Open Access Article
Open Access ArticleNavigating the dichotomy of reactive oxygen, nitrogen, and sulfur species: detection strategies and therapeutic interventions
Prayasee
Baruah
 ,
Dikshaa
Padhi
,
Hariharan
Moorthy
,
Dikshaa
Padhi
,
Hariharan
Moorthy
 ,
Madhu
Ramesh
,
Madhu
Ramesh
 and
Thimmaiah
Govindaraju
and
Thimmaiah
Govindaraju
 *
*
Bioorganic Chemistry Laboratory, New Chemistry Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur P.O., Bengaluru, Karnataka 560064, India. E-mail: tgraju@jncasr.ac.in
First published on 17th January 2025
Abstract
Reactive oxygen, nitrogen and sulfur species (RONSS) collectively encompasses a variety of energetically dynamic entities that emerge as inherent characteristics of aerobic life. This broad category includes reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive sulfur species (RSS). A conundrum arises from the indispensable role of RONSS in redox signalling, while its overproduction in the mitochondria poses deleterious effects. This imbalance leads to biomolecular damage and contributes to neurodegenerative disorders, cancer, cardiovascular diseases and inflammation. Notably, the differential roles of RONSS across various diseases can be strategically exploited for therapeutic interventions. Timely, precise, and sensitive detection methods are indispensable for elucidating the spatiotemporal dynamics of RONSS and evaluating disease pathogenesis and progression. By monitoring RONSS levels, we can discern early markers of disease onset, enabling proactive intervention strategies for effective disease management. Therapeutic interventions targeting oxidative/nitrosative stress in disease pathologies have proven to be effective treatment routes in the mitigation of different diseases. This review aims to offer a comprehensive overview of the functional implications and delicate balance of RONSS in disease conditions, and advances made in detection strategies over the years while offering therapeutic strategies to tackle their adverse effects. A special emphasis is focussed on neurodegenerative disorders and cancer with case studies using RONSS-targeted chemical probes and prodrugs.
1. Introduction
The paradoxical dependency of aerobic organisms on oxygen is an intriguing phenomenon because while essential for survival, an excess of oxygen can have catastrophic consequences. This dichotomy of oxygen both nurtures and terminates life.1,2 Reactive oxygen species (ROS) is a major class of reactive species that includes a variety of chemical compounds resulting from the partial reduction of oxygen.3,4 In addition to ROS, RNS have also been extensively studied for their impact on redox homeostasis. In the 20th century, the field of redox biology primarily focused on metal ions, ROS, and RNS.5–7 Collectively, RONSS exert a dichotomous influence on health and disease, playing pivotal yet contradictory roles. In addition, alkyl (R), alkoxy (RO), peroxyl (RO2) radicals, and carbonyl oxides (R1R2COO) can engage in various unimolecular and bimolecular chemical processes. However, research on these is still in nascent stages and outside the scope of this review.RONSS plays a pivotal role in maintaining optimal well-being through cellular signalling and transduction, protein phosphorylation, maintenance of ion channels, and biosynthesis of thyroid hormones (THs) by the thyroid gland. RONSS has also been utilized as part of therapeutic methods, especially in the annihilation of cancer cells. When oxidants exceed an organism's antioxidant defences, it can cause damage to cellular organelles (such as mitochondria) and biomolecules (like DNA, proteins, and lipids). This can lead to the onset of various disease conditions, including oxidative stress, inflammation, neurodegeneration, cancer, cardiovascular, liver, and kidney diseases (Fig. 1). Therefore, detection and neutralisation of RONSS are of significance in different pathological condtions.8–12
Detection strategies for RONSS are vital for identifying oxidative and nitrosative stress in disease contexts, enabling timely diagnosis and monitoring of disease progression. To effectively sense or treat RONSS in various diseases, achieving both sensitivity and specificity is essential. This requires strategies that target RONSS selectively while minimizing off-target effects. One approach is to design RONSS-specific molecules, such as fluorogenic/calorimetric probes, that activate fluorescence or colorimetric changes only in the presence of a specific RONSS type (e.g., H2O2, HOCl, or ONOO−). This selectivity relies on tailored chemical reactions that specifically recognize individual RONSS, thereby reducing cross-reactivity. Additionally, to enhance probe reliability, these molecules should be stable under physiological conditions, resistant to environmental factors such as pH variations, serum, and temperature, which can otherwise lead to non-specific signals.13,14 For therapeutic applications, redox-active drugs can be designed to respond only to pathological RONSS levels, sparing normal cells. For instance, prodrugs activated by elevated concentrations of specific RONSS in cancer cells can provide selective cytotoxicity.15 Nanotechnology further advances specificity by using targeted nanocarriers conjugated with antibodies or ligands that recognize disease-specific markers, allowing precise delivery of RONSS-sensitive probes or therapeutic agents to affected tissues.16 Additionally, enzyme-mimicking nanozymes designed to scavenge RONSS offer selective neutralization of RONSS in diseased tissues.17 This approach combines diagnostic and therapeutic functions, providing potential for both monitoring and mitigating RONSS-related damage. These advancements enable more accurate, targeted approaches to detecting and treating RONSS-associated diseases.
2. The RONSS cycle: homeostasis
RONSS are generated endogenously as part of normal cellular physiology under pathological conditions or triggered by external environmental stressors such as air pollution, smoking, and pathogens. The mitochondrial respiratory chain is the primary source of cellular ROS, generated during ATP generation through normal oxygen metabolism.18 Oxygen readily accepts electrons from cellular oxidative metabolism, generating ROS like superoxide anion (O2˙−), hydroxyl (HO˙), and hydrogen peroxide (H2O2). Various processes like electron transport uncoupling and enzyme system activities (e.g., cytochrome P450, xanthine oxidoreductase, inflammatory enzymes), and accumulation of unbound metal ions of Fe, Cu, and Mn can exacerbate ROS production.19 NADPH Oxidase (NOX), certain membrane-bound proteins and the endoplasmic reticulum (ER) are also involved in the production of ROS. Nitric oxide (NO) is enzymatically produced by nitric oxide synthase (NOS) through a series of redox reactions. The biosynthesis of NO is the primary source of RNS production in biological systems through its rapid interaction with various ROS.20 For example, NO with O2˙− reacts at a diffusion-controlled rate to form the peroxynitrite anion (ONOO−) and its conjugated acid, peroxynitrous acid (ONOOH). NO2 is generated in an aqueous environment, through the isomerization of ONOOH.The primary source of RSS is the mitochondrial sulfide oxidation pathway. At least four RSS are produced during the process of mitochondrial sulfide oxidation: persulfide, glutathione (GSH), thiosulfate, and sulfite. RSS are difficult to measure due to their high reactivity and instability.21 However, over the past decade, there has been a renewed interest in the detection methods for H2S and related RSS, largely attributed to the growing recognition of their pivotal and intricate roles in the maintenance of health.22
In the body, defence against oxidative stress or excess ROS involves the synergistic action of enzymatic and non-enzymatic antioxidants. The endogenous enzymatic antioxidants include superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione reductase (GR), which serve as the first line of defence against RONSS.23 Non-enzymatic hydrophilic antioxidants include Nrf2-Keap 1 pathway, urate, ascorbate, GSH, and flavonoids, while lipophilic radical antioxidants comprise tocopherol, carotenoid, and ubiquinol. Glutathione S-transferases (GSTs), thioredoxin system, peroxiredoxins, and the paraoxonase family (PON1, PON2, PON3) detoxify xenobiotics and counteract cellular toxins.24 Antioxidant formulations are also therapeutically used to combat oxidative stress associated with different disorders.
3. RONSS in healthy organism
There has been a growing appreciation of the role of ROS as mediators of intracellular signalling and regulation of numerous physiological and biological responses, such as growth factor signalling, cell division, differentiation, and adaptive responses necessary to maintain the living system.25 ROS is known to interact with various proteins and regulate cell proliferation and apoptosis, via oxidation of redox-active residues such as tyrosine, histidine or cysteine, thus creating intra- or inter-protein bridges and modulating protein functions. The first-degree oxidation of cysteine residues catalysed by H2O2 within proteins serves as a reversible signal transduction mechanism in living cells.26 ROS inhibit phosphatases, while simultaneously activating an extensive range of apoptotic protein kinases belonging to the Src family, small G proteins, the Ras family, c-Jun, and the p38 mitogen-activated protein kinase (MAPK) pathways.27 Furthermore, ROS are known to transmit cytoplasmic signals to the nucleus by influencing the activities of transcription factors that regulate gene expression. In counter-response to oxidative stress, cancer cells induce the transcription of antioxidant enzymes to regulate intracellular ROS levels.Conversely, ROS have also been investigated for their therapeutic potential, especially in cancer management. High levels of ROS target cancer cells through the initiation of programmed cell death (PCD), facilitated by a group of proteases known as caspases, which are cysteine-dependent and aspartate-directed, culminating in the formation of apoptotic bodies. Monoclonal antibodies such as rituximab are used for the treatment of B cell lymphomas whose mechanism of action involves the suppression of BCL2 and p38MAPK signalling pathways, which in turn increases ROS levels and triggers apoptosis (Fig. 2).28 The application of ROS as therapeutics is also extended to neurodegenerative diseases. The photodynamic therapy (PDT) is a strategy targeting diseases through controlled and localized ROS generation. In a report, photooxygenation of amyloid β (Aβ) was achieved using peptide-based porphyrin supramolecular self-assembly (PKNPs) for Alzheimer’s disease (AD) treatment.29 The Aβ-responsive structural transformation of PKNPs was designed for selective photooxygenation of Aβ enabling controlled ROS production. PKNPs as activatable photosensitizers offer targeted therapy with enhanced blood–brain barrier (BBB) permeability and Aβ-driven disassembly. Smart peptide-based metallo-nanodrugs were utilized for effective PDT with a high ROS generation capacity, making it a next-generation smart nanomedicine for cancer therapy.30 Hypoxia has been observed in both cancer and neurodegeneration and thus development of probes for its detection is an emerging field.31 Oxygen therapy has also been reported to act on many pathological aspects of AD such as Aβ metabolism, tau phosphorylation, neuroinflammation and neuronal apoptosis.32 These advancements showcase the potential of controllable and localized ROS generation in various therapeutic applications.
RNS such as NO, nitrite (NO2−), nitrate (NO3−) and 3-nitrotyrosine and others are involved in cellular signalling, immune response, and vasodilation. Additionally, NO is instrumental in the immune system response mechanism to infections.33 In the nervous system, NO functions as a neurotransmitter and is involved in the transmission of signals between neurons. Furthermore, ONOO− may exhibit antimicrobial properties and contribute to the immune response against pathogens.34 A balanced synergy exists in the production of RNS, which is attributed to immune responses against the activation of macrophages in tumor cells, or to proinflammatory cytokines.
Unlike ROS and RNS which are often associated with oxidative stress and cellular damage, RSS have predominantly been recognized for their beneficial roles, particularly their antioxidant properties. Studies have shown a significant correlation between the expression of trans-sulfuration enzymes, namely cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), which are integral to the endogenous production of H2S, and lifespan.35 Cysteine persulfidation serves as a protective mechanism against overoxidation induced by excessive ROS, and this process is conserved across various species, including mice and humans. H2S is an essential signaling molecule in cellular processes and regulates numerous physiological functions such as blood vessel dilation, inflammation regulation, and neurotransmission.36 H2S influences protein function through a process known as sulfhydration, where it modifies cysteine residues in target proteins. While genetic studies suggest a potential relationship between RSS and senescence, the reason why reduced levels of RSS trigger cellular senescence remains unclear.37 The chemistry and biological understanding of H2S is still in its nascent stages, with several unresolved questions.
4. RONSS in diseases and their detection
RONSS, particularly ROS and RNS, play a crucial role in the pathogenesis of various diseases by inducing oxidative/nitrosative stress and causing subsequent biomolecular damage. The detection and therapeutic modulation of these species are crucial for devising advanced strategies to alleviate their deleterious effects.4.1 Metals, RONSS and their implications in diseases
Metals play a pivotal role in numerous biological processes, nevertheless, an imbalance in metal homeostasis can instigate the production of ROS, leading to oxidative stress. Fe, while indispensable for physiological processes, can prove detrimental when present in excess as labile iron pool (LIP). Fe ions engage in the Fenton reaction with H2O2, culminating in the generation of highly reactive HO˙.38 Similarly, Cu2+ instigates oxidative stress by catalysing the Fenton-type reaction. In the presence of O2− or biological reductants such as ascorbic acid or GSH, Cu2+ is reduced to Cu+ in a cyclic redox process. This results in the generation of HO˙ through the decomposition of H2O2. Notably, both Cu and Fe have been shown to form inclusion complexes with Aβ peptides and proteins to accelerate their aggregation and produce excessive ROS, implicated in AD.3,39 Ferroptosis/oxytosis, is an Fe-dependent form of cell death that involves lipid peroxidation by ROS produced by Fe3+/Fe2+ redox cycle.40,41 Transferrin receptor imports Fe as Fe2+, reduced by Six Transmembrane Epithelial Antigen of Prostate 3 (STEAP3) to form lipid radicals (R˙) and initiate lipid peroxidation. GSH, through the glutamate cysteine antiporter, plays a crucial role in regulating ferroptosis via GPX4 (Fig. 3).11 Cuproptosis is a Cu-dependent cell death involves lipoylated enzymes accumulation and loss of Fe–S cluster proteins.42 FDX1 converts Cu2+ to Cu+, promoting cell death through dihydrolipoamide S-acetyltransferase (DLAT) lipoylation and Fe–S cluster inhibition. Cu+ binds to lipoylated DLAT, triggering aggregation and cell death (Fig. 3). These metal ion-dependent forms of cell death underscore the intricate interplay between metal homeostasis, redox biology and cell fate highlighting their potential as therapeutic targets in various diseases.4.2 RONSS in neurodegeneration
The brain is supplied with a substantial amount of oxygen (>20%, through blood supply) for its optimal function. It is susceptible to oxidative stress and subsequent damage due to relatively low levels of antioxidant enzymes and high levels of catalytic transition metals which trigger ROS and RNS. This vulnerability can lead to a variety of neurodegenerative disorders, including AD, PD, HD, and ALS.Elevated levels of oxidative stress in AD reduces antioxidants levels such as vitamins C and E, uric acid, SOD, CAT, GPX, which eventually affect neurons, contributing to cognitive impairment and memory deficits and as such many antioxidants are known to act as inhibitors of AD.
4.2.1.1 Probes targeting RONSS in AD. A multitude of probes have been deployed for the detection of diverse classes of RONSS abundant in the brain as biomarkers in AD. Concurrently, therapeutic interventions aimed at mitigating RONSS have become integral components of contemporary AD treatment paradigms. Probes often contain functional groups that react selectively with RONSS, leading to measurable changes in their fluorescence properties. For instance, some probes undergo oxidation, aggregation or cleavage in the presence of specific RONSS, resulting in a quantifiable fluorescent signal.48 These chemical interactions allow for the accurate detection and quantification of RONSS levels within biological systems.12 Fluorescent probes can identify target compounds through weak molecular interactions or chemical reactions. Typically, molecular fluorescent probes consist of a fluorophore, a linker, and a recognition group. Traditional design strategies offer optical recognition for detection by interacting with analytes, typically forming a lock-and-key type of interaction. However, activity-based fluorescent probes that function via chemical bonding are more ideal for real-time imaging of ROS and RNS. The main sensing mechanisms include photoinduced electron transfer (PeT), intramolecular charge transfer (ICT), fluorescence resonance energy transfer (FRET), excited-state intramolecular proton transfer (ESIPT), and aggregation-induced emission (AIE).49
In AD, different probes based on different mechanisms have been designed for detection of RONSS. HOCl produced in macrophages and phagocytes neutralize pathogens such as viruses and bacteria. The production of HOCl from H2O2 is catalysed by MPO enzyme. The overexpression of MPO, Aβ plaques and its inclusion complexes of Cu2+ and Fe3+ result in the production of excess HOCl in the brain, which aggravate oxidative stress and inflammation in AD, thus acting as a potential biomarker.50 In this context, we have developed a molecular probe for the unambiguous detection of elevated HOCl levels in the brains of AD double transgenic mice (Fig. 5(a)).8 The coumarin–morpholine conjugate (CM2) probe acts as a turn on fluorescent probe for the specific detection of HOCl produced and proximally localized with amyloid plaques. In the presence of HOCl, the non-fluorescent thioamide probe CM2 undergoes regioselective transformation to fluorescent amide probe CM1 with remarkable selectivity and sensitivity (90-fold fluorescence enhancement, 0.32 quantum yield, and detection limit: 0.17 μM) (Fig. 5(b)). Confocal microscopy imaging validated the excellent detection capability of the probe in the cortex and hippocampus regions of the AD transgenic mice brain tissues (Fig. 5(c) and (d)). This study established and validated HOCl produced and proximally localized with amyloid plaques as a new combination biomarker with potential to be qualified for inclusion in the National Institute on Aging and Alzheimer's Association (NIA-AA) research framework 2024 designated list of biomarkers (ATN) for reliable AD diagnosis.
 | ||
| Fig. 5 Detection of RONSS and associated oxidative stress. (a) Structure of CM2 and its HOCl detection mechanism. (b) Fluorescence response of CM2 to various ROS. (c) Confocal microscopy images of hippocampus (left) and cortex (right) of the CM2 administered WT and AD mouse brain and (d) corresponding quantification of HOCl (green) with Aβ plaques (red). Scale bar: 150 μm. Reproduced with permission from ref. 8. Copyright 2019 American Chemical Society. (e) Structure of 3-HF-OMe and (f) its mechanism of sensing. (g) Fluorescence emission spectra of 3-HF-OMe in presence of Aβ42 oligomers and Aβ42 aggregates in presence of ONOO−. Reproduced with permission from ref. 51. Copyright 2018 American Chemical Society. (h) Schematic representation for the conversion of QCy-BA to QCy-DT in presence of H2O2. (i) Fluorescence response of QCy-BA to various ROS. (j) Fluorescence microscopy of MRC5 cells incubated with QCy-BA in absence and presence of H2O2. Scale bar: 20 μm. Reproduced with permission from ref. 9. Copyright 2016 Royal Society of Chemistry. (k) Schematic representation of the fluorescence amplification of CRANAD 88 in response to H2O2 and Aβ. (l) Fluorescence response of CRANAD-88 on addition of H2O2 and Aβ. Reproduced with permission from ref. 52. Copyright 2016 Springer Nature. (m) Conversion of DNPOCy to NIR emitting Cy dye in response to H2S. Fluorescence response of DNPOCy on addition of different ROS (n) and H2S (o). Reproduced with permission from ref. 53. Copyright 2014 Royal Society of Chemistry. | ||
Aβ instigates the production of NOS in microglial cells, which in turn generates NO and ONOO−. These RNS released from microglial cells damage biomolecules through nitrosylation causing neuronal dysfunction and cell death. In fact, an elevated levels of nitrosylated proteins were detected in the neurons located in the cortex and hippocampus regions of the AD patients’ brains. This observation suggests the potential utility of ONOO− as a diagnostic marker for AD.54 A dual-functional probe (3-HF-Ome) was developed for detecting ONOO− using the fluorescence property of 3-hydroxyflavone (3-HFs) that works via the excited state intramolecular proton transfer (ESIPT) mechanism (Fig. 5(e) and (f)).51 ESIPT results in normal and phototautomeric species with distinct emissions upon excitation, which can be observed in the fluorescence emission spectra of 3-HF-OMe. The presence of Aβ42 oligomers and aggregates with ONOO− results in distinct fluorescence emission by 3-HF-OMe, demonstrating its robust sensing capabilities (Fig. 5(g)). Wang et al. developed BTNPO, an innovative two-photon fluorescent probe adept at tracking the distribution and alterations of Aβ aggregates and ONOO−via two distinct fluorescence channels.55 BTNPO consists of a benzothiazole-naphthalene derivative, chosen for its structural similarity to thioflavin T, which serves as the recognition group for Aβ aggregates and oxindole which acts as the recognition group for ONOO−. Through co-imaging the dynamics of Aβ aggregates and ONOO− levels in brain slices across various ages, it was revealed that elevated levels of ONOO− manifested earlier than Aβ aggregates in the brains of AD mice.
Stimuli-responsive fluorescent probes are widely utilized in detecting the physiological and pathological states of living systems. We have developed a boronic acid-functionalized quinone-cyanine probe (QCy-BA) in combination with AT-rich DNA (exogenous or endogenous cellular DNA), termed QCy-BA⊂DNA, acts as a stimuli-responsive Near-Infrared (NIR) fluorescence probe for measuring the cellular levels and homeostasis of H2O2.4 The potential role of H2O2 in causing oxidative stress in AD is well established. Deposits of Aβ plaques, NFTs, and their metal-inclusions catalyse the production of ˙OH from H2O2 through the Fenton reaction. Upon exposure to cellular H2O2, QCy-BA transforms into QCy-DT, a one-donor, two-acceptor (D2A) system that binds to the cellular DNA and displays switch-on NIR fluorescence (Fig. 5(h)–(j)). QCy-BA can detect both normal and elevated levels of H2O2 produced by GOx-mediated oxidation of glucose, through EGF/Nox pathways, and post-genotoxic stress in primary and senescent cells. A curcumin analogue CRANAD-88 was designed by coupling a CRANAD-58 as Aβ recognition moiety and borate as H2O2-selective chemical moieties for the detection of H2O2 in AD (Fig. 5(k)).52CRANAD-88 detects H2O2 with NIR fluorescence (NIRF) in transgenic AD model APP/PS1 mice brain samples containing Aβ plagues (Fig. 5(l)).
In addition to ROS and RNS, emerging reports suggest a role for RSS, specifically H2S, in neurological diseases. H2S has been implicated in the regulation of memory in numerous studies, leading to a hypothesis that the characteristic memory decline in AD may be related to decreased levels of H2S in the brain. There have been conflicting reports on reduced levels of free H2S in AD patients as compared to healthy controls, however, alteration in the total sulfide concentrations (acid-labile and bound) was observed in AD patients.56 We have developed a highly sensitive ratiometric NIR fluorescence and colorimetric probe, DNPOCy for H2S detection, that reacts rapidly with H2S in an aqueous medium to release the cyanine dye, Cy-quinone which displays NIR emission at λem = 555 nm (Fig. 5(m)–(o)).53
4.2.1.2 Therapeutic strategies targeting RONSS for mitigating AD. In the field of therapeutics, several molecules with RONSS scavenging properties have been developed as a therapeutic route towards AD. Aβ in the presence of redox-active metals such as Cu2+ and Fe3+ can produce ROS leading to oxidative stress. We have developed a peptidomimetic inhibitor P6 combining a Cu-binding natural tripeptide of human origin, GHK, and hybrid peptoid with Aβ aggregation inhibition ability. (Fig. 6(a)).57,58P6 complexes and maintain Cu2+ in a redox-dormant state preventing ROS production, exhibiting potent inhibition of Aβ and Cu2+ induced toxicity in cells. In a recent development, a series of short peptides (PNGln) conjugated with a dual-functional fluorophoric amino acid (NGln) was developed as anti-AD candidates, with the lead peptide, P2NGln, exhibiting strong Cu2+ affinity and stabilizing the ion in a redox-inactive state. P2NGln reduces ROS-mediated cytotoxicity induced by Cu2+, and inhibits both Cu-dependent and independent Aβ42 fibrillation and associated toxicity while suppressing liposaccharide-induced ROS and RNS production in microglial cells.59 We have developed hybrid multifunctional modulators (HMMs) by combining the structural and functional elements of metal chelator clioquinol (Clq, one of the earliest drug candidates enter clinical trial for AD) and the natural antioxidant epigallocatechin gallate (EGCG) found in green tea.47 We found that the lead HMM, TGR86 (Fig. 6(b)) complexes Cu2+ halting its redox cycle and averting the production of harmful ROS. TGR86 rescued PC12 cells from Aβ/Aβ-Cu2+-induced neurotoxicity by preventing ROS generation. It protected biomolecules such as proteins, DNA, and lipids from damage inflicted by ROS. Furthermore, it disrupted the interaction of toxic Aβ species with mitochondria, thereby preventing mitochondrial damage. Inspired by GHK, We have designed small multifunctional modulators (MFMs) that modulate H2O2-induced ROS generation and quenches total nitrite concentration in LPS-treated cells (Fig. 6(c)–(e)).60 While GHK and P6 modulate Cu2+/Aβ-Cu2+-associated toxicity, the lead analogue MFM4 complexes both Cu2+ and Fe3+ in their free form or in their Aβ-bound states, disrupting their redox cycles and preventing excessive ROS production. This, in turn, ameliorates oxidative DNA damage and mitochondrial dysfunction and modulates Nrf2 protein signaling under oxidative stress conditions by eliminating toxic stress elements. In another natural product-inspired strategy, the berberine derivative, Ber-D (Fig. 6(f)) was developed, which has been shown to alleviate metal-dependent and independent Aβ toxicity.10 Notably, Ber-D shows a remarkable ability to quench intracellular ROS, reduce protein oxidation, and inhibit DNA damage, lipid peroxidation, and mitochondrial damage, thereby protecting cells from apoptosis (Fig. 6(g)). Additionally, multi-targeted flavonoids with potent antioxidant properties have been shown to be effective against multiple pathways of AD.61 We have designed synthesized small molecules with the capability to modulate the neuroinflammatory pathway. A small molecule, M3, (Fig. 6(h)) rationally designed by integrating pharmacophores for metal chelation, antioxidant onto Aβ42 aggregation modulating naphthalene monoimide (NMI) scaffold was found to modulate metal dependent Aβ toxicity and reduce oxidative stress, as monitored by the Nrf2 signalling pathway.62M3 effectively reduces microglial hyperactivation and neuroinflammation as monitored by bio-AFM analysis (Fig. 6(i)).
 | ||
| Fig. 6 Therapeutic strategies to modulate oxidative and metal dyshomeostasis in AD. Structure of (a) P6, (b) TGR 86 (c) MFM-4. (d) In cellulo ROS quenching assay of MFM 1-4 and GHK. (e) % Nitrite concentration measured in cell media upon treatment with MFM 1–4 or GHK. Reproduced with permission from ref. 60. Copyright 2019 American Chemical Society. (f) Structure of Ber-D. (g) Quantification of ROS generation by DCFDA assay in PC12 cells incubated with Aβ42, Cu2+ and Ber-D. Reproduced with permission from ref. 10. Copyright 2020 Cell Press. (h) Structure of M3, (i) Microglial activation monitored by both Bio-AFM and confocal microscope reproduced with permission from ref. 62. Copyright 2022 American Chemical Society. (j) Structure of lead bipyridyl multifunctional modulator, compound 5, (k) NO levels (RNS) by Griess assay reproduced with permission from ref. 63. Copyright 2022 Royal Society of Chemistry. (l) Nrf2 activation by TA. Scale bar: 30 μm (m) TA binds to activator site of GPX4. (n) Western blot to monitor enhancement in GPX4 levels in presence of TA. Reproduced with permission from ref. 11. Copyright 2023 Royal Society of Chemistry. (o) Structure of GCTR. (p) IF imaging to monitor decline in GPX4 levels in presence of Aβ + Fe3+ and its enhancement on co-treatment with GCTR. Scale bar: 25 μm. Reproduced with permission from ref. 64. Copyright 2024 Elsevier. (q) The co-assembly of nanodrug Ang-AIE NCs, (r) Size change of NCs treated with PBS and H2O2 for various times. Reproduced with permission from ref. 65 Copyright Nature (s) Gracilin A derivatives decreased ROS and NO produced by microglia. Reproduced with permisiion from ref. 66. Copyright American chemical society. (t) Itaconate acts as a crucial anti-inflammatory metabolite, which engages Nrf2 to modulate type I interferons and limit inflammation. Adapted from ref. 67. Copyright 2018 Springer Nature (u) Structure of CPUY192018. (v) Ratio of GSH/GSSG were measured in HK-2 cells. Reproduced with permission from ref. 68. Copyright 2019 Elsevier. | ||
We designed and synthesized bipyridyl-based multifunctional modulators showing ROS and RNS scavenging properties, as examined from in vitro and in cellulo studies.63 In SH-SY5Y cells, ROS was generated using H2O2, wherein the lead dopamine functionalized bipyridyl compound 5 (Fig. 6(j)) effectively scavenged ROS, and rescued cells from oxidative stress and nitrosative stress (Fig. 6(k)). Neuroinflammation becomes a significant factor under oxidative stress conditions and its modulation is a major therapeutic approach in AD.69 Ferroptosis and AD exhibit common pathological characteristics, which include an abnormal accumulation of Fe, elevated levels of ROS, increased lipid peroxidation, and diminished levels of antioxidant enzyme GPX4. The natural polyphenol, tannic acid (TA), a known antioxidant offers a comprehensive approach to simultaneous mitigation of ferroptosis and AD.11TA was identified as a Fe-chelator, ROS scavenger, and a potent inhibitor of Aβ42 and tau aggregation. TA effectively modulates Fe-dyshomeostasis, harmful ROS, highly toxic Fe-induced Aβ42 aggregates, inhibits mitochondrial damage, and activates Nrf2 (Fig. 6(l)) and thereby combating AD. In addition to inhibiting ferroptosis through conventional Fe-chelation and preventing ROS production and lipid peroxidation, TA binds to the activator site of GPX4 (Fig. 6(m)), enhancing the activity and cellular levels of the enzyme (Fig. 6(n)), thereby strengthening the inherent antioxidant mechanism. The combined approach of Nrf2-GPX4 activation and ferroptosis inhibition introduces a conceptually novel and integrated strategy for effectively targeting the GPX4-ferroptosis-AD axis to tackle AD pathology. The dual therapeutic nature of polyphenols inspired the design and synthesis of gallic acid-CDP hybrids where the lead molecule, GCTR synergistically combats both ferroptosis and amyloid toxicity (Fig. 6(o)).64GCTR enhances the expression of GPX4 (Fig. 6(p)) and modulates Fe3+-induced liquid–liquid phase separation of tau, thereby fostering the development of small molecule-based novel therapeutics for AD. Furthermore, a polycatechol PLDP, comprising L-DOPA, was designed to possess antioxidant, anti-amyloid aggregation, and ferroptosis inhibition properties. Interestingly, while L-DOPA itself acts as a pro-oxidant in the presence of Fe, PLDP functioned as an antioxidant.70
A near-infrared-II aggregation-induced emission (AIE) nanotheranostic was engineered for targeted AD therapy. Upon activation by ROS, the release of two therapeutic AIE molecules was precisely controlled (Fig. 6(q)).65 One molecule disrupted Aβ fibril formation, degraded existing fibrils and prevented reaggregation while the other scavenged ROS (Fig. 6(r)), restored redox balance thereby reversing neurotoxicity and enhancing cognitive function in a female AD mouse model. We present a polydopamine–ruthenium (PDA–Ru) nanosystem, which functions concurrently as a near-infrared photothermal therapy (PTT) agent, ROS scavenger, and H2O2 catalyst. This multifunctional approach holds promise as a therapeutic strategy for AD and other neurodegenerative disorders.71 Gracilin A derivatives, synthesized via pharmacophore-directed retrosynthesis, have demonstrated significant neuroprotective properties (Fig. 6(s)) They were particularly effective in modulating the expression of antioxidant genes (CAT, GPx, SODs, and Nrf2), reducing cytokine release (IL-1β, IL-6, GM-CSF, TNF-α) and other ROS/RNS, highlighting their potential as promising candidates for AD therapy.66 The critical role of oxidative stress in AD has been recognized by researchers recently, and natural antioxidants have been demonstrated to have anti-AD activity in animal models, such as Ginkgo biloba extract, soy isoflavones, lycopene, and so on.72 In the field of cancer therapeutics too, the development of novel tools has led to the utilization of nonenzymatic antioxidants, activators of Nrf2, inhibitors of NOX, and vitamins, as oxidative stress plays a role in the progression of cancer. For example, fumaric acid esters serve as Nrf2 activators that operate by modifying sensor cysteines of KEAP1, such as dimethyl fumarate (DMF). DMF operates by alkylating Cys151 of KEAP1, thereby disrupting the association between KEAP1 and Nrf2. The endogenous metabolite itaconate is often necessary for the activation of Nrf2, which operates via the alkylation of KEAP1. Itaconate acts as a critical anti-inflammatory metabolite, which engages Nrf2 to modulate type I interferons and limit inflammation (Fig. 6(t)).67 The therapeutic efficacy of CPUY192018, a small-molecule inhibitor of the KEAP1-Nrf2 pathway in renal inflammation was evaluated. CPUY192018 significantly activates the Nrf2-based cytoprotective system and thus, protects the kidney from inflammatory injury (Fig. 6(u) and (v)).68 Expanding the use of the dicyanomethylene-4H-chromone (DCM) chromophore, a novel NIR theranostic prodrug, DCM-S-CPT, for cancer chemotherapy in mice was explored making use of the abundance of the antioxidant GSH in cancer cells.73 DCM-S-CPT links the DCM fragment to the camptothecin (CPT) drug through a disulfide linker, suppressing both fluorescence and cytotoxicity. However, cancer cells with excess GSH selectively cleave the disulfide bond, releasing the cytotoxic drug and the fluorophore from PEG-PLA-loaded nanoparticles. The production of ROS by NOX is accompanied by the formation of SO2−. Inflammation-triggered macrophages generate NO, which combines with SO2− to form ONOO−, aiding in tumor cell death. Tumor cells express NOS isoforms, affecting progression based on type and stage. Human colorectal cancer and inflammatory diseases link inducible iNOS activity with p53 alterations, suggesting RNS trigger cancer-related gene mutations. Research using iNOS knockout mice implies NO from iNOS plays a role in melanoma carcinogenesis initiation and promotion.74 Besides ROS and RNS, the association of RSS with oxidative stress arises from their consumption of thiols, raising ROS levels directly or indirectly. The selective oxidation holds promise for antimicrobial and cancer therapies.75
4.2.2.1 Probes targeting RONSS in other neurodegenerative disorders. A dual-responsive fluorescent probe Mito-VS possesses high mitochondrion-targeting capabilities and can simultaneously measure mitochondrial viscosity and H2S (Fig. 7(a)).80 Mito-VS is initially nonfluorescent due to free intramolecular rotation between dimethylaniline and pyridine, but an increase in viscosity restricts this rotation, resulting in the release of intense red fluorescence. Liu et al. engineered Mito NIRHV, a near-infrared (NIR) fluorescent probe, to discern mitochondrial viscosity and H2O2 levels in the brains of PD mice (Fig. 7(b)).81 The probe consisted of a p-pinacolborylbenzyl moiety which has high selectivity toward H2O2, a quaternarized quinoline unit for targeting mitochondria and a lipophilic π-conjugated thiophene-bridge extending NIR emission and a twistable ethylene responding to environmental viscosity. Huang et al. devised BT-NH, a fluorescent probe meticulously crafted for the specific and remarkably sensitive detection of NO within the brains of PD animal models (Fig. 7(c)). Its structure was inspired from diaminofluorescein and benzobis(1,2,5-thiadiazole) structures which harbour precision and sensitivity for NO.82 Liu et al. pioneered NIR-PNs, a class of near-infrared probes capable of ultra-fast (within seconds) and highly selective detection of ONOO− across various PD animal models, underscoring their utility in rapid and specific detection assays (Fig. 7(d)).83 For synthesizing NIR-PNs, dicyanoisophorone-based NIR fluorophore with a donor–π–acceptor structure was chosen for its biocompatibility, NIR emission, and photostability whereas p-aminophenol receptors were integrated as ONOO− reactive sites. Upon reacting with ONOO−, p-aminophenol groups cleave to release p-benzoquinone, restoring the fluorescence of the probe. CuxO nanoparticle clusters (NCs) were developed to functionally mimic the activities of SOD, CAT and GPX4, which inhibited neurotoxicity in a cellular model of PD (Fig. 7(e)).76 RNS contribute to lipid peroxidation and protein dysfunction through the formation of S-nitrosothiols, impairing the brain function in PD patients.84 A decrease in the levels of H2S is observed in the striatum and plasma of PD animal models.85
 | ||
| Fig. 7 (a) Design of dual-responsive fluorescent probe Mito-VS for detection of viscosity and H2S. Reproduced with permission from ref. 80. Copyright 2018 American Chemical Society. (b) Design of dual-responsive fluorescent probe Mito-NRHV for detection of viscosity and H2S. Reproduced with permission from ref. 81. Copyright 2020 Royal Society of Chemistry. (c) Design of BT-NH for the specific detection of NO within the brains of PD animal models. Reproduced with permission from ref. 82. Copyright 2019 Elsevier. (d) Design of NIR-PNs, a class of near-infrared probes for ultra-fast and highly selective detection of ONOO− across various PD animal models. Reproduced with permission from ref. 83. Copyright 2020 American Chemical Society. (e) Design of CuxO nanoclusters. Reproduced with permission from ref. 76. Copyright 2019 American Chemical Society. (f) Design strategy of TCAB for the precise detection of H2S and H2O2 simultaneously. Reproduced with permission from ref. 86. Copyright 2020 American Chemical Society. (g) Design strategy for HCy-ONO that offers dual turn-on fluorescence responses for H2Sn and O2˙− in separate windows. Reproduced with permission from ref. 87. Copyright 2019 American Chemical Society. (h) Design strategy for BOD-NH-SC, an (ESIPT)-based ‘AND’ logic system probe, tailored for imaging thiols and ONOO− in cellular environments. Reproduced with permission from ref. 88. Copyright 2021 Wiley. (i) Design strategy for TFP for simultaneous determination of mitochondrial H2O2 and ATP changes in two well-separated fluorescence channels. Reproduced with permission from ref. 89. Copyright 2020 American Chemical Society. (j) Structure of TGP18 and schematic showing its binding to GQ and subsequent cancer cell death. (k) Immunofluorescence images of A549 cells showing nuclear translocation of Nrf2 (green) upon treatment with TGP18. Scale bar: 10 μm. (l) Immunofluorescence images to assess DNA damage response (DDR) as a result of treatment with TGP18 or UV exposure. Reproduced with permission from ref. 75. Copyright 2020 Ivyspring International Publisher. Reproduced with permission from ref. 77. Copyright 2020 Ivy spring International Publisher. (m) Structure of TCA and TCZ and their dual targeting nature causing cancer cell death. (n) Localization of TCA and TCZ (green) in MDAMB-231 cells by colocalizing with the Alexa 647-conjugated Nucleolin (red) antibody at 1 h. Reproduced with permission from ref. 86. Copyright 2022 American Chemistry Society. | ||
The implication of RONSS with diverse pathological role is also evident in HD, Wilson's disease, psychiatric ailments, and schizophrenia, showcasing diverse roles.
4.2.2.2 Therapeutic strategies for mitigating RONSS in other neurodegenerative disorders. A study on high-dose antioxidant therapy combining vitamin C (3000 mg per day) and vitamin E (3200 IU per day) suggests this approach may slow the progression of PD.90 A multicenter, randomized, placebo-controlled, double-blind trial with parallel-group and dosage-ranging design further investigated the impact of Coenzyme Q10, an endogenous antioxidant at 300, 600, and 1200 mg daily compared to placebo wherein a dose-dependent improvement of physiological symptions was observed.91 Intraventricular injections of iron chelators desferrioxamine and deferasirox were shown to prevent dopamine depletion in the striatum in a 6-OHDA rat model of PD.92 In MPTP-induced mouse models, melatonin administration helped protect the nigrostriatal pathway from oxidative stress, neurotoxicity, and degeneration caused by the toxin, with similar findings in 6-OHDA models.93 BN82451, a brain-penetrable compound for Huntington's disease, exhibits neuroprotective effects by safeguarding mitochondria against oxidative damage.94 Another innovative antioxidant, XJB-5-131, which targets mitochondria, was found to substantially reduce mitochondrial DNA oxidative damage and slow disease progression in Huntington's disease animal models.95 Dietary flavonoids like rutin, myricetin, and hesperidin also show benefits in Huntington's disease by activating the neuroprotective and cytoprotective Nrf2 pathways.79
In ALS mouse models, resveratrol, a SIRT1 activator, provided mitochondrial protection and symptomatic improvement.96 Furthermore, a novel acylaminoimidazole derivative, WN1316, which selectively reduces oxidative stress-induced cell death and inflammation by upregulating Nrf2 and boosting GSH, showed efficacy in enhancing motor function and extending survival in SOD1H46R- and SOD1G93A-transgenic mice.97 Edavarone (MCI-186) acts a free radical scavenger of both oxidative and nitrosative stress and exhibits therapeutic potency against ALS.98
4.3 RONSS in cancer
The high mitochondrial metabolism observed in cancer cells leads to an increase in RONSS levels, which in turn promotes tumorigenesis and the accumulation of mutations, thereby facilitating faster growth and spread. The role of ROS in tumorigenesis was first proposed in the 1990s.99 In p53-heterozygous mice, the deletion of Brca1 results in increased ROS and the formation of mammary tumors.100 The control of oxidative stress by Brca1 involves Nrf2. Growth factors and cytokines such as Interferon (IFN), Tumor Necrosis Factor (TNF), and macrophages stimulate the production of H2O2 and NO in tumor cells. The activation of Erk1/2 by ROS enhances survival, growth, and motility in various cancers including ovarian, breast, melanoma, and leukemia. The Kirsten rat sarcoma viral oncogene homolog (K-Ras) triggers the activation of the MAPK/Erk1/2 pathway, which is linked to cell proliferation. Ovarian cancer cells with high levels of ROS exhibit elevated Erk1/2 due to persistent ubiquitination and loss of MKP3, thereby enhancing Erk1/2 activity.1014.4 RONSS in inflammation
The immune system initiates a defense mechanism in response to any pathological assault, including inflammation. ROS is involved in regulating the inflammatory response through key regulators such as Nrf2, NF-κB, and TNFR1 pathways. In disease conditions, mitochondrial ROS triggers the activation of tissue macrophage inflammasomes, which mediate inflammation. It has been reported that NALP and NLRP inflammasomes are regulated by mitochondrial ROS through MAPK/Erk1/2 pathways.109 ROS also regulates the infiltration of cells to the site of inflammation by facilitating the extravasation of inflammatory macrophages. A gradient of H2O2 created around the wound signals the infiltration of macrophages to the injury site. Endothelial junctions serve as barriers to the extravascular infiltration of inflammatory cells. ROS regulates occludin interactions through GSH. The recruitment of neutrophils is regulated by ROS through the regulated expression of IL1β. Microbes increase extracellular ROS, triggering IL1β expression to infiltrate neutrophils and clear the microbial load. There are a few reports suggesting that mitochondrial ROS regulates endothelial cell migration and angiogenesis, which are essential for the inflammatory response. The regulation of inflammation by ROS and oxidative stress is mediated by various redox-sensitive transcription factors and inflammatory cytokines. NF-κB, a redox-sensitive transcription factor, is involved in regulating inflammatory processes in response to ROS. Mitochondrial ROS is known to promote TNFα-mediated NF-κB activation and inflammatory response.110 NOX-dependent ROS regulates the differentiation of macrophages to M2 phenotypes and continues the production of NO, which is required for the bactericidal activity of M2 macrophages. Tang et al. designed an activatable fluorescent nanoprobe (TPE–IPB–PEG) with AIE emission characteristics that were used to accurately image ONOO− at the site of inflammation in bacterially infected mice (Fig. 8(a) and (b)).111TPE-IPB consists of a tetraphenylethene (TPE) moiety as an AIEgen, an imine group as an emission mediator, and a phenylboronate as a recognition site for ONOO−. Formulated with a lipid–PEG matrix, TPE-IPB nanoprobes (TPE-IPB-PEG) are non-fluorescent in aqueous solution but emit intense yellow fluorescence upon reaction with ONOO− at pH 7.4. Subsequently, Wang and coworkers introduced a fluorescent probe, PAM–BN–PB for the rapid imaging of H2O2 in mice afflicted with peritonitis, utilizing phenanthroimidazole, benzonitrile, and benzyl boronic ester components (Fig. 8(c)).112 Additionally, they developed HOTN, a tetrastyrene-based AIE fluorescent probe capable of visualizing signals of HOCl in mouse models of peritonitis, arthritis, and liver cancer.113 In another study, Pinkment, a functionalized synthetic derivative of peroxyresorufin-1, was developed aimed at visualizing ONOO− in LPS-induced peritonitis mouse models, enabling assessment of the therapeutic efficacy of indomethacin using resorufin as the fluorophore and a boronic ester as the ONOO− recognition moiety (Fig. 8(d)–(f)).114 Furthermore, Chang et al. presented MITO-CC, a two-photon ratiometric fluorescence probe for the detection of ONOO− in cells and inflammation mouse models (Fig. 8(g)).115MITO-CC featured a rhodamine derivative for ONOO− recognition and coumarin for energy donation, enabling ratiometric detection of ONOO−. | ||
| Fig. 8 Fluorescent probes for detecting RIS in inflammatory and liver, kidney and cardiovascular diseases. (a) Design of fluorescent nanoprobe TPE–IPB–PEG with AIE emission characteristics to accurately image ONOO− at the site of inflammation in bacterially infected mice (b). Reproduced with permission from ref. 111. Copyright 2016 Royal Society of Chemistry. (c) Design of PAM–BN–PB for the rapid imaging of H2O2 in mice afflicted with peritonitis. Reproduced with permission from ref. 112. Copyright 2017 American Chemical Society. (d) Design of Pinkment, a functionalized synthetic derivative of peroxyresorufin-1, for visualizing ONOO− in LPS-induced peritonitis mouse models. (e) Fluorescence spectra of Pinkment on interaction with ONOO−. (f) Confocal imaging of RAW 264.7 macrophages treated with LPS (1 μg mL−1, 24 h) and then loaded with Pinkment 8 (20 μM, 30 min). The cell nuclei were stained using Hoechst 33342. Scale bar = 100 μm. Reproduced with permission from ref. 114. Copyright 2020 Royal society of Chemistry. (g) Design of MITO-CC, a two-photon ratiometric fluorescence probe for the detection of ONOO− in cells and inflammation mouse models. Reproduced with permission from ref. 115. Copyright 2017 American Chemical Society. (h) Design of A2, a fluorescent probe enabling visualization of ONOO− flux in diabetic rat kidney tissue. Reproduced with permission from ref. 116. Copyright 2016 Elsevier. (i) Design of probe LW-OTf for detecting and imaging O2˙− and ONOO− involved in drug involved liver injury (DILI). Reproduced with permission from ref. 117. Copyright 2021 Royal Society of Chemistry. (j) Design of DT-Gal, a galactosyl(azido) naphthalimide-based fluorogenic probe as a liver cell-specific probe for imaging H2S in HepG2 liver cancer cells. Reproduced with permission from ref. 118. Copyright 2015 Royal Society of Chemistry. | ||
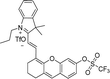
4.5 RONSS in cardiovascular, liver and kidney disease
Physiologically, the RONSS regulates the activities of vascular smooth muscle cells, influencing their contraction, relaxation, and development. Recent studies suggest that ROS may directly affect cardiac contractility, in addition to its indirect effects. There are reports indicating that ROS could enhance the inotropic effect of substances such as ET-1 by activating redox signalling pathways like Erk1/2. The activation of MAPK, particularly p38 MAP kinase, may precede the overproduction of ROS, which is associated with heart failure.119 ROS plays a significant role in coronary artery disease by mediating the creation of oxidized LDL and activating matrix metalloproteinases (MMP), leading to plaque rupture. An increase in ROS levels due to D-amino acid oxidase may result in systolic heart failure without ventricular hypertrophy in rat hearts. Furthermore, ROS contributes to the formation of thrombus and other cardiovascular diseases. Patients with heart failure have been found to exhibit reduced NO production, increased nitrotyrosine formation, and increased iNOS expression.120 The role of RSS in cardiovascular disease remains unclear. However, down-regulated H2S pathways have been implicated in vascular disorders.The kidney and liver are organs that are highly susceptible to oxidative damage. During damage, there is a surge in ischemia-related RONSS components such as ONOO− and HOCl, while antioxidant enzymes decline. This phenomenon is observed in renal tissue following ischemia and nephrotoxicity, as well as in sepsis-induced kidney damage. Overproduction of NO from the activation of iNOS during inflammation leads to the formation of O2−, contributing to the vicious cycle of oxidative stress and chronic inflammation associated with Chronic Kidney Disease (CKD). Guo et al. developed A2, a fluorescent probe enabling visualization of ONOO− flux in diabetic rat kidney tissue (Fig. 8(h)).116A2 rapidly responded with turn-on fluorescence (within seconds) and boasted ultrahigh sensitivity (detection limit: <2 nM), successfully visualizing endogenous ONOO− in RAW264.7 macrophages and oxygen-glucose-deprived endothelial cells and indicated elevated ONOO− concentration in diabetic kidney tissue (Fig. 8(h)). In chronic liver diseases, there is an elevation in oxidative stress which affects protein expression patterns. James et al. developed a molecular probe (LW-OTf) for detecting and imaging two biomarkers associated with drug-involved liver injury (DILI) (Fig. 8(i)). Initially, the ROS superoxide (O2˙−), selectively activates near-infrared fluorescence (NIRF) by generating the fluorophore LW-OH. This hemicyanine fluorophore is then oxidized by RNS peroxynitrite (ONOO−), cleaving it to release the xanthene derivative LW-XTD, which is detected using two-photon excitation fluorescence (TPEF). Alternatively, LW-OTf can be cleaved by ONOO− to form non-fluorescent LW-XTD-OTf, which reacts with O2˙− to produce the fluorescent LW-XTD. By combining NIRF and TPEF, LW-OTf enables the differential and simultaneous detection of ROS and RNS in DILI through two optically orthogonal channels.117 Meanwhile, addressing the biological significance of H2S, DT-Gal, a galactosyl(azido) naphthalimide-based fluorogenic probe, was developed (Fig. 8(j)).118 Targeting liver cells with its galactosyl unit and reacting selectively with H2S via its azido group, DT-Gal demonstrated its potential as a liver cell-specific probe for imaging H2S in HepG2 liver cancer cells, offering insights into the role of abnormal H2S levels in liver disease pathogenesis. Redox-sensitive factors such as Egr-1, NF-κB, AP-1, and kinases respond to these changes, driving cellular alterations and triggering hepatocyte apoptosis, which is a part of liver damage. The role of RSS in kidney and liver diseases remains unclear, as does the exact mechanism by which RONSS causes damage to these organs. All probes mentioned in this review have been summarized in Table 1.
| S. no | Name of molecule | Structure | RONSS detected | Disease | LOD | Ref. |
|---|---|---|---|---|---|---|
| 1 | CM2 |

|
HOCl | AD | 0.17 μM | 8 |
| 2 | QCy-BA |

|
H2O2 | Cancer | 5.3 μM | 9 |
| 3 | 3-HF-OMe |

|
ONOO− | AD | 65.5 nM | 51 |
| 4 | BTNPO |

|
ONOO− | AD | — | 51 |
| 5 | CRANAD 88 |

|
H2O2 | AD | 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nM nM |
52 |
| 6 | DNPOCY |

|
H2S | AD | — | 53 |
| 7 | MITO-VS |

|
H2S | AD | — | 80 |
| 8 | Mito NIRHV |

|
H2O2 | AD | 3 nM | 81 |
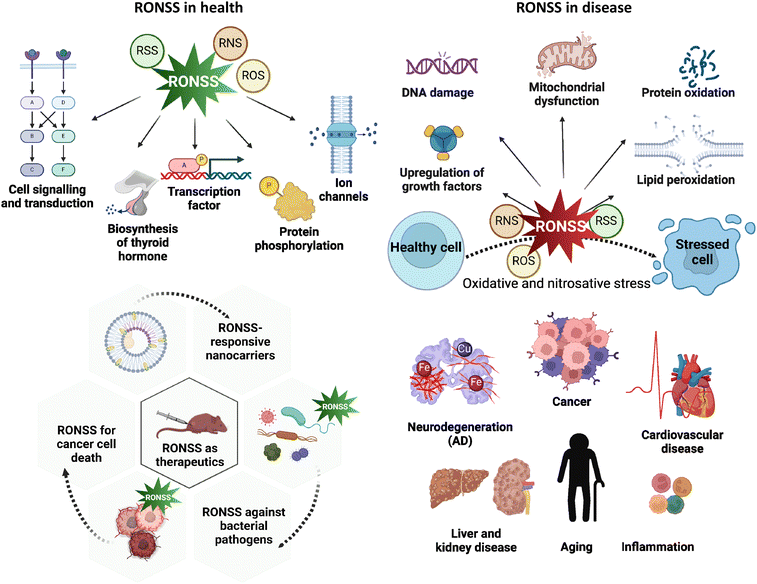
| 9 | BT-NH |
|
NO | PD | 6.96 nM | 82 |
| 10 | NIR-PNs |

|
ONOO− | PD | 4.59 to 18.9 nM | 83 |
| 11 | TCAB |

|
H2S, H2O2 | Cancer and other diseases | 0.044 and 0.058 μM | 86 |
| 12 | HCY-ONO |

|
O2˙−, SO2 | Cancer, inflammation, cardiovascular disease | 90 and 100 nM | 87 |
| 13 | BOD-NH-SC |

|
NO, H2S | Various diseases | 31 nM | 88 |
| 14 | TFP |

|
H2O2 | Mitochondrial stress | 0.4–10 μM | 89 |
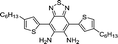
| 15 | MPIBA |

|
SO2, ClO− | Cancer | 3.5 nM and 12.5 nM | 102 |
| 16 | TPE-IPB |

|
ONOO− | Inflammation and other diseases | ≈100 μM | 111 |
| 17 | PAM–BN–PB |

|
H2O2 | Cancer, diabetes and neurodegeneration | 148 nM | 112 |
| 18 | HOTN |
|
HOCl | Inflammation, cancer | 0.108 μM | 113 |
| 19 | Pinkment probe |

|
ONOO− | Cancer | Not reported | 114 |
| 20 | MITO-CC |

|
ONOO− | Cancer and inflammation | 11.30 nM | 115 |
| 21 | A2 |

|
ONOO− | Diabetes | 2 nM | 116 |
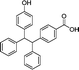
| 22 | LW-OTf |
|
O2˙−, ONOO− | Drug induced liver injury | 46.5 nM and 38.2 nM | 117 |
| 23 | DT-GAL |

|
H2S | Liver injury and cancer | 0.78 μM | 118 |
5. Conclusions and outlook
The father of medicine, Greek physician Hippocrates rightly said “Anything in excess is opposed to nature”. Since their discovery more than a century ago, RONSS has long been viewed with the glasses of a critique, their role in physiology mostly being associated with detrimental effects and the progression of numerous diseases. Generation of RONSS is an intrinsic evolutionary trait, in the complex metabolic pathway of aerobic respiration and they play vital roles in the maintenance of optimum health, especially in cell signalling, transduction and immune response pathways. Excessive ROS production can however damage cellular components, including DNA, proteins, and lipids, leading to various chronic diseases such as cancer, neurodegenerative disorders, and cardiovascular diseases. Similarly, dysregulated production of RNS, can lead to the formation of toxic molecules like ONOO−, contributing to tissue damage, mitochondrial dysfunction, and chronic inflammation, implicated in several pathological conditions, including atherosclerosis, sepsis, and autoimmune diseases. While RSS, especially H2S contributes to cardiovascular health, neuroprotection, and anti-inflammatory responses, their dysregulation or excessive production can lead to tissue damage, and inflammation, and exacerbate disease conditions ranging from cancer to neurodegenerative diseases.The precise detection of RONSS is critical for understanding their complex biological functions and contributions to disease progression. The development of improved probe sensitivity and specificity will enable real-time, in vivo monitoring of these species at the cellular and subcellular levels, offering insights into their roles in pathophysiology. Additionally, the integration of these probes with imaging technologies and nanotechnology could lead to more precise diagnostics and targeted therapeutic interventions, potentially revolutionizing the management of oxidative stress-related disorders. Future advancements in RONSS therapeutics will rely on designing precise and selective molecular tools tailored to specific disease conditions. Probes for detecting RONSS must address disease-specific contexts. For example, in cancer, oxidative and nitrosative stress fluctuates within a heterogeneous tumor microenvironment influenced by hypoxia and overexpressed enzymes like nitroreductases. In contrast, neurodegenerative disorders involve chronic oxidative stress in neurons, necessitating probes with higher selectivity and long-term stability. Moreover, BBB permeability is a critical factor for targeting neurodegeneration, which is not essential for all cancer types. RONSS-targeting theranostics for cancer often integrate detection with therapeutic functions, such as ROS-triggered drug release. In contrast, neurodegeneration probes prioritize neuroprotection, incorporating antioxidants to mitigate oxidative damage while monitoring RONSS. While both aim to detect oxidative stress, their designs differ in addressing BBB permeability, species dynamics, target localization, and functional demands.
The future of therapeutics targeting RONSS lies in the development of more precise and selective agents that can modulate these reactive species to treat various diseases. Advances in antioxidant therapies will focus on fine-tuning redox balance rather than simply quenching these species, preserving their signaling roles while mitigating their harmful effects. Novel small molecules, gene therapies, and nanomedicine approaches could offer targeted delivery of these therapeutics, enhancing efficacy and reducing side effects. Additionally, the use of RSS-based therapies, such as H2S donors, may emerge as promising treatments for conditions like neurodegenerative diseases, cardiovascular disorders, and cancer, where redox imbalance plays a critical role.
Despite massive research being directed towards the RONSS paradigm, their mechanism of action in many aspects of wellbeing and ailment remain poorly understood. Also, except for ROS and to an extent, RNS, the understanding of other species is still in infancy. Extensive research is required to lift the fog on how to arrive at the golden mean and deal best with this double-edged sword and tune the oxidant/antioxidant balance to maintain optimum health, longevity as well as fine-tune oxidative stress for therapeutic avenues.
Author contributions
TG conceptualized and supervised the manuscript, PB wrote the draft, DP wrote Section 3, HH wrote Section 4.1, MR wrote Section 4.4. Figures were made by PB with help from all other authors. All authors reviewed and edited the final draft of the review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank JNCASR, core grant (CRG/2020/004594), Science and Engineering Research Board (SERB), New Delhi, India, for the funding. DP, HH and MR thank DST India, CSIR and UGC for the student fellowships.References
- H. Sies, C. Berndt and D. P. Jones, Annu. Rev. Biochem., 2017, 86, 715–748 CrossRef CAS PubMed
.
-
H. Sies, in Introductory Remarks ed. H. B. T.-O. S. Sies, Academic Press, London, 1985, pp. 1–8 Search PubMed
.
-
S. Samanta, M. Ramesh and T. Govindaraju, Alzheimer's Disease: Recent Findings in Pathophysiology, Diagnostic and Therapeutic Modalities, The Royal Society of Chemistry, 2022, pp. 1–34 Search PubMed
.
- J.-M. Noël and F. Kanoufi, Curr. Opin. Electrochem., 2022, 35, 101071 CrossRef
.
- J. Medrano-Macías, A. C. Flores-Gallegos, E. Nava-Reyna, I. Morales, G. Tortella, S. Solís-Gaona and A. Benavides-Mendoza, Plants, 2022, 11 Search PubMed
.
- G. D. Puglia, Plant Growth Regul., 2024, 103, 9–32 CrossRef CAS
.
- I. Pérez-Torres, M. E. Soto, V. Castrejón-Tellez, M. E. Rubio-Ruiz, L. Manzano Pech and V. Guarner-Lans, Oxid. Med. Cell. Longevity, 2020, 2020, 8819719 Search PubMed
.
- S. Samanta and T. Govindaraju, ACS Chem. Neurosci., 2019, 10, 4847–4853 CrossRef CAS PubMed
.
- N. Narayanaswamy, S. Narra, R. R. Nair, D. K. Saini, P. Kondaiah and T. Govindaraju, Chem. Sci., 2016, 7, 2832–2841 RSC
.
- K. Rajasekhar, S. Samanta, V. Bagoband, N. A. Murugan and T. Govindaraju, iScience, 2020, 23, 101005 CrossRef CAS PubMed
.
- P. Baruah, H. Moorthy, M. Ramesh, D. Padhi and T. Govindaraju, Chem. Sci., 2013, 14, 9427–9438 RSC
.
- L. Wu, A. C. Sedgwick, X. Sun, S. D. Bull, X.-P. He and T. D. James, Acc. Chem. Res., 2019, 52, 2582–2597 CrossRef CAS PubMed
.
- P. Gao, W. Pan, N. Li and B. Tang, Chem. Sci., 2019, 10, 6035–6071 RSC
.
- R. B. Ferreira, L. Fu, Y. Jung, J. Yang and K. S. Carroll, Nat. Commun., 2022, 13, 5522 CrossRef CAS PubMed
.
- X. Peng and V. Gandhi, Ther. Delivery, 2012, 3, 823–833 CrossRef CAS PubMed
.
- X. Li, Y. Hu, X. Zhang, X. Shi, W. J. Parak and A. Pich, Nat. Commun., 2024, 15, 8172 CrossRef CAS PubMed
.
- R. Das, J. Hardie, B. P. Joshi, X. Zhang, A. Gupta, D. C. Luther, S. Fedeli, M. E. Farkas and V. M. Rotello, JACS Au, 2022, 2, 1679–1685 CrossRef CAS PubMed
.
- D. A. Butterfield and B. Halliwell, Nat. Rev. Neurosci., 2019, 20, 148–160 CrossRef CAS PubMed
.
- B. Commoner, J. Townsend and G. E. Pake, Nature, 1954, 174, 689–691 CrossRef CAS PubMed
.
- K. Chachlaki, J. Garthwaite and V. Prevot, Nat. Rev. Endocrinol., 2017, 13, 521–535 CrossRef CAS PubMed
.
- G. I. Giles, M. J. Nasim, W. Ali and C. Jacob, Antioxidants, 2017, 6, 38–42 CrossRef PubMed
.
- H. M. Smith and M. D. Pluth, JACS Au, 2023, 3, 2677–2691 CrossRef CAS PubMed
.
- O. M. Ighodaro and O. A. Akinloye, Alexandria J. Med., 2018, 54, 287–293 CrossRef
.
- P. R. Augusti, A. Quatrin, S. Somacal, G. M. Conterato, R. Sobieski, A. R. Ruviaro, L. H. Maurer, M. M. Duarte, M. Roehrs and T. Emanuelli, J. Clin. Biochem. Nutr., 2012, 51, 42–49 CrossRef CAS PubMed
.
- H. Sies, V. V. Belousov, N. S. Chandel, M. J. Davies, D. P. Jones, G. E. Mann, M. P. Murphy, M. Yamamoto and C. Winterbourn, Nat. Rev. Mol. Cell Biol., 2022, 23, 499–515 CrossRef CAS PubMed
.
- D. Garrido Ruiz, A. Sandoval-Perez, A. V. Rangarajan, E. L. Gunderson and M. P. Jacobson, Biochemistry, 2022, 61, 2165–2176 CrossRef CAS PubMed
.
- W. Zhang and H. T. Liu, Cell Res., 2002, 12, 9–18 CrossRef PubMed
.
- S. Alas, C. Ng and B. Bonavida, Clinm Cancer Res., 2002, 8, 836–845 CAS
.
- Z. Liu, M. Ma, D. Yu, J. Ren and X. Qu, Chem. Sci., 2020, 11, 11003–11008 RSC
.
- S. Li, Q. Zou, Y. Li, C. Yuan, R. Xing and X. Yan, J. Am. Chem. Soc., 2018, 140, 10794–10802 CrossRef CAS PubMed
.
- A. L. D. Wallabregue, H. Bolland, S. Faulkner, E. M. Hammond and S. J. Conway, J. Am. Chem. Soc., 2023, 145, 2572–2583 CrossRef CAS PubMed
.
- C. Yang, Q. Yang, Y. Xiang, X.-R. Zeng, J. Xiao and W.-D. Le, Neural Regener. Res., 2023, 18, 57–63 CrossRef CAS PubMed
.
- H.-L. Zhou, R. Zhang, P. Anand, C. T. Stomberski, Z. Qian, A. Hausladen, L. Wang, E. P. Rhee, S. M. Parikh, S. A. Karumanchi and J. S. Stamler, Nature, 2019, 565, 96–100 CrossRef CAS PubMed
.
- G. Ferrer-Sueta and R. Radi, ACS Chem. Biol., 2009, 4, 161–177 CrossRef CAS PubMed
.
- D. L. Miller and M. B. Roth, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 20618–20622 CrossRef CAS PubMed
.
- B. D. Paul and S. H. Snyder, Nat. Rev. Mol. Cell Biol., 2012, 13, 499–507 CrossRef CAS PubMed
.
- X. Ni, S. S. Kelly, S. Xu and M. Xian, Acc. Chem. Res., 2021, 54, 3968–3978 CrossRef CAS PubMed
.
- J. Prousek, Pure Appl. Chem., 2007, 79, 2325–2338 CrossRef CAS
.
- K. Rajasekhar and T. Govindaraju, RSC Adv., 2018, 8, 23780–23804 RSC
.
- S. J. Dixon, K. M. Lemberg, M. R. Lamprecht, R. Skouta, E. M. Zaitsev, C. E. Gleason, D. N. Patel, A. J. Bauer, A. M. Cantley, W. S. Yang, B. Morrison 3rd and B. R. Stockwell, Cell, 2012, 149, 1060–1072 CrossRef CAS PubMed
.
- P. Maher, A. Currais and D. Schubert, Cell Chem. Biol., 2020, 27, 1456–1471 CrossRef CAS PubMed
.
- P. Tsvetkov, S. Coy, B. Petrova, M. Dreishpoon, A. Verma, M. Abdusamad, J. Rossen, L. Joesch-Cohen, R. Humeidi, R. D. Spangler, J. K. Eaton, E. Frenkel, M. Kocak, S. M. Corsello, S. Lutsenko, N. Kanarek, S. Santagata and T. R. Golub, Science, 2022, 375, 1254–1261 CrossRef CAS PubMed
.
- S. Samanta, K. Rajasekhar, M. Ramesh, N. A. Murugan, S. Alam, D. Shah, J. P. Clement and T. Govindaraju, Adv. Ther., 2021, 4, 2000225 CrossRef CAS
.
- M. Ramesh and T. Govindaraju, Chem. Sci., 2022, 13, 13657–13689 RSC
.
- H. Arora, M. Ramesh, K. Rajasekhar and T. Govindaraju, Bull. Chem. Soc. Jpn., 2020, 93, 507–546 CrossRef CAS
.
- K. Rajasekhar, M. Chakrabarti and T. Govindaraju, Chem. Commun., 2015, 51, 13434–13450 RSC
.
- K. Rajasekhar, K. Mehta and T. Govindaraju, ACS Chem. Neurosci., 2018, 9, 1432–1440 CrossRef CAS PubMed
.
- T. Ueno and T. Nagano, Nat. Methods, 2011, 8, 642–645 CrossRef CAS PubMed
.
- Y. Geng, Z. Wang, J. Zhou, M. Zhu, J. Liu and T. D. James, Chem. Soc. Rev., 2023, 52, 3873–3926 RSC
.
- J. Ke, P. Zhao, J. Li and Q. Fu, J. Mater. Chem. B, 2022, 10, 8744–8749 RSC
.
- A. C. Sedgwick, W.-T. Dou, J.-B. Jiao, L. Wu, G. T. Williams, A. T. A. Jenkins, S. D. Bull, J. L. Sessler, X.-P. He and T. D. James, J. Am. Chem. Soc., 2018, 140, 14267–14271 CrossRef CAS PubMed
.
- J. Yang, J. Yang, S. H. Liang, Y. Xu, A. Moore and C. Ran, Sci. Rep., 2016, 6, 35613 CrossRef CAS PubMed
.
- D. Maity, A. Raj, P. K. Samanta, D. Karthigeyan, T. K. Kundu, S. K. Pati and T. Govindaraju, RSC Adv., 2014, 4, 11147–11151 RSC
.
- S. Ghatak, T. Nakamura and S. A. Lipton, Front. Neural Circuits, 2023, 17, 1099467 CrossRef CAS PubMed
.
- P. Wang, L. Yu, J. Gong, J. Xiong, S. Zi, H. Xie, F. Zhang, Z. Mao, Z. Liu and J. S. Kim, Angew. Chem., Int. Ed., 2022, 61, e202206894 CrossRef CAS PubMed
.
- E. Disbrow, K. Y. Stokes, C. Ledbetter, J. Patterson, R. Kelley, S. Pardue, T. Reekes, L. Larmeu, V. Batra, S. Yuan, U. Cvek, M. Trutschl, P. Kilgore, J. S. Alexander and C. G. Kevil, Alzheimer's Dementia, 2021, 17, 1391–1402 CrossRef CAS PubMed
.
- K. Rajasekhar, C. Madhu and T. Govindaraju, ACS Chem. Neurosci., 2016, 7, 1300–1310 CrossRef CAS PubMed
.
- K. Rajasekhar, N. Narayanaswamy, N. A. Murugan, G. Kuang, H. Ågren and T. Govindaraju, Sci. Rep., 2016, 6, 23668 CrossRef CAS PubMed
.
- S. Mandal, Y. V. Suseela, S. Samanta, B. Vileno, P. Faller and T. Govindaraju, ACS Med. Chem. Lett., 2024, 15, 1376–1385 CrossRef CAS PubMed
.
- S. Samanta, K. Rajasekhar, V. Babagond and T. Govindaraju, ACS Chem. Neurosci., 2019, 10, 3611–3621 CrossRef CAS PubMed
.
- S. Park, M. Kim, Y. Lin, M. Hong, G. Nam, A. Mieczkowski, J. Kardos, Y.-H. Lee and M. H. Lim, Chem. Sci., 2023, 14, 9293–9305 RSC
.
- M. Ramesh, C. Balachandra, P. Andhare and T. Govindaraju, ACS Chem. Neurosci., 2022, 13, 2209–2221 CrossRef CAS PubMed
.
- D. Padhi, C. Balachandra, M. Ramesh and T. Govindaraju, Chem. Commun., 2022, 58, 6288–6291 RSC
.
- D. Padhi, P. Baruah, M. Ramesh, H. Moorthy and T. Govindaraju, Redox Biol., 2024, 71, 103119 CrossRef CAS PubMed
.
- J. Wang, P. Shangguan, X. Chen, Y. Zhong, M. Lin, M. He, Y. Liu, Y. Zhou, X. Pang, L. Han, M. Lu, X. Wang, Y. Liu, H. Yang, J. Chen, C. Song, J. Zhang, X. Wang, B. Shi and B. Z. Tang, Nat. Commun., 2024, 15, 705 CrossRef CAS PubMed
.
- R. Alvariño, E. Alonso, M. E. Abbasov, C. M. Chaheine, M. L. Conner, D. Romo, A. Alfonso and L. M. Botana, ACS Chem. Neurosci., 2019, 10, 4102–4111 CrossRef PubMed
.
- M. Dodson, R. Castro-Portuguez and D. D. Zhang, Redox Biol., 2019, 23, 101107 CrossRef CAS PubMed
.
- M.-C. Lu, J. Zhao, Y.-T. Liu, T. Liu, M.-M. Tao, Q.-D. You and Z.-Y. Jiang, Redox Biol., 2019, 26, 101266 CrossRef CAS PubMed
.
- H. Moorthy, L. P. Datta, S. Samanta and T. Govindaraju, ACS Appl. Mater. Interfaces, 2022, 14, 56535–56547 CrossRef CAS PubMed
.
- H. Moorthy, M. Ramesh, D. Padhi, P. Baruah and T. Govindaraju, Mater. Horiz., 2024, 11, 3082–3089 RSC
.
- Y. Liu, Y. Chen, Y. Gong, H. Yang and J. Liu, ACS Appl. Nano Mater., 2023, 6, 5384–5393 CrossRef CAS
.
- Y. Wang, Y. Huang, A. Ma, J. You, J. Miao and J. Li, J. Agric. Food Chem., 2024, 72, 11854–11870 CrossRef CAS PubMed
.
- X. Wu, X. Sun, Z. Guo, J. Tang, Y. Shen, T. D. James, H. Tian and W. Zhu, J. Am. Chem. Soc., 2014, 136, 3579–3588 CrossRef CAS PubMed
.
- M. Iciek, A. Bilska-Wilkosz, M. Kozdrowicki and M. Górny, Biosci. Rep., 2022, 42, BSR20221006 CrossRef CAS PubMed
.
- Y. V. Suseela, P. Satha, N. A. Murugan and T. Govindaraju, Theranostics, 2020, 10, 10394–10414 CrossRef CAS PubMed
.
- C. Hao, A. Qu, L. Xu, M. Sun, H. Zhang, C. Xu and H. Kuang, J. Am. Chem. Soc., 2019, 141, 1091–1099 CrossRef CAS PubMed
.
- R. N. Kalaria, G. E. Maestre, R. Arizaga, R. P. Friedland, D. Galasko, K. Hall, J. A. Luchsinger, A. Ogunniyi, E. K. Perry, F. Potocnik, M. Prince, R. Stewart, A. Wimo, Z. X. Zhang and P. Antuono, Lancet Neurol., 2008, 7, 812–826 CrossRef PubMed
.
- G. Cenini, A. Lloret and R. Cascella, Oxid. Med. Cell. Longevity, 2019, 2019, 2105607 Search PubMed
.
- E. O. Olufunmilayo, M. B. Gerke-Duncan and R. M. D. Holsinger, Antioxidants, 2023, 12, 517 CrossRef CAS PubMed
.
- S.-J. Li, Y.-F. Li, H.-W. Liu, D.-Y. Zhou, W.-L. Jiang, J. Ou-Yang and C.-Y. Li, Anal. Chem., 2018, 90, 9418–9425 CrossRef CAS PubMed
.
- S. Li, P. Wang, W. Feng, Y. Xiang, K. Dou and Z. Liu, Chem. Commun., 2020, 56, 1050–1053 RSC
.
- M. Weng, X. Yang, Y. Ni, C. Xu, H. Zhang, J. Shao, N. Shi, C. Zhang, Q. Wu, L. Li and W. Huang, Sens. Actuators, B, 2019, 283, 769–775 CrossRef CAS
.
- Q. Sun, J. Xu, C. Ji, M. S. S. Shaibani, Z. Li, K. Lim, C. Zhang, L. Li and Z. Liu, Anal. Chem., 2020, 92, 4038–4045 CrossRef CAS PubMed
.
- Y.-Q. Yuan, Y.-L. Wang, B.-S. Yuan, X. Yuan, X.-O. Hou, J.-S. Bian, C.-F. Liu and L.-F. Hu, Brain, Behav., Immun., 2018, 67, 77–90 CrossRef CAS PubMed
.
- A. Gaspar, M. J. Matos, J. Garrido, E. Uriarte and F. Borges, Chem. Rev., 2014, 114, 4960–4992 CrossRef CAS PubMed
.
- L. Yang, Y. Zhang, X. Ren, B. Wang, Z. Yang, X. Song and W. Wang, Anal. Chem., 2020, 92, 4387–4394 CrossRef CAS PubMed
.
- M. Gao, X. Zhang, Y. Wang, Q. Liu, F. Yu, Y. Huang, C. Ding and L. Chen, Anal. Chem., 2019, 91, 7774–7781 CrossRef CAS PubMed
.
- T. Zhu, N. Ren, X. Liu, Y. Dong, R. Wang, J. Gao, J. Sun, Y. Zhu, L. Wang, C. Fan, H. Tian, J. Li and C. Zhao, Angew. Chem., Int. Ed., 2021, 60, 8450–8454 CrossRef CAS PubMed
.
- Z. Wu, M. Liu, Z. Liu and Y. Tian, J. Am. Chem. Soc., 2020, 142, 7532–7541 CrossRef CAS PubMed
.
- S. Fahn, Ann. Neurol., 1992, 32, S128–S132 CrossRef PubMed
.
- C. W. Shults, D. Oakes, K. Kieburtz, M. F. Beal, R. Haas, S. Plumb, J. L. Juncos, J. Nutt, I. Shoulson, J. Carter, K. Kompoliti, J. S. Perlmutter, S. Reich, M. Stern, R. L. Watts, R. Kurlan, E. Molho, M. Harrison, M. Lew and Parkinson Study Group, Arch. Neurol., 2002, 59, 1541–1550 CrossRef PubMed
.
- D. T. Dexter, S. A. Statton, C. Whitmore, W. Freinbichler, P. Weinberger, K. F. Tipton, L. Della Corte, R. J. Ward and R. R. Crichton, J. Neural Transm., 2011, 118, 223–231 CrossRef CAS PubMed
.
- L.-Y. Su, H. Li, L. Lv, Y.-M. Feng, G.-D. Li, R. Luo, H.-J. Zhou, X.-G. Lei, L. Ma, J.-L. Li, L. Xu, X.-T. Hu and Y.-G. Yao, Autophagy, 2015, 11, 1745–1759 CrossRef CAS PubMed
.
- P. E. Chabrier and M. Auguet, CNS Drug Rev., 2007, 13, 317–332 CrossRef CAS PubMed
.
- Z. Xun, S. Rivera-Sánchez, S. Ayala-Peña, J. Lim, H. Budworth, E. M. Skoda, P. D. Robbins, L. J. Niedernhofer, P. Wipf and C. T. McMurray, Cell Rep., 2012, 2, 1137–1142 CrossRef CAS PubMed
.
- S. Han, J.-R. Choi, K. Soon Shin and S. J. Kang, Brain Res., 2012, 1483, 112–117 CrossRef CAS PubMed
.
- K. Tanaka, T. Kanno, Y. Yanagisawa, K. Yasutake, S. Inoue, N. Hirayama and J.-E. Ikeda, PLoS One, 2014, 9, e87728 CrossRef PubMed
.
- I. I. C. Chio and D. A. Tuveson, Trends Mol. Med., 2017, 23, 411–429 CrossRef CAS PubMed
.
- Y. Huo, P. Selenica, A. H. Mahdi, F. Pareja, K. Kyker-Snowman, Y. Chen, R. Kumar, A. Da Cruz Paula, T. Basili, D. N. Brown, X. Pei, N. Riaz, Y. Tan, Y.-X. Huang, T. Li, N. J. Barnard, J. S. Reis-Filho, B. Weigelt and B. Xia, npj Breast Cancer, 2021, 7, 45 CrossRef CAS PubMed
.
- S. S. Sabharwal and P. T. Schumacker, Nat. Rev. Cancer, 2014, 14, 709–721 CrossRef CAS PubMed
.
- E. L. Mills, D. G. Ryan, H. A. Prag, D. Dikovskaya, D. Menon, Z. Zaslona, M. P. Jedrychowski, A. S. H. Costa, M. Higgins, E. Hams, J. Szpyt, M. C. Runtsch, M. S. King, J. F. McGouran, R. Fischer, B. M. Kessler, A. F. McGettrick, M. M. Hughes, R. G. Carroll, L. M. Booty, E. V. Knatko, P. J. Meakin, M. L. J. Ashford, L. K. Modis, G. Brunori, D. C. Sévin, P. G. Fallon, S. T. Caldwell, E. R. S. Kunji, E. T. Chouchani, C. Frezza, A. T. Dinkova-Kostova, R. C. Hartley, M. P. Murphy and L. A. O’Neill, Nature, 2018, 556, 113–117 CrossRef CAS PubMed
.
- K. Dou, Q. Fu, G. Chen, F. Yu, Y. Liu, Z. Cao, G. Li, X. Zhao, L. Xia, L. Chen, H. Wang and J. You, Biomaterials, 2017, 133, 82–93 CrossRef CAS PubMed
.
- M. Wang, M. Chang, C. Li, Q. Chen, Z. Hou, B. Xing and J. Lin, Adv. Mater., 2022, 34, 2106010 CrossRef CAS PubMed
.
- W. Zhang, X. Hu, Q. Shen and D. Xing, Nat. Commun., 2019, 10, 1704 CrossRef PubMed
.
- I. Okamoto, H. Miyaji, S. Miyata, K. Shitomi, T. Sugaya, N. Ushijima, T. Akasaka, S. Enya, S. Saita and H. Kawasaki, ACS Omega, 2021, 6, 9279–9290 CrossRef CAS PubMed
.
- A. M. Fleming, Y. Ding and C. J. Burrows, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 2604–2609 CrossRef CAS PubMed
.
- S. Cogoi, A. Ferino, G. Miglietta, E. B. Pedersen and L. E. Xodo, Nucleic Acids Res., 2018, 46, 661–676 CrossRef CAS PubMed
.
- Y. Venkata Suseela, P. Sengupta, T. Roychowdhury, S. Panda, S. Talukdar, S. Chattopadhyay, S. Chatterjee and T. Govindaraju, ACS Bio Med Chem Au, 2022, 2, 125–139 CrossRef CAS PubMed
.
- K. Lingappan, Curr. Opin. Toxicol., 2018, 7, 81–86 CrossRef PubMed
.
- A. Mongirdienė, L. Skrodenis, L. Varoneckaitė, G. Mierkytė and J. Gerulis, Biomedicines, 2022, 10, 602–607 CrossRef PubMed
.
- Z. Song, D. Mao, S. H. P. Sung, R. T. K. Kwok, J. W. Y. Lam, D. Kong, D. Ding and B. Z. Tang, Adv. Mater., 2016, 28, 7249–7256 CrossRef CAS PubMed
.
- Y. Chen, X. Shi, Z. Lu, X. Wang and Z. Wang, Anal. Chem., 2017, 89, 5278–5284 CrossRef CAS PubMed
.
- X. Han, Y. Ma, Y. Chen, X. Wang and Z. Wang, Anal. Chem., 2020, 92, 2830–2838 CrossRef CAS PubMed
.
- M. Weber, H.-H. Han, B.-H. Li, M. L. Odyniec, C. E. F. Jarman, Y. Zang, S. D. Bull, A. B. Mackenzie, A. C. Sedgwick, J. Li, X.-P. He and T. D. James, Chem. Sci., 2020, 11, 8567–8571 RSC
.
- D. Cheng, Y. Pan, L. Wang, Z. Zeng, L. Yuan, X. Zhang and Y.-T. Chang, J. Am. Chem. Soc., 2017, 139, 285–292 CrossRef CAS PubMed
.
- J. Miao, Y. Huo, Q. Liu, Z. Li, H. Shi, Y. Shi and W. Guo, Biomaterials, 2016, 107, 33–43 CrossRef CAS PubMed
.
- L. Wu, J. Liu, X. Tian, R. R. Groleau, S. D. Bull, P. Li, B. Tang and T. D. James, Chem. Sci., 2021, 12, 3921–3928 RSC
.
- D.-T. Shi, D. Zhou, Y. Zang, J. Li, G.-R. Chen, T. D. James, X.-P. He and H. Tian, Chem. Commun., 2015, 51, 3653–3655 RSC
.
- P. Pacher, J. S. Beckman and L. Liaudet, Physiol. Rev., 2007, 87, 315–424 CrossRef CAS PubMed
.
- M. V. Irazabal and V. E. Torres, Cells, 2020, 9, 1342 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2025 |