Open Access Article
Open Access ArticleDevelopment of shotgun metabolomic profile analysis for detecting canine visceral leishmaniasis using flow-through pinhole paper spray mass spectrometry†
Emmanuel Dadzie
Akuffu‡
ab,
Riley
Ferguson‡
a,
Jonathan N.
Chilaka
 a,
Mônica
Duarte da Silva
a,
Hianka J.
Costa de Carvalho
a,
Kingsley
Badu
b and
Abraham K.
Badu-Tawiah
a,
Mônica
Duarte da Silva
a,
Hianka J.
Costa de Carvalho
a,
Kingsley
Badu
b and
Abraham K.
Badu-Tawiah
 *a
*a
aDepartment of Chemistry and Biochemistry, The Ohio State University, Columbus, Ohio 43210, USA. E-mail: badu-tawiah.1@osu.edu
bDepartment of Theoretical and Applied Biology- Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
First published on 23rd June 2025
Abstract
Visceral leishmaniasis (VL) is a neglected infectious disease that can be transmitted between dogs and humans. In this work, a novel flow-through pinhole paper spray mass spectrometry (MS) technique is described to detect metabolites that might be up- or down-regulated during canine VL infection. The flow-through pinhole paper spray MS allows direct analysis of dried samples prepared in paper substrates, without prior sample pre-treatment. Here, clinical canine serum samples from ten dogs positives for VL were prepared in embossed hydrophobic paper substrates in the lab. Similar dried serum samples from uninfected control dogs were prepared in embossed paper. Partial least squares discriminant analysis was performed on positive-ion mode full mass spectra recorded from the 20 samples, which showed significant separation between VL positive and negative samples. Volcano plots confirmed the presence of several up- and down-regulated species in the infected dog samples. Results from this work demonstrate detectable metabolomic changes can be measured during canine VL from dried serum samples without sample preparation.
Introduction
Visceral leishmaniasis (VL) is a vector-borne disease (caused mainly by leishmania (L.) infantum) with serious public health concerns. This disease is transmitted through the bite of infected female sandflies during the blood meal.1,2 VL is also a neglected zoonotic (it can spread between animals and humans) infectious disease with its presence directly linked to poverty, while social, environmental, and climatic factors affect the epidemiology of the disease.3,4 While over a billion people reside in regions where leishmaniasis is prevalent and are at risk of infection, most humans who contract the parasite remain asymptomatic throughout their lives. Each year, it is estimated that there are 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 new cases of VL and over 1 million new cases of cutaneous leishmaniasis (CL)5 with less than 20
000 new cases of VL and over 1 million new cases of cutaneous leishmaniasis (CL)5 with less than 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cases of VL were reported in 2023.6 The relationship between canine VL (CVL) and human VL makes the control of the disease difficult. Indeed, the domestic dog is the main reservoir of L. infantum in urban areas.7 It is not uncommon to find dogs living in conglomerations in low- and middle-income countries, where the disease is prevalent. Such conditions can attract the vector, which can transmit the parasite in the community, from dogs to humans. Thus, controlling CVL is important, something that is commonly achieved by culling of infected dogs and by using insecticide collars.8
000 cases of VL were reported in 2023.6 The relationship between canine VL (CVL) and human VL makes the control of the disease difficult. Indeed, the domestic dog is the main reservoir of L. infantum in urban areas.7 It is not uncommon to find dogs living in conglomerations in low- and middle-income countries, where the disease is prevalent. Such conditions can attract the vector, which can transmit the parasite in the community, from dogs to humans. Thus, controlling CVL is important, something that is commonly achieved by culling of infected dogs and by using insecticide collars.8
Aside from the control of vector and animal reservoir hosts, strategies that can enable early diagnosis and surveillance are important to promptly monitor and manage VL. Early detection will enable timely treatment, which can be expected to reduce the prevalence of the disease. Due to limited resources in endemic regions, methods that can enable sample collection from remote areas will offer significant impact by enabling a centralized platform for asymptomatic infection. Traditionally, CVL has been diagnosed using rapid diagnostic tests (RDT),9–11 immunofluorescence assays (IFA),12,13 enzyme-linked immunosorbent assays (ELISA),14,15 and polymerase chain reactions (PCR).16–18 RDT is best suited for in-field screening since it can be delivered to remote areas, and it is low-cost. However, the sensitivity of this diagnostic method is limited and does not allow early-stage disease detection.9 The remaining diagnostic techniques (i.e., IFA, ELISA, and PCR) are often used in clinical settings since extensive sample preparation is required. Take PCR as an example; the DNA extraction process is time-consuming, coupled with a laborious amplification process. This process limits the applicability of these methods in large-scale CVL screening. The animal can be transported for testing, but this only increases the cost of analysis and the inconvenience of the process. The alternative strategy is to collect samples remotely for subsequent analysis at a later time. While this might work for PCR since the DNA analyte is stable at room temperature, cold storage is required to ship proteins often analyzed in IFA and ELISA. Cold-chain shipping is particularly difficult due to the requirement to sustain a regulated temperature during transit, a task that becomes even more challenging in remote areas.19
In addition to DNA and protein analysis, changes in metabolites have been used to diagnose VL.20,21 Parasites like L. infantum derive most of their nutrients from their hosts. This process intimately links the metabolism of the parasite to that of their hosts, providing a detectable change in the metabolite profile of infected dogs or people. Traditionally, such metabolite changes are analyzed using nuclear magnetic resonance (NMR),22,23 liquid chromatography mass spectrometry (LC-MS),24,25 and gas chromatography mass spectrometry (GC-MS).26 NMR is robust, nondestructive, and reproducible, but it requires highly purified samples in high concentrations due to limited sensitivity. Both LC-MS and GC-MS are highly sensitive and robust, but they also require sample preparation, and analysis time can be long. These factors limit the implementation of these traditional methods in resource-limited settings. Recently, ambient mass spectrometry (MS) was introduced by Prof. R. Graham Cooks to enable direct analysis of complex mixtures without prior sample preparation.27 This technology has enabled messy samples like whole blood and serum to be analyzed on standalone mass spectrometers, including portable instruments, without front-end chromatographic separation.28,29 This capability has been achieved through a plethora of ambient ionization methods, including desorption electrospray ionization (DESI),30 direct analysis in real-time (DART),31 and paper spray ionization,32 just to mention a few. Unlike DESI and DART, paper spray ionization does not require nitrogen or helium nebulizer gases,32–34 making it more suitable for field analysis in resource-limited settings.
Recently, our research group introduced pinhole paper spray, which utilized salinized hydrophobic paper to collect and store biofluids onto embossed wells created in the paper substrate.35 Extensive prior studies36–39 have shown that analytes stored in the hydrophobic paper are stabilized at room temperature for extended time periods without the need for cold storage. We propose that the embossed hydrophobic paper substrates can be used to collect samples from remote locations where VL is endemic. The dried samples present in the paper substrate can then be shipped without cold-chain restriction to a central facility where a standalone mass spectrometer can be used to analyze the samples directly from the paper substrate. We demonstrate this capability by analyzing 20 clinical canine samples prepared in the lab using our embossed hydrophobic paper substrates. Using the pinhole paper spray MS data, we performed partial least squares discriminant analysis (PLS-DA) to differentiate the ten infected samples from the ten uninfected samples. This ambient paper spray MS method based on metabolite profiling has the potential to support surveillance strategies in CVL endemic regions by allowing sample collection, room temperature storage, and direct analysis using existing mass spectrometers without any front-end modification.
Experimental section
Preparation of embossed hydrophobic paper
An embossment plate made of two pairs of master plate molds (one male, projecting mold, and one female, intaglio mold, Fig. S1†) was designed from AutoCAD 2021 software (AutoDesk, San Rafael, CA) and utilized for paper embossment. Whatman Grade 3 chromatography filter paper circles were cut using a laser cutter (FSL Muse Core Desktop CO2 Laser Cutter, Full Spectrum Laser, Las Vegas, NV) to a diameter of 130 mm. These pre-cut paper circles were first spritzed with an aqueous/ethanol solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol/water, % v/v) followed by firmly hand-pressing the paper between both male and female molds for a minimum of 5 min. The embossed paper was then air-dried and then subjected to silane vapor reactions under vacuum. In this way, the embossed Whatman 3 chromatographic filter papers were treated chemically with methyl-trichlorosilane in a vacuum desiccator to produce the hydrophobic paper used for sample collection and MS analysis.
1 ethanol/water, % v/v) followed by firmly hand-pressing the paper between both male and female molds for a minimum of 5 min. The embossed paper was then air-dried and then subjected to silane vapor reactions under vacuum. In this way, the embossed Whatman 3 chromatographic filter papers were treated chemically with methyl-trichlorosilane in a vacuum desiccator to produce the hydrophobic paper used for sample collection and MS analysis.
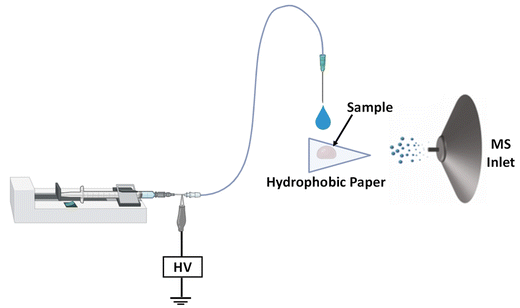
Flow-through pinhole paper ionization
Canine serum samples were provided by our collaborator from the Laboratory of Leishmaniasis at the Universidade Federal de Minas Gerais (UFMG), Brazil. Dried serum spheroids were prepared by pipetting 10 μL of liquid canine serum samples onto the embossed hydrophobic paper substrate and drying overnight at room temperature. Once dry, each well of the embossed paper substrate was punched using a standard hole puncher. The punched well containing the dried serum was then inverted 180° and placed onto the surface of triangular hydrophilic paper, which served as the paper spray substrate for analyte ionization via an electrospray-like mechanism. To enable metabolite extraction from the dried serum, we created a hole at the bottom of the embossed hydrophobic paper using a sharp 16-gauge hypodermic sterile needle (see Fig. S2 and S3† for a visual representation of the pinhole paper spray MS set up). This pinhole provided a channel to guide the spray solvent through the crushed three-dimensional dried serum spheroid, facilitating in situ extraction processes. After this, the assembly of the two paper substrates (i.e., embossed paper with sample/pinhole placed on a hydrophilic paper triangle, 10 mm base × 16 mm height) is placed in front of a mass spectrometer and analyzed via a novel flow-through pinhole paper spray as shown in Fig. 1. Here, the spray solvent (methanol/water, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v, with 0.1% formic acid) was pumped at 25 μL min−1 from a syringe through a fused silica capillary (100 μm ID) toward the paper assembly containing the sample. An optimized direct current (DC) high voltage of +6 kV was applied to the metal needle of the syringe, which caused electrosprayed charged microdroplets to be generated toward the paper. This approach is contrary to the traditional paper spray experiment where the spray solvent and high voltage are directly applied to the paper substrate. This novel flow-through paper spray experiment forms part of our ongoing effort to stabilize paper spray signal as well as to make it high throughput. By floating the paper substrate, an array of samples can be arranged on a moving stage in a straightforward manner to improve the reproducibility and speed of the analysis. For the current work, it required a 90 s delay time for the spray solvent to completely wet the hydrophilic paper triangle and begin to generate secondary charged microdroplets containing the extracted metabolites and transfer them toward the proximal mass spectrometer.
20, v/v, with 0.1% formic acid) was pumped at 25 μL min−1 from a syringe through a fused silica capillary (100 μm ID) toward the paper assembly containing the sample. An optimized direct current (DC) high voltage of +6 kV was applied to the metal needle of the syringe, which caused electrosprayed charged microdroplets to be generated toward the paper. This approach is contrary to the traditional paper spray experiment where the spray solvent and high voltage are directly applied to the paper substrate. This novel flow-through paper spray experiment forms part of our ongoing effort to stabilize paper spray signal as well as to make it high throughput. By floating the paper substrate, an array of samples can be arranged on a moving stage in a straightforward manner to improve the reproducibility and speed of the analysis. For the current work, it required a 90 s delay time for the spray solvent to completely wet the hydrophilic paper triangle and begin to generate secondary charged microdroplets containing the extracted metabolites and transfer them toward the proximal mass spectrometer.
Mass spectrometry
Mass spectra were recorded using a Thermo Scientific Finnegan LTQ linear ion trap mass spectrometer (San Jose, CA). MS parameters used were as follows: 200 °C capillary temperature, and three microscans. Spray voltage was kept at 6 kV for all experiments. The distance of the hydrophilic paper triangle tip to the MS inlet was kept at 5 mm. Thermo Fisher Scientific Xcalibur 2.2 SP1 software was applied for MS data collecting and processing. The paper substrate was positioned with its tip parallel to the mass spectrometer inlet using a copper alligator clip, which was subjected to a continuous supply of primary charged microdroplets from the spray solvent contained in the syringe.Sample collection and ethical clearance
Clinical canine serum samples were donated by our collaborator from Laboratory of Leishmaniasis at UFMG, Brazil. The samples were previously collected and tested for VL by immunofluorescence assay and PCR at the Laboratory of Leishmaniasis at UFMG, Brazil. The protocol for collecting clinical samples was approved by the Ethics Committee on the Use of Animals from the University of Minas Gerais (protocol number 198/2014). Samples were stored at −80 °C in a freezer and transported in dry ice to our laboratory under an Import Permit from the Centers of Diseases Control and Prevention (CDC) (PHS Permit number 20230302-0910A). Obtained serum were aliquoted, kept at −80 °C in a freezer, and thawed right before experiments.Statistical data analysis
Mass spectral data were extracted into Microsoft Excel using Xcalibur 2.2 SP1 software provided by Thermo Fisher Scientific. Average of three replicates was exported to MetaboAnalyst, where the data was auto-scaled and normalized, after which PLS-DA, t-test, and fold change analyses were performed.Chemicals and reagents
All solvents (methanol (99.9%, HPLC grade), ethyl acetate (≥99.5%), pure ethyl alcohol (200 proof, anhydrous, ≥99.5%), acetonitrile (HPLC plus grade), and methyltrichlorosilane (≥99.9%) were obtained from Sigma Aldrich (St Louis, MO)). A 1.0 mg mL−1 standard solution of cocaine was acquired from Cerilliant (Round Rock, TX, USA). 18.2 MΩ water was obtained from a Milli-Q water purification system (Millipore, Billerica, MA, USA). Potassium hydroxide pellets (ACS certified), Whatman chromatography filter paper (grade 1) and Whatman chromatography filter paper (grade 3) were purchased from Whatman (Little Chalfont, England). All experiments were performed in accordance with the guidelines of the Office of Responsible Research Practices (ORRP).Results and discussion
Optimization of flow-through pinhole paper mass spectrometry
The initial experiments involved the optimization of spray solvent and voltage for our flow-through pinhole paper spray. We used a methanolic solution of cocaine for this optimization study. Preliminary experiments showed 25 μL min−1 flowrate provided stable signal in less than 90 s, so we used this flowrate to optimize the voltage and solvent. For cocaine desorption from the paper substrate with primary charged microdroplets from the spray solvent, we observed that increasing water content in a methanol/water solvent system generally improved ion signal derived from the secondary charged microdroplets generated from the tip of the hydrophilic paper triangle (Fig. S4†). However, the pinhole paper spray signal peaked when methanol/water (80/20, v/v, with 0.1% formic acid) was used, thus we used this spray system for the rest of our experiments. The optimized spray solvent composition is controlled by the solubility of the analyte and evaporation rate. Higher methanol content might be good for extracting hydrophobic compounds, but such solvent might evaporate too quickly, which will reduce analyte extraction. Next, we checked spray voltage, for which we observed an increase in signal with applied DC voltage (Fig. S5†), but we chose +6 kV as our optimized value, as this spray voltage generated minimal electrical discharge. The difference between the typical 5 kV and our optimized 6 kV for this flow-through paper spray might be due to the change in setup configuration in which the paper substrate is floating in front of a grounded mass spectrometer inlet. This configuration can reduce the overall electric field shape and strength and might require a higher applied spray voltage to allow the generation of secondary charged microdroplets from the paper substrate. On the contrary, applying the voltage directly to the paper substrate, which is positioned in front of a grounded mass spectrometer, will require an overall lower voltage to generate high electric fields.Analysis of clinical canine serum samples
We applied the pinhole flow-through paper spray MS platform to analyze 20 canine samples freshly collected and sent from Brazil. These samples were analyzed by immunofluorescence assay by our collaborators, with ten of them diagnosed to be positive with CVL and the remaining ten samples being negative. For analysis of these samples, 10 μL dried serum spheroids were prepared in embossed hydrophobic paper substrates and subsequently analyzed via the new pinhole flow-through paper spray MS method after placing the dried samples onto a hydrophilic paper triangle. Metabolite extraction and in situ ionization was achieved with methanol/water (80/20, v/v, with 0.1% formic acid) spray solvent. Full positive-ion mode mass spectra of metabolite profiles were analyzed by partial least squares discriminant analysis (PLS-DA) to differentiate the infected samples from uninfected ones. Initial experiments showed positive-ion mode results to give better differentiation than results from negative-ion mode (Fig. S6†).Fig. 2A shows PLS-DA analysis based on data collected from positive-ion mode pinhole flow-through paper spray MS for the 20 samples. As shown, the ten CVL infected serum samples can be distinguished from the ten uninfected samples. Typical positive-ion mode flow-through paper spray MS spectra recorded from infected and uninfected canine serum samples are provided in Fig. S7.† The top 15 differentiation ions are summarized in Fig. S8,† which showed that 13 of these species were upregulated in the infected samples. We generated the volcano plot shown in Fig. 2B to confirm if indeed the differentiating species were upregulated. Here, y-values representing −log10(p-value), that are greater than 1 are considered significant. In this case, any species with x-values [log2(FC)] > 1 are deemed upregulated (top right corner) in the infected samples, and those with x-values < −1 are deemed downregulated (top left corner). The volcano plot generated using these typical parameters, with significant fold change (FC), revealed three upregulated species: m/z 214, 347, and 570. Two of these ions (m/z 347 and 570) were ranked among the top 8 differentiating species in the PLS-DA analysis. These statistical results show that the pinhole flow-through paper spray MS experiment can be employed to analyze dried samples collected onto hydrophobic paper substrates. Further analyses are needed to characterize the specific identities of the upregulated species, but the current profiling mode was demonstrated to be sufficient in differentiating infected samples from uninfected ones. Note: other species with log2 (FC) values less than 1 might have been considered among the top 15 compounds in the PLS-DA analysis. The volcano plot only considers species with significant fold change, in which species with x-values > 1 (upregulated species) and x-values < 1 (down regulated), are all above the −log10(p-value) > 1 cutoff.
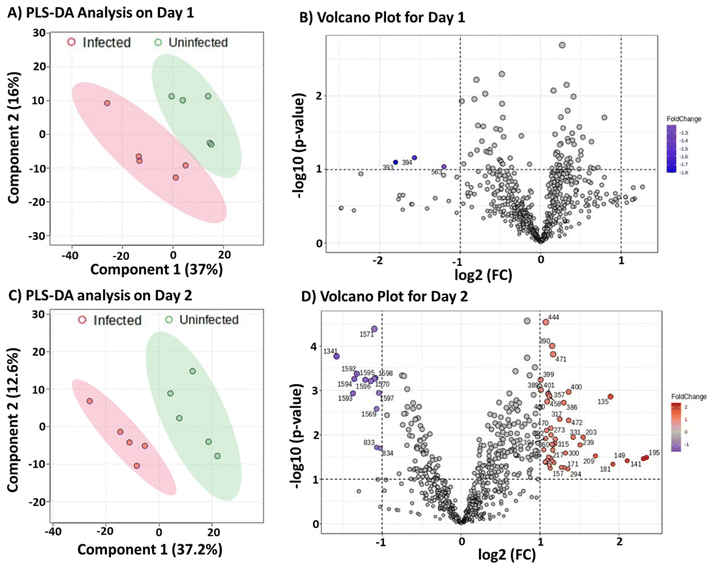
Importance of instrument variability
At the beginning of this project, due to instrument conditions, we analyzed five of the infected samples and five of the uninfected ones on the same day. Then on a separate day, we repeated this experiment by analyzing additional five infected and five uninfected samples together to complete the analysis of all 20 clinical canine serum samples. We then applied the same PLS-DA analysis to differentiate the two groups independently. Fig. 3A shows the PLS-DA data derived from analyzing the first ten samples on Day 1, which showed good separation between the infected and uninfected groups. The volcano plot derived from these samples (Fig. 3B) shows three species (m/z 393, 394, and 563) that are downregulated in the infected samples as indicated by the significant fold changes (FC) in the left upper corner. Similarly, Fig. 3C shows the PLS-DA data obtained from analyzing the second ten samples on Day 2. This time, the separation between the infected and uninfected groups was larger, and this is supported by a volcano plot analysis (Fig. 3D), which showed a multitude of species that are upregulated and down regulated in the infected samples. These results reproduced data shown in Fig. 2, where the ten infected serum samples were differentiated from the ten uninfected samples.The surprise came when we combined the two infected samples analyzed on two different days into a single data set for PLS-DA analysis. We attempted to build a model that could differentiate the combined infected samples from the combined uninfected samples. Fig. 4A shows that the combined data from infected serum samples could not be differentiated from the combined data from uninfected samples, although these were the same samples successfully differentiated in Fig. 2 and reproduced in Fig. 3. We hypothesized that the overlap observed in Fig. 4A might be due to significant instrument variabilities when comparing data from two different days. If this is the case, then the data generated in a single day for both infected and uninfected combined should be more similar and could be differentiated from the combined data from the second day. Indeed, PLS-DA analysis (Fig. 4B) showed significant separation of data collected from the two days, even though the combined data are from distinct groups. That is, we combined data from infected and uninfected samples on Day 1 to be a single data set. Similarly, Day 2 data represented a combination of MS results derived from infected and uninfected samples in Day 2. These results suggest significant instrument variations when the experiments are performed on different days. We do not expect such instrument variability to be specific to our pinhole flow-through paper MS experiment but can be present in most other ambient mass spectrometry experiments. As shown earlier, such instrument variabilities can be overcome by analyzing all samples in the same day. The fact that the infected and uninfected serum samples can be differentiated by comparing the positive-ion mode ion profiles, despite potential instrument variabilities, strongly suggests inherent differences in metabolites present during CVL infection.
Our findings on MS variability confirm what is already known, although not typically reported in the literature. For example, in constructing a calibration curve, the data must be collected in quick successions and in a single day to avoid large variations. Internal standards help, but even here combining data collected in two different days typically fails to give satisfactory linear regression analysis. For ambient MS, where the experiment is performed in the open laboratory environment, other factors like environmental and chemical noise can affect day-to-day variability. The method proposed has potential for high throughput automated analysis, which will enable >300 samples to be run in triplicates in a single day when considering the 90 s delay time. Such capability will minimize instrument variability in real-world application. Our samples were stored at −80 °C until the day of the analysis, therefore we do not expect sample degradation to account for the differences observed. The volcano plot, which represents graphical display of differences underlying partial least squares discriminant analysis, were noted to be markedly different for the two days. This difference is, however, not related to instrument variability since the species were characterized according to fold changes (up- or down-regulated in the infected samples). Rather, the number of species detected in each day is related to the level of the leishmania infection, which can be correlated with antibody titer. The antibody titers for the samples analyzed in each day were determined via immunofluorescence assay (IFA), via a dilution experiment. The more a sample can be diluted and still provide IFA signal, the higher the antibody titer. The results from this experiment are summarized in Tables S1 and S2,† which showed that samples analyzed in Day 2 had higher antibody titer, which signifies a more advanced infection. This may explain why more metabolites were detected in samples analyzed in Day 2 (Fig. 3D) than samples analyzed in Day 1 (Fig. 3B).
Conclusions
In summary, we have demonstrated the use of embossed hydrophobic paper substrates to prepare dried serum samples for subsequent direct analysis via a novel flow-through pinhole paper spray MS platform. The method was used to analyze 20 canine samples, 10 of which were positive for canine visceral leishmaniasis while the remaining 10 were negative. Positive-ion mode full mass spectra recorded from these clinical samples were subjected to partial least squares discriminant analysis, which showed statistically significant separation between infected and uninfected groups. The flow-through pinhole paper spray MS analysis was reproduced on two other days, all showing significant separation between samples infected with canine visceral leishmaniasis versus those that are not infected. Volcano plot analyses confirmed significant fold changes for several species detected in the infected serum samples. Characterization of the discriminating metabolites is the subject of ongoing investigation, but we suspect these to fall with the class of lipids, amino acids, and fatty acids. Our data suggest that instrument variability can be significant for samples that are not analyzed in the same day. The variability reported here is not meant to affect data comparison. Notice how the individual decisions made on three different days (Days 1 and 2 in Fig. 3, and data in Fig. 2) were all comparable. That is, all samples were correctly classified in all three experiments. The caution highlighted is for situations where data is combined from two different days. Overall, the ability to use paper substrates to collect samples and analyze directly from the paper substrate without any pre-treatment has potential to reduce instrument requirements for metabolomic analysis, which has important implications in how analytical measurements can be achieved in resource-limited settings.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available in the ESI of this article.†Acknowledgements
This work was supported by National Science Foundation (award number CHE-2305057). EDA and KB thank the U.S. National Institute of Allergy and Infectious Disease (award number R01-AI 143809) for support. We thank Dr Hélida M. de Andrade from the Laboratory of Leishmaniasis at the Universidade Federal de Minas Gerais, Belo Horizonte-MG, Brazil, for donating the canine serum samples used in these experiments.References
- R. Gálvez, A. Montoya, F. Fontal, L. Martínez De Murguía and G. Miró, Controlling phlebotomine sand flies to prevent canine Leishmania infantum infection: A case of knowing your enemy, Res. Vet. Sci., 2018, 121, 94–103 CrossRef PubMed.
- P. Desjeux, Leishmaniasis: current situation and new perspectives, Comp. Immunol., Microbiol. Infect. Dis., 2004, 27, 305–318 CrossRef CAS PubMed.
- Pan American Health Organization: Leishmaniasis. https://www.paho.org/en/topics/leishmaniasis (accessed 2025-01-07).
- J. Alvar, S. Yactayo and C. Bern, Leishmaniasis and poverty, Trends Parasitol., 2006, 22, 552–557 CrossRef PubMed.
- World Health Organization. Leishmaniasis. https://www.who.int/health-topics/leishmaniasis/the-global-epidemiology-of-leishmaniasis#tab=tab_1 (accessed 2025-01-07).
- World Health Organization . The Global Health Observatory Number of cases of visceral leishmaniasis reported,https://www.who.int/data/gho/data/indicators/indicator-details/GHO/number-of-cases-of-visceral-leishmaniasis-reported (accessed 2025-01-07).
- R. J. Quinnell and O. Courtenay, Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis, Parasitology, 2009, 136, 1915–1934 CrossRef CAS PubMed.
- M. S. Duthie and C. Petersen, Could canine visceral leishmaniosis take hold in the UK?, Vet. Rec., 2019, 184, 438–440 CrossRef PubMed.
- P. Srivastava, K. Gidwani, A. Picado, G. Van der Auwera, P. Tiwary, B. Ostyn, J. Dujardin, M. Boelaert and S. Sundar, Molecular and serological markers of Leishmania donovani, infection in healthy individuals from endemic areas of Bihar, India, Trop. Med. Int. Health, 2013, 18(5), 548–554 CrossRef PubMed.
- L. M. A. Braz, R. Tahmasebi, P. M. Hefford and J. A. L. Lindoso, Visceral Leishmaniasis diagnosis: A rapid test is a must at the hospital bedside, Clinics, 2020, 75, 1–2 CrossRef PubMed.
- G. Herrera, A. Castillo, M. S. Ayala, C. Flórez, O. Cantillo-Barraza and J. D. Ramirez, Evaluation of four rapid diagnostic tests for canine and human visceral Leishmaniasis in Colombia, BMC Infect. Dis., 2019, 19(1), 747 CrossRef PubMed.
- M. E. F. Brito, M. G. Mendonça, Y. M. Gomes, M. L. Jardim and F. G. C. Abath, Dynamics of the antibody response in patients with therapeutic or spontaneous cure of American cutaneous leishmaniasis, Trans. R. Soc. Trop. Med. Hyg., 2001, 95, 203–206 CrossRef CAS PubMed.
- R. Szargiki, E. Alcântara De Castro, E. Luz, W. Kowalthuk, Â. M. Machado and V. Thomaz-Soccol, Comparison of Serological and Parasitological Methods for Cutaneous Leishmaniasis Diagnosis in the State of Paraná, Brazil, Braz. J. Infect. Dis., 2009, 13(1), 47–52 CrossRef PubMed.
- D. Kumar, B. Khanal, P. Tiwary, S. L. Mudavath, N. K. Tiwary, R. Singh, K. Koirala, M. Boelaert, S. Rijal and S. Sundar, Comparative evaluation of blood and serum samples in rapid Immunochromatographic tests for visceral Leishmaniasis, J. Clin. Microbiol., 2013, 51(12), 3955–3959 CrossRef PubMed.
- L. F. de A. Paz, A. da Silva, H. R. F. da Silva, M. P. Cavalcanti, V. M. F. de Lima, M. R. O. d. C. Beltrão, M. B. A. Silva, O. P. de Melo Neto, Z. M. Medeiros and W. J. T. d. Santos, Diagnostic Potential for the Detection of Canine Visceral Leishmaniasis of an ELISA Assay Based on the Q5 Recombinant Protein: A Large-Scale and Comparative Evaluation Using Canine Sera with a Positive Diagnosis from the Dual-Path-Platform (DPP) Test, Vet. Sci., 2023, 10(10), 1–12 Search PubMed.
- L. Galluzzi, M. Ceccarelli, A. Diotallevi, M. Menotta and M. Magnani, Real-time PCR applications for diagnosis of leishmaniasis, Parasites Vectors, 2018, 11(1), 273 CrossRef PubMed.
- F. Hossain, P. Ghosh, M. A. A. Khan, M. S. Duthie, A. C. Vallur, A. Picone, R. F. Howard, S. G. Reed and D. Mondal, Real-time PCR in detection and quantitation of Leishmania donovani for the diagnosis of visceral leishmaniasis patients and the monitoring of their response to treatment, PLoS One, 2017, 12(9), 1–16 Search PubMed.
- R. C. Silva, V. B. Richini-Pereira, M. Kikuti, P. M. Marson and H. Langoni, Detection of Leishmania (L.) infantum in stray dogs by molecular techniques with sensitive species-specific primers, Vet. Q., 2017, 37(1), 23–30 CrossRef PubMed.
- S. Lee, D. S. Kulyk, S. O. Afriyie, K. Badu and A. K. Badu-Tawiah, Malaria Diagnosis Using Paper-Based Immunoassay for Clinical Blood Sampling and Analysis by a Miniature Mass Spectrometer, Anal. Chem., 2022, 94(41), 14377–14384 CrossRef CAS PubMed.
- H. Qin, J. Zhang, K. Dong, D. Chen, D. Yuan and J. Chen, Metabolic characterization and biomarkers screening for visceral leishmaniasis in golden hamsters, Acta Trop., 2022, 225, 106222 CrossRef CAS PubMed.
- D. Yuan, J. Chen, Z. Zhao and H. Qin, Metabolomics analysis of visceral leishmaniasis based on urine of golden hamsters, Parasites Vectors, 2023, 16(1), 304 CrossRef CAS PubMed.
- S. D. Lamour, B. Choi, H. C. Keun, I. Müller and J. Saric, Metabolic characterization of Leishmania major infection in activated and nonactivated macrophages, J. Proteome Res., 2012, 11(8), 4211–4222 CrossRef CAS PubMed.
- M. Arjmand, A. Madrakian, G. Khalili, A. Najafi Dastnaee, Z. Zamani and Z. Akbari, Metabolomics-based study of logarithmic and stationary phases of promastigotes in Leishmania major by 1H NMR spectroscopy, Iran. Biomed. J., 2016, 20(2), 77–83 Search PubMed.
- R. T'Kindt, A. Jankevics, R. A. Scheltema, L. Zheng, D. G. Watson, J. C. Dujardin, R. Breitling, G. H. Coombs and S. Decuypere, Towards an unbiased metabolic profiling of protozoan parasites: optimisation of a Leishmania sampling protocol for HILIC-orbitrap analysis, Anal. Bioanal. Chem., 2010, 398(5), 2059–2069 CrossRef PubMed.
- G. D. Westrop, R. A. M. Williams, L. Wang, T. Zhang, D. G. Watson, A. M. Silva and G. H. Coombs, Metabolomic analyses of Leishmania reveal multiple species differences and large differences in amino acid metabolism, PLoS One, 2015, 10(9), e0136891 CrossRef PubMed.
- D. P. De Souza, E. C. Saunders, M. J. McConville and V. A. Likić, Progressive peak clustering in GC-MS Metabolomic experiments applied to Leishmania parasites, Bioinformatics, 2006, 22(11), 1391–1396 CrossRef CAS PubMed.
- A. K. Badu-Tawiah, L. S. Eberlin, Z. Ouyang and R. G. Cooks, Chemical aspects of the extractive methods of ambient ionization mass spectrometry, Annu. Rev. Phys. Chem., 2013, 64, 481–505 CrossRef CAS PubMed.
- M. E. King, M. Lin, M. Spradlin and L. S. Eberlin, Advances and Emerging Medical Applications of Direct Mass Spectrometry Technologies for Tissue Analysis, Annu. Rev. Anal. Chem., 2023, 16(1), 1–25 CrossRef CAS PubMed.
- D. T. Snyder, C. J. Pulliam, Z. Ouyang and R. G. Cooks, Miniature and Fieldable Mass Spectrometers: Recent Advances, Anal. Chem., 2016, 88(1), 2–29 CrossRef CAS PubMed.
- Z. Takáts, J. M. Wiseman, B. Gologan and R. G. Cooks, Mass spectrometry sampling under ambient conditions with desorption electrospray ionization, Science, 2004, 306(5695), 471–473 CrossRef PubMed.
- R. B. Cody, J. A. Laramée and H. D. Durst, Versatile New Ion Source for the Analysis of Materials in Open Air under Ambient Conditions, Anal. Chem., 2005, 77(8), 2297–2302 CrossRef CAS PubMed.
- H. Wang, J. Liu, R. G. Cooks and Z. Ouyang, Paper Spray for Direct Analysis of Complex Mixtures Using Mass Spectrometry, Angew. Chem., Int. Ed., 2010, 49(5), 877–880 CrossRef CAS PubMed.
- A. J. Grooms, B. J. Burris and A. K. Badu-Tawiah, Mass spectrometry for metabolomics analysis: Applications in neonatal and cancer screening, Mass Spectrom. Rev., 2022, e21826 Search PubMed.
- S. Lee, K. Chintalapudi and A. K. Badu-Tawiah, Clinical Chemistry for Developing Countries: Mass Spectrometry, Annu. Rev. Anal. Chem., 2021, 14(1), 437–465 CrossRef CAS PubMed.
- B. S. Frey, D. R. Heiss and A. K. Badu-Tawiah, Embossed Paper Platform for Whole Blood Collection, Room Temperature Storage, and Direct Analysis by Pinhole Paper Spray Mass Spectrometry, Anal. Chem., 2022, 94(10), 4417–4425 CrossRef CAS PubMed.
- E. L. Rossini, D. S. Kulyk, E. Ansu-Gyeabourh, T. Sahraeian, H. R. Pezza and A. K. Badu-Tawiah, Direct analysis of doping agents in raw urine using hydrophobic paper spray mass spectrometry, J. Am. Soc. Mass Spectrom., 2020, 31(6), 1212–1222 CrossRef CAS PubMed.
- D. E. Damon, K. M. Davis, C. R. Moreira, P. Capone, R. Cruttenden and A. K. Badu-Tawiah, Direct Biofluid Analysis using Hydrophobic Paper Spray Mass Spectrometry, Anal. Chem., 2016, 88(3), 1878–1884 CrossRef CAS PubMed.
- B. S. Frey, D. E. Damon, D. M. Allen, J. Baker, S. Asamoah and A. K. Badu-Tawiah, Protective Mechanism of Dried Blood Spheroids: Stabilization of Labile Analytes in Whole Blood, Plasma and Serum, Analyst, 2021, 146, 6780–6787 RSC.
- D. E. Damon, M. Yin, D. M. Allen, Y. S. Maher, C. J. Tanny, S. Oyola-Reynoso, B. L. Smith, S. Maher, M. M. Thuo and A. K. Badu-Tawiah, Blood Spheroids for Dry-State Room Temperature Stabilization of Microliter Blood Samples, Anal. Chem., 2018, 90(15), 9353–9358 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5an00451a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |