Open Access Article
Open Access ArticleWhy there is no evidence that pyridine killed the English crabs†
Alex T.
Ford
 *a,
Mark F.
Fitzsimons
*a,
Mark F.
Fitzsimons
 b and
Crispin
Halsall
b and
Crispin
Halsall
 c
c
aInstitute of Marine Sciences, University of Portsmouth, Ferry Road, Portsmouth, PO4 9LY, UK. E-mail: alex.ford@port.ac.uk
bMarine Institute, University of Plymouth, Drake Circus, Plymouth, PL4 8AA, UK
cLancaster Environment Centre, Lancaster University, Bailrigg, Lancaster LA1 4YQ, UK
First published on 5th August 2024
Abstract
The North East coast of England experienced a mass mortality event in late 2021 affecting millions of crabs and lobsters. The die-off coincided with the redevelopment of one of the UK's flagship ports, prompting local scientists to suggest the remobilization of dredged industrial contaminants as a cause. A multi-agency investigation found no definitive causal factor; however, re-evaluation of data by consultants drew a different conclusion, linking the industrial compound pyridine to the crustacean deaths. Authors of an unpublished study subsequently claimed that their data demonstrated pyridine to be exceptionally toxic and that their modeling explained the coastal distribution of washups. These data were presented to a cross-party Environmental, Fisheries and Rural Affairs (EFRA) committee in the UK parliament and led to the commissioning of an independent panel to review the data. This panel was also unable to identify a definitive cause, but found that a major role for pyridine was ‘very unlikely’. Unfortunately, the debate has been highly politicised, with misleading information aired by the two leading political parties. Here, several members of that independent review panel refute the pyridine link to the mass mortality, based on both reported data and the known chemistry and behaviour of this molecule, and highlight where the science has been misrepresented by the media.
Environmental significanceWe challenge the hypothesis that the industrial compound pyridine was responsible for a mass mortality event that killed large numbers of crustaceans along 70 km of the English coastline. This event made international news and has been the subject of investigative documentaries and independent reviews. It links with broader topics such as SDGs on water quality and life below water, as well as science communication and trust in science/scientists more generally. The manuscript carefully goes through a series of 5 key questions refuting the hypothesis that an industrial compound called pyridine caused a mass die-off of crustacean life across 70 km of English coastline. This is significant as it details how mistrust in government agency data, partisan politics and arguably overconfidence by the media in research, which had not undergone peer review and appropriate scientific scrutiny, played a role. |
1 Background
In autumn 2021, the North East coast of England experienced a mass mortality event that affected millions of crabs and lobsters across a 70 km stretch of coastline.1 This event severely impacted the local inshore fishery, making international news and, more recently, notable shifts in benthic invertebrate ecosystems. The die-off coincided with the dredging and redevelopment of one of the UK's flagship ports, in the Tees estuary, prompting local scientists and environmental campaigners to suggest the remobilization of industrial contaminants as a cause, and opposition politicians to call for the suspension of Teesside redevelopment. Following an investigation by multiple government agencies, no definitive causal factor was determined, although it was speculated, based on satellite data, that the cause may have been an algal bloom.1 Re-evaluation of Environment Agency data by consultants, funded by the fishing industry, drew a different conclusion and proposed the industrial compound pyridine as a cause.2 Pyridine was widely used in industrial processes and historically manufactured in the area. The Environment Agency urged extreme caution in overextrapolating their pyridine data within the biota, however, as the methodology had not been optimized and a limited number of crabs were analysed.These developments led to speculation in the media that there had been a cover-up by the UK government. For example, an article in the Guardian newspaper on the 6th June 2022 entitled “The dead shellfish littering our beaches tell you a lot about safety and secrecy in Britain” started with the opening line:
“With every passing week, it looks more like a cover-up. The repeated mass strandings of crabs and lobsters on the coast of north-east England, and the ever less plausible explanations provided by the government, are the outward signs of an undersea disaster and a grim new politics3”.
The Times newspaper in January 2023 ran with the headlines “Cover-up claim over shellfish mass deaths off Teesside4” and “DEFRA won't dredge up truth about toxic seas5”.
Authors of an unpublished study in 2022, led by several universities in North East England, further pushed the pyridine hypothesis when they announced that their investigation had demonstrated pyridine to be exceptionally toxic to crustaceans, while their coastal modeling explained the distribution of dead crustaceans along the coastline.6 In their study, they reported that pyridine:
“is highly toxic to C. pagurus [edible crabs], ranging from distinctive patterns of acute toxicity to observable behavioural responses at much lower concentrations. The observed behaviours under acute exposure correspond with reports from the fishing community and other coastal users during the mass mortalities, specifically that moribund animals were presenting as twitching and paralysed6”.
The data were presented to a cross-party Environmental, Fisheries and Rural Affairs (EFRA) committee in the UK parliament in October 2022 and led the government to commission an independent review of the available evidence.7 This independent panel was commissioned in early December 2022 and was tasked with reviewing all the available evidence and trying to determine the cause of the mass mortality event (see terms of ref. 8). Their report was published on the 20th January 2023 and the panel, like the government agencies before them, was unable to identify a definitive cause, but found that a major role for pyridine was ‘very unlikely’.9 Unfortunately, the debate became highly politicised, with misleading information aired by the two largest political parties. Here, several members of that independent review panel refute the pyridine link to the mass mortality and highlight where the science has been misrepresented by the media. In doing so, we explain why pyridine did not kill the Teesside crabs. We do this by answering the following 5 questions.
2 How strong is the evidence that pyridine was found in high concentrations in crab tissues?
Original data from a joint report1 by the UK Environment Agency (EA) and the Centre for Environment, Fisheries and Aquaculture Sciences (CEFAS) found elevated concentrations of pyridine in crabs (Table 1). However, the results between the impacted and reference locations were not significantly different (Mann Whitney T Test; p = 0.2). The EA urged caution as they were using a non-optimised semi-quantitative gas chromatography-mass spectrometry (GC-MS) methodology and stated that “This is unlikely to be very accurate, but we don't have the data to confirm this”. However, some felt there was sufficient evidence to warrant further investigation,6 given that pyridine was an industrial compound, manufactured and discharged at the location.| Vicinity of the mortality event | Concentration (mg kg−1) | Reference locations | Concentration (mg kg−1) |
|---|---|---|---|
| Saltburn | 439 | Norfolk Wash | 195 |
| Bran sands | 255 | Norfolk Wash | 3 |
| Seaton | 204 | St Mary's Lighthouse | 78 |
| Runswick | 20 | Penzance | 35 |
| Mean (SD) | 229.5 (172.3) | Mean | 77.8 (84.0) |
This methodology was recently revisited by a CEFAS study, which further developed the analytical method to take account of potential matrix effects associated with the sampling and analysis of crustacean tissue samples and marine sediments. This study confirmed that the Environment Agency was correct to be cautious in their interpretation of the elevated pyridine results.10 Reanalysis of crustacean samples that had originally returned high indicative pyridine levels with the EA method (3–429 mg kg−1; Table 1) demonstrated very low concentrations of the chemical (<0.02–0.077 mg kg−1, over 3 orders of magnitude lower). Analysis of additional crab samples, unrelated to the events, also demonstrated the presence of pyridine at very low levels (<0.02–0.139 mg kg−1). Therefore, the original rationale and justification for pursuing pyridine as a causal factor are substantially weakened.
3 Is pyridine ‘exceptionally’ toxic to crustaceans?
Evidence presented to the EFRA inquiry7 and repeated in the UK parliament, across news media and several special documentaries was that pyridine was “exceptionally toxic”. As evidence, the EFRA committee were told:“…we [authors of Eastabrook et al.6] undertook our rigorous scientific work that was done to international accredited standards, we found that pyridine was not just toxic, but exceptionally toxic7”.
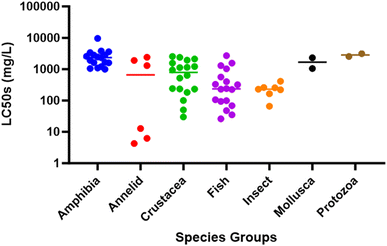
All chemical compounds will be acutely toxic when presented to aquatic organisms at sufficiently high concentrations. What makes them exceptionally toxic is that they do not need to be present at particularly high concentrations to cause mortality, which is where comparative data become important. Searching archived lethal toxicity data (US EPA Ecotoxicology Knowledgebase: https://cfpub.epa.gov/ecotox/; ESI Date Table†) on pyridine hydrochloride (CAS-no: 110-86-1) reveals that the LC50s for crustaceans (50–2550 mg L−1; median ∼900 mg L−1) and fish (26–1560 mg L−1; median ∼400 mg L−1) are comparatively higher (i.e., less toxic) compared to other many ubiquitous known industrial legacy contaminants. In fact, the levels are similar to those for caffeine, a ubiquitous compound found in wastewater, so classifying pyridine as exceptionally toxic to crustaceans, or any aquatic organisms, is questionable.
The results of toxicology experiments conducted on edible crabs by Eastabrook et al. (unpublished preprint6), and presented as evidence to the EFRA inquiry, are reproduced in Table 2. These results are particularly revealing as they highlight some of the issues that can arise from modeling toxicology with a small number of replicates (n = 3 per treatment). Eastabrook et al.6 reported that:
| Time (hours) | LC10 mg L−1 | LC20 mg L−1 | LC50 mg L−1 | NOEC mg L−1 | LOEC mg L−1 | R 2 |
|---|---|---|---|---|---|---|
| 24 | 17.38 | 18.11 | 19.44 | 20 | 50 | 0.69 |
| 72 | 0.066 | 0.26 | 2.75 | 20 | 50 | 0.37 |
“We extrapolate our findings in crabs to the other decapod species impacted by the 2021 autumn mass mortalities. Our LC50 data indicates that C. pagurus is more susceptible to pyridine toxicity than other commonly used bioassay organisms (fish and non-decapod crustaceans)6”.
In this instance, the “No” and “Lowest Observed Effects Concentration” (NOECs and LOECs) for both 24 and 72 hours were 20 and 50 mg L−1, respectively, and above the modeled LC50s. Based on existing published literature, the LOECs in Eastabrook et al.,6 if accurate, would be at the lower end of what had been published for LC50s in crustaceans (see Fig. 1). Their modeled LC10 [lethal dose predicted to kill 10% of the sampled population] was 0.066 mg L−1, which is over 750 times lower than the calculated LOEC. Typically, extrapolation would not be performed past the NOECs and LOECs; however, in this study, the LC10s were used to model crab mortality 70 km down the coastline. For example, the EFRA committee were told:
“We modeled based upon the rate of pyridine on the same scale CEFAS used, so a total release of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 000 litres. We ran the model using the tides and the currents affecting the coast on each given day that the dredging campaign took place and for the following weeks. The model clearly showed that pyridine in water would be transported all the way down to Whitby and Robin Hood's Bay. From that we could pull out toxicity values based upon what we had generated in my laboratory. We could then make reasonably sound estimates of the extent of population loss from the crabs and lobsters in that given area…. Based upon our work, we predicted that half of the crab population would have died in the first 24 hours. Moving further down the coast to Runswick Bay, we predicted that 30% would have died within 24 hours. Then moving right the way down to Whitby, we believed that we would have lost 10% as a minimum of the population there. Therefore, we have shown that pyridine is extremely toxic.7”
000 litres. We ran the model using the tides and the currents affecting the coast on each given day that the dredging campaign took place and for the following weeks. The model clearly showed that pyridine in water would be transported all the way down to Whitby and Robin Hood's Bay. From that we could pull out toxicity values based upon what we had generated in my laboratory. We could then make reasonably sound estimates of the extent of population loss from the crabs and lobsters in that given area…. Based upon our work, we predicted that half of the crab population would have died in the first 24 hours. Moving further down the coast to Runswick Bay, we predicted that 30% would have died within 24 hours. Then moving right the way down to Whitby, we believed that we would have lost 10% as a minimum of the population there. Therefore, we have shown that pyridine is extremely toxic.7”
The R2 value for the regression analysis was only 0.37, indicating the modeled best fit equation was not strong at 72 h. Unusually for lethality testing, the LC50 values were modeled not using probit analysis on the proportion (%) of the population that died, but using a binary regression model on individual crabs being alive [1] or dead [0], which meant after 72 h, the model predicted, with confidence intervals, that the crabs [and not a proportion of the population] were between 0 and 1 and thus neither alive nor dead. Therefore, in answer to the question “is pyridine exceptionally toxic to crustaceans” the answer from existing data is no. We would argue that having modelled LC50s below the ‘no observed effect concentrations’ makes these data unreliable. Furthermore, extrapolating down to LC10s to help explain mortality events 70 km down a coastline is questionable.
4 Has pyridine ever been recorded at concentrations likely to cause acute toxicity?
The Environment Agency has been monitoring water in the Tees estuary using GC-MS screening, which includes pyridine, as part of their surveillance programme since 2011.1,9 The highest recorded concentration of pyridine was 2.4 μg L−1, at a time when it was being actively manufactured and discharged under license by the EA. This concentration maximum for 2012 is approximately 375![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times lower than the median LC50 values for crustaceans. Following the mass mortality event, there was no pyridine measured in the water (i.e., below the limits of detection). Therefore, it is highly unlikely for pyridine to have reached acute concentrations without detection, which persisted for 70 km down the coastline.
000 times lower than the median LC50 values for crustaceans. Following the mass mortality event, there was no pyridine measured in the water (i.e., below the limits of detection). Therefore, it is highly unlikely for pyridine to have reached acute concentrations without detection, which persisted for 70 km down the coastline.
5 Does pyridine adsorb to sediments?
Pyridine (C5H5N) is a monoaromatic molecule and can be regarded as a derivative of benzene, where a sp2-hybridised nitrogen atom replaces a CH unit. Pyridine is highly soluble in water, unlike benzene. This enhanced solubility in water stems from the presence of an electronegative N atom, which polarises the molecule and withdraws electron density from the ring. Some physico-chemical properties of pyridine are shown in Table 3. As a water-soluble, low molecular weight, monoaromatic chemical, pyridine possesses a very low octanol–water partition coefficient (Kow) and organic carbon–water partitioning coefficient (Koc); the latter effectively describes the partitioning of a chemical between water and the organic component of soil or sediments. As a comparison, benzene, which does not have a propensity to partition or sorb to sediments, has a Koc of ∼55, while pyridine is lower still with a Koc of ∼7 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.84 in Table 3). A low molecular weight, polycyclic aromatic hydrocarbon, like naphthalene, has a much higher Koc of ∼1000 and hence is commonly present associated with sediments in industrial ports, etc. This is not the case for pyridine.
0.84 in Table 3). A low molecular weight, polycyclic aromatic hydrocarbon, like naphthalene, has a much higher Koc of ∼1000 and hence is commonly present associated with sediments in industrial ports, etc. This is not the case for pyridine.
| Property | Value | Reference |
|---|---|---|
| Molecular mass | 79.10 g mol−1 | Sax N. I., Lewis R. J., 1987 (ref. 11) |
| Physical state | Liquid | Sax N. I., Lewis R. J., 1987 (ref. 11) |
| Boiling point | 115.5 °C | Weast R. C. (ed.), 1985 (ref. 12) |
| Solubility in water at 20 °C | Very soluble | Sax and Lewis, 1987 (ref. 11) |
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow Kow |
0.64/1.04 | Verschueren K., 1983 (ref. 13) |
Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc Koc |
0.84 | Roy W. R., Griffin R. A., 1985 (ref. 14) |
| pKa | 5.19 | Reinhardt C. F., Brittelli M. R., 1981 (ref. 15) |
Pyridine is a weak base in aqueous solution, much weaker than ammonia or saturated amines (pKa ∼10). This affects the extent to which pyridine can be protonated (giving rise to the cationic pyridinium ion) and attached and bonded to the surface exchange sites of particles, which are negatively charged. At a pH of 8.1, which is in the range expected for UK coastal seawater, the ratio of the protonated pyridinium ion [C5H6N]+ to neutral pyridine [C5H5N] would be 0.0011. At the same pH, the saturated amine, trimethylamine, (CH3)3N, would have a protonated to unprotonated ratio of 41.0122. As such, only a negligible percentage of pyridine could adsorb to particulate matter at seawater pH. Even in deeper, marine sediments where anoxic conditions might prevail, with a relatively low pH of ∼6, the proportion of the ‘stickier’ protonated pyridinium cation will still be low (∼12%), given that the pKa of pyridine is 5.19. Disturbance of old sediments through dredging operations will therefore not give rise to marked re-partitioning or desorption of pyridine back into the dissolved phase, as there is a negligible sorbed quantity of pyridine in the first place. Alkylated varieties of pyridine, or other higher molecular weight N-containing polycyclic compounds like quinoline (2-ring) or acridine (3-ring), have higher Koc values than pyridine and will show stronger partitioning tendencies to sediment. Historically, these chemicals, alongside pyridine, would have been released as waste through industrial coal-processing activities (carbonisation, gasification, and liquefaction).16 Accurate modeling of active sediment movement processes, such as dredging and disposal of estuarine sediments, would need to account for the low propensity of pyridine to adsorb to particulate matter.
In marine and estuarine systems, the environmental residence time of pyridine is considered to be short, on the order of days (water column) to months (sediments).17 Given its high aqueous solubility, volatilisation loss from surface waters will be less pronounced compared to other volatile chemicals such as benzene. Microbial biodegradation is considered to be a key loss or transformation process for pyridine. Historical pyridine released from past coal processing activities in the Teesside area will have long dispersed and/or been biochemically transformed and degraded over the ensuing decades. Evidence from the literature indicates that pyridine residues present in sediments, even anoxic sediments, undergo biodegradation but at varying rates and influenced by the redox conditions pertinent to a given location.18,19 Initial biotransformation of pyridine gives rise to hydroxylated-derivatives, i.e., metabolites of catabolic driven processes by microorganisms.18–21 These compounds exhibit toxicity akin to pyridine (and higher molecular weight analogues of pyridine), but they are generally transient in the environment (shorter half-lives than pyridine) and their concentrations are low relative to pyridine itself.18–21 This infers that the likelihood of biotransformation products of pyridine being a casual factor is highly unlikely, especially given the low or non-detectable concentrations of pyridine measured in the environment at the time.
The independent panel report10 concluded that maintenance and capital dredging were both ‘highly’ and ‘exceptionally unlikely’, respectively, to be the cause of the mass die-off event in November 2021, the latter on the basis that capital dredging had not yet taken place around the time of the crab die-off. The joint investigation by the government agencies found no evidence for pyridine in the sediments or water around the time of the mortality event.1 The EFRA committee and the independent panel were presented with further data commissioned by the North East Fishing Collective from 2022, where pyridine was detected in 15 of 24 sediments from the Tees estuary.20 One explanation provided for the mass mortality event was that the dredging had tapped into an unknown reservoir of highly contaminated sediments containing pyridine.7 For example, the EFRA committee were told:
“Simply by the fact that we are measuring pyridine in the majority of our samples, in the Tees estuary and in the spoil zone, is a significant red flag that must be taken further, because it implies in those deeper sediments, where there is no oxygen present, you have a reservoir of pyridine there. What we believe we were measuring in our surface sediments was effectively the diffusion or the percolation from that pyridine reserve. It was coming to the surface and being interacted with in that destruction zone, and that is what we were measuring. Again, simply the fact that we were measuring pyridine in the sediments, given what we know about the chemistry, is of great concern…. We know the discharge of pyridine will have been reaching the whole way down that coast. We have even seen that from the sediment. You speak to the fisheries when they put their pots out at Whitby and they are finding out their pots are being covered in fine sediment. That never happened before. You are not just getting water being transported carrying pyridine; you are also getting the sediment itself.7”
The independent panel concluded that it was very unlikely that pyridine released from sediments could have caused the unusual mortality of crabs in the region.9 They based this upon the fact that pyridine concentrations that were measured in sediment samples (for example, the highest measured concentration of 42 μg kg−1 (ref. 22)) were too low, along with the total dredged and disposed mass of sediment (∼150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes). Therefore, it was predicted that a total of <10 kg (or <10.2 L) of ‘released’ pyridine at either the dredged area and/or the offshore disposal site could be released.9 This quantity is far too low to be toxicologically significant, even if pyridine was retained in a stratified water layer (e.g., associated with a salt wedge estuarine system).9 In response to the independent report, Newcastle University issued a press release stating that:
000 tonnes). Therefore, it was predicted that a total of <10 kg (or <10.2 L) of ‘released’ pyridine at either the dredged area and/or the offshore disposal site could be released.9 This quantity is far too low to be toxicologically significant, even if pyridine was retained in a stratified water layer (e.g., associated with a salt wedge estuarine system).9 In response to the independent report, Newcastle University issued a press release stating that:
“…the dismissal of pyridine involvement also ignores the chemistry of the molecule, including its propensity to adsorb to sediment particles and its capacity to remain for many years in the environment if protected from oxygen. The report also overlooks the fact that we detected pyridine in surface sediment fully 7 months after the mass die-offs, and that we have been prevented from taking sediment core samples to quantify pyridine levels in the deeper sediment.23”
In contrast to claims of ignoring the chemistry, it was these chemical properties of pyridine and consideration of more realistic volumes that, in part, indicate the unlikelihood of this compound causing the mass mortality event. The claim that pyridine strongly binds to sediments and thus could form sufficient ‘reservoirs’ of the compound to cause acute toxicity is unlikely given its characteristics. Similarly, these same properties make this particular compound unlikely to have drifted down the coast ‘adsorbed to sediment particles’ again in sufficient quantities to cause mass mortality.
6 Could pyridine hang around long enough and at sufficient concentrations to cause acute mortality across 70 km of coastline?
We highlight that it is likely the modeled lethal toxicity values generated by Eastabrook et al.6 overestimated the toxicity of pyridine (see question 2). However, making the assumption that the LC50s are accurate, the independent panel report highlighted that the coastal modelling undertaken was deficient in two aspects: (1) an unrealistically large volume of substance was injected into the model simulation and (2) the model was approximately a factor of 10 more dispersive than most comparator observational estimates of coastal dispersion.9 The report states:“This modelling was based on release of large volumes of pyridine (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 000 L at two sites, or a total of 19.6 tonnes). Even with the large input of pyridine, the modelling demonstrates that pyridine concentrations in the water column are too low to cause mortality in crabs. For example, the highest concentration range of pyridine modelled in a seawater plume from both the dredged area of the Teesmouth and the spoil disposal site was between ∼1 to 10 μg L−1. That is ∼6 to 60-fold lower than the estimated 72 hours LC10 value for edible crab and ∼2000-fold lower than the estimated 24 hours LC10 value (reported by Eastabrook et al., 2022). While the pyridine modelling was undertaken to illustrate the geographical spread and duration of a potential plume rather than representing the absolute concentrations, it is evident that a very significant emission of pyridine (i.e. greater than >20
000 L at two sites, or a total of 19.6 tonnes). Even with the large input of pyridine, the modelling demonstrates that pyridine concentrations in the water column are too low to cause mortality in crabs. For example, the highest concentration range of pyridine modelled in a seawater plume from both the dredged area of the Teesmouth and the spoil disposal site was between ∼1 to 10 μg L−1. That is ∼6 to 60-fold lower than the estimated 72 hours LC10 value for edible crab and ∼2000-fold lower than the estimated 24 hours LC10 value (reported by Eastabrook et al., 2022). While the pyridine modelling was undertaken to illustrate the geographical spread and duration of a potential plume rather than representing the absolute concentrations, it is evident that a very significant emission of pyridine (i.e. greater than >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 000 L) would be required to achieve concentrations in seawater close to those that resulted in acute toxicity.9”
000 L) would be required to achieve concentrations in seawater close to those that resulted in acute toxicity.9”
Therefore, to summarise, the LC10s generated by Eastabrook et al.6 are over 750 times lower than their own derived lowest observed effect concentrations (LOECs) and the authors' own coastal modelling used an excessive volume (2 × 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L) of pyridine, which is 200× higher than estimates based on sediment data.9 Even with these excessive volumes for the dredged area and disposal site, the predicted pyridine concentrations of 1 to 10 μg L−1 in the vicinity of the dredging or disposal are ∼6 to 60 fold lower than their derived LC10s for 72 h. With the pyridine concentrations even close to the dredge and/or disposal locations not reaching acute toxicity thresholds, evidence that it could be concentrated enough to cause acute toxicity 70 km down the coast becomes very weak.
000 L) of pyridine, which is 200× higher than estimates based on sediment data.9 Even with these excessive volumes for the dredged area and disposal site, the predicted pyridine concentrations of 1 to 10 μg L−1 in the vicinity of the dredging or disposal are ∼6 to 60 fold lower than their derived LC10s for 72 h. With the pyridine concentrations even close to the dredge and/or disposal locations not reaching acute toxicity thresholds, evidence that it could be concentrated enough to cause acute toxicity 70 km down the coast becomes very weak.
7 Conclusions
We conclude that pyridine did not kill the Teesside crabs because there is no credible evidence that it was present in sufficient concentrations to cause acute toxicity and a localized die-off. The sampling that led to the hypothesis that pyridine was responsible was done using a very limited number of crabs (n = 4 per location) and using a methodology that was not validated. When this methodology was optimized and samples were reanalyzed, the caution recommended over their accuracy was vindicated. It therefore becomes even less credible that a die-off could have occurred across at least 70 km of coastline. The evidence presented to the EFRA committee and across media outlets claiming that pyridine was ‘exceptionally toxic’ to crustaceans is not substantiated by the published literature. Crabs were presented as uniquely sensitive to pyridine but again the literature suggests otherwise. The unpublished data presented to the committee is not without several limitations in the study design, as highlighted. Unfortunately, the crab mortality event became highly politicized, highlighting a potential lack of trust in the data presented by government agencies. The latest polls put trust in politicians (9%) at a 40 year low and the lowest since records began in 1983.24 It is therefore conceivable that trust in the data presented by government agencies may well have diminished due to speculation about central government interference. The data were presented to the EFRA committee in the context of university scientists vs. government scientists when the data and interpretations had not undergone rigorous scrutiny and peer review. The media were also quick to present the data with an unusual level of certainty. This presents a problem for those serving on the EFRA committee and for wider society, who need politicians to make informed decisions based on the best available science. Progress in understanding the cause of the crustacean mortality was not helped by the partisan behaviour of politicians and some members of the EFRA committee openly questioning the independence of the panel they requested be set up. Furthermore, the independent panel did not state or imply that dredging of historically contaminated harbours is safe and not without impact, as was presented by some politicians.Through several investigations, neither the EA, CEFAS nor the independent panel of scientists (CMEP) were able to identify a casual factor. The CMEP categorized parasites and/or disease as ‘as likely as not’ on the basis that crustaceans had presented a pathology (tremors and twitching) seen in other mass mortality events with crustaceans and the fact that there had not been widespread monitoring of novel viral/bacterial pathogens.9 These mass mortality events with crustaceans caused by disease were also known to occur in areas with multiple stressors (e.g., eutrophication, legacy contaminants and urban runoff). The panel reported that the likelihood category “would move to either very unlikely (<10% probability) if results of molecular screening were confirmed as negative; or very likely (>10% probability) if a broad diagnostic screen of these potential pathogens proved positive9”. The independent report by CMEP concluded that “It is possible that a combination of factors led to the unusual mortality, rather than one of the causal factors considered in this report9”. However, we feel that elevating pyridine disproportionally, among the thousands of chemical compounds present, is not justified when there is no evidence it was present at close to toxic concentrations, and it was detected at similar concentrations in other areas of the United Kingdom. As members of the independent panel that investigated the data available on the mass die-off event, we share the frustration of not finding a causal factor. It is therefore imperative that we improve our coastal monitoring so that we can better understand our changing environment.
Data availability
Data for this article were partly retrieved from the US EPA ECOTOX knowledgebase https://cfpub.epa.gov/ecotox/ and have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- DEFRA, Joint agency investigation into Teesside and Yorkshire Coast Crab and Lobster mortalities, Department for Environment, Food and Rural Affairs, London, 2022, May. 16, available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1082129/Joint_agency_investigation_into_Teesside_and_Yorkshire_Coast_Crab_and_Lobster_mortalities.pdf Search PubMed.
- T. Deere-Jones, Mass Mortality of marine species along N.E coast of England: (2nd Report) updated review of relevant data provided by DEFRA agencies and others, Briefing Paper to Whitby Commercial Fishing Association, 2022, Mar, available from: https://whitbylobsterhatchery.co.uk/storage/pdf/NEFC-MASS-MORTALITY-REPORT-TDJ-(MARCH-2022).pdf Search PubMed.
- The Guardian, The dead shellfish littering our beaches tell you a lot about safety and secrecy in Britain, The Guardian, 2022 Jun 6, available from: https://www.theguardian.com/commentisfree/2022/jun/06/dead-shellfish-beaches-safety-secrecy-britain.
- The Times, Cover-up claim over shellfish mass deaths off Teesside, The Times, 2023 Jan 14, available from: https://www.thetimes.co.uk/article/cover-up-claim-over-shellfish-mass-deaths-off-teesside-2l7lpczjd.
- The Times, DEFRA won't dredge up truth about toxic seas, The Times. 2023 Jan 23, available from: https://www.thetimes.co.uk/article/defra-wont-dredge-up-truth-about-toxic-seas-fzc38sqd5.
- C. L. Eastabrook, M. Morales Maqueda, C. Vagg, J. Idomeh, T. Nasif-Whiteshone, P. Lawrence, et al., Determining the toxicity and potential for environmental transport of pyridine using the brown crab Cancer pagurus (L.), bioRxiv, 2022, DOI:10.1101/2022.11.17.516169.
- EFRA, Sealife Mortality off the North East Coast - Oral evidence, 2022 Oct 25, available from: https://committees.parliament.uk/event/15058/formal-meeting-oral-evidence-session/.
- DEFRA, Assessment of unusual crustacean mortality in the north east of England in 2021 and 2022, Terms of Reference for the Crustacean Mortality Expert Panel (CMEP), available from: https://www.gov.uk/government/publications/assessment-of-unusual-crustacean-mortality-in-the-north-east-of-england-in-2021-and-2022/terms-of-reference-for-the-crustacean-mortality-expert-panel-cmep Search PubMed.
- G. Henderson, E. Bresnan, J. Brooke, K. Davidson, M. Dearnaley, M. Fitzsimons, et al., Independent Expert Assessment of Unusual Crustacean Mortality in the North-east of England in 2021 and 2022, Department for Environment, Food and Rural Affairs, London, 2023. available from: https://www.gov.uk/government/publications/assessment-of-unusual-crustacean-mortality-in-the-north-east-of-england-in-2021-and-2022.
- DEFRA, Development, validation, and application of a fully quantitative method for the determination of pyridine in crustacean tissues (and application of the same method in sediments), Department for Environment, Food and Rural Affairs, London, 2023. p. 29, available from: https://randd.defra.gov.uk/ProjectDetails?ProjectId=21344.
- N. I. Sax and R. J. Lewis. Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold Company, New York, NY, 1987. p. 982 Search PubMed.
- CRC Handbook of Chemistry and Physics, ed. R. C. Weast, CRC Press, Inc., Boca Raton, FL, 1985, p. 462 Search PubMed.
- K. Verschueren. Handbook of Environmental Data on Organic Chemicals, Van Nostrand Reinhold Company, New York, NY, 2nd edn, 1983, pp. 1035–1038 Search PubMed.
- W. R. Roy and R. A. Griffin, Mobility of organic solvents in water-saturated soil materials, Environ. Geol. Water Sci., 1985, 7, 241–247 CrossRef CAS.
- C. F. Reinhardt and M. R. Brittelli. Patty's industrial hygiene and toxicology, Toxicology, John Wiley and Sons, New York, NY, 3rd edn, 1981, vol. 2A, pp. 2727–2732 Search PubMed.
- K. V. Padoley, S. N. Mudliar and R. A. Pandey, Heterocyclic nitrogenous pollutants in the environment and their treatment options – an overview, Bioresour. Technol., 2008, 99(10), 4029–4043 CrossRef CAS PubMed.
- G. K. Sims, E. J. O'Loughlin and R. L. Crawford, Degradation of pyridines in the environment, Crit. Rev. Environ. Sci. Technol., 1989, 19(4), 309–340 CAS.
- S. Fetzner, Bacterial degradation of pyridine, indole, quinoline, and their derivatives under different redox conditions, Appl. Microbiol. Biotechnol., 1998, 49, 237–250 CrossRef CAS.
- J. P. Kaiser, Y. Feng and J.-M. Bollag, Microbial metabolism of pyridine, quinoline, acridine, and their derivatives under aerobic and anaerobic conditions, Microbiol. Rev., 1996, 60, 483–498 CrossRef CAS PubMed.
- H. Liu, H. Yang, X. Yin, S. Wang, S. Fang and H. Zhang, A novel pbd gene cluster responsible for pyrrole and pyridine ring cleavage in Rhodococcus ruber A5, J. Hazard. Mater., 2024, 15(464), 132992 CrossRef PubMed.
- V. Časaitė, R. Stanislauskienė, J. Vaitekūnas, D. Tauraitė, R. Rutkienė, R. Gasparavičiūtė and R. Meškys, Microbial degradation of pyridine: a complete pathway in Arthrobacter sp. strain 68b deciphered, Appl. Environ. Microbiol., 2020, 86(15), e00902–e00920 CrossRef PubMed.
- North East Fishing Collective, North East Research Group Investigative Report v1.3, 2022 Oct. p. 41 Search PubMed.
- Newcastle University, Loss of sealife Teesside report, 2023, available from: https://www.ncl.ac.uk/press/articles/latest/2023/01/lossofsealifeteessidereport/ Search PubMed.
- IPSOS, Ipsos Veracity Index 2023: Trust in professions survey, 2023, available from: https://www.ipsos.com/en-uk/ipsos-trust-in-professions-veracity-index-2023.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00006d |
| This journal is © The Royal Society of Chemistry 2024 |