Tetraphenylpyrazine-based chiral deep-blue dyes with high brightness for energy delivery†
Xiang
He‡
a,
Canze
Zheng‡
a,
Xin
Deng
a,
Yingjuan
Hong
a,
Miao
Meng
 a,
Chunxuan
Qi
b,
Hai-Tao
Feng
a,
Chunxuan
Qi
b,
Hai-Tao
Feng
 b,
Ming
Chen
b,
Ming
Chen
 *a and
Ben Zhong
Tang
c
*a and
Ben Zhong
Tang
c
aCollege of Chemistry and Materials Science, Jinan University, Guangzhou 510632, China. E-mail: chenming@jnu.edu.cn
bAIE Research Center, Shaanxi Key Laboratory of Photochemistry, College of Chemistry and Chemical Engineering, Baoji University of Arts and Sciences, Baoji 721013, China
cSchool of Science and Engineering, Shenzhen Institute of Aggregate Science and Technology, The Chinese University of Hong Kong, Shenzhen (CUHK-SZ), Guangdong, China
First published on 30th May 2024
Abstract
The search for deep-blue dyes with high luminescence efficiency in the aggregate state and high brightness is particularly important in optoelectronic application. Herein, based on a typical aggregation-induced emission (AIE) unit of tetraphenylpyrazine (TPP), four dyes were prepared by connecting it with chiral binaphthyl units via an acetylenic bond. The use of the TPP unit is critical to aggregation-enhanced emission (AEE) and deep-blue emission, whereas the merging of the binaphthyl unit is beneficial for molecular conjugation to remarkably improve absorption, photoluminescence (PL) quantum efficiency and brightness. Besides, the binaphthyl unit can serve as an axially chiral source to endow the deep-blue dyes with chirality. It was revealed that attaching a longer alkoxy chain to the dyes facilitates the formation of helical self-assemblies determined by chiral transfer but leads to an aggregation-caused emission (ACQ) effect. Moreover, using these deep-blue dyes as energy donors for energy delivery, other emissions and pure white light emission can be generated effectively via an energy transfer process based on a rational mixture of donors and acceptors in PMMA films. The device fabricated by coating this film onto a blue LED also emitted distinct and stable white light. This work provides a flexible way to design high-performance blue materials for potential applications.
Introduction
Organic emitters have attracted tremendous attention in various applications such as optoelectronic devices, fluorescent probes and smart materials for their good processability, flexible molecular design and low toxicity.1 However, most of the organic luminogens such as pyrene, naphthalene and perylene bisimide are conjugated molecules with a planar conformation. They are prone to form π–π stacking upon aggregation to quench emission, which affects their practical application.2 The emergence of aggregation-induced emission (AIE) or aggregation-enhanced emission (AEE) molecules will help resolve the aggregation-caused quenching (ACQ) effect found in traditional dyes.3 The intrinsic property of AIE or AEE dyes is their twisted and flexible molecular conformation, which enables them to relax in the excited state in solution to annihilate or weaken the emission, while the conformational changes can be supressed to turn-on or enhance the emission by aggregation.4 On this basis, many AIE and AEE dyes have been developed to show extremely high photoluminescence (PL) quantum efficiencies in the aggregate state, which is much desirable to meet diversified applications.5 However, conformational torsion will exert some negative effect on their photo-physical behaviour. In particular, the π electronic cloud overlapping between the inner units is weakened to decrease molecular conjugation, which is unfavourable for absorption.6 It suggests that a good light-harvesting ability is another key factor to determine their applications.7 Thus, designing high-brightness AIE or AEE dyes by a combination of high PL quantum efficiencies and strong absorptions is challenging.Furthermore, despite their potential to exhibit AIE or AEE effects based on their structural design, many fluorescent dyes eventually show a quenched fluorescence emission behaviour in the aggregate state.8 For example, Tang reported two diphenyldibenzofulvene-based dyes, which exhibited strong emissions in the crystal state but almost quenched emissions due to unformed aggregation.9 This indicates that the aggregation-state structure has a profound effect on emission behaviour. However, it is still a challenge to artificially control the aggregate-state behaviour to deeply understand this relationship. Recently, some studies have revealed that for designing high-efficiency photothermal conversion agents, attaching long alkyl chains to the dyes can space the molecules in the aggregate state while vigorous intramolecular motions can take place after excitation due to less steric restriction to boost the non-radiative energy dissipation.10 Thus, it is particularly interesting to use such a strategy to investigate the effect of side chain length on the aggregate-state behaviour of dyes as well as their emission properties, which is instructive to design the luminescent materials with desirable functions and applications.
The search for high-performance blue materials is always important in the optoelectronic field, as they can not only realize one of the three primary colours but also act as hosts to excite other emissions.11 However, the drawbacks of low luminescence efficiency and bad light purity still hamper their applications. Tetraphenylpyrazine (TPP) belongs to a class of heterocycle-based AIE cores, which shows the merits of facile synthesis, good stability, tunable electronic property, rich nitrogen site, etc.12 Many works have demonstrated the superiority of TPP in designing functional materials. For example, the AIE molecules with large two-photon absorption cross sections can be realized by using TPP as an acceptor to induce intramolecular charge transfer.13 A metal–organic framework with TPP as the ligand can fluorescently recognize arginine due to the specific hydrogen bond interaction between the guanidine group in arginine and the nitrogen atom in TPP.14 It is also reported that TPP is favourable to boost the intersystem crossing to enhance the photosensitization by supressing the internal conversion caused by the intramolecular motions in a photosensitizer design.15 The use of TPP in developing blue materials is also promising. By decorating TPP with phenanthroimidazole-based group, an AIE emitter with sky blue emission can be generated with its fabricated light-emitting device showing a high external quantum yield of 4.85%.16 Moreover, TPP can be used to fabricate a blue-emissive amphiphilic organic cage to capture diketopyrrolopyrrole via a host–guest interaction, while their combined roles can contribute to a white light emission.17 Much recently, we fabricated microfibers with a TPP-based AIE dye, which show a high PL quantum efficiency, narrow deep-blue emissions and good photo-stability. The fibers show a good waveguide effect with a low optical loss coefficient and can light up the other emissions in the microscale.18 Although much efforts have been devoted to design blue materials based on such a building block, achieving blue AIE or AEE dyes with high brightness is still desirable.
In this work, based on a typical AIE unit of TPP, we prepared four dyes (two pairs of chiral enantiomers) named TPP-C2(R), TPP-C2(S), TPP-C6(R) and TPP-C6(S) by connecting chiral binaphthyl units with an acetylenic linker (Chart 1). The utilization of the TPP unit is crucial to the AEE effect and deep-blue emission due to its intrinsic property. The merging of binaphthyl units can obviously increase the molecular conjugation to determine a high light absorption ability >8 × 104 cm−1 L mol−1, a PL quantum efficiency of ∼72% and brightness >5.5 × 104 cm−1 L mol−1, which was particularly desirable in the practical applications. Moreover, derived from the binaphthyl units, the molecular chirality was successfully achieved via chiral transfer, as observed from the CD spectra. The molecular chirality can transfer to their self-assembled aggregates. In particular, in TPP-C6(R) and TPP-C6(S) with a longer alkoxy chain, the self-assembled fibers with an opposite helix can be formed, which can be observed using a scanning electron microscope (SEM) and a fluorescence microscope. However, we found that increasing the length of side groups can transform the AEE effect to the ACQ effect, suggesting that the emission behaviour is closely related to the aggregate-state structure affected by this factor. These dyes were further used as energy donors to excite the other emissions. We demonstrate that based on a rational mixture of these deep-blue dyes with other fluorescent dyes (Dye-G, Dye-O and Dye-R) in PMMA, the distinct green, orange and red emissions can be obtained, respectively, with an energy transfer efficiency close to 100%. The PL quantum efficiencies of these emissions were much better than their unity without a donor, indicating a superiority of utilizing them for energy delivery and magnifying emission. Moreover, by using these dyes as donors, the pure white light with a Commission International de L’Eclairage (CIE) coordinate of (0.33, 0.33) and a PL quantum efficiency approaching 100% can generate through a controlled energy transfer effect. After coating the film onto the blue LED, the resulting device can give distinct and stable white light emissions. Thus, this work provides a strategy to design chiral dyes with deep-blue emissions and high brightness for potential optoelectronic applications.
Results and discussion
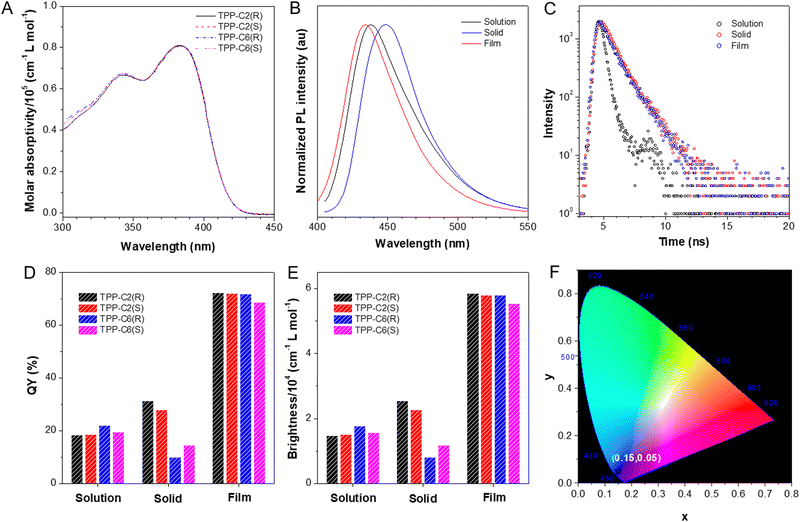
We designed four fluorescent dyes named TPP-C2(R), TPP-C2(S), TPP-C6(R) and TPP-C6(S) by linking the TPP unit and the chiral binaphthyl unit through a Sonogashira coupling reaction, while the length of the alkoxy chain appending to the binaphthyl unit is tunable (Chart 1). Our design principle is as follows: (1) the incorporation of AIE-active TPP unit would endow the molecules with blue emissions and high luminescence efficiencies, while the binaphthyl unit connected by the acetylene bond would remarkably enhance the molecular conjugation to improve the absorptivity, which collectively render them ideal luminescent materials; (2) the binaphthyl unit can not only be used as a conjugation group but also act as an axially chiral source to endow the resulting blue dyes with chirality; (3) the different lengths of alkoxy chain would make the aggregate-state behaviour of dyes adjustable to affect their luminescence and self-assembly properties. The synthetic routes of these dyes are shown in ESI.† After successive reactions, the products were obtained via purification by column chromatography with their structures well characterized by 1H and 13C NMR and high-resolution mass spectroscopies (Fig. S1–S12, ESI†). The thermal stability of these molecules was investigated by thermogravimetric analysis (TGA) at a heating rate of 10 °C under nitrogen. The results indicated that the temperatures at their 5% weight loss were all ∼390 °C, indicating good stability resistant to thermal decomposition irrespective of the alkoxy chain length (Fig. S13, ESI†).The UV-Vis spectra indicated that these dyes almost possessed an identical absorption profile in THF (Fig. 1A). The long-wavelength absorption at 383 nm was ascribed to the extended conjugation by the molecular design, while the short-wavelength absorption at 344 nm was determined by the local excitation such as the TPP unit in the molecules. Moreover, their molar absorptivity (ε) has increased to more than 8 × 104 cm−1 L mol−1 when compared to TPP (2.4 × 104 cm−1 L mol−1) (Table S1, ESI†).12a This suggested that the combination of TPP with the binaphthyl unit has remarkably improved the light-harvesting ability. Moreover, in comparison to the solutions, a red-shift effect by ∼12 and ∼18 nm was found in the powders and doped PMMA films, respectively, which was probably due to the increased intermolecular interaction caused by the aggregation or changed molecular conformations affected by the external environment (Fig. S14, ESI†). The PL spectra showed that they all have an emission maximum at 438 nm in the solutions, while a slight red-shift effect by ∼12 nm can be found in their powders (Fig. 1B and S15, ESI†). This is in accordance with the UV-Vis spectral results that the intermolecular interaction increased to some extent due to the aggregate effect. However, the emission of PMMA films was a bit bluer than that of the solutions. This is because their structural relaxation after excitation has been remarkably reduced by the rigid media, which affected the molecular levels.19 These dyes possessed short-lived fluorescence emissions as recorded by the transient PL spectra (Fig. 1C and S16, ESI†). Moreover, their PL quantum yield (ΦF) in the solutions, powders and films was evaluated by an integrating sphere. The results indicated that the ΦF values of TPP-C2(R) and TPP-C2(S) in the solutions were 18.3% and 18.7%, respectively, which were much smaller than that in the powders (31.3% and 28.0%) (Fig. 1D and Table S1, ESI†). Thus, the AEE effect was realized in TPP-C2(R) and TPP-C2(S), which was attributed to the introduction of the TPP unit. Because TPP is AIE-active, it may endow the resulting molecules with a high conformational freedom, which will provide structural relaxation in the excited state in the solution to weaken the emission, while such relaxation can be restricted in the aggregate state to enhance the emission.12a However, the emission of the dyes can be tuned by the aggregate-state behaviour affected by the appended alkoxy chains. For example, TPP-C6(R) and TPP-C6(S) possessed similar ΦF values to TPP-C2(R) and TPP-C2(S) in the solutions. However, their ΦF values dropped to 10.0% and 14.6%, respectively in the powders. Thus, increasing the length of side groups makes them suffer from the ACQ effect. This was because the much longer alkoxy chains in TPP-C6(R) and TPP-C6(S) may provide much flexible surroundings while the excited-state intramolecular motions were still easy to take place to boost the non-radiative transition. The ΦF values of all dyes were recorded as high as ∼72% in the films. This confirmed that a rigid environment can be formed by PMMA to prohibit the intramolecular motions to further boost the emissions.19 Normally, the brightness (εΦF) is an important parameter to evaluate the luminescent materials by a combinative consideration of their absorption and emission properties. Here, the brightness of these dyes in these films was calculated to be more than 5.5 × 104 cm−1 L mol−1, which were superior to the most reported conventional dyes and AIE dyes (Fig. 1E and Table S1, ESI†).20 Moreover, the Commission International de L’Eclairage (CIE) coordinates of these films were (0.15, 0.05) (Fig. 1F and Table S1, ESI†). All these suggested that these dyes are deep-blue materials with excellent brightness, which were particularly desirable in the practical applications.
Moreover, to provide more insights into the photophysical properties of these dyes, their excited-state decay rates were evaluated using the formula of kr = ΦF/τ and knr = (1 − ΦF)/τ, where kr, knr and τ are the radiative and non-radiative decay rates, and excited-state lifetime, respectively. Their calculated kr values in the solutions were ∼0.36 × 109 S−1, which were much larger than that in the powder (0.12–0.28 × 109 S−1) (Table S2, ESI†). It was corresponding to the possible formation of excimers caused by the binaphthyl unit in the aggregate state, which impaired the radiative transition process. Moreover, the knr values of these dyes in the solutions were 1.15–1.67 × 109 S−1. However, in the powders, the knr values of TPP-C2(R) and TPP-C2(S) reduced remarkably as the excited-state intramolecular motions were prohibited by the aggregation. The much obvious reduction in knr than kr from the aggregate to solution state can lead to the AEE effect. By comparison, the knr values of TPP-C6(R) and TPP-C6(S) were nearly as twice as that of TPP-C2(R) and TPP-C2(S) and close to that of their solutions. This suggested that the introduction of long alkoxy chains into the molecules can facilitate the intramolecular motions to quench the emission even when they were in the aggregate state. Besides, a much larger kr value of ∼0.61 × 109 S−1 can be found in their films in comparison to the solutions and powders. This may be due to two factors: (1) in PMMA films, the molecular conjugation may increase due to the conformational change and (2) the molecules were diluted by the films, while the formation of excimers may be effectively prohibited. Furthermore, the PMMA matrix also played a positive role in supressing the non-radiative transition as reflected by their much reduced knr value of ∼0.25 × 109 S−1, suggesting that this rigid matrix was pretty evident to restrict the intramolecular motions in the excited state.
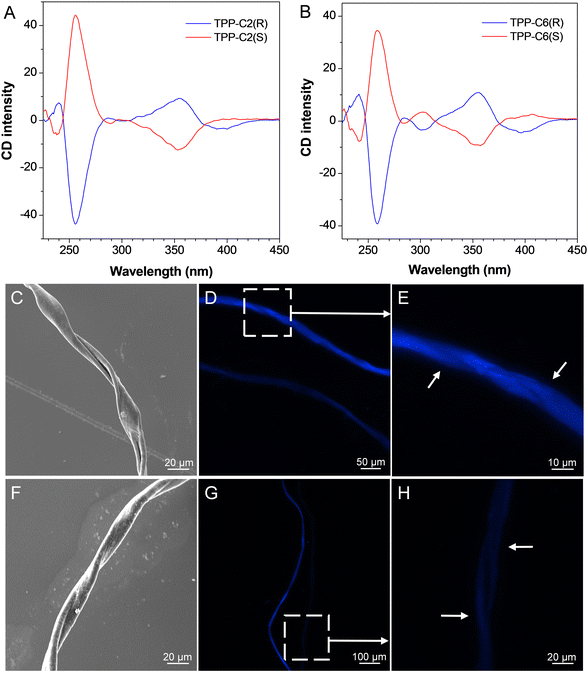
In this work, we employed the binaphthyl unit not only because of the role in strengthening the molecular conjugation but also in consideration of a chiral source to bestow the dyes with chirality. To check this, their circular dichroism (CD) spectroscopies in the solutions were investigated. As shown in Fig. 2A, the CD spectra of TPP-C2(R) and TPP-C2(S) in the solutions showed distinct Cotton effects and a mirror-image relationship at a wavelength in the range from 230 to 450 nm. The CD signals at ∼260 nm were attributed to the binaphthyl moiety, whereas the CD bands centered at ∼390 nm and 350 nm were mainly decided by the whole molecule and TPP unit, respectively, which were corresponding to the absorption peaks in the UV-Vis spectra. This indicated that the chirality of the binaphthyl unit has successfully transferred from the part to the whole, and two chiral enantiomers with deep-blue emissions and AEE property were obtained. Besides, a very similar phenomenon was observed in the CD spectra of TPP-C6(R) and TPP-C6(S) though they experienced an ACQ effect (Fig. 2B).
Many studies indicated that the molecular chirality may aid in the self-assembly to definite morphologies such as vesicles, spheres and fibers.21 Would such self-assembly behaviour be achieved by our dyes? To answer this question, we prepared the samples with TPP-C2(R) and TPP-C2(S) dissolved in a CHCl3/CH3CN (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture. Because CHCl3 and CH3CN separately served as good and poor solvents, this moderate mixture may induce the formation of self-assemblies with the time. However, there was nothing detectable in the solutions by naked eyes during a continuous 5-day observation. On day 5, the sample was extracted and casted onto the silica plate, followed by studying using a scanning electron microscope (SEM). The results indicated that there were almost no definite self-assemblies, which can be observed in the image, suggestive of a worse self-assembly behaviour (Fig. S17, ESI†). In sharp contrast, a much clear structure can be formed when TPP-C6(R) and TPP-C6(S) were dissolved in the same mixture. For TPP-C6(R), the explicit fibers with a diameter of ∼15 µm can be found by SEM, which were uniformly right-handed (Fig. 2C). A similar phenomenon can be observed using a fluorescence microscope under UV light excitation, while the deep-blue fibers with a specific helix can be discerned (Fig. 2D and E). Moreover, although the fibers of similar sizes can be generated by TPP-C6(S) under the same condition, they were almost left-handed, which were confirmed by both SEM and fluorescence microscopy (Fig. 2F–H). This suggested that the molecular chirality has transferred to their self-assembled aggregates through a hierarchical approach, which ensured an efficient outcome for the generation of highly functional self-assembled structures.22 In other words, the length of alkoxy chains appended to the chiral molecules played a vital role in this transfer behaviour.
1) mixture. Because CHCl3 and CH3CN separately served as good and poor solvents, this moderate mixture may induce the formation of self-assemblies with the time. However, there was nothing detectable in the solutions by naked eyes during a continuous 5-day observation. On day 5, the sample was extracted and casted onto the silica plate, followed by studying using a scanning electron microscope (SEM). The results indicated that there were almost no definite self-assemblies, which can be observed in the image, suggestive of a worse self-assembly behaviour (Fig. S17, ESI†). In sharp contrast, a much clear structure can be formed when TPP-C6(R) and TPP-C6(S) were dissolved in the same mixture. For TPP-C6(R), the explicit fibers with a diameter of ∼15 µm can be found by SEM, which were uniformly right-handed (Fig. 2C). A similar phenomenon can be observed using a fluorescence microscope under UV light excitation, while the deep-blue fibers with a specific helix can be discerned (Fig. 2D and E). Moreover, although the fibers of similar sizes can be generated by TPP-C6(S) under the same condition, they were almost left-handed, which were confirmed by both SEM and fluorescence microscopy (Fig. 2F–H). This suggested that the molecular chirality has transferred to their self-assembled aggregates through a hierarchical approach, which ensured an efficient outcome for the generation of highly functional self-assembled structures.22 In other words, the length of alkoxy chains appended to the chiral molecules played a vital role in this transfer behaviour.
The S0 geometries of these dyes were optimized by B3LYP/6-31G(d,p), Grimme's DFT-D3.23 The results indicated that these conformations were highly twisted, particularly in the TPP and binaphthyl moieties (Fig. S18, ESI†). Thus, the incorporation of the TPP unit may weaken the π–π stacking effect while restricting the intramolecular motions in the aggregate state to facilitate the emission. The twist between two naphthyl rings in the binaphthyl unit in the enantiomers was opposite with a dihedral angle larger than 70°. The remarkable conformational difference may ensure the chiral property of dyes due to the mirror symmetry. The electronic structure analysis suggested that the highest occupied molecular orbitals (HOMOs) of these dyes were mainly distributed on the central binaphthyl units, part of the peripheral TPP unit and the connected acetylenic bonds (Fig. 3). In contrast, more electronic clouds were found in the pyrazine rings of TPP besides the above-mentioned regions in their lowest unoccupied molecular orbitals (LUMOs). The successive electronic cloud distribution on the whole molecules suggested that a good conjugation effect has been realized due to a good planarity between the naphthyl rings and the phenyl rings connected by the acetylenic bonds. Moreover, good electronic cloud overlapping between the HOMOs and LUMOs manifested that local state excitation is dominant in the excitation behaviour. Both factors can contribute to a high molar absorptivity and luminescence efficiency of the dyes. Moreover, we found that the molecular conformation as well as the electronic structure were less affected by the length of alkoxy chains, suggesting that such role exerted a negligible effect on their photo-physical properties at a molecular level.
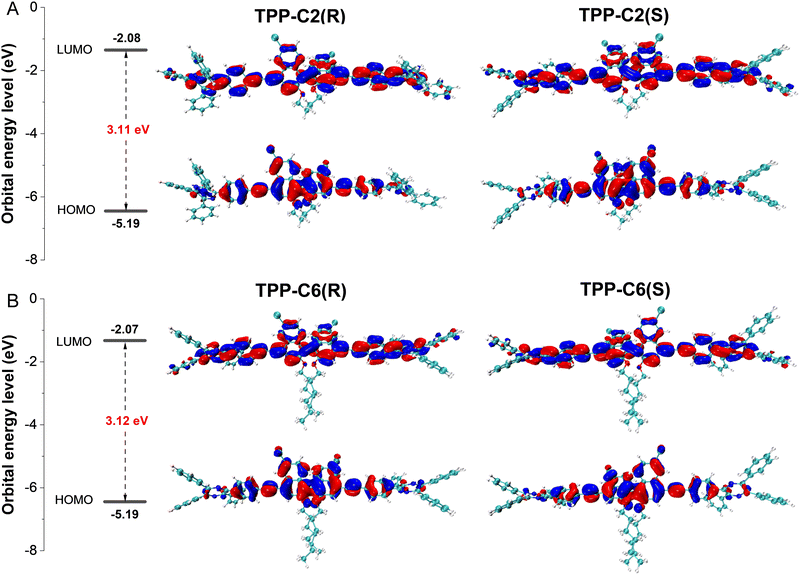 | ||
| Fig. 3 Orbital distribution on S0 conformations of (A) TPP-C2(R) and TPP-C2(S), and (B) TPP-C6(R) and TPP-C6(S) optimized by B3LYP/6-31G(d,p), Grimme's DFT-D3 and their energy levels. | ||
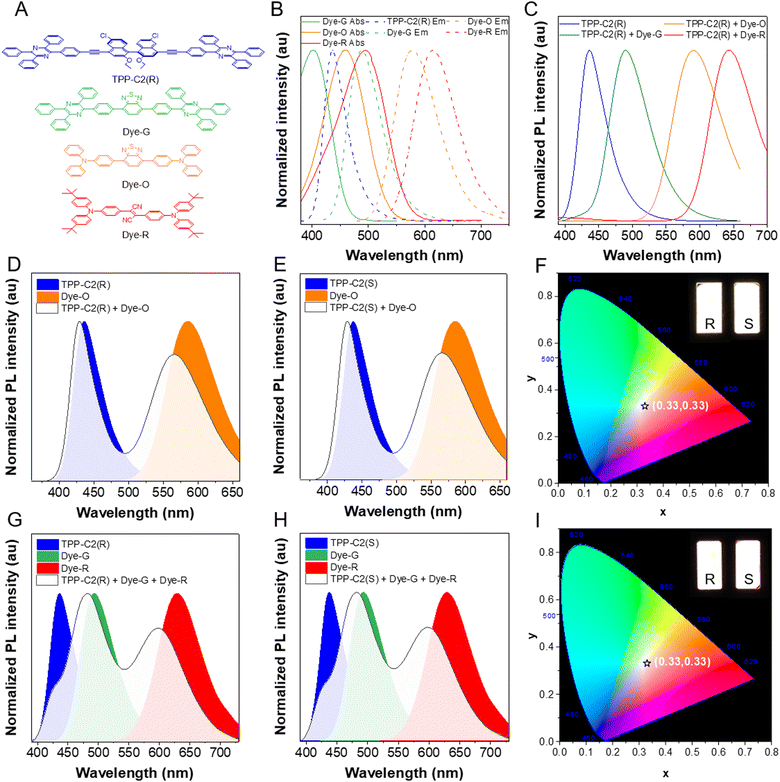
With the high-brightness deep-blue dyes in the hand, we expected to utilize them as an energy host to excite the other emissions. In this work, three fluorescent dyes named Dye-G, Dye-O and Dye-R were adopted, with their structures shown in Fig. 4A. Most of these dyes have been proved to show the AIE effect with green, orange and red emissions, respectively, and possess a high luminescence efficiency.24 We thus synthesized them according to the procedure reported in the literature with their structures well characterized by NMR spectroscopy (see ESI†). The PL study indicated that they showed emission maximums at 488, 578 and 614 nm in the films, while their absorption maximums were located at 401, 460 and 494 nm in THF, respectively (Fig. 4B and S19, ESI†). Note that the absorptions of these dyes have good overlapping with the emission of deep-blue dyes, indicating a theoretical possibility for the energy transfer from the donors to the acceptors. Indeed, when they are doped into deep-blue dyes with PMMA as a matrix, their emissions can originate remarkably with the increase in doping concentration accompanied by the decrease in blue light intensities. Moreover, the energy transfer efficiency was calculated using the formula of η = 1 − (IDA/ID), where ID is the emission intensity of donor initially, and IDA is the emission intensity of donor after doping with the acceptor.25 Our studies revealed that with a suitable mixing ratio, almost all the energies can be transferred from the donors to the acceptors after excitation (Fig. 4C and Fig. S20, ESI†). For example, when the ratio of TPP-C2(R) between Dye-G, Dye-O and Dye-R reached 2 wt%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt%, 7.3 wt%
1 wt%, 7.3 wt%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 wt%, and 16.5 wt%
0.9 wt%, and 16.5 wt%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 wt%, very high η of 93.5%, 99.9% and 97.9% can be realized, respectively (Table S3, ESI†). Because TPP-C2(S), TPP-C6(R) and TPP-C6(S) behaved similarly in the PMMA films, they all possessed a similar energy transfer process with these dopants. Moreover, the average PL quantum efficiencies of these acceptors were recorded as high as 67.1%, 99.1% and 54.8%, respectively, which were much better than their pure state, suggesting that it was attractive to magnify the emissions of other dyes through an energy transfer approach with our deep-blue dyes as energy donors (Table S3, ESI†).26
0.8 wt%, very high η of 93.5%, 99.9% and 97.9% can be realized, respectively (Table S3, ESI†). Because TPP-C2(S), TPP-C6(R) and TPP-C6(S) behaved similarly in the PMMA films, they all possessed a similar energy transfer process with these dopants. Moreover, the average PL quantum efficiencies of these acceptors were recorded as high as 67.1%, 99.1% and 54.8%, respectively, which were much better than their pure state, suggesting that it was attractive to magnify the emissions of other dyes through an energy transfer approach with our deep-blue dyes as energy donors (Table S3, ESI†).26
White light plays an essential role in the illumination in our daily life.27 The white light is always realized by a partial energy transfer from the blue host to the other guest, while a mixed blue emission and other emissions can generate a consecutive spectrum similar to the visible light region in the sunlight.28 In this regard, TPP-C2(R) or TPP-C2(S) was taken as a host to excite Dye-O. While the ratio of TPP-C2(R)/TPP-C2(S)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Dye-O were all 1 wt%
Dye-O were all 1 wt%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 wt%, the distinct white light emissions comprising the blue light from the donor and the orange light from the acceptor can emerge with their PL quantum efficiencies approaching 100% (Fig. 4D and E and Table S3, ESI†). The white emission can also be obtained by a controllable mixture of TPP-C2(R) or TPP-C2(S) with Dye-G and Dye-R in PMMA if the emission from the individual was balanced (Fig. 4G and H). However, their luminescence efficiencies have discounted to some extent based on this approach (Table S3, ESI†). Moreover, the CIE coordinates of these films were recorded as (0.33, 0.33), which matched well with the standard of pure white light proposed by the International Commission on illumination (Fig. 4F and I). TPP-C6(R) and TPP-C6(S) also played a similar role in generating white light (Fig. S21, ESI†). All these indicated that the high-quality white light emission can be realized readily with our dyes as hosts. Finally, to demonstrate the potential applications, the white light PMMA film containing TPP-C2(R) and Dye-O was coated on a blue LED (Fig. 5A–C). It was clear that the strong white light can be generated with the LED as an excitation source (Fig. 5D–F). Moreover, as reflected by the fluorescence spectra, less changes in colour and emission intensities can be found during 5 days, indicating a good stability in the use (Fig. S22, ESI†). Thus, it was promising to fabricate it as a device for potential illuminations.
0.3 wt%, the distinct white light emissions comprising the blue light from the donor and the orange light from the acceptor can emerge with their PL quantum efficiencies approaching 100% (Fig. 4D and E and Table S3, ESI†). The white emission can also be obtained by a controllable mixture of TPP-C2(R) or TPP-C2(S) with Dye-G and Dye-R in PMMA if the emission from the individual was balanced (Fig. 4G and H). However, their luminescence efficiencies have discounted to some extent based on this approach (Table S3, ESI†). Moreover, the CIE coordinates of these films were recorded as (0.33, 0.33), which matched well with the standard of pure white light proposed by the International Commission on illumination (Fig. 4F and I). TPP-C6(R) and TPP-C6(S) also played a similar role in generating white light (Fig. S21, ESI†). All these indicated that the high-quality white light emission can be realized readily with our dyes as hosts. Finally, to demonstrate the potential applications, the white light PMMA film containing TPP-C2(R) and Dye-O was coated on a blue LED (Fig. 5A–C). It was clear that the strong white light can be generated with the LED as an excitation source (Fig. 5D–F). Moreover, as reflected by the fluorescence spectra, less changes in colour and emission intensities can be found during 5 days, indicating a good stability in the use (Fig. S22, ESI†). Thus, it was promising to fabricate it as a device for potential illuminations.
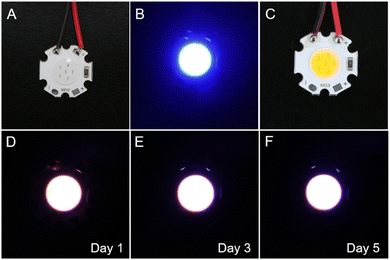 | ||
| Fig. 5 (A)–(C) Fabrication of while light-emitting device based on blue LEDs by coating a TPP-C2(R)/Dye-O film. (D)–(F) Exhibition of white light emission by the device in 5 days. | ||
Conclusions
In this work, we have fabricated TPP-based chiral deep-blue dyes by a combination of TPP and binaphthyl units connected by acetylenic bonds. Such a concept can not only endow the molecules with the AEE effect decided by the TPP unit but also remarkably strengthen the molecular conjugation to improve the light absorption ability and luminescence efficiency. This was reflected by ε of more than 8 × 104 cm−1 L mol−1 in the solutions and ΦF of ∼72% in the PMMA films, which can lead to high brightness of more than 5.5 × 104 cm−1 L mol−1. Moreover, the CIE coordinates of these films were (0.15, 0.05), indicating good deep-blue materials for potential applications. The incorporated binaphthyl units can also serve as chiral sources to endow the deep-blue dyes with axial chirality, which was confirmed by the CD spectra. The length of the appended alkoxy chains has an evident effect on self-assemble behaviour, and a longer side chain may induce the formation of definite helical fibers with an opposite direction in the chiral enantiomers, as monitored by SEM and fluorescence microscopy. However, increasing the length of side groups may lead to conversion from AEE to ACQ, as affected by the aggregate-state behaviour caused by such a factor. Moreover, these dyes can be utilized as energy donors to excite other emissions. With their rational mixture with other fluorescent dyes in the PMMA matrix, almost all the energies can be transferred from the donors to the acceptors while a magnified emission can be realized in the acceptors. We also found that the pure white light emission with a CIE coordinate of (0.33, 0.33) can be readily obtained by controllable energy transfer from the donors to the acceptors with PL quantum yields of films approaching 100%. Finally, after coating the films onto the blue LED, the while light-emitting device was fabricated, which showed distinct emissions and good stability. All these indicated that they hold great promise in the optoelectronic application.Author contributions
M. C. conceived and designed the experiment, and write the paper. X. H. and Y. H. synthesized and characterized the materials. X. H. and C. Z. carried out the fluorescence spectra, SEM and fluorescence microscope tests, etc. X. D. conducted the theoretical calculation. C. Q. and H. T. F. carried out the transient PL spectra and absolute quantum yield test. M. M. and B. Z. T. provided suggestions in the study and manuscript. All authors approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is partially supported by the National Natural Science Foundation of China (22275072), the Project of Science and Technology of Guangzhou (2024A04J3712), Shenzhen Key Laboratory of Functional Aggregate Materials (ZDSYS20211021111400001), the Science Technology Innovation Commission of Shenzhen Municipality (KQTD20210811090142053 and JCYJ20220818103007014), Research Foundation of Education Department of Shaanxi Province (20JS005) and Scientific and Technological Innovation Team of Shaanxi Province (2022TD-36).Notes and references
- (a) X.-H. Zhu, J. Peng, Y. Cao and J. Toncali, Chem. Soc. Rev., 2011, 40, 3509–3524 RSC; (b) J. Song, H. Lee, E. G. Jeong, K. C. Choi and S. Yoo, Adv. Mater., 2020, 32, 1907539 CrossRef CAS PubMed; (c) G. M. Farinola and R. Ragni, Chem. Soc. Rev., 2011, 40, 3467–3482 RSC; (d) S. Ma, S. Du, G. Pan, S. Dai, B. Xu and W. Tuan, Aggregate, 2021, 2, e96 CrossRef CAS; (e) Y. Liu, C. Li, Z. Ren, S. Yan and M. R. Bryce, Nat. Rev. Mater., 2018, 3, 18020 CrossRef CAS.
- (a) E. A. Chandross and C. J. Dempster, J. Am. Chem. Soc., 1970, 92, 3586–3593 CrossRef CAS; (b) J. M. G. Martinho, J. Phys. Chem., 1989, 93, 6687–6692 CrossRef CAS; (c) K. Stavrou, A. Danos, T. Hama, T. Hatakeyama and A. Monkman, ACS Appl. Mater. Interfaces, 2021, 13, 8643–8655 CrossRef CAS PubMed; (d) B. R. Crenshaw and C. Weder, Adv. Mater., 2005, 17, 1471–1476 CrossRef CAS.
- (a) J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed; (b) J. Yang, M. Fang and Z. Li, Aggregate, 2020, 1, 6–18 CrossRef; (c) M. Fang, J. Yang and Z. Li, Prog. Mater. Sci., 2022, 125, 100914 CrossRef CAS; (d) G. R. Suman, M. Pandey and A. S. J. Chakravarthy, Mater. Chem. Front., 2021, 5, 1541–1584 RSC; (e) Y. Song, G. Pan, C. Zhang, C. Wang, B. Xu and W. Tian, Mater. Chem. Front., 2023, 7, 5104–5119 RSC; (f) N. Jiang, D. Zhu, Z. Su and M. R. Bryce, Mater. Chem. Front., 2021, 5, 60–75 RSC.
- (a) J. Li, M. Zhao, J. Huang, P. Liu, X. Luo, Y. Zhang, C. Yan, W.-H. Zhu and Z. Guo, Coordin. Chem. Rev., 2022, 473, 214813 CrossRef CAS; (b) F. Zhang, H. Xie, B. Guo, C. Zhu and J. Xu, Polym. Chem., 2022, 13, 8–13 RSC; (c) Z. Yang, Z. Mao, Z. Xie, Y. Zhang, S. Liu, J. Zhao, J. Xu, Z. Chi and M. P. Aldred, Chem. Soc. Rev., 2017, 46, 915–1016 RSC; (d) Q. Li, Y. Li, T. Min, J. Gong, L. Du, D. L. Phillips, J. Liu, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, R. T. K. Kwok, C. L. Ho, K. Li, J. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 132, 9557–9564 CrossRef; (e) M. Chen, Z. Zhang, R. Lin, J. Liu, M. Xie, X. He, C. Zheng, M. Kang, X. Li, H.-T. Feng, J. W. Y. Lam, D. Wang and B. Z. Tang, Chem. Sci., 2024 10.1039/D3SC06886B; (f) T. Zang, Y. Xie, S. Su, F. Liu, Q. Chen, J. Jing, R. Zhang, G. Niu and X. Zhang, Angew. Chem. Int. Ed., 2020, 132, 10089–10093 CrossRef; (g) X. Tian, H. Wang, S. Cao, Y. Liu, F. Meng, X. Zheng and G. Niu, J. Mater. Chem. C, 2023, 11, 5987–5994 RSC.
- (a) X. Cai and B. Liu, Angew. Chem., Int. Ed., 2020, 59, 9868–9886 CrossRef CAS PubMed; (b) F. Hu, S. Xu and B. Liu, Adv. Mater., 2018, 30, 1801350 CrossRef PubMed; (c) D. D. La, S. V. Bhosale, L. A. Jones and S. V. Bhosale, ACS Appl. Mater. Interfaces, 2018, 10, 12189–12216 CrossRef CAS PubMed; (d) E. Ubba, Y. Tao, Z. Yang, J. Zhao, L. Wang and Z. Chi, Chem. – Asian J., 2018, 13, 3106–3121 CrossRef CAS PubMed; (e) F. Rizzo and F. Cucinotta, Isr. J. Chem., 2018, 58, 888–974 CrossRef; (f) Q. Wei, N. Fei, A. Islam, T. Lei, L. Hong, R. Peng, X. Fan, L. Chen, P. Gao and Z. Ge, Adv. Opt. Mater., 2018, 6, 1800512 CrossRef; (g) R. Xu, D. Dang, Z. Wang, Y. Zhou, Y. Xu, Y. Zhao, X. Wang, Z. Yang and L. Meng, Chem. Sci., 2022, 13, 1270–1280 RSC; (h) W. Ma, Y. Wang, Y. Xue, M. Wang, C. Lu, W. Guo, Y.-H. Liu, D. Shu, G. Shao, Q. Xu, S. Tu and H. Yan, Chem. Sci., 2024, 15, 4019–4030 RSC.
- (a) Y. Li, Y. Tang, W. Hu, Z. Wang, X. Li, X. Lu, S. Chen, W. Huang and Q. Fan, Adv. Sci., 2023, 10, 2204695 CrossRef CAS PubMed; (b) S. Liu, H. Ou, Y. Li, H. Zhang, J. Liu, X. Lu, R. T. K. Kwok, J. W. Y. Lam, D. Ding and B. Z. Tang, J. Am. Chem. Soc., 2020, 142, 15146–15156 CrossRef CAS PubMed.
- (a) K. Terayama, S. Habuchi and T. Michinobu, J. Polym. Sci., 2023, 61, 2276–2291 CrossRef CAS; (b) M. Nakamura, I. Kanetani, M. Gon and K. Tanaka, Angew. Chem., Int. Ed., 2024 DOI:10.1002/ange.202404178; (c) Y. Gui, K. Chen, Y. Sun, Y. Tan, W. Luo, D. Zhu, Y. Xiong, D. Yan, D. Wang and B. Z. Tang, Chin. J. Chem., 2023, 41, 1249–1259 CrossRef CAS.
- (a) L. Qian, B. Tong, J. Shen, J. Shi, J. Zhi, Q. Dong, F. Yang, Y. Dong, J. W. Y. Lam, Y. Liu and B. Z. Tang, J. Phys. Chem. B, 2009, 113, 9098–9103 CrossRef CAS PubMed; (b) C. Zheng, Q. Zang, H. Nie, W. Huang, Z. Zhao, A. Qin, R. Hu and B. Z. Tang, Mater. Chem. Front., 2018, 2, 180–188 RSC; (c) Z. Qiu, W. Zhao, M. Cao, Y. Wang, J. W. Y. Lam, Z. Zhang, X. Chen and B. Z. Tang, Adv. Mater., 2018, 2, 1803924 CrossRef PubMed.
- X. Luo, J. Li, C. Li, L. Heng, Y. Q. Dong, Z. Liu, Z. Bo and B. Z. Tang, Adv. Mater., 2011, 23, 3261–3265 CrossRef CAS PubMed.
- (a) Z. Zhao, C. Chen, W. Wu, F. Wang, L. Du, X. Zhang, Y. Xiong, X. He, Y. Cai, R. T. K. Kwok, J. W. Y. Lam, X. Gao, P. Sun, D. L. Phillips, D. Ding and B. Z. Tang, Nat. Commun., 2019, 10, 768 CrossRef CAS PubMed; (b) S. Liu, X. Zhou, H. Zhang, H. Qu, J. W. Y. Lam, Y. Liu, L. Shi, D. Ding and B. Z. Tang, J. Am. Chem. Soc., 2019, 141, 5359–5368 CrossRef CAS PubMed.
- (a) S. Rana, S. R. Nayak, S. Patel and S. Vaidyanathan, J. Mater. Chem. C, 2024, 12, 765–818 RSC; (b) Z. Zhang, D. Dou, R. Xia, P. Wu, E. Spuling, K. Wang, J. Cao, B. Wei, X. Li, J. Zhang, S. Bräse and Z. Wang, Sci. Adv., 2023, 9, eadf4060 CrossRef PubMed; (c) P. Sun, D. Liu, F. Zhu and D. Yan, Nat. Photonics, 2023, 17, 264–272 CrossRef CAS; (d) X. Wang, G. Zhao, T. Gao, G. Zhang, H. Chen, T. Zhou, Z. Zhang, W. Tian, W. Jiang and Y. Sun, J. Mater. Chem. C, 2024 10.1039/D3TC04808J; (e) Z. Feng, Z. Cheng, Z. Su, L. Wan, S. Ge, H. Liu, X. Ma, F. Liu and P. Lu, Adv. Opt. Mater., 2024 DOI:10.1002/adom.202302839; (f) B. Zhou, Z. Qi, M. Dai, C. Xing and D. Yan, Angew. Chem., Int. Ed., 2023, 135, e202309913 CrossRef.
- (a) M. Chen, L. Li, H. Nie, J. Tong, L. Yan, B. Xu, J. Z. Sun, W. Tian, Z. Zhao, A. Qin and B. Z. Tang, Chem. Sci., 2015, 6, 1932–1937 RSC; (b) M. Chen, A. Qin, J. W. Y. Lam and B. Z. Tang, Coordin. Chem. Rev., 2020, 422, 213472 CrossRef CAS; (c) M. Chen, J. Liu, F. Liu, H. Nie, J. Zeng, G. Lin, A. Qin, M. Tu, Z. Kai, H. H. Y. Sung, I. D. Williams, J. W. Y. Lam and B. Z. Tang, Adv. Funct. Mater., 2019, 29, 1903834 CrossRef; (d) M. Chen, R. Chen, Y. Shi, J. Wang, Y. Cheng, Y. Li, X. Gao, Y. Yan, J. Z. Sun, A. Qin, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Adv. Funct. Mater., 2018, 28, 1704689 CrossRef; (e) P. Meti, F. Mateen, D. Y. Hwang, Y.-E. Lee, S.-K. Hong and Y.-D. Gong, Dyes Pigm., 2022, 202, 110221 CrossRef CAS; (f) X.-D. Ying, J.-X. Chen, D.-Y. Tu, Y.-C. Zhuang, D. Wu and L. Shen, ACS Appl. Mater. Interfaces, 2021, 13, 6421–6429 CrossRef CAS PubMed; (g) L. Yao, K. Fu and G. Liu, ACS Appl. Mater. Interfaces, 2023, 15, 40817–40827 CrossRef CAS PubMed; (h) G. Gong, H. Wu, T. Zhang, Z. Wang, X. Li and Y. Xie, Mater. Chem. Front., 2021, 5, 5012–5023 RSC; (i) H.-Q. Zheng, L. Zhang, M. Lu, X. Xiao, Y. Yang, Y. Cui and G. Qian, Research, 2022, 9869510 CAS; (j) H. F. Pananilath, C. Govind, T. D. Thadathilanickal and V. Karunakaran, Phys. Chem. Chem. Phys., 2023, 25, 26575–26587 RSC.
- M. Chen, H. Nie, B. Song, L. Li, J. Z. Sun, A. Qin and B. Z. Tang, J. Mater. Chem. C, 2016, 4, 2901–2908 RSC.
- H.-Q. Yin, X.-Y. Wang and X.-B. Yin, J. Am. Chem. Soc., 2019, 141, 15166–15172 CrossRef CAS PubMed.
- C. Li, J. Liu, Y. Hong, R. Lin, Z. Liu, M. Chen, J. W. Y. Lam, G.-H. Ning, X. Zheng, A. Qin and B. Z. Tang, Angew. Chem., Int. Ed., 2022, 61, e202202005 CrossRef CAS PubMed.
- H. Wu, Y. Pan, J. Zeng, L. Du, W. Luo, H. Zhang, K. Xue, P. Chen, D. L. Phillips, Z. Wang, A. Qin and B. Z. Tang, Adv. Optical Mater., 2019, 7, 1900283 CrossRef.
- H.-T. Feng, X. Zheng, X. Gu, M. Chen, J. W. Y. Lam, X. Huang and B. Z. Tang, Chem. Mater., 2018, 30, 1285–1290 CrossRef CAS.
- H. Rao, Z. Liu, M. Chen, C. Zheng, L. Xu, J. Liu, J. W. Y. Lam, B. Li, X. Yang and B. Z. Tang, Aggregate, 2024, 5, e453 CrossRef CAS.
- (a) M. Tang, J. Wen, Y. Sun, Q. Hou, X. Cai, W. He, X. Xie, H. Ding, F. Li, L. Zheng, Y. Shi and Q. Cao, Adv. Funct. Mater., 2024 DOI:10.1002/adfm.202314130; (b) M. Dong, L. Liao, C. Li, Y. Mu, Y. Huo, Z.-M. Su and F. Liang, J. Mater. Chem. C, 2024, 12, 443–448 RSC.
- (a) A. H. Ashoka, I. O. Aparin, A. Reisch and A. S. Klymchenko, Chem. Soc. Rev., 2023, 52, 4525–4548 RSC; (b) G. Jiang, H. Liu, H. Liu, G. Ke, T.-B. Ren, B. Xiong, X.-B. Zhang and L. Yuan, Angew. Chem. Int. Ed., 2024, 136, e202315217 CrossRef.
- (a) Y. Xia, A. Hao and P. Xing, ACS Nano, 2023, 17, 21993–22003 CrossRef PubMed; (b) J. Kwon, K. H. Park, W. J. Choi, N. A. Kotov and J. Yeom, Acc. Chem. Res., 2023, 56, 1359–1672 CrossRef CAS PubMed; (c) S. Huang, H. Yu and Q. Li, Adv. Sci., 2021, 8, 2002132 CrossRef PubMed; (d) H. Li, X. Mao, H. Wang, Z. Geng, B. Xiong, L. Zhang, S. Liu, J. Xu and J. Zhu, Macromolecules, 2020, 53, 4214–4223 CrossRef CAS; (e) H. Li, E. J. Cornel, Z. Fan and J. Du, Chem. Sci., 2022, 13, 14179–14190 RSC.
- (a) J. Zhang, Q. Liu, W. Wu, J. Peng, H. Zhang, F. Song, B. He, X. Wang, H. H. Y. Sung, M. Chen, B. S. Li, S. H. Liu, J. W. Y. Lam and B. Z. Tang, ACS Nano, 2019, 13, 3618–3628 CrossRef CAS PubMed; (b) Y. S. Lee, Self-Assembly and Nanotechnology: a Force Balance Approach, John Wiley & Sons, 2008 CrossRef.
- (a) M. J. Frisch, et al.Gaussian 16, Gaussian, Inc., Wallingford, CT, 2016 Search PubMed; (b) S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed; (c) S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- (a) L. Pan, Y. Cai, H. Wu, F. Zhou, A. Qin, Z. Wang and B. Z. Tang, Mater. Chem. Front., 2018, 2, 1310–1316 RSC; (b) K. R. J. Thomas, J. T. Lin, M. Velusamy, Y.-T. Tao and C.-H. Chuen, Adv. Funct. Mater., 2004, 14, 83–90 CrossRef CAS; (c) W. Qin, N. Alifu, J. W. Y. Lam, Y. Cui, H. Su, G. Liang, J. Qian and B. Z. Tang, Adv. Mater., 2020, 32, 2000364 CrossRef CAS PubMed.
- (a) Q. Song, S. Goia, J. Yang, S. C. L. Hall, M. Staniforth, V. G. Stavros and S. Perrier, J. Am. Chem. Soc., 2021, 143, 382–389 CrossRef CAS PubMed; (b) B. Song, L. Zhang, J. Sun, J. W. Y. Lam and B. Z. Tang, Angew. Chem., Int. Ed., 2023, 62, e202302543 CrossRef CAS PubMed.
- (a) P. Q. Nhien, J.-H. Tien, T. T. K. Cuc, T. M. Khang, N. T. Trung, B. T. B. Hue, J. I. Wu and H.-C. Lin, J. Mater. Chem. C, 2022, 10, 18241–18257 RSC; (b) N. T. Trung, P. Q. Nhien, T. T. K. Cuc, C.-H. Wu, B. T. B. Hue, J. I. Wu, Y.-K. Li and H.-C. Lin, ACS Appl. Mater. Interfaces, 2023, 15, 15353–15366 CrossRef CAS PubMed; (c) P. Q. Nhien, T. T. K. Cuc, T. M. Khang, C.-H. Wu, B. Z. B. Hue, J. I. Wu, B. W. Mansel, H.-L. Chen and H.-C. Lin, ACS Appl. Mater. Interfaces, 2020, 12, 47921–47938 CrossRef CAS PubMed; (d) P. Q. Nhien, H.-K. Chang, T. T. K. Cuc, T. M. Khang, C.-H. Wu, B. T. B. Hue, J. I. Wu and H.-C. Lin, Sens. Actuators, B, 2022, 372, 132634 CrossRef CAS.
- (a) Z. Chen, C.-L. Ho, L. Wang and W.-Y. Wong, Adv. Mater., 2020, 32, 1903269 CrossRef CAS PubMed; (b) H. Zhang, Q. Su and S. Chen, Nat. Commun., 2020, 11, 2826 CrossRef CAS PubMed.
- (a) N.-C. Chiu, K. T. Smith and K. C. Stylianou, Coordin. Chem. Rev., 2022, 459, 214441 CrossRef CAS; (b) Y. Huang, E.-L. Hsiang, M.-Y. Deng and S.-T. Wu, Light: Sci. Appl., 2020, 9, 105 CrossRef CAS PubMed; (c) B. Kumari, R. Dahiwadkar and S. Kanvah, Aggregate, 2022, 3, e191 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc01374c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |