Open Access Article
Open Access ArticlePrecise molecular design for a twisted pyrene-thiophene based mechanofluorochromic probe with large Stokes shift and feasibility study towards security ink and re-writable papers†
Ram Prasad
Bhatta
a,
Sumit
 a,
Vishal
Kachwal
a,
Vandana
Vishwakarma
b,
Angshuman
Roy Choudhury
a,
Vishal
Kachwal
a,
Vandana
Vishwakarma
b,
Angshuman
Roy Choudhury
 b and
Inamur Rahaman
Laskar
b and
Inamur Rahaman
Laskar
 *a
*a
aDepartment of Chemistry, Birla Institute of Technology and Science (BITS), Pilani Campus, Pilani, Rajasthan 333031, India. E-mail: ir_laskar@pilani.bits-pilani.ac.in
bDepartment of Chemical Sciences, Indian Institute of Science Education and Research (IISER), Mohali, Sector 81, S. A. S. Nagar, Manauli PO, Mohali, Punjab 140306, India
First published on 23rd July 2024
Abstract
The creation of MFC-active smart molecules by tuning functionality has received considerable attention owing to its versatile applicability. Pyrene-based twisted donor–acceptor (D–A) dyes (PySS and PySP) have been synthesized and characterized. Here, the pyrene is directly connected with thiophene, and this unit is further linked terminally to photoactive species (thiophene/pyridine) via a four-carbon unit conjugated spacer. These molecules show excellent solvatochromic properties, with a substantial shifting of the emission wavelength (PySS- 147 nm and PySP- 130 nm). The lowest transition state contains a significant contribution from ICT characteristics, as evidenced by spectral analysis and computational calculations. Moreover, these are identified as ‘aggregation-induced enhanced emission’ (AIEE) active compounds and exhibit mechanofluorochromism (MFC). By grinding, PySS and PySP display MFC features with 50 nm and 54 nm red shifting, respectively. Interestingly, PySS shows a gradual emission change from green (510 nm) to orange emission (578 nm) by gradually changing the pressure with a hydraulic press (0 to 12.5 tons). The single crystal structure of both compounds was investigated to understand the structure–property relationship for MFC. The crystal packing shows that the twisted molecules (dihedral angle between pyrene and thiophene is 59.36° and 56.93° for PySS and PySP, respectively) are loosely bound with several weak interactions (C–H⋯π, C–π⋯H, H⋯H, C–H⋯O). Interestingly, it was observed that two molecules in a unit cell are arranged in an antiparallel fashion; these molecular pairs are linearly connected to another pair axially, forming a long one-dimensional chain-type arrangement. On applying pressure, these twisted molecular pairs may slowly planarize, leading the molecules to come closer, thus changing the molecular interaction and the emission properties. A feasibility study of the potentiality of using these compounds in data encryption–decryption and security ink has also been demonstrated.
Introduction
Mechanofluorochromic (MFC) materials are a class of smart stimuli-responsive materials in which the emission properties of the material change upon the application of stress.1 Organic mechanofluorochromic (MFC) materials (that change their emission under anisotropic and isotropic pressure) have attracted great attention in recent years due to their promising applications in sensing pressure, storage devices, security inks, three-dimensional (3D) printing, etc.2–5 Stimuli-responsive materials with tunable emission and reversible switching are highly demanding for different applications, such as security encryption devices, deformation detectors, fluorescent probes, switches, and optoelectronic devices.6,7 The process of protein folding and unfolding, as well as the movement of cells, can be influenced by pressure in biology.8 Due to the influence of the aggregation-caused quenching (ACQ) effect, most of the conventional organic chromophores exhibit weakened or even totally quenched emission in the aggregated state, which limits the development and application of MFC materials to a greater extent.9,10 A new opportunity to overcome the ACQ problem and develop durable solid-state luminescent materials is presented through the generation of ‘aggregation-induced emission’ (AIE) compounds.11 Compounds with AIE properties usually possess a twisted geometry. Stimuli-responsive organic materials with AIE characteristics would be an interesting platform to explore the structure–property relationship for future designing MFC compounds.In several reports, it has been observed that AIE active organic compounds exhibit distinct responses in emission properties upon mechanical grinding (anisotropic) and hydrostatic pressure (isotropic).1 The isotropic force is applied uniformly by the diamond anvil cell (DAC). The mechanochromic luminescence performance of AIEgens can be explained as a synergetic process associated with the changing of the molecular conformations, the molecular packing forms and weak intermolecular interactions (such as C–H⋯π, C–H⋯O, and C–H⋯N hydrogen bonds).6,12,13 The majority of mechanochromic luminescent materials have loose and metastable packing, and are believed to be critical factors in achieving structural transformation and pressure-dependent multicolor emissions. After grinding or compressing physically, these weak intermolecular interactions are altered, resulting in the variation of the stacking mode and the molecular conformation, and finally causing the emission color change.14–16
In 2012, Tian et al.17 reported mechanochromic luminescence of a 9,10-distyrylanthracene (DSA) derivative 9,10-bis[(E)-2-(pyridin-2-yl)vinyl]anthracene (BP2VA). This molecule showed extraordinary luminescence properties: grinding and applying external pressure changed the photoluminescence (PL) of the BP2VA powder from green to red color. With the external pressure increasing from 0 to 8 GPa, the emission of BP2VA powder showed a gradual red shift from green to red distinctly. There are many reports found from carbon nanodots, metal–organic frameworks (MOFs), covalent organic frameworks (COFs), etc. which enriched the chemistry of MFC materials.18–23 The majority of high-contrast solid-state MFC materials include tetraphenyl ethylene (TPE),24–26 triphenylamine (TPA),27–29 anthracene,30–32 carbazole, phenothiazine, stilbene, spiropyrans, etc.33–39 Pyrene is a highly planar and conjugated molecule. Hence, it is prone to form a dimer structure due to strong π–π stacking during the aggregation process.40,41 In 2020, Xiong et al.13 synthesized (4-(phenothiazin-10-yl)phenyl)(pyren-1-yl)-methanone (Py-BP-PTZ) which exhibits AIEE, polymorphism and mechanochromic properties. It shows gradually red shifted emission under high pressure by DAC, and the planar pyrene derivative changes emission mainly due to planarization under high pressure and change of the molecular packing mode with changing intermolecular interactions. Very recently (2024), Eldhose et al.42 reported an MFC property of AIE active pyrene-based compounds and investigated the molecular packing and explained the role of molecular interaction for luminescence behavior. Pyrene-based molecules are enriched in optical properties because of a highly electron-rich polyaromatic system. Some of the pyrene-based materials with high contrast solid-state emission are reported for MFC properties, but there still remain many challenges in terms of controllable approaches to yield high-quality pyrene-based protocols, morphological modulation, unclear structure–function relationships, etc.43,44 The pyrene derivatives reported to date show some degree of fluorescent quenching in the solid state.45,46 Developing pyrene-based fluorescent molecules with good solid-state emission properties is still a challenge.
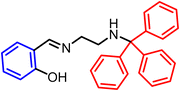
In 2017, our group (Pasha et al.) reported an AIE active salicylaldehyde-based Schiff base that exhibited a reversible change of emission color gradually with applying pressure slowly.47 The reported molecule (shown below) has twisted D–A-type features with ICT characteristics.
After analyzing the packing of the crystal structure, it was found that two molecules form a pair aligned in an opposite manner with a definite weak interaction across the two molecules. Each of the pairs is connected axially forming a long linear chain and these chains are connected horizontally through intermolecular interactions resulting in a molecular sheet. To determine the molecular structure that is responsible for demonstrating a particular way of molecular arrangement described above, more study is required with consideration of the appropriate molecular structure.
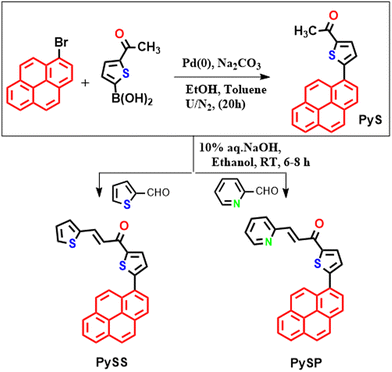
Recently, a pyrene and acyl-thiophene connected D–A type molecule [1-(5-(pyren-1-yl)thiophen-2-yl)ethan-1-one (PySY)] has been synthesized48 and the dihedral angle was observed to be ∼48° (pyrene and thiophene plane). The compound was found to be sensitive to shearing force (by grinding) with hypsochromic emission shifting (yellow to green). But it does not respond to mechanofluorochromism with applying pressure (compressive force). To make the present compound sensitive towards pressure imposed mechanofluorochromism, mimicking our previous work,48 a four-unit conjugated spacer attached to the thiophene side (of the pyridine-acyl substituted thiophene) along with some photoactive species connected terminally. Here, two compounds (PySS and PySP, shown below) have been synthesized, their MFC properties (shearing and compressive forces) were studied, and the feasibility study towards data encryption–decryption and security inks was checked.
Experimental section
Materials
We purchased 1-bromopyrene, palladium(0), thiophene-2-aldehyde and pyridine-2-aldehyde from TCI chemicals. 5-Acetyl thiophene-2-boronic acid was bought from Alfa Aesar. Inorganic salts such as sodium carbonate, potassium carbonate, sodium hydroxide, and potassium hydroxide, etc. were obtained from Merck, and UV-graded solvents like tetrahydrofuran (THF), hexane, cyclohexane, carbon tetrachloride (CCl4), toluene, acetone, N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), dichloromethane (DCM), ethanol and methanol were purchased from the Spectrochem company. All these chemicals were used without any further purification.Measurement and characterization
1H NMR and 13C NMR spectra were recorded using a 400 MHz Bruker NMR spectroscope. High-resolution mass spectra (HRMS) were recorded on an Agilent Q-TOF LC-MS spectrometer in positive electrospray ionization (ESI) mode. UV-VIS absorption spectra were recorded using a Shimadzu spectrophotometer (models UV-1800 and 2550), and solid-state UV-VIS absorption spectra were recorded by a Shimadzu UV-2450, UV-VIS spectrophotometer in absorption mode. Steady-state photoluminescence (PL) spectra were recorded on a Horiba Jobin Yvon spectrofluorometer (FluoroMax-4 and Horiba ‘Fluoro Log’ Spectrofluorometer). Powder X-ray analysis was performed using a Rigaku Mini-Flex II diffractometer with incident Cu-Kα (λ = 1.54 Å) radiation. Raman analysis was performed by using a HORIBA, AIST-NT (Model: Labram HR Evolution, Omega Scope). The quantum yields of the compounds were obtained on a Quanta-Phi (F-3029) accessory connected to the FluoroMax-4. The lifetime of the compounds was recorded on a Horiba Delta Flex 01. Thermogravimetric analysis (TGA) was performed by TGA-50, SHIMADZU equipment at 10 °C min−1, under a nitrogen atmosphere. A differential scanning calorimetry (DSC) study was performed by using a DSC-60 plus instrument from SHIMADZU equipment at 10 °C min−1, under a nitrogen atmosphere. A model- “Apreo LoVac” FE-SEM was used to investigate the morphology of all forms of isolated compounds.Single crystal X-ray diffraction data was collected on Rigaku XtaLABmini X-ray diffractometer equipped with Mercury CCD detector, graphite monochromatic Mo-Kα radiation (λ = 0.71073 Å) at 100.0 (2) K using ω scans (see supporting documents for details).Synthesis of PySS and PySP
The synthetic scheme of the pyrene thiophene-based compounds (PySS and PySP) is summarized below (Scheme 1).Initially, the compound 1-(5-(pyren-1-yl)thiazol-2-yl)ethan-1-one (PyS) was synthesized by following an earlier method reported by our group.48 To synthesize PySS, PyS (0.3 gm, 0.92 mmol) in ethanol (4 ml) was stirred for 30 min at 50 °C and cooled to (10–15) °C, then to the clear solution of reaction mass, NaOH (10% aq. solution, 1 ml) was added slowly and the reaction mixture was further stirred for 10–15 min followed by adding thiophene-2-aldehyde (0.155 gm, 1.38 mmol). Then, the reaction mass was stirred for 10–12 h at 40–50 °C. The reaction mass temperature was slowly cooled to RT, then water (3.0 ml) was added which resulted in precipitate formation, and the reaction mass was filtered, washed with chilled ethanol, and the obtained product was dried for 12 h under vacuum. A dried product (350 mg, 90% yield) was obtained. A similar process was followed to synthesize PySP. The synthesized compounds were characterized by NMR and HRMS (Fig. S1–S9, ESI†).
PyS (1-(5-(pyren-1-yl)thiazol-2-yl)ethan-1-one)
1H NMR (400 MHz, chloroform-d) δ/ppm: 8.4696–8.4928 (d, 1H, J = 9.28 Hz), 8.2745–8.2228 (m, 3H), 8.1776–8.1275 (m, 3H), 8.1078–8.0567 (m, 2H), 7.8692–7.8596 (d, 1H, J = 3.84 Hz), 7.4373–7.4277 (d, 1H, J = 3.84 Hz), 2.6862 (s, 3H). 13C NMR (100 MHz, chloroform-d) δ/ppm: 190.72, 151.54, 132.72, 131.77, 131.43, 130.85, 128.97, 128.91, 128.52, 128.37, 128.08, 127.28, 126.35, 125.76, 125.43, 124.65, 124.36, 26.74. LC-HRMS (ESI): found m/z: 327.0837 [M + H]+. Calculated m/z: 326.0835.PySS ((E)-1-(5-(pyren-1-yl)thiophen-2-yl)-3-(thiophen-2-yl)prop-2-en-1-one)
1H NMR (400 MHz, chloroform-d) δ 8.52 (d, J = 9.3 Hz, 1H), 8.25 (td, J = 7.9, 2.7 Hz, 3H), 8.15 (dd, J = 9.7, 7.2, 2.9 Hz, 4H), 8.12–8.03 (m, 2H), 8.01 (d, J = 3.9 Hz, 1H), 7.48 (dd, J = 4.4, 2.1 Hz, 2H), 7.43 (d, J = 3.6 Hz, 1H), 7.34 (d, J = 15.3 Hz, 1H), 7.15 (dd, J = 5.0, 3.6 Hz, 1H). 13C NMR (100 MHz, chloroform-d) δ/ppm: 181.44, 151.61, 145.67, 140.28, 136.28, 132,19, 132.08, 131.74, 131.41, 130.84, 129.09, 128.91, 128.88, 128.58, 128.54, 128.42, 128.37, 128.13, 127.29, 126.35, 125.76, 125.43, 125.01, 124.69, 124.64, 124.42, 120.33. LC-HRMS (ESI): found m/z: 421.0715 [M + H]+, calculated m/z: 420.0643.PySP (E)-1-(5-(pyren-1-yl)thiophen-2-yl)-3-(pyridin-2-yl)prop-2-en-1-one
1H NMR (400 MHz, chloroform-d) δ 8.75 (dt, J = 4.6, 1.5 Hz, 1H), 8.54 (d, J = 9.3 Hz, 1H), 8.29–8.21 (m, 3H), 8.20–8.10 (m, 5H), 8.13–8.03 (m, 2H), 7.90 (d, J = 15.1 Hz, 1H), 7.79 (td, J = 7.7, 1.8 Hz, 1H), 7.57–7.51 (m, 1H), 7.50 (d, J = 3.8 Hz, 1H), 7.35 (ddd, J = 7.6, 4.7, 1.2 Hz, 1H). 13C NMR (101 MHz, chloroform-d) δ 182.13, 153.12, 152.26, 150.24, 145.72, 142.02, 136.98, 132.98, 131.79, 131.42, 130.85, 129.21, 128.91, 128.59, 128.57, 128.41, 128.16, 127.31, 126.38, 125.80, 125.76, 125.48, 125.16, 125.03, 124.73, 124.66, 124.52, 124.44. LC-HRMS (ESI): found m/z: 416.1131 [M + H]+, calculated m/z: 415.1031.Preparation of AIEE solution
An “aggregation-induced emission enhancement” (AIEE) study of the prepared probe molecules (PySS and PySP) was performed. A stock solution of compounds (10−4 M in THF) was prepared first and then 0.5 ml of each probe solution was taken in a glass vial of 5 ml volume. For preparing the 0% water fraction, 4.5 ml of THF, for the 20% water fraction, 1.0 ml water and 3.5 ml THF, for the 40% water fraction, 2.0 ml of water and 2.5 ml THF, for the 60% water fraction, 3.0 ml water and 1.5 ml THF, and for the 90% water fraction, 4.5 ml water was added. The total volume was made to 5 ml for all the vials.Computational studies
The DFT-based computational calculations were performed by the Gaussian 16 package,49–51 using the ωB97X-D exchange–correlation functional.52 The 6-311+G(d,p) basis set was employed to consider polarization and diffusion effects in the molecules. All the computational study was done in a gaseous state. The optimized structure with the HOMO–LUMO distribution is shown in Fig. 2.Results and discussion
Synthesis of solid-sate emitters
The synthetic scheme of pyrene thiophene-based compounds is summarized in Scheme 1. Initially, PyS was synthesized by using the earlier reported method of our group.48PySS and PySP were synthesized using the Claisen–Schmidt condensation reaction between PyS and the corresponding aldehyde (Scheme 1). The synthesized compound was characterized by NMR and HRMS (Fig. S1–S9, ESI†). The molecular structure of both compounds was determined by single-crystal X-ray diffraction studies (Fig. 3). Thermogravimetric analysis (TGA) of the molecules shows that these molecules are stable up to 320 °C (Fig. S26, ESI†).Photophysical properties
The UV-VIS absorption spectra of the synthesized compounds (PySS and PySP) were recorded in THF (10 μM) solution. It shows absorption peaks at two major regions, intense and sharp peaks observed at ∼230–260 nm and broad vibronic peaks at ∼300–400 nm (Fig. 1). The intense absorption peak at 242 nm corresponds to the 1π–π* transition of pyrene moieties in both compounds.48 The absorption band in the long-wavelength region (300–400 nm) can be attributed to a mixed 3π–π* transition with a charge transfer (CT) character that belongs to the push–pull chalcone π-system.53–56 Solid state absorption of PySS and PySP was recorded; PySS shows a broad and red-shifted absorption tailing up to 600 nm and similarly, for the PySP case, absorption tailing up to 560 nm (Fig. S10, ESI†). The emission spectra of the powder sample of both compounds were recorded at excitation, λmax = 400 nm, and the emission peak was observed at 510 nm for PySS and 531 nm for PySP (Fig. S10, ESI†). In the dilute solution of THF (10−4 M), emission maxima were observed at 509 nm and 520 nm for PySS and PySP, respectively (Fig. 1).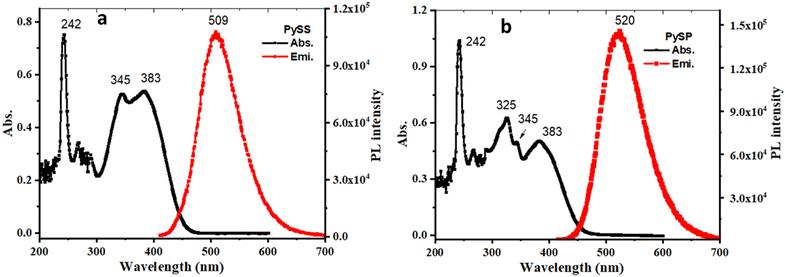 | ||
| Fig. 1 Absorption spectra (black line) with 10 μM concentration and emission (red line) with a concentration of 100 μM of PySS (a) and PySP (b) in THF. | ||
The solvatochromic properties of PySS and PySP were investigated with UV-VIS absorption and photoluminescence (PL) spectroscopy in solvents with varying polarities (Fig. S11 and S12, ESI†), and the corresponding data are summarized in Tables S1 and S2 (ESI†). It was observed that with increasing polarity of the solvent, continuous red-shifting of the emission band occurs (Fig. S11 and S12, ESI†). To better understand the solvatochromic behavior, the relationship between the solvent polarity parameter (Δf) and the Stokes’ shift (Δν) based on the Lippert–Mataga equation was investigated. As shown in (Fig. S13a, ESI†), PySS displays a linear dependence of Δν on Δf, which suggests that solvents with strong polarity are able to stabilize the excited state via the reorientation of the solvent molecules,57 resulting in a lowered energy of the system and the bathochromically shifted emission maximum. In the case of PySP (Fig. S13b, ESI†), relatively more deviation was observed from the linearity. The protic polar solvent (MeOH) deviates more because of the presence of a pyridine ring, and the nitrogen possibly forms a hydrogen bond with MeOH. From the literature, we found that solvatochromic properties were observed mainly because of the ICT (intramolecular charge transfer) and TICT (twisted intramolecular charge transfer) character of the molecules.55 Here, we observed that PySS and PySP are highly sensitive towards the solvent polarity, for PySS ∼ 147 nm shifting from hexane to methanol and in the case of PySP ∼ 130 nm red shift from hexane to DMSO (Fig. S11 and S12, ESI†). The formation of an excimer and intramolecular charge transfer (ICT) are two possibilities that can account for the emission changes. Pyrene has a rigid planar system; it tends to form an excimer at high concentrations.58,59 The packing diagram of PySS (Fig. 7) clearly shows the absence of any pyrene–pyrene interaction, thus excluding the possibility of the formation of any excimer. However, the packing diagram of PySP (Fig. 8) shows the weak pyrene–pyrene interaction, which indicates the possibility of the formation of an excimer. To examine whether an excimer was formed in this system, the PL spectra of PySP in DCM solution with increasing concentrations (10−2 to 10−6 M) were recorded (Fig. S14, ESI†). However, no clear peak shifting was observed, indicating that no excimer was formed. Therefore, it is speculated that the lowest emitting state consists of ICT characters for PySS and PySP. To verify this fact, computational calculations of pyrene thiophene in its optimized lowest energy gas phase were performed using the Gaussian 16 package. The frontier orbital plots of the HOMO and LUMO are shown in Fig. 2. The HOMO of PySS was mainly distributed on pyrene and immediately connected to the thiophene moiety, whereas the LUMO of PySS was majorly shifted towards the second thiophene through the π-electron bridge. In the case of PySP, it was observed that the HOMO is located on the pyrene and immediately connected to the thiophene and the LUMO is located on the pyridine-carbonyl side of the molecule. Since the distributions of the HOMO and LUMO were separated in the donor–acceptor system, this emission will be consistent with the proposed ICT character.
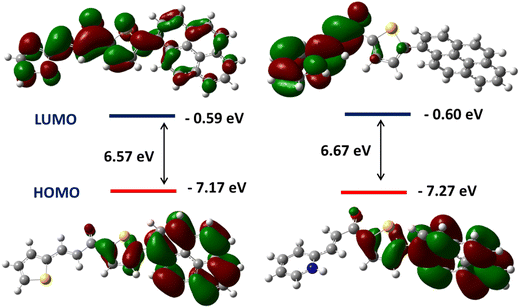 | ||
| Fig. 2 The spatial electron distributions of the HOMOs and LUMOs of PySS (left) and PySP (right), which were calculated using the ωB97X-D/6-311++G(d,P) level by the Gaussian 16 package. | ||
It was observed from the optimized structure of both the molecules that the dihedral angle between the pyrene and attached thiophene is in the range of 50–52°. The observed twisted structure hints at the possibility of ‘Aggregation-induced Emission Enhanced’ (AIEE) behaviour of PySS and PySP. To check the AIEE behavior, a 100 μM tetrahydrofuran solution of PySS and PySP was prepared as a stock solution. Then, AIEE solutions were prepared by varying the water fraction (v/v%) from 0% to 90% (details given in the Experimental section). In the case of PySS (Fig. S15, ESI†), the PL intensity slightly decreases with redshifted emission observed until 60% water fraction (fw), and then a sudden rise of PL intensity was observed. In the case of PySP (Fig. S16, ESI†), the PL intensity decreases up to 40% water and then slightly increases up to 70% water fraction, and then a sharp increment is observed. The extended molecular conjugations for exciton movements are responsible for the redshifted emission upon aggregation.60,61 From the single crystal analysis (Fig. 7 and 8), PySS and PySP have many intermolecular interactions and short contacts (Table S4, ESI†) between the neighbouring molecules. These contacts will be involved in restricting rotational motion, facilitating the excited state molecules to be relaxed in a radiative pathway.
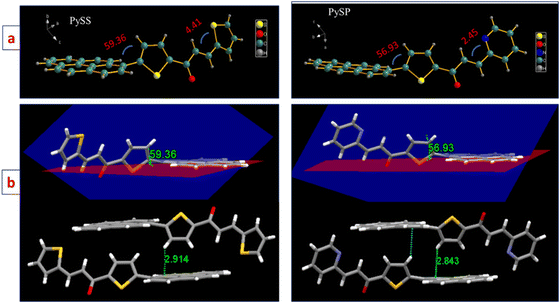
Single-crystal X-ray structures were investigated to comprehend solid-state AIEE, and MFC behavior for these fluorophores. Single crystal structures of PySS and PySP were grown in the DCM/Hexane system by a slow diffusion process (CCDC- for PySS- 2349027 and PySP- 2349045†). In the crystal structure of PySS, pyrene and thiophene are in different planes having a dihedral angle of 59.36°, and the unit cell consists of two molecules facing opposite to each other (see left side of Fig. 3). Similarly, in the case of PySP, the pyrene and thiophene planes are different with a dihedral angle of 56.93°, and unit cell molecules are facing opposite to each other (right side of Fig. 3). The angle between the plane of two thiophene rings in the case of PySS is at 23.54° and the plane of the thiophene and pyridine rings in the case of PySP is at 20.45°.
Mechanofluorochromic properties of PySS and PySP
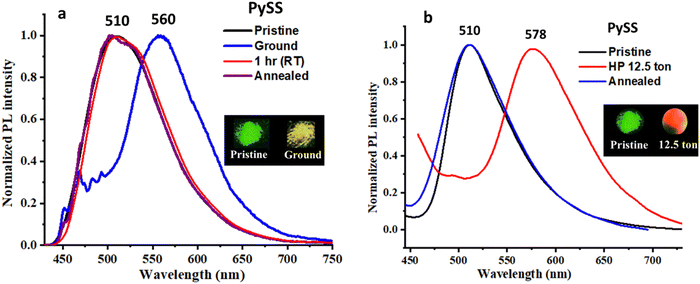
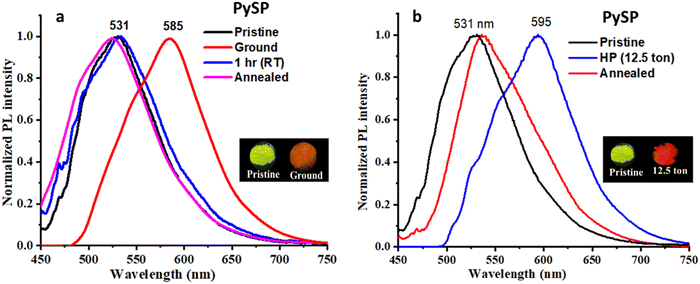
Initially, the solid-state emission properties of both compounds were recorded in powder form. Interestingly, both molecules were emissive in the solid state with a reasonably high quantum yield: compound {λmax, absolute quantum yield (% Φf)}: PySS (510 nm, 24.13); PySP (531 nm, 19.01). The MFC features of these molecules were studied by grinding (using a mortar pestle) and by applying compressive force (using hydraulic pallet press). After grinding, PySS and PySP show large bathochromic emission shifts. It was observed that for the case of PySS, the emission shifted 50 nm from green to yellow (Fig. 4a), and for the case of PySP, the emission shifted 54 nm from greenish yellow to orange emission (Fig. 5a). The reversibility of ground samples of PySS and PySP was checked without giving any stimuli at ambient temperature. The original emission of both the compounds returned within 1 h. As no external stimuli were required to bring these to their original state, the compounds can be used as self-reversible MFC materials.62Interestingly, the MFC behavior of PySS and PySP was reversible on fumigation with methanol vapor/heating at 70–80 °C for 1–2 min, and the same platform can be reused more than five times (Fig. S17 and S18, ESI†). These compounds are highly sensitive to a shearing force, and a clear change of emission could be observed by simply rubbing with a metal spatula (Fig. S29, ESI†). Another efficient feature of PySS and PySP is their responsiveness towards hydraulic pressure (compressive force). Pristine samples of PySS and PySP, after applying hydraulic pressure of 12.5 tons, showed an increment of the bathochromically shifted emission of 68 nm (PySS case Fig. 4b) and 64 nm (PySP case, Fig. 5b), respectively.
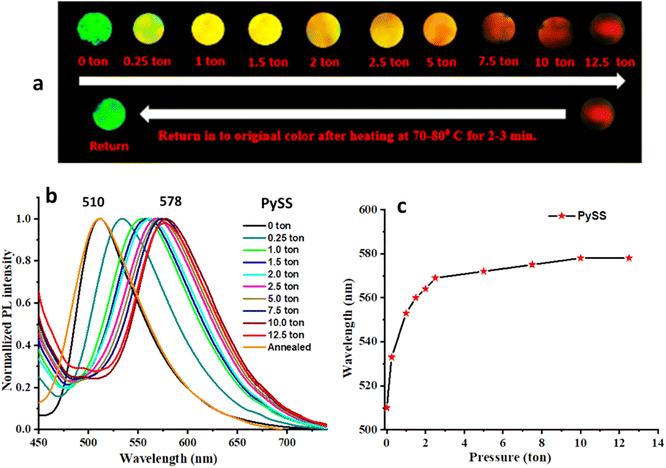
On slowly changing the pressure with a hydraulic press from 0 to 12.5 tons, the original green emission of PySS is gradually transformed through variable intermediate emission colors (yellow, yellow-orange, orange) and finally changed to orange red (λmax = 510 nm → 578 nm). It slowly reverts into the pristine form after releasing pressure within 24 h at ambient conditions and returns within 2–3 min with heating at 60–70 °C (Fig. 6). We have shown the images to describe the recovery process with heating (Fig. S30a, ESI†) and without any external trigger (Fig. S30b, ESI†). It was observed that with gradually increasing pressure (0 to 12.5 tons applied for 2 min each), the emission wavelength increases continuously (Fig. 6c). The solid-state UV-VIS absorption spectra were recorded at different pressures (0 to 12.5 tons) and it was observed that by increasing the pressure, the absorption at ∼445 nm gradually decreased along with the observation of a new redshifted peak at ∼505 nm (Fig. S19, ESI†).
This observation hints that the pyrene-attached twisted thiophene ring (supported by SXRD) becomes planar with the pyrene plane, facilitating the approaching of molecules with each other under compression, thus decreasing intermolecular distances and hence increasing interactions.63 The lifetime study and absolute quantum yield measurement of the pristine sample, ground sample, and compressed (12.5 tons) sample were recorded for PySS and PySP (Table S3, ESI†). It was found that the excited state lifetime increases after mechanical grinding and compressive force for both compounds (Fig. S20, ESI†). The quantum efficiency (QY) of both compounds is increased after grinding, while the QY decreases after pressing (12.5 tons) (Table S3, ESI†). The powder XRD diffractograms of the pristine powder of PySS and PySP exhibited many sharp and strong scattered peaks, supporting good crystallinity with ordered molecular packing (Fig. S21, ESI†). However, the ground powder showed less intensity with some of the peaks disappearing implying their less crystalline or semi-crystalline nature. From the DSC, PySS shows the melting point at 190.5 °C and 191.5 °C for the pristine and ground phases, respectively (Fig. S23, ESI†). Similarly, PySP shows a melting temperature at 207.9 °C and 208.7 °C (Fig. S24, ESI†), for the pristine and ground samples, respectively. After having DSC data of the ground form of both compounds, we did not observe any phase transition which might be because of the highly reversible nature of the ground form of the compounds. The melting point of both compounds was recorded, and the melting range is 188–192 °C for PySS and 207–210 °C for PySP. The compounds (PySS and PySP) were filled in melting capillaries and the emission was captured before and after melting under UV (365 nm) excitation (Fig. S25, ESI†). After melting, the bathochromic emission shifting for both compounds was observed, which supports the phase transition results in bathochromic emission shifting.39 TGA analysis of PySS and PySP showed that both compounds are thermally stable up to ∼320 °C (Fig. S26, ESI†). As already discussed in photophysical properties, the synthesized molecules show ICT characteristics. The new emission band, which is gradually red-shifted with pressure, originated from the ICT state.64 This is also evident from the change in UV-VIS absorption spectra of PySS with increasing pressure (Fig. S19, ESI†). We also performed the Raman spectra of PySS with different amounts of hydraulic pressure (Fig. S27, ESI†), and there was no significant change found in the spectra; it was only observed that the peak intensity reduced at higher pressure. This means that there might be a possibility that at high pressure the intermolecular distance decreases, thus the molecules approach closer upon compression leading to the π–π stacking interactions.65 The FESEM images of both samples were recorded (Fig. S28, ESI†). The pristine form shows a rod and sheet-type morphology, and after applying a shearing force (ground form) non-uniform micro-ranged particles formed.
The mechanism behind the gradual change of emission color of PySS and PySP was investigated by applying compressive force. The packing diagram shows that two molecules are arranged in an anti-parallel orientation, forming a molecular pair with two interactions. This molecular pair further extended axially to a long-range one-dimensional chain-type arrangement. In the case of PySS, two molecules interacted through π-electrons of one carbon of the pyrene ring with the H of the thiophene ring (π(C27)⋯H1 = 2.796 Å) forming a molecular pair and it is further extended through H-bonded interaction (H26⋯O1 = 2.685 Å) to another molecular pair in a one-dimensional fashion. These molecular pairs are extended in one direction linearly and form a one-dimensional chain (Fig. 7a). Each of these one-dimensional chains interacts sidewise with the neighboring parallel and similar chain held with a total of four different interactions (Fig. 7b and Table S4, ESI†). Similarly, for the case of PySP (Fig. 8a), two molecules connected through C–H⋯π interaction (C26⋯H11 = 2.843 Å) and further extended linearly through the hydrogen of pyrene with the pyridine ring hydrogen (H3⋯H20 = 2.329 Å) forming a one-dimensional chain. This one-dimensional chain is further connected sidewise to the neighboring parallel chain through a total of three different interactions (Fig. 8b and Table S4, ESI†). From the crystal packing, it was observed that planar pyrene rings are separated with twisted thiophene and pyridine. Thus, it is presumed that by the application of compressive force the rigid twisted thiophene ring becomes planar and comes closer forming a molecular pair of pyrenes (see packing in Fig. S22, ESI†). A similar kind of molecular packing was observed for the AIE active salicylaldehyde-based Schiff base reported by our group,47 which was also given reversible MFC properties under hydraulic pressure.
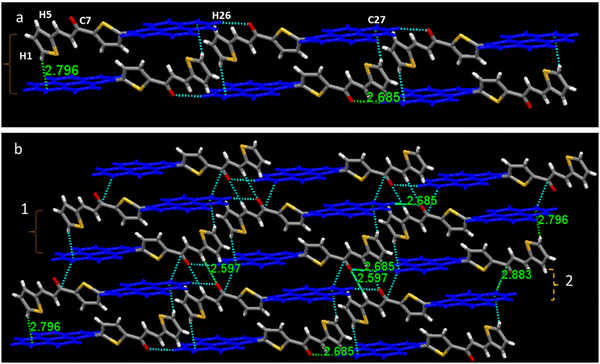 | ||
| Fig. 7 (a) Crystal packing of PySS showing molecule faces opposite to each other and extended in one direction and (b) crystal packing of PySS with intermolecular interactions between molecules. | ||
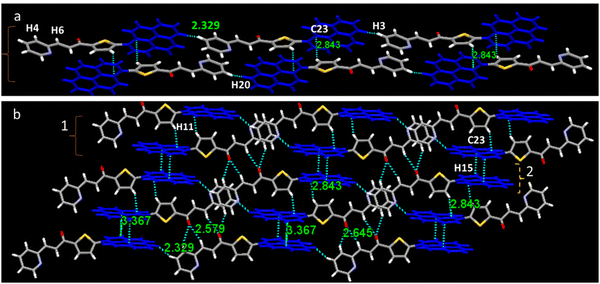 | ||
| Fig. 8 (a) Crystal packing of PySS showing interactions and short contacts between molecules and (b) crystal packing of PySP with intermolecular interactions between molecules. | ||
It is noted that in both the cases (PySS and PySP), the MFC properties were reversible under the application of shearing force and hydraulic pressure, which is contradictory to the earlier report of our group,47 where the emission change by shearing force is not reversible. It was observed that in the case of shearing force, the emission properties are reversible within 1.0 h at ambient conditions. Surprisingly, the reversibility process takes longer time for the hydraulic press one (∼24 h at ambient conditions). From the crystal packing, it is speculated that molecular pairs that help to form a regular arrangement of crystals will mostly be intact under shearing force. So, after releasing pressure the ground sample recovers its original emission. On the other hand, it was observed from the crystal packing that flat pyrene rings are arranged in such a way that with the application of compressive force (hydraulic press), planarization of the molecule increases, and thus the molecules become close to each other. This enhances the π–π stacking interactions that ultimately result in a decrease in emission intensity with red-shifting.43 Upon releasing the pressure, the intermolecular interactions get loosened, and thus the molecules return to their original position (green emission) slowly.
Feasibility study for the application of PySS and PySP for reversible data storage and VOCs
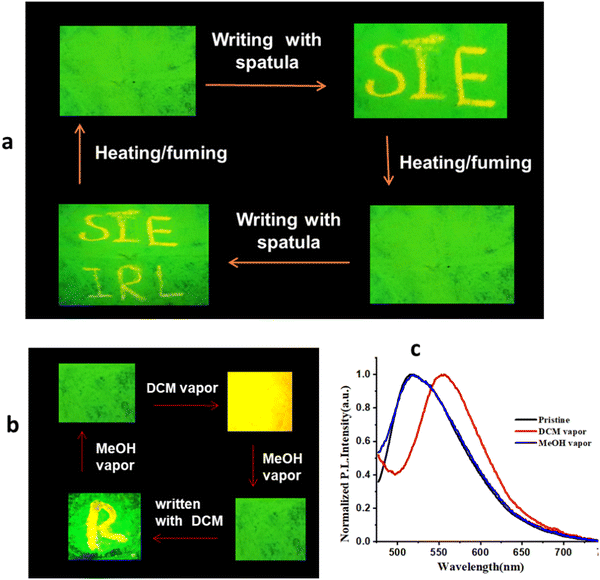
The reversible color-switching features of PySS and PySP offer a promising reusable and secured platform for anticounterfeiting applications. An economical paper strip-based device is fabricated by dispersing green emitting solid (PySS) on a Whatman filter paper, demonstrated in security writing and piezo patterning applications.60 A needle spatula was used to write on the paper, and after writing the emission color changed from green to yellow (Fig. 9), which happens because of the stress arising from the MFC property. This phenomenon is reversible; after heating/fuming with MeOH, its emission color returned to its original form (green emission). It may be useful in security data storage applications. Interestingly, if we expose the DCM vapor to the paper film of PySS, it converts its emission color from green to yellow. On the probe-impregnated filter paper, the letter R was written with DCM and exposed to MeOH vapor; its emission color returned to its original emission (Fig. 9b). Similarly, PySP was coated on filter paper and used for re-writable paper and DCM spot sensors (Fig. S29, ESI†). This way, reversible MFC properties of the probe could be useful for rewritable paper and sensing of volatile organic compounds.Conclusion
Pyrene-thiophene based high-contrast MFC materials (PySS and PySP), which are sensitive to both grinding and compressive force, have been strategically designed and synthesized. Furthermore, PySS and PySP were found to be responsive towards solvent polarity with a large Stokes shift (147 nm and 130 nm). Interestingly, PySS gives a tunable color change by slowly increasing the hydraulic force (compressive), which is reversible in nature. By solvent fumigation or heating, it could be reconverted into its original emission within 2 min for grinding and compressive force. The sensitive MFC properties have been employed to demonstrate security ink and data storage devices. The packing diagram of both compounds showed a head–tail pair followed by a linear chain axially and it leads to a sheet with a layer-based structure. The present study and the earlier studies47 support that the following arrangement of the molecules in the crystal would be responsible for the resulting highly sensitive and tunable emission by compressive force. Certainly, this work will inspire the finer designing of compounds that will result in more precise and controllable reversible tunable emission color.Ethics statement
There are no ethical issues in this work.Data availability
All the data are available in the ESI† and raw data will be made available to readers when requested.Conflicts of interest
The authors have no conflict of interest to declare.Acknowledgements
RPB gratefully acknowledges Birla Institute of Technology and Science (BITS) Pilani, Pilani campus, Rajasthan (India) for the fellowship. VV thanks CSIR for SRF. The ‘UGC-sponsored Special Assistance Program (F.540/14/DRS/2007, SAP-I)’ and DST-FIST programs in the chemistry department are acknowledged for instrumental support. We are also thankful to the Department of Physics, BITS Pilani, Pilani campus for the X-ray powder diffraction facility sponsored by DST-FIST. We are obliged to the central instrument facility of BITS Pilani for NMR, TGA, DSC and other instrumentation facilities. ARC and VV thank IISER Mohali for the “XtaLab mini” single crystal X-ray facility at the Department of Chemical Sciences, IISER Mohali.References
- V. Kachwal and I. R. Laskar, Mechanofluorochromism with Aggregation-Induced Emission (AIE) Characteristics: A Perspective Applying Isotropic and Anisotropic Force, Top. Curr. Chem., 2021, 379(4), 28 CrossRef CAS PubMed.
- Y. Hong, L. Meng, S. Chen, C. W. T. Leung, L.-T. Da, M. Faisal, D.-A. Silva, J. Liu, J. W. Y. Lam and X. Huang, Monitoring and inhibition of insulin fibrillation by a small organic fluorogen with aggregation-induced emission characteristics, J. Am. Chem. Soc., 2012, 134(3), 1680–1689 CrossRef CAS.
- M. Chen, R. Chen, Y. Shi, J. Wang, Y. Cheng, Y. Li, X. Gao, Y. Yan, J. Z. Sun and A. Qin, Malonitrile-functionalized tetraphenylpyrazine: aggregation-induced emission, ratiometric detection of hydrogen sulfide, and mechanochromism, Adv. Funct. Mater., 2018, 28(6), 1704689 CrossRef.
- H. Wang, X. Ji, Z. A. Page and J. L. Sessler, Fluorescent materials-based information storage, Mater. Chem. Front., 2020, 4(4), 1024–1039 RSC.
- A. Li, Z. Ma, J. Wu, P. Li, H. Wang, Y. Geng, S. Xu, B. Yang, H. Zhang and H. Cui, Pressure-Induced Wide-Range Reversible Emission Shift of Triphenylamine-Substituted Anthracene via Hybridized Local and Charge Transfer (HLCT) Excited State, Adv. Opt. Mater., 2018, 6(3), 1700647 CrossRef.
- Y. Sun, Z. Lei and H. Ma, Twisted aggregation-induced emission luminogens (AIEgens) contribute to mechanochromism materials: a review, J. Mater. Chem. C, 2022, 10(40), 14834–14867 RSC.
- C. Xing, Z. Qi, B. Zhou, D. Yan and W. H. Fang, Solid-State Photochemical Cascade Process Boosting Smart Ultralong Room-Temperature Phosphorescence in Bismuth Halides, Angew. Chem., 2024, 136(21), e202402634 CrossRef.
- C. Bustamante, L. Alexander, K. Maciuba and C. M. Kaiser, Single-Molecule Studies of Protein Folding with Optical Tweezers, Annu. Rev. Biochem., 2020, 89, 443–470 CrossRef CAS.
- Y. Hong, J. W. Lam and B. Z. Tang, Aggregation-induced emission, Chem. Soc. Rev., 2011, 40(11), 5361–5388 RSC.
- Z. Zhao, B. He and B. Z. Tang, Aggregation-induced emission of siloles, Chem. Sci., 2015, 6(10), 5347–5365 RSC.
- B. Z. Tang, X. Zhan, G. Yu, P. P. S. Lee, Y. Liu and D. Zhu, Efficient blue emission from siloles, J. Mater. Chem., 2001, 11(12), 2974–2978 RSC.
- W. Z. Yuan, Y. Tan, Y. Gong, P. Lu, J. W. Y. Lam, X. Y. Shen, C. Feng, H. H. Y. Sung, Y. Lu, I. D. Williams, J. Z. Sun, Y. Zhang and B. Z. Tang, Synergy between Twisted Conformation and Effective Intermolecular Interactions: Strategy for Efficient Mechanochromic Luminogens with High Contrast, Adv. Mater., 2013, 25(20), 2837–2843 CrossRef CAS.
- Y. Xiong, J. Huang, Y. Liu, B. Xiao, B. Xu, Z. Zhao and B. Z. Tang, High-contrast luminescence dependent on polymorphism and mechanochromism of AIE-active (4-(phenothiazin-10-yl)phenyl)(pyren-1-yl)methanone, J. Mater. Chem. C, 2020, 8(7), 2460–2466 RSC.
- C. Li, X. Luo, W. Zhao, C. Li, Z. Liu, Z. Bo, Y. Dong, Y. Q. Dong and B. Z. Tang, Switching the emission of tetrakis(4-methoxyphenyl)ethylene among three colors in the solid state, New J. Chem., 2013, 37(6), 1696–1699 RSC.
- P. Galer, R. C. Korošec, M. Vidmar and B. Šket, Crystal Structures and Emission Properties of the BF2 Complex 1-Phenyl-3-(3,5-dimethoxyphenyl)-propane-1,3-dione: Multiple Chromisms, Aggregation- or Crystallization-Induced Emission, and the Self-Assembly Effect, J. Am. Chem. Soc., 2014, 136(20), 7383–7394 CrossRef CAS.
- M. Chakraborty and M. Chakravarty, Variation in solvato-, AIE-and mechano-fluorochromic behavior for furanyl and thiophenyl-substituted anthranyl π-conjugates: the role of tiny flanking donor groups, Mater. Adv., 2021, 2(19), 6418–6427 RSC.
- Y. Dong, B. Xu, J. Zhang, X. Tan, L. Wang, J. Chen, H. Lv, S. Wen, B. Li, L. Ye, B. Zou and W. Tian, Piezochromic Luminescence Based on the Molecular Aggregation of 9,10-Bis((E)-2-(pyrid-2-yl)vinyl)anthracene, Angew. Chem., Int. Ed., 2012, 51(43), 10782–10785 CrossRef CAS.
- J. Li, S. Yuan, J.-S. Qin, L. Huang, R. Bose, J. Pang, P. Zhang, Z. Xiao, K. Tan, A. V. Malko, T. Cagin and H.-C. Zhou, Fluorescence Enhancement in the Solid State by Isolating Perylene Fluorophores in Metal–Organic Frameworks, ACS Appl. Mater. Interfaces, 2020, 12(23), 26727–26732 CrossRef CAS PubMed.
- X. Li, Q. Gao, J. Wang, Y. Chen, Z.-H. Chen, H.-S. Xu, W. Tang, K. Leng, G.-H. Ning, J. Wu, Q.-H. Xu, S. Y. Quek, Y. Lu and K. P. Loh, Tuneable near white-emissive two-dimensional covalent organic frameworks, Nat. Commun., 2018, 9(1), 2335 CrossRef.
- X. Wang, J. Zhang, X. Mao, Y. Liu, R. Li, J. Bai, J. Zhang, C. Redshaw, X. Feng and B. Z. Tang, Intermolecular Hydrogen-Bond-Assisted Solid-State Dual-Emission Molecules with Mechanical Force-Induced Enhanced Emission, J. Org. Chem., 2022, 87(13), 8503–8514 CrossRef CAS PubMed.
- C. Liu, G. Xiao, M. Yang, B. Zou, Z.-L. Zhang and D.-W. Pang, Mechanofluorochromic Carbon Nanodots: Controllable Pressure-Triggered Blue- and Red-Shifted Photoluminescence, Angew. Chem., Int. Ed., 2018, 57(7), 1893–1897 CrossRef CAS PubMed.
- Y.-J. Ma, X. Fang, G. Xiao and D. Yan, Dynamic Manipulating Space-Resolved Persistent Luminescence in Core–Shell MOFs Heterostructures via Reversible Photochromism, Angew. Chem., Int. Ed., 2022, 61(2), e202114100 CrossRef CAS PubMed.
- G. Xiao, B. Zhou, X. Fang and D. Yan, Room-Temperature Phosphorescent Organic-Doped Inorganic Frameworks Showing Wide-Range and Multicolor Long-Persistent Luminescence, Research, 2021, 2021, 9862327 CAS.
- J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts, Adv. Mater., 2014, 26(31), 5429–5479 CrossRef CAS.
- B. Valeur, Molecular Fluorescence, Encyclopedia of Applied Physics, 2009, pp. 477–531 Search PubMed.
- J. Xiong, K. Wang, Z. Yao, B. Zou, J. Xu and X.-H. Bu, Multi-Stimuli-Responsive Fluorescence Switching from a Pyridine-Functionalized Tetraphenylethene AIEgen, ACS Appl. Mater. Interfaces, 2018, 10(6), 5819–5827 CrossRef CAS.
- W. Yang, C. Li, M. Zhang, W. Zhou, R. Xue, H. Liu and Y. Li, Aggregation-induced emission and intermolecular charge transfer effect in triphenylamine fluorophores containing diphenylhydrazone structures, Phys. Chem. Chem. Phys., 2016, 18(40), 28052–28060 RSC.
- J. Jia and H. Zhao, Structure-dependent reversible mechanochromism of D-π-A triphenylamine derivatives, Tetrahedron Lett., 2019, 60(3), 252–259 CrossRef CAS.
- M. Fang, J. Yang, Q. Liao, Y. Gong, Z. Xie, Z. Chi, Q. Peng, Q. Li and Z. Li, Triphenylamine derivatives: different molecular packing and the corresponding mechanoluminescent or mechanochromism property, J. Mater. Chem. C, 2017, 5(38), 9879–9885 RSC.
- M. Yoshizawa and J. K. Klosterman, Molecular architectures of multi-anthracene assemblies, Chem. Soc. Rev., 2014, 43(6), 1885–1898 RSC.
- B. Sun, X. Yang, L. Ma, C. Niu, F. Wang, N. Na, J. Wen and J. Ouyang, Design and Application of Anthracene Derivative with Aggregation-Induced Emission Charateristics for Visualization and Monitoring of Erythropoietin Unfolding, Langmuir, 2013, 29(6), 1956–1962 CrossRef CAS.
- J. Zhao, Z. Chi, Z. Yang, Z. Mao, Y. Zhang, E. Ubba and Z. Chi, Recent progress in the mechanofluorochromism of distyrylanthracene derivatives with aggregation-induced emission, Mater. Chem. Front., 2018, 2(9), 1595–1608 RSC.
- Y. Gu, K. Wang, Y. Dai, G. Xiao, Y. Ma, Y. Qiao and B. Zou, Pressure-Induced Emission Enhancement of Carbazole: The Restriction of Intramolecular Vibration, J. Phys. Chem. Lett., 2017, 8(17), 4191–4196 CrossRef CAS PubMed.
- X. Mei, K. Wei, G. Wen, Z. Liu, Z. Lin, Z. Zhou, L. Huang, E. Yang and Q. Ling, Carbazole-based diphenyl maleimides: Multi-functional smart fluorescent materials for data process and sensing for pressure, explosive and pH, Dyes Pigm., 2016, 133, 345–353 CrossRef CAS.
- Y. Takeda, H. Mizuno, Y. Okada, M. Okazaki, S. Minakata, T. Penfold and G. Fukuhara, Hydrostatic Pressure-Controlled Ratiometric Luminescence Responses of a Dibenzo[a,j]phenazine-Cored Mechanoluminophore, ChemPhotoChem, 2019, 3(12), 1203–1211 CrossRef CAS.
- Y. Yang, A. Li, Z. Ma, J. Liu, W. Xu, Z. Ma and X. Jia, Dibenzo[a,c]phenazine-phenothiazine dyad: AIEE, polymorphism, distinctive mechanochromism, high sensitivity to pressure, Dyes Pigm., 2020, 181, 108575 CrossRef CAS.
- S. Ghosh, H. Bhambri, A. K. Singh, S. K. Mandal, L. Roy and P. S. Addy, A convenient route to a vinylogous dicyano aryl based AIEgen with switchable mechanochromic luminescence properties, Chem. Commun., 2023, 59(30), 4463–4466 RSC.
- X. Meng, G. Qi, X. Li, Z. Wang, K. Wang, B. Zou and Y. Ma, Spiropyran-based multi-colored switching tuned by pressure and mechanical grinding, J. Mater. Chem. C, 2016, 4(32), 7584–7588 RSC.
- Y. Xu, K. Wang, Y. Zhang, Z. Xie, B. Zou and Y. Ma, Fluorescence mutation and structural evolution of a π-conjugated molecular crystal during phase transition, J. Mater. Chem. C, 2016, 4(6), 1257–1262 RSC.
- J. M. Robertson and J. White, 72. The crystal structure of pyrene. A quantitative X-ray investigation, J. Chem. Soc., 1947, 358–368 RSC.
- M. Fang, J. Yang and Z. Li, Light emission of organic luminogens: Generation, mechanism and application, Prog. Mater. Sci., 2022, 125, 100914 CrossRef CAS.
- E. V. Varghese, C.-Y. Yao and C.-H. Chen, Investigation of Mechanochromic Luminescence of Pyrene-based Aggregation-Induced Emission Luminogens: Correlation between Molecular Packing and Luminescence Behavior, Chem. – Asian J., 2024, 19(1), e202300910 CrossRef CAS PubMed.
- Y. Xiong, J. Huang, Y. Liu, B. Xiao, B. Xu, Z. Zhao and B. Z. Tang, High-contrast luminescence dependent on polymorphism and mechanochromism of AIE-active (4-(phenothiazin-10-yl)phenyl)(pyren-1-yl)methanone, J. Mater. Chem. C, 2020, 8(7), 2460–2466 RSC.
- X. Feng, X. Wang, C. Redshaw and B. Z. Tang, Aggregation behaviour of pyrene-based luminescent materials, from molecular design and optical properties to application, Chem. Soc. Rev., 2023, 52, 6715–6753 RSC.
- M. M. Islam, Z. Hu, Q. Wang, C. Redshaw and X. Feng, Pyrene-based aggregation-induced emission luminogens and their applications, Mater. Chem. Front., 2019, 3(5), 762–781 RSC.
- P. Sonar, M. S. Soh, Y. H. Cheng, J. T. Henssler and A. Sellinger, 1,3,6,8-tetrasubstituted pyrenes: solution-processable materials for application in organic electronics, Org. Lett., 2010, 12(15), 3292–3295 CrossRef CAS.
- S. S. Pasha, H. R. Yadav, A. R. Choudhury and I. R. Laskar, Synthesis of an aggregation-induced emission (AIE) active salicylaldehyde based Schiff base: study of mechanoluminescence and sensitive Zn(II) sensing, J. Mater. Chem. C, 2017, 5(37), 9651–9658 RSC.
- R. P. Bhatta, V. Kachwal, C. Climent, M. Joshi, P. Alemany, A. R. Choudhury and I. R. Laskar, Tunable emission in the visible range from a single organic fluorophore through time-controlled morphological evolution, J. Mater. Chem. C, 2023, 11(33), 11399–11408 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, et al., Gaussian 09, Revision A.01, Gaussian. Inc., Wallingford CT, 2009, vol. 121, pp. 150–166 Search PubMed.
- W. J. Hehre, R. Ditchfield and J. A. Pople, Self—consistent molecular orbital methods. XII. Further extensions of Gaussian—type basis sets for use in molecular orbital studies of organic molecules, J. Chem. Phys., 1972, 56(5), 2257–2261 CrossRef CAS.
- M. Frisch, G. Trucks, H. B. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, G. Petersson and H. Nakatsuji, Gaussian 16, Gaussian, Inc., Wallingford, CT, 2016 Search PubMed.
- J.-D. Chai and M. Head-Gordon, Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections, Phys. Chem. Chem. Phys., 2008, 10(44), 6615–6620 RSC.
- Y.-B. Gong, P. Zhang, Y.-R. Gu, J.-Q. Wang, M.-M. Han, C. Chen, X.-J. Zhan, Z.-L. Xie, B. Zou, Q. Peng, Z.-G. Chi and Z. Li, The Influence of Molecular Packing on the Emissive Behavior of Pyrene Derivatives: Mechanoluminescence and Mechanochromism, Adv. Opt. Mater., 2018, 6(16), 1800198 CrossRef.
- E. V. Varghese, C. Y. Yao and C. H. Chen, Investigation of Mechanochromic Luminescence of Pyrene-based Aggregation-Induced Emission Luminogens: Correlation between Molecular Packing and Luminescence Behavior, Chem. – Asian J., 2024, 19(1), e202300910 CrossRef CAS.
- S.-W. Yang, A. Elangovan, K.-C. Hwang and T.-I. Ho, Electronic polarization reversal and excited state intramolecular charge transfer in donor/acceptor ethynylpyrenes, J. Phys. Chem. B, 2005, 109(35), 16628–16635 CrossRef CAS PubMed.
- A. D'Aléo, A. Karapetyan, V. Heresanu, M. Giorgi and F. Fages, Tuning solid-state emission properties of pyrene-containing chalcone derivatives, Tetrahedron, 2015, 71(15), 2255–2259 CrossRef.
- Z. Huang, F. Tang, F. He, L. Kong, J. Huang, J. Yang and A. Ding, Pyrene and triphenylamine substituted cyanostyrene and cyanostilbene derivatives with dual-state emission for high-contrast mechanofluorochromism and cell imaging, Org. Chem. Front., 2022, 9(19), 5118–5124 RSC.
- Z. Zhao, S. Chen, J. W. Y. Lam, Z. Wang, P. Lu, F. Mahtab, H. H. Y. Sung, I. D. Williams, Y. Ma, H. S. Kwok and B. Z. Tang, Pyrene-substituted ethenes: aggregation-enhanced excimer emission and highly efficient electroluminescence, J. Mater. Chem., 2011, 21(20), 7210–7216 RSC.
- Y. Kim, J. Bouffard, S. E. Kooi and T. M. Swager, Highly Emissive Conjugated Polymer Excimers, J. Am. Chem. Soc., 2005, 127(39), 13726–13731 CrossRef CAS.
- M. Chakraborty and M. Chakravarty, Varied optical features and mechanofluorochromism in dicyanovinyl-vs. cyanoacrylicacid-linked twisted π-conjugates: A potential reusable platform for security applications, Mater. Today Chem., 2024, 35, 101836 CrossRef CAS.
- G. Niu, X. Zheng, Z. Zhao, H. Zhang, J. Wang, X. He, Y. Chen, X. Shi, C. Ma, R. T. K. Kwok, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, K. S. Wong, P. Wang and B. Z. Tang, Functionalized Acrylonitriles with Aggregation-Induced Emission: Structure Tuning by Simple Reaction-Condition Variation, Efficient Red Emission, and Two-Photon Bioimaging, J. Am. Chem. Soc., 2019, 141(38), 15111–15120 CrossRef CAS.
- P. S. Hariharan, V. K. Prasad, S. Nandi, A. Anoop, D. Moon and S. P. Anthony, Molecular engineering of triphenylamine based aggregation enhanced emissive fluorophore: structure-dependent mechanochromism and self-reversible fluorescence switching, Cryst. Growth Des., 2017, 17(1), 146–155 CrossRef CAS.
- X. Wang, Q. Liu, H. Yan, Z. Liu, M. Yao, Q. Zhang, S. Gong and W. He, Piezochromic luminescence behaviors of two new benzothiazole–enamido boron difluoride complexes: intra- and inter-molecular effects induced by hydrostatic compression, Chem. Commun., 2015, 51(35), 7497–7500 RSC.
- Q. Qi, J. Qian, X. Tan, J. Zhang, L. Wang, B. Xu, B. Zou and W. Tian, Remarkable Turn-On and Color-Tuned Piezochromic Luminescence: Mechanically Switching Intramolecular Charge Transfer in Molecular Crystals, Adv. Funct. Mater., 2015, 25(26), 4005–4010 CrossRef CAS.
- A. Li, P. Li, Y. Geng, S. Xu, H. Zhang, H. Cui and W. Xu, Investigation of supramolecular interaction in 4,4′-bipyridine crystal by hydrostatic pressure spectroscopies, Spectrochim. Acta, Part A, 2018, 202, 70–75 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2349027 and 2349045. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4tc02262a |
| This journal is © The Royal Society of Chemistry 2024 |