Efficient thermally activated delayed fluorophores featuring multi-donor arms and a π-extended acceptor core†
Bo
Liu
ab,
Si-Wei
Chen
ab,
Wen-Cheng
Chen
 *ab,
Longjiang
Xing
ab,
Ji-Hua
Tan
ab,
Wei-Le
Wu
ab,
Xiao-Long
Liu
*ab,
Longjiang
Xing
ab,
Ji-Hua
Tan
ab,
Wei-Le
Wu
ab,
Xiao-Long
Liu
 ab,
Jia-Xiong
Chen
ab,
Hao-Li
Zhang
ab,
Jia-Xiong
Chen
ab,
Hao-Li
Zhang
 *c and
Yanping
Huo
*c and
Yanping
Huo
 *abd
*abd
aGuangdong Provincial Laboratory of Chemistry and Fine Chemical Engineering Jieyang Center, Jieyang, 515200, P. R. China. E-mail: wencchen@gdut.edu.cn; yphuo@gdut.edu.cn
bSchool of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou, 510006, P. R. China
cState Key Laboratory of Applied Organic Chemistry (SKLAOC), Key Laboratory of Special Function Materials and Structure Design (MOE), College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: haoli.zhang@lzu.edu.cn
dAnalytical & Testing Center, Guangdong University of Technology, Guangzhou, 510006, P. R. China
First published on 16th April 2024
Abstract
Thermally activated delayed fluorophores often face challenges related to low solid-state luminescence efficiency and inefficient spin–flip processes, compromising their performances in organic light-emitting diodes. Herein, we introduce two new thermally activated delayed fluorophores, namely 4CzAQ and 4CzDP, based on a π-extended acceptor core (acenaphtho[1,2-b]quinoxaline/dibenzo[a,c]phenazine) and four electron-donating carbazolyl arms. These new emitters feature a propeller-like sterically demanding configuration, which mitigates emission quenching in the solid state. Crucially, this design strategy results in a dense charge-transfer excited-state alignment and strategically introduces locally excited triplet states from the acceptor within the alignment to facilitate spin flipping by a spin–vibronic coupling mechanism. The luminescence properties and spin-flipping efficiency can be finely tuned by varying the acceptor unit, depending on its π-conjugation extension and energy level. A device doped with 10 wt% of the optimal emitter, 4CzAQ, achieves a remarkable electroluminescence performance with an external quantum efficiency of 26.8% and a peak at 555 nm. The combined steric and electronic modulation arising from this molecular design offers a compelling strategy to address critical challenges in thermally activated delayed fluorophores.
Introduction
Thermally activated delayed fluorescence (TADF) materials have drawn increasing attention and demonstrated their versatility in applications ranging from bioimaging,1 sensing,2 photocatalysis,3 and lasers,4 to optoelectronic devices.5,6 Particularly, TADF compounds have become a subject of interest in the organic light-emitting diode (OLED) field, specifically for displays and lighting.7,8 In contrast to conventional phosphors, TADF emitters stand out for their ability to fully utilize electrically formed singlet and triplet excitons without relying on noble metals,9–11 highlighting their practical advantages, including notable efficiency, cost-effectiveness, and environmentally friendly nature. Despite blue and green TADF materials having been well developed, achieving comparable efficiencies in longer-wavelength TADF emitters remains a challenge because of the energy gap law,12–14 resulting in increased non-radiative transition and lower luminescence efficiency.Enhancing molecular rigidity is a proven method to diminish molecular rotation and vibration, consequently inhibiting non-radiative processes.15 Thus, much effort has been devoted to developing new TADF materials with long-wavelength using π-extended rigid frameworks.16–18 However, employment of extended π-conjugated motifs often leads to strong interchromophore interactions in the solid state, resulting in severe emission quenching and hindering electroluminescence (EL) performance improvement.19 To mitigate emission quenching, the emissive materials must be dispersed within a suitable host material at low concentrations (<5 wt%).20–22 Nevertheless, this method also brings about additional issues, such as insufficient host-to-guest energy transfer,23 imbalanced charge transport,6 and the need for precise regulation of the evaporation rate during device fabrication.
Another approach to enhance the EL performance involves the promotion of reverse intersystem crossing (RISC). This compensates for excitons leaking through intersystem crossing (ISC), allowing more excitons to remain in the S1 state, ready for fluorescence. Nevertheless, a typical twisted donor (D)–acceptor (A)-based TADF compound has a predominating charge transfer (CT) characterized S1 and T1 states (1CT and 3CT, respectively).24,25 As per the EI-Sayed's rule, 3CT → 1CT spin flipping is theoretically forbidden as the spin–orbit coupling (SOC) matrix element between S1 and T1 with similar orbital features is extremely small.26–28 This results in significant triplets accumulating at high excitation densities, leading to detrimental exciton losses.29,30
Theoretically, fine-tuning the D/A structure allows the placement of the locally excited (LE) triplet state of the donor (3LED) or acceptor (3LEA) between or near 1CT and 3CT, promoting spin-flipping efficiency. As majority of the donors in twisted D–A-based TADF emitters are of limited conjugation with high 3LED energy levels, modulating 3LEA is more practical for developing long-wavelength TADF emitters. Our previous research14 and those of other groups31–34 have underscored the crucial importance of the triplet energy level position of the acceptor component in achieving high efficiency in long-wavelength TADF systems. Besides, recent studies have indicated that introducing multiple donor moieties, typically carbazole derivatives, in TADF emitters significantly promotes their RISC rate constant (kRISC).35–37 This is attributed to the generation of charge-resonance hybrid triples and the emergence of a dense manifold of triplet states, offering rich spin-flipping channels through a second-order spin–vibronic coupling (SVC) mechanism.38 Currently, most multi-carbazole-based TADF compounds rely on small-conjugated acceptors, such as benzonitrile39–41 and triazine derivatives,42–44 and achieving long-wavelength emission with EL peak of over 550 nm in this type of TADF system remains sporadic.45,46
In consideration of these insights, we propose that coupling multiple donors to a rigid, π-extended acceptor would be a potent strategy for creating high-performance long-wavelength TADF emitters. In this approach, the rigid, π-extended acceptor would provide a reasonably low-lying 3LEA that approaches the 1CT and 3CT manifolds to invoke the RISC process. Incorporating multi-donor units not only enriches the density of CT excited states, thereby further facilitating spin flipping through SVC-assisted RISC processes, but also acts as bulky protective arms to mitigate emission quenching. The proof-of-the-concept TADF emitters, namely 8,9,10,11-tetra(9H-carbazol-9-yl)acenaphtho[1,2-b]quinoxaline (4CzAQ) and 10,11,12,13-tetra(9H-carbazol-9-yl)dibenzo[a,c]phenazine (4CzDP), showcase long-wavelength emission of over 550 nm and mitigatory quenching behavior in the solid state. Furthermore, the new emitters showed decent kRISC values of over 105 s−1. Through a systematic structure–property relationship study, we observed that TADF properties can be finely tuned by adjusting the conjugation and electron-accepting capacity of the acceptor units, significantly influencing emission color and exciton dynamics. As a result, we obtained a high external quantum efficiency (EQE) of 26.8%, marking one of the highest efficiencies reported for multi-carbazole-based TADF OLEDs.
Results and discussion
Synthesis and characterization
The chemical structures of the new TADF emitters, 4CzAQ and 4CzDP, are illustrated in Fig. 1. These molecules are characterized by a quadruple carbazole decoration combined with a π-extended acceptor core, composed of acenaphtho[1,2-b]quinoxaline (AQ) or dibenzo[a,c]phenazine (DP), respectively. The synthetic routes to 4CzAQ and 4CzDP are outlined in Scheme S1 (ESI†). Typically, synthesizing sterically demanding π-extended conjugated molecules presents challenges due to low coupling yields. To overcome this issue, the new multi-donor TADF emitters were synthesized through steric hindrance–insensitive nucleophilic aromatic substitution (SNAr) reactions36 utilizing a multiply fluorinated π-extended pyrazine. This pyrazine can be readily obtained via the condensation reaction47 of 3,4,5,6-tetrafluorobenzene-1,2-diamine and aromatic diketone.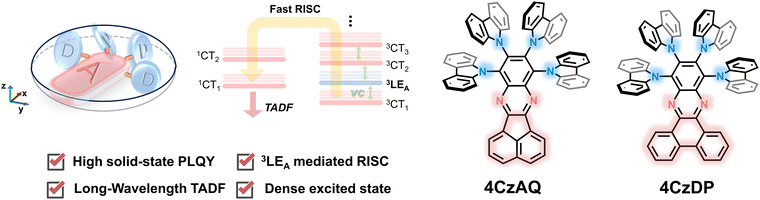 | ||
| Fig. 1 The proposed molecular design for the long-wavelength TADF molecules with multi-donor arms and a π-extended acceptor core. | ||
4CzAQ and 4CzDP were purified using temperature-gradient vacuum sublimation, followed by comprehensive characterization using NMR, mass spectroscopies, and crystallographic analyses. Their highest occupied molecular orbital (HOMO) energy levels (EHOMO) were determined as −5.44 and −5.47 eV, respectively, based on the half-wave potentials from cyclic voltammetry curves (Fig. S1, ESI†). Additionally, the lowest unoccupied molecular orbital (LUMO) energy levels were calculated from EHOMO − Eg (optical energy gap estimated from the absorption onset) to be −2.92 and −3.15 eV, respectively. 4CzAQ and 4CzDP exhibit superior decomposition temperatures (5% weight loss) of 445.5 and 501.1 °C (Fig. S2 and Table S1, ESI†), respectively, which are thermally stable for fabricating vacuum-deposited OLEDs.
Crystallographic analysis
The multi-donor cluster is believed to effectively shield the π-extended AQ and DP moieties from strong interchromophore interactions, thereby ensuring favorable solid-state luminescence properties. The influence of the quadruple carbazole decoration on molecular conformation and intermolecular interactions has been investigated through crystallographic analysis (Fig. 2). 4CzAQ and 4CzDP were initially dissolved in CH2Cl2 due to their relatively high solubility (>5 mg mL−1), and then subjected to an anti-solvent (MeOH) vapor diffusion process to yield single crystals. Both new compounds exhibit a propeller-like bulky configuration, with D–A dihedral angles ranging from 53° to 67° (Fig. 2a). The densely packed carbazole motifs within the molecule can interlock with each other via steric hindrance and π interaction,48 resulting in a stable conformation beneficial for suppressing non-radiative transitions.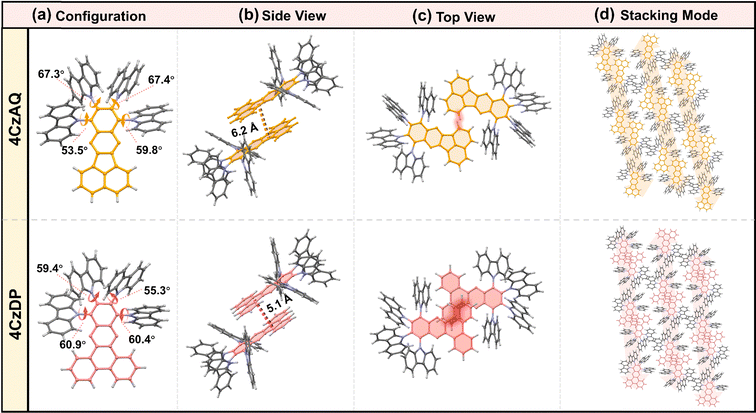 | ||
| Fig. 2 Single crystal structures of 4CzAQ and 4CzDP: (a) configuration; the side view (b) and top view (c) of two molecular dimers; (d) single crystal stacking mode of two molecules. | ||
It is observed that every two molecules of 4CzAQ and 4CzDP form an anti-symmetric head-to-head dimeric structure (Fig. 2b). This arrangement arises due to the less sterically demanding nature of the terminal part of AQ/DP (i.e., the fused naphthyl and phenanthryl of AQ and DP, respectively). Due to DP's more extended π conjugation compared to AQ, intermolecular interactions become more pronounced, resulting in a shorter centroid–centroid distance between two adjacent AQ/DP units (Fig. 2b). Fortunately, the interchromophore interactions of both emitters are relatively weak, as evidenced by the limited intermolecular overlap (Fig. 2c), which helps preserve favorable solid-state luminescence, particularly for 4CzAQ. Typically, reported multi-donor TADF emitters are based on acceptors with limited conjugation, often exhibiting a ball-like molecular configuration with minimal intermolecular contacts.49 While this feature is desirable for maintaining excellent solid-state luminescence properties, it impedes carrier transport in solid film-based organic EL devices.50 The present multi-carbazole decorated π-extended molecular design can overcome this trade-off by fostering suitable intermolecular contacts that facilitate a carrier hopping channel via π-extended acceptors (Fig. 2d).
Theoretical calculations
Theoretical calculations based on density functional theory (DFT) were performed to elucidate the electronic structures (Fig. 3 and Table S2, ESI†). Electrostatic potential mapping highlights the significance of the two sp2-hybridized nitrogen atoms within the pyrazine segment as the primary electron-withdrawing components of AQ/DP, accentuated in red in Fig. 3a. Notably, in contrast to AQ, DP exhibits a larger extension of the red surface to its fused phenanthrene moiety, possibly due to its heightened degree of conjugation. This observation suggests DP's superior electron-accepting capabilities relative to AQ, as evidenced by its deeper LUMO level (−2.21 vs. −2.03 eV). Consequently, this results in stronger intramolecular charge transfer (ICT) and a narrower energy gap in 4CzDP (Fig. 3b).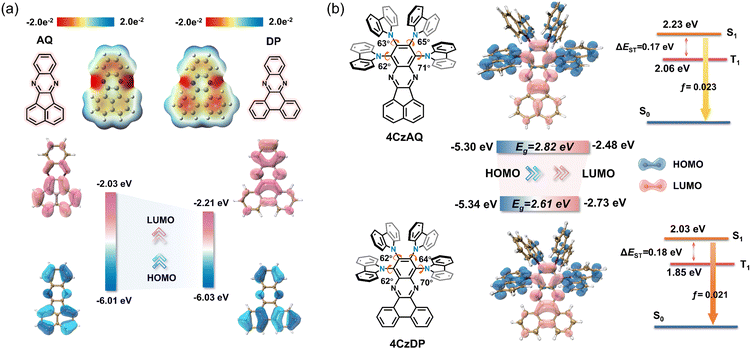 | ||
| Fig. 3 (a) The electrostatic potential surfaces and HOMO/LUMO of AQ and DP. (b) Simulated optimized geometry, HOMO and LUMO distributions and single triplet energy level of 4CzAQ and 4CzDP. | ||
In general, most twisted D-A type TADF emitters exhibit near-zero oscillator strength (f) due to their perpendicular D–A structure, which results in compromised photoluminescence quantum yields (PLQYs), especially pronounced in long-wavelength emitters.22 However, in our case, both 4CzAQ and 4CzDP demonstrate moderate-to-large D–A twisting angles just exceeding 60°. Although the HOMOs and LUMOs of 4CzAQ and 4CzDP mainly localize on the donor units and the π-extended acceptor, respectively, there is still discernible HOMO/LUMO overlap on the terminal fused benzene ring of the acceptors, resulting in the non-vanishing f values of 0.023 and 0.021 for 4CzAQ and 4CzDP. Furthermore, the nearly separated characteristics of the frontier molecular orbitals contribute to minimizing the exchange energy, resulting in reduced ΔESTs of 0.17 and 0.18 eV for 4CzAQ and 4CzDP, respectively.
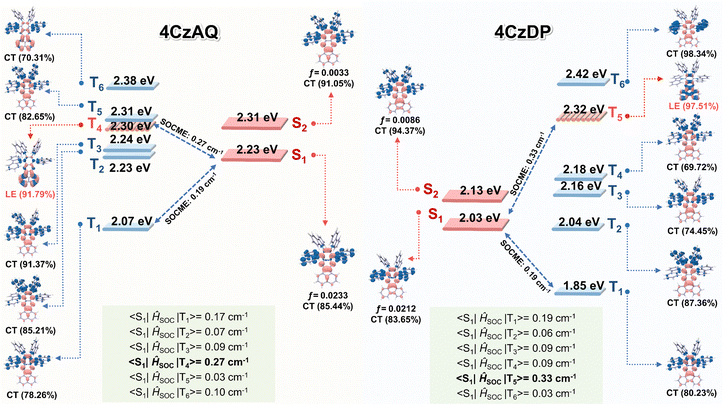
Natural transition orbital (NTO) analyses were performed to gain insights into the excited-state characteristics of 4CzAQ and 4CzDP. As shown in Fig. 4, for the S1 and T1 states of both compounds, the holes and electrons are well distributed within the carbazole segments and the acceptor core, indicating a typical CT character of S1 (1CT) and T1 states (3CT). Other excited states, such as T2, T3, etc., exhibit CT or hybridized local and charge-transfer (HLCT) characteristics featuring slightly different hole distribution patterns and are close in energy. Notably, the T4 state of 4CzAQ and the T5 state of 4CzDP exhibit features of LE character, with electron and hole density primarily localized on the acceptor unit, indicating they are 3LEA states. This is confirmed by their energy levels being equivalent to the T1 state of the corresponding acceptor (Fig. S3, ESI†). Consequently, the 3LEA states are closely aligned in energy with the 1CT and the dense 3CT manifolds. In this scenario, despite the S1 and T1 excitations having comparable CT character, rendering direct SOC inefficient, the closely situated upper triplet excited states provide a viable RISC channel mediated by an SVC mechanism, in which the higher-order SOC for 3LEA–1CT in thermal equilibrium plays a key role.51 As a result, the SOC matrix element values of 〈1CT|ĤSOC|3LEA〉 for 4CzAQ (3LEA = T4) and 4CzDP (3LEA = T5) are as high as 0.27 and 0.33 cm−1, respectively, surpassing those of the corresponding 〈S1|ĤSOC|T1〉 (0.17 and 0.19 cm−1).
Photophysical properties
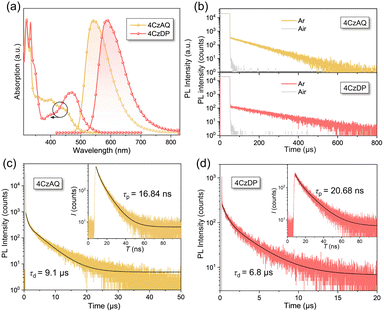
Fig. 5a illustrates the UV-vis absorption and photoluminescence (PL) spectra in toluene (10−5 mol L−1). The prominent absorption band observed below 370 nm is attributed to the π–π* transition of the carbazole and AQ/DP fragments. The bands with lower intensity ranging from 370 to 530 nm stem from the ICT transition. Notably, the ICT band of 4CzDP is significantly stronger and redshifted compared to that of 4CzAQ, consistent with the calculation results indicating DP's stronger acceptor character. Consequently, 4CzDP exhibits longer-wavelength emission than 4CzAQ (595 vs. 545 nm). Solvent-dependent PL spectra are presented in Fig. S4 (ESI†). The PL of both emitters undergoes distinct bathochromic shifts with increasing solvent polarity. In nonpolar n-hexane, the PL profiles of 4CzAQ and 4CzDP display delicate structures, gradually tuned to broader and featureless profiles in more polar solvents, indicative of typical ICT characteristics in excited states.52We observed a distinct delayed component in the emission of both emitters after Ar bubbling, whereas the delayed fluorescence was completely quenched under air-equilibrated conditions (Fig. 5b), indicating the involvement of oxygen-sensitive triplet excitons. The PL decays of the two emitters comprise prompt fluorescence on a nanosecond scale and delayed fluorescence on a microsecond scale (Fig. 5c). The corresponding prompt (τPF) and delayed (τDF) lifetimes were fitted to be 16.84 ns/9.1 μs for 4CzAQ and 20.68 ns/6.8 μs for 4CzDP, respectively (Table 1). Furthermore, the contribution of delayed components increases with increasing temperatures (Fig. S5, ESI†), confirming the thermally accelerated RISC processes inherent in TADF.45 The short τDF values for 4CzAQ and 4CzDP indicate a fast RISC process. However, due to the moderate D–A twisting angles, both in toluene and doped films, 4CzAQ and 4CzDP exhibit relatively large ΔEST values of 0.26/0.23 and 0.25/0.30 eV, respectively, as determined from the fluorescence and phosphorescence spectra (Fig. S6, ESI†). Combined with the theoretical calculations discussed above, it is envisioned that the 3LEA state may play a pivotal role in the RISC process.53
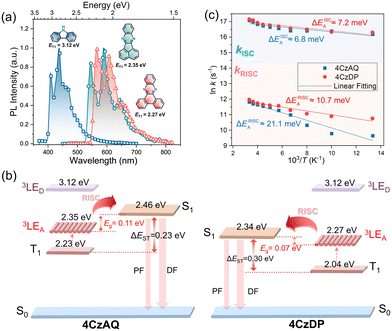
To verify this hypothesis, exciton dynamics were further investigated. First, the T1 energy levels of carbazole, AQ, and DP were obtained from their phosphorescence spectra (Fig. 6a), and an energy level diagram of the new emitters and their components is plotted in Fig. 6b. It can be observed that the energy gap between the 3LEA and the S1 of the corresponding emitter is smaller than that of ΔEST (0.11 and 0.07 eV for 4CzAQ and 4CzDP, respectively), suggesting that 3LEA can serve as a potential mediator for spin flipping. Given carbazole's high T1 energy level, 3LED is unlikely to participate in the exciton RISC process. Next, the temperature dependencies of kISC and kRISC were analyzed. The kRISC rates as a function of temperature are plotted in Fig. 6c. The ISC and RISC activation energies (EAISC and EARISC) were obtained by linearly fitting the plots according to the Arrhenius dependence.54,55 It was observed that while the EAISC values of 4CzAQ (6.8 meV) and 4CzDP (7.2 meV) are nearly identical, the EARISC values are quite different. Specifically, 4CzDP exhibits a smaller EARISC of 10.7 meV, notably smaller than that of 4CzDP (21.1 meV). This disparity can be attributed to the smaller energy gap between 3LEA and S1 of 4CzDP. The lower EARISC of 4CzDP suggests a lower energy barrier for spin flipping. Consequently, 4CzDP (kRISC = 1.57 × 105 s−1) demonstrates a higher kRISC than 4CzAQ (1.37 × 105 s−1). Thus, despite the relatively large ΔEST of the molecule, an efficient RISC process can be achieved by utilizing 3LEA as an accelerating intermediate.56 This underscores the significance of the molecular design featuring a π-extended acceptor in a multi-donor TADF-type emitter.
The solid-state photophysical properties were investigated with doped films in a 4,4'-bis(N-carbazolyl)-1,1'-biphenyl (CBP) host matrix. As illustrated in Fig. S7 (ESI†), with the increasing doping level from 5 to 50 wt%, the PL peak of the 4CzAQ film exhibited a slight redshift from 535 to 554 nm (19 nm), while the shift for 4CzDP was 31 nm (from 581 to 612 nm). Interestingly, compared to reported long-wavelength TADF emitters, 4CzAQ demonstrates an insensitivity to doping concentration. The PLQY of the 4CzAQ doped film (10 wt% in CBP) was estimated to be 92%, whereas that of 4CzDP was only 58% (see Table 2). Notably, 4CzAQ displays more obvious insensitivity than 4CzDP, attributed to its loosely packed mode, which is conducive to achieving high EL performances.
| Emitter | Φ PL (%) | Φ p (%) | Φ d (%) | k F (×107 s−1) | k ISC (×107 s−1) | k RISC (×105 s−1) | k nr (×107 s−1) |
|---|---|---|---|---|---|---|---|
| 4CzAQ | 92 | 63 | 29 | 4.39 | 2.20 | 1.37 | 0.38 |
| 4CzDP | 58 | 34 | 24 | 2.62 | 3.19 | 1.57 | 1.90 |
Electroluminescence properties
To evaluate the EL performances of the proposed TADF compounds, a series of OLEDs were fabricated. The device structure comprised indium tin oxide (ITO)/2,3,6,7,10,11-hexacyano-1,4,5,8,9,12-hexaazatriphenylene (HAT-CN, 10 nm)/1,1-bis[(di-4-alkylamino)phenyl]cyclohexane (TAPC, 35 nm)/4,4′,4′′tri(N-carbazolyl)-triphenylamine (TCTA, 10 nm)/1,3-bis(9H-carbazol-9-yl)benzene (mCP, 10 nm)/CBP: x wt% 4CzAQ or 4CzDP (20 nm)/1,3,5-tri[(3-pyridyl)-phen-3-yl]benzene (TmPyPB, 45 nm)/LiF (1 nm)/Al (x = 5, 10, 15, and 20). In this setup, ITO and Al were respectively utilized as the anode and cathode. HAT-CN acted as the hole-injection layer, TAPC as the hole-transporting layer, TCTA as the exciton-blocking layer, mCP as both the electron and exciton-blocking layer, and CBP as the hole-blocking layer. Furthermore, TmPyPB was employed as the electron-transporting layer, while LiF functioned as the electron injection layer. The efficiency calibration factors, obtained from the angular distribution of the EL intensity of the OLEDs (Fig. S8, ESI†), were 0.983 and 0.913 for 4CzAQ and 4CzDP, respectively.As shown in Fig. S9 and Table S3 (ESI†), when the doping level increased from 5 to 20 wt%, the EL peak of 4CzAQ experienced a redshift from 550 to 564 nm (14 nm), while that of 4CzDP underwent a larger redshift from 594 to 616 nm (22 nm), consistent with the PL results (Fig. S7, ESI†). The EL performances of 4CzAQ OLEDs improved with increased x, reaching a maximum at x = 10. The 10 wt% doped 4CzAQ OLED achieved a superior EQEmax of 26.8%, more than twice that of the 4CzDP counterpart (11.7%), as shown in Fig. 7 and Table 3. Even under higher doping levels of 20 wt%, its EQEmax remained at a high level of over 17%. Conversely, although 4CzDP exhibited a higher kRISC than 4CzAQ, it suffered from stronger interchromophore interactions, resulting in lower luminescence quantum efficiency, thereby compromising EL performances. The device performances of representative multi-carbazole TADF compounds are concisely summarized in Fig. 7d and Table S4 (ESI†). Remarkably, our new TADF materials demonstrate state-of-the-art EQE values,57–59 particularly in the long-wavelength region with EL peaks exceeding 550 nm. These results affirm the efficacy of our molecular design tactics in constructing highly efficient long-wavelength TADF emitters.
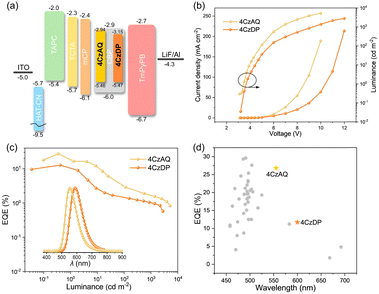 | ||
| Fig. 7 (a) Device structures and energy level diagrams (unit: eV); (b) current density and luminance versus driving voltage characteristics (inset: Electroluminescence spectra at 100 cd m−2) for 4CzAQ and 4CzDP; (c) external quantum efficiency versus luminance characteristics; (d) EQEmax comparison in terms of EL peaks with reported OLEDs based on multi-carbazole derivatives (references for the plotted data are given in Table S4, ESI†) | ||
| Dopant | V on (V) | λ EL (nm) | CIEc (x, y) | CEmaxd (cd A−1) | PEmaxe (lm W−1) | EQEf (%) |
|---|---|---|---|---|---|---|
| a Turn-on voltage at 1 cd m−2. b EL peak wavelength at 100 cd m−2. c CIE 1931 coordinates measured at 100 cd m−2. d Maximum current efficiency. e Maximum power efficiency. f External quantum efficiency values at maximum. | ||||||
| 4CzAQ | 3.7 | 555 | (0.43, 0.55) | 60.5 | 57.5 | 26.8 |
| 4CzDP | 3.8 | 600 | (0.55, 0.44) | 25.4 | 22.7 | 11.7 |
Conclusion
In summary, this work presents two new TADF molecules, 4CzAQ and 4CzDP, developed through a strategic multi-donor coupled π-extended acceptor design. These materials are efficiently synthesized with decent yields and exhibit steric-demanding configurations that alleviate emission quenching, facilitating good luminescence efficiency. Notably, 4CzAQ exhibits a higher PLQY of 92% due to its negligible interchromophore interaction. We reveal that the integration of the π-extended AQ/DP and carbazole units not only provides large molecular conjugation and intense ICT for long-wavelength emission with peaks of over 550 nm, but also offers a dense excited-state alignment with a 3LEA state as a crucial accelerating intermediate that facilitates RISC via the SVC mechanism. We also highlight the importance of the energy gap between the 3LEA and 1CT states in tuning the rate of RISC; the smaller the gap, the lower the activation energy barrier for RISC. Because 4CzAQ exhibits a superior PLQY and a relatively high kRISC, its 10 wt% doped OLED showed a high EQEmax of 26.8% with an EL peak at 555 nm. The combined steric and electronic effects of the present molecular design provide an efficient approach for tackling challenges in long-wavelength TADF EL devices.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We sincerely acknowledge the financial support provided by the National Natural Science Foundation of China (Grant no. U2001222, U22A20399, U23A20594).Notes and references
- F. Fang, L. Zhu, M. Li, Y. Song, M. Sun, D. Zhao and J. Zhang, Adv. Sci., 2021, 8, 2102970 CrossRef CAS PubMed.
- X. Li, G. Baryshnikov, C. Deng, X. Bao, B. Wu, Y. Zhou, H. Ågren and L. Zhu, Nat. Commun., 2019, 10, 731 CrossRef CAS PubMed.
- M. A. Bryden and E. Zysman-Colman, Chem. Soc. Rev., 2021, 50, 7587–7680 RSC.
- T. Zhang, Z. Zhou, X. Liu, K. Wang, Y. Fan, C. Zhang, J. Yao, Y. Yan and Y. S. Zhao, J. Am. Chem. Soc., 2021, 143, 20249–20255 CrossRef CAS PubMed.
- G. Chen, J. Wang, W.-C. Chen, Y. Gong, N. Zhuang, H. Liang, L. Xing, Y. Liu, S. Ji, H.-L. Zhang, Z. Zhao, Y. Huo and B. Z. Tang, Adv. Funct. Mater., 2023, 33, 2211893 CrossRef CAS.
- J.-H. Tan, J.-M. Jin, W.-C. Chen, C. Cao, R. Wang, Z.-L. Zhu, Y. Huo and C.-S. Lee, ACS Appl. Mater. Interfaces, 2022, 14, 53120–53128 CrossRef CAS PubMed.
- R. Mac Ciarnáin, H. W. Mo, K. Nagayoshi, H. Fujimoto, K. Harada, R. Gehlhaar, T. H. Ke, P. Heremans and C. Adachi, Adv. Mater., 2022, 34, 2201409 CrossRef PubMed.
- N. B. Kotadiya, P. W. M. Blom and G.-J. A. H. Wetzelaer, Nat. Photonics, 2019, 13, 765–769 CrossRef CAS.
- S. O. Jeon, K. H. Lee, J. S. Kim, S.-G. Ihn, Y. S. Chung, J. W. Kim, H. Lee, S. Kim, H. Choi and J. Y. Lee, Nat. Photonics, 2021, 15, 208–215 CrossRef CAS.
- Q. Liu, W.-C. Chen, R. Zhang, H. Wei, B. Liu, J.-M. Jin, Y. Liu, Z. Ye, J.-X. Chen, S. Ji, H.-L. Zhang and Y. Huo, Dyes Pigm., 2023, 212, 111125 CrossRef CAS.
- B. Liu, W.-C. Chen, R. Zhang, Q. Liu, H. Wei, W.-L. Wu, L. Xing, R. Wang, Y. Liu, S. Ji, H.-L. Zhang and Y. Huo, Dyes Pigm., 2023, 216, 111314 CrossRef CAS.
- R. Englman and J. Jortner, Mol. Phys., 1970, 18, 145–164 CrossRef CAS.
- Y. Xiao, H. Wang, Z. Xie, M. Shen, R. Huang, Y. Miao, G. Liu, T. Yu and W. Huang, Chem. Sci., 2022, 13, 8906–8923 RSC.
- H. U. Kim, T. Kim, C. Kim, M. Kim and T. Park, Adv. Funct. Mater., 2023, 33, 2208082 CrossRef CAS.
- H.-Y. Zhang, H.-Y. Yang, M. Zhang, H. Lin, S.-L. Tao, C.-J. Zheng and X.-H. Zhang, Mater. Horiz., 2022, 9, 2425–2432 RSC.
- H.-Y. Yang, H. Zhang, M. Zhang, X. Fan, H. Lin, S.-L. Tao, C.-J. Zheng and X.-H. Zhang, Chem. Eng. J., 2022, 448, 137717 CrossRef CAS.
- Y. Yuan, Y. Hu, Y.-X. Zhang, J.-D. Lin, Y.-K. Wang, Z.-Q. Jiang, L.-S. Liao and S.-T. Lee, Adv. Funct. Mater., 2017, 27, 1700986 CrossRef.
- S. Wang, X. Yan, Z. Cheng, H. Zhang, Y. Liu and Y. Wang, Angew. Chem., Int. Ed., 2015, 54, 13068–13072 CrossRef CAS PubMed.
- H. Wang, J.-X. Chen, X.-C. Fan, Y.-C. Cheng, L. Zhou, X. Zhang, J. Yu, K. Wang and X.-H. Zhang, ACS Appl. Mater. Interfaces, 2023, 15, 1685–1692 CrossRef CAS PubMed.
- U. Balijapalli, Y.-T. Lee, B. S. B. Karunathilaka, G. Tumen-Ulzii, M. Auffray, Y. Tsuchiya, H. Nakanotani and C. Adachi, Angew. Chem., Int. Ed., 2021, 60, 19364–19373 CrossRef CAS PubMed.
- J.-L. He, F.-C. Kong, B. Sun, X.-J. Wang, Q.-S. Tian, J. Fan and L.-S. Liao, Chem. Eng. J., 2021, 424, 130470 CrossRef CAS.
- J. Fan, J. Miao, N. Li, Y. Zeng, C. Ye, X. Yin and C. Yang, J. Mater. Chem. C, 2022, 10, 10255–10261 RSC.
- J. Pu, X. Nie, D. Li, X. Peng, W. Qiu, W. Li, D. Li, G. Sun, C. Shen, S. Ji, D. Cao and S.-J. Su, Chem. Eng. J., 2023, 471, 144508 CrossRef CAS.
- L. Zhou, H. Wang, Y.-Z. Shi, X.-C. Fan, J.-X. Chen, K. Wang, J. Yu and X.-H. Zhang, Chem. Eng. J., 2022, 440, 135775 CrossRef CAS.
- J.-X. Chen, H. Wang, Y.-F. Xiao, K. Wang, M.-H. Zheng, W.-C. Chen, L. Zhou, D. Hu, Y. Huo, C.-S. Lee and X.-H. Zhang, Small, 2022, 18, 2201548 CrossRef CAS PubMed.
- M. A. El-Sayed, J. Chem. Phys., 1963, 38, 2834–2838 CrossRef CAS.
- Q. Zhang, H. Kuwabara, W. J. Potscavage, S. Huang, Y. Hatae, T. Shibata and C. Adachi, J. Am. Chem. Soc., 2014, 136, 18070–18081 CrossRef CAS PubMed.
- W. Zeng, T. Zhou, W. Ning, C. Zhong, J. He, S. Gong, G. Xie and C. Yang, Adv. Mater., 2019, 31, 1901404 CrossRef PubMed.
- V. T. N. Mai, V. Ahmad, M. Mamada, T. Fukunaga, A. Shukla, J. Sobus, G. Krishnan, E. G. Moore, G. G. Andersson, C. Adachi, E. B. Namdas and S.-C. Lo, Nat. Commun., 2020, 11, 5623 CrossRef CAS PubMed.
- R. Chen, Y. Xu, Z. Zhou, H. Wang, Y. Jia, Q. Chang, P. Jin, B. Yin, C. Li and C. Zhang, Phys. Rev. Appl., 2024, 21, 014039 CrossRef CAS.
- J.-X. Chen, Y.-F. Xiao, K. Wang, D. Sun, X.-C. Fan, X. Zhang, M. Zhang, Y.-Z. Shi, J. Yu, F.-X. Geng, C.-S. Lee and X.-H. Zhang, Angew. Chem., Int. Ed., 2021, 60, 2478–2484 CrossRef CAS PubMed.
- S. Kothavale, W. J. Chung and J. Y. Lee, J. Mater. Chem. C, 2022, 10, 6043–6049 RSC.
- J. U. Kim, I. S. Park, C.-Y. Chan, M. Tanaka, Y. Tsuchiya, H. Nakanotani and C. Adachi, Nat. Commun., 2020, 11, 1765 CrossRef CAS PubMed.
- H. Lee, R. Braveenth, S. Muruganantham, C. Y. Jeon, H. S. Lee and J. H. Kwon, Nat. Commun., 2023, 14, 419 CrossRef CAS PubMed.
- H. S. Kim, J. Y. Lee, S. Shin, W. Jeong, S. H. Lee, S. Kim, J. Lee, M. C. Suh and S. Yoo, Adv. Funct. Mater., 2021, 31, 2104646 CrossRef CAS.
- L.-S. Cui, A. J. Gillett, S.-F. Zhang, H. Ye, Y. Liu, X.-K. Chen, Z.-S. Lin, E. W. Evans, W. K. Myers, T. K. Ronson, H. Nakanotani, S. Reineke, J.-L. Bredas, C. Adachi and R. H. Friend, Nat. Photonics, 2020, 14, 636–642 CrossRef CAS.
- P. K. Samanta, D. Kim, V. Coropceanu and J.-L. Brédas, J. Am. Chem. Soc., 2017, 139, 4042–4051 CrossRef CAS PubMed.
- M. K. Etherington, J. Gibson, H. F. Higginbotham, T. J. Penfold and A. P. Monkman, Nat. Commun., 2016, 7, 13680 CrossRef CAS PubMed.
- D. Zhang, X. Song, A. J. Gillett, B. H. Drummond, S. T. E. Jones, G. Li, H. He, M. Cai, D. Credgington and L. Duan, Adv. Mater., 2020, 32, 1908355 CrossRef CAS PubMed.
- H. Noda, H. Nakanotani and C. Adachi, Sci. Adv., 2018, 4, eaao6910 CrossRef PubMed.
- B. Madushani, M. Mamada, K. Goushi, T. B. Nguyen, H. Nakanotani, H. Kaji and C. Adachi, Sci. Rep., 2023, 13, 7644 CrossRef CAS PubMed.
- J.-R. Cha, C. W. Lee and M.-S. Gong, Dyes Pigm., 2017, 140, 399–406 CrossRef CAS.
- T. Matulaitis, P. Imbrasas, N. A. Kukhta, P. Baronas, T. Bučiūnas, D. Banevičius, K. Kazlauskas, J. V. Gražulevičius and S. Juršėnas, J. Phys. Chem. C, 2017, 121, 23618–23625 CrossRef CAS.
- C. S. Oh, H. L. Lee, S. H. Han and J. Y. Lee, Chem. – Eur. J., 2019, 25, 642–648 CrossRef CAS PubMed.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- X. Zhou, Y. Xiang, S. Gong, Z. Chen, F. Ni, G. Xie and C. Yang, Chem. Commun., 2019, 55, 14190–14193 RSC.
- X. Zhang, H. Wang, J.-X. Chen, L. Zhou, X.-Y. Hao, J. Yu, K. Wang and X.-H. Zhang, Mater. Chem. Front., 2024, 8, 1120–1127 RSC.
- W.-C. Chen, M.-H. Zheng, Y.-L. Wu, R.-J. Wang, J.-M. Jin, S.-W. Chen, B. Liu, J.-X. Chen, Y. Huo and S. Ji, Chem. Eng. J., 2024, 480, 148314 CrossRef CAS.
- M. Mamada, H. Katagiri, C.-Y. Chan, Y.-T. Lee, K. Goushi, H. Nakanotani, T. Hatakeyama and C. Adachi, Adv. Funct. Mater., 2022, 32, 2204352 CrossRef CAS.
- W. Chen, Y. Yuan, G. Wu, H. Wei, L. Tang, Q. Tong, F. Wong and C. Lee, Adv. Opt. Mater., 2014, 2, 626–631 CrossRef CAS.
- H. L. Lee, J. Kang, J. Lim, S. C. Kim, S. O. Jeon and J. Y. Lee, Nat. Commun., 2023, 14, 4818 CrossRef CAS PubMed.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899–4032 CrossRef PubMed.
- T. Hosokai, H. Matsuzaki, H. Nakanotani, K. Tokumaru, T. Tsutsui, A. Furube, K. Nasu, H. Nomura, M. Yahiro and C. Adachi, Sci. Adv., 2017, 3, e1603282 CrossRef PubMed.
- L. Skhirtladze, K. Lietonas, A. Bucinskas, D. Volyniuk, M. Mahmoudi, O. Mukbaniani, K. L. Woon, A. Ariffin and J. V. Grazulevicius, J. Mater. Chem. C, 2022, 10, 4929–4940 RSC.
- T. Serevičius, R. Skaisgiris, I. Fiodorova, G. Kreiza, D. Banevičius, K. Kazlauskas, S. Tumkevičius and S. Juršėnas, J. Mater. Chem. C, 2021, 9, 836–841 RSC.
- H. Noda, X.-K. Chen, H. Nakanotani, T. Hosokai, M. Miyajima, N. Notsuka, Y. Kashima, J.-L. Brédas and C. Adachi, Nat. Mater., 2019, 18, 1084–1090 CrossRef CAS PubMed.
- F.-M. Xie, H.-Z. Li, K. Zhang, H.-Y. Wang, Y.-Q. Li and J.-X. Tang, ACS Appl. Mater. Interfaces, 2023, 15, 39669–39676 CrossRef CAS PubMed.
- J. H. Yun, K. H. Lee, H. Jeong and J. Y. Lee, J. Mater. Chem. C, 2022, 10, 10950–10956 RSC.
- U. Balijapalli, M. Tanaka, M. Auffray, C.-Y. Chan, Y.-T. Lee, Y. Tsuchiya, H. Nakanotani and C. Adachi, ACS Appl. Mater. Interfaces, 2020, 12, 9498–9506 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, characterization, photophysical data, and other additional information. CCDC 2224330 and 2224332. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4tc00988f |
| This journal is © The Royal Society of Chemistry 2024 |