. Shi, X. Zhang, C. Li, Y. Zhao, R. Li, G. I. N. Waterhouse and T. Zhang, Electrochemical Oxidation of Concentrated Benzyl Alcohol to High-purity Benzaldehyde via Superwetting Organic-solid-water Interfaces, Sci. Adv., 2024, 10, eadn0947 CrossRef CAS PubMed
.
- C. Lucky and M. Schreier, Mind the Interface: The Role of Adsorption in Electrocatalysis, ACS Nano, 2024, 18, 6008–6015 CrossRef CAS PubMed
.
- E. Fabbri and T. J. Schmidt, Oxygen Evolution Reaction—The Enigma in Water Electrolysis, ACS Catal., 2018, 8, 9765–9774 CrossRef CAS
.
- T. Wang, X. Cao and L. Jiao, Progress in Hydrogen Production Coupled with Electrochemical Oxidation of Small Molecules, Angew. Chem., Int. Ed., 2022, 16, e202213328 Search PubMed
.
- D. Antón-García, E. Edwardes Moore, M. A. Bajada, A. Eisenschmidt, A. R. Oliveira, I. A. C. Pereira, J. Warnan and E. Reisner, Photoelectrochemical Hybrid Cell for Unbiased CO2 Reduction Coupled to Alcohol Oxidation, Nat. Synth., 2022, 1, 77–86 CrossRef
.
- J. Li, H. Li, K. Fan, J. Y. Lee, W. Xie and M. Shao, Electrocatalytic Nitrate Reduction to Ammonia Coupled with Organic Oxidation, Chem. Catal., 2023, 3, 100638 CrossRef CAS
.
- A. G. Breuhaus-Alvarez, Q. Cheek, J. J. Cooper, S. Maldonado and B. M. Bartlett, Chloride Oxidation as an Alternative to the Oxygen-Evolution Reaction on HxWO3 Photoelectrodes, J. Phys. Chem. C, 2021, 125, 8543–8550 CrossRef CAS
.
- Z. Xu and E. Kovács, Beyond Traditional Synthesis: Electrochemical Approaches to Amine Oxidation for Nitriles and Imines, ACS Org. Inorg. Au, 2024, 4, 471–484 CrossRef CAS PubMed
.
- Z. Li, Y. Yan, S.-M. Xu, H. Zhou, M. Xu, L. Ma, M. Shao, X. Kong, B. Wang, L. Zheng and H. Duan, Alcohols Electrooxidation Coupled with H2 Production at High Current Densities Promoted by a Cooperative Catalyst, Nat. Commun., 2022, 13, 147 CrossRef CAS PubMed
.
- T. Wang, X. Cao, H. Qin, X. Chen, J. Li and L. Jiao, Integrating Energy-saving Hydrogen Production with Methanol Electrooxidation over Mo Modified Co4N Nanoarrays, J. Mater. Chem. A, 2021, 9, 21094–21100 RSC
.
- Y. Li, X. Wei, L. Chen, J. Shi and M. He, Nickel-molybdenum Nitride Nanoplate Electrocatalysts for Concurrent Electrolytic Hydrogen and Formate Productions, Nat. Commun., 2019, 10, 5335 CrossRef PubMed
.
- Z. G. Schichtl, S. K. Conlin, H. Mehrabi, A. C. Nielander and R. H. Coridan, Characterizing Sustained Solar-to-Hydrogen Electrocatalysis at Low Cell Potentials Enabled by Crude Glycerol Oxidation, ACS Appl. Energy Mater., 2022, 5, 3863–3875 CrossRef CAS
.
- C. C. L. McCrory, S. Jung, I. M. Ferrer, S. M. Chatman, J. C. Peters and T. F. Jaramillo, Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices, J. Am. Chem. Soc., 2015, 137, 4347–4357 CrossRef CAS PubMed
.
- M. T. Bender, X. Yuan and K.-S. Choi, Alcohol Oxidation as Alternative Anode Reactions Paired with (Photo)electrochemical Fuel Production Reactions, Nat. Commun., 2020, 11, 4594 CrossRef CAS PubMed
.
- W.-J. Liu, L. Dang, Z. Xu, H.-Q. Yu, S. Jin and G. W. Huber, Electrochemical Oxidation of 5-Hydroxymethylfurfural with NiFe Layered Double Hydroxide (LDH) Nanosheet Catalysts, ACS Catal., 2018, 8, 5533–5541 CrossRef CAS
.
- M. T. Bender and K. Choi, Electrochemical Oxidation of HMF via Hydrogen Atom Transfer and Hydride Transfer on NiOOH and the Impact of NiOOH Composition, ChemSusChem, 2022, 15, e202200675 CrossRef CAS PubMed
.
- L. Chen, C. Yu, X. Song, J. Dong, J. Mu and J. Qiu, Integrated Electrochemical and Chemical System for Ampere-level Production of Terephthalic Acid Alternatives and Hydrogen, Nat. Commun., 2024, 15, 8072 CrossRef CAS PubMed
.
- F. Wang and S. S. Stahl, Electrochemical Oxidation of Organic Molecules at Lower Overpotential: Accessing Broader Functional Group Compatibility with Electron−Proton Transfer Mediators, Acc. Chem. Res., 2020, 53, 561–574 CrossRef CAS PubMed
.
- Y. Huang, R. Yang, G. Anandhababu, J. Xie, J. Lv, X. Zhao, X. Wang, M. Wu, Q. Li and Y. Wang, Cobalt/Iron(Oxides) Heterostructures for Efficient Oxygen Evolution and Benzyl Alcohol Oxidation Reactions, ACS Energy Lett., 2018, 3, 1854–1860 CrossRef CAS
.
- T. Mallat and A. Baiker, Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts, Chem. Rev., 2004, 104, 3037–3058 CrossRef CAS
.
- K. Sato, M. Aoki, J. Takagi and R. Noyori, Organic Solvent- and Halide-Free Oxidation of Alcohols with Aqueous Hydrogen Peroxide, J. Am. Chem. Soc., 1997, 119, 12386–12387 CrossRef CAS
.
- Q. Wei, C. Yu, X. Song, Y. Zhong, L. Ni, Y. Ren, W. Guo, J. Yu and J. Qiu, Recognition of Water-Induced Effects toward Enhanced Interaction between Catalyst and Reactant in Alcohol Oxidation, J. Am. Chem. Soc., 2021, 143, 6071–6078 CrossRef CAS PubMed
.
- C. M. Crombie, R. J. Lewis, R. L. Taylor, D. J. Morgan, T. E. Davies, A. Folli, D. M. Murphy, J. K. Edwards, J. Qi, H. Jiang, C. J. Kiely, X. Liu, M. S. Skjøth-Rasmussen and G. J. Hutchings, Enhanced Selective Oxidation of Benzyl Alcohol via In Situ H2O2 Production over Supported Pd-Based Catalysts, ACS Catal., 2021, 11, 2701–2714 CrossRef CAS
.
- S. Marx and A. Baiker, Beneficial Interaction of Gold and Palladium in Bimetallic Catalysts for the Selective Oxidation of Benzyl Alcohol, J. Phys. Chem. C, 2009, 113, 6191–6201 CrossRef CAS
.
- C. Parmeggiani and F. Cardona, Transition Metal Based Catalysts in the Aerobic Oxidation of Alcohols, Green Chem., 2012, 14, 547 RSC
.
- J. C. F. Rodríguez-Reyes, C. M. Friend and R. J. Madix, Origin of the Selectivity in the Gold-mediated Oxidation of Benzyl Alcohol, Surf. Sci., 2012, 606, 1129–1134 CrossRef
.
- S. H. Pang, A. M. Román and J. W. Medlin, Adsorption Orientation-Induced Selectivity Control of Reactions of Benzyl Alcohol on Pd(111), J. Phys. Chem. C, 2012, 116, 13654–13660 CrossRef CAS
.
- C. D. Pina, E. Falletta and M. Rossi, Update on Selective Oxidation Using Gold, Chem. Soc. Rev., 2012, 41, 350–369 RSC
.
- M. Haruta, Size- and Support-dependency in the Catalysis of Gold, Catal. Today, 1997, 36, 153–166 CrossRef CAS
.
- B. Xu, R. J. Madix and C. M. Friend, Achieving Optimum Selectivity in Oxygen Assisted Alcohol Cross-Coupling on Gold, J. Am. Chem. Soc., 2010, 132, 16571–16580 CrossRef CAS PubMed
.
- J. K. Nørskov, T. Bligaard, A. Logadottir, S. Bahn, L. B. Hansen, M. Bollinger, H. Bengaard, B. Hammer, Z. Sljivancanin, M. Mavrikakis, Y. Xu, S. Dahl and C. J. H. Jacobsen, Universality in Heterogeneous Catalysis, J. Catal., 2002, 209, 275–278 CrossRef
.
- A. O. Pereira and C. R. Miranda, Atomic Scale Insights into Ethanol Oxidation on Pt, Pd and Au Metallic Nanofilms: A DFT with van der Waals Interactions, Appl. Surf. Sci., 2014, 288, 564–571 CrossRef CAS
.
- E. Nowicka, S. Althahban, T. D. Leah, G. Shaw, D. Morgan, C. J. Kiely, A. Roldan and G. J. Hutchings, Benzyl Alcohol Oxidation with Pd-Zn/TiO2: Computational and Experimental Studies, Sci. Technol. Adv. Mater., 2019, 20, 367–378 CrossRef CAS PubMed
.
- A. Savara, C. E. Chan-Thaw, J. E. Sutton, D. Wang, L. Prati and A. Villa, Molecular Origin of the Selectivity Differences between Palladium and Gold–Palladium in Benzyl Alcohol Oxidation: Different Oxygen Adsorption Properties, ChemCatChem, 2017, 9, 253–257 CrossRef CAS
.
- Y. Hu, Z. Shen, B. Li, S. Li, J. Yue, G. Zhao, M. Muhler and X. Wang, Solvent Effects on Photocatalytic Anaerobic Oxidation of Benzyl Alcohol over Pt-Loaded Defective SrTiO3 Nanoparticles, ACS Appl. Nano Mater., 2021, 4, 9254–9264 CrossRef CAS
.
- M. Valter, B. Wickman and A. Hellman, Solvent Effects for Methanol Electrooxidation on Gold, J. Phys. Chem. C, 2021, 125, 1355–1360 CrossRef CAS
.
- R. Ali, K. Nour, A. Al-warthan and M. R. H. Siddiqui, Selective Oxidation of Benzylic Alcohols Using Copper-Manganese Mixed Oxide Nanoparticles as Catalyst, Arabian J. Chem., 2015, 8, 512–517 CrossRef CAS
.
- L. P. Hammett, The Effect of Structure upon the Reactions of Organic Compounds. Benzene Derivatives, J. Am. Chem. Soc., 1937, 59, 96–103 CrossRef CAS
.
- A. Mahdavi-Shakib, J. Sempel, L. Babb, A. Oza, M. Hoffman, T. N. Whittaker, B. D. Chandler and R. N. Austin, Combining Benzyl Alcohol Oxidation Saturation Kinetics and Hammett Studies as Mechanistic Tools for Examining Supported Metal Catalysts, ACS Catal., 2020, 10, 10207–10215 CrossRef CAS
.
- G. Kumar, L. Tibbitts, J. Newell, B. Panthi, A. Mukhopadhyay, R. M. Rioux, C. J. Pursell, M. Janik and B. D. Chandler, Evaluating Differences in the Active-site Electronics of Supported Au Nanoparticle Catalysts Using Hammett and DFT Studies, Nat. Chem., 2018, 10, 268–274 CrossRef CAS PubMed
.
- N. L. Weinberg and H. R. Weinberg, Electrochemical Oxidation of Organic Compounds, Chem. Rev., 1968, 68, 449–523 CrossRef CAS
.
- T. Suga, N. Shida and M. Atobe, Au-catalyzed Electrochemical Oxidation of Alcohols Using an Electrochemical Column Flow Cell, Electrochem. Commun., 2021, 124, 106944 CrossRef CAS
.
- Y. Zhao, X. Li, J. M. Schechter and Y. Yang, Revisiting the Oxidation Peak in the Cathodic Scan of the Cyclic Voltammogram of Alcohol Oxidation on Noble Metal Electrodes, RSC Adv., 2016, 6, 5384–5390 RSC
.
- Q. Hua, N. M. Alghoraibi, X. Chen and A. A. Gewirth, Enhanced Methanol Oxidation Using Polymer-Incorporated Rough Pt Electrodes, ACS Catal., 2023, 13, 10683–10693 CrossRef CAS
.
- M. S. Ureta-Zañartu, C. Berríos, T. González, F. Fernández, D. Báez, R. Salazar and C. Gutiérrez, Electrocatalytic Oxidation of Alcohols at Gold Electrodes in Alkaline Media, Int. J. Electrochem. Sci., 2012, 7, 8905–8928 CrossRef
.
- Y. Kwon, S. C. S. Lai, P. Rodriguez and M. T. M. Koper, Electrocatalytic Oxidation of Alcohols on Gold in Alkaline Media: Base or Gold Catalysis?, J. Am. Chem. Soc., 2011, 133, 6914–6917 CrossRef CAS PubMed
.
- A. Chen and J. Lipkowski, Electrochemical and Spectroscopic Studies of Hydroxide Adsorption at the Au(111) Electrode, J. Phys. Chem. B, 1999, 103, 682–691 CrossRef CAS
.
- S. Yang and D. G. H. Hetterscheid, Redefinition of the Active Species and the Mechanism of the Oxygen Evolution Reaction on Gold Oxide, ACS Catal., 2020, 10, 12582–12589 CrossRef CAS
.
- A. M. Verma, L. Laverdure, M. M. Melander and K. Honkala, Mechanistic Origins of the pH Dependency in Au-Catalyzed Glycerol Electro-oxidation: Insight from First-Principles Calculations, ACS Catal., 2022, 12, 662–675 CrossRef CAS
.
- F. Song, L. Bai, A. Moysiadou, S. Lee, C. Hu, L. Liardet and X. Hu, Transition Metal Oxides as Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Solutions: An Application-Inspired Renaissance, J. Am. Chem. Soc., 2018, 140, 7748–7759 CrossRef CAS PubMed
.
- M. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith and S. W. Boettcher, Cobalt–Iron (Oxy)hydroxide Oxygen Evolution Electrocatalysts: The Role of Structure and Composition on Activity, Stability, and Mechanism, J. Am. Chem. Soc., 2015, 137, 3638–3648 CrossRef CAS PubMed
.
- M. B. Stevens, C. D. M. Trang, L. J. Enman, J. Deng and S. W. Boettcher, Reactive Fe-Sites in Ni/Fe (Oxy)hydroxide Are Responsible for Exceptional Oxygen Electrocatalysis Activity, J. Am. Chem. Soc., 2017, 139, 11361–11364 CrossRef CAS PubMed
.
- H. Huang, C. Yu, X. Han, H. Huang, Q. Wei, W. Guo, Z. Wang and J. Qiu, Ni, Co Hydroxide Triggers Electrocatalytic Production of High-purity Benzoic Acid over 400 mA cm−2, Energy Environ. Sci., 2020, 13, 4990–4999 RSC
.
- M. Fleischmann, K. Korinek and D. Pletcher, The Kinetics and Mechanism of the Oxidation of Amines and Alcohols at Oxide-covered Nickel, Silver, Copper, and Cobalt Electrodes, J. Chem. Soc., Perkin Trans. 2, 1972, 1396 RSC
.
- M. K. Goetz, M. T. Bender and K.-S. Choi, Predictive Control of Selective Secondary Alcohol Oxidation of Glycerol on NiOOH, Nat. Commun., 2022, 13, 5848 CrossRef CAS PubMed
.
- V. T. T. Phan, Q. P. Nguyen, B. Wang and I. J. Burgess, Oxygen Vacancies Alter Methanol Oxidation Pathways on NiOOH, J. Am. Chem. Soc., 2024, 146, 4830–4841 CrossRef CAS PubMed
.
- P. Rodriguez, Y. Kwon and M. T. M. Koper, The Promoting Effect of Adsorbed Carbon Monoxide on the Oxidation of Alcohols on a Gold Catalyst, Nat. Chem., 2012, 4, 177–182 CrossRef CAS PubMed
.
- N. Pittayaporn, A. Therdthianwong and S. Therdthianwong, Au/C Catalysts Promoted with Ni for Glycerol Electrooxidation in Alkaline Media, J. Appl. Electrochem., 2018, 48, 251–262 CrossRef CAS
.
- P. Chakthranont, J. Kibsgaard, A. Gallo, J. Park, M. Mitani, D. Sokaras, T. Kroll, R. Sinclair, M. B. Mogensen and T. F. Jaramillo, Effects of Gold Substrates on the Intrinsic and Extrinsic Activity of High-Loading Nickel-Based Oxyhydroxide Oxygen Evolution Catalysts, ACS Catal., 2017, 7, 5399–5409 CrossRef CAS
.
- A. L. Strickler, M. Escudero-Escribano and T. F. Jaramillo, Core-Shell Au@Metal-Oxide Nanoparticle Electrocatalysts for Enhanced Oxygen Evolution, Nano Lett., 2017, 17, 6040–6046 CrossRef CAS PubMed
.
- E. A. Mayeda, L. L. Miller and J. F. Wolf, Electrooxidation of Benzylic Ethers, Esters, Alcohols, and Phenyl Epoxides, J. Am. Chem. Soc., 1972, 94, 6812–6816 CrossRef CAS
.
- M. Fleischmann, K. Korinek and D. Pletcher, The Oxidation of Organic Compounds at a Nickel Anode in Alkaline Solution, J. Electroanal. Chem. Interfacial Electrochem., 1971, 31, 39–49 CrossRef CAS
.
- J. Qiu, D. Boskin, D. Oleson, W. Wu and M. Anderson, Plasmon-enhanced Electrochemical Oxidation of 4-(hydroxymethyl)benzoic acid, J. Chem. Phys., 2022, 157, 081101 CrossRef CAS PubMed
.
- X. Kang, M. Li, J. Li, K. Wang, D. Han and J. Gong, Solubility Measurement and Thermodynamic Correlation of 4-(Hydroxymethyl) Benzoic Acid in Nine Pure Solvents and Two Binary Solvent Mixtures at (283.15–323.15) K, J. Chem. Eng. Data, 2021, 66, 2114–2123 CrossRef CAS
.
- W. Chen, C. Xie, Y. Wang, Y. Zou, C.-L. Dong, Y.-C. Huang, Z. Xiao, Z. Wei, S. Du, C. Chen, B. Zhou, J. Ma and S. Wang, Activity Origins and Design Principles of Nickel-Based Catalysts for Nucleophile Electrooxidation, Chem, 2020, 6, 2974–2993 CAS
.
- R. Wu, Q. Meng, J. Yan, H. Liu, Q. Zhu, L. Zheng, J. Zhang and B. Han, Electrochemical Strategy for the Simultaneous Production of Cyclohexanone and Benzoquinone by the Reaction of Phenol and Water, J. Am. Chem. Soc., 2022, 144, 1556–1571 CrossRef CAS PubMed
.
- L. Luo, Z. Wang, X. Xiang, D. Yan and J. Ye, Selective Activation of Benzyl Alcohol Coupled with Photoelectrochemical Water Oxidation via a Radical Relay Strategy, ACS Catal., 2020, 10, 4906–4913 CrossRef CAS
.
- X. Pan, N. Zhang, X. Fu and Y.-J. Xu, Selective Oxidation of Benzyl Alcohol over TiO2 Nanosheets with Exposed {001} Facets: Catalyst Deactivation and Regeneration, Appl. Catal., A, 2013, 453, 181–187 CrossRef CAS
.
- C.-J. Li, G.-R. Xu, B. Zhang and J. R. Gong, High Selectivity in Visible-light-driven Partial Photocatalytic Oxidation of Benzyl Alcohol into Benzaldehyde over Single-crystalline Rutile TiO2 Nanorods, Appl. Catal., B, 2012, 115–116, 201–208 CrossRef CAS
.
- A. Mahdavi-Shakib, J. Sempel, M. Hoffman, A. Oza, E. Bennett, J. S. Owen, A. Rahmani Chokanlu, B. G. Frederick and R. N. Austin, Au/TiO2-Catalyzed Benzyl Alcohol Oxidation on Morphologically Precise Anatase Nanoparticles, ACS Appl. Mater. Interfaces, 2021, 13, 11793–11804 CrossRef CAS PubMed
.
- H. Li, F. Qin, Z. Yang, X. Cui, J. Wang and L. Zhang, New Reaction Pathway Induced by Plasmon for Selective Benzyl Alcohol Oxidation on BiOCl Possessing Oxygen Vacancies, J. Am. Chem. Soc., 2017, 139, 3513–3521 CrossRef CAS PubMed
.
- F. A. L. Laskowski, M. R. Nellist, J. Qiu and S. W. Boettcher, Metal Oxide/(oxy)hydroxide Overlayers as Hole Collectors and Oxygen-Evolution Catalysts on Water-Splitting Photoanodes, J. Am. Chem. Soc., 2019, 141, 1394–1405 CrossRef CAS PubMed
.
- A. M. Hilbrands, M. K. Goetz and K.-S. Choi, C–C Bond Formation Coupled with C–C Bond Cleavage during Oxidative Upgrading of Glycerol on a Nanoporous BiVO4 Photoanode, J. Am. Chem. Soc., 2023, 145, 25382–25391 CrossRef CAS PubMed
.
- C. H. Choi, K. Chung, T.-T. H. Nguyen and D. H. Kim, Plasmon-Mediated Electrocatalysis for Sustainable Energy: From Electrochemical Conversion of Different Feedstocks to Fuel Cell Reactions, ACS Energy Lett., 2018, 3, 1415–1433 CrossRef CAS
.
- C. Wang, X.-G. Nie, Y. Shi, Y. Zhou, J.-J. Xu, X.-H. Xia and H.-Y. Chen, Direct Plasmon-Accelerated Electrochemical Reaction on Gold Nanoparticles, ACS Nano, 2017, 11, 5897–5905 CrossRef CAS PubMed
.
- R. Kamarudheen, G. W. Castellanos, L. P. J. Kamp, H. J. H. Clercx and A. Baldi, Quantifying Photothermal and Hot Charge Carrier Effects in Plasmon-Driven Nanoparticle Syntheses, ACS Nano, 2018, 12, 8447–8455 CrossRef CAS PubMed
.
- N. B. Schorr, M. J. Counihan, R. Bhargava and J. Rodríguez-López, Impact of Plasmonic Photothermal Effects on the Reactivity of Au Nanoparticle Modified Graphene Electrodes Visualized Using Scanning Electrochemical Microscopy, Anal. Chem., 2020, 92, 3666–3673 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2025 |