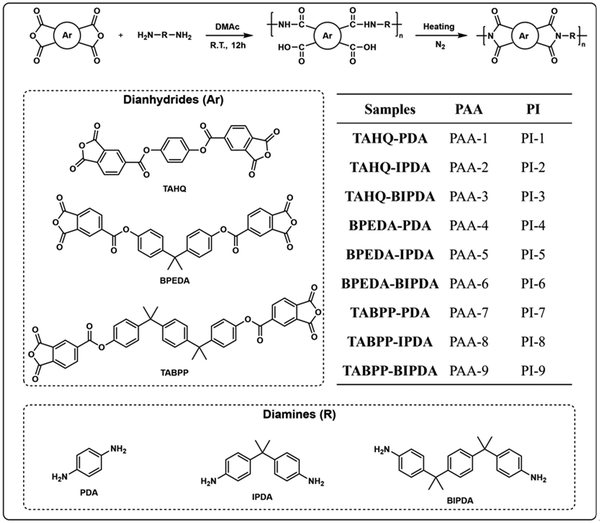
A comprehensive study on the effect of molecular chain flexibility on the low-temperature curing ability of polyimides†
Shan
Huang‡
ab,
Yao
Zhang‡
a,
Xingwang
Lai
a,
Xialei
Lv
*a,
Jinhui
Li
 *a,
Siyao
Qiu
a,
Guoping
Zhang
*a,
Siyao
Qiu
a,
Guoping
Zhang
 *a and
Rong
Sun
*a and
Rong
Sun
 a
a
aShenzhen International Innovation Institutes of Advanced Electronic Materials, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China. E-mail: xl.lv@siat.ac.cn; jh.li@siat.ac.cn; gp.zhang@siat.ac.cn
bDepartment of Nano Science and Technology Institute, University of Science and Technology of China, Suzhou, 215123, China
First published on 27th November 2023
Abstract
As stringent demands for PI materials with low-temperature curable properties have increased in the high-frequency communication era, the introduction of flexible structures has gained prominence for enhancing molecular chain mobility. However, systematic studies on the effect of flexible structures on low-temperature curing ability remain limited. In this work, we designed a new dianhydride monomer with isopropylidene and ester groups named TABPP. Low-temperature curable polyimides with different molecular chain flexibility were prepared by choosing different diamines and anhydrides. Surprisingly, the degree of imidization was not straightforwardly aligned with molecular chain flexibility. An exemplar instance was PI-9-200, exhibiting the greatest flexibility among the samples, yet possessing the lowest degree of imidization (ID) of 68.59%. Based on the analysis of experimental results and front-line orbital energy levels, it could be seen that the mismatch between the ID and the flexibility may originate from the influence of electronic effects of the monomers. Notably, when enough flexible structures were introduced into the polyimide backbone, the effect of increasing the free volume appeared to outweigh the influence of incomplete imidization, thereby favoring the preparation of low-temperature curable PI films with outstanding dielectric properties. In particular, the dielectric constant of the prepared low-temperature curable PI films was as low as 2.50, which is the best performance among the low-temperature curable PI films. This work throws new light on the correlation between flexibility and low-temperature curing ability and offers fresh perspectives on the preparation of low-temperature curable PIs with excellent dielectric properties.
Introduction
For decades, Moore's law has provided crucial guidance to the semiconductor industry. However, in recent years, Moore's law has encountered limitations, necessitating new approaches to further develop advance integrated circuits.1,2 Advanced package was beginning to emerge and fan-out wafer-level packaging (FOWLP) stood out among many advanced packaging formats due to its more I/O counts and lower cost.3,4 Polyimide (PI) has emerged as a crucial material in the realm of FOWLP, specifically as the redistribution layer (RDL), owing to its excellent comprehensive performances such as stable thermal properties, outstanding mechanical properties and shielding effects on α particles.5–7 Nonetheless, traditional PI often demands curing at high temperatures surpassing 350 °C, which poses challenges such as wafer warpage, thereby limiting its applications within semiconductor manufacturing processes.8–12 Therefore, with the development of advanced packaging and increasing wafer size, low-temperature curable polyimide has attracted widespread attention from researchers and industries, necessitating the preparation of low-temperature curable PIs with excellent comprehensive performance.In order to effectively reduce the curing temperature, designing special polyimide structures with autocatalytic effects, including nitrogen heterocycles and flexible structures, is a popular approach to obtain low-temperature curable polyimides. Nitrogen heterocycles, functioning as base catalysts, could increase the nucleophilicity of diamines to attack carbonyl carbon, thereby facilitating the acylation reaction and ultimately reducing the curing temperature.13–17 Artem'eva et al. illuminated the influence of pyrimidine structure on thermal imidization, revealing that the presence of a pyrimidine ring diminished the energetic barrier of imidization and expedited the resynthesis of partially damaged polyamide acid induced by thermocyclization.15 Concurrently, integration of flexible structures within diamines or dianhydrides would disrupt the conjugated system of molecular backbones and improve molecular chain mobility. Consequently, softer monomers or molecular chains could contribute to the reduction of curing temperatures.18–22 For example, Ghosh et al. introduced flexible siloxane structures to prepare PIs with flexible segments at 180 °C, achieved by reacting two diamines with amino-propyl terminated polydimethylsiloxane.18 Meanwhile, Leu et al. ingeniously introduced flexible groups and large side group naphthalene into the monomer to increase chain activity and break the conjugated system of molecular backbone, effectively lowering the curing temperature to 180 °C.20 However, a reasonable constitutive relationship between the flexibility of the structure and the low-temperature curing ability is lacking. Additionally, studies on novel flexible dianhydride monomers remain scarce, constraining the design of low-temperature curable PIs. Furthermore, the advent of fifth-generation (5G) mobile communication technology has escalated the operation spectrum bandwidth into the high-frequency GHz range.23 To avoid the increase of transmission loss at high frequencies, low-temperature curable PIs applied to RDL must preserve favorable dielectric properties.24,25 In comparison to polar nitrogen heterocyclic structures, which can detrimentally impact dielectric properties, flexible structures exhibit a capacity for increased free volume, conducive to enhanced dielectric properties. Therefore, it is necessary to systematically investigate the effect of molecular chain flexibility on low-temperature curable PIs.
In this work, we synthesized a novel dianhydride containing an ester-based structure by the acid chloride reaction, named (1,4-phenylenebis(propane-2,2-diyl))bis(4,1-phenylene) bis(1,3-dioxo-1,3-dihydroisobenzofuran-5-carboxylate) (TABPP). We carefully selected three commercial diamines and two commercial dianhydrides for the polymerization process. To delve deeper into the relationship between the PI structure and low-temperature curing ability, a series of PI films with similar structures but different molecular chain flexibility were prepared at curing temperatures of 200 °C and 350 °C. By systematically comparing the degree of imidization, comprehensive properties of the low-temperature curable PI films and electronic effects of monomers, the complex relationship between low-temperature curing ability and molecular chain flexibility was revealed. In addition, the dielectric properties of low-temperature curable PIs were significantly improved. Notably, PI-8-200 displayed an exceptional dielectric constant of 2.50, outperforming previously reported low-temperature curable PIs. In essence, this work provides a novel strategy for achieving low-temperature curable PIs with excellent dielectric properties.
Experimental section
Synthesis of PAAs
Synthesis of PIs
5 mL of the obtained PAA solution was poured onto clean and transparent glass plates of size 10 cm × 10 cm, and the glass plates were spin-coated evenly for 30 s at 800 rpm by using a homogenizer. Then the spin-coated glass plates with PAAs were soft-baked at 80 °C for 5 min. After soft-baking, the spin-coated glass plates covered with PAAs were heated and cured in the procedure of a heating rate of 5 °C min−1 under a nitrogen environment. The temperature was maintained at 100 °C for 1 h, 200 °C for 1 h, 300 °C for 1 h and 350 °C for 1 h, and the prepared films were named PI-1-350 to PI-9-350. Besides, the heating program of the low-temperature curable PIs was maintained at 100 °C for 1 h and 200 °C for 3 h at a heating rate of 5 °C min−1, and the prepared films were named PI-1-200 to PI-9-200. The cured glass plates covered with PI films were placed in deionized water, and the PI films could be removed from the glass plates after 2 h. Subsequently, the peeled PI films were baked at 120 °C for 6 h to obtain dry and flat PI films.Results and discussion
Infrared analysis and theoretical calculations
The FTIR spectra of the prepared PI films are presented in Fig. 1 and Fig. S3 (ESI†), showcasing the characteristic bands of the imide ring (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric stretching at around 1780 cm−1, C
O asymmetric stretching at around 1780 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric stretching at approximately 1720 cm−1 and stretching vibration of C–N at about 1380 cm−1). Meanwhile, the characteristic absorption bands corresponding to the methyl group could be clearly found at about 2900 cm−1. These demonstrated the successful preparation of PI films cured at 200 °C. Additionally, the FT-IR spectra of the samples exhibited inconspicuous characteristic peaks of polyamic acid (C
O symmetric stretching at approximately 1720 cm−1 and stretching vibration of C–N at about 1380 cm−1). Meanwhile, the characteristic absorption bands corresponding to the methyl group could be clearly found at about 2900 cm−1. These demonstrated the successful preparation of PI films cured at 200 °C. Additionally, the FT-IR spectra of the samples exhibited inconspicuous characteristic peaks of polyamic acid (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of CONH at around 1640 cm−1 and C–NH deformation at about 1540 cm−1), which signified a comparatively low presence of residual amic acid segments. In order to visually elucidate the disparities in the flexibility of molecular chains, the end-to-end distance (Lend-to-end) and total chain length (L0) of the PI segment simulated were calculated utilizing the reported method.26 The computed values for Lend-to-end, L0 and Lend-to-end/L0 are shown in Table S1 (ESI†), where Lend-to-end/L0 served as an indicator of flexibility in the repeating unit of PI. A diminished Lend-to-end/L0 value showed heightened tendencies toward bending and molecular chain rotation, thereby signifying an augmented degree of flexibility. It is clear that the incorporation of the isopropylidene group into the monomer could foster suppleness of PI molecular chains. PI-9 was proved to have the most flexible structure among all the samples, stood in stark contrast to PI-1, with a comparatively inflexible configuration. Moreover, to evaluate the low-temperature curing ability, the degree of imidization (ID) was calculated by determining the integral area of the characteristic bands of the imide ring (near 1380 cm−1) and the benzene ring (near 1500 cm−1) as an internal standard, according to previous reports.27–30 Specifically, PI cured at 350 °C was considered to undergo relatively complete imidization. “ID” served as a metric to gauge the degree of thermal imidization of the low-temperature curable PI samples in comparison to their counterparts cured at 350 °C. This work revealed that the ID of PI films cured at 200 °C did not correspond proportionally to the flexibility of the molecular chains. Specifically, PI-2-200 displayed a lower ID value of 79.97% compared to PI-1-200, despite having a more flexible molecular structure. Additionally, even though PI-9-200 possessed the most flexible structure among all the samples, its ID was merely 68.6%. These unexpected results suggested that low temperature curing ability and flexibility are not positively correlated. Therefore, it became imperative to consider other factors that might influence the thermal imidization process in polyimides.
O stretching of CONH at around 1640 cm−1 and C–NH deformation at about 1540 cm−1), which signified a comparatively low presence of residual amic acid segments. In order to visually elucidate the disparities in the flexibility of molecular chains, the end-to-end distance (Lend-to-end) and total chain length (L0) of the PI segment simulated were calculated utilizing the reported method.26 The computed values for Lend-to-end, L0 and Lend-to-end/L0 are shown in Table S1 (ESI†), where Lend-to-end/L0 served as an indicator of flexibility in the repeating unit of PI. A diminished Lend-to-end/L0 value showed heightened tendencies toward bending and molecular chain rotation, thereby signifying an augmented degree of flexibility. It is clear that the incorporation of the isopropylidene group into the monomer could foster suppleness of PI molecular chains. PI-9 was proved to have the most flexible structure among all the samples, stood in stark contrast to PI-1, with a comparatively inflexible configuration. Moreover, to evaluate the low-temperature curing ability, the degree of imidization (ID) was calculated by determining the integral area of the characteristic bands of the imide ring (near 1380 cm−1) and the benzene ring (near 1500 cm−1) as an internal standard, according to previous reports.27–30 Specifically, PI cured at 350 °C was considered to undergo relatively complete imidization. “ID” served as a metric to gauge the degree of thermal imidization of the low-temperature curable PI samples in comparison to their counterparts cured at 350 °C. This work revealed that the ID of PI films cured at 200 °C did not correspond proportionally to the flexibility of the molecular chains. Specifically, PI-2-200 displayed a lower ID value of 79.97% compared to PI-1-200, despite having a more flexible molecular structure. Additionally, even though PI-9-200 possessed the most flexible structure among all the samples, its ID was merely 68.6%. These unexpected results suggested that low temperature curing ability and flexibility are not positively correlated. Therefore, it became imperative to consider other factors that might influence the thermal imidization process in polyimides.
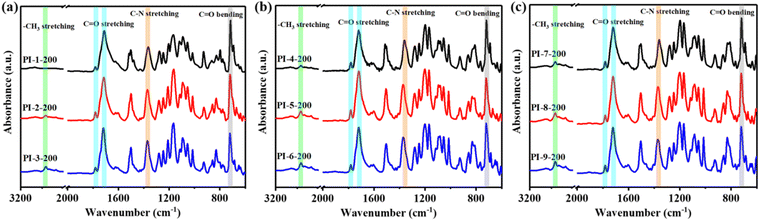 | ||
| Fig. 1 FT-IR spectra of (a) PI films with anhydride TAHQ cured at 200 °C, (b) PI films with anhydride BPEDA cured at 200 °C and (c) PI films with anhydride TABPP cured at 200 °C. | ||
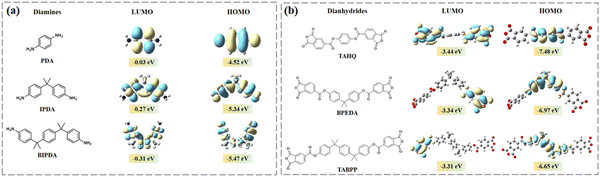
The formation of the imide ring is intricately linked to the nucleophilic attack of the amino groups in the diamine on the dianhydride.15,31,32 In order to further analyze the relationship between the degree of imidization and monomer structure, the front-line orbital energy levels of the used diamines and dianhydrides were calculated, as illustrated in Fig. 2 and Table S2 (ESI†). Evidently, the introduction of flexible structures significantly influenced the electronic effects within the monomers. The HOMO (highest occupied molecular orbital) of diamine PDA was observed to be shallower at −4.52 eV, indicating a stronger electron-donating ability compared to IPDA with a HOMO level of −5.34 eV, and BIPDA at −5.47 eV. Similarly, dianhydride TAHQ exhibited a deeper LUMO (lowest unoccupied molecular orbital) at −3.44 eV, signifying a stronger electron-withdrawing ability than BPEDA (−3.34 eV) and TABPP (−3.31 eV). The interplay of both flexibility and electronic effects may account for the observed trends in ID. The excessive introduction of flexible structures could adversely affect the reactivity of monomers, consequently diminishing the low-temperature curing effect. For instance, the cyclization reaction of PI-2-200 was negatively affected due to the poorer electron-donating ability of IPDA, resulting in a lower ID compared to that of PI-1-200. Conversely, PI-3-200 showed a higher ID than PI-1-200 and PI-2-200, which suggested that the beneficial effect of increased mobility of molecular chains outweighed the detrimental effect of decreased monomer reactivity. Furthermore, due to the reduction in nucleophilicity and electrophilicity of monomers, the nucleophilic attack became more difficult in PI-9-200, leading to the lowest ID among the samples. The molecular weight (Mn) of the resulting PAA correlated well with the theoretical calculations above, as presented in Table S3 (ESI†). PAA-1 had the highest molecular weight (Mn = 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 777 Da), while PAA-9 displayed the lowest molecular weight (Mn = 38
777 Da), while PAA-9 displayed the lowest molecular weight (Mn = 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 159 Da) among the samples. These findings underscored the importance of considering flexibility and electronic effects of monomers simultaneously in the structure design of low-temperature curable PIs.
159 Da) among the samples. These findings underscored the importance of considering flexibility and electronic effects of monomers simultaneously in the structure design of low-temperature curable PIs.
The XRD patterns of PI films are illustrated in Fig. S4 (ESI†), serving as a means to investigate the packing states of molecular chains. The resulting PI films exhibited an amorphous nature, as evidenced by the broad diffraction peak observed in the XRD patterns. The d-spacing values were calculated using Bragg's law and are shown in Table 1 and Table S4 (ESI†). In general, a conspicuous correlation was established between the degree of imidization and the corresponding d-spacing values. Specifically, a higher content of imide rings within the PI structure corresponded to a reduction in the observed d-spacing. Therefore, low-temperature curable PI films showed larger d-spacing values than the corresponding films cured at 350 °C. Furthermore, the presence of flexible structures also exerted a discernible influence on the d-spacing values. For instance, despite PI-6-200 showcasing a higher ID of 90.38% compared to PI-5-200 (ID = 75.38%), its d-spacing surpassed that of PI-5-200, which could be attributed to the enhanced molecular chain flexibility of PI-6-200.
| Sample name | ID (%) | d-Spacing (Å) | CTE (ppm K−1) | Dichroic ratio | Dianhydride | Diamine |
|---|---|---|---|---|---|---|
| PI-1-200 | 85.66 | 4.23 | 7.48 | 2.131 | TAHQ | PDA |
| PI-2-200 | 79.97 | 5.53 | 50.15 | 2.091 | TAHQ | IPDA |
| PI-3-200 | 90.95 | 5.31 | 55.79 | 2.058 | TAHQ | BIPDA |
| PI-4-200 | 89.01 | 5.52 | 54.61 | 2.060 | BPEDA | PDA |
| PI-5-200 | 75.38 | 5.63 | 70.04 | 2.036 | BPEDA | IPDA |
| PI-6-200 | 90.38 | 5.49 | 76.57 | 2.024 | BPEDA | BIPDA |
| PI-7-200 | 88.11 | 5.02 | 72.04 | 2.031 | TABPP | PDA |
| PI-8-200 | 88.81 | 5.39 | 72.46 | 2.029 | TABPP | IPDA |
| PI-9-200 | 68.59 | 5.65 | 69.42 | 2.037 | TABPP | BIPDA |
Mechanical and thermal properties
The mechanical properties, including tensile strength (σmax), elongation at break (εb) and Young's modulus (E), of the PI films were thoroughly investigated via DMA, as illustrated in Fig. 3, Table 2 and Fig. S5 (ESI†). Generally, the incorporation of a flexible structure proved beneficial for enhancing the elongation at break while negatively impacting the modulus of PI. Consequently, PI-1-350 exhibited the highest modulus (E = 8.66 GPa) but the lowest elongation at break (εb = 5.7%) among them due to its extremely rigid structure. However, it is important to note that the relationship between flexibility and mechanical properties did not universally apply to all the samples. In particular, PI-9-350 with a more flexible structure surprisingly displayed poorer toughness (εb = 12.5%) due to poor film-forming ability resulting from the reduced reactivity of monomers. Conversely, PI-3-350 showed excellent mechanical properties (εb = 53.8%) due to its balanced flexibility and monomer reactivity. These findings suggest that an appropriate introduction of flexibility structures was more conducive to the preparation of PI films with favorable mechanical properties. In addition, it is worth highlighting that the tensile strength of low-temperature curable PI films outperformed that of their corresponding films cured at 350 °C, where PI-1-200 exhibited superior tensile strength exceeding 235 MPa. This discrepancy could be attributed to the fact that high curing temperatures may disrupt the regular arrangement of molecular chains, thereby weakening the intermolecular forces and subsequently compromising the tensile strength of the films.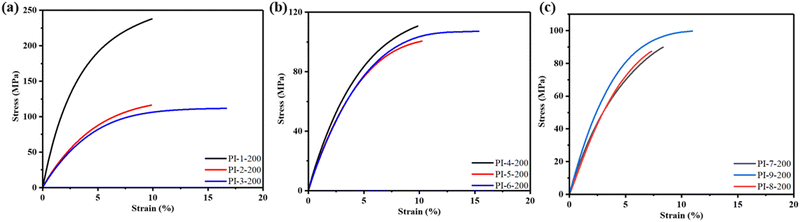 | ||
| Fig. 3 Typical stress–strain curves of (a) PI films with anhydride TAHQ cured at 200 °C, (b) PI films with anhydride BPEDA cured at 200 °C and (c) PI films with anhydride TABPP cured at 200 °C. | ||
| Sample name | Mechanical propertiesa | Thermal properties | ||||||
|---|---|---|---|---|---|---|---|---|
| σ max [MPa] | ε b [%] | E [GPa] | T d,5% [°C] | T d,10% [°C] | T d,30% [°C] | T HRI [°C] | ||
| a The mechanical properties were measured by DMA at room temperature. b T HRI = 0.49 × [Td,5% + 0.6 × (Td,30% − Td,5%)]; Td,5%, Td,10% and Td,30% were the corresponding decomposition temperatures of 5%, 10% and 30% weight loss, respectively. | ||||||||
| PI-1-200 | 238 | 9.9 | 8.00 | 452 | 511 | 568 | 256 | |
| PI-2-200 | 116 | 9.9 | 2.92 | 440 | 457 | 520 | 239 | |
| PI-3-200 | 112 | 16.7 | 2.65 | 445 | 458 | 504 | 235 | |
| PI-4-200 | 111 | 9.8 | 2.78 | 439 | 456 | 491 | 230 | |
| PI-5-200 | 101 | 10.2 | 2.30 | 435 | 450 | 479 | 226 | |
| PI-6-200 | 107 | 15.3 | 2.44 | 440 | 454 | 480 | 227 | |
| PI-7-200 | 90 | 8.3 | 2.52 | 445 | 452 | 476 | 227 | |
| PI-8-200 | 87 | 7.3 | 1.66 | 433 | 444 | 466 | 222 | |
| PI-9-200 | 86 | 7.5 | 2.03 | 440 | 452 | 474 | 226 | |
The thermal stability of the synthesized PI films was evaluated using TGA. As depicted in Table 2, Fig. S6 and Table S5 (ESI†), the PI films showed high Td,5% values above 433 °C. To further reflect the thermal stability of the samples, the heat resistance index (THRI) was calculated.33,34 Apparently, all the samples showed a heat resistance index higher than 220 °C. Consequently, PI-1-350 displayed the highest thermal stability among the synthesized samples due to the most rigid molecular backbone, with Td,5%, Td,10% and THRI values of 504 °C, 526 °C and 269 °C, respectively. Conversely, PI-9-350 showed the loosest molecular chains, which weakened the intermolecular forces, resulting in the lowest thermal stability among the samples cured at 350 °C. For low-temperature curable PI films, their thermal stability was slightly lower than that cured at 350 °C due to a lower degree of cross-linking and ID. However, in this work, the ID of low-temperature curable PIs did not strictly align with the observed thermal stability. Except for PI-1-200, the thermal stability of the other PI films cured at 200 °C was close to that of the corresponding PI films cured at 350 °C. This indicated that the flexibility of the molecular chains may exert a greater influence on thermal stability.
The coefficient of thermal expansion (CTE) of the PIs was measured by TMA, and the results are presented in Table 1, Fig. S7 and Table S4 (ESI†). Remarkably, regardless of the curing temperature (200 °C or 350 °C), the trend in CTE values of the PI films closely mirrored the changes in molecular chain flexibility. Specifically, PI-1-200 stood out with the lowest CTE, registering at 7.48 ppm K−1 among the low-temperature curable samples. This notable feature could primarily be attributed to the rigid structure of the diamine monomer PDA employed.35–38 This observation could be attributed to the presence of more flexible and rotatable groups that disrupted the ordered and close packing of the molecular chains, thereby influencing the CTE values of the PI films.39,40 However, it is essential to recognize that the CTE of the samples was not solely determined by molecular chain flexibility. For instance, despite PI-9-200 exhibiting a more flexible structure compared to PI-7-200 and PI-8-200, its CTE was marginally less than that of the latter two. This phenomenon could be ascribed to the molecular arrangement of flexible PIs, which minimized intermolecular repulsion during temperature fluctuations, consequently mitigating the CTE. A heightened degree of orientation might be helpful to the reduced CTE. And the dichroic ratio (R) was calculated using polarized IR to analyze the in-plane orientation of the PI films based on previous reports (as shown in Fig. S8 and S9, ESI†).41 Apparently, the trend of R was consistent with the variation of CTE. The decrease in the dichroic ratio indicated a more disordered arrangement of molecular chains and increased the CTE of the PI films. Most of the low-temperature curable PI films exhibited higher orientation and lower CTE compared to the corresponding PI films cured at 350 °C. This may be attributed to the increased mobility of the molecular chains at high curing temperatures, which disrupted the regular arrangement of the PIs. It is noteworthy that the high orientation of PI-1-200 (R = 2.131) resulted in its low CTE with a value of 7.48 ppm K−1.
The glass transition temperature (Tg) of the PI films was determined by DSC and TMA, and the resulting Tg values are presented in Table S6 (ESI†). The corresponding DSC curves for the PIs are illustrated in Fig. S10 (ESI†). Unfortunately, the PI films cured at 350 °C showed indistinct Tg, which might be attributed to the heightened degree of crosslinking of PIs cured at high temperatures, consequently diminishing the mobility of units within the molecular chains and leading to less conspicuous alterations in the heat capacity. Nonetheless, a notable trend was seen when examining the Tg values of low-temperature curable PIs. With the incorporation of the flexible structure, there was a clear decrease in Tg values of PI films cured at 200 °C with the flexibility of molecular chain, suggesting that rigidity and flexibility are important factors affecting Tg. As a supplement, the Tg values measured by TMA exhibited a similar rule, although some samples failed to obtain Tg owing to the inability to withstand high temperature thermal expansion. These collective findings underscored the substantial impact of the flexible structure on Tg values.
Dielectric properties
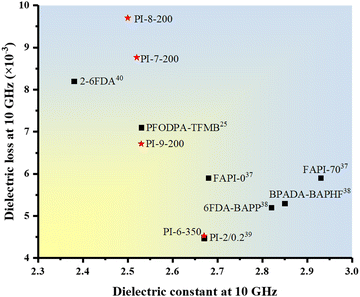
With the rapid advancement of 5G high-frequency communications, the development of polyimides as potential candidates for advanced packaging applications necessitated the reduction of their dielectric constant (Dk) and dielectric loss (Df) to mitigate the high transmission loss at high frequencies. Therefore, the dielectric properties of PI films at 10 GHz were measured as illustrated in Fig. 4, Table 3 and Table S7 (ESI†). For the PI films cured at 350 °C, the Dk values correlated well with the flexibility of the molecular chains. PI-1-350 exhibited a high Dk with a value of 3.47 due to its rigid structure, leading to a smaller free volume. In contrast, PI-9-350 with a more flexible structure exhibited excellent dielectric properties with a Dk of 2.68. Additionally, PI-2-350 and PI-4-350 showed extremely similar Dk values owing to their comparable length of repeating units. These results demonstrated that the Dk of PI films could be finely modulated by controlling the flexibility. However, the dielectric constant of low-temperature curable PIs was affected by multiple factors rather than only flexibility. A decrease in ID would lead to an increase in the number of polar groups, which deteriorated the dielectric constant of PIs. Indeed, the dielectric constant of the polymer is intimately linked to polarizability according to the Clausius–Mossotti equation. The lower ID indicated that a proportion of amidic acid fragments were not converted to imide rings, resulting in increased polarizability. Consequently, it is generally believed that the dielectric constant of low-temperature curable PIs was higher than that of their counterparts cured at 350 °C. For instance, PI-1-200 showed an increased Dk value of 3.66 compared to PI-1-350. However, as flexibility increased, the beneficial effect of increased free volume outweighed the detrimental effect of decreased ID on dielectric constant. Hence, even though PI-9-200 had the lowest ID of 68.59% among the samples, it exhibited excellent dielectric properties (Dk = 2.53). Consequently, some samples, such as PI-7-200, PI-8-200 and PI-9-200, displayed a lower dielectric constant than their counterparts cured at 350 °C. PI-8-200 had the lowest dielectric constant among the samples, with a value of only 2.50, achieved through a clever balance between ID and flexibility. As shown in Fig. 5, the Dk of the low-temperature curable PI films with TABPP was lower than most of the reported low dielectric constant PI films,25,42–45 indicating the potential application of the novel anhydride. This finding provides a new approach for enhancing dielectric properties in advanced packaging applications.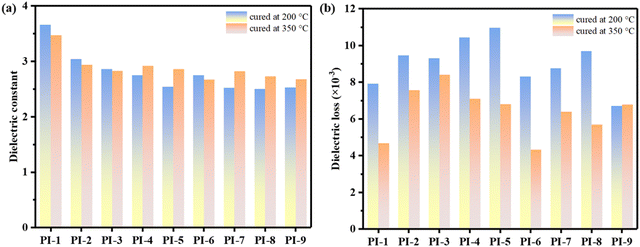 | ||
| Fig. 4 (a) Dielectric constant of the synthesized PIs at 10 GHz and (b) dielectric loss of the synthesized PIs at 10 GHz. | ||
| Sample name | Dielectric propertiesa | Hydrophilicity | Transmittanceb (%) | ||
|---|---|---|---|---|---|
| D k | D f (×10−3) | WA (%) | WCA (°) | ||
| a 10 GHz at room temperature. b Transmittance at 500 nm. | |||||
| PI-1-200 | 3.66 | 7.92 | 1.71 | 66.1 | 79.2 |
| PI-2-200 | 3.04 | 9.46 | 1.62 | 68.1 | 87.8 |
| PI-3-200 | 2.86 | 9.30 | 1.65 | 73.5 | 89.3 |
| PI-4-200 | 2.75 | 10.44 | 1.57 | 68.0 | 88.3 |
| PI-5-200 | 2.54 | 10.97 | 1.42 | 69.0 | 89.8 |
| PI-6-200 | 2.75 | 8.31 | 1.2 | 73.2 | 89.9 |
| PI-7-200 | 2.52 | 8.76 | 1.22 | 70.1 | 89.2 |
| PI-8-200 | 2.50 | 9.70 | 1.08 | 80.0 | 90.3 |
| PI-9-200 | 2.53 | 6.71 | 1.04 | 80.9 | 90.5 |
Meanwhile, PI films cured at 350 °C showed a low dielectric loss below 8.5‰ due to the presence of the ester group,26,46 with PI-6-350 displaying a remarkably low dielectric loss at only 4.53‰, surpassing that of most reported PI films (see Fig. 5). However, the relationship between the PI structure and dielectric loss remains unclear. Some studies proposed that the rigidity of molecular chains would lead to a low dielectric loss,42 while others attributed the low dielectric loss to the long repeating unit (low imide group content) of polyimides.43 Notably, these explanations did not align with the observed trend of dielectric loss in this work. Dielectric loss may not be determined by a single factor, but the combined effect of the length (imide group content) and rigidity of the repeating unit and so on. Two prominent examples that highlighted the complex relationship were PI-1-200 and PI-9-200. In the case of PI-1-200, the higher dielectric loss compared to PI-1-350 could likely be attributed to incomplete imidization, resulting in reduced molecular chain rigidity and enhanced dipole deflection. Conversely, PI-9-200 exhibited a significantly lower dielectric loss compared to the corresponding sample cured at 350 °C, which may arise from the decrease in the imide ring content. This intriguing phenomenon warranted further in-depth investigation in future research to unravel the underlying mechanisms.
Hydrophilicity
The hydrophilicity of the synthesized PI films was evaluated using the water contact angle (WCA) and water absorption (WA). As displayed in Fig. 6 and Fig. S11 (ESI†), the WCA values of the PI films cured at 350 °C and 200 °C fell within the ranges of 66.5° to 84.2° and 66.1° to 80.9°, respectively. Clearly, the WCA values of the low-temperature curable PI films were lower than those of the PI films cured at 350 °C. This could be attributed to an increase of residual polar groups such as carboxyl groups resulting from incomplete imidization. Moreover, as the flexibility of the molecular chains increased, the water contact angle of the films gradually increased, while the water absorption gradually decreased. This trend could be explained by the more complex movement of the molecular chains resulting from the introduction of flexible structures. The increased molecular chain mobility reduced the penetration of water molecules into the film and also reduced the available adsorption sites for water molecules. Consequently, the PI films with higher molecular chain flexibility exhibited reduced water absorption and higher water contact angles. It is noteworthy that PI-9-200 exhibited the best hydrophobicity (WCA = 80.9°, WA = 1.04%) among the low-temperature curable samples despite having the lowest ID. This observation suggested that the favorable effect of the flexible structure on hydrophobicity outweighed the unfavorable effect of the residual polar groups on hydrophobicity. Therefore, the controlled introduction of flexible structures in low-temperature curable PIs could lead to promising materials with excellent hydrophilicity properties for various applications.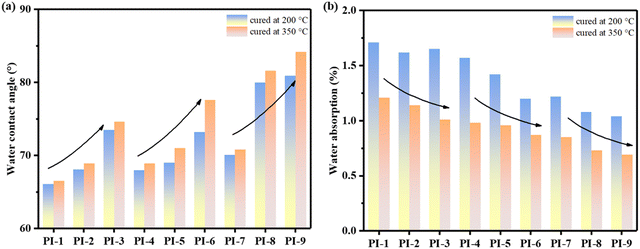 | ||
| Fig. 6 (a) The water contact angle of the synthesized PI films and (b) water absorption of the synthesized PI films. | ||
Optical properties
Traditional PI films were typically yellow or even brown in color due to their conjugated aromatic backbone and the formation of charge transfer complexes (CTCs).47 However, the synthesized low-temperature curable PI films in this work exhibited advantageous optical properties, as shown in the images in Fig. 7 and UV-visible spectra in Fig. S12 (ESI†). The transmittance at 500 nm (T500) of the PI films cured at 200 °C and 350 °C ranged from 79.2% to 90.5% and 54.7% to 84.3%, respectively. Notably, the low-temperature curable PI films demonstrated superior optical transparency compared to films cured at 350 °C. This difference in optical transparency could be attributed to the higher curing temperature at 350 °C, which led to increased cross-linking of the molecular chains and enhanced the CTC effect, resulting in reduced optical transparency of the films. Furthermore, the transparency of the PI films matched the flexibility of molecular chains. The introduction of a flexible molecular segment contributed to weakening the conjugation effect and hindering the transfer of charge within the molecular chain, which improved the optical transparency of the PI films. As a result, PI-9-200 exhibited a satisfactory optical transparency with a T500 of 90.5% due to the more flexible structure.Solubility
The solubility of the synthesized PI films was assessed using various polar solvents, and the results are summarized in Table S8 (ESI†). Thermal cure PI films are often insoluble in polar solvents, primarily due to the highly crosslinking and rigid imide ring. In contrast, PIs with more open or disordered molecular chains are often more soluble, where a larger d-spacing typically indicates a less closely packed molecular chain. Based on the calculated d-spacing values mentioned earlier, it is evident that the d-spacing values of PI films cured at 200 °C were larger than those of their counterparts cured at 350 °C. This suggested that lowering the curing temperature is advantageous for improving the solubility of PIs. Therefore, low-temperature curable PI films demonstrated better solubility, which could be attributed to the lower degree of cross-linking and weaker intermolecular forces due to incomplete imidization. Besides, the introduction of flexible structures was also beneficial for improving the solubility of PIs. The incorporation of the isopropylidene group in the molecular chain introduced distortions, resulting in a more flexible structure and looser molecular chains, which consequently weaken the intermolecular forces. As a result, PI-9-200 with a large d-spacing value of 5.65 Å showed the best solubility among the samples due to its highly flexible structure and low ID, signifying its promising potential for advanced packaging applications.Conclusions
In summary, this study successfully prepared a series of low-temperature curable PIs with varying flexibility, aiming to elucidate the intricate relationship between flexibility and low-temperature curing ability. First, a key discovery was that heightened molecular chain flexibility did not necessarily translate into superior low-temperature curing ability. This reduction in ID of PI-9-200 could be attributed to the diminished reactivity of monomers due to excessive molecular chain flexibility. Second, this work endowed low dielectric constants with low-temperature curable PI films. Remarkably, the judicious introduction of flexible structures realized outstanding dielectric properties (Dk = 2.50–2.53 and Df = 6.71–9.70‰), surpassing those of previously reported low-temperature curable PI films. The present work was focused on exploring the comprehensive effect of molecular chain flexibility on low-temperature curable PIs, providing new insight into the preparation of low-temperature curable PIs with excellent performance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (61904191, 62174170, and 62304141), Guangdong Basic and Applied Basic Research Foundation (2022B1515120037 and 2023A1515010766), the Key R&D Project of Guangdong Province (2020B010180001), Guangdong Jointed Funding (2020A1515110934), the Key Laboratory of Guangdong Province (2014B030301014), the SIAT Innovation Program for Excellent Young Researchers (E2G030) and the National Key R&D Project from Minister of Science and Technology of China (2017ZX02519).Notes and references
- S. Y. Zhang, X. Y. Xu, T. S. Lin and P. He, J. Mater. Sci.: Mater. Electron., 2019, 30(15), 13855–13868 CrossRef CAS.
- J. H. Lau, IEEE Trans. Compon. Packaging Manuf. Technol., 2022, 12(2), 228–252 Search PubMed.
- T. Braun, K. F. Becker, O. Hoelck, S. Voges, R. Kahle, M. Dreissigacker and M. Schneider-Ramelow, Micromachines, 2019, 10(5), 342 CrossRef.
- C. H. Lee, B. Huang, J. See, L. Prenger, Y. M. Lin, W. L. Chiu, O. H. Lee and K. N. Chen, IEEE Trans. Compon. Packaging Manuf. Technol., 2022, 12(4), 692–699 CAS.
- I. Gouzman, E. Grossman, R. Verker, N. Atar, A. Bolker and N. Eliaz, Adv. Mater., 2019, 31(18), 1807738 CrossRef.
- Y. J. Wan, G. Li, Y. M. Yao, X. L. Zeng, P. L. Zhu and R. Sun, Compos. Commun., 2020, 19, 154–167 CrossRef.
- I. H. Tseng, P. N. Hsu, W. Y. Hsu, D. P. Tran, B. T. H. Lin, C. C. Chang, K. N. Tu and C. Chen, Results Phys., 2021, 31, 105048 CrossRef.
- J. W. Baek, W. S. Yang, M. J. Hur, J. C. Yun and S. J. Park, Mater. Sci. Semicond. Process., 2019, 91, 392–398 CrossRef CAS.
- K. Fukuka and M. Ueda, Polym. J., 2008, 40(4), 281–296 CrossRef.
- R. J. Iredale, C. Ward and I. Hamerton, Prog. Polym. Sci., 2017, 69, 1–21 CrossRef CAS.
- J. Kusunoki and T. Hirano, J. Photopolym. Sci. Technol., 2005, 18(2), 321–325 CrossRef CAS.
- T. Sasaki, J. Photopolym. Sci. Technol., 2016, 29(3), 379–382 CrossRef CAS.
- Y. Sui, J. Li, T. Wang, D. Sun, C. Huang, F. Zhang, L. Shan, F. Niu, G. Zhang and R. Sun, Polymer, 2021, 218, 123514 CrossRef CAS.
- C. Li, Y. Wang, Y. Yin, Y. Li, J. Li, D. Sun, J. Lu, G. Zhang and R. Sun, Polymer, 2021, 228, 123963 CrossRef CAS.
- V. N. Artemeva, V. V. Kudryavtsev, E. M. Nekrasova, V. P. Sklizkova, G. V. Lyubimova, I. V. Hofman, V. P. Borovik and O. P. Shkurko, Russ. Chem. B, 1994, 43(3), 387–390 CrossRef.
- M. J. Hu, H. Q. Chen, M. X. Wang, G. Liu, C. H. Chen, G. T. Qian and Y. H. Yu, J. Polym. Sci., 2021, 59(4), 329–339 CrossRef CAS.
- K. I. Fukukawa and M. Ueda, Polym. J., 2006, 38(5), 405–418 CrossRef CAS.
- A. Ghosh, S. Banerjee, H. Komber, K. Schneider, L. Häußler and B. Voit, Eur. Polym. J., 2009, 45(5), 1561–1569 CrossRef CAS.
- X. Li, P. Zhang, J. Dong, F. Gan, X. Zhao and Q. Zhang, Composites, Part B, 2019, 177, 107401 CrossRef CAS.
- T. S. Leu and C. S. Wang, J. Polym. Sci., Part A: Polym. Chem., 2001, 39(23), 4139–4151 CrossRef CAS.
- X. T. Han, Y. Tian, L. H. Wang, C. F. Xiao and B. Q. Liu, Eur. Polym. J., 2007, 43(10), 4382–4388 CrossRef CAS.
- Y. B. Zhuang, J. G. Seong and Y. M. Lee, Prog. Polym. Sci., 2019, 92, 35–88 CrossRef CAS.
- Y. X. Ding, H. B. Duan, M. H. Xie, R. C. Mao, J. J. Wang and W. B. Zhang, Resour., Conserv. Recycl., 2022, 182, 106339 CrossRef CAS.
- Y. H. Li, G. H. Sun, Y. Zhou, G. M. Liu, J. Wang and S. H. Han, Prog. Org. Coat., 2022, 172, 107103 CrossRef CAS.
- W. F. Peng, H. Y. Lei, L. H. Qiu, F. Bao and M. J. Huang, Polym. Chem., 2022, 13(26), 3949–3955 RSC.
- Y. C. Chen, Y. C. Lin, C. C. Kuo, M. Ueda and W. C. Chen, Polymer, 2022, 256, 1251844 Search PubMed.
- C. Huang, J. Li, D. Sun, R. Xuan, Y. Sui, T. Li, L. Shang, G. Zhang, R. Sun and C. P. Wong, J. Mater. Chem. C, 2020, 8(42), 14886–14894 RSC.
- Y. Zhai, Q. Yang, R. Q. Zhu and Y. Gu, J. Mater. Sci., 2008, 43(1), 338–344 CrossRef CAS.
- C. A. Pryde, J. Polym. Sci., Part A: Polym. Chem., 1993, 31(4), 1045–1052 CrossRef CAS.
- R. W. Snyder, B. Thomson, B. Bartges, D. Czerniawski and P. C. Painter, Macromolecules, 1989, 22(11), 4166–4172 CrossRef CAS.
- M. M. Koton, T. K. Meleshko, V. V. Kudryavtsev, P. P. Netchaev, Y. V. Kamzolkina and N. N. Bogorad, Vysokomol. Soedin., Ser. A, 1982, 24(4), 715–721 CAS.
- V. M. Svetlichnyi, N. G. Antonov, B. V. Chernitsa, V. M. Denisov, A. I. Koltsov, V. V. Kudryavtsev and M. M. Koton, Vysokomol. Soedin., Ser. A, 1986, 28(11), 2412–2418 CAS.
- Z. Liu, X. L. Fan, M. Y. Han, H. Li, J. L. Zhang, L. X. Chen, Q. J. Zhu and J. W. Gu, Chinese J. Chem., 2023, 41(8), 939–950 CrossRef CAS.
- K. P. Ruan and J. W. Gu, Macromolecules, 2022, 55(10), 4134–4145 CrossRef CAS.
- M. Hasegawa, Y. Hoshino, N. Katsura and J. Ishii, Polymer, 2017, 111, 91–102 CrossRef CAS.
- M. Hasegawa and K. Koseki, High Perform. Polym., 2006, 18(5), 697–717 CrossRef CAS.
- W. Chen, F. L. Liu, M. Ji and S. Y. Yang, High Perform. Polym., 2017, 29(5), 501–512 CrossRef CAS.
- Y. L. Lei, Y. J. Shu, J. H. Peng, Y. J. Tang and J. C. Huo, e-Polymers, 2016, 16(4), 295–302 CrossRef CAS.
- Y. Y. Tian, L. B. Luo, Q. Q. Yang, L. J. Zhang, M. Wang, D. F. Wu, X. Wang and X. Y. Liu, Polymer, 2020, 188, 122100 CrossRef CAS.
- J. H. Jou and P. T. Huang, Macromolecules, 1991, 24(13), 3796–3803 CrossRef CAS.
- S. I. Matsuda and S. J. Ando, J. Polym. Sci., Part A: Polym. Phys., 2003, 41(4), 418–428 CrossRef CAS.
- J. J. He, H. X. Yang, F. Zheng and S. Y. Yang, Polymers, 2022, 14(3), 649 CrossRef CAS PubMed.
- C. C. Kuo, Y. C. Lin, Y. C. Chen, P. H. Wu, S. Ando, M. Ueda and W. C. Chen, ACS Appl. Polym. Mater., 2021, 3(1), 362–371 CrossRef CAS.
- H. M. Li, X. M. Wang, Y. Z. Gong, H. B. Zhao, L. Tao, Y. Y. Peng, K. Ma, Z. B. Liu, Z. Z. Hu and D. Dastan, RSC Adv., 2023, 13(11), 7585–7596 RSC.
- M. Zhong, X. M. Wu, C. Shu, Y. L. Wang, X. Y. Huang and W. Huang, React. Funct. Polym., 2021, 169, 105065 CrossRef CAS.
- M. Hasegawa, T. Saito and Y. Tsujimura, Polym. Adv. Technol., 2020, 31(3), 389–406 CrossRef CAS.
- Y. Y. Liu, Y. K. Wang and D. Y. Wu, J. Appl. Polym. Sci., 2022, 139(28), e52604 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3tc03070a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |