Open Access Article
Open Access ArticleSeparation of coal combustion residue for critical element extraction and other bulk uses†
Kanishk Kumar
Karan
ab,
R. Ebhin
Masto
 *ab,
Hridesh
Agarwalla
ab,
Siddharth
Bari
b,
Manish
Kumar
ab,
P.
Gopinathan
ab,
Bodhisatwa
Hazra
ab,
Sujan
Saha
ab and
Sudip
Maity
ab
*ab,
Hridesh
Agarwalla
ab,
Siddharth
Bari
b,
Manish
Kumar
ab,
P.
Gopinathan
ab,
Bodhisatwa
Hazra
ab,
Sujan
Saha
ab and
Sudip
Maity
ab
aAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, Uttar Pradesh, India
bCSIR-Central Institute of Mining and Fuel Research, Digwadih Campus, Dhanbad-828108, Jharkhand, India. Tel: +91-326-2388339(O) Tel: +91-326-2382908(R) Tel: +91-9431542415E-mail: mastocfri@yahoo.com; ebhinmasto@cimfr.nic.in
First published on 14th November 2023
Abstract
The demand for critical and rare earth elements is surging and coal combustion residue could be an alternate source of critical elements. Data on the concentration of critical and rare earth elements (REYs) in different size fractions of fly ash would help in segregation of the ash. This study was conducted with the objective of examining the possibility of separation of coal ash into a size fraction useful for element extraction and the rest for bulk uses like cement, concrete, landfill, roads, embankments, etc. The concentration of critical elements, their partitioning in different size ash particles (>500 to <25 μm), and their chemical association were determined for a coal fly ash sample from Talcher, India. The total REY concentration in the ash varied between 440 and 529 mg kg−1, wherein the contents were relatively higher for Nd (75–103 mg kg−1) followed by Ce (58.3–88.7 mg kg−1), La (41.6–80.3 mg kg−1), Sm (39.0–79.3 mg kg−1), and Y (38.4–49.3 mg kg−1). The REY outlook coefficient of the raw ash (1.03) is more than 0.7 and accordingly this fly ash can be considered as an interesting source of rare earth elements. This factor was further enhanced to 2.3 in the coarse ash particles of size > 250 μm. Sequential extraction showed that most of the rare and critical elements are associated with the alumino-silicate matrix. The Al2O3 content of this ash is relatively high (25%), so there is scope for co-extraction of Al along with the rare earth elements. The ash disposal and utilization policy should consider the separation and preservation of the coarse ash fraction (>250 μm) for the extraction of critical and rare earth elements.
Environmental significanceGainful utilization of large volumes of fly ash is a benign process for environmental sustainability. Coal combustion residues are used in landfills, buildings, cement concrete, and other industrial applications. However, the valuable critical elements present in it should not be permanently buried in such bulk disposal sites. This study is an attempt to separate a portion of fly ash that could be utilized for critical elements and the rest for bulk disposal. We have found that the coarse fraction of the ash carries important rare earth elements in higher concentrations. Segregation of the coarse fraction is made more environmentally friendly by following a simple physical separation process. About 90% of the ash with a relatively low concentration of critical rare elements can be disposed in bulk. This is an initial finding and needs to be validated across different ash varieties. |
1. Introduction
There is a growing demand for rare earth and critical elements as many countries are shifting from conventional to new clean energy technologies. The primary mineral supply of these rare and critical elements is limited, alternate secondary sources like fly ash are being explored. Coal ash could be a secondary source for the extraction of critical elements (Ge, Ga, U, V, Se, rare earth elements, Y, Sc, Nb, Au, Ag, and Re), and base metals (Al and Mg). As rare earth elements (REEs) are non-volatile, during the burning of coal in thermal power plants, most of the rare earth elements are enriched in fly ash.1 The concentrations of these economically important elements in coal ash are sometimes comparable with their respective concentration in conventional ores and even higher.2 Dai and Finkelman3 reported that Ge, Se, and V were extracted from coal ash in industrial scale plants. Pilot (Ga, Al, Si, and Mg) and laboratory (rare earth elements (REEs), Nb, Zr, and Ti) scale processes were reported for the extraction of critical elements from coal ash. Global fly ash production is about 800 MT and is mainly used in concrete or concrete based products (37.13%), structural fill/embankments (18.06%), blended cement (10.4%), and cement raw materials (8.29%).4 In India, about 271 million tons of coal fly ash are generated every year, of which 95.5% is utilized mainly in cement industries, landfills, bricks, soil amendment etc.5 Fly ash with valuable critical elements should not be dumped in landfills or permanently blocked in building materials.6 The characterization of fly ash for the content and mode of occurrence of economically important critical elements is needed, such data will help in developing suitable policies for ash utilization and disposal. Identification of the enrichment pattern of critical elements in size fractions of ash will help in the separation of the enriched ash portion suitable for extraction.Franus et al.7 reported 500–600 mg kg−1 of total REEs in some Polish fly ash, a similar concentration (630 mg kg−1) was reported by Wu et al.8 for a fly ash sample from the Guizhou province of China. Blissett et al.9 compared six fly ash samples from the UK and Poland, and found that the concentration of REEs varied from 246 to 481 mg kg−1. Though the concentration of REYs in fly ash is low, few studies have reported on the enrichment of the concentration by adopting physical and chemical processes. Physical beneficiation, particularly by size separation of ash, is an easier way to isolate the ash fractions enriched with critical elements. In a circulated fluidized boiler fly ash, most of the REYs are concentrated in the <96 μm fraction.10 In some pond ash from the Kentucky power plant, the REEs were enriched in <200 mesh size fly ash fractions.11 The enrichment of Ga, Ge, and V in finer ash particles was reported by Lanzerstorfer et al.;12 accordingly, air classification could be a potential beneficiation process. The amounts of Nd and Co in some Polish coal ash are higher than the Clarke value and are mostly associated with the magnetic fraction; their segregation in different grain size fractions of the ash was not significant.6 The preferential accumulation of critical elements in specific size fractions or magnetic phases of ash is an interesting phenomenon that aids in the beneficiation of ash. Chemically, the critical elements are concentrated in the aluminosilicate glass fractions and this phase should be targeted for the extraction of REEs from fly ash.13 Ash particles with a glassier phase have more REEs on their surface.14 REEs are mostly dispersed in the aluminosilicate or as micro-particles in a large aluminosilicate particle. The REEs are also chemically bound with phosphates, sulfates, CaO/Ca-rich aluminosilicates, titanites, and MnO2.
Studies on the content of critical elements in different particle size fractions of the ash are limited. The understanding of the mode of occurrence of critical elements in ash is also important for their enrichment and extraction. Talcher coalfield is an important coal reserve of India which produces about 145 MT of coal for use in the power industry. About 50–60 MT of coal ash is produced annually from these coals. Large quantities of fly ash are used for bulk applications like cement, concrete, landfills, road, embankment, etc. wherein the critical elements are permanently locked in building materials and landfills. The objective of this study is to assess the concentration of rare and critical elements in Talcher fly ash, and their mode of occurrence. Yet another objective is to evaluate the enrichment of critical elements in particle size fractions of the fly ash, so that the enriched fraction could be separated and preserved for critical element extraction and the lean fraction with low concentration of critical elements could be used for bulk applications. To achieve this objective, the fly ash sample was separated into different particle size fractions by using a set of sieves and the critical element concentration in each size fraction was assessed. To determine the chemical mode of occurrence of the critical elements, a sequential extraction scheme was followed.
2. Materials and methods
2.1 Materials
The coal fly ash was collected from a pulverized coal power plant in Talcher, India. In the pulverized coal fired plant, grounded coal (∼100 μm) is injected into the furnace for steam generation; after coal burning, the fly ash is collected through electrostatic precipitators. Fly ash samples were collected from the silo of a coal fired power plant on a daily basis for 7 days continuously. Fly ash particle size separation was performed using a set of sieves from 500 to 25 microns.152.2 Analytical methods
The fly ash samples were air-dried and processed by following standard methods. For the determination of basic properties like pH, EC, and loss on ignition (LOI), the air-dried ash sample was homogenized and passed through a 2 mm sieve. Loss on ignition was determined as per the ASTM D7348 method.16 Fly ash pH and EC were measured by following the method of Dhyan et al.17 The pH of the ash was measured in ash water slurry (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5) using a pH electrode (Thermo Scientific, USA, Model: Orion Star A214). For determining the electrical conductivity, the ash water slurry was allowed to settle for 24 hours, the EC of the supernatant was measured using an EC meter (Eutech, Netherlands, Model: PCS Tester 35).
2.5) using a pH electrode (Thermo Scientific, USA, Model: Orion Star A214). For determining the electrical conductivity, the ash water slurry was allowed to settle for 24 hours, the EC of the supernatant was measured using an EC meter (Eutech, Netherlands, Model: PCS Tester 35).
An X-ray fluorescence spectrophotometer (Rigaku WD XRF ZSX Primus IV) was used to measure the concentration of major oxides in fly ash. About 1 g of fly ash sample and 0.1 g of stearic acid were mixed and ground using a mortar and pestle. The mixture was pelletized between boric acid (14.5 g) layers by using a 30-ton pellet press. The resultant pellet (40 mm diameter) was used for XRF analysis.
X-ray diffraction (XRD) analysis of the powdered fly ash was performed using an automated high-resolution θ–θ multipurpose X-ray diffractometer (XRD) with expert system Guidance Software (model: SmartLab X-ray Diffractometer, make: Rigaku, Japan). The X-ray intensities of the samples were collected in the 2θ range of 10 to 80° using Cu Kα radiation with a 3 kW sealed X-ray tube, CBO optics, and a D/teX Ultra 250 silicon strip detector. An X-ray amorphous sample holder was used for sample loading and the scan was made in continuous mode with a scan speed of 10.40182° min−1 and step size of 0.01°. Analysis was done using PDXL2 software and ICDD data bank for mineral identification.
For FTIR analysis, fly ash samples were dried at 105 °C in a hot air oven for 1 hour. Dried fly ash samples (1 mg) and KBr (200 mg) powder were crushed in an agate mortar and pelletized. The pellets were analyzed in the FTIR instrument (Shimadzu, IR Affinity-1S-00303) in absorbance mode at wave numbers between 400 and 4000 cm−1.
Field emission scanning electron microscopy - FESEM (Carl Zeiss Model Supra 55) studies were also carried out to analyze the micromorphology of the fly ash particles. Carbon tape was pasted on a metal stub to increase conductivity onto which the air-dried sample was mounted. The sample was pasted to the tape and then the sputter coating with gold was done in a controlled manner in a sputter coater to prevent charge buildup on the surface. A secondary electron detector (SE2) was used to take the backscattered image of the sample.
ASTM protocol (ASTM, 2021a) was followed for the determination of the concentration of critical elements in fly ash. The air-dried fly ash samples were heated at 750 °C in a muffle furnace for 2 hours to drive out the unburnt carbon. After ashing, the samples were ground using a clean agate mortar. About 0.1 g of the ashed sample was digested in a hydrothermal autoclave vessel using a concentrated HCl, HF and HNO3 mixture at 130 °C for 2 hours. After cooling, the sample was again digested with 92 mL of 1.5% H3BO3 for 1 hour at 130 °C. After cooling, the sample was diluted to 250 mL and the concentration of critical elements in the solution was determined using ICP-OES (Thermo Scientific, iCAP 6000 series). For quality control, different fly ash CRMs like SRM 2690, SRM 2691, SRM 2689 and BCR 176R were digested and analyzed. The recovery of most of the elements from these CRMs is between 85 and 115%.
2.3 Sequential extraction procedure
The chemical fractionation scheme proposed by Van Herck and Vandecasteele18 was followed for the sequential extraction of critical elements. Five grams of fly ash sample were sequentially extracted with water, 1 M MgCl2, and 1 M sodium acetate solutions to obtain the water soluble, ion exchangeable, and carbonate bound fractions, respectively. For the determination of the metal oxide bound fraction, the ash residue obtained after the extraction of the carbonate bound fraction was digested with hydroxylamine hydrochloride and acetic acid. The resultant residue was digested with H2O2 and HNO3 to obtain the organically bound fraction and those associated with oxidizable minerals like sulfides. Finally, a portion of the residue left after the organic fraction was digested in a hydrothermal reactor as detailed in Section 2.2 to obtain the elements associated with the aluminosilicate fraction. The critical elements' contents in the extracted and digested solutions obtained in each step were analyzed using ICP-OES (Thermo Scientific, iCAP 6000 series).3. Results and discussion
3.1 Basic properties of fly ash
The loss on ignition of the fly ash, a measure of the unburnt carbon content, varied from 0.31 to 0.65% (ESI Table 1†). In general, the unburnt carbon content in fly ash varies up to 15%, depending on the combustion efficiency of the boiler. The unburnt carbon content in this fly ash is within the requirement for its use for cement and concrete industries (<5%). In terms of critical elements, the larger surface area of unburnt carbon aids in the adsorption of volatile elements on the ash surface.15 The fly ash was slightly acidic (pH 5.75–6.6) (ESI Table 1†). The pH of the ash influences its reactivity, environmental impact, and suitability for use in construction, agriculture, environmental remediation, and other industries. Acidic fly ash tends to release potentially toxic elements in the environment during ash weathering. The electrical conductivity (EC) varied from 140 to 759 μS cm−1. Fly ash pH and EC depend on the chemical and mineral composition of the ash. The major chemical composition (Table 1, ESI Table 1†) of the ash could be arranged in the order: SiO2 > Al2O3 > Fe2O3 > Na2O > K2O > CaO > P2O5 > MgO > SO3. The Al2O3 content of the ash was comparatively higher (24.63–26.13%). Extraction of alumina from coal fly ash, particularly ashes with Al2O3 content around 30%, was reported earlier.10,19–21 The concentration of oxides in different particle size fractions of the ash was studied with an objective of segregation of ash for critical element extraction and other bulk uses, like cement, concrete, land fill, roads, embankments, etc. The ash yields under different size fractions are presented in ESI Fig. 1;† the major portion of the ash particles is <74 μm. The finer fractions are generally more in the fly ash from coal fired power plants.10,22,23 As per the Indian standards for the requirement of fly ash properties for use as a pozzolana for the production of cement and concrete, the sum of the concentration SiO2 + Al2O3 + Fe2O3 should be greater than 70%. In the fly ash studied, this value varied from 90% in the coarse fraction to 93% in the finer fractions, so this ash is suitable for bulk use in the cement and concrete sector. The distribution of major oxides in different size fractions was almost uniform and was not enriched in any size fraction (Table 1). Based on the basic chemical properties, it is evident that this ash could be used in cement and concrete industries and for the extraction of Al, as the Al2O3 content is about 25%.| Al2O3 (%) | CaO (%) | Fe2O3 (%) | K2O (%) | MgO (%) | Na2O (%) | P2O5 (%) | SO3 (%) | SiO2 (%) | TiO2 (%) | MnO (%) | V2O5 (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw ash | 26.1 | 1.53 | 4.53 | 1.71 | 0.58 | 0.31 | 0.63 | 0.17 | 59.8 | 1.80 | 0.07 | 0.04 |
| >500 μm | 23.2 | 1.64 | 4.91 | 1.56 | 0.76 | 0.35 | 0.79 | 0.40 | 62.6 | 1.58 | 0.06 | 0.03 |
| 250–500 μm | 25.6 | 0.83 | 6.47 | 1.50 | 0.64 | 0.06 | 0.54 | 0.18 | 60.2 | 1.79 | 0.04 | 0.03 |
| 212–250 μm | 26.6 | 0.58 | 3.69 | 1.51 | 0.50 | 0.06 | 0.52 | 0.12 | 62.5 | 1.70 | 0.04 | 0.03 |
| 150–212 μm | 26.3 | 0.63 | 5.64 | 1.38 | 0.52 | 0.05 | 0.53 | 0.08 | 60.8 | 1.84 | 0.03 | 0.03 |
| 74–150 μm | 26.9 | 0.56 | 4.72 | 1.34 | 0.49 | 0.04 | 0.47 | 0.07 | 61.4 | 1.83 | 0.03 | 0.03 |
| 43–74 μm | 27.1 | 0.50 | 4.04 | 1.29 | 0.48 | 0.05 | 0.45 | 0.08 | 62.1 | 1.79 | 0.02 | 0.03 |
| 37–43 μm | 27.0 | 0.48 | 3.84 | 1.27 | 0.49 | 0.05 | 0.50 | 0.08 | 62.3 | 1.74 | 0.02 | 0.03 |
| 25–37 μm | 26.8 | 0.47 | 3.81 | 1.27 | 0.49 | 0.05 | 0.51 | 0.08 | 62.6 | 1.74 | 0.03 | 0.03 |
| <25 μm | 26.7 | 0.49 | 4.12 | 1.33 | 0.48 | 0.05 | 0.54 | 0.08 | 62.2 | 1.81 | 0.06 | 0.03 |
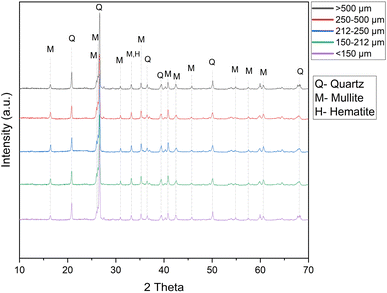
Regarding the ash mineralogy, it is apparent that during combustion of coal, some of the minerals in coal undergo direct transformation while some of the minerals are either fragmented or remain in their original form. Quartz, mullite, and hematite are the major minerals present in fly ash (Fig. 1). During coal combustion, the illite and kaolinite minerals originally present in the coal are converted into aluminosilicate glass and mullite due to the high temperature.24 Hematite in fly ash is generally transformed from the pyrite mineral originally present in coal.25 The abundance of quartz, mullite, and hematite mineral did not vary between the size fractions of the ash (Fig. 1). The mineralogy study of the fly ash showed the abundance of alumino-silicate phases in the fly ash, which generally encapsulate most of the critical elements.
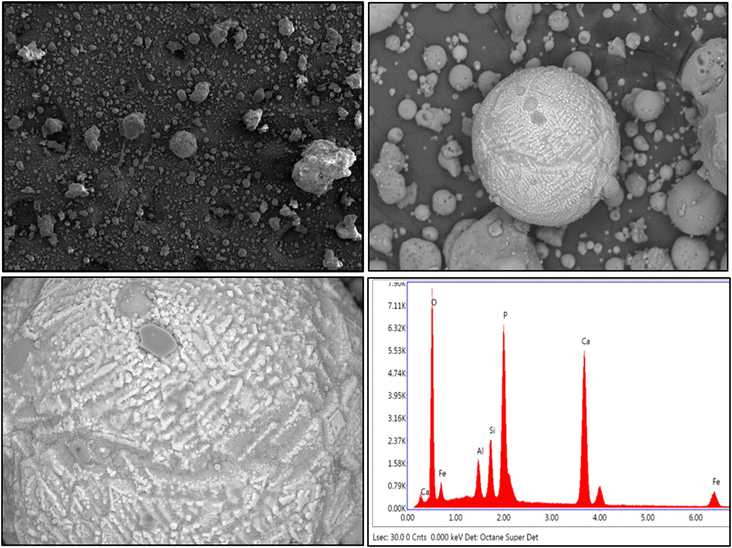
The presence of aluminosilicate cenospheres is further confirmed by the SEM image (Fig. 2). There are irregular shaped particles with blunt edges probably comprising of the aluminosilicate glass matrix. The formation of these minerals is due to the melting and decomposition of particles during the combustion of coal.26 The EDS spectra of aluminosilicate glass showed that Fe, Ca, and P are also present in the melt. It is expected that the volatilized elements are deposited on the surface of the cenosphere and other ash particles during the combustion process, whereas non-volatile critical elements are impregnated in the glassy matrix.
The results of the FTIR analysis are shown in ESI Fig. 3.† The peak at 1080–1091 cm−1 is due to the asymmetric stretching of Si (Al)–O–Si. This peak arises particularly due to the glassy silicate and its frequency varies slightly depending on the state of hydration, non-bridging oxygen concentration, and aluminum content. The peaks in the finer fractions of fly ash tend to widen, possibly due to the presence of more amorphous silica and the state of hydration in the finer fractions. The peak around 780–800 cm−1 is associated with the bending vibrations in silicate tetrahedra. FTIR spectra showed the dominance of Si–O functional groups and are not affected by the particle size fraction of the ash.
3.2 Critical elements in fly ash
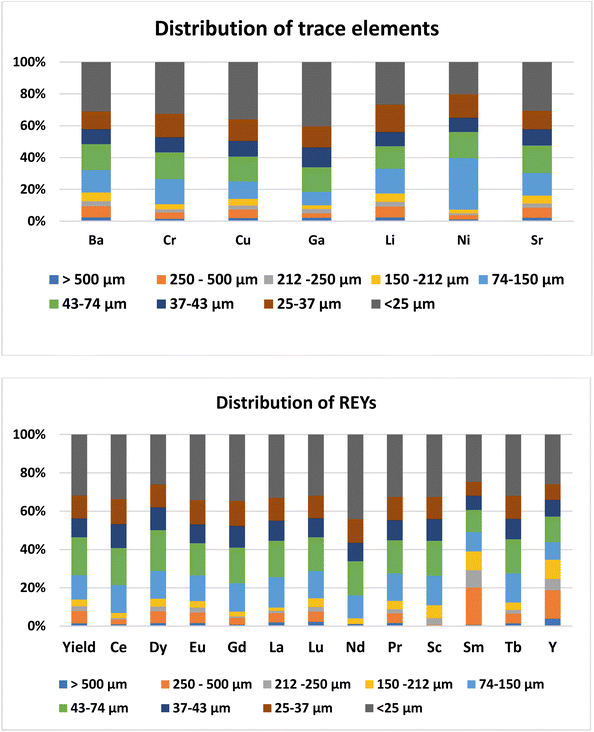
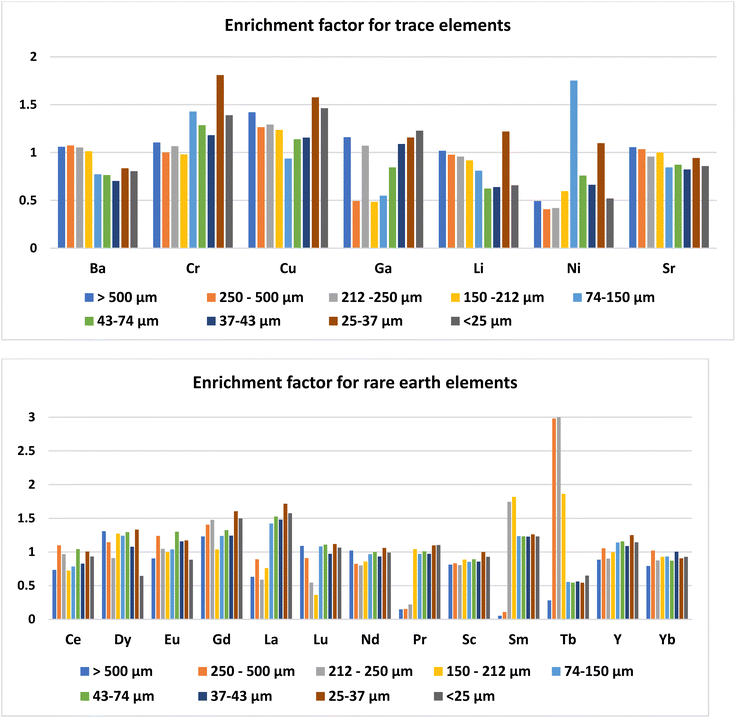
The concentration of trace elements in the ash was not affected by the day of ash sampling (ESI Table 2†). Among the trace elements, the concentration was higher for Ba (465–756 mg kg−1) followed by Sr (141–172 mg kg−1) and Cr (56.8–157 mg kg−1). Concentrations of all the other trace elements were less than 100 mg kg−1 (Table 2). The trace elements in Talcher coal are derived from the sediment present near the coal basin.27 Contents of all the elements, except Cr, were less than the world coal ash average. The concentration of trace elements in different size fractions of fly ash was almost uniform (Fig. 3), except that Ni was slightly enriched in the 74–150 μm size particles. Enrichment of elements in different size fractions was calculated as the ratio of elemental concentration in the size fraction to the elemental concentration in bulk ash (Fig. 4). Except for Ni, Cr and Cu, the enrichment of all the trace elements was less than 1.5. For Ni, the enrichment factor was 1.75 in the 74–150 μm size fraction with a concentration of 142 mg kg−1. The Ba content was comparatively higher in the coarse fraction of ash of size > 150 μm (Table 2), probably due to its volatilization properties.28 Ba and Sr are mostly enriched in the coarse fraction, due to their low volatilization during combustion.29 The concentrations of Ba, Cr and Sr were higher in the ash, however below the threshold for economical extraction.| Raw ash | >500 μm | 250–500 μm | 212–250 μm | 150–212 μm | 74–150 μm | 43–74 μm | 37–43 μm | 25–37 μm | <25 μm | |
|---|---|---|---|---|---|---|---|---|---|---|
| Trace elements (mg kg− 1 ) | ||||||||||
| Ba | 515 | 546 | 553 | 542 | 522 | 398 | 394 | 362 | 430 | 415 |
| Cr | 105 | 116 | 105 | 112 | 103 | 150 | 135 | 124 | 190 | 146 |
| Cu | 70.4 | 100 | 89.0 | 91.0 | 87.0 | 65.9 | 80.2 | 81.4 | 111 | 103 |
| Ga | 32.4 | 37.6 | 16.0 | 34.7 | 15.7 | 17.8 | 27.4 | 35.3 | 37.5 | 39.8 |
| Li | 50.1 | 51.0 | 49.0 | 48.0 | 46.0 | 40.6 | 31.2 | 32.0 | 61.1 | 33.0 |
| Ni | 81.0 | 40.0 | 33.0 | 34.0 | 32.0 | 142 | 61.6 | 53.7 | 88.9 | 42.1 |
| Sr | 142 | 150 | 147 | 136 | 142 | 120 | 124 | 117 | 134 | 122 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||
| Rare earth elements (mg kg− 1 ) | ||||||||||
| Ce | 88.7 | 65.1 | 97.5 | 85.7 | 64.2 | 69.5 | 92.6 | 73.4 | 89.3 | 82.8 |
| Dy | 5.5 | 7.20 | 6.30 | 5.00 | 7.00 | 6.83 | 7.12 | 5.93 | 7.33 | 3.55 |
| Er | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| Eu | 2.1 | 1.90 | 2.60 | 2.20 | 2.10 | 2.18 | 2.73 | 2.43 | 2.46 | 1.86 |
| Gd | 23.5 | 28.9 | 33.0 | 34.7 | 24.4 | 29.1 | 31.1 | 29.2 | 37.7 | 35.2 |
| Ho | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| La | 41.6 | 26.3 | 37.1 | 24.5 | 31.6 | 59.2 | 63.5 | 61.6 | 71.4 | 65.5 |
| Lu | 1.10 | 1.20 | 1.00 | 0.60 | 0.40 | 1.19 | 1.22 | 1.07 | 1.23 | 1.17 |
| Nd | 101 | 103 | 83.2 | 80.8 | 86.7 | 97.6 | 101 | 94.2 | 107 | 100 |
| Pr | 54.2 | 8.10 | 8.40 | 12.0 | 56.5 | 52.6 | 54.7 | 52.8 | 59.5 | 59.8 |
| Sc | 24.0 | 19.5 | 20.0 | 19.3 | 21.3 | 21.3 | 23.9 | 22.2 | 21.4 | 24.8 |
| Sm | 39.0 | 2.10 | 4.30 | 68.0 | 70.9 | 48.2 | 48.1 | 47.9 | 49.2 | 48.0 |
| Tb | 16.0 | 4.50 | 47.7 | 48.1 | 29.8 | 8.91 | 8.72 | 8.99 | 8.71 | 10.4 |
| Tm | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| Y | 38.4 | 34.0 | 40.5 | 34.6 | 38.3 | 43.8 | 44.4 | 41.8 | 48.0 | 43.9 |
| Yb | 4.80 | 3.80 | 4.90 | 4.20 | 4.46 | 4.48 | 4.18 | 4.82 | 4.35 | 4.46 |
| REY | 440 | 305 | 386 | 420 | 438 | 430 | 469 | 433 | 498 | 472 |
| LREY | 325 | 204 | 231 | 271 | 310 | 309 | 343 | 312 | 359 | 341 |
| MREY | 86 | 77 | 130 | 125 | 102 | 97 | 102 | 98 | 113 | 106 |
| HREY | 5.90 | 5.00 | 5.90 | 4.80 | 4.50 | 2.28 | 2.58 | 2.48 | 2.56 | 2.64 |
| Critical | 163 | 150 | 180 | 171 | 165 | 148 | 155 | 146 | 165 | 156 |
| Uncritical | 158 | 65.4 | 82.8 | 139 | 183 | 189 | 197 | 191 | 218 | 209 |
| Excessive | 94.6 | 70.1 | 103 | 90.5 | 68.7 | 71.8 | 95.2 | 75.9 | 91.8 | 85.5 |
| C outl | 1.03 | 2.30 | 2.18 | 1.23 | 0.90 | 0.78 | 0.79 | 0.76 | 0.76 | 0.75 |
Day-wise concentration of REY is presented in ESI Table 3.† For the REYs, the concentration (Table 2) was higher for La (41.6–80.3 mg kg−1) followed by Sm (39.0–79.3 mg kg−1), Ce (58.3–88.7 mg kg−1), Nd (75–103 mg kg−1), and Y (38.4–49.3 mg kg−1). The concentration of the rest of the REYs was less than 48 mg kg−1. The concentrations of REYs are almost same in all the particle sizes of the ash, except that Tb was enriched (two times) in coarser particles of size > 212 μm (Fig. 3). Seredin and Dai1 classified REYs geochemically, and divided the rare earth elements into light REY (LREY: La, Ce, Pr, Nd, Sm), medium REY (MREY: Eu, Gd, Tb, Dy, Y), and heavy REY (HREY: Ho, Er, Tm, Yb, Lu) as well as into three commercial groups based on their industrial demand: critical (Nd, Eu, Tb, Dy, Y, and Er), uncritical (La, Pr, Sm, and Gd), and excessive (Ce, Ho, Tm, Yb, and Lu). In our study, the concentration was higher for LREY (325 mg kg−1) followed by MREY (86 mg kg−1), and HREY (5.9 mg kg−1). The critical REY concentration varied from 82 to 125 mg kg−1, which is much lower than the concentration of REE ores. However, the total content of REYs in fly ash is not important, but the ratio of critical to excessive elements termed as the outlook coefficient is an important measure for assessing the suitability of the ash. Though the concentrations of some of the REYs (Dy, Eu, Gd, La, Lu, Nd, Pr, Sc, Sm, and Tb) are higher than the world coal ash average, the Coutl is 1.03. The outlook coefficient increased up to 2.3 in the coarse particles of the ash (Table 2), which is predominantly due to the enrichment of Tb in coarse fractions (Fig. 4). The coefficient was more than 2 for the particle size greater than 250 μm. The quantum of ash with particle size > 250 μm is about 10%. Thus the 10% coarse fraction may be segregated and preserved for the extraction of valuable rare earth elements, and the rest of the ash can be designated for bulk uses like cement, concrete, landfill, road, embankments, etc.
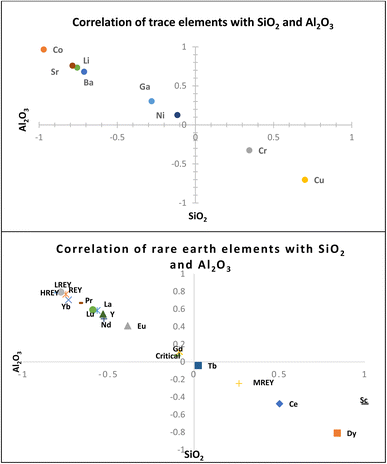
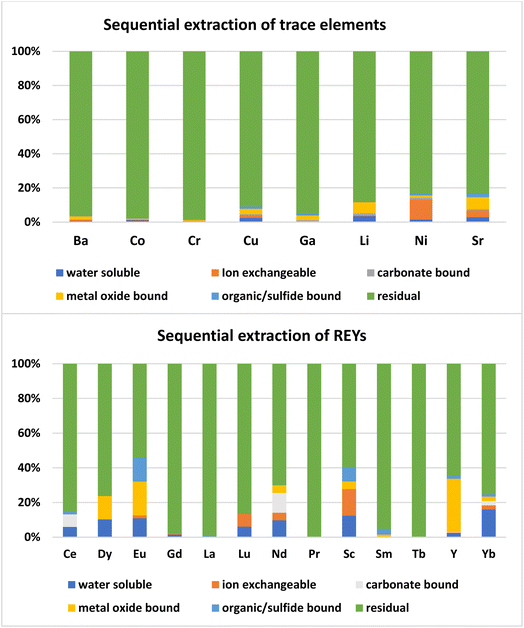
Most of the REYs showed a significant correlation with SiO2 except Dy, Ce, Sc, and MREYs which showed a significant correlation with Al2O3. The correlation of trace and rare earth elements with SiO2 indicates their association in a glassy phase of the fly ash which is further confirmed by the sequential extraction data. As per the results of sequential extraction experiments, most (>90%) of the trace elements are associated with the residual aluminum silicate fraction of the fly ash (Fig. 6). The correlation study also showed that most of the trace elements (Sr, Ba, Co, Li, Ga, and Ni) are positively correlated (P < 0.01) with SiO2 (Fig. 5). In general, the solubility of these elements is poor due to their association with silicate glass.30 About 5–10% of Sr, Ni, Li, and Cu are associated with the ion exchangeable and metal oxide bound fractions. From the correlations, it appears that most of the elements are closely associated with the Si–Al matrix of the ash.
Most of the REYs are associated with the Al-Si residual fraction, followed by the metal oxide form, organic or sulfide, ion exchangeable form, water soluble, and carbonate bound fractions. About 84% of REYs were present in the residual form (Fig. 6) while 4–5% in the metal oxide or water-soluble form; and 1–2% in the sulfide and ion exchangeable forms. Earlier studies also showed that REYs in fly ash are present in the aluminosilicate glass phase, carbonate minerals, and phosphate minerals.23,31 During the combustion of coal the REYs get converted into their respective oxides, phosphates, carbonates and sulfates, and get incorporated into the glassy phase of fly ash.14 Thus, most of the REYS in coal ash occur in the crystalline and amorphous phases of the aluminosilicate minerals of coal ash. Accordingly, the extraction strategy should focus on solubilizing the alumino silicate phases of the ash.
4. Conclusion
Rare earth elements in fly ash have the potential for recycling and decreasing the dependency on mining and extraction from their primary ores. Therefore, the evaluation and segregation of fly ash for the extraction of rare earth elements is needed, otherwise these valuable elements along with the bulk ash get disposed in ash lagoons or get locked in bulk utilization projects like cement, concrete, landfill, road, and embankments. The total REY concentration in the Talcher ash was 440 mg kg−1, with an outlook coefficient of 1.03, whereas in the coarse fraction (>250 μm) of ash, the total concentration was 305 mg kg−1 with an outlook coefficient of 2.30. Thus, the coarse fraction of ash is to be preserved for the extraction of critical rare earth elements. Sequential chemical extraction showed that most of these rare earth elements occur in the aluminosilicate phase of coal ash. Accordingly, the extraction strategy should focus on solubilizing the alumino-silicate phases of the ash with a target on co-extraction of alumina and rare earth elements. The ash disposal and utilization policy should encourage the separation and preservation of the enriched ash fraction for the extraction of critical and rare earth elements.Author contributions
KKK: formal analysis, investigation, result interpretation and validation, writing-original draft preparation; REM: funding acquisition, conceptualization, methodology, supervision, writing-reviewing and editing; HA: resources, visualization; SB: formal analysis, data curation; MK: resources; PG: resources; BH: formal analysis; SS: formal analysis; SM: resources.Conflicts of interest
The authors declare that there were no conflicts of interest in this study.Acknowledgements
This research was funded by the Science and Engineering Research Board (SERB), New Delhi, India, (grant no. CRG/2021/002884). The authors would like to thank Director CSIR-CIMFR for supporting this publication. The insights provided by the two anonymous reviewers are greatly acknowledged.References
- V. V. Seredin and S. Dai, Coal deposits as potential alternative sources for lanthanides and yttrium, Int. J. Coal Geol., 2012, 94, 67–93 CrossRef CAS
.
- V. V. Seredin and R. B. Finkelman, Metalliferous coals: a review of the main genetic and geochemical types, Int. J. Coal Geol., 2008, 76, 253–289 CrossRef CAS
.
- S. Dai and R. B. Finkelman, Coal as a promising source of critical elements: progress and future prospects, Int. J. Coal Geol., 2018, 186, 155–164 CrossRef CAS
.
- A. R. K. Gollakota, V. Volli and C.-M. Shu, Progressive utilisation prospects of coal fly ash: a review, Sci. Total Environ., 2019, 672, 951–989 CrossRef CAS PubMed
.
-
CEA, Report on fly ash generation at coal/lignite based thermal power stations and its utilization in the country for the year 2021–22, Central Electricity Authority, Civil Design Division, Ministry of Power, New Delhi, 2022 Search PubMed
.
- E. Strzałkowska, Rare earth elements and other critical elements in the magnetic fraction of fly ash from several Polish power plants, Int. J. Coal Geol., 2022, 258, 104015 CrossRef
.
- W. Franus, M. M. Wiatros-Motyka and M. Wdowin, Coal fly ash as a resource for rare earth elements, Environ. Sci. Pollut. Res., 2015, 22, 9464–9474 CrossRef CAS PubMed
.
- L. Wu, L. Ma, G. Huang, J. Li and H. Xu, Distribution and Speciation of Rare Earth Elements in Coal Fly Ash from the Qianxi Power Plant, Guizhou Province, Southwest China, Minerals, 2022, 12, 1089 CrossRef CAS
.
- R. S. Blissett, N. Smalley and N. A. Rowson, An investigation into six coal fly ashes from the United Kingdom and Poland to evaluate rare earth element content, Fuel, 2014, 119, 236–239 CrossRef CAS
.
- C. Zhou, C. Li, W. Li, J. Sun, Q. Li, W. Wu and G. Liu, Distribution and preconcentration of critical elements from coal fly ash by integrated physical separations, Int. J. Coal Geol., 2022, 261, 104095 CrossRef CAS
.
- J. C. Hower, J. G. Groppo, K. R. Henke, U. M. Graham, M. M. Hood, P. Joshi and D. V. Preda, Ponded and landfilled fly ash as a source of rare earth elements from a Kentucky power plant, Coal Combustion and Gasification Products, 2017, 9, 1–21 CrossRef
.
- C. Lanzerstorfer, Fly ash from coal combustion: dependence of the concentration of various elements on the particle size, Fuel, 2018, 228, 263–271 CrossRef CAS
.
- A. Kolker, C. Scott, J. C. Hower, J. A. Vazquez, C. L. Lopano and S. Dai, Distribution of rare earth elements in coal combustion fly ash, determined by SHRIMP-RG ion microprobe, Int. J. Coal Geol., 2017, 184, 1–10 CrossRef CAS
.
- M. Y. Stuckman, C. L. Lopano and E. J. Granite, Distribution and speciation of rare earth elements in coal combustion by-products via synchrotron microscopy and spectroscopy, Int. J. Coal Geol., 2018, 195, 125–138 CrossRef CAS
.
- F. Xu, S. Qin, S. Li, J. Wang, Q. Lu and J. Xing, Distribution, occurrence mode, and extraction potential of critical elements in coal ashes of the Chongqing Power Plant, J. Cleaner Prod., 2022, 342, 130910 CrossRef CAS
.
- ASTM, ASTM D7348-21, Standard Test Methods for Loss on Ignition (LOI) of Solid Combustion Residues, ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA, 19428-2959 USA, 2021 Search PubMed.
-
S. Dhyan, P. Chhonkar and B. Dwivedi, Manual on soil, plant and water analysis, Westville Publishing House, 2005 Search PubMed
.
- P. Van Herck and C. Vandecasteele, Evaluation of the use of a sequential extraction procedure for the characterization and treatment of metal containing solid waste, Waste Manage., 2001, 21, 685–694 CrossRef CAS PubMed
.
- Y. Shi, K.-x. Jiang and T.-a. Zhang, Cleaner extraction of alumina from coal fly ash: baking-electrolysis method, Fuel, 2020, 273, 117697 CrossRef CAS
.
- N. Nayak and C. R. Panda, Aluminium extraction and leaching characteristics of Talcher Thermal Power Station fly ash with sulphuric acid, Fuel, 2010, 89, 53–58 CrossRef CAS
.
- X.-l. Fan, S.-q. Lv, J.-l. Xia, Z.-y. Nie, D.-r. Zhang, X. Pan, L.-z. Liu, W. Wen, L. Zheng and Y.-d. Zhao, Extraction of Al and Ce from coal fly ash by biogenic Fe3+ and H2SO4, Chem. Eng. J., 2019, 370, 1407–1424 CrossRef CAS
.
- R. Lin, M. Stuckman, B. H. Howard, T. L. Bank, E. A. Roth, M. K. Macala, C. Lopano, Y. Soong and E. J. Granite, Application of sequential extraction and hydrothermal treatment for characterization and enrichment of rare earth elements from coal fly ash, Fuel, 2018, 232, 124–133 CrossRef CAS
.
- J. Pan, C. Zhou, C. Liu, M. Tang, S. Cao, T. Hu, W. Ji, Y. Luo, M. Wen and N. Zhang, Modes of occurrence of rare earth elements in coal fly ash: a case study, Energy Fuels, 2018, 32, 9738–9743 CrossRef CAS
.
- G. Liu, S. V. Vassilev, L. Gao, L. Zheng and Z. Peng, Mineral and chemical composition and some trace element contents in coals and coal ashes from Huaibei coal field, China, Energy Convers. Manage., 2005, 46, 2001–2009 CrossRef CAS
.
- B. K. Saikia, J. C. Hower, N. Islam, A. Sharma and P. Das, Geochemistry and petrology of coal and coal fly ash from a thermal power plant in India, Fuel, 2021, 291, 120122 CrossRef CAS
.
- C. Li, C. Zhou, W. Li, W. Zhu, J. Shi and G. Liu, Enrichment of critical elements from coal fly ash by the combination of physical separations, Fuel, 2023, 336, 127156 CrossRef CAS
.
- P. Gopinathan, M. Jha, A. K. Singh, A. Mahato, T. Subramani, P. K. Singh and V. Singh, Geochemical characteristics, origin and forms of sulphur distribution in the Talcher coalfield, India, Fuel, 2022, 316, 123376 CrossRef CAS
.
- S. Dai, L. Zhao, J. C. Hower, M. N. Johnston, W. Song, P. Wang and S. Zhang, Petrology, mineralogy, and chemistry of size-fractioned fly ash from the Jungar power plant, Inner Mongolia, China, with emphasis on the distribution of rare earth elements, Energy Fuels, 2014, 28, 1502–1514 CrossRef CAS
.
- P. Jiang, J. Chen, Y. Li, X. Li, X. Qi, J. Wang, P. Chen, W. Liu and R. Wang, Partitioning and Migration of Trace Elements during Coal Combustion in Two Coal-Fired Power Plants in Hefei City, Anhui Province, Eastern China, Minerals, 2023, 13, 152 CrossRef CAS
.
- P. Liu, Q. Wang, H. Jung and Y. Tang, Speciation, Distribution, and Mobility of Hazardous Trace Elements in Coal Fly Ash: Insights from Cr, Ni, and Cu, Energy Fuels, 2020, 34, 14333–14343 CrossRef CAS
.
- G.-q. Wu, T. Wang, J.-w. Wang, Y.-s. Zhang and W.-p. Pan, Occurrence forms of rare earth elements in coal and coal gangue and their combustion products, J. Fuel Chem. Technol., 2020, 48, 1498–1505 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3va00186e |
| This journal is © The Royal Society of Chemistry 2024 |