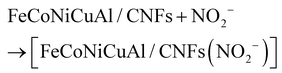Nanofibers decorated with high-entropy alloy particles for the detection of nitrites†
Wang
Yang
,
Wanchen
Xie
,
Chongtao
Zhang
,
Fang
Duan
 ,
Shuanglong
Lu
,
Shuanglong
Lu
 and
Mingliang
Du
and
Mingliang
Du
 *
*
Key Laboratory of Synthetic and Biological Colloids, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, P. R. China. E-mail: du@jiangnan.edu.cn
First published on 20th November 2024
Abstract
Excessive residues of nitrite can pose a serious threat to human health, making the establishment of an efficient and effective electrochemical sensor for nitrite detection highly necessary. Herein, we report on a sensor based on nitrogen-doped carbon nanofibers, with FeCoNiCuAl high-entropy alloy (HEAs) nanoparticles in situ grown on the carbon fibers through a confinement effect. The FeCoNiCuAl/CNF sensor is capable of electrochemically detecting nitrite using both differential pulse voltammetry (DPV) and amperometric (I–t) methods. The DPV detection offers a linear range of 0.1–5000 μM and 5000–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μM, with sensitivities of 150.6 μA mM−1 cm−2 and 80.1 μA mM−1 cm−2 and a detection limit of 0.023 μM (S/N = 3). The I–t detection covers a range of 1–10
000 μM, with sensitivities of 150.6 μA mM−1 cm−2 and 80.1 μA mM−1 cm−2 and a detection limit of 0.023 μM (S/N = 3). The I–t detection covers a range of 1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μM, with a sensitivity of 337.84 μA mM−1 cm−2 and a detection limit of 0.12 μM. Moreover, the sensor exhibits excellent anti-interference properties, stability, and reproducibility, providing feasibility for nitrite detection in real-world environments.
000 μM, with a sensitivity of 337.84 μA mM−1 cm−2 and a detection limit of 0.12 μM. Moreover, the sensor exhibits excellent anti-interference properties, stability, and reproducibility, providing feasibility for nitrite detection in real-world environments.
Introduction
Nitrite is an inorganic chemical substance widely found in food and the environment. It is mainly used in food processing, the pharmaceutical industry and industrial buildings.1 Nitrite can be used as a food additive for food preservation and coloring and can also be used as a key intermediate product for sewage treatment. Excessive nitrite is very harmful to the human body, which can lead to anemia, lactic acidosis, blue baby syndrome and even cancer.2–4 Eutrophication and the increased concentration of nitrates in water bodies affect the microbial activity and environmental conditions in the nitrogen cycle, leading to an increase in nitrite concentration, which in turn poses potential risks to aquatic ecosystems and human health. Therefore, it is very important to explore a method that can accurately detect the content of nitrite in food and the environment.Many detection methods have been established, such as fluorescence spectroscopy,5 chemiluminescence,6 spectrophotometry,7 capillary electrophoresis,8 Raman spectroscopy,9 chromatography,10 molecular absorption spectroscopy11 and the electrochemical method.12–14 However, many of these methods have the disadvantages of high cost, time-consuming, a complex sample preparation process and the need for skilled personnel to operate in specific practical detection applications, which limit their further practical application and cannot be used for rapid on-site detection and analysis.15 Relatively speaking, electrochemical detection technology has attracted the attention of researchers due to its advantages of simple operation, low cost, fast analysis speed, high sensitivity and low detection limit.16 The determination of nitrites also faces challenges in electrochemistry due to the lack of suitable ionophores or stable enzyme systems. Therefore, the development of new working electrode catalysts and the improvement of the design of electrochemical sensors are hot topics for improving the electrocatalytic detection performance in the future.
Electrochemical sensors for detection can be classified into enzymatic and non-enzymatic categories, based on the utilization of biological enzymes as the molecular recognition components. Enzymatic sensors, while being highly sensitive, face challenges such as the instability of enzymes, complex storage requirements, and sensitivity to environmental parameters like pH and temperature. Conversely, non-enzymatic sensors are gaining considerable interest due to their rapid response times, excellent stability and reproducibility, as well as their low limits of detection.17 To date, materials including noble metals, metal compounds, carbon nanomaterials, and their composites have demonstrated potential as effective materials for the development of nitrite-specific non-enzymatic sensors.18–21 However, precious metal materials are expensive and easy to deactivate, which greatly limit their practical detection applications. Transition metal materials have gradually become alternative materials for traditional electrocatalytic materials due to their abundant reserves, low price, high catalytic activity and stability, such as recently reported α-Fe2O3–ZnO, CeVO@f-CNF, and FeCl3/MIL-101(Cr) materials.22–24 Metal materials are usually combined with carbon materials to form composite materials, thus inheriting the advantages of the two materials. Interestingly, the composite material can also be endowed with some new excellent characteristics due to the synergistic effect, such as Co3O4/rGO, Mn2O/rGO, TiO2/rGO and other materials. The combination of metal oxides and reduced graphene oxide can significantly reduce the stacking of graphene sheets and effectively inhibit the self-aggregation of metal oxide particles, which is beneficial to increase the electroactive surface area of the modified electrode.25–28 In recent years, high-entropy alloy (HEA) materials have shown an extremely wide range of applications in the field of electrochemistry.29,30 Due to the synergistic effect of five or more different elements in the single-phase solid solution of high-entropy alloys, it has a unique electronic structure and can adjust the catalytically active sites by selecting element configuration and composition optimization, which helps to improve the electrocatalytic performance.31–34 High-entropy alloy materials have excellent catalytic performance in the field of electrochemical catalysis, including the carbon dioxide reduction reaction, electrolytic water, the nitridation reaction and the alcohol oxidation reaction.35–39 However, compared with other electrochemical applications, there are few studies on the application of high-entropy alloys to electrochemical sensing catalytic materials. In recent years, the application of FeCoNiCuMn/NCNS in the ORR and nitrite detection has been reported, which proves the feasibility of HEAs in this field.40 Therefore, we hope to prepare a high-entropy alloy single-phase solid solution and carbon nanomaterial composite material to give full play to the synergistic advantages of the two, so as to be applied to the electrochemical detection of nitrite.
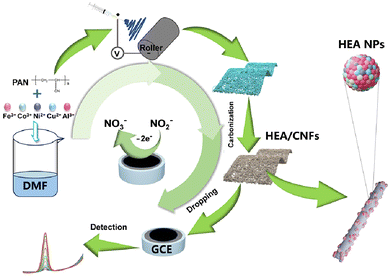
In this work, we present a novel approach for the in situ growth of HEA nanoparticles on carbon fibers, achieved through confinement within nitrogen-doped carbon nanofibers. The ratio of raw materials and fiber size were controlled by electrospinning technology, and the formed FeCoNiCuAl HEA single-phase solid solution was uniformly distributed on one-dimensional carbon nanofibers after high temperature treatment under an argon atmosphere. By compounding HEA NPs on carbon fibers, the two have a synergistic effect. Carbon nanofibers limit the growth size of HEA NPs and effectively hinder the self-aggregation of metals to make them uniform in size. We selected four commonly utilized transition metals—iron (Fe), cobalt (Co), nickel (Ni), and copper (Cu)—as well as aluminum (Al), a metal with high natural abundance. The unique low electronegativity of Al, combined with the lattice distortion effect inherent to high-entropy alloys (HEAs), has induced a significant alteration in the electronic structure of the formed HEA solid solution. This alteration is conducive to the adsorption of nitrite ions, thereby facilitating the essential redox reactions for sensing applications. Moreover, the amalgamation of nitrogen-enriched carbon fiber substrates with HEAs yields a composite material with an enhanced surface area. This increased surface area endows the material with a multitude of adsorption sites and electrocatalytically active sites, which significantly augment the detection capabilities for nitrite ions, offering a robust platform for the advancement of sensitive and efficient sensing technologies.
Experiments
Materials
Iron(III) acetylacetonate (C15H21FeO6, 98%), nickel(II) acetylacetonate (C10H14NiO4, 95%), cobaltic(III) acetylacetonate (C15H21CoO6, 98%), cupric(II) acetylacetonate (C10H14CuO4), aluminum(III) acetylacetonate (C15H21AlO6), polyacrylonitrile (PAN, (C3H3N)n, average Mw 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and sodium nitrite (NaNO2, 99.99%) were purchased from Shanghai Maclean Biochemical Technology Co. N,N-Dimethylformamide (DMF) was purchased from Shanghai ShuoXu Technology Co. All chemical reagents were used directly without further purification.
000) and sodium nitrite (NaNO2, 99.99%) were purchased from Shanghai Maclean Biochemical Technology Co. N,N-Dimethylformamide (DMF) was purchased from Shanghai ShuoXu Technology Co. All chemical reagents were used directly without further purification.
Instruments
Scanning electron microscopy (SEM, Hitachi, S-4800, Japan) and transmission electron microscopy (TEM, JEOL, JEM-2100F, Japan) were used to check the apparent and microscopic morphology of the synthesized materials. Scanning TEM (STEM, FEI, Tecnai G2 F30S-Twin, America) was used to observed the lattice of individual high-entropy alloy particles and the distribution of elements in a region. The crystal structures of the synthesized materials were characterized by X-ray diffraction (XRD, Bruker AXS D2 PHASER, Germany) with Cu Kα radiation (λ = 1.5406 Å) at a scan speed of 5° min−1 in the range of 30° to 90°. X-ray photoelectron spectrometry (XPS, Kratos, Axis Ultra DLD, UK) was used to study the surface chemical states of the products.Preparation of electrochemical sensors
The glassy carbon electrode (GCE, diameter 3 mm) was meticulously polished using a gradation of alumina powders with particle sizes of 1.0 μm and 0.05 μm. Subsequently, it was thoroughly rinsed with ethanol, followed by deionized water, and finally dried using a stream of nitrogen gas. A precise 3 mg aliquot of the synthesized FeCoNiCuAl/CNF powder was introduced into a solution comprising 20 μL of Nafion solution and 980 μL of an ultrapure water–isopropanol mixture with a volumetric ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The resulting suspension was then thoroughly mixed to ensure homogeneity through ultrasonication. A precise 3 μL aliquot of this suspension was applied uniformly onto the surface of the glassy carbon electrode, followed by air drying in preparation for testing. Electrochemical characterization was conducted using a CHI660E workstation from Shanghai Chenhua Instrument Co., Ltd. Cyclic voltammetry (CV) measurements were performed over a potential range of 0.4 to 1.2 V, while electrochemical impedance spectroscopy (EIS) was conducted across a frequency spectrum of 1000 kHz to 0.1 Hz.
3. The resulting suspension was then thoroughly mixed to ensure homogeneity through ultrasonication. A precise 3 μL aliquot of this suspension was applied uniformly onto the surface of the glassy carbon electrode, followed by air drying in preparation for testing. Electrochemical characterization was conducted using a CHI660E workstation from Shanghai Chenhua Instrument Co., Ltd. Cyclic voltammetry (CV) measurements were performed over a potential range of 0.4 to 1.2 V, while electrochemical impedance spectroscopy (EIS) was conducted across a frequency spectrum of 1000 kHz to 0.1 Hz.
Results and discussion
Morphology and crystal structure characterization
The synthesis route to high-entropy alloy nanoparticles supported by carbon fibers is illustrated in Scheme 1. A precursor solution is prepared by dissolving metal salts, which is then subjected to electrospinning. Subsequent high-temperature graphitization is carried out to uniformly grow metal particles within the carbon nanofibers. The size of the metal particles can be controlled to prevent self-aggregation by adjusting the dimensions of the carbon fibers. The synthesized material is characterized using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) to ensure the uniform dispersion and desired properties of the nanoparticles. As shown in Fig. 1a, carbon nanofibers with a diameter of about 100–200 nm are disorderedly arranged after high temperature graphitization. Due to the confinement of nanofibers, the in situ synthesized FeCoNiCuAl high-entropy alloy nanoparticles (HEA NPs) are uniformly distributed on the surface of carbon nanofibers and coated with a carbon layer. These metal nanoparticles have diameters between 20 and 50 nm (Fig. 1b). The high-resolution TEM (HRTEM) images of HEA NPs showed lattice fringes of 0.178 nm and 0.212 nm, corresponding to the (200) and (111) crystal planes of HEA NPs (Fig. 1c, d and e). Fig. 1f shows that the metal nanoparticles decorated on the carbon fibers were uniformly loaded with five metal elements, which proved the formation of FeCoNiCuAl HEA NPs. The metal particles wrapped by the carbon layer are beneficial for the stability of the material for electrocatalytic. In addition, inductively coupled plasma optical emission spectroscopy (ICP-OES) test results also show that the atomic content of the five metal elements is between 5% and 35%. The configurational entropy formula is . The configurational entropies of FeCoNi alloy, FeCoNiCu alloy and FeCoNiAl alloy are 1.1R, 1.34R and 1.38R, which belong to medium entropy alloys (MEAs). The configurational entropy of FeCoNiCuAl HEA NPs is calculated to be 1.57R, which is completely consistent with the definition of high entropy alloys.
. The configurational entropies of FeCoNi alloy, FeCoNiCu alloy and FeCoNiAl alloy are 1.1R, 1.34R and 1.38R, which belong to medium entropy alloys (MEAs). The configurational entropy of FeCoNiCuAl HEA NPs is calculated to be 1.57R, which is completely consistent with the definition of high entropy alloys.
The crystal structure of the FeCoNiCuAl HEA supported on carbon nanofibers (CNFs) was characterized by X-ray diffraction (XRD) analysis. For comparative purposes, FeCoNiCu/CNFs, FeCoNiAl/CNFs, and FeCoNi/CNFs were also synthesized. Fig. 2a illustrates that the prominent diffraction peaks observed in the FeCoNiCuAl/CNF sample at 44.0°, 51.2°, and 75.1° can be attributed to the (111), (200), and (220) crystal planes of a face-centered cubic (fcc) lattice, respectively. The dimensions of the lattice fringes observed are consistent with the findings depicted in Fig. 1c. These results confirm that the synthesized FeCoNiCuAl HEA exhibits a stable fcc phase, forming a solid solution. The FeCoNi alloy manifests additional diffraction peaks, indicating a complex crystal structure that encompasses both face-centered cubic (fcc) and body-centered cubic (bcc) phases, rather than forming a uniform single-phase solid solution. Intriguingly, the incorporation of Cu and Al, which possess larger atomic radii, into the FeCoNi alloy lattice induces lattice expansion and an increase in the interplanar spacing. This results in a lattice distortion within the high-entropy alloy (HEA), as indicated by the shift of the FeCoNiCuAl HEA's diffraction peaks towards a lower 2θ angle in comparison to those of the FeCoNiCu and FeCoNiAl alloys. This observation suggests that the alloy's crystal structure is significantly influenced by the addition of Cu and Al. In contrast to the FeCoNi alloy, the introduction of these elements facilitates the formation of a stable, single-phase fcc solid solution HEA. The chemical constitution and valence states of the FeCoNiCuAl/CNF composite were meticulously examined through X-ray photoelectron spectroscopy (XPS). This analysis revealed the presence of a diverse elemental array, including Fe, Co, Ni, Cu, Al, C, N, and O. The high-resolution C 1s XPS spectrum, as illustrated in Fig. S2a,† exhibits three peaks at binding energies of 284.8, 285.3, and 286.3 eV. These peaks are ascribed to the C–C, C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups, respectively, providing insight into the carbonaceous components of the composite. The N 1s XPS spectrum, featured in Fig. S2b,† delineates two peaks at 398.9 and 401.25 eV, which are indicative of pyridinic-N and graphitic-N species. This observation underscores the successful incorporation of nitrogen into the carbon nanofiber framework, resulting in a nitrogen-doped carbon structure. Such nitrogen doping is instrumental in augmenting the active site inventory of the nanomaterial, thereby enhancing its electrochemical performance.41 Additionally, it endows the carbon fibers with increased surface polarity, which is pivotal for bolstering their affinity for reactant molecules, consequently amplifying their catalytic efficacy.42,43 The XPS spectra in Fig. 2b reveal the Fe 2p region with peaks at 707.4, 709.4, 711.1, 716.65, 720.5, 722.5, and 724.2 eV, corresponding to various iron oxidation states, including Fe0, Fe2+, and Fe3+, along with satellite features. Fig. 2c shows the Co 2p region with peaks at 777.8, 779.4, 792.8, and 794.4 eV, indicative of Co0 and Co2+ states, complemented by satellite peaks at 783.4, 788.7, and 796.6 eV. The Cu 2p region in Fig. 2d exhibits peaks at 932.85, 934.45, 936.45, 952.6, 954.2, and 956.2 eV, reflecting the presence of Cu0, Cu+, and Cu2+ oxidation states. The XPS analysis in Fig. 2e identifies peaks for Ni at 852.7, 854.6, 870, and 871.9 eV, corresponding to Ni0 and Ni2+ states in both 2p3/2 and 2p1/2 orbitals. Fig. 2f shows an Al 2p peak at 74.9 eV, indicating a predominant Al3+ state. This suggests aluminum's role in the high-entropy alloy as an electron donor to other metals, due to its lower electronegativity (1.61) compared to Fe (1.83), Co (1.88), Ni (1.91), and Cu (1.90). This electron transfer potentially optimizes the alloy's electronic structure for improved catalytic activity.
O functional groups, respectively, providing insight into the carbonaceous components of the composite. The N 1s XPS spectrum, featured in Fig. S2b,† delineates two peaks at 398.9 and 401.25 eV, which are indicative of pyridinic-N and graphitic-N species. This observation underscores the successful incorporation of nitrogen into the carbon nanofiber framework, resulting in a nitrogen-doped carbon structure. Such nitrogen doping is instrumental in augmenting the active site inventory of the nanomaterial, thereby enhancing its electrochemical performance.41 Additionally, it endows the carbon fibers with increased surface polarity, which is pivotal for bolstering their affinity for reactant molecules, consequently amplifying their catalytic efficacy.42,43 The XPS spectra in Fig. 2b reveal the Fe 2p region with peaks at 707.4, 709.4, 711.1, 716.65, 720.5, 722.5, and 724.2 eV, corresponding to various iron oxidation states, including Fe0, Fe2+, and Fe3+, along with satellite features. Fig. 2c shows the Co 2p region with peaks at 777.8, 779.4, 792.8, and 794.4 eV, indicative of Co0 and Co2+ states, complemented by satellite peaks at 783.4, 788.7, and 796.6 eV. The Cu 2p region in Fig. 2d exhibits peaks at 932.85, 934.45, 936.45, 952.6, 954.2, and 956.2 eV, reflecting the presence of Cu0, Cu+, and Cu2+ oxidation states. The XPS analysis in Fig. 2e identifies peaks for Ni at 852.7, 854.6, 870, and 871.9 eV, corresponding to Ni0 and Ni2+ states in both 2p3/2 and 2p1/2 orbitals. Fig. 2f shows an Al 2p peak at 74.9 eV, indicating a predominant Al3+ state. This suggests aluminum's role in the high-entropy alloy as an electron donor to other metals, due to its lower electronegativity (1.61) compared to Fe (1.83), Co (1.88), Ni (1.91), and Cu (1.90). This electron transfer potentially optimizes the alloy's electronic structure for improved catalytic activity.
 | ||
| Fig. 2 (a) XRD patterns of FeCoNiCuAl/CNFs, FeCoNiCu/CNFs, FeCoNiAl/CNFs and FeCoNi/CNFs; XPS spectroscopic analysis of (b) Fe 2p, (c) Co 2p, (d) Cu 2p, (e) Ni 2p and (f) Al 2p. | ||
Sensor performance in CV tests and sensing mechanisms
The electrochemical nitrite sensing capabilities of the FeCoNiCuAl/CNF composite were evaluated using a three-electrode setup. The FeCoNiCuAl/CNF-modified glassy carbon electrode (FeCoNiCuAl/CNFs/GCE) served as the working electrode, with a platinum electrode acting as the counter electrode and a saturated calomel electrode (SCE) as the reference. Cyclic voltammetry (CV) measurements were conducted in a 0.1 M phosphate-buffered saline (PBS) solution at pH 7 across various samples. As depicted in Fig. S3,† in the absence of nitrite, all electrodes exhibited negligible peak current signals, but the background current for the GCE modified with the composite material was markedly higher than that of the unmodified GCE. Upon the introduction of 2 mM nitrite into the PBS solution, as illustrated in Fig. 3a, the pristine GCE demonstrated a minimal anodic peak current. In contrast, the FeCoNi/CNFs/GCE, FeCoNiAl/CNFs/GCE, FeCoNiCu/CNFs/GCE, and FeCoNiCuAl/CNFs/GCE electrodes exhibited pronounced anodic peak currents, which increased progressively. This observation indicates that the electrocatalytic oxidation of nitrite to nitrate occurs effectively at the modified electrode surfaces. Compared to the lower anodic peak current of CNFs/GCE, the incorporation of metals has been proven to increase the number of catalytically active sites, thereby enhancing the anodic peak current. Moreover, the FeCoNiCuAl HEA exhibits the most significant enhancement in electrocatalytic performance due to the synergistic effects of multiple metals. This enhancement is attributed to the synergistic interactions among the multiple metal components. CNFs serve as a scaffold, facilitating the growth of HEA nanoparticles (NPs) on their surface. Moreover, the CNFs augment the electron transfer capabilities of the HEA NPs and shield them from corrosion. The integration of HEAs with CNFs not only enhances electron transport, but also enriches the active site centers available for nitrite catalysis.Electrochemical impedance spectroscopy (EIS) is a crucial technique for assessing the electrode's electron transfer kinetics during the electrochemical reaction process. It provides valuable insights into the charge transfer resistance and the overall electrochemical behavior of the modified electrode. Fig. 3b presents the Nyquist plots for the bare GCE, CNFs/GCE, FeCoNi/CNFs/GCE, FeCoNiAl/CNFs/GCE, FeCoNiCu/CNFs/GCE, and FeCoNiCuAl/CNFs/GCE. From Fig. 3b, it is evident that the FeCoNiCuAl/CNFs/GCE exhibits the lowest charge transfer resistance (Rct), which signifies its superior electron transfer capability. This enhanced electron transfer ability can accelerate the rate of electron exchange and substantially decrease the electrode's interfacial resistance, thereby optimizing the electrochemical performance.
To achieve sensitive detection performance, the impact of PBS solutions with varying pH on electrochemical testing was initially investigated. Fig. S4† illustrates that the cyclic voltammetry (CV) measurements yielded the highest peak current in a 0.1 M PBS solution at pH 7. Consequently, this pH 7 PBS solution was selected as the electrolyte for all subsequent electrochemical tests. Fig. 3c depicts the CV curves of the FeCoNiCuAl/CNFs/GCE sensor in response to different concentrations of nitrite. Within the concentration range of 0–20 mM, the peak current increased linearly with the nitrite concentration, with the linear regression equation of Ip (μA) = 9.3758 + 25.069C (mM) (R2 = 0.999) (Fig. 3d). This indicates that the FeCoNiCuAl/CNFs/GCE sensor exhibits a significant current signal response to nitrite over a broad concentration range.
Fig. 3e illustrates the CV curves at various scan rates, showing that as the scan rate (v) increases, the peak current also increases, and the corresponding oxidation potential shifts to higher values. Moreover, a linear relationship between the peak current and the square root of the scan rate (v1/2) is observed, with the equation Ipa (μA) = 10.072 + 6.894v1/2 (mV s−1)1/2 (Fig. 3f). This suggests that the oxidation of nitrite at the sensor is a diffusion-controlled process. The presence of only an anodic oxidation peak in the CV indicates that the reaction is irreversible. Additionally, the anodic peak potential (Epa) exhibits a linear relationship with the logarithm of the scan rate (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v), with a linear regression equation of Epa (V) = 0.0227
v), with a linear regression equation of Epa (V) = 0.0227![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + 0.7003, (R2 = 0.995) (Fig. S5†). The number of electrons transferred during the electrocatalytic reaction can be determined using the formula:
v + 0.7003, (R2 = 0.995) (Fig. S5†). The number of electrons transferred during the electrocatalytic reaction can be determined using the formula:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v. The calculated value of nα for the nitrite oxidation reaction is approximately 1.1 (rounded to 1), indicating that the rate-determining step in the oxidation process of nitrite on the FeCoNiCuAl/CNFs/GCE surface involves a single-electron transfer event. Consequently, the reaction mechanism for the electrocatalytic oxidation can be described by the following equation:
v. The calculated value of nα for the nitrite oxidation reaction is approximately 1.1 (rounded to 1), indicating that the rate-determining step in the oxidation process of nitrite on the FeCoNiCuAl/CNFs/GCE surface involves a single-electron transfer event. Consequently, the reaction mechanism for the electrocatalytic oxidation can be described by the following equation:| 2NO2 + H2O → NO3− + NO2− + 2H+ |
| NO2− + H2O → NO3− + 2H+ + 2e− |
Initially, nitrite ions adsorb onto the FeCoNiCuAl/CNF material, forming an intermediate complex [FeCoNiCuAl/CNFs(NO2−)]. The fibers of FeCoNiCuAl/CNFs, with their high aspect ratio and substantial surface area, coupled with the uniform dispersion of high-entropy alloy nanoparticles (HEA NPs) as active sites on the fiber surface, greatly facilitate the adsorption of nitrite ions onto the material for the subsequent reaction. Subsequently, the adsorbed NO2− on FeCoNiCuAl/CNFs loses an electron under the catalysis of HEA NPs and is oxidized to NO2. The NO2 then undergoes a disproportionation reaction with water to produce NO3− and NO2−, with the NO2− subsequently participating in further reactions to generate NO3−. Thus, the ultimate oxidation product of nitrite on the FeCoNiCuAl/CNF material is nitrate.
Sensor performance with DPV and amperometry
Differential pulse voltammetry (DPV) is a widely utilized electroanalytical technique for the detection of trace substances. The DPV curves of the FeCoNiCuAl/CNFs/GCE sensor for various concentrations of nitrite are depicted in Fig. 4a, with detection ranges of 0.1–5000 μM and 5000–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μM. The linear regression equations for these two ranges are Ipa1 = 0.64683 + 0.01064C (R2 = 0.998) and Ipa2 = 25.81817 + 0.00573C (R2 = 0.997), as shown in Fig. 4b. The dual linear ranges are attributed to the sufficient active sites available on the electrode surface for nitrite adsorption and reaction at low concentrations. However, at higher concentrations, the reaction products begin to occupy the material surface, hindering the oxidation process and thus reducing the sensor's sensitivity. Notably, the sensor still exhibits excellent detection performance in the range of 0–200 μM, as illustrated in Fig. S6.† The calculated sensitivities of the FeCoNiCuAl/CNFs/GCE sensor are 150.6 μA mM−1 cm−2 and 80.1 μA mM−1 cm−2, with the detection limit (LOD) of 0.023 μM (S/N = 3). Fig. S7† displays the DPV curves of five different electrodes modified with the same amounts of FeCoNiCuAl/CNFs and tested with the same concentration of nitrite, showing a coefficient of variation (CV) of 2.24% for the anodic peak current, indicating good reproducibility of the prepared FeCoNiCuAl/CNFs/GCE. Fig. S8† presents the CV curves of five different electrodes, with a CV of only 0.44%, further confirming the material's excellent reproducibility. The sensor's stability was also evaluated, with Fig. S9 and S10† showing the current peak values from DPV and CV measurements of the same concentration of nitrite over several weeks. It was observed that the peak current eventually stabilized, with a decline of 7.4% and 4.0% in the DPV and CV measurements over a 28-day period, respectively, indicating good long-term stability. In practical applications, interference related to the target analyte can affect the sensor's performance, thus assessing the sensor's interference resistance is necessary. First, a selection of common simple substances including NH4Cl, CuCl2, K2SO4, KCl, KNO3, and NaAc were tested. The concentration of the interfering substances was ten times that of the nitrite concentration. Fig. S11† shows that the fluctuation in peak current is minimal, indicating that these substances have little impact on the detection of nitrites. Subsequently, some common electrochemical detection substances, such as ascorbic acid (AA), uric acid (UA), glucose (Glu), as well as heavy metals Pb2+ and Cd2+, were selected as interfering substances to further verify the selectivity of the material. As shown in Fig. S12,† these interfering substances have some impact on DPV testing, slightly altering the peak intensity and position.
000 μM. The linear regression equations for these two ranges are Ipa1 = 0.64683 + 0.01064C (R2 = 0.998) and Ipa2 = 25.81817 + 0.00573C (R2 = 0.997), as shown in Fig. 4b. The dual linear ranges are attributed to the sufficient active sites available on the electrode surface for nitrite adsorption and reaction at low concentrations. However, at higher concentrations, the reaction products begin to occupy the material surface, hindering the oxidation process and thus reducing the sensor's sensitivity. Notably, the sensor still exhibits excellent detection performance in the range of 0–200 μM, as illustrated in Fig. S6.† The calculated sensitivities of the FeCoNiCuAl/CNFs/GCE sensor are 150.6 μA mM−1 cm−2 and 80.1 μA mM−1 cm−2, with the detection limit (LOD) of 0.023 μM (S/N = 3). Fig. S7† displays the DPV curves of five different electrodes modified with the same amounts of FeCoNiCuAl/CNFs and tested with the same concentration of nitrite, showing a coefficient of variation (CV) of 2.24% for the anodic peak current, indicating good reproducibility of the prepared FeCoNiCuAl/CNFs/GCE. Fig. S8† presents the CV curves of five different electrodes, with a CV of only 0.44%, further confirming the material's excellent reproducibility. The sensor's stability was also evaluated, with Fig. S9 and S10† showing the current peak values from DPV and CV measurements of the same concentration of nitrite over several weeks. It was observed that the peak current eventually stabilized, with a decline of 7.4% and 4.0% in the DPV and CV measurements over a 28-day period, respectively, indicating good long-term stability. In practical applications, interference related to the target analyte can affect the sensor's performance, thus assessing the sensor's interference resistance is necessary. First, a selection of common simple substances including NH4Cl, CuCl2, K2SO4, KCl, KNO3, and NaAc were tested. The concentration of the interfering substances was ten times that of the nitrite concentration. Fig. S11† shows that the fluctuation in peak current is minimal, indicating that these substances have little impact on the detection of nitrites. Subsequently, some common electrochemical detection substances, such as ascorbic acid (AA), uric acid (UA), glucose (Glu), as well as heavy metals Pb2+ and Cd2+, were selected as interfering substances to further verify the selectivity of the material. As shown in Fig. S12,† these interfering substances have some impact on DPV testing, slightly altering the peak intensity and position.
Amperometry (I–t) is a commonly employed electrochemical method for determining the concentration of substances by measuring the current generated at the electrode under a specific potential. Initially, the FeCoNiCuAl/CNFs/GCE sensor's suitability for amperometric testing was assessed by examining the chronoamperometric responses at various potentials. Nitrite was added to a 0.1 M PBS solution to achieve a concentration of 2 mM, and different potentials near the peak potential from DPV were selected to test the current–voltage relationship (Fig. S13†). It was evident that the current at 0.76 V was the highest and relatively stable, with a sensor response time of approximately 1.9 seconds, indicating that the FeCoNiCuAl/CNFs/GCE sensor can detect nitrite by amperometry with a rapid response. Subsequent tests were conducted at 0.76 V. Fig. 4c illustrates the relationship between the response current and time upon successive additions of nitrite in the PBS solution, showing a clear gradient. Fig. 4d demonstrates a good linear relationship between the current and nitrite concentration in the range of 1–1000 μM, with the equation Ip (μA) = 0.4747 + 0.02388C (R2 = 0.999), and a distinct linear response is still observed at low nitrite concentrations of 1–10 μM. The calculated sensor sensitivity is 337.84 μA mM−1 cm−2, with a limit of detection (LOD) of 0.12 μM (S/N = 3). Additionally, Fig. 4e presents the I–t curve for nitrite concentrations ranging from 1 to 10 mM, which also exhibits a good linear relationship (Fig. S14†).
The stability of the FeCoNiCuAl/CNFs/GCE in a PBS solution with 2 mM nitrite was tested using amperometry. As shown in Fig. S15,† the current remained stable for over 1800 seconds, with a loss of approximately 6.6%, confirming the sensor's excellent stability. To assess the interference resistance, various interfering substances, including NH4Cl, KNO3, NaAc, KCl, K2SO4, and CuCl2, were added to a PBS solution containing 0.5 mM nitrite at concentrations ten times that of the nitrite. Fig. 4f shows that the response current did not change significantly upon the sequential addition of these interferents. Subsequently, we selected a range of common electrochemical test substances, including ascorbic acid (AA), uric acid (UA), glucose (Glu), as well as the heavy metals Cd2+ and Pb2+, for testing, as depicted in Fig. S16.† Upon the addition of Glu, AA, and UA, there was a slight alteration in the curve, indicating that these three substances have a modest impact on the current–time testing of nitrites. Table 1 compares the performance of the FeCoNiCuAl/CNFs/GCE with recently reported sensors, highlighting its lower detection limit, broader detection range, and the capability to be tested using both DPV and I–t methods, offering significant advantages in detection.
| Electrode | Method | Linear range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|
| OMCF | Amperometry | 1–6000 | 0.1 | 1 |
| MCO/GCE | Amperometry | 5–3000 | 0.95 | 4 |
| FeCl3/MIL-101(Cr)/GCE | Amperometry | 2.5–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
0.21 | 24 |
| FeCoNiCuMn/NCNS/GCE | Amperometry | 2–1000 | 0.14 | 40 |
| CuO@NF-1 | Amperometry | 1–4250 | 28.7 | 44 |
| C–A Zn/Co–Fe PNSs@CC | Amperometry | 1.25–4000 | 0.44 | 45 |
| 3D MoS2/2D C3N4/GCE | DPV | 0.1–1100 | 0.065 | 46 |
| Fe3O4@SiO2/MGCE | DPV | 10–1000 | 3.33 | 47 |
| ZrCu-MOF-818/ILs/GCE | DPV | 6–5030 | 0.148 | 48 |
| AuNPs/GCE | DPV | 10–3800 | 33 | 49 |
| Ag–CeO2@C | DPV | 40–500 | 4.3 | 50 |
| SnO2/Pt/Ti/SiO2/Si | DPV | 10–400 | 1.7 | 51 |
| Au/NiO/rGO/SPCE | DPV | 1–500 | 0.2 | 52 |
| FeCoNiCuAl/CNFs/GCE | DPV | 0.1–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.023 | This work |
| Amperometry | 1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.12 |
Given the superior electrochemical detection performance, selectivity, and stability of the FeCoNiCuAl/CNFs/GCE towards nitrite, we further explored its feasibility for practical applications. We selected tap water, Taihu Lake water (Wuxi City, China), and locally purchased milk as test samples. After sample pretreatment, nitrite was added via the standard addition method to achieve a concentration of 100 μM. Following sample preparation, nitrite was introduced to a final concentration of 100 μM using the standard addition method. Subsequent analysis was conducted using both DPV and I–t techniques. The DPV method yielded concentrations of 97.2 μM, 96.4 μM, and 95.7 μM for the three samples, with recovery rates ranging from 95.7% to 97.2% (Table S1†). The I–t method determined concentrations of 100.4 μM, 93.0 μM, and 92.0 μM, respectively, and the recovery rates spanned from 92.0% to 100.4% (Table S2†). These results demonstrate the reliability and accuracy of the applied analytical methods for nitrite detection in various samples. The high recovery rates and satisfactory practical application capabilities of the FeCoNiCuAl/CNFs/GCE demonstrate its potential for the actual detection of nitrite, confirming its viability in real-world scenarios.
Conclusions
In summary, by utilizing nitrogen-doped carbon fibers as a substrate and employing electrospinning technology, FeCoNiCuAl high-entropy alloy nanoparticles (HEA NPs) were in situ synthesized through the confinement effect of nanofibers, constructing an electrochemical sensor. The multi-metallic synergy, lattice distortion, and high-entropy effect of the high-entropy alloy endow it with a plethora of unique active sites, thereby exhibiting superior electrochemical sensing performance. The FeCoNiCuAl/CNF sensor can electrochemically detect nitrite using both DPV and I–t methods. The DPV detection provides a linear range of 0.1–5000 μM and 5000–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μM, with sensitivities of 150.6 μA mM−1 cm−2 and 80.1 μA mM−1 cm−2 and a detection limit of 0.023 μM (S/N = 3). The I–t detection range is 1–10
000 μM, with sensitivities of 150.6 μA mM−1 cm−2 and 80.1 μA mM−1 cm−2 and a detection limit of 0.023 μM (S/N = 3). The I–t detection range is 1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μM, with a sensitivity of 337.84 μA mM−1 cm−2 and a detection limit of 0.12 μM (S/N = 3). Moreover, this sensor demonstrates excellent anti-interference properties, good stability, and reproducibility, offering feasibility for nitrite detection in practical environments. This work provides a novel approach for designing high-entropy alloy material-based electrochemical sensors, thereby expanding their application scope in the field of electrochemical sensing.
000 μM, with a sensitivity of 337.84 μA mM−1 cm−2 and a detection limit of 0.12 μM (S/N = 3). Moreover, this sensor demonstrates excellent anti-interference properties, good stability, and reproducibility, offering feasibility for nitrite detection in practical environments. This work provides a novel approach for designing high-entropy alloy material-based electrochemical sensors, thereby expanding their application scope in the field of electrochemical sensing.
Data availability
Data will be made available on request. All data used in this article are publicly available.Conflicts of interest
The authors declare no competing financial interest.References
- Z. Zhang, T. Zhang, D. Yang, Y. Yang, X. Zhao, Y. Fan, J. Zhang and J.-H. Yang, J. Environ. Chem. Eng., 2024, 12, 112218 CrossRef CAS.
- H. Wang, X. Wang and J. Cheng, ACS Nano, 2022, 16, 14849–14859 CrossRef CAS PubMed.
- F. Hosseini, M. Majdi, S. Naghshi, F. Sheikhhossein, K. Djafarian and S. Shab-Bidar, Clin. Nutr., 2021, 40, 3073–3081 CrossRef CAS.
- A. Ramesh, P. K. Sahu, S. Duvvuri and C. Subrahmanyam, Inorg. Chem., 2024, 63, 9941–9952 CrossRef CAS PubMed.
- G. Daneshvar Tarigh and F. Shemirani, Talanta, 2014, 128, 354–359 CrossRef CAS PubMed.
- C. Prolo, N. Rios, L. Piacenza, M. N. Álvarez and R. Radi, Free Radicals Biol. Med., 2018, 128, 59–68 CrossRef CAS PubMed.
- P. Singh, M. K. Singh, Y. R. Beg and G. R. Nishad, Talanta, 2019, 191, 364–381 CrossRef CAS PubMed.
- N. Kamilova, Z. Kalaycıoğlu and A. Gölcü, ACS Omega, 2023, 8, 5097–5102 CrossRef CAS PubMed.
- P. Zheng, S. Kasani, X. Shi, A. E. Boryczka, F. Yang, H. Tang, M. Li, W. Zheng, D. E. Elswick and N. Wu, Anal. Chim. Acta, 2018, 1040, 158–165 CrossRef CAS PubMed.
- H. S. Lim, S. J. Lee, E. Choi, S. B. Lee, H. S. Nam and J. K. Lee, Food Chem., 2022, 382, 132280 CrossRef CAS PubMed.
- C. Liu, S. Gao, X. Han, Y. Tian, J. Ma, W. Wang, X.-W. Chen, M.-L. Chen and Y. Zhang, Spectrochim. Acta, Part A, 2024, 317, 124423 CrossRef CAS PubMed.
- B. Li, T. Meng, X. Xie, X. Guo, Q. Li, W. Du, X. Zhang, X. Meng and H. Pang, Mater. Today Chem., 2023, 33, 101747 CrossRef CAS.
- H. Hu, F. Hu, X. Wang and X. Shi, J. Environ. Chem. Eng., 2024, 12, 112858 CrossRef CAS.
- Y. Sun, H. Xu, D. Zhou, C. Xia, W. Liu, A. Cui, Z. Wang, W. Zheng, G. Shan, J. Huang and X. Wang, Small, 2024, 20, 2309357 CrossRef CAS PubMed.
- T. Zhe, R. Li, Q. Wang, D. Shi, F. Li, Y. Liu, S. Liang, X. Sun, Y. Cao and L. Wang, Sens. Actuators, B, 2020, 321, 128452 CrossRef CAS.
- J. Baranwal, B. Barse, G. Gatto, G. Broncova and A. Kumar, Chemosensors, 2022, 10, 363 CrossRef CAS.
- J. F. Tan, A. Anastasi and S. Chandra, Curr. Opin. Electrochem., 2022, 32, 100926 CrossRef CAS.
- Z. Yang, X. Zhou, Y. Yin and W. Fang, Anal. Lett., 2021, 54, 2826–2850 CrossRef CAS.
- H. Song, Z. Cheng, H. Hu, Z. Li, H. Nan, N. Ma, G. Wang, T. He, L. Wang, Y. Lu, X. Wei and L. Huo, Electrochim. Acta, 2024, 498, 144643 CrossRef CAS.
- N. Ibrahim, M. A. Hefnawy, S. A. Fadlallah and S. S. Medany, Food Chem., 2025, 462, 140962 CrossRef CAS PubMed.
- G. Lang, B. Feng, X. Chen, Z. Zhou, Z. Zhao, Q. Deng, Z. Jiang and J. Feng, Talanta, 2024, 275, 126133 CrossRef CAS PubMed.
- R. Ahmad, Abdullah, M. T. Rehman, M. F. AlAjmi, S. Alam, K. S. Bhat, P. Mishra and B.-I. Lee, Nanomaterials, 2024, 14, 706 CrossRef CAS PubMed.
- D. D. Khandagale and S.-F. Wang, Food Chem., 2024, 459, 140353 CrossRef CAS.
- Y. Dong, F. Liu, S. Liu, F. Shen and S. Peng, ACS Appl. Nano Mater., 2024, 7, 16620–16629 CrossRef CAS.
- Z. Cheng, H. Song, X. Zhang, X. Cheng, Y. Xu, H. Zhao, S. Gao and L. Huo, Sens. Actuators, B, 2022, 355, 131313 CrossRef CAS.
- H. Liu, K. Guo, J. Lv, Y. Gao, C. Duan, L. Deng and Z. Zhu, Sens. Actuators, B, 2017, 238, 249–256 CrossRef CAS.
- N. Jaiswal, I. Tiwari, C. W. Foster and C. E. Banks, Electrochim. Acta, 2017, 227, 255–266 CrossRef CAS.
- X. Li, S. Hou, C. Xie and G. Fan, Int. J. Electrochem. Sci., 2018, 13, 315–323 CrossRef CAS.
- J.-T. Ren, L. Chen, H.-Y. Wang and Z.-Y. Yuan, Chem. Soc. Rev., 2023, 52, 8319–8373 RSC.
- H. Li, J. Lai, Z. Li and L. Wang, Adv. Funct. Mater., 2021, 31, 2106715 CrossRef CAS.
- E. P. George, D. Raabe and R. O. Ritchie, Nat. Rev. Mater., 2019, 4, 515–534 CrossRef CAS.
- Y. F. Ye, Q. Wang, J. Lu, C. T. Liu and Y. Yang, Mater. Today, 2016, 19, 349–362 CrossRef CAS.
- V. Verma, C. H. Belcher, D. Apelian and E. J. Lavernia, Prog. Mater. Sci., 2024, 142, 101245 CrossRef CAS.
- J. Liang, G. Cao, M. Zeng and L. Fu, Chem. Soc. Rev., 2024, 53, 6021–6041 RSC.
- H. Huang, J. Zhao, H. Guo, B. Weng, H. Zhang, R. A. Saha, M. Zhang, F. Lai, Y. Zhou, R.-Z. Juan, P.-C. Chen, S. Wang, J. A. Steele, F. Zhong, T. Liu, J. Hofkens, Y.-M. Zheng, J. Long and M. B. J. Roeffaers, Adv. Mater., 2024, 36, 2313209 CrossRef CAS PubMed.
- H. Li, H. Huang, Y. Chen, F. Lai, H. Fu, L. Zhang, N. Zhang, S. Bai and T. Liu, Adv. Mater., 2023, 35, 2209242 CrossRef CAS.
- C. Yu, X.-w. Wang, W.-x. He, Z.-y. Zheng, X.-j. Dang and Y.-f. Zhang, Surf. Interfaces, 2024, 46, 104084 CrossRef CAS.
- Y.-f. Yu, W. Zhang, F.-l. Sun, Q.-j. Fang, J.-k. Pan, W.-x. Chen and G.-l. Zhuang, Mol. Catal., 2022, 519, 112141 CrossRef CAS.
- M. Li, C. Huang, H. Yang, Y. Wang, X. Song, T. Cheng, J. Jiang, Y. Lu, M. Liu, Q. Yuan, Z. Ye, Z. Hu and H. Huang, ACS Nano, 2023, 17, 13659–13671 CrossRef CAS PubMed.
- P. Gao, S. Zhao, X. Qu, X. Qian, F. Duan, S. Lu, H. Zhu and M. Du, Electrochim. Acta, 2022, 432, 141160 CrossRef CAS.
- L.-L. Li, Y. Zhao, L.-J. Pan, J.-B. Xu and Y. Shi, Rare Met., 2024, 43, 2184–2192 CrossRef CAS.
- T. Li, X. An and D. Fu, Energy Fuels, 2023, 37, 8160–8179 CrossRef CAS.
- Y.-Y. Wang, T. Sun, J. Wang, A.-J. Xu and B. Xue, Microchem. J., 2024, 197, 109830 CrossRef CAS.
- J. Deng, X. Ren, H. Yang, T. Qiu, Z. Wang, Y. Zhang, C. Miao, O. Fontaine, Y. Zhu and S. Chen, Microchem. J., 2024, 200, 110337 CrossRef CAS.
- T. Zhe, S. Shen, F. Li, R. Li, M. Li, K. Ma, K. Xu, P. Jia and L. Wang, Food Chem., 2023, 402, 134228 CrossRef CAS PubMed.
- L. Wang, Z. Fan, F. Yue, S. Zhang, S. Qin, C. Luo, L. Pang, J. Zhao, J. Du, B. Jin and H. Zhang, Food Chem., 2024, 430, 137027 CrossRef CAS PubMed.
- M. Zhang, Y. Yang and W. Guo, Food Chem., 2024, 430, 137004 CrossRef CAS PubMed.
- L. Zhang, Y. Han, M. Sun and S. Li, Food Chem., 2024, 456, 140023 CrossRef CAS PubMed.
- H. Shi, L. Fu, F. Chen, S. Zhao and G. Lai, Environ. Res., 2022, 209, 112747 CrossRef CAS PubMed.
- K. Zhao, Z. Zhang, Y. Zhou and X. Lin, Molecules, 2024, 29, 2644 CrossRef CAS PubMed.
- C. Lete, M. Chelu, M. Marin, S. Mihaiu, S. Preda, M. Anastasescu, J. M. Calderón-Moreno, S. Dinulescu, C. Moldovan and M. Gartner, Sens. Actuators, B, 2020, 316, 128102 CrossRef CAS.
- K. Xu, Q. Chen, Y. Zhao, C. Ge, S. Lin and J. Liao, Sens. Actuators, B, 2020, 319, 128221 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4an01246a |
| This journal is © The Royal Society of Chemistry 2025 |