Open Access Article
Open Access ArticleIdentification of plasticizers using thermal desorption dielectric barrier discharge ionization mass spectrometry
Qiao
Lu†
 ab,
Xiaokang
Guan†
b,
Xue
You†
b and
Renato
Zenobi
ab,
Xiaokang
Guan†
b,
Xue
You†
b and
Renato
Zenobi
 *bc
*bc
aClinical Molecular Diagnostic Center of Taihe Hospital, and Hubei Key Laboratory of Wudang Local Chinese Medicine Research, Hubei University of Medicine, Shiyan 442000, China
bDepartment of Chemistry and the MOE Key Laboratory of Spectrochemical Analysis & Instrumentation, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China
cDepartment of Chemistry and Applied Biosciences, ETH Zurich, CH-8093 Zurich, Switzerland. E-mail: zenobi@org.chem.ethz.ch
First published on 7th May 2025
Abstract
Plasticizers, which are extensively utilized in the manufacturing of plastic products, have garnered significant attention due to their potential toxicity and the consequent health and environmental risks they pose. Traditional methods for detecting plasticizers, such as gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS), are often time-consuming and require complex sample preparation. In response to these challenges, this study introduces an innovative approach for the rapid and sensitive identification of seven common plasticizers using thermal desorption dielectric barrier discharge ionization mass spectrometry (TD-DBDI-MS). The proposed technique significantly reduces background interference by employing nitrogen as the discharge gas, thereby enabling the detection of characteristic quasi-molecular ions ([M + H]+) and their fragments, which are crucial for structural elucidation. The method exhibits remarkable sensitivity, with a limit of detection as low as 0.1 ppm for diethyl phthalate and a linear dynamic range of orders of magnitude. Furthermore, it allows for expedited screening of various plastic products, including cling wrap, packaging bags, and centrifuge tubes, with each sample analyzed in less than 30 seconds. This study underscores the efficacy of TD-DBDI-MS as a rapid, sensitive, and user-friendly methodology for the preliminary screening of plasticizers in a wide range of materials.
Introduction
Plasticizers are widely used as additives in plastic manufacturing to enhance flexibility and durability, and they are also frequently incorporated into industrial and household products because of their distinct physicochemical characteristics.1 Plasticizers are prevalent in diverse environmental contexts and are frequently detected in various biological samples and materials.2 However, they can potentially migrate from plastic products into the environment3 and can enter the human body through multiple routes, including ingestion and dermal contact, posing diverse health risks.4Consequently, the development of rapid, sensitive, and reliable methods for identifying and detecting plasticizers in plastic products is important.5 Currently, several extraction techniques are employed for isolating plasticizers from matrix materials prior to detection, including solid-phase extraction,6 ultrasound-assisted extraction,7 and solid-phase microextraction.8 While these methods are capable of extracting trace amounts of plasticizers from matrix materials, they are inevitably susceptible to contamination from ambient air and experimental equipment during various stages of sample processing, including sampling, extraction, purification, concentration, and sample injection. Traditional analytical techniques, such as gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS), offer high sensitivity and accuracy but are often time-consuming, labor-intensive, and require complex sample preparation.9 These limitations hinder their application in rapid screening and on-site detection scenarios.
To address these challenges, two main approaches have been developed. First, a semi-closed/closed sample chamber and clean gas were used to reduce the exposure time of samples to air.10 Second, employing atmospheric pressure ionization mass spectrometry (API-MS) enabled the direct introduction of solid or liquid samples into the ion source without the need for extensive sample preparation or chromatographic separation.11 In this fashion, we are able to circumvent traditional sample pretreatment processes such as extraction and purification, which creates significant advantages in terms of simplicity and speed. For example, Krieger et al. adopted a direct inlet probe-atmospheric pressure chemical ionization-MS technology for non-destructive analysis of plastic products and were able to directly detect plasticizers from plastic products.12 Rothenbacher et al. used direct analysis in real time-MS (DART-MS) to directly analyze phthalic acid esters in polyvinyl chloride (PVC) toys.13 The complete analysis of a single sample takes only about 8 minutes. The helium temperature was set at 130 °C, and most samples were not damaged, enabling rapid non-destructive analysis of children's plastic toys. Sisco et al. used thermal desorption-DART-MS to analyze trace pollutants. They changed the traditional DART design and ran the sample desorption process in a closed tube, which reduced interference from the laboratory environment.14 Driffield and colleagues developed an innovative analytical approach utilizing a dual-purpose solid probe that functioned simultaneously as a sampling interface and a corona ionization source coupled with quadrupole time-of-flight mass spectrometry (Q-TOF MS). This integrated system was effectively employed for the rapid screening and identification of plasticizer compounds migrating from food-contact gasket materials.15 Jecklin et al. used a floating atmospheric pressure afterglow discharge-MS technology to identify di-(2-ethylhexyl) phthalate (DEHP) and dibutyl phthalate (DBP) in PVC food packaging, and each sample takes less than 30 seconds.16 There is basically no requirement for the type of sample material, and both liquid and solid (soluble or insoluble) polymers and particles can be handled.
In recent years, dielectric barrier discharge ionization (DBDI) has garnered significant attention since its introduction as an ambient ionization source for MS in 2007.17 This interest has stemmed from its notable advantages, including high ionization efficiency, relatively soft ionization characteristics, low energy consumption, and the potential for miniaturization, as well as its compatibility with various ambient inlet MS instruments.18 Owing to these features, DBDI has found extensive applications across diverse fields, such as biochemical analysis, environmental monitoring, food safety, forensic authentication, real-time monitoring of reaction intermediates, and mass spectrometry imaging (MSI).19,20 Its widespread utilization underscores its versatility and adaptability in scientific research and analytical practice.
This study introduces a novel approach based on thermal desorption-DBDI-MS (TD-DBDI-MS) to analyze seven common plasticizers, all of which are commonly used in industrial manufacturing.21 The TD-DBDI-MS system integrates a custom-built DBDI source with a Q-TOF MS, enabling rapid and sensitive detection of plasticizers. The DBDI source, configured in an in-line geometry, utilizes nitrogen as both the carrier and discharge gas, and it is coupled to a closed sample cell, which effectively reduces background interference from ambient air and plastic tubing. This design enhances molecule/ion transmission efficiency and provides characteristic fragmentation patterns, facilitating both molecular weight determination and structural identification. The system demonstrated excellent sensitivity, with a limit of detection (LOD) of 0.1 ppm for DEP. The combination of a tungsten needle direct sampling approach and TD-DBDI-MS allowed for rapid screening, with analyses completed in under 30 seconds per sample. Cling wrap, packaging bags, and centrifuge tubes could be analyzed. This study highlights the potential of TD-DBDI-MS as a fast, sensitive, and user-friendly method for the preliminary screening of plasticizers in plastic products and holds substantial promise for applications in real-time on-site detection, contributing to improved safety and regulatory compliance in the use of plastic products.
Experimental
Materials
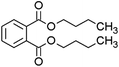
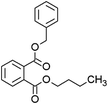
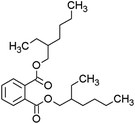
Seven kinds of plasticizers – dimethyl phthalate (DMP), diethyl phthalate (DEP), dibutyl phthalate (DBP), butyl benzyl phthalate (BBP), dibutoxyethyl phthalate (DBEP), di-(2-ethylhexyl) phthalate (DEHP) and diisononyl phthalate (DINP) – were purchased from Aladdin Reagent Company (Shanghai, China). Acetonitrile (CAN, C2H3N, HPLC) was purchased from China Pharmaceutical Group Chemical Reagent Co., Ltd. Nitrogen (N2, 99.999%) was purchased from Xinhang Gas Company (Xiamen, China). The tungsten needles used in this study were sourced from Shenzhen Ji Chen Electronics Co., Ltd (Shenzhen, China) and featured a tip diameter of 1 μm. All aqueous solutions were prepared using ultrapure water (18.2 MΩ cm) from a Milli-Q ultrapure water system (Millipore, MA, USA). The solvent was an ACN/H2O (50/50 v/v) mixture.Sample introduction system and ionization source
A syringe pump (XMSP-3C, Nanjing Ximai Nano Co., Nanjing, China) was employed to deliver the samples to the thermal desorption cell (constructed from stainless steel) at a predetermined flow rate. The liquid sample was subsequently vaporized using heated nitrogen gas (N2) at 250 °C, which was supplied through a stainless steel pipe at a fixed flow rate of 0.7 L min−1. In this closed system, N2 served as both the carrier gas and the discharge gas, effectively preventing the introduction of ambient gases and minimizing interference from plasticizers present in the plastic tubing. The thermal desorption cell was directly connected to the DBDI source.For the ion source, we employed a laboratory-built DBDI source configured in an “in-line geometry”, as detailed in our previous publication.22–24 This design is compatible with any MS system equipped with an atmospheric pressure (AP) interface. In brief, the setup consists of a quartz glass capillary (inner diameter [i.d.] = 1.1 mm, outer diameter [o.d.] = 1.5 mm, and length [l] = 30.0 mm) acting as the dielectric barrier between two electrodes. The capillary is coupled to the MS system via a custom-built Teflon inlet. A copper ring (i.d. = 1.6 mm, o.d. = 3.0 mm, and l = 8 mm) tightly wrapped around the quartz capillary serves as the high-voltage electrode, while a stainless-steel capillary (i.d. = 0.6 mm and o.d. = 1.0 mm) connected to a thermal desorption cell is inserted into the quartz capillary, functioning as both the ground electrode and the sampling capillary. An alternating high voltage of approximately 3.5 kV at a frequency of 40 kHz is applied to the electrodes to generate a DBD plasma within the capillary. This plasma ionizes gaseous sample molecules carried by the nitrogen (N2) gas. The design significantly enhances molecule/ion transmission efficiency while effectively isolating air-related interferences. It is important to note that safety precautions, such as warning signs and proper insulation of cables and connectors, are strongly recommended. A schematic of the experimental setup, including all relevant parameters, is shown in Fig. 1.
Mass spectrometry
The analysis was performed using a Q-TOF MS system (API-TOF, TOFWERK AG, Thun, Switzerland). The MS was operated in positive ion mode with a mass-to-charge (m/z) range of 7–560. The instrument parameters were set as follows: capillary voltage at 0 V, capillary temperature at 200 °C, and TOF extraction rate at 16 kHz. Raw data were initially processed using Tofware version 3.2.1 (TOFWERK AG, Thun, Switzerland), and the resulting mass spectra were subsequently exported and analyzed using OriginPro 16 (OriginLab, Northampton, MA, USA).Results and discussion
To evaluate the feasibility of the instrumental setup, we first analyzed the background MS signal. In Fig. 2, the blue curve represents the background MS signal obtained using DBDI-MS with air as the discharge gas, while the red curve corresponds to the background signal when nitrogen (N2) was used as the discharge gas. As evident from the results, background signals from air pollutants were significantly reduced, by about 90% for DBP, when employing the TD-DBDI-MS system with N2 as the discharge gas. | ||
| Fig. 2 Mass spectra of the background obtained by TD-DBDI-MS when lab air was used as the discharge gas (blue curve) and when N2 was used as the discharge gas (red curve). | ||
In this experiment, seven typical plasticizer standards (DMP, DEP, DBP, BBP, DBEP, DEHP, and DINP) were analyzed and identified by TD-DBDI-MS. A 5 μL aliquot of each standard solution, with a concentration of 100 ppm, was introduced into the thermal desorption cell using a micro-sampler. The plasticizer solution was volatilized by heated N2 gas, ionized by the DBD plasma, and subsequently detected using a Q-TOF mass spectrometer. Fig. 3 shows the mass spectra of these seven plasticizers obtained through TD-DBDI-MS.
As shown in Fig. 3(a), the mass spectrum of DMP displays characteristic peaks at m/z = 195 and m/z = 163. In agreement with the literature,25 the peak at m/z = 195 is attributed to the quasi-molecular ion [M + H]+ of DMP, whereas the peak at m/z = 163 corresponds to the fragment ion formed through the loss of a methoxy radical (–OCH3˙) from the ionized DMP molecule. The mass spectrum of DEP in Fig. 3(b) shows three significant peaks: the quasi-molecular ion [M + H]+ at m/z = 223, the fragment ion [M − C2H4O]+ at m/z = 177 resulting from ethoxy radical elimination, and the protonated phthalic anhydride ion [M − C4H8OH]+ at m/z = 149 following complete alkyl group loss. This fragmentation pattern is consistent across the analyzed plasticizers. In Fig. 3(c), DBP shows its quasi-molecular ion [M + H]+ at m/z = 279 and the characteristic protonated phthalic anhydride ion at m/z = 149 [M − C8H16OH]+. Similarly, BBP in Fig. 3(d) exhibits its quasi-molecular ion [M + H]+ at m/z = 313 and the protonated phthalic anhydride ion at m/z = 149 [M − C11H15O]+. Fig. 3(e–g) reveal the quasi-molecular ions [M + H]+ of DBEP, DEHP, and DINP at m/z equal to 367, 391, and 419, respectively. Notably, the protonated phthalic anhydride ion peak at m/z = 149 appears consistently in all spectra, serving as a characteristic fragment for the presence of phthalate plasticizers. Additional fragment peaks specific to each plasticizer molecule were also identified.
It should be noted that we attempted to turn off the hot source during detection of standards, yet fragmentation still occurred. This indicates that the fragmentation originates from the source itself rather than from thermal decomposition prior to ionization. Previous studies suggest that humidified nitrogen may help achieve softer ionization.26 Due to the absence of tandem mass spectrometry capabilities in our experimental setup, fragment ion peak assignments were verified using mass spectral data from the NIST Chemistry WebBook (https://webbook.nist.gov/chemistry/). Table 1 lists a comprehensive summary of the seven plasticizers analysed, including their names, molecular formulae, molecular structures, molecular weights, quasi-molecular ions, and characteristic fragment ions (m/z).
Based on our experimental data and comparison with literature,27,28 we propose a plausible fragmentation mechanism for plasticizer molecules. The ionization process initiates when DEP molecules enter the plasma region of the DBDI source. Subsequent proton exchange reactions occur with [(H2O)n+1 + H]+ generated in the plasma, forming the quasi-molecular ion [M + H]+. This ion undergoes sequential fragmentation, initially losing two alkyl branches to yield the protonated phthalate ion [M − C4H7]+, followed by dehydration to generate the characteristic protonated phthalic anhydride ion at m/z = 149. Alternative fragmentation pathways involve direct interaction of DEP molecules with plasma-generated reactive species, including He˙, N4˙+, N2˙+, O2˙+, NO˙+, etc., forming radical cations M˙+. These radical cations can subsequently lose ethoxy radicals to produce [M − OR]+ ions, which undergo structural rearrangement and alkyl side chain elimination to ultimately form phthalic anhydride ions. A similar fragmentation pattern was observed for the plasticizer molecules of phthalate esters. Therefore, the plasma-based DBDI source can not only ionize the excimer ions of the plasticizer molecules but also obtain a certain degree of fragment ions. This characteristic proves particularly valuable for rapid substance identification, as it provides both molecular weight information and structural insights through characteristic fragmentation patterns.
To evaluate the potential of this method for real sample detection, we first selected disposable droppers as experimental samples. Plastic droppers from the laboratory were cut into 1 cm2 square pieces and immersed in 5 mL of acetonitrile (ACN) for 0.5, 1, 2 and 4 hours, respectively. The extracts were injected at a flow rate of 200 μL min−1 using a syringe pump and subsequently analyzed by TD-DBDI-MS to identify the plasticizers present in the droppers and evaluate the influence of extraction time on efficiency. In this experiment, plasticizers were identified using the fingerprint spectra of the standards provided in Table 1. As shown in Fig. 4, three distinct quasi-molecular ion peaks of different plasticizer molecules were detected: a peak at m/z = 279 corresponding to the [M + H]+ of DBP, a peak at m/z = 313 corresponding to the [M + H]+ of BBP, and a peak at m/z = 419 corresponding to the [M + H]+ of DINP. Additionally, the primary fragment ion peak of the plasticizers at m/z = 149 was observed. Furthermore, as the extraction time increased from 0.5 hours to 4 hours, the mass spectrometric signals of the detected plasticizer molecules gradually intensified. This indicates that the plasticizers progressively migrated from the disposable droppers into the acetonitrile solution. We further assessed the sensitivity of our method for detecting plasticizer molecules. A series of DEP standard solutions with concentrations ranging from 1 ppm to 100 ppm were prepared, and 5 μL aliquots of each solution were introduced into the thermal desorption cell. MS data were subsequently acquired to construct a calibration curve for DEP. The linear regression equation was y = 0.57x + 0.28, where x represents the concentration of DEP and y corresponds to the MS peak intensity. The regression coefficient (R2) was determined to be 0.9978, indicating excellent linearity. The LOD was calculated to be 0.1 ppm (S/N = 3). The noise level was determined by averaging a 100-data-point baseline segment adjacent to the m/z = 223 peak in the mass spectrum.
Subsequently, we utilized the tungsten needle direct sampling technique29,30 coupled with the TD-DBDI-MS method to conduct a rapid screening and analysis of plasticizers in three commonly used laboratory plastic products: cling wrap, package bags, and centrifuge tubes. Surface sampling was conducted by making a 1 cm linear scratch on the sample using a tungsten needle, which was then directly inserted into the thermal desorption cell for thermal evaporation. The resulting vapors were transported by the carrier gas into the DBDI source for ionization, followed by analysis in the MS. As depicted in Fig. 5(a), the mass spectra of cling wrap revealed the presence of [M + H]+ peaks corresponding to DMP, DBP, DBEP, DEHP, and DINP, along with their respective fragment ions. Similarly, Fig. 5(b) illustrates that the same suite of plasticizer molecules was identified in the package bags. In contrast, Fig. 5(c) demonstrates that the centrifuge tubes contained a more limited range of plasticizers, with only the [M + H]+ peaks of DBP, BBP, DBEP, and DEHP, as well as their associated fragment ions, being detected. The unidentified peaks observed in the mass spectra could potentially correspond to molecular ions or fragmentation products derived from either additives or macromolecular components present in the materials.
 | ||
| Fig. 5 Mass spectra of (a) cling wrap, (b) packaging bags, and (c) centrifuge tubes using tungsten needle direct sampling combined with the TD-DBDI-MS method. | ||
Conclusions
In this paper, we demonstrate the effectiveness of TD-DBDI-MS as a rapid, sensitive, and user-friendly method for the identification and screening of plasticizers in various plastic products. By utilizing nitrogen as the discharge gas, this closed device significantly reduces background interference, enabling the detection of characteristic quasi-molecular ions and their fragments, which are useful for structural elucidation. The method exhibited remarkable sensitivity, with an LOD as low as 0.1 ppm for standard DEP and a linear regression coefficient of 0.9978. The TD-DBDI-MS system allows for the expedited analysis of plastic products, including cling wrap, packaging bags, and centrifuge tubes, with each sample analysed in less than 30 seconds. This rapid screening capability, along with the ability to detect multiple plasticizers simultaneously and a simple or no sample preparation procedure, makes the method particularly well-suited for real-time on-site detection applications and underscores the method's potential for environmental monitoring, food safety, and public health protection.Data availability
Data will be made available on request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by funding from the Natural Science Foundation of China (no. 22304050), the Hubei Provincial Natural Science Foundation of China (no. 2023AFB271), and the Faculty Development Grants from Hubei University of Medicine (no. 2022QDJZR007).References
- Y.-Q. Huang, Y. Zeng, J.-L. Mai, Z.-S. Huang, Y.-F. Guan and S.-J. Chen, Environ. Sci. Technol., 2024, 58, 3098–3107 CAS.
- L. Zhang, Y. He, L. Jiang, Y. Shi, L. Hao, L. Huang, M. Lyu and S. Wang, Environ. Res., 2024, 263, 120007 CrossRef CAS PubMed.
- L. Arfaeinia, S. Dobaradaran, F. Nasrzadeh, S. Shamsi, Y. Poureshgh and H. Arfaeinia, Microchem. J., 2020, 155, 104719 CrossRef CAS.
- K.-Y. Yuan, Y.-H. Gu, Y.-H. Pei, S.-Y. Yu, T.-Z. Li, T. Feng, Y. Liu, J. Tian, X. Miao, J. Xiong, M. Hu and B.-F. Yuan, J. Hazard. Mater., 2025, 486, 136970 CrossRef CAS PubMed.
- L. Bernard, D. Bourdeaux, B. Pereira, N. Azaroual, C. Barthélémy, C. Breysse, P. Chennell, R. Cueff, T. Dine, T. Eljezi, F. Feutry, S. Genay, N. Kambia, M. Lecoeur, M. Masse, P. Odou, T. Radaniel, N. Simon, C. Vaccher, C. Verlhac, M. Yessaad, B. Décaudin and V. Sautou, Talanta, 2017, 162, 604–611 CrossRef CAS PubMed.
- H. Chen, W. Mao, Y. Shen, W. Feng, G. Mao, T. Zhao, L. Yang, L. Yang, C. Meng, Y. Li and X. Wu, Environ. Sci. Pollut. Res., 2019, 26, 24609–24619 CrossRef CAS PubMed.
- I. Notardonato, C. Protano, M. Vitali and P. Avino, Food Anal. Methods, 2019, 12, 2562–2571 CrossRef.
- M. A. Moreira, L. C. André and Z. D. L. Cardeal, Food Chem., 2015, 178, 195–200 CrossRef CAS PubMed.
- K. Poitou, T. Rogez-Florent, M. Lecoeur, C. Danel, R. Regnault, P. Vérité, C. Monteil and C. Foulon, Toxics, 2021, 9, 200 CrossRef CAS PubMed.
- J. Fernández-Arribas, S. Callejas-Martos, A. Balasch, T. Moreno and E. Eljarrat, Anal. Bioanal. Chem., 2024, 416, 6957–6972 CrossRef PubMed.
- T. Xu, H. Li, P. Dou, Y. Luo, S. Pu, H. Mu, Z. Zhang, D. Feng, X. Hu, T. Wang, G. Tan, C. Chen, H. Li, X. Shi, C. Hu and G. Xu, Adv. Sci., 2024, 11, 2306659 CrossRef CAS PubMed.
- S. Krieger and O. J. Schmitz, Rapid Commun. Mass Spectrom., 2014, 28, 1862–1870 CrossRef CAS PubMed.
- T. Rothenbacher and W. Schwack, Rapid Commun. Mass Spectrom., 2009, 23, 2829–2835 CrossRef CAS PubMed.
- E. Sisco, T. P. Forbes, M. E. Staymates and G. Gillen, Anal. Methods, 2016, 8, 6494–6499 RSC.
- M. Driffield, E. Bradley, L. Castle, A. Lloyd, M. Parmar, D. Speck, D. Roberts and S. Stead, Rapid Commun. Mass Spectrom., 2015, 29, 1603–1610 CrossRef CAS PubMed.
- M. C. Jecklin, G. Gamez and R. Zenobi, Analyst, 2009, 134, 1629–1636 RSC.
- N. Na, M. Zhao, S. Zhang, C. Yang and X. Zhang, J. Am. Soc. Mass Spectrom., 2007, 18, 1859–1862 CrossRef CAS PubMed.
- A. Begley and R. Zenobi, J. Mass Spectrom., 2023, 58, e4910 CrossRef CAS PubMed.
- M. Bouza, J. García-Martínez, B. Gilbert-López, S. Brandt, J. F. García-Reyes, A. Molina-Díaz and J. Franzke, Anal. Chem., 2023, 95, 854–861 CrossRef CAS PubMed.
- Q. Lu, X. Guan, X. You, Z. Xu and R. Zenobi, Anal. Chem., 2021, 93, 14694–14700 CrossRef CAS PubMed.
- A. D. Godwin, Applied plastics engineering handbook, Elsevier, 2024, pp. 595–618 Search PubMed.
- Q. Lu, Z. Xu, X. You, S. Ma and R. Zenobi, Anal. Chem., 2021, 93, 6232–6238 CrossRef CAS PubMed.
- X. Zhao, X. Guan, Q. Lu, X. Yan and R. Zenobi, Analyst, 2024, 149, 5034–5040 RSC.
- M. M. Nudnova, L. Zhu and R. Zenobi, Rapid Commun. Mass Spectrom., 2012, 26, 1447–1452 CrossRef CAS PubMed.
- P. Gerbaux, J. De Winter, R. Flammang, V. S. Nguyen and M. T. Nguyen, Int. J. Mass Spectrom., 2010, 290, 127–132 CrossRef CAS.
- L. Gyr, F. D. Klute, J. Franzke and R. Zenobi, Anal. Chem., 2019, 91, 6865–6871 CrossRef CAS PubMed.
- S. H. Jeon, Y. P. Kim, Y. Kho, J. H. Shin, W. H. Ji and Y. G. Ahn, J. Anal. Methods Chem., 2018, 2018, 9470254 Search PubMed.
- S. Cheng, W. Cao, X. Zhao, N. Li, Z. Ouyang, Q. Ma and X. Ma, Int. J. Mass Spectrom., 2019, 444, 116176 CrossRef CAS.
- Q. Lu, R. Lin, C. Du, Y. Meng, M. Yang, R. Zenobi and W. Hang, Anal. Chem., 2020, 92, 5921–5928 CrossRef CAS PubMed.
- X. Guan, Q. Lu, X. Zhao, X. Yan and R. Zenobi, Anal. Chem., 2023, 95, 12470–12477 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |