Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synchrotron-based infrared microspectroscopy unveils the biomolecular response of healthy and tumour cell lines to neon minibeam radiation therapy†
R.
González-Vegas
 a,
O.
Seksek
b,
A.
Bertho
a,
O.
Seksek
b,
A.
Bertho
 cd,
J.
Bergs
e,
R.
Hirayama
f,
T.
Inaniwa
fg,
N.
Matsufuji
fg,
T.
Shimokawa
cd,
J.
Bergs
e,
R.
Hirayama
f,
T.
Inaniwa
fg,
N.
Matsufuji
fg,
T.
Shimokawa
 fg,
Y.
Prezado
cdhi,
I.
Yousef
fg,
Y.
Prezado
cdhi,
I.
Yousef
 j and
I.
Martínez-Rovira
j and
I.
Martínez-Rovira
 *a
*a
aPhysics Department, Universitat Autònoma de Barcelona (UAB), 08193 Cerdanyola del Vallès, Barcelona, Spain. E-mail: Immaculada.Martinez@uab.cat
bIJCLab, French National Centre for Scientific Research, 91450 Orsay, France
cInstitut Curie, Université PSL, CNRS UMR3347, Inserm U1021, Signalisation Radiobiologie et Cancer, 91400 Orsay, France
dUniversité Paris-Saclay, CNRS UMR3347, Inserm U1021, Signalisation Radiobiologie et Cancer, 91400 Orsay, France
eRadiology Department, Charité-Universitätsmedizin Berlin, 10117 Berlin, Germany
fDepartment of Charged Particle Therapy Research, Institute for Quantum Medical Science, National Institutes for Quantum Science and Technology (QST), 4-9-1 Anagawa, Inage-ku, Chiba-shi 263-8555, Japan
gDepartment of Accelerator and Medical Physics, QST, 4-9-1 Anagawa, Inage-ku, Chiba-shi 263-8555, Japan
hNew Approaches in Radiotherapy Lab, Center for Research in Molecular Medicine and Chronic Diseases (CIMUS), Instituto de Investigación Sanitaria de Santiago de Compostela (IDIS), University of Santiago de Compostela, Santiago de Compostela, A Coruña 15706, Spain
iOportunius Program, Galician Agency of Innovation (GAIN), Xunta de Galicia, Santiago de Compostela, A Coruña, Spain
jMIRAS Beamline, ALBA Synchrotron, 08209 Cerdanyola del Vallès, Barcelona, Spain
First published on 13th December 2024
Abstract
Radioresistant tumours remain complex to manage with current radiotherapy (RT) techniques. Heavy ion beams were proposed for their treatment given their advantageous radiobiological properties. However, previous studies with patients resulted in serious adverse effects in the surrounding healthy tissues. Heavy ion RT could therefore benefit from the tissue-sparing effects of minibeam radiation therapy (MBRT). To investigate the potential of this combination, here we assessed the biochemical response to neon MBRT (NeMBRT) through synchrotron-based Fourier transform infrared microspectroscopy (SR-FTIRM). Healthy (BJ) and tumour (B16-F10) cell lines were subjected to seamless (broad beam) neon RT (NeBB) and NeMBRT at HIMAC. SR-FTIRM measurements were conducted at the MIRAS beamline of ALBA Synchrotron. Principal component analysis (PCA) permitted to assess the biochemical effects after the irradiations and 24 hours post-irradiation for the different RT modalities and doses. For the healthy cells, NeMBRT resulted in the most dissimilar spectral signatures from non-irradiated cells early after irradiations, mainly due to protein conformational modifications. Nevertheless, most of the damage appeared to recover one day post-RT; conversely, protein- and nucleic acid-related IR bands were strongly affected by NeBB 24 hours after treatment, suggesting superior oxidative damage and nucleic acid degradation. Tumour cells appeared to be less sensitive to NeBB than to NeMBRT shortly after RT. Still, after one day, both NeBB and the high-dose NeMBRT regions yielded important spectral modifications, suggestive of cell death processes, protein oxidation or oxidative stress. Lipid-associated spectral changes, especially due to the NeBB and NeMBRT peak groups for the tumour cell line, were consistent with reactive oxygen species attacks.
1 Introduction
Cancer is one of the leading causes of death in the world. Accordingly, radiotherapy (RT) has established itself as one of the most important therapeutic options against these diseases: approximately 50–70% of cancer patients will receive RT treatment during the duration of their disease. The last decades have seen significant technological advances in RT, related to greater precision in tumour delineation, improved dose conformation and reduced toxicities to normal tissues, all of which have led to better treatment outcomes. Still, the management of some cancer variants remains difficult to address with conventional RT techniques. An example would be the treatment of radioresistant tumours, which are generally associated with a poor prognosis.For that matter, the use of heavy ion beams (e.g. carbon or neon beams) was proposed to cope with these challenging scenarios. They exhibit a superior biological effectiveness and a lower oxygen enhancement ratio with respect to other beams, suggesting heavy ions to be adequate for the treatment of radioresistant malignancies. In the period between 1977 and 1992, a few hundred cancer patients (including disease types such as malignant gliomas, salivary gland carcinomas, bone sarcomas, and others) received neon RT, in some cases combined with photon or helium RT.1,2 However, some patients experienced severe adverse effects, which led to the cessation of the use of neon beams in a clinical setting.
The quest for novel RT modalities that widen the therapeutic window has resulted in a paradigm shift from the traditional approaches, leading to changes in temporal schemes, dose rates or spatial dose distributions. Concerning the latter point, minibeam radiation therapy (MBRT) has emerged as a promising alternative to conventional RT. Firstly proposed in 2006,3 MBRT consists in a spatial dose modulation, employing arrays of beamlets, 0.5–1.0 mm wide and separated by a center-to-center (c-t-c) distance of 1–4 mm. These beam characteristics have been shown to increase normal tissue dose tolerances using several types of beams.4–6 Regarding tumour control, MBRT has proven to be, at least, as equally effective as conventional RT.6–8
In this context, a promising reinvented use of neon beams could be realised in combination with MBRT (NeMBRT). Previous Monte Carlo studies evaluated the dosimetric feasibility of this technique.9,10 Their findings showed that NeMBRT could provide a high peak-to-valley dose ratio (PVDR) with low valley doses and reduced linear energy transfer (LET) values in normal tissues (favourable for their sparing). These results guided a subsequent biological study in which the normal tissue response to seamless (broad beam) neon RT (NeBB) and NeMBRT was evaluated.11 Severe damage was observed in mice subjected to NeBB, including cutaneous ulceration and epidermal necrosis. On the contrary, NeMBRT-treated mice only presented mild hair loss and erythema without ulceration. Thus, authors concluded that NeMBRT offered a gain in healthy tissue preservation compared to NeBB, regardless of the high peak doses.
Despite these promising results, the full biological basis of NeMBRT (and MBRT in general) has yet to be fully disentangled. The main mechanisms suggested to explain the response of both healthy and tumour tissues to these novel RT approaches are: differential vascular effects, particularly the great impact of spatial dose fractionation on immature vessels;12 the migration of stem cells from valley to peak dose regions in normal tissues in favour of repair processes;13 the immune system activation as antitumour response;14 cell signalling mechanisms (e.g. bystander/cohort and abscopal effects);15,16 and the direct and indirect effects of reactive oxygen species (ROS).17
One possible approach to grasp the remaining biochemical mechanisms underlying MBRT would be having recourse to synchrotron radiation-based Fourier transform infrared microspectroscopy (SR-FTIRM). This non-destructive modality is based on the vibrational excitation of molecular bonds due to their interaction with infrared (IR) light. SR-FTIRM is an excellent tool for interrogating biological materials and studying their biochemical structure and modifications without altering them.18 Also, the use of synchrotron IR light provides an excellent signal-to-noise ratio with the high spatial resolution required in single-cell analyses. SR-FTIRM has proven useful to uncover the biomolecular response to innovative RT modalities, such as proton therapy,19,20 RT combined with nanoparticles,21–23 X-ray microbeam RT,24 proton MBRT (pMBRT)25 or FLASH-RT.26
Therefore, and given that the alliance of heavy ions with MBRT is a promising alternative to current therapeutic practices for certain treatments, the present SR-FTIRM study is aimed at providing new insights into the biomolecular rationale that underlies NeMBRT. To this end, both healthy and tumour cell lines subjected to NeBB and NeMBRT were evaluated through SR-FTIRM at different doses and post-irradiation time-points.
2 Experimental section
2.1 Sample preparation & irradiations
BJ human foreskin fibroblasts (ATCC®-CRL-2522™) and B16-F10 mouse skin melanoma (ATCC®-CRL-6475™) cell lines were purchased from ATCC. Both cell lines were cultured in high glucose DMEM medium (Gibco™, LifeTechnologies SAS, Courtaboeuf, France) supplemented with 10% fetal calf serum, 1% penicillin–streptomycin (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units per mL each), 1 mM GlutaMAX™, 1 mM sodium pyruvate and 10 mM HEPES. The conditions of the incubation chamber were set to 37 °C, 95% humidity and 5% CO2. Samples were directly grown onto 0.5 mm-thick IR transparent CaF2 coverglasses (Crystran Ltd) attached to the slides of Thermo Scientific™Nunc™Lab-Tek™ flasks; 1 mL of cell suspension was seeded in each coverglass (at a concentration of 5 × 104 cells per mL) so as to obtain a 75–80% confluence rate on the day of irradiations.
000 units per mL each), 1 mM GlutaMAX™, 1 mM sodium pyruvate and 10 mM HEPES. The conditions of the incubation chamber were set to 37 °C, 95% humidity and 5% CO2. Samples were directly grown onto 0.5 mm-thick IR transparent CaF2 coverglasses (Crystran Ltd) attached to the slides of Thermo Scientific™Nunc™Lab-Tek™ flasks; 1 mL of cell suspension was seeded in each coverglass (at a concentration of 5 × 104 cells per mL) so as to obtain a 75–80% confluence rate on the day of irradiations.
RT experiments were carried out at the Heavy-Ion Medical Accelerator in Chiba (HIMAC, National Institutes for Quantum Science and Technology, Chiba, Japan). At HIMAC, heavy ion species ranging from helium to argon can be accelerated for their medical use.27 Neon beams of 230 MeV/u (energy at the exit of the accelerator) and LET of 45 keV/μm (value at the target position28) were used to perform NeBB and NeMBRT irradiations, delivering mean (physical) doses of 2, 4 and 8 Gy. In spatially fractionated RT, the previous values refer to the mean doses of NeMBRT lateral profiles. The specific peak and valley doses were (respectively): 6.9 ± 0.7 Gy and 0.11 ± 0.01 Gy (2 Gy mean dose), 16 ± 2 Gy and 0.20 ± 0.02 Gy (4 Gy mean dose), and 29 ± 3 Gy and 0.35 ± 0.04 Gy (8 Gy mean dose). Minibeams were generated by means of a divergent 10 cm-thick multislit brass collimator, attached at the exit of the accelerator (Fig. 1). Slits were 700 μm-wide and they were separated by a c-t-c distance of 3500 μm. Dosimetry was accomplished by using Gafchromic™EBT3 films attached to the IR slides containing the samples, which allowed to guarantee irradiation quality, to localize the NeBB field, and to distinguish between NeMBRT peak and valley regions (also allowing to select the cells corresponding to these groups during SR-FTIRM measurements, see Fig. 1).
The cell lines were fixated at two different time-points post-RT: following irradiations (henceforth labelled as 0h) and 24 hours later (henceforth labelled as 24h). Early after RT, medium of one half of the flasks containing the samples was removed and slides were rinsed twice with phosphate-buffered saline. Subsequently, samples were incubated for 1 hour at room temperature with 10% formalin neutral buffered solution (Sigma-Aldrich). Then, any residual phosphate ions were washed out after 3 rounds of ultrapure water rinsing, and samples were dried out at room temperature for posterior SR-FTIRM analyses. The other half of the flasks were incubated for one day, after which the same procedure as described above was applied for their fixation.
2.2 SR-FTIRM measurements
Samples subjected to both RT treatments were submitted to SR-FTIRM measurements at the MIRAS beamline of ALBA Synchrotron. The end-station is equipped with a Hyperion 3000 microscope coupled to a Vertex 70 spectrometer (Bruker Optics GmbH, Germany). A mercury cadmium telluride detector, cooled with liquid nitrogen, enabled the acquisition of the IR data. Single masking aperture sizes (IR beam size) were set to 16 × 16 μm2 (BJ cell line) and 9 × 9 μm2 (B16-F10 cell line) for single cell measurements; the different aperture sizes were chosen due to the size differences between the two cell lines. Over 125 cells were randomly selected from each sample and irradiation condition (Control, BB, MBpeak and MBvalley). IR spectra of the cells were acquired using the transmission measurement mode of the microscope. Single cell IR measurements were collected in the 3800–1000 cm−1 mid-IR range, with a spectral resolution of 4 cm−1; 256 scans coupled with 40 kHz scanning velocity lead to an exposure time of 1 min per spectrum. Background spectra were collected every 5 samples to compensate for varying ambient conditions in the beamline during the measurements, under the same acquisition parameters as previously described.2.3 Data analysis
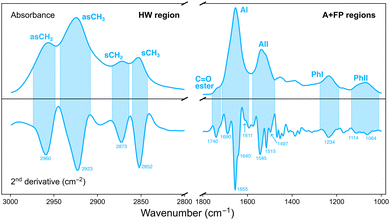
Analysis of the IR data was performed with the open source software Quasar (version 1.9).29 Principal component analysis (PCA) was used as an unsupervised, multivariate method to investigate the effect of the various irradiation configurations on samples according to their different IR biochemical signatures. PCA was performed in two separate spectral regions (Fig. 2):• Amides and fingerprint (A + FP, 1800–1000 cm−1). The 1800–1400 cm−1 range originates from vibrational modes of proteins and peptides,30 and is composed of two main IR bands: the 1710–1590 cm−1 spectral range is associated to the Amide I (AI) band and arises from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations and, to a lesser extent, from out-of-phase CN stretching, CCN deformation and NH in-plane bending; the 1585–1478 cm−1 spectral region is assigned to the Amide II (AII) band, arising from in-plane NH bending and CN stretching vibrations. The low-frequency region (1350–1000 cm−1) results from carbohydrates and sugar-phosphate vibrations, providing information on the conformations of the nucleic acids backbone.31
O stretching vibrations and, to a lesser extent, from out-of-phase CN stretching, CCN deformation and NH in-plane bending; the 1585–1478 cm−1 spectral region is assigned to the Amide II (AII) band, arising from in-plane NH bending and CN stretching vibrations. The low-frequency region (1350–1000 cm−1) results from carbohydrates and sugar-phosphate vibrations, providing information on the conformations of the nucleic acids backbone.31
• Higher wavenumber (HW, 3000–2800 cm−1). Arises from stretching vibrations of C–H groups (methyl and methylene), present in the hydrocarbon acyl chains of membrane lipids.32
Multivariate analysis in each region was performed on second-derivative, vector normalised IR spectra. Differentiation was accomplished by using a Savitzky–Golay filter (9 points window for the HW region; 19–25 points window for the A + FP regions) and made it possible to overcome baseline artifacts in the data, as well as to resolve overlapping IR bands.25,33 Prior to PCA, second-derivative IR spectra were unit vector normalised.
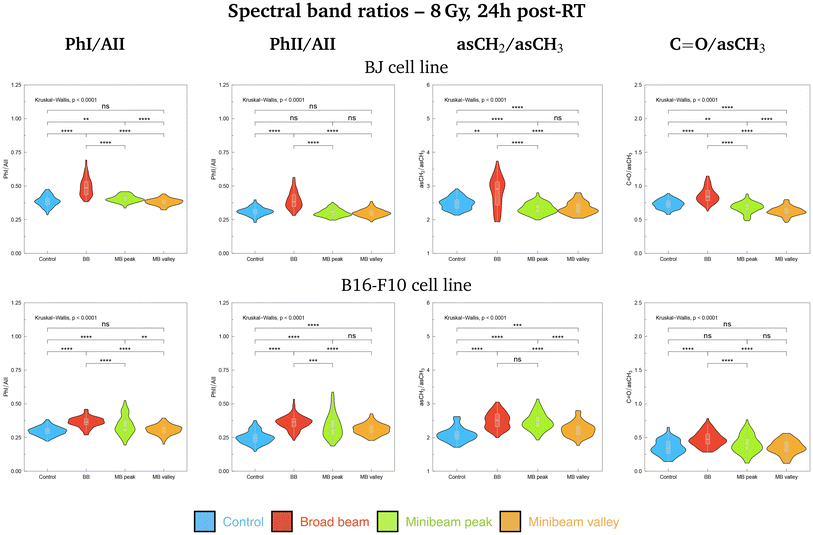
Additionally, violin plots showing the probability density of several spectral band ratios of interest were generated for both cell lines, used as markers of biochemical modifications: the Phosphate I (1280–1185 cm−1) to Amide II (1585–1478 cm−1), PhI/AII; the Phosphate II (1140–1010 cm−1) to Amide II (1585–1478 cm−1), PhII/AII; the asymmetric methylene (2948–2900 cm−1) to asymmetric methyl (2978–2947 cm−1), asCH2/asCH3; and the carbonyl ester (1760–1725 cm−1) to asymmetric methyl (2978–2947 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/asCH3. The Kruskal–Wallis test was employed to assess the global significance between groups and, if differences were statistically significant, a Dunn test with the Bonferroni adjustment was used to perform pairwise comparisons. Statistical analysis was conducted with the software R (version 4.3.2).34
O/asCH3. The Kruskal–Wallis test was employed to assess the global significance between groups and, if differences were statistically significant, a Dunn test with the Bonferroni adjustment was used to perform pairwise comparisons. Statistical analysis was conducted with the software R (version 4.3.2).34
3 Results & discussion
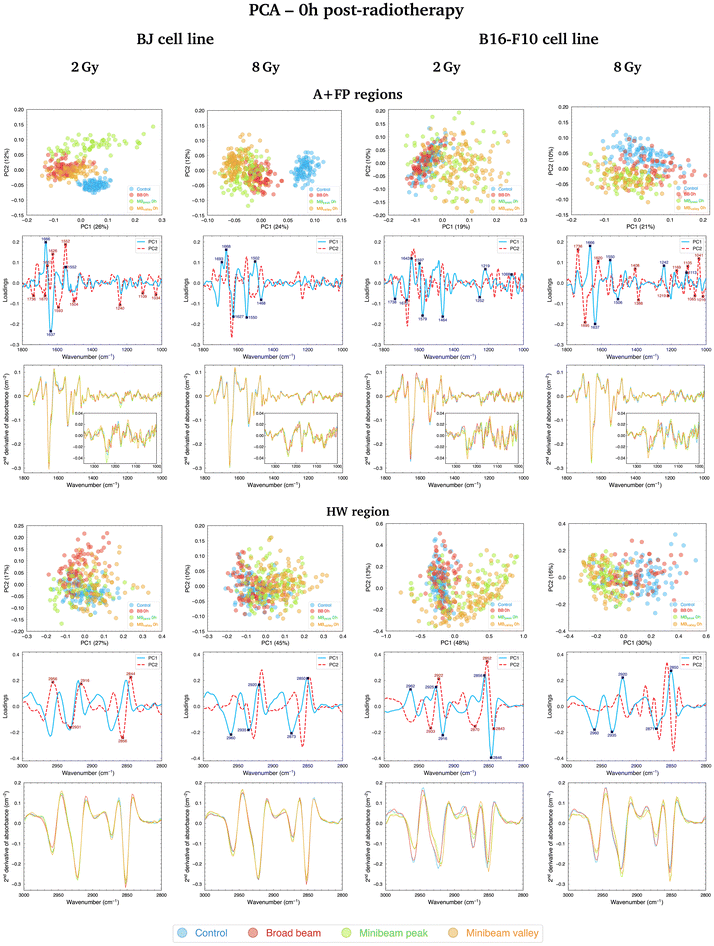
PCA results for samples fixated at 0h and 24h post-irradiations are presented in sections 3.1 and 3.2, respectively. Results for the A + FP and HW spectral regions are reported separately. Discussion covers both the BJ and B16-F10 cell lines irradiated with the three doses studied. Data for the intermediate dose of 4 Gy (Fig. S1†), as well as the figures with the average absorbance spectra for all irradiation configurations (Fig. S2 and S3†) are included in the ESI.†3.1 PCA at 0h post-RT
Regarding the changes in the FP region, the main contributions to data separation are encountered in the 1250–1220 cm−1 spectral range, arising from asymmetric stretching vibrations of the PO2− band, named PhI (1280–1185 cm−1).38 Changes in this band were dose-dependent and primarily contributed to differentiate Control and RT-treated samples, but also helped to separate NeBB and NeMBRT groups. PhI modifications are considered indicative of RT-induced DNA damage39 and could be related to strand cleavage and chromatin fragmentation due to DNA breakages,19 DNA condensation and degradation,40 or oxidative stress.41 Additional bands in the low-frequency region also contribute to data splitting, with the main ones being the A-form DNA (1180–1160 cm−1)38 and the 1140–1010 cm−1 spectral range, named PhII.38 The latter region mainly arises from PO2− symmetric stretching modes and C–O furanose vibrations. Modifications of these bands might be indicative of DNA-associated alterations upon RT modalities, such as rearrangements of nucleic acids structures,42 increased DNA breakages,43 and base stacking and pairing alterations.44 These changes are mainly associated to the BB and MBpeak groups.
PCA results in the A + FP regions for the B16-F10 cell line fixated at 0h post-treatment are shown in Fig. 3 (top, right; 2 Gy and 8 Gy) and Fig. S1† (top, left; 4 Gy). NeBB remains proximal to Control, while NeMBRT clusters depart from these groups. For the 2 Gy, the main peaks explaining the separation of NeMBRT clusters are assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl ester band (1760–1725 cm−1, arising from stretching vibrations of cellular phospholipids41), substructures of AI and AII, including the 1600–1589 cm−1 spectral region arising from NH2 vibrations of amine groups,37 and the CH2 bending modes of the acyl chains of lipids (peak near 1464 cm−1),30 as well as to several contributions in the FP region from the PhI and PhII bands. The peak near 1579 cm−1 is assigned to in-plane bending vibrations of the NH2 group of AII and the ring of the adenine and cytosine, and could reflect a degree of damage to these base pairs.39 Most of the intra-group variability of the MBpeak and MBvalley groups comes from differences in the β-turn, α-helix and β-sheet substructures of AI. Regarding the intermediate and high doses, most of the observed separation of RT-treated groups from Control is due to contributions from the anti-parallel β-sheet (1705–1685 cm−1)37 and the β-turn of AI, the α-helix of AII, the PhI band (mainly from contributions of the B-form DNA in the 1225–1220 cm−1 range38), the symmetric phosphodiester stretching of the DNA backbone and the C–O furanose. These spectral features, particularly those in the FP region, mainly contribute to the separation of NeMBRT peak and valley groups from the other clusters. Additionally, the peaks in the 1410–1385 cm−1 spectral region arise from complex vibrational modes of the COO− and CH3 groups present in the fatty acids, proteins and amino acids;36,45 alterations in this region are associated with the effects of the MBpeak and MBvalley groups.
O carbonyl ester band (1760–1725 cm−1, arising from stretching vibrations of cellular phospholipids41), substructures of AI and AII, including the 1600–1589 cm−1 spectral region arising from NH2 vibrations of amine groups,37 and the CH2 bending modes of the acyl chains of lipids (peak near 1464 cm−1),30 as well as to several contributions in the FP region from the PhI and PhII bands. The peak near 1579 cm−1 is assigned to in-plane bending vibrations of the NH2 group of AII and the ring of the adenine and cytosine, and could reflect a degree of damage to these base pairs.39 Most of the intra-group variability of the MBpeak and MBvalley groups comes from differences in the β-turn, α-helix and β-sheet substructures of AI. Regarding the intermediate and high doses, most of the observed separation of RT-treated groups from Control is due to contributions from the anti-parallel β-sheet (1705–1685 cm−1)37 and the β-turn of AI, the α-helix of AII, the PhI band (mainly from contributions of the B-form DNA in the 1225–1220 cm−1 range38), the symmetric phosphodiester stretching of the DNA backbone and the C–O furanose. These spectral features, particularly those in the FP region, mainly contribute to the separation of NeMBRT peak and valley groups from the other clusters. Additionally, the peaks in the 1410–1385 cm−1 spectral region arise from complex vibrational modes of the COO− and CH3 groups present in the fatty acids, proteins and amino acids;36,45 alterations in this region are associated with the effects of the MBpeak and MBvalley groups.
Comparison of cluster separation for the two cell lines revealed some key differences in the response to treatment modalities at this time-point. The healthy cell line resulted sensitive to both types of treatment: both NeBB and NeMBRT clusters showed clearly distinct from Control group. Also, some differences between MBpeak and MBvalley were noticeable at this time-point. On the contrary, the tumour cell line seemed less sensitive to NeBB, with this group being quite close to Control for the three doses, whereas NeMBRT groups were already well distinct from the Control cluster as for the healthy cell line. The relative contribution of the FP spectral region (with respect to the amides region) to data segregation also seemed to be greater for the tumour cell line than for the healthy one.
PCA results for the B16-F10 cell line in the HW region early after irradiations are depicted in Fig. 3 (bottom, right; 2 Gy and 8 Gy) and Fig. S1† (bottom, left; 4 Gy). The treatment effects are in the same line for the three doses: Control and NeBB groups are proximate to each other, while NeMBRT clusters spread away from them. Inspection of the loadings indicates that the main bands modified due to NeMBRT are the asCH3, sCH3 and sCH2. Specifically, the effects of NeMBRT on the methyl and methylene modes resulted in an intensity increase of the asCH2/asCH3 spectral band ratio (data not shown), and was already observed in cells with longer acyl chain lengths as a consequence of oxidative stress processes.32,46
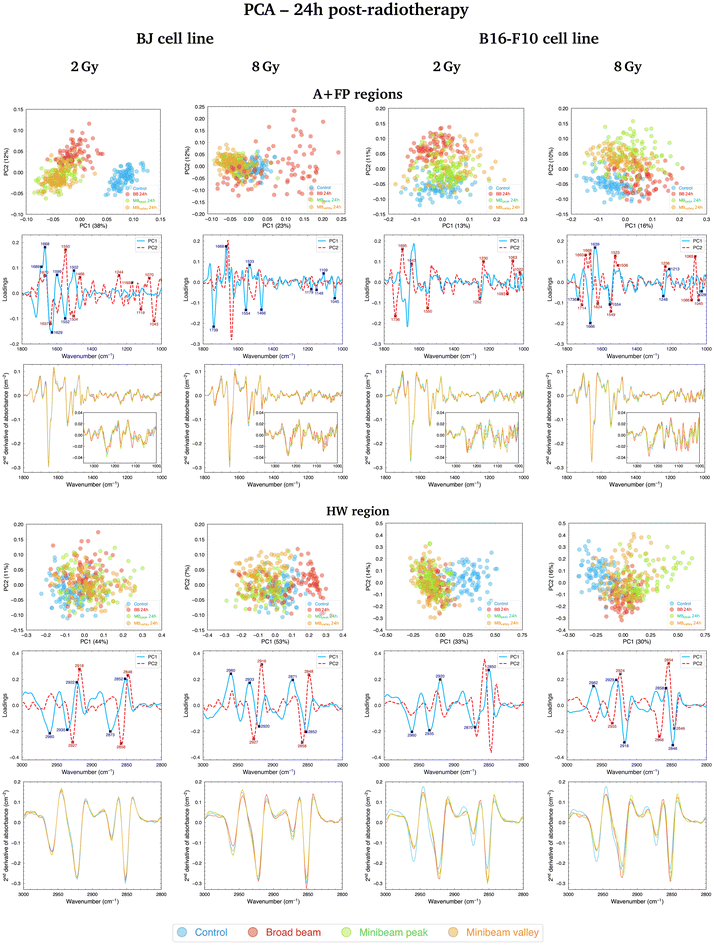
3.2 PCA at 24h post-RT
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl ester spectral range is particularly relevant in the separation of NeBB for the highest dose. Alterations of this band have been previously identified as hallmarks of cell death and oxidative damage,47 supported by the intensity increase of the C
O carbonyl ester spectral range is particularly relevant in the separation of NeBB for the highest dose. Alterations of this band have been previously identified as hallmarks of cell death and oxidative damage,47 supported by the intensity increase of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/asCH3 spectral band ratio41 for NeBB (Fig. 5, top). On the other hand, the MBpeak and MBvalley groups generally resulted in a decrease of the C
O/asCH3 spectral band ratio41 for NeBB (Fig. 5, top). On the other hand, the MBpeak and MBvalley groups generally resulted in a decrease of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/asCH3 ratio with respect to Control. Other bands, associated to the proteins, are also taking an important role in data splitting: the β-turn, α-helix, and β-sheet substructures of AI. These bands mainly contribute to segregate NeBB from the rest of the groups for the intermediate and high doses. Changes in cellular proteins are often associated with modifications in their distribution during cell death, oxidative stress, or to denaturation/oxidation of existing proteins,24,47 also in agreement with the observed alterations of the C
O/asCH3 ratio with respect to Control. Other bands, associated to the proteins, are also taking an important role in data splitting: the β-turn, α-helix, and β-sheet substructures of AI. These bands mainly contribute to segregate NeBB from the rest of the groups for the intermediate and high doses. Changes in cellular proteins are often associated with modifications in their distribution during cell death, oxidative stress, or to denaturation/oxidation of existing proteins,24,47 also in agreement with the observed alterations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl ester group due to NeBB. Additional bands were also particularly affected by NeBB, such as the α-helix of AII or the CH2 bending modes; modifications of the latter band have been associated with an altered conformation of lipid chain packing.24
O carbonyl ester group due to NeBB. Additional bands were also particularly affected by NeBB, such as the α-helix of AII or the CH2 bending modes; modifications of the latter band have been associated with an altered conformation of lipid chain packing.24
Considering the modifications in the FP region, important contributions to data separation are encountered. For 2 Gy, conformational changes in the 1250–1040 cm−1 spectral range resulted in a separation between NeBB and NeMBRT clusters, with contributions from bands associated to the A-form DNA (peaks near 1240 cm−1 and 1170 cm−1), the ribose stretching (peak near 1115 cm−1) and the C–O stretching modes of the nucleic acids backbone and furanose (1070–1035 cm−1).38,39 The mentioned bands primarily contribute to the separation of the NeBB group; NeMBRT peak and valley IR signatures in this low-frequency region were closer to those of the Control. These band modifications reflect a degree of conformational changes and rearrangements in the structure of the nucleic acids after NeBB treatment, and might have resulted from DNA degradation or condensation,40 base alterations in the RNA,44 or even oxidative damage.41 Loadings for the 4 Gy and 8 Gy also indicate a contribution from the 1250–1000 cm−1 spectral region, mainly contributing to NeBB segregation from the other clusters. Interestingly, NeBB spectra exhibit a high intensity increase of the PhI/AII and PhII/AII spectral band ratios for the 8 Gy irradiations (Fig. 5, top), previously associated to increased DNA single- and double-strand breaks,19 or to oxidative stress.41
Cluster separation after PCA followed clear trends for BJ cells fixated at the two analysed time-points. Early after irradiations, NeMBRT-treated cells showed the most dissimilar IR signatures compared to the Control group. Conversely, the analysis at 24h post-RT revealed that the irradiation-induced damage due to NeBB appeared to be more persistent than that due to NeMBRT; this was reflected in the MBpeak and MBvalley groups being closer to the non-irradiated cells. Also, differences between MBpeak and MBvalley dose regions one day post-treatment were much more less noticeable than early after RT. At 24h, certain spectral markers became more important than at 0h for the segregation of NeBB from the other groups: the 1045–1035 cm−1 spectral region (C–O stretching modes of nucleic acids backbone and furanose); the band near 1637 cm−1 assigned to the AI β-sheet; and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl ester band near 1740 cm−1, particularly for the highest dose. These spectral signatures might reflect unrecovered oxidative damage due to NeBB one day after irradiations;41,47 conversely, NeMBRT clusters being close to Control at 24h post-treatment reflect that a certain degree of the radiation-induced damage has already been recovered.
O carbonyl ester band near 1740 cm−1, particularly for the highest dose. These spectral signatures might reflect unrecovered oxidative damage due to NeBB one day after irradiations;41,47 conversely, NeMBRT clusters being close to Control at 24h post-treatment reflect that a certain degree of the radiation-induced damage has already been recovered.
Fig. 4 (top, right; 2 Gy and 8 Gy) and Fig. S1† (top, right; 4 Gy) show the PCA for B16-F10 cells in the A + FP regions, where the three RT groups separate from the non-irradiated sample; it is noteworthy that for 8 Gy irradiations there is minimal overlap between groups, with MBpeak being less proximate to Control than the rest of configurations. The main spectral bands contributing to group separation are assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl ester, substructures of the AI and AII, the PhI and PhII bands, and the C–O furanose vibrations of Z-form DNA (1030–1015 cm−1).38 Modifications of the C
O carbonyl ester, substructures of the AI and AII, the PhI and PhII bands, and the C–O furanose vibrations of Z-form DNA (1030–1015 cm−1).38 Modifications of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl ester band contribute to the separation of the irradiated groups from Control, especially of the NeBB group, suggesting superior oxidative damage;47 again, an increase of the C
O carbonyl ester band contribute to the separation of the irradiated groups from Control, especially of the NeBB group, suggesting superior oxidative damage;47 again, an increase of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/asCH3 spectral band ratio (Fig. 5, bottom) is consistent with cells being under oxidative stress. Additionally for 8 Gy irradiations, the appearance of peak near 1714 cm−1 in the spectral region associated to the C
O/asCH3 spectral band ratio (Fig. 5, bottom) is consistent with cells being under oxidative stress. Additionally for 8 Gy irradiations, the appearance of peak near 1714 cm−1 in the spectral region associated to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester suggests that this group is becoming non-hydrogen bonded after oxidative damage,36,47 and contributes to differentiate NeMBRT from Control and NeBB clusters. Protein modifications essentially explain the separation of NeBB for the lowest dose and of MBpeak for the highest dose from the rest of the groups, with the anti-parallel β-sheet, β-turn and α-helix of AI, as well as the α-helix of AII, highly contributing to the separation of these groups. This might be suggestive of different conformational modifications after irradiations, such as protein oxidation.36 Spectral variations of the sugar-phosphate backbone bands in the low-frequency region by the BB and MBpeak groups are also consistent with oxidative stress.36,45 Regarding the PhI/AII and PhII/AII spectral band ratios (Fig. 5, bottom), an intensity increase for all irradiated groups compared to Control was detected. This behaviour is consistent with the results observed for pMBRT irradiations in a different tumour cell line,25 and might result from strand or chromatin cleavage after DNA fragmentation, or from oxidative stress.19,41
O ester suggests that this group is becoming non-hydrogen bonded after oxidative damage,36,47 and contributes to differentiate NeMBRT from Control and NeBB clusters. Protein modifications essentially explain the separation of NeBB for the lowest dose and of MBpeak for the highest dose from the rest of the groups, with the anti-parallel β-sheet, β-turn and α-helix of AI, as well as the α-helix of AII, highly contributing to the separation of these groups. This might be suggestive of different conformational modifications after irradiations, such as protein oxidation.36 Spectral variations of the sugar-phosphate backbone bands in the low-frequency region by the BB and MBpeak groups are also consistent with oxidative stress.36,45 Regarding the PhI/AII and PhII/AII spectral band ratios (Fig. 5, bottom), an intensity increase for all irradiated groups compared to Control was detected. This behaviour is consistent with the results observed for pMBRT irradiations in a different tumour cell line,25 and might result from strand or chromatin cleavage after DNA fragmentation, or from oxidative stress.19,41
Comparing the two time-points analysed, we observed that NeBB-treated B16-F10 cells remained close to Control at 0 h after treatment, while NeMBRT groups gave rise to spectral variations allowing them to be differentiated from the previous groups. At 24h post-RT, differences due to NeBB caused this group to separate further from the non-irradiated cells. However, NeMBRT-treated samples remained well distinct from the Control group, particularly for 8 Gy, with apparent differences between all irradiation configurations.
Furthermore, a difference in the clustering of RT modalities was observed for both cell lines one day after irradiations: NeMBRT clusters were always more proximate to Control than the NeBB group for the healthy cell line, whereas the three irradiation configurations remained differentiated from Control for the tumour cells, especially the BB and MBpeak groups.
In this spectral region, the effects of the RT modalities on the IR spectra of BJ cells fixated at the two time-points were similar. For 2 Gy, the most different group from Control at 0h post-RT was NeBB, with NeMBRT groups being closer to untreated cells, but one day after RT they also became distinguishable from the Control group. For 4 Gy, RT-treated cells were always well distinct from non-irradiated cells, but 24h after treatment slight differences between NeMBRT groups and NeBB emerged. Lastly, for 8 Gy, NeMBRT clusters clearly separated from Control and NeBB groups just after treatment; at 24h post-RT, NeBB was also distinct from Control and NeMBRT groups, emerging differences between MBpeak and MBvalley groups as well.
Differences in the IR spectral features of B16-F10 cells subjected to the different RT modalities are also noticeable in Fig. 4 (bottom, right; 2 Gy and 8 Gy) and Fig. S1† (bottom, right; 4 Gy). For the lowest dose, there is an overlap between NeBB and NeMBRT groups, which separate from the Control cluster. For the intermediate and high doses, the four data clusters are well-separated, with the MBpeak being the most dissimilar group from Control. The most important contributions separating non-irradiated from irradiated groups come from the asCH3, asCH2 and sCH2 stretching modes. Differences in the asCH2 and sCH2 IR bands also allow to differentiate between BB, MBpeak and MBvalley groups. Higher absorbances of the methylene bands, along with a concomitant decrease of the CH3 stretching modes, resulted in an increase of the asCH2/asCH3 spectral band ratio (Fig. 5, bottom) for the irradiated groups, especially for the BB and MBpeak. This behaviour suggests the activation of oxidative stress processes leading to the observed structural alterations. This would also explain the differences between BB and MBpeak with respect to MBvalley, since in the high-LET regions of heavy ion beams a greater recombination of certain ROS occurs (in particular, of hydrogen peroxideH2O2). Therefore, the concentration of H2O2 in the valleys would decrease with respect to that in the peaks, resulting in less H2O2-induced damage (often considered a good candidate to explain MBRT efficacy).17,48 This behaviour is also consistent with our previous results observed for pMBRT in a rat glioma cell line.25
In this spectral region, NeMBRT appeared to induce greater effects on the C–H bands of B16-F10 cells than NeBB early after treatment, reflected by the separation of the groups at that time-point. But one day after RT, NeBB-treated tumour cells also underwent modifications that resulted in their separation from the Control group; despite this, NeMBRT still showed the largest IR differences compared to non-irradiated cells, particularly the MBpeak group.
4 Conclusions
In this study, SR-FTIRM allowed to assess the biochemical response of healthy (BJ) and tumour (B16-F10) cell lines to NeMBRT, a promising novel RT approach that combines the superior radiobiological properties of neon ions with the normal-tissue protection effects of MBRT. The use of synchrotron infrared light allowed to provide key information about subtle modifications in IR bands associated to lipids, proteins, nucleic acids and carbohydrates at the single cell level with a high signal-to-noise ratio. Despite the limited availability of synchrotron sources, this work is a relevant proof-of-concept study in view of the future availability of laser-based sources covering the mid-IR range.Multivariate data analysis methods enabled to uncover the differential effects of NeBB and NeMBRT on both cell lines at 0h and 24h post-treatment. In general, the early impact of NeMBRT on the IR signatures of samples resulted in greater dissimilarities from the spectral pattern of the non-irradiated group than those due to NeBB, for both cell lines. NeMBRT-induced alterations in the 1800–1000 cm−1 spectral region might result from protein oxidation, nucleic acid rearrangements and/or oxidative stress early after irradiations; modifications of lipid-related spectral bands were also suggestive of lipid chain conformations or ROS attacks, especially in the tumour cell line. Nonetheless, the role of the repair mechanisms that NeMBRT has already been shown to activate was evident 24h after treatment: the IR signatures of the healthy samples subjected to NeMBRT were closer to those of the Control group. On the other hand, NeBB-treated healthy cells presented the most differing IR characteristics from the non-irradiated sample at this time-point, showing that NeBB-induced damage still persisted one day following RT; spectral alterations due to NeBB were consistent with enhanced oxidative stress. In contrast, the impact of both treatment modalities on the tumour cell line was more similar at 24h post-RT: the spectral features associated to lipids, proteins, nucleic acids and carbohydrates were highly affected by the BB and MBpeak groups at this time-point, with the latter configuration generally being the most dissimilar from Control. Modifications of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the proteins and the C–H bonds present in the phospholipids, mainly by the BB and MBpeak configurations, may be related to protein oxidation mechanisms, oxidative damage or cell death processes. However, further biological studies would be necessary to fully disentangle the radiobiological rationale underlying NeMBRT.
O groups of the proteins and the C–H bonds present in the phospholipids, mainly by the BB and MBpeak configurations, may be related to protein oxidation mechanisms, oxidative damage or cell death processes. However, further biological studies would be necessary to fully disentangle the radiobiological rationale underlying NeMBRT.
Author contributions
RGV: formal analysis, investigation, visualization, writing (original draft); OS: investigation; AB: investigation; JB: investigation; RH: investigation; TI: investigation; NM: investigation; TS: investigation; YP: conceptualization, methodology, investigation, funding acquisition; IY: conceptualization, methodology, investigation, funding acquisition, writing (review and edit); IMR: project administration, conceptualization, methodology, investigation, funding acquisition, validation, data curation, writing (original draft). All authors reviewed the manuscript.Data availability
Research data will be stored and made available in the CORA research data repository (https://dataverse.csuc.cat).Conflicts of interest
The authors have no conflicts of interest to disclose.Acknowledgements
This study was supported by the Spanish Ministry of Science, Innovation and Universities (grants RYC2018-024043-I, PID2020-114079RA-I00 and PRE2021-097298), by the Spanish Association Against Cancer (IDEAS21849MART) and by the Catalan Agency for Management of University and Research Grants (2021 SGR 00607). This research was also funded by the Particle Therapy Cooperative Group (PTCOG), project funding 2019. The research was also partially funded by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement no. 817908). J. B. gratefully acknowledges the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, grant number GRK2260 BIOQIC). Infrared experiments were performed at MIRAS beamline of ALBA Synchrotron Light Source Facility with the collaboration of ALBA staff. Authors would also like to acknowledge the granted beam time for radiotherapy irradiations at HIMAC.References
- D. E. Linstadt, J. R. Castro and T. L. Phillips, Int. J. Radiat. Oncol., Biol., Phys., 1991, 20, 761–769 CrossRef CAS PubMed.
- J. R. Castro, D. E. Linstadt, J.-P. Bahary, P. L. Petti, I. Daftari, J. Collier, P. H. Gutin, G. Gauger and T. L. Phillips, Int. J. Radiat. Oncol., Biol., Phys., 1994, 29, 647–655 CrossRef CAS.
- F. A. Dilmanian, Z. Zhong, T. Bacarian, H. Benveniste, P. Romanelli, R. Wang, J. Welwart, T. Yuasa, E. M. Rosen and D. J. Anschel, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 9709–9714 CrossRef CAS.
- Y. Prezado, M. Dos Santos, W. Gonzalez, G. Jouvion, C. Guardiola, S. Heinrich, D. Labiod, M. Juchaux, L. Jourdain, C. Sebrie and F. Pouzoulet, Sci. Rep., 2017, 7, 17295 CrossRef CAS.
- C. Lamirault, V. Doyère, M. Juchaux, F. Pouzoulet, D. Labiod, R. Dendale, A. Patriarca, C. Nauraye, M. Le Dudal, G. Jouvion, D. Hardy, N. E. Massioui and Y. Prezado, Sci. Rep., 2020, 10, 13511 CrossRef CAS PubMed.
- V. Kundapur, M. Mayer, R. N. Auer, A. Alexander, S. Weibe, M. J. Pushie and G. Cranmer-Sargison, Radiat. Res., 2022, 198, 162–171 CAS.
- M. Sotiropoulos, E. Brisebard, M. L. Dudal, G. Jouvion, M. Juchaux, D. Crépin, C. Sebrie, L. Jourdain, D. Labiod, C. Lamirault, F. Pouzoulet and Y. Prezado, Clin. Transl. Radiat. Oncol., 2021, 27, 44–49 CAS.
- C. Lamirault, E. Brisebard, A. Patriarca, M. Juchaux, D. Crepin, D. Labiod, F. Pouzoulet, C. Sebrie, L. Jourdain, M. Le Dudal, D. Hardy, L. De Marzi, R. Dendale, G. Jouvion and Y. Prezado, Radiat. Res., 2020, 194, 715–723 CAS.
- C. Peucelle, I. Martínez-Rovira and Y. Prezado, Med. Phys., 2015, 42, 5928–5936 CrossRef CAS PubMed.
- W. González and Y. Prezado, Med. Phys., 2018, 45, 2620–2627 CrossRef.
- Y. Prezado, R. Hirayama, N. Matsufuji, T. Inaniwa, I. Martínez-Rovira, O. Seksek, A. Bertho, S. Koike, D. Labiod, F. Pouzoulet, L. Polledo, N. Warfving, A. Liens, J. Bergs and T. Shimokawa, Cancers, 2021, 13, 1–14 CrossRef.
- S. Sabatasso, J. A. Laissue, R. Hlushchuk, W. Graber, A. Bravin, E. Bräuer-Krisch, S. Corde, H. Blattmann, G. Gruber and V. Djonov, Int. J. Radiat. Oncol., Biol., Phys., 2011, 80, 1522–1532 CrossRef PubMed.
- J. W. Hopewell and K.-R. Trott, Radiother. Oncol., 2000, 56, 283–288 CrossRef CAS.
- A. Bertho, L. Iturri, E. Brisebard, M. Juchaux, C. Gilbert, R. Ortiz, C. Sebrie, L. Jourdain, C. Lamirault, G. Ramasamy, F. Pouzoulet and Y. Prezado, Int. J. Radiat. Oncol., Biol., Phys., 2023, 115, 426–439 CrossRef.
- A. Bertho, L. Iturri and Y. Prezado, Ionizing Radiation and the Immune Response - Part A, Academic Press, 2023, vol. 376, pp. 37–68 Search PubMed.
- A. J. Johnsrud, S. V. Jenkins and R. J. Griffin, in Spatially Fractionated, Microbeam and FLASH Radiation Therapy, ed. H. Zhang and N. A. Mayr, IOP Publishing, 2023, pp. 2–18 Search PubMed.
- R. Dal Bello, T. Becher, M. C. Fuss, M. Krämer and J. Seco, Front. Phys., 2020, 8, 564836 CrossRef.
- M. J. Baker, J. Trevisan, P. Bassan, R. Bhargava, H. J. Butler, K. M. Dorling, P. R. Fielden, S. W. Fogarty, N. J. Fullwood, K. A. Heys, C. Hughes, P. Lasch, P. L. Martin-Hirsch, B. Obinaju, G. D. Sockalingum, J. Sulé-Suso, R. J. Strong, M. J. Walsh, B. R. Wood, P. Gardner and F. L. Martin, Nat. Protoc., 2014, 9, 1771–1791 CrossRef CAS.
- E. Lipiec, K. R. Bambery, P. Heraud, C. Hirschmugl, J. Lekki, W. M. Kwiatek, M. J. Tobin, C. Vogel, D. Whelan and B. R. Wood, J. Mol. Struct., 2014, 1073, 134–141 CrossRef CAS.
- E. Lipiec, B. R. Wood, A. Kulik, W. M. Kwiatek and G. Dietler, Anal. Chem., 2018, 90, 7644–7650 CrossRef CAS PubMed.
- I. Yousef, O. Seksek, S. Gil, Y. Prezado, J. Sulé-Suso and I. Martínez-Rovira, Analyst, 2016, 141, 2238–2249 RSC.
- I. Martínez-Rovira, O. Seksek, J. Puxeu, J. Gómez, M. Kreuzer, T. Dučić, M. J. Ferreres, M. Artigues and I. Yousef, Analyst, 2019, 144, 5511–5520 RSC.
- I. Martínez-Rovira, O. Seksek, I. Dokic, S. Brons, A. Abdollahi and I. Yousef, Analyst, 2020, 145, 2345–2356 RSC.
- M. Sharma, J. C. Crosbie, L. Puskar and P. A. W. Rogers, Int. J. Radiat. Biol., 2012, 89, 79–87 CrossRef.
- R. González-Vegas, I. Yousef, O. Seksek, R. Ortiz, A. Bertho, M. Juchaux, C. Nauraye, L. DeMarzi, A. Patriarca, Y. Prezado and I. Martínez-Rovira, Sci. Rep., 2024, 14, 11973 CrossRef.
- I. Martínez-Rovira, P. Montay-Gruel, B. Petit, R. J. Leavitt, R. González-Vegas, P. Froidevaux, M. Juchaux, Y. Prezado, I. Yousef and M.-C. Vozenin, Radiother. Oncol., 2024, 196, 110238 CrossRef.
- T. Kamada, in Carbon-Ion Radiotherapy, ed. H. Tsujii, T. Kamada, T. Shirai, K. Noda, H. Tsuji and K. Karasawa, Springer Japan, Tokyo, 2014, pp. 17–22 Search PubMed.
- H. Yasuda and K. Fujitaka, Radiat. Prot. Dosim., 2001, 94, 275–280 CrossRef CAS.
- M. Toplak, S. T. Read, C. Sandt and F. Borondics, Cells, 2021, 10, 2300 CrossRef PubMed.
- A. Barth, Biochim. Biophys. Acta, Bioenerg., 2007, 1767, 1073–1101 CrossRef CAS PubMed.
- B. H. Stuart, Infrared Spectroscopy: Fundamentals and Applications, Wiley, 2004 Search PubMed.
- A. Derenne, T. Claessens, C. Conus and E. Goormaghtigh, in Infrared Spectroscopy of Membrane Lipids, ed. G. C. K. Roberts, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, pp. 1074–1081 Search PubMed.
- I. Martínez-Rovira, O. Seksek and I. Yousef, Analyst, 2019, 144, 6352–6364 RSC.
- R Core Team, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, 2023 Search PubMed.
- W. André, C. Sandt, P. Dumas, P. Djian and G. Hoffner, Anal. Chem., 2013, 85, 3765–3773 CrossRef PubMed.
- N. Gault, O. Rigaud, J.-L. Poncy and J.-L. Lefaix, Int. J. Radiat. Biol., 2005, 81, 767–779 CrossRef CAS PubMed.
- C. Petibois and G. Déléris, Trends Biotechnol., 2006, 24, 455–462 CrossRef CAS.
- M. Banyay, M. Sarkar and A. Gräslund, Biophys. Chem., 2003, 104, 477–488 CrossRef CAS PubMed.
- K. Sofińska, N. Wilkosz, M. Szymoński and E. Lipiec, Molecules, 2020, 25, 561 CrossRef PubMed.
- G. Birarda, D. E. Bedolla, E. Mitri, S. Pacor, G. Grenci and L. Vaccari, Analyst, 2014, 139, 3097–3106 RSC.
- B. Vileno, S. Jeney, A. Sienkiewicz, P. Marcoux, L. Miller and L. Forró, Biophys. Chem., 2010, 152, 164–169 CrossRef CAS.
- N. Gault and J.-L. Lefaix, Radiat. Res., 2003, 160, 238–250 CrossRef CAS PubMed.
- A. D. Meade, C. Clarke, H. J. Byrne and F. M. Lyng, Radiat. Res., 2010, 173, 225–237 CrossRef CAS.
- E. Lipiec, G. Birarda, J. Kowalska, J. Lekki, L. Vaccari, A. Wiecheć, B. Wood and W. Kwiatek, Radiat. Phys. Chem., 2013, 93, 135–141 CrossRef CAS.
- C. Petibois and G. Déléris, Analyst, 2004, 129, 912–916 RSC.
- F. S. Ruggeri, C. Marcott, S. Dinarelli, G. Longo, M. Girasole, G. Dietler and T. P. J. Knowles, Int. J. Mol. Sci., 2018, 19, 2582 CrossRef PubMed.
- H.-Y. N. Holman, M. C. Martin, E. A. Blakely, K. Bjornstad and W. R. Mckinney, Biopolymers, 2000, 57, 329–335 CrossRef CAS.
- T. A. M. Masilela and Y. Prezado, Med. Phys., 2023, 50, 5115–5134 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: PCA for 4 Gy irradiations, average raw IR absorbance spectra. See DOI: https://doi.org/10.1039/d4an01038h |
| This journal is © The Royal Society of Chemistry 2025 |