Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nitrogen isotopes in herbaria document historical nitrogen sewage pollution in the Mersey Estuary, England†
Freya C.
Alldred
 *a,
Darren R.
Gröcke
*a,
Darren R.
Gröcke
 *a,
Samuel E.
Jackson
*a,
Samuel E.
Jackson
 b and
Geraldine
Reid
b and
Geraldine
Reid
 c
c
aDepartment of Earth Sciences, Durham University, South Road, Durham, DH1 3LE, UK. E-mail: freya.alldred@durham.ac.uk; d.r.grocke@durham.ac.uk
bDepartment of Mathematical Sciences, Durham University, Stockton Road, Durham, DH1 3LE, UK
cWorld Museum, National Museums Liverpool, William Brown Street, Liverpool, L3 8EN, UK
First published on 29th April 2024
Abstract
A macroalgae (seaweed) herbarium nitrogen isotope (δ15N) record is produced for the River Mersey and Liverpool South Docks (England) between 1821 and 2018. A modern macroalgae δ15N record was also produced from September 2022. The herbaria δ15N record shows a stark difference from 1821 to the present. Lower δ15N in the early 1800s is attributed to agricultural and raw sewage pollution. From 1970 to the present the herbaria samples record very elevated δ15N values – peaking in 1978 at +31‰. The 1989 Water Act and privatisation of water companies in the UK had limited impact on the herbarium δ15N record but indicated a dominance of sewage nitrogen in the River Mersey. Macroalgae δ15N has become even more elevated since the last herbaria sample in 2013. The herbaria and modern data record some of the highest seaweed δ15N values (and therefore, sewage nitrogen pollution) recorded to date. This study highlights a novel use of herbaria macroalgae to document past changes in nitrogen pollution in estuarine environments. More poignantly it highlights that the River Mersey – Mersey Estuary is heavily polluted with sewage nitrogen and requires immediate action to resolve this environmental issue.
Environmental significanceStable nitrogen isotope ratios (δ15N) in macroalgae are an under-utilised tool in the UK for studying coastal and estuarine pollution. Nitrogen loading from effluent and industrial sources produce distinct nitrogen isotope signatures. Herbaria are an untapped resource for understanding anthropogenic modification in the environment. This study uses macroalgae herbaria to identify broad nitrogen pollution changes over the past 200 years for the Mersey Estuary, UK. This is the first study of its kind to use herbarium macroalgae to investigate nitrogen pollution. Herbaria and modern macroalgae δ15N indicate that the Mersey Estuary has been dominantly affected by sewage for over 200 years. |
Introduction
Wastewater discharge in UK rivers and coastal environments is becoming more frequent which is leading to a decline in water quality.1–3 In February 2024, there are no rivers in England that have good overall health status and only 15% of rivers are of good ecological health status.1–3 This is in stark contrast to Scotland which has ∼57% of its rivers with good (or better) overall health status.4 Policy changes to permit combined sewer overflows (CSOs) to discharge raw sewage at times of peak flow have been detrimental to the ecological health of UK rivers.5 Many wastewater treatment facilities are inadequate to cope with current population levels and have had minimal investment for decades, causing the number and frequency of CSOs to increase.5 This environmental crisis was graphically highlighted in the BBC Two documentary, Our Troubled Rivers.6 Discharge during periods of low flow in rivers increases the residence time of the pollutants, enhancing the detrimental impact it has on the environment. Excessive nutrient loads are responsible for phytoplankton, algal and macrophyte blooms, which subsequently reduce oxygen contents leading to eutrophication of water bodies.7–9 The current environmental issues in England rivers are exacerbated by budget cuts in the Environment Agency, which has hindered their ability to monitor, designate and prosecute water companies over illegal discharges of sewage.5,10,11 It is also hampered by an unwillingness from privatised water companies to openly share data on discharge amounts and dates, resulting in the public taking on the responsibility to report and monitor wastewater discharges.12,13 There is widespread concern nationally over the effects that wastewater (i.e., raw and treated sewage) release has had on riverine and coastal environments.Sewage effluent that reaches the coastal ecosystem can be identified through nitrogen isotope (δ15N) analysis of organisms living in that environment (e.g., macroalgae, mussels and fish).14–17 Macroalgae (seaweed) is less-frequently used as a bio-monitor to trace nitrogen sources, especially in the UK.17,18 Macroalgae takes up nitrogen (e.g., ammonium and nitrate) with minimal nitrogen isotope fractionation and therefore, can be used to discriminate the nitrogen source in the marine environment.19 Different nitrogen sources (e.g., fertilisers, raw and treated sewage) have distinct isotopic averages. Treated sewage effluent is often identified through δ15N values greater than +7‰ in macroalgae.17,20,21 On the other hand, nitrogen pollution derived from industrial chemical processes (e.g., artificial fertilisers) produces δ15N values near the atmospheric nitrogen value, ≈0‰.22–24 Although there are numerous studies that have used macroalgae δ15N to identify sewage pollution, there are only a handful of studies that exist for the UK despite the ongoing sewage pollution crisis.17,21,25,26
Museum herbarium collections contain a vast amount of ecological and environmental information that is often underutilised in the study of recent anthropogenic change.27,28 Only recently have researchers considered herbaria as a tool to track biogeographical, environmental, and climatological changes.28–30 Although herbaria relate to all forms of dried materials, such as vascular plants, macroalgae, bryophytes, lichens, and fungi, 82% of current research has focussed on accessing vascular plant collections.29 Herbaria studies have dominantly been used to record population densities, distribution, and organism morphology to infer environmental conditions, but more recently this has extended to include DNA sequencing and chemical analyses.27,29,31 Macroalgae herbaria are starting to be used to investigate the marine environment although they remain under-utilised on samples prior to the 20th century.29,32,33 Recently, Miller et al.32 used macroalgae herbaria and nitrogen isotopes to reconstruct past upwelling trends along the Californian coast – this approach was adopted since that is the dominant environmental mechanism affecting nitrogen isotopes in that region.
In this study, we used macroalgae specimens collected and stored in the herbarium at the World Museum, National Museums Liverpool to reconstruct historical nitrogen pollution in the Mersey Estuary and Liverpool Docks. Herbaria specimens from the Mersey Estuary were assessed for their suitability for destructive sampling. Only those where there was adequate material available to have a portion removed without damaging the scientific integrity of the specimen for future research were used in this study. Very delicate small specimens, for example, were avoided and for pre-1900 specimens only one sample per year was permitted for sampling: again, to preserve the herbarium collection for future research. The collection has been generated relatively consistently from the same region between 1821 and 1860 and from the 1960s to the present day. Sampling gaps occurred during World War I and II, and may be a common artefact in herbarium records around Europe for this time interval. Although this is unfortunate from a scientific perspective, the herbaria record available will still allow us to reconstruct changes in nitrogen pollution from the Industrial Revolution to the modern sewage era. The River Mersey and Mersey Estuary has witnessed significant anthropogenic changes over the past 200 years and thus, is an ideal natural setting to assess the use of macroalgae δ15N from herbaria as a proxy for reconstructing historical nitrogen pollution.
The River Mersey and estuary
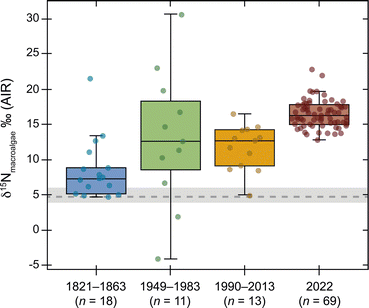
The River Mersey has a catchment area of ∼1800 mi2 and includes Manchester, Lancashire and Cheshire in the north-west of England.34 It flows for 69 miles before widening into the Mersey Estuary that stretches for almost 16 miles (Fig. 1).35 The large metropolitan city of Liverpool is located at the mouth of the River Mersey (Fig. 1), where large tidal ranges (>10 m) cause strong currents and large sand banks.36 The Mersey Basin has grown in population size from ∼500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (1821) to >5 million (2021); this estimate includes other nearby population centres such as Manchester, Liverpool, and Salford.37–39 This region is serviced by the private water company, United Utilities Group PLC. The River Mersey and Mersey Estuary have had a long history of pollution and poor water quality since the early 1800s.36,38 Nitrate plumes originating in the River Mersey have seriously impacted Liverpool Bay since the 1960s.34,40 Public outcry in the 1980s incentivised the launch of the Mersey Basin Campaign.38,41 The aim was to clean up the polluted River Mersey after it was described as an “affront to the standards a civilised society should demand” by the then Secretary of State for the Environment, Lord Heseltine.42 Unfortunately, pollution issues still exist in the River Mersey. For example, data extracted from The Rivers Trust Sewage Map43 show that in 2021 the River Mersey catchment area experienced over 212
000 (1821) to >5 million (2021); this estimate includes other nearby population centres such as Manchester, Liverpool, and Salford.37–39 This region is serviced by the private water company, United Utilities Group PLC. The River Mersey and Mersey Estuary have had a long history of pollution and poor water quality since the early 1800s.36,38 Nitrate plumes originating in the River Mersey have seriously impacted Liverpool Bay since the 1960s.34,40 Public outcry in the 1980s incentivised the launch of the Mersey Basin Campaign.38,41 The aim was to clean up the polluted River Mersey after it was described as an “affront to the standards a civilised society should demand” by the then Secretary of State for the Environment, Lord Heseltine.42 Unfortunately, pollution issues still exist in the River Mersey. For example, data extracted from The Rivers Trust Sewage Map43 show that in 2021 the River Mersey catchment area experienced over 212![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h of effluent discharge prior to processing through 12–24 h (for reference, a calendar year = 8760 h).
000 h of effluent discharge prior to processing through 12–24 h (for reference, a calendar year = 8760 h).
The River Mersey and Mersey Estuary macroalgae herbaria δ15N record spans 197 years consisting of 70 macroalgae specimens collected between 1821 (Enteromorpha compressa, Liverpool) and 2018 (Polysiphonia stricta, Queens Dock)—including the time gap previously mentioned (see the ESI†). Many different macroalgae species have been used to generate the δ15N record, because a range of specimen types are collected for herbaria; these are often selected based on casual observation, ecological monitoring, identification and taxonomy, or more frequently because of their fragility, rarity, and beauty on herbarium paper. A brown seaweed is less often collected, such as Fucus vesiculosus (bladder wrack), because it is not as eye-catching as a delicate red seaweed and is very abundant around the UK coastline. The same macroalgae species are often not routinely collected for herbaria and hence, generating a long-term species-specific δ15N record will not be possible. We suspect that this will be an inherent issue with many herbarium collections around the world. Irrespective of the use of different macroalgae species the δ15N record produced in this study reveals consistent and significant changes over the past 200 years that can be related to societal changes and a major neoliberalism event in 1989 in the UK:44 the privatisation of water companies.
F. vesiculosus collected from Eastham in 1978 recorded the most elevated δ15N value of +30.6‰, whereas the lowest δ15N value was −4.1‰ collected from Otterspool in 1968 (also F. vesiculosus) (see the ESI†). δ15N values of macroalgae above +20‰ are very rare in the literature45 and suggest extreme environmental conditions; in this case, we interpret these elevated δ15N values as a result of continued, voluminous release of sewage into the River Mersey. Four herbaria samples from Birkdale (along the coastline north of Liverpool) were also analysed and exhibit a range between +2.9‰ and +9.1‰ spanning the time interval, 1936–1938; these data are not discussed any further in this study. To place the herbaria δ15N record in the context of the present-day environment, a series of macroalgae samples from the Mersey Estuary and South Docks were collected in September 2022 (see the ESI†). Many different macroalgae species were collected from the South Docks and produced an average δ15N value of +10.6‰ ± 3.2‰ (n = 27) (Fig. 2). However, macroalgae δ15N from the Mersey Estuary average +16.5‰ ± 2.0‰ (n = 50) and +15.5‰ ± 2.7‰ (n = 22) for Fucus sp. and Ulva sp., respectively (Fig. 3). The herbaria δ15N data from the Mersey Estuary and the South Docks will be discussed separately. Due to the limited sample size, we have grouped the herbaria δ15N data into age ranges as discussed below.
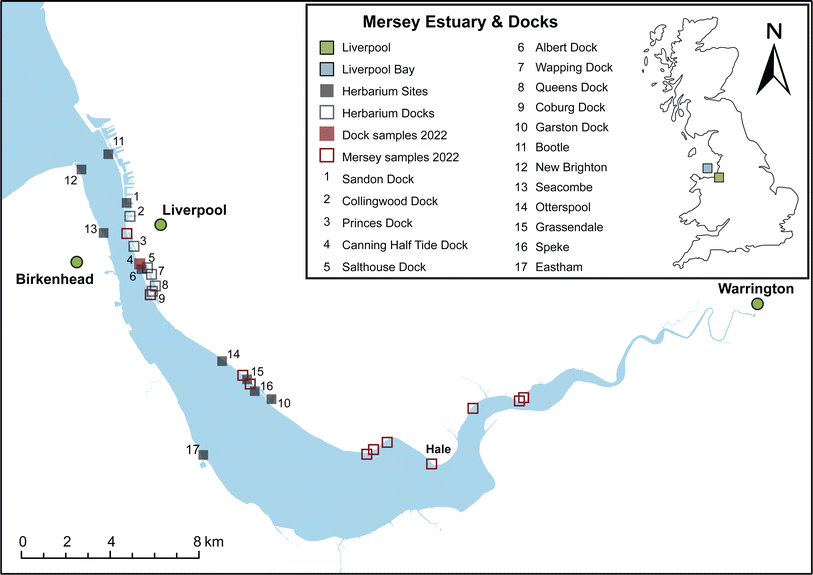
 | ||
| Fig. 2 Herbaria and modern nitrogen isotope record for the South Docks of Liverpool. Herbarium data points are shown in lilac (n = 23); the modern nitrogen isotope range is provided as a boxplot collected in September 2022 (n = 27). The grey box represents the “natural” isotopic range (+4‰ to +6‰) for the North Atlantic25 and the dashed line represents the global nitrate δ15N value.51 | ||
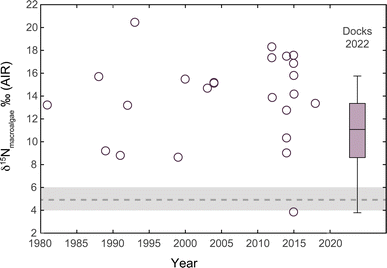
 | ||
| Fig. 3 Herbaria and modern nitrogen isotope record for the Mersey Estuary between 1821 and 2013 (n = 42) and 2022 (n = 69). There is a significant statistical difference between 1821–1863 and 1990–2013 and 2022. There is no statistical difference for the dataset 1949−1983 due to the large range in δ15N, which spans the entire range of the whole dataset of this study. The grey box represents the “natural” isotopic range (+4‰ to +6‰) for the North Atlantic25 and the dashed line represents the global nitrate δ15N value.51 | ||
The South Docks herbaria record
The South Docks in Liverpool are an interconnected system,46 and therefore the herbaria macroalgae δ15N record is being treated as a single composite record. Fig. 2 shows the herbaria South Docks δ15N record between 1981 and 2018. An 1846 herbaria sample collected from Princes Dock is the only dock specimen collected during the 1800s and so this sample has been omitted from further discussion (F. vesiculosus, δ15N = +8.2‰). δ15N values show no clear trend in the South Docks over the 40-year period (Fig. 2). The South Docks herbaria record has an average δ15N value of +13.7‰ ± 3.8‰ (n = 23), which is significantly more elevated (p value > 0.003) than the 2022 dock average (+10.6‰ ± 3.1‰, n = 27).Allen et al.46 reported low public opinion of dock water quality in the 1990s. Efforts to limit mixing between the river and docks were obtained through the installation of a pump in 1992 to replenish water levels, thus reducing turnover rates of the dock seawater to between 6 and 12 months.34,46,47 A reduced replenishment rate may be the reason behind dock herbaria not reflecting similar macroalgae δ15N values recorded in the Mersey Estuary. The lower δ15N average of the dock herbaria and modern macroalgae compared to the Mersey Estuary suggests two potential mechanisms: (a) increased industry-sourced nitrogen pollution entering the docks (e.g., drains and road runoff); and/or (b) water filtering (i.e., cleansing) by the presence of the bivalve, Mytilus edulis (blue mussel).47 Since the mid-1980s there have been several reports of blue mussel colonies thriving in the docks; they were introduced to Graving Dock as part of a bio-filtration experiment and naturally settled in Albert Dock and subsequently throughout the South Dock system.34,47 The duration it would take for blue mussels to filter the volume of water in Albert Dock (170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3) is calculated to be every 4 days. Scaled up to include the entire South Dock complex (1.28 million m3) the duration to filter all that water would be ∼30 days assuming that blue mussel density is consistent throughout the docks.47,48 Nitrogen is consumed by microalgae and phytoplankton in the docks which are consumed by filtering blue mussels resulting in lower macroalgae δ15N values. This is consistent with a blue mussel experiment showing that water nitrate and blue mussel tissue are more depleted in δ15N when nitrate concentrations in the water are high.49 Dock water samples analysed in this study indicate low to moderately high nitrate concentration ranges from 0.01 to 11.6 mg l−1 (n = 13, January–July 2023). Therefore, blue mussels, or other filtering organisms, should be considered as a natural bio-remediator in ports/harbours/docks, but they should not be considered as a solution without rectifying the cause.
000 m3) is calculated to be every 4 days. Scaled up to include the entire South Dock complex (1.28 million m3) the duration to filter all that water would be ∼30 days assuming that blue mussel density is consistent throughout the docks.47,48 Nitrogen is consumed by microalgae and phytoplankton in the docks which are consumed by filtering blue mussels resulting in lower macroalgae δ15N values. This is consistent with a blue mussel experiment showing that water nitrate and blue mussel tissue are more depleted in δ15N when nitrate concentrations in the water are high.49 Dock water samples analysed in this study indicate low to moderately high nitrate concentration ranges from 0.01 to 11.6 mg l−1 (n = 13, January–July 2023). Therefore, blue mussels, or other filtering organisms, should be considered as a natural bio-remediator in ports/harbours/docks, but they should not be considered as a solution without rectifying the cause.
The modern and herbaria macroalgae δ15N record (Fig. 2) indicates that dock water quality has remained relatively consistent since its redevelopment in the 1970s and introduction of the blue mussel ecosystem in the 1980s. Whilst the dock herbaria and modern macroalgae δ15N records show less elevated values compared to the Mersey Estuary, the docks still record elevated signatures (+13.7‰ and +10.6‰, respectively). Such elevated macroalgae δ15N would suggest that the dock water still contains anthropogenic nitrogen sourced from raw and/or treated sewage. Since the Mersey Estuary is the primary source of seawater for the docks, a sewage nitrogen δ15N signature is unavoidable.
Mersey Estuary, 1821–1863: Victorians and raw sewage
Between 1821 and 1863, herbaria δ15N values ranged between +4.7‰ (Ectocarpus granulosus 1863 and Enteromorpha compressa 1853) and +21.5‰ (Enteromorpha intestinalis 1849) (Fig. 3). The majority of the herbaria specimens from this period have δ15N values that fall between +4.7‰ and +8.8‰ (n = 14) with four other results greater than +11‰. A single specimen (E. intestinalis) from Bootle in 1849 recorded a δ15N value of +21.5‰. This time interval records an average δ15N value of +8.3‰ ± 4.1‰ (n = 18) or with exclusion of the 1849 Bootle sample, +7.5‰ ± 2.6‰ (n = 17). Fig. 3 also shows a global average deep-water δ15N value of +4.8‰ and the expected range (+4‰ to +6‰) for macroalgae in the North Atlantic.16,50,51Although the 1821–1863 Mersey Estuary herbaria record has a limited amount of δ15N data (n = 18) and information regarding their precise sampling location and precise date, the δ15N range is significantly different from the 1990–2013 (p value < 0.02, n = 13) and 2022 (p-value = 1.6 × 10−7, n = 72) datasets; it is not significantly different from the 1949–1983 dataset (p value > 0.1, n = 11). During the 1800s the Mersey Basin and Mersey Estuary were dominantly influenced by industrial activities: cotton, alkali and hydrogen-chloride production, shipbuilding and transport of coal.36,38 However, industry of this kind preceded the discovery and explosion of the use of nitrogen gas in industrial processes. For example, agricultural fertilisers made from nitrogen gas (δ15N ≈ 0‰) were not generated until the discovery of the Haber–Bosch process and large-scale processing in 1913.52,53 Therefore, industrial processes would not be the cause behind lower δ15N values compared to the other time intervals in this study.
River pollution in England was rife during the Victorian Era.54 The main route of sewage/wastewater disposal in the 19th century was to cast it into rivers and/or cesspits, with the latter infiltrating into the hydrological system.55 Between 1847 and 1858 an 80-mile-long sewer network with 48 outflows discharging into the River Mersey was constructed, focusing sewage waste in the river.38,56 Sewage waste in England reached such a level that it caused several national cholera outbreaks;57 the largest of these outbreaks occurred in 1849. It is interesting to observe that the 1849 cholera outbreak coincides with the most elevated δ15N value (Bootle, +21.5‰) in our herbaria record and Liverpool having the highest cholera mortality rate in large towns reported for England (Liverpool, 11.3 deaths per 1000 people).57 Even after the cholera outbreaks there are reports of major sewage issues; for example, the Great Stink of London in 1858 when the River Thames was so heavily contaminated it sparked the conception of the modern-day sewerage system.58
Principally, we postulate that the input of raw sewage (e.g., not treated, and hence not denitrified59) was the cause behind the 1821–1863 δ15N values recorded for the Mersey Estuary. Raw human sewage would have a similar δ15N value to the diet they were consuming60 and based on a modern-day equivalent dietary value this would represent a range between +4‰ and +8‰.61 Although raw sewage can explain the δ15N record, agricultural practices cannot be excluded.38,56 In addition, leaching and weathering of soil-derived nitrogen from fields using organic fertilisers (i.e., manure) may also be contributing to the δ15N signature.24 To our knowledge no soil-nitrogen isotope studies exist for Merseyside. In addition, no water quality data (i.e., nitrate concentration) exist this far back in time for this region, unlike the River Thames, London.39,62,63 Although the contribution of nitrogen sources is uncertain the macroalgae δ15N record from 1821–1863 is most likely caused by raw sewage (e.g., human and animal husbandry), and it is evident that it was different from the modern Mersey Estuary record (1990–present).
The ∼100 year gap from 1863 is unfortunate since it would have been interesting to understand whether the macroalgae δ15N record would follow an increasing trend or shift suddenly. There is however some information pertaining to water quality from historical records during this time interval. No “waterweeds” (i.e., macroalgae) were present in the Mersey Estuary between the 1870s and 1900s which can be attributed to water pollution.39 Furthermore, from the 1900s unregulated sewage and industrial discharges persisted until the 1950s when wastewater discharge permits were first introduced.64,65
Mersey Estuary 1949–1989: divergent nitrogen pollution sources
The herbaria δ15N record for the 1970s is more elevated in comparison to the δ15N record for the 1800s. The Mersey macroalgae herbaria record from 1949 to 1983 shows the greatest range in δ15N (34.7‰) with an average value of +13.0‰ ± 9.2‰ (n = 11). The lowest δ15N value of −4.1‰ recorded in F. vesiculosus occurred in 1968 at Otterspool, and the most elevated δ15N value at +30.6‰ (also by F. vesiculosus) occurred a decade later in Eastham. It is difficult to accurately determine or constrain the cause behind the large variation in δ15N between 1949 and 1983. It records one of the most elevated macroalgae δ15N values (i.e., caused by sewage/denitrification) ever recorded to date, but is immediately followed by a negative δ15N value indicative of industrial pollution. Due to the large range in δ15N for this time interval it is not significantly different from any of the age range groups assigned to this data set (see Fig. 3). Since the majority of the δ15N values in this time interval are elevated, removing the lowest two δ15N values causes the average value to increase to +16.2‰ ± 6.9‰. Standard processes in wastewater treatment plants cause the sewage effluent to become enriched in 15N due to denitrification.66 This time interval has very elevated δ15N values in comparison to background “natural” macroalgae,16 thus implying it is heavily influenced by nitrogen pollution created by wastewater treatment plants.66Decades of historical releases of sewage and industrial pollution led to a serious decline in the health status of UK water bodies. Massive cuts in funding and spending during the 1970s and 1980s exacerbated specifically the impact of sewage pollution on the environment making it a national problem that needed addressing.41 The Control of Pollution Act 197467 introduced the requirement that local stakeholders had to apply for permits to discharge sewage effluent and industrial waste or face prosecution. This was heralded as a major legal improvement although it was slow to be implemented nationally.68 In the 1970s the Mersey Estuary was considered the most polluted estuary in the UK receiving significant wastewater inputs that subsequently led to high nitrate plumes in Liverpool Bay.34,38,41,56,64 High nutrient fluxes as a result of sewage wastewater discharges caused biochemical oxygen demand (BOD) in the late 1960s to average 20 mg l−1 which is indicative of very polluted water. The enriched δ15N values observed in herbaria collected for this interval corroborate the input of denitrified sewage wastewater (Fig. 3).34 Growing public demand for improved water quality at the peak of the Mersey pollution crisis in the mid to late 1980s saw the establishment of the Mersey Basin Campaign.41,69 At the same time public concern over environmental quality was growing across the nation.41 The Conservative Government and the then Prime Minister, Margaret Thatcher, actioned the privatisation of water companies in England and Wales through the Water Act 1989.70 Even then, as it is now, the privatisation of water companies was primarily focused on macro-economic policies – to inject much-needed cash into an infrastructure that had received little investment for decades.41,71 With a failing regulatory system between DEFRA (Department for Environment, Food and Rural Affairs), the Environment Agency and Ofwat (Water Services Regulation Authority) there has been a continuous lack of investment from the water industry in keeping up with population growth and protecting the environment.71
Mersey Estuary, 1990–2013: the start of the sewage era
The herbaria δ15N record depicted in Fig. 3 indicates that the privatisation of water companies had an impact on the macroalgae nitrogen isotope signature in the Mersey Estuary. It resulted in more stability in herbaria δ15N values (average = +12.3‰ ± 2.5‰, n = 13) excluding one outlier, +4.9‰ (F. vesiculosus, 1997, Grassendale). Although the record is more stable, the δ15N values are elevated in comparison to a background “natural” macroalgae range (+4‰ to +6‰). The herbaria δ15N values are significantly more elevated than those in the 1821–1863 period (p value < 0.02, n = 13), which is interpreted as a result of wastewater treatment processes that elevate the 15N content of sewage effluent. It is proposed that the stability of δ15N in this time interval was a result of stricter controls on discharges, monitoring and increased chemical processing (i.e., ammonia) in wastewater treatment plants.25,61,72,73During this time interval, stricter regulations were implemented by the European Union on the UK government to address water quality issues. This legally enforced that the level of nitrate released into freshwater and marine environments be set to a maximum limit of 50 mg l−1 (or 11.3 mg l−1 of nitrate N).65 Environmental action, regulations and investment around the Mersey Basin led to the reduction of BOD from 40 mg l−1 in the 1960s to ∼7 mg l−1 in the River Mersey in the early 2000s;34 the Mersey Estuary is still classified as eutrophic.
Mersey Estuary, 2022: a peak in the sewage era?
The macroalgae δ15N data from September 2022 are very elevated (+16.5‰ ± 1.9‰) and significantly different from those of all previous time intervals, except for 1949–1983. δ15N values are consistently more elevated with the lowest value at +12.8‰ and the highest value of +22.8‰.34,69 Elevated δ15N values >+16‰ are rarely recorded in macroalgae studies45,74 (Gröcke et al., unpub. data), and only a handful of studies have recorded values >20‰,21,25,45,74 and none previously in the UK. Although BOD assessments indicate that the Mersey Estuary has improved, the Environment Agency reports that it is “not achieving good status” due to industry,63 with no indication of water detriment from the water industry. The Environment Agency also gives “low confidence” that targets to reach “good” nitrogen levels will be achieved by 2027.75,76 The herbaria δ15N data indicate that the dominant ‘nitrogen’ pollution signal in the Mersey Estuary is caused by wastewater (i.e., sewage) and not by nitrogen chemical pollutants (i.e., artificial fertilisers). Overall, recent herbaria and modern macroalgae δ15N datasets suggest that denitrified sewage input into the waters is extensive and well above the “natural” range expected for a coastal and estuarine environment in the North Atlantic.Although there is a time-gap between the 1990−2013 and 2022 δ15N datasets there is a clear and significant increase. Since 2012 the Environment Agency has been aware of illegal sewage discharges by United Utilities and has been accused of “knowingly permitting” such discharges to occur.10 DEFRA states that CSOs are only permitted to discharge during periods of heavy, continuous rainfall and will be tightened to permit discharges only where “there is no adverse ecological impact” by 2050.77 Although there is abundant social media evidence that water company regulations on discharging are not being adhered to,78 additional evidence was presented in a recent BBC Panorama report documenting how pollution incidents are being covered up.79
The Liverpool sewerage infrastructure is one of the oldest sewerage systems in the UK,80 and discharges predominantly through CSOs (i.e., 84% of outlets to the River Mersey and Mersey Estuary). We interpret the elevated macroalgae δ15N values as directly sourced from these CSOs which routinely discharge sewage effluent directly into the river. For example, in 2022 the River Mersey received a total of 3346 hours of sewage dumping (i.e., 4.6 months)43,81,82 dominantly occurring around Manchester and Warrington.43,81–83 Although nitrate assimilation can lead to elevated residual nitrate δ15N values, its impact is relatively minor in the order of 5‰ to 8‰.84 Thus, denitrification processes must be a primary cause for generating elevated δ15N recorded in macroalgae. There are four potential mechanisms and sources:
(1) denitrified sewage effluent from wastewater treatment plants. It is well documented that wastewater treatment processes that use anaerobic conditions can elevate effluent δ15N values above +10‰;85
(2) increasing nitrogen productivity and deposition of nitrate as a consequence of elevated wastewater effluent discharges. Nitrogen consumption in the water profile via surface water productivity (i.e., phytoplankton) will preferentially remove 14N, resulting in more elevated nitrate δ15N values in the water column. Burial of the nitrate and nitrogen-bound organic matter will also remove 14N in preference to 15N causing an increase in δ15N.
(3) denitrified groundwater nitrate. A 1999 study on groundwater nitrate indicated that sewage effluent was leaking into the aquifer beneath Liverpool (Whitehead et al. 1999) – this was based on nitrate δ15N and microbiological analyses. However, the sample with the most elevated E. coli and faecal streptococci contents was not analysed for nitrate δ15N. The other samples in that study ranged between −11.9‰ and +13.2‰ (average = +6.8‰ ± 8.7‰), and therefore, it would seem that aquifer discharge is not a cause for elevated δ15N in the Mersey Estuary. Further research is required to fully ascertain the effect of groundwater nitrate on this system; and
(4) denitrification in estuarine sediment. The tidal range for the Mersey Estuary is large (between 4 m to 10 m). Due to the volume of water being replenished daily it is reasonable to state that the water column would be well-oxygenated and, hence, increase the depth of the suboxic layer in the sediment. Nitrification of wastewater ammonia would occur in the water column, and nitrification in the sediment profile will produce elevated δ15N pore-water ammonia values as nitrate is reduced in concentration.86,87 Subsequent denitrification in the sediment profile is therefore a function of oxygen supply and penetration, as well as nitrate replenishment from the water column into the sediment profile. Although the Mersey Estuary has the largest total inorganic nitrogen load (3959 × 106 moles per year) in an estuary in the UK,88 further research on nitrification and denitrification in Mersey Estuary sediment is required to understand its impact on the system.
Of the four options above, the most preferred explanation as a cause for the elevated δ15N values in macroalgae in the Mersey Estuary is due to (1) and (2)—increased denitrified and raw wastewater sewage effluent causing high productivity and eutrophication in the River Mersey and Mersey Estuary. At present, water companies are undeterred by fines imposed by regulatory bodies1 and have no incentive to change current operational practices (i.e., releasing effluent during dry periods). Funding cuts to the Environment Agency have also limited their ability to properly monitor, respond, assess and impose fines on water companies in breach of environmental standards.1,10,11 The decline in England river water quality (and subsequently, estuaries and coastal settings) is exacerbated by a continued, significant lack of investment from water companies to improve and repair their infrastructure to accompany increases in population and corresponding changes in present/future climate conditions; illegal discharging from CSOs has become so frequent as to become the ‘norm’.89 Although England water companies have until 2050 to achieve infrastructure upgrades and compliance, by that time the environmental damage to rivers, estuaries and coastal settings may be irreversible.77 Continued nitrogen isotope monitoring of macroalgae will show whether improvements are made in the future or whether the Mersey Estuary will remain an environment impacted by sewage pollution in the ‘sewage era’.
Conclusion
The Mersey Estuary has been identified as a heavily polluted environment since the early 1800s. Museum herbaria provide an excellent source of material to reconstruct historical pollution. Despite the number of available herbaria samples for this study, it has been possible to depict broadscale nitrogen isotope changes and trends in the Mersey Estuary since 1821.A macroalgae herbaria δ15N dataset from 1813 to 2013 reveals three major nitrogen pollution episodes: (1) 1821–1863 is dominated by raw sewage; (2) 1949–1983 is influenced by industrial, agricultural and treated sewage processes; and (3) 1990–2013 records treated and raw sewage pollution termed the ‘sewage era’. Although BOD has decreased from very elevated levels in the 1960s, the River Mersey−Mersey Estuary still contains a significant proportion of nitrogen sewage pollution as interpreted using δ15N macroalgae values. Privatisation of water companies in England in 1989 was driven by economics and not environmental sustainability. Its impact on herbaria δ15N values was to stabilise the variability (and hence, the influence of varying nitrogen pollution sources). The modern macroalgae δ15N record reflects increasing sewage nitrogen pollution into the River Mersey – through denitrified wastewater effluent and release of raw sewage. The very elevated δ15N values are interpreted as a consequence of limited regulation, underinvestment, and legislation changes.
Poor water quality is an incessant problem in the UK—the elevated macroalgae δ15N values recorded in both herbaria and modern samples from the Mersey Estuary highlight an ongoing failure to reduce nitrogen pollution in our hydrological environments. This study demonstrates the usefulness of macroalgae δ15N in determining the nitrogen pollution source into the Mersey Estuary over the past 200 years and, hence, is indicative of a whole catchment source problem. The Mersey Estuary δ15N record showcases how the nitrogen cycle has been severely influenced by human processes that have had a lasting impact on our riverine/estuarine environments. Large-scale nitrogen pollution from wastewater treatment plants is transforming the nitrogen cycle in UK rivers, estuaries and coastlines. This study highlights that the ‘sewage era’ is upon us and necessitates a critical shift in increased private investment into wastewater treatment infrastructure, stricter and immediate prosecution of policy breakers and a re-evaluation of environmental monitoring methods and policy.
Materials and methods
A total of 72 herbaria macroalgae specimens were sampled at the World Museum, Liverpool, providing a sample record from 1821 to 2018. The macroalgae specimens are from multiple locations and different macroalgae species for the Mersey Estuary and Liverpool South Docks (see Fig. 1); specific sample details are provided in the ESI.† A scalpel was used to remove the longest macroalgae thallus tips, which represent the last growth of the sample for all macroalgae specimens. The sample was then transferred into microcentrifuge tubes until processing for stable isotope analysis. It has been reported that nitrogen isotopic ratios are not impacted by paper type and so herbaria are assumed to be comparable to specimens pressed on modern acid-free paper.32 Even with this knowledge we still inspected every herbarium sample to prove that no sample had herbarium paper attached. Herbarium specimens were compared to a modern geospatial sample set from the Mersey Estuary collected in September 2022. This sample set was collected for comparison with the herbaria samples – most of which were also collected in the summer season. Fifty F. vesiculosus and 22 Ulva sp. modern specimens were sampled from the Mersey Estuary in addition to 27 specimens collected from the South Docks (Fig. 1). Dock samples include 22 Ulva sp., nine Cladophora sp. and two Callithamnion corymbosum. Modern specimens were dried in an oven between 45 and 60 °C – this drying method has no impact on the bulk signature of macroalgae stable isotope ratios. Once dried, sub-samples of macroalgae and the most recent growing tip of Fucus sp. were weighed between 1.1 and 1.5 mg into tin capsules for stable isotope analysis. All nitrogen isotope analyses were carried out in the Stable Isotope Biogeochemistry Laboratory (SIBL) at Durham University. Nitrogen isotopes are reported against atmospheric nitrogen (AIR) and accuracy is continuously monitored with both in-house and international standards. In-house standards are calibrated against international standards (e.g., IAEA-600, IAEA-N1, and IAEA-N2). Analytical uncertainty is typically ±0.1‰ (1 sd) for replicate analyses of our standards. Most herbaria specimens only had enough material for one analysis due to strict museum limitations on sample destruction. For further details on analytical methods see Bailes and Grocke21 and Grocke et al.21,26Conflicts of interest
The authors declare there are no conflicts of interest to declare.Acknowledgements
This research formed part of the MSc by Research dissertation of FCA, which was funded by the Stephen Mills Scholarship from the Department of Earth Sciences, Durham University. DRG and FCA wish to thank the staff at the World Museum for making our visits enjoyable. The Canal and River Trust kindly provided information and guidance about sampling around the Mersey Estuary and Liverpool South Docks. The fieldtrips to Liverpool were a pleasure for DRG and FCA – the people of this region were friendly, accommodating and often stopped to speak to us when we were collecting samples. DRG wishes to thank Durham University and Sea-Changers for funding his transition into macroalgae research. Additional funding for this project was covered by the Stable Isotope Biogeochemistry Laboratory (SIBL). Two anonymous reviewers greatly improved our manuscript, so thank you for your valuable insights and time.References
- Environment Agency, Water and sewerage company performance on pollution hits new low, https://www.gov.uk/government/news/water-and-sewerage-company-performance-on-pollution-hits-new-low, accessed 4 March 2024.
- J. Bevan, The state of our waters: the facts, https://environmentagency.blog.gov.uk/2020/10/02/the-state-of-our-waters-the-facts/, accessed 14 March 2023.
- Environmental Audit Committee, Water Quality in Rivers: Fourth Report of Session 2021–22, London, 2022, https://publications.parliament.uk/pa/cm5802/cmselect/cmenvaud/74/summary.html, accessed 6 March 2024 Search PubMed.
- The Rivers Trust, State of Our Rivers, https://theriverstrust.org/rivers-report-2024, accessed 1 March 2024 Search PubMed.
- E. Gill, B. Horton, J. Gilbert, S. Riisnaes and E. Partridge, Storm Overflow Evidence Project Final Report Prepared for: Water UK, https://assets.publishing.service.gov.uk/media/6182bad4e90e07197867ecd4/storm-overflows-evidence-project.pdf, accessed 6 March 2024 Search PubMed.
- BBC, Paul Whitehouse and Our Troubled Rivers, https://www.bbc.co.uk/programmes/m001jw74, accessed 20 February 2024 Search PubMed.
- R. Bermejo, N. Golden, E. Schrofner, K. Knöller, O. Fenton, E. Serrão and L. Morrison, Biomass and nutrient dynamics of major green tides in Ireland: Implications for biomonitoring, Mar. Pollut. Bull., 2022, 172, 113318 CrossRef PubMed.
- P. M. Glibert, Eutrophication, harmful algae and biodiversity—Challenging paradigms in a world of complex nutrient changes, Mar. Pollut. Bull., 2017, 124, 591–606 CrossRef CAS PubMed.
- V. H. Smith, Eutrophication of Freshwater and Coastal Marine Ecosystems A Global Problem, Environ. Sci. Pollut. Res., 2003, 10, 126–139 CrossRef CAS PubMed.
- House of Commons, Oral Evidence: Work of the Environment Agency, HC 221, Environment, Food and Rural Affairs Committee, https://committees.parliament.uk/oralevidence/12822/html/, 2023.
- S. Lovett, Environment Agency funding cut by 50% over past decade as sewage spills rise, analysis shows, The Independent, https://www.independent.co.uk/climate-change/news/water-pollution-sewage-environment-agency-funding-b2154848.html#, 2022, accessed 2 March 2024.
- H. Briggs, Citizen scientists join fight to clean up rivers, BBC Science & Environment, https://www.bbc.co.uk/news/science-environment-63747838, 2022, accessed 4 March 2024.
- J. Woodward, To clean up England’s rivers we need to know how much sewage is dumped – but water firms won’t tell us, University of Manchester, https://www.manchester.ac.uk/discover/news/sewage-water-firms-wont-tell-us/, accessed 4 March 2024.
- J. Tucker, N. Sheats, A. E. Giblin, C. S. Hopkinson and J. P. Montoya, Using stable isotopes to trace sewage-derived material through Boston Harbor and Massachusetts Bay, Mar. Environ. Res., 1999, 48, 353–375 CrossRef CAS.
- S. D. Costanzo, M. J. Oõdonohue, W. C. Dennison, N. R. Loneraganà and M. Thomas, A New Approach for Detecting and Mapping Sewage Impacts, Mar. Pollut. Bull., 2001, 42, 149–156 CrossRef CAS PubMed.
- C. Savage and R. Elmgren, Macroalgal (Fucus vesiculosus) δ15N values trace decrease in sewage influence, Ecol. Appl., 2004, 14, 517–526 CrossRef.
- J. Samper-Villarreal, Strengths and challenges of δ15N to identify anthropogenic nutrient loading in coastal systems, Isot. Environ. Health Stud., 2020, 56, 700–712 CrossRef CAS PubMed.
- R. García-Seoane, J. A. Fernández, R. Villares and J. R. Aboal, Use of macroalgae to biomonitor pollutants in coastal waters: Optimization of the methodology, Ecol. Indicat., 2018, 84, 710–726 CrossRef.
- R. Cohen and P. Fong, Experimental evidence supports the use of δ15N content of the opportunistic green macroalga Enteromorpha intestinalis to determine nitrogen sources to estuaries, J. Phycol., 2005, 41, 229–448 CrossRef.
- D. Xue, J. Botte, B. De Baets, F. Accoe, A. Nestler, P. Taylor, O. Van Cleemput, M. Berglund and P. Boeckx, Present limitations and future prospects of stable isotope methods for nitrate source identification in surface- and groundwater, Water Res., 2009, 43, 1159–1170 CrossRef CAS PubMed.
- I. R. Bailes and D. R. Gröcke, Isotopically labelled macroalgae: A new method for determining sources of excess nitrogen pollution, Rapid Commun. Mass Spectrom., 2020, 34, e8951 CrossRef CAS PubMed.
- L. Orlandi, E. Calizza, G. Careddu, P. Carlino, M. L. Costantini and L. Rossi, The effects of nitrogen pollutants on the isotopic signal (Δ15N) of Ulva lactuca: Microcosm experiments, Mar. Pollut. Bull., 2017, 115, 429–435 CrossRef CAS PubMed.
- A. S. Bateman and S. D. Kelly, Fertilizer nitrogen isotope signatures, Isot. Environ. Health Stud., 2007, 43, 237–247 CrossRef CAS PubMed.
- T. H. E. Heaton, Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: A review, Chem. Geol. (Isotope Geoscience Section), 1986, 59, 87–102 CrossRef CAS.
- F. C. Alldred, D. R. Gröcke, C. Y. Leung, L. P. Wright and N. Banfield, Diffuse and concentrated nitrogen sewage pollution in island environments with differing treatment systems, Sci. Rep., 2023, 13, 4838 CrossRef CAS PubMed.
- D. R. Gröcke, B. Racionero-Gómez, J. W. Marschalek and H. C. Greenwell, Translocation of isotopically distinct macroalgae: A route to low-cost biomonitoring?, Chemosphere, 2017, 184, 1175–1185 CrossRef PubMed.
- G. H. Pyke and P. R. Ehrlich, Biological collections and ecological/environmental research: A review, some observations and a look to the future, Biol. Rev., 2010, 85, 247–266 CrossRef PubMed.
- A. M. Lister, S. J. Brooks, P. B. Fenberg, A. G. Glover, K. E. James, K. G. Johnson, E. Michel, B. Okamura, M. Spencer, J. R. Stewart, J. A. Todd, E. Valsami-Jones and J. Young, Trends Ecol. Evol., 2011, 26, 153–154 CrossRef PubMed.
- C. Lavoie, Biological collections in an ever changing world: Herbaria as tools for biogeographical and environmental studies, Perspect. Plant Ecol. Evol. Systemat., 2013, 15, 68–76 CrossRef.
- M. R. Pie, S. P. Batke, J. Reyes-Chávez and T. Dallimore, Fern and lycophyte niche displacement under predicted climate change in Honduras, Plant Ecol., 2022, 223, 613–625 CrossRef.
- C. C. Davis, The herbarium of the future, Trends Ecol. Evol., 2022, 38, 412–423 CrossRef PubMed.
- E. A. Miller, S. E. Lisin, C. M. Smith and K. S. Van Houtan, Herbaria macroalgae as a proxy for historical upwelling trends in Central California, Proc. R. Soc. B, 2020, 287, 20200732 CrossRef CAS PubMed.
- B. Alfonso, M. Sansón, C. Sangil, F. J. Expósito, J. P. Díaz and J. C. Hernández, Herbarium macroalgae specimens reveal a rapid reduction of thallus size and reproductive effort related with climate change, Mar. Environ. Res., 2021, 174, 105546 CrossRef PubMed.
- S. J. Hawkins, K. A. O’Shaughnessy, L. A. Adams, W. J. Langston, S. Bray, J. R. Allen, S. Wilkinson, K. Bohn, N. Mieszkowska and L. B. Firth, Recovery of an urbanised estuary: Clean-up, de-industrialisation and restoration of redundant dock-basins in the Mersey, Mar. Pollut. Bull., 2020, 156, 111150 CrossRef CAS PubMed.
- Mersey Basin Campaign, River Mersey, https://www.merseybasin.org.uk/#:%7E:text=TheMerseyBasinCampaignbegan,MerseyBasinCampaignin2010 Search PubMed.
- N. Ritchie-Noakes, Liverpool's Historic Waterfront: The World's First Mercantile Dock System, H.M. Stationary Office, London, 1984 Search PubMed.
- ONS, Census 2021, https://www.ons.gov.uk/visualisations/censuspopulationchange/E08000003/, accessed 7 October 2023.
- L. R. Burton, The Mersey Basin: An historical assessment of water quality from an anecdotal perspective, Sci. Total Environ., 2003, 314–316, 53–66 CrossRef CAS PubMed.
- L. R. Burton, A. Howard and B. Goodall, Construction of a historical water pollution index for the Mersey Basin, Area, 2003, 35, 438–448 CrossRef.
- P. G. W. Jones and S. M. Haq, The Distribution of Phaeocystis in the Eastern Irish Sea, J. Mar. Sci., 1963, 28, 8–20 Search PubMed.
- S. Meredith, Water Privatisation: the dangers and the benefits, Long. Range Plan., 1992, 25, 72–81 CrossRef.
- BBC, River Mersey should be sewage free by 2030, says metro mayor, 2021, https://www.bbc.co.uk/news/uk-england-merseyside-59406932, last accessed 3 March 2024.
- The Rivers Trust, Sewage in Our Rivers, 2024, https://theriverstrust.org/sewage-map, accessed 7 March 2024.
- B. Jessop, Neoliberalization, uneven development, and Brexit: further reflections on the organic crisis of the British state and society, Eur. Plann. Stud., 2018, 26, 1728–1746 CrossRef.
- M. L. Dailer, R. S. Knox, J. E. Smith, M. Napier and C. M. Smith, Using δ15N values in algal tissue to map locations and potential sources of anthropogenic nutrient inputs on the island of Maui, Hawai'i, USA, Mar. Pollut. Bull., 2010, 60, 655–671 CrossRef CAS PubMed.
- J. R. Allen, S. B. Wilkinson and S. J. Hawkins, Redeveloped docks as artificial lagoons: The development of brackish-water communities and potential for conservation of lagoonal species, Aqua. Conserv.: Mar. Fresh. Ecosyst., 1995, 5, 299–309 CrossRef.
- S. B. Wilkinson, W. Zheng, J. R. Allen, N. J. Fielding, V. C. Wanstall, G. Russell and S. J. Hawkins, Water quality improvements in Liverpool docks: The role of filter feeders in algal and nutrient dynamics, Mar. Ecol., 1996, 17, 197–211 CrossRef CAS.
- R. M. Connolly, D. Gorman, J. S. Hindell, T. N. Kildea and T. A. Schlacher, High congruence of isotope sewage signals in multiple marine taxa, Mar. Pollut. Bull., 2013, 71, 152–158 CrossRef CAS PubMed.
- R. J. Pruell, B. K. Taplin, A. J. Oczkowski, J. S. Grear, W. G. Mendoza, A. R. Pimenta, A. R. Hanson and K. M. Miller, Nitrogen isotope fractionation in a continuous culture system containing phytoplankton and blue mussels, Mar. Pollut. Bull., 2022, 150, 110745 CrossRef PubMed.
- I. G. Viana and A. Bode, Stable nitrogen isotopes in coastal macroalgae: Geographic and anthropogenic variability, Sci. Total Environ., 2013, 443, 887–895 CrossRef CAS PubMed.
- D. M. Sigman, M. A. Altabet, D. C. McCorkle, R. Francois and G. Fischer, The δ15N of nitrate in the Southern Ocean: Nitrogen cycling and circulation in the ocean interior, J. Geophys. Res.: Oceans, 2000, 105, 19599–19614 CrossRef CAS.
- J. Galloway, A. Leach, J. W. Erisman and A. Bleeker, Nitrogen: the historical progression from ignorance to knowledge, with a view to future solutions, Soil Res., 2017, 55, 417–424 CrossRef.
- P. Cao, C. Lu and Z. Yu, Historical nitrogen fertilizer use in agricultural ecosystems of the contiguous United Stages during 1850-2015: application rate, timing and fertiliser types, Earth Syst. Sci. Data, 2018, 10, 969–984 CrossRef.
- L. Rosenthal, The River Pollution Dilemma in Victorian England: Nuisance Law versus Economic Efficiency, Routledge, London, 1st edn, 2014 Search PubMed.
- M. Hughes, The Victorian London sanitation projects and the sanitation of projects, Int. J. Proj. Manag., 2013, 31, 682–691 CrossRef.
- E. Porter, Pollution in Four Industrialised Estuaries: Studies in Relation to Changes in Population and Industrial Development; Four Case Studies Undertaken for the Royal Commission on Environmental Pollution, H.M. Stationary Office, London, 1973, https://archive.org/details/pollutioninfouri0000port Search PubMed.
- R. J. Davenport, M. Satchell and L. M. W. Shaw-Taylor, Cholera as a ‘sanitary test’ of British cities, 1831–1866, Hist. Fam., 2019, 24, 404–438 CrossRef PubMed.
- S. Halliday, The Great Stink of London: Sir Joseph Bazalgette and the Cleansing of the Victorian Metropolis, Sutton Publishing, 1999 Search PubMed.
- H. S. Lee and B. Liao, Anaerobic membrane bioreactors for wastewater treatment: Challenges and opportunities, Water Environ. Res., 2021, 93, 993–1004 CrossRef CAS PubMed.
- R. E. B. Reid, B. E. Crowley and R. J. Haupt, The prospects of poop: a review of past achievements and future possibilities in faecal isotope analysis, Biol. Rev., 2023, 98, 2091–2113 CrossRef CAS PubMed.
- M. I. Bird, J. Haig, S. Ulm and C. Wurster, A carbon and nitrogen isotope perspective on ancient human diet in the British Isles, J. Archaeol. Sci., 2022, 137, 105516 CrossRef CAS.
- M. J. Whelan, C. Linstead, F. Worrall, S. J. Ormerod, I. Durance, A. C. Johnson, D. Johnson, M. Owen, E. Wiik, N. J. K. Howden, T. P. Burt, A. Boxall, C. D. Brown, D. M. Oliver and D. Tickner, Is water quality in British rivers “better than at any time since the end of the Industrial Revolution”, Sci. Total Environ., 2022, 843, 157014 CrossRef CAS PubMed.
- N. J. K. Howden, T. P. Burt, F. Worrall, M. J. Whelan and M. Bieroza, Nitrate concentrations and fluxes in the River Thames over 140 years (1868–2008): Are increases irreversible?, Hydrol. Processes, 2010, 24, 2657–2662 CrossRef CAS.
- P. D. Jones, The Mersey Estuary - Back from the dead? Solving a 150-Year Old Problem, Water Environ. J., 2000, 14, 124–130 CrossRef CAS.
- OFWAT, The Development of the Water Industry in England and Wales, 2006, https://www.ofwat.gov.uk/publication/the-development-of-the-water-industry-in-england-and-wales/ Search PubMed.
- K. M. Rogers, Stable carbon and nitrogen isotope signatures indicate recovery of marine biota from sewage pollution at Moa Point, New Zealand, Mar. Pollut. Bull., 2003, 46, 821–827 CrossRef CAS PubMed.
- UK Government, Control of Pollution Act 1974, UK Government, London, 1974, https://www.legislation.gov.uk/ukpga/1974/40 Search PubMed.
- W. Howarth, Water Pollution: Improving the Legal Controls, J. Environ. Law, 1989, 1, 25–37 CrossRef.
- S. Kidd and D. Shaw, The Mersey Basin and its River Valley Initiatives: An appropriate model for the management of rivers?, Local Environ., 2000, 5, 191–209 CrossRef.
- UK Government, The Water Act, UK Government, 1989, https://www.legislation.gov.uk/ukpga/1989/15/contents Search PubMed.
- A. Schaefer, Corporate greening and changing regulatory regimes: The UK water industry, Bus. Strat. Environ., 2009, 18, 320–333 CrossRef.
- DEFRA, Sewage Treatment in the UK: UK Implementation of the EC Urban Waste Water Treatment Directive, London, 2002, https://www.gov.uk/government/publications/sewage-treatment-in-the-uk-2002 Search PubMed.
- K. Bakker, Neoliberalizing nature? market environmentalism in water supply in England and Wales, Ann. Assoc. Am. Geogr., 2005, 95, 542–565 CrossRef.
- S. Van Wynsberge, F. Antypas, M. Brisset, A. Desnues, L. Jamet, L. Lagourgue, C. Payri, T. Jauffrais and H. Lemonnier, A new set of N isotopic reference values for monitoring Ulva green tides in coral reef ecosystems, Mar. Pollut. Bull., 2024, 200, 116152 CrossRef CAS PubMed.
- G. Aertebjerg, J. Carstensen, K. Dahl, J. Hansen, K. Nygaard, B. Rygg, K. Sørensen, G. Severinsen, S. Casartelli, W. Schrimpf, C. Schiller and J. N. Druon, Eutrophication in Europe's Coastal Waters, Copenhagen, 2001 Search PubMed.
- Environment Agency, Mersey Water Body, https://environment.data.gov.uk/catchment-planning/WaterBody/GB531206908100, accessed 3 March 2024.
- DEFRA, Consultation on the Government's Storm Overflows Discharge Reduction Plan, 2022, https://assets.publishing.service.gov.uk/media/65115ef02f404b000dc3d823/Government_response_to_the_overflows_consultation.pdf, last accessed, 6 March 2024 Search PubMed.
- J. Hobson and B. Holmes, BBC Online, 2023, https://www.bbc.co.uk/news/uk-england-merseyside-66455794#, last accessed 3 March 2024.
- BBC Panorama, The Water Pollution Cover-Up, 2023, https://www.bbc.co.uk/programmes/m001t4g5, last accessed 6 March 2024.
- National Rivers Authority, The Mersey Estuary: a Report on Environmental Quality, Harlequin Colourprint, Bristol, 1995 Search PubMed.
- United Utilities, Storms Overflow Report, Warrington, 2022, https://www.unitedutilities.com/corporate/responsibility/environment/, last accessed 7 April 2023 Search PubMed.
- L. Thorp, More than 147 days' worth of raw sewage dumped in Merseyside water last year, Liverpool Echo, 2023, https://www.liverpoolecho.co.uk/news/liverpool-news/more-147-days-worth-raw-26698627#, last accessed 6 March 2024.
- Top of the Poops, Pollution Summary, 2022, https://top-of-the-poops.org/waterway/united-utilities/river-mersey, accessed 6 March 2024.
- F. Fripiat, A. Martinez-Garcia, S. Fawcett, P. Kemeny, A. Studer, S. Smart, F. Rubach, S. Oleynik, D. Sigman and G. Haug, The isotope effect of nitrate assimilation in the Antarctic Zone: Improved estimates and paleoceanographic implications, Geochim. Cosmochim. Acta, 2019, 247, 261–279 CrossRef CAS.
- T. Onodera, K. Komatsu, A. Kohzu, G. Kanaya, M. Mizuochi and K. Syutsubo, Evaluation of stable isotope ratios (δ15N and δ18O) of nitrate in advanced sewage treatment processes: Isotopic signature in four process types, Sci. Total Environ., 2021, 762, 144120 CrossRef CAS PubMed.
- M. F. Lehmann, D. M. Sigman, D. C. McCorkle, J. Granger, S. Hoffmann, G. Cane and B. G. Brunelle, The distribution of nitrate 15N/14N in marine sediments and the impact of benthic nitrogen loss on the isotopic composition of oceanic nitrate, Geochim. Cosmochim. Acta, 2007, 71, 5384–5404 CrossRef CAS.
- M. Alkhatib, M. F. Lehmann and P. A. del Giorgio, The Nitrogen Isotope Effect of Benthic Remineralisation-Nitrification-Denitrification Coupling in an Estuarine Environment, Biogeosciences, 2012, 9, 1633–1646 CrossRef CAS.
- D. B. Nedwell, L. F. Dong, A. Sage and G. J. C. Underwood, Variations of the Nutrients Loads to the Mainland U.K. Estuaries: Correlation with Catchment Areas, Urbanisation and Coastal Eutrophication, Estuar. Coast Shelf Sci., 2002, 54, 951–970 CrossRef CAS.
- T. Giakoumis and N. Voulvoulis, Combined sewer overflows: relating event duration monitoring data to wastewater systems' capacity in England, Environ. Sci. (Camb.), 2023, 9, 707–722 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00015c |
| This journal is © The Royal Society of Chemistry 2024 |