Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Detection of β-D-glucuronidase activity in environmental samples using 4-fluorophenyl β-D-glucuronide and 19F NMR†
Aleksandra
Teriosina
a,
Igor L.
Barsukov
b,
Alan
Cartmell
c,
Andrew K.
Powell
d,
Andrew V.
Stachulski
 *e and
Edwin A.
Yates
*e and
Edwin A.
Yates
 *b
*b
aSchool of Biological Sciences, University of Liverpool, Crown Street, Liverpool, L69 7ZB, UK
bDepartment of Biochemistry, Cell and Systems Biology, ISMIB, University of Liverpool, Crown St., Liverpool L69 7ZB, UK. E-mail: eayates@liverpool.ac.uk
cDepartment of Biology, University of York, Heslington, York, YO10 5DD, UK
dSchool of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, Byrom Street, Liverpool, L3 3AF, UK
eDepartment of Chemistry, University of Liverpool, Liverpool L69 7ZD, UK
First published on 10th February 2025
Abstract
Common methods for establishing the presence of enteric bacteria polluting water supplies, or in other samples, rely on detecting the hydrolysis of model glucuronide substrates by glucuronidases to release a phenolic product quantifiable by absorbance or fluorescence. Substrates include the β-D-glucuronides of p-nitrophenol, and umbelliferyl or quercetin derivatives. One limitation is that it may be difficult or impossible to quantify the released phenolic moiety in samples that are strongly coloured or, that contain fluorescent compounds. Exploiting the sensitivity available from the 19F nucleus to changes in chemical environment which can be detected by 19F NMR spectroscopy, and the almost complete absence of 19F from naturally-occurring samples containing organic matter, which provides background-free signals, we propose a model substrate; 4-fluorophenyl β-D-glucuronide (4FP-glucuronide). The 19F NMR chemical shift position of 4FP-glucuronide changes from −121.0 ppm upon hydrolysis to release 4-fluorophenol, at −124.9 ppm (at pH 6.8), enabling detection of β-glucuronidase activity. We illustrate the use of this substrate with environmental samples from forest soil, standing water, and mud from cattle pasture. Each of these would challenge conventional methods, owing to their opacity or the presence of coloured organic material. The technique enables detection of glucuronidases, a widely-used proxy for enteric bacteria, extending the scope of testing beyond water to include environmental and other challenging samples.
1. Introduction
The provision of water free of significant levels of potentially pathogenic microorganisms, especially enteric bacteria, is a milestone in the history of Public Health that stands alongside vaccination in significance. Despite this apparent success, pressures on land use for agriculture combined with the challenges of sewage and water management, result in the pollution of rivers and water courses worldwide; a situation that also threatens the ecological balance.1 Faecal coliform contamination of water courses can arise from a variety of sources which include sewage spills, agricultural or urban run-off, wild animals and storm over-flows. The bacteria that are most commonly cited include Escherichia coli, as well as Streptococci, Clostridium and Klebsiella species2 although many others have been detected (see, for example, Table 2 in ref. 2). The bacterial load of water courses can vary widely; for example, the counts (quoted as most probable number: MPN) of E. coli in a variety of water sources in the Dangme West District of Ghana ranged from 0–50/100 mL MPN,3 that of drinking water in Hanoi, Vietnam, has been recorded as 2.4 × 103/100 mL MPN,4 while that from the river Beiyun, Beijing, China, varied between 103 and 105/L MPN (102–104/100 mL MPN).5Clearly, the ability to test the safety of water ideally includes an assessment of the level of any contamination. Also crucial is the capacity to assess the potential sources of that contamination, including detecting the presence of bacteria in environmental samples that may not necessarily be taken directly from water supplies, but which could originate from the surrounding catchment area or wider environment. The presence of enteric bacteria may be established by a number of means, requiring a variety of equipment and resources that include direct bacterial culture, MALDI-TOF analysis, a raft of immunological techniques and PCR-based approaches.6–9 One widely used proxy for detecting contamination with enteric gut bacteria is the presence of β-glucuronidase enzymes (GUS) capable of hydrolysing model β-D-glucuronides to their constituent D-glucuronate and phenolic moieties. Beta-D-glucuronides are D-glucuronic acid derivatives in which the β-linked aglycone moiety is typically a phenolic compound. Beta-D-glucuronides are formed in mammalian organs, particularly the liver, kidney and large intestine, to increase the solubility, and hence the rate of clearance of phenolic compounds, especially those of a dietary origin but, they serve a similar role with xenobiotics. Beta-glucuronidase enzymes (GUS) are produced by a wide range of gut bacterial classes; Firmicutes, Bacteroidetes, Verrucomicrobiota and Probacteria, and 279 GUS isoforms have been identified and grouped into six structural categories.10 These can include those capable of hydrolysing the glucuronides of naturally-occurring phenolic compounds, such as bilibrubin, steroid hormones (estrone, estradiol, testosterone and androsterone diol) and neurostransmitters (dopamine and serotonin), as well as around one hundred xenobiotics11 that include anti-cancer drugs and opioids, as well as dietary substances (reviewed10). It has also been established that there is considerable variation between individuals regarding GUS enzymes in animals and humans.12–14 Given these observations, it is unsurprising that there is little evidence of a direct correlation between simple bacterial numbers and disease type or severity and, indeed, while numerous enzymes capable of hydrolysing the model substrate, 4-methylumbelliferyl β-D-glucuronide (4MU) for example, have been identified,15 extensive diversity in the structure, function and cellular localization of GUS enzymes has also been noted.16
Despite these caveats, a method of screening that can identify the presence per se of such bacteria and thereby serve as an indicator of potential contamination, and that could complement the existing 4MU-based assay17 would have practical value. In particular, samples whose physical state, such as opacity resulting from extensive colouration arising from the presence of organic matter, that effectively precludes standard procedures could be more easily amenable to analysis.
Several tests have been devised for the detection of GUS enzymes. Often, these are based on the release of the phenolic product from a glucuronide resulting in a change of retention by HPLC.18 Common forms of such glucuronides are p-nitrophenyl β-D-glucuronide,19 4-methylumbelliferyl β-D-glucuronide20 and quercetin derivatives.21 For some of these, the excitation and emission maxima (e.g. 360 and 450 nm at pH 7 for 4-MU) change as a function of pH.22 Here, we address the challenge of assessing samples that are characterized by high optical opacity, hence display significant background absorbance, which limits the feasibility and sensitivity of conventional testing. One route by which background signals can be eliminated is to exploit the properties of the 19F nucleus in NMR spectroscopy in a suitable 19F-containing glucuronide substrate. In addition to the simple spectra that it provides, the 19F nucleus (spin 1/2 nucleus) is very sensitive to changes in chemical environment and, since there is almost no naturally-occurring fluorine in biological systems, it also provides background-free signals unaffected by whatever complex organic milieu is under observation.23
2. Results and discussion
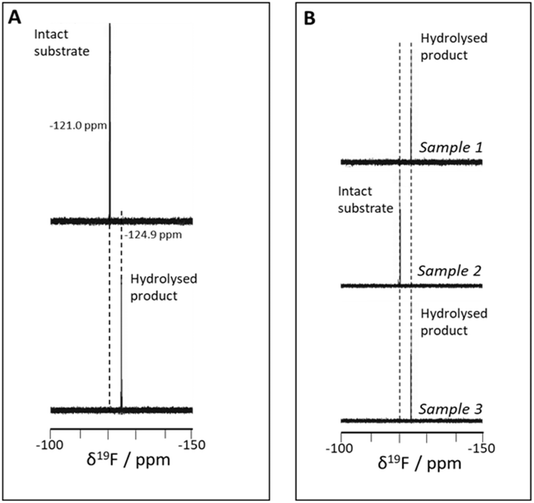
During our recent programme focusing on the synthesis, properties and degradation of model glucuronides,24–26 we prepared the β-D-glucuronide of 4-fluorophenol via the β-anomer of tetraacetylated methyl ester of D-glucuronic acid as the glycosyl donor, based on a published method24 (see ESI†). We reasoned that, owing to the sensitivity of the 19F nucleus to its chemical environment, it may be possible to distinguish between the intact glucuronide and the hydrolysed form, in which free 4-fluorophenol would be the species detected (Scheme 1). We observed that, indeed, the 19F NMR chemical shift of the intact glucuronide is distinct from that of the free phenol (−121.5 vs. −124.9 ppm under buffered (pH 6.8) conditions, Fig. 1A) released by hydrolysis. This provides a potential approach by which enzymatic hydrolysis by β-glucuronidases could be monitored using 19F NMR in environmental samples, free of complicating background signals and irrespective of the optical opacity of the sample. This latter property precludes accurate conventional detection which uses, for example, the absorbance at 401 nm of the enolate (at pH 11.3) form of the released product when employing p-nitrophenyl β-D-glucuronide27 or absorbance/emission at 350/450 nm of 4-methylumbelliferyl β-D-glucuronide.20The potential of the method to provide a means of detecting hydrolysis of the glucuronide in environmentally-derived samples was then explored using three samples that would, owing to their optical opacity, be intractable with standard glucuronide enzyme assays.
2.1 4-fluorophenyl β-D-glucuronide is a suitable substrate for the detection of β-glucuronidase activity in environmentally derived samples
The 4-fluorophenyl glucuronide is stable in aqueous buffered (1/4 strength phosphate buffered saline; NaCl 35 mM, pH 6.8) solution (Fig. 1A, upper), while the hydrolysed product in the same buffer (Fig. 1A, lower) exhibits a signal with clearly distinct 19F NMR chemical shift value, shifted upfield by 3.9 ppm from −121.0 to −124.9 ppm.The method was explored using three environmentally-derived samples: one of damp, deciduous forest soil (Sample 1), standing water from a forest pond (Sample 2), and water from a puddle on land in use as cattle pasture (Sample 3). The 19F NMR spectra of these, following incubation with the substrate, are shown in Fig. 1B, in which samples 1 and 3 (Fig. 1B, upper and lower) clearly show complete conversion to product (−124.9 ppm) while the standing water (Sample 2) (Fig. 1B, middle) shows no product (intact glucuronide remains, at −121.0 ppm).
In principle, any 19F-containing glucuronide could have been employed for this purpose but, the glucuronide of the simple phenol, 4-fluorophenol, the para-substituted analogue of phenol, is straightforward to prepare (see ESI, 1–8†). The reaction provides β-D-selectivity in good yield (69% for the coupling step) and, since the pKa of 4-fluorophenol is 9.95,28 avoids the risk of low yield that can be associated with low pKa phenols. This route also avoids heavy metal salts employed in the traditional Koenigs–Knorr synthesis to achieve (1,2-trans configuration) β-D-glycosidic bond formation, although the 4-fluorophenyl β-D-glucuronide should also be accessible via several alternatives, including Schmidt imidate donors.
The 19F nucleus, with 100% abundance and 87% the sensitivity of the 1H nucleus, is extremely rare in biological systems, but highly sensitive to changes in chemical environment on account of its electronic configuration, 1s22s22p5. In the present system, this sensitivity provides an upfield shift of 3.9 ppm following hydrolysis, clearly separating the signals from glucuronide and released phenol (Fig. 1A). There have been attempts to form a comprehensive theoretical basis for predicting the chemical shifts in this class of molecules29 which derive from earlier work in substituted aromatic systems,28 and postulate the donation of p-electrons from fluorine into the π electrons of the aromatic system (p–π donation).
Practical subtleties regarding the method developed here are the need to add buffer to eliminate any pH dependent chemical shift changes, and to centrifuge the sample prior to addition of the glucuronide. Centrifugation precipitates solid material but, this does not necessarily render the sample transparent and amenable to conventional spectroscopic assays, owing to residual colouration from dissolved organic matter. Indeed, after centrifugation, the environmental samples retained significant absorbance values (>0.25, 1 cm pathlength) in the range 350–450 nm, in which conventional spectroscopic measurements are made. The purpose of centrifuging the sample is two-fold; first, it was found that, if added without prior centrifugation, the glucuronide may bind any solid matter, thereby causing the 19F NMR signals to broaden or disappear (data not shown) and, second, removal of solid material renders the sample more physically homogeneous, making shimming the sample in the NMR magnet more straightforward. The samples were buffered to preclude chemical shift changes caused by pH differences, with phosphate buffered saline at 1/4 strength rather than full strength (137 mM NaCl), also to facilitate shimming. To confirm reproducibility, four independent measurements on one separate environmental sample provided an average value of 96.6% hydrolysis (3.4% intact glucuronide) with a standard deviation of 0.6% (see ESI, 9–18†).
2.2 Detection limit for the hydrolysed product under standard recording conditions (64 scans)
As with most direct spectroscopic methods, there is a trade-off between signal to noise ratio, the number of scans (effectively the time taken to record the spectrum) and the quantity (concentration) of sample. In the case of NMR, there are several other potential factors, including the magnetic field strength and the precise operating parameters such as the relaxation delay. Under the conditions employed here (a standard 5 mm NMR tube, 600 MHz cryoprobe, 0.70 mL volume and 0.1 mg (∼30 mmoles) of sample, 64 scans) the signal of the product in cases where low conversion of substrate to product had occurred were investigated. To provide samples with appropriately small signals, we utilised the bacterial cell lysates of two Bacteroides species, B. intestinalis and B. thetaiotaomicron which generated only low levels of products (∼2%) with which to explore the effective limit of detection under the standardized recording conditions employed here. The spectra of these samples provided clear signals of product, consistently at −124.9 ppm with signal to noise ratios (Fig. 2A and B, insets) of ∼3 to 1 and correspond to detection of a 10's of μM solutions (10 s of nmoles).3. Conclusions
The samples, taken on the same day within a distance of 200 m of each other from temperate rain forest (British National Vegetation Classification W11) and neighbouring farmland in North Wales, UK, provided both β-D-glucuronidase positive and negative results. Unsurprisingly, the sample from cattle pasture (Sample 3), exposed to regular contact with bovine faecal matter, was positive. Perhaps more surprisingly, given that GUS activity is often taken as a proxy for the presence of enteric bacteria, the forest soil sample (Sample 1) was also positive. The presence of bacterially-derived GUS cannot be excluded but, plants, fungi and bacteria that employ plant-derived materials as carbon sources could provide an alternative source of suitable enzymes, highlighting that caution needs to be exercised when employing simple proxy measurements to infer the presence of particular bacterial classes. Other enzymes that may be present include those from plants with specificities against, for example, xylans containing β-linked D-glucuronic acid30 or, from fungi such as Aspergillus niger, that contain β-glucuronidases.31 It is important to note therefore that, although hydrolysis of the substrate constitutes β-glucuronidase activity by definition, it cannot be assumed that such activity arises from bacterial GUS enzymes alone. Hydrolysis of this substrate could also result from other carbohydrate hydrolysing enzymes in addition to those of faecal origin.14The present paper provides an additional method for the detection of glucuronidase activity which is suitable in cases where conventional techniques would be hampered by opacity or the presence of coloured organic matter. This extends the scope of testing beyond water to include environmental and other challenging samples. It also provides a complementary approach to a range of other techniques by which bacteria can be detected which employ, for example, biosensors,32 optical chips33 and chemiluminescence.34 The method described here is suitable for use in an assay format, in which the ultimate signal to noise ratio will depend on many factors including the particular state of the samples under test but, since it this an NMR-based method, it is possible to optimise detection though increased scans and the use of higher magnetic field strength.
4 Materials and methods
4.1 Preparation of the substrate, 4-fluorophenyl β-D-glucuronide
The substrate, 4-fluorophenyl β-D-glucuronide, was prepared as part of our on-going programme in which a series of dietary glucuronides have been synthesized.24–26 Briefly, the synthesis of 4-fluorophenyl β-D-glucuronide, an unnatural analogue of the glucuronides of the simple phenols 4-methylphenol (p-cresol) and 4-methoxyphenol reported in ref. 24 and ref. 26 respectively, employed the β-anomeric tetraacetate intermediate of the methyl ester of D-glucuronic acid as donor. It utilized trimethylsilyl trifluoromethanesulfonate catalysis, with 4-fluorophenol as acceptor in dichloromethane, based on, ref. 24 to form the protected glucuronide intermediate (compound (1) in ESI†) which, following de-protection, provided the product (compound (2) in ESI†) in 69% yield in the coupling step (for full details and characterization, see ESI, 1–8†).4.2 Hydrolysis of the substrate by environmental samples
Test samples (final PBS; NaCl 37 mM, pH 6.8) were prepared by mixing 0.5 mL of the test material with 0.5 mL 75 mM PBS, pH 6.8 containing 10% (v/v) D2O, and spinning in a benchtop centrifuge for 2 min, at 13.5 × 103 rpm, in a 5 cm rotor. (Note that to employ the method as a detection system, as is the case in his instance, samples should be incubated overnight (18 h, as here) but, if it were to be used in an assay format for comparison of activities or rates, then incubation times appropriate for the enzymes level in the system under study would need to be employed, and an excess of substrate would need to be provided; all of which would have to be optimized for the particular system under investigation.) A calibration curve (ESI 9†, inset) shows that the peak area of the 19F NMR signal of 4-fluorphenol is linear in the range 0.01 to 0.1 mg. Following incubation, 0.7 mL of the supernatant was then taken for NMR analysis. Controls consisted of untreated 4-fluorophenyl β-D-glucuronide (0.3 μmoles, in 0.70 mL of 1/4 strength phosphate buffered saline (35 mM NaCl PBS, pH 6.8) containing 10% D2O) – the no treatment control, and 0.3 μmoles 4-fluorophenol in the same buffer – the hydrolysed control.4.3 Bacterial culture and lysate preparation
As a source available from our recent study with known lysate activity,26 bacterial samples (Bacteroides intestinalis; DSM17393 and Bacteroides thetaiotaomicron; ATCC 29148) were grown anaerobically in BHI media supplemented with haematin (120 μg ml−1) to mid-late log phase (OD ca. 0.8). Five mL of culture medium were removed, spun and washed (2×) with PBS (5 ml) before resuspension in 1 ml of PBS. Cells were sonicated (Sonics Vibracell, with ultrasound probe for 2 × 15 s at 25% power). Twenty μL of lysed bacterial supernatant was added to the glucuronide (5 μL, 1 mg ml−1) in PBS and incubated overnight (37 °C).4.4 19F NMR spectroscopy
One-dimensional 19F NMR NMR spectra were recorded with proton decoupling (Bruker, zgfhigqn30.2) on a Bruker Ascend 600 MHz instrument in 5 mm tubes (0.7 ml volume) at 300 K, recording 64 scans. Note that where the limit of detection is approached, improved signal to noise can be obtained by increasing the number of scans (N) providing signal to noise proportional to N1/2. Spectra were processed using Bruker TopSpin software.Data availability
The datasets supporting this article have been included or uploaded as part of the ESI.†Author contributions
A. T.: investigation, formal analysis, experimental write-up. I. L. B: investigation, formal analysis. A. C.: supervision, editing of MS, investigation, formal analysis. A. K. P.: investigation, formal analysis, editing of MS A. V. S.: project administration, supervision, investigation, formal analysis, editing of MS E. A. Y.: project conception and administration, supervision, investigation, formal analysis, experimental write-up, editing of MS.Conflicts of interest
We have no conflicts of interest to declare.Acknowledgements
A. T. was a Master's in Biological Science project student (2023–2024) at the University of Liverpool.References
- I. Stevenson, S. Thackeray and E. Ransome, Delivering Biodiversity: Priority Actions for Fresh Water, British Ecological Society, London, UK, 2024 Search PubMed.
- S. Some, R. Mondal, D. Mitra, D. Jain, D. Verma and S. Das, Energy Nexus, 2021, 1, 100008 CrossRef CAS.
- S. T. Odonkor and T. Mahami, Int. J. Microbiol., 2020, 2534130 Search PubMed.
- S. C. Crosby, N. C. Spiller, K. E. Tietz, J. R. Cooper and P. J. Fraboni, Environ. Monit. Assess., 2019, 19, 745 CrossRef PubMed.
- L. Chen, L. F. Li, X. S. Zhi, P. Zhang, Y. Dai, Y. C. Xiao and Z. Y. Shen, xuanjing kexue, 2019, 40, 633–639 Search PubMed.
- K. Gilbride, Molecular Methods for the Detection of Waterborne Pathogens. Waterborne Pathogens, Elsevier, Amsterdam, The Netherlands, 2014, pp. 231–290 Search PubMed.
- R. A. Deshmukh, K. Joshi, S. Bhand and U. Roy, Microbiologyopen, 2016, 6, 901–922 CrossRef PubMed.
- B. Pang, C. Zhao, X. Song, K. Xu, J. Wang and Y. Liu, et al. , Anal. Biochem., 2018, 542, 58–62 CrossRef CAS PubMed.
- A. F. Maheux, D. K. Boudreau, M. A. Bisson, V. Dion-Dupont, S. Bouchard and M. Nkuranga, et al. , Appl. Environ. Microbiol., 2014, 80, 4074–4084 CrossRef PubMed.
- S. Gai, R. Sun, R. Singh, S. Y. So, C. T. Y. Chan, T. Savidge and M. Hu, Drug Discov. Today, 2022, 27, 103316 CrossRef PubMed.
- M. M. Elmassy, S. Kim and B. Busby, PLoS One, 2021, 16, e0244876 CrossRef PubMed.
- C. Ebuzoeme, I. Etim, A. Ikimi, J. Song, T. Du and M. Hu, et al. , Pharmaceutics, 2021, 13, 1043 CrossRef CAS PubMed.
- Z. Xiang, H. Zhu, B. Yang, H. Fan, J. Guo and J. Liu, et al. , Sci. Rep., 2020, 10, 16628 CrossRef CAS PubMed.
- M. Mroczynska, M. Galecka, P. Szachta, D. Kamoda, Z. Libudzisz and D. Roszak, Pol. J. Microbiol., 2013, 62, 319–325 CAS.
- Overview of structure-function relationships of glucuronidases. Glycoside Hydrolases, in Foundations and Frontiers in Enzymology, ed. S. B. Mohapatra and N. Manoj, in A. Goyal and K. Sharma, Academic Press, Elsevier, 2023, ch. 12, pp. 255–274 Search PubMed.
- R. M. Pollet, E. H. D'Agostino, W. G. Walton, Y. Xu, M. S. Little and K. A. Biernat, et al. , Structure, 2017, 25, 967–977 CrossRef CAS PubMed.
- H. Nelis and S. van Pouke, Water, Air, Soil Pollut., 2000, 123, 43–52 CrossRef CAS.
- B. Sperker, M. Schick and H. K. Kroemer, J. Chromatogr. B:Biomed. Sci. Appl., 1996, 685, 181–184 CrossRef CAS PubMed.
- S. Uhlig, L. Ivanova and C. K. Faeste, J. Agric. Food Chem., 2013, 61, 2006–2012 CrossRef CAS PubMed.
- A. H. Farnleitner, L. Hocke, C. Beiwl, G. G. Kavka and R. L. Mach, Water Res., 2002, 36, 975–981 CrossRef CAS PubMed.
- C. Tong, G. Cai, Q. Wei, Y. Cao, Y. Chen and S. Shi, Microchem. J., 2022, 174, 107104 CrossRef CAS.
- D. W. Fink and W. R. Koehler, Anal. Chem., 1970, 42, 990–993 CrossRef CAS.
- H. Chen, S. Viel, F. Ziarelli and L. Peng, Chem. Soc. Rev., 2013, 42, 7971–7982 RSC.
- J. A. London, E. C. S. Wang, I. L. Barsukov, E. A. Yates and A. V. Stachulski, Carbohydr. Res., 2021, 499, 108225 CrossRef CAS PubMed.
- M. K. Fraser, A. Gorecka, E. A. Yates, J. A. Iggo, K. Baj and A. V. Stachulski, Org. Chem. Front., 2024, 11, 2720 RSC.
- A. Gorecka, H. Schacht, M. K. Fraser, A. Teriosina, J. A. London and I. L. Barsukov, et al. , ACS Omega, 2024, 10, 1419–1428 CrossRef PubMed.
- J.-J. Max, F. Meddleb-Mouelhi, M. Beauregarde and C. Chapados, Appl. Spectrosc., 2012, 66, 1433–1441 CrossRef CAS PubMed.
- A. W. Shepard, J. Am. Chem. Soc., 1965, 87, 2410–2420 CrossRef.
- C. Kasireddy, J. G. Bann and K. R. Mitchell-Koch, Phys. Chem. Chem. Phys., 2015, 17, 30606–30612 RSC.
- M. Derba-Macleuch, M. Mitra, M. Hedenström, X. Liu, M. L. Gandla and F. R. Barbut, et al. , New Phytol., 2023, 238, 297–312 CrossRef PubMed.
- T. E. Gottshalk, J. E. Nielsen and P. Rasmussen, Appl. Microbiol. Biotechnol., 1996, 45, 240–244 CrossRef PubMed.
- L. Zhang, Y. Chen, N. Cheng, Y. Xu, K. Huang, Y. Luo, P. Wang, D. Duan and W. Xu, Anal. Chem., 2017, 89, 10194 CrossRef CAS PubMed.
- W. Wu, B. T. T. Nguyen, P. Y. Liu, G. Cai, S. Feng and Y. Shi, et al. , Sens. Actuators, B, 2022, 368, 132198 CrossRef CAS.
- W. Wu, B. T. T. Nguyen, P. Y. Liu, G. Cai, S. Feng and Y. Shi, et al. , Biosens. Bioelectron., 2022, 215, 114594 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ay01723d |
| This journal is © The Royal Society of Chemistry 2025 |