 Open Access Article
Open Access ArticlePotent BODIPY-based photosensitisers for selective mitochondrial dysfunction and effective photodynamic therapy†
Edward R. H.
Walter‡
 ab,
Peter Kam-Keung
Leung‡
ab,
Peter Kam-Keung
Leung‡
 cd,
Lawrence Cho-Cheung
Lee
cd,
Lawrence Cho-Cheung
Lee
 bc,
Kenneth Kam-Wing
Lo
bc,
Kenneth Kam-Wing
Lo
 *cd and
Nicholas J.
Long
*cd and
Nicholas J.
Long
 *a
*a
aDepartment of Chemistry, Imperial College London, Molecular Sciences Research Hub, London, W12 0BZ, UK. E-mail: n.long@imperial.ac.uk
bLaboratory for Synthetic Chemistry and Chemical Biology Limited, Units 1503-1511, 15/F, Building 17 W, Hong Kong Science Park, New Territories, Hong Kong, P. R. China
cDepartment of Chemistry, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong, P. R. China. E-mail: bhkenlo@cityu.edu.hk
dState Key Laboratory of Terahertz and Millimetre Waves, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong, P. R. China
First published on 5th September 2024
Abstract
The development of new and improved mitochondria-targeting photosensitisers (PSs) for photodynamic therapy (PDT) remains highly desirable, due to the critical role the mitochondria play in maintaining healthy cellular function. Here, we report the design, synthesis, photophysical properties and biological characterisation of a series of di-iodinated BODIPY-based PSs, BODIPY-Mito-I-n, for mitochondria-targeted PDT applications. Six BODIPY-Mito-I-n analogues were synthesised in good yields, with fast reaction times of between 30 and 60 min under mild conditions. The di-iodination of the BODIPY scaffold enabled highly efficient population of the triplet state, leading to high singlet oxygen (1O2) photosensitisation efficiencies (ΦΔ = 0.55–0.65). All BODIPY-Mito-I-n compounds exhibited very high photocytotoxic activity towards HeLa cells, with IC50,light values of between 1.30 and 6.93 nM, due to photoinduced 1O2 generation. Notably, the poly(ethylene glycol) (PEG)-modified BODIPY-Mito-I-6 showed remarkably lower dark cytotoxicity (IC50,dark = 6.68–7.25 μM) than the non-PEGylated analogues BODIPY-Mito-I-1 to BODIPY-Mito-I-5 (IC50,dark = 0.58–1.09 μM), resulting in photocytotoxicity indices up to 2120. Mechanistic studies revealed that BODIPY-Mito-I-6 induced reactive oxygen species overproduction and mitochondrial dysfunction in cells upon irradiation, leading to significant cell death through a combination of apoptosis and necrosis. It is anticipated that our design will contribute to the development of more effective mitochondria-targeting PSs for cancer therapy.
Introduction
Mitochondria are small subcellular organelles involved in a wide range of essential biological processes.1,2 Mitochondria display a negative membrane potential (MMP, Δψm),3 as a consequence of a proton gradient across the inner mitochondrial membrane.4 Therefore, lipophilic cations such as triphenylphosphonium (TPP+) preferentially accumulate in the mitochondria over other cellular compartments.5 Additionally, the MMP is known to be significantly elevated in cancer and cardiovascular disease,6 enabling TPP+-based compounds to be utilised in mitochondrial imaging7,8 and therapeutic applications.9Photodynamic therapy (PDT) is a minimally invasive and emerging cancer therapy that has been approved clinically for many different types of cancer.10 PDT utilises photosensitisers (PSs), compounds that produce reactive oxygen species (ROS) such as singlet oxygen (1O2) upon irradiation with light.11 Importantly, targeting PSs to cancer cells results in a highly localised treatment, minimising the side effects for the patient.
Over the last decade a wide range of mitochondria-targeting PSs have been reported, including boron-dipyrromethenes (BODIPYs),12,13 cyanines,14 hemicyanines,15 porphyrins,16 cyclometallated iridium(III) complexes,17,18 and a promising iridium(III)–BODIPY conjugate for triple-negative breast cancer cells.19 Such an approach is a highly effective cancer therapy, due to the important role of the mitochondria in energy production and modulating cellular function.
Halogenated BODIPYs are widely used as PSs due to their high photostability, tuneable absorption wavelengths and efficient population of the triplet excited states via intersystem crossing (ISC).20–22 Furthermore, the BODIPY structure can be easily modified to fine-tune photophysical properties and facilitate the introduction of functional handles, such as targeting units,7,8 water-solubilising groups,23 and other therapeutic modalities for synergistic therapy approaches.24–26
The utilisation of halogenated BODIPYs with a TPP+ moiety for developing mitochondria-targeting PSs in PDT applications remains relatively unexplored.27–31 Examples in the literature show promise; for example, 1BDP-TPP developed by Guo and co-workers displaying an encouraging photocytotoxicity index (PI; IC50,dark/IC50light) of 720 in MCF-7 cells (Fig. 1A).29 Additionally, Huang and co-workers improved the solubility of BDP-L2 solubility through the addition of a triethylene glycol linker (Fig. 1B).30
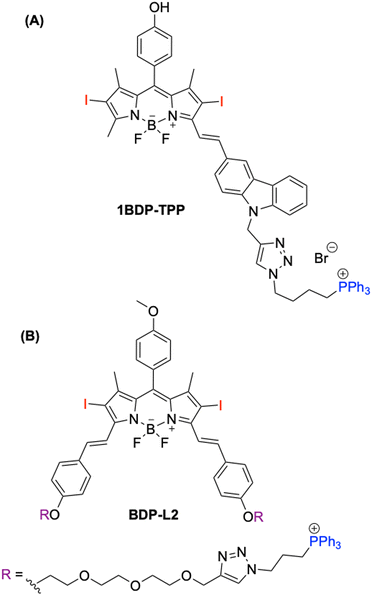 | ||
| Fig. 1 Literature examples of TPP+-based BODIPY PSs. (A) 1BDP-TPP developed by Guo and co-workers29 and (B) BDP-L2 developed by Huang and co-workers.30 | ||
However, no detailed studies have been carried out to fine tune these photophysical characteristics by varying the alkyl/aryl moiety on the phosphonium cation, in an attempt to further enhance the PI values. As such, compounds displaying both a large PI value and excellent water solubility remain highly sought-after. Furthermore, through the modification of substituents on the phosphonium cation, results are directly transferable to facilitate the development of a new generation of mitochondria-targeting compounds for imaging and therapy.
In our previous work we reported a series of biocompatible fluorescent BODIPY-based compounds, BODIPY-Mito-n, bearing cyclohexyl or phenyl functionalities.32 Additionally, analogues possessing improved water solubility were developed through the incorporation of di(ethylene glycol) (DEG) and poly(ethylene glycol) (PEG) moieties (Fig. 2A). All compounds exhibited high mitochondrial specificity (Pearson's correlation coefficients (PCCs) between 0.76 and 0.96) and excellent MMP-sensitivity. However, PEGylated (n = 7) BODIPY-Mito-6 was found to have the highest MMP-sensitive localisation, with a 75% decrease in the fluorescence intensity following MMP depolarisation.
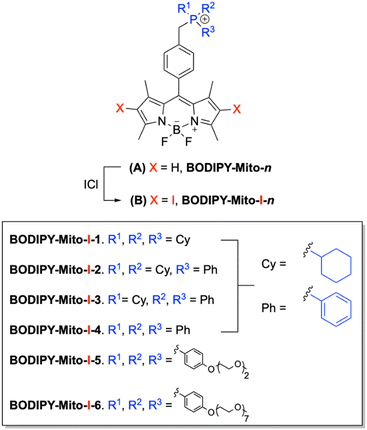 | ||
| Fig. 2 (A) Structures of fluorescent BODIPY-Mito-n32 and (B) their di-iodinated counterparts, BODIPY-Mito-I-n, described in this study. | ||
We report herein the design, synthesis and characterisation of a series of di-iodinated BODIPY-based PSs, BODIPY-Mito-I-n (Fig. 2B). These PSs were designed based on our previously developed versatile BODIPY scaffolds.32 However, di-iodination promotes 1O2 production as a result of ISC to the triplet excited states, and enables such probes to be used as mitochondria-targeting PSs instead of fluorescent imaging agents.20–22 Analogues BODIPY-Mito-I-1 to BODIPY-Mito-I-4 vary by the number of cyclohexyl- and phenyl moieties on the phosphonium cation, to gain a deeper understanding on how this modulates their dark cytotoxicity and photocytotoxicity. However, one major limitation of current PSs is poor water solubility due to aggregation in aqueous media.33 Therefore, analogues incorporating DEG (BODIPY-Mito-I-5) and PEG (BODIPY-Mito-I-6) functionalities were developed in an attempt to improve water solubility and lower dark cytotoxicity to achieve an enhanced phototherapeutic efficacy.
Results and discussion
Design and synthesis of BODIPY-Mito-I-n analogues
Compounds BODIPY-Mito-I-n were synthesised in a one-step reaction from their BODIPY-Mito-n precursors, using iodine monochloride under mild conditions and fast reaction times of between 30 and 60 min (Scheme 1). Following purification by silica gel column chromatography, BODIPY-Mito-I-n analogues were all isolated in good yields of between 60 and 76%. Furthermore, the incorporation of DEG and PEG moieties for BODIPY-Mito-I-5 and BODIPY-Mito-I-6, respectively, significantly enhanced aqueous solubility.Di-iodination of the β-pyrrolic position of BODIPY (1) prior to functionalisation with TPP+ was also attempted (Scheme 2). However, it was found that alkylation of (2), previously synthesised in a procedure from Zhang and co-workers,35 with triphenylphosphine resulted in the removal of the iodine atoms, and the partial regeneration of the non-iodinated compound (1).
Photophysical and photochemical properties of BODIPY-Mito-I-n analogues
The photophysical properties of BODIPY-Mito-I-n analogues were recorded in phosphate-buffered saline (PBS) (pH = 7.4) and acetonitrile (Table 1, Fig. 3 and Fig. S1, S2, ESI†) for comparison with our previously reported BODIPY-Mito-n series.32| Compound | Medium | λ abs/nm (ε/104 M−1 cm−1) | λ ex/nm | λ em/nm | Φ em |
|---|---|---|---|---|---|
| a [BODIPY] = 10 μM, 298 K. Quantum yields (Φ) ± 20% were measured using rhodamine 6G in ethanol (Φ488nm = 0.94)34 as the standard. b Very weak emission was observed. c The extinction coefficient (ε) in CH3CN was not reported. | |||||
| This work | |||||
| BODIPY-Mito-I-1 | PBS | 514 (2.10), 542 (2.61) | 535 | 575b | <0.001 |
| CH3CN | 532 (6.81) | 534 | 553 | 0.018 | |
| BODIPY-Mito-I-2 | PBS | 514 (2.13), 544 (2.50) | 535 | 579b | <0.001 |
| CH3CN | 532 (7.09) | 535 | 553 | 0.019 | |
| BODIPY-Mito-I-3 | PBS | 514 (2.09), 542 (2.49) | 532 | 576b | <0.001 |
| CH3CN | 532 (7.02) | 535 | 552 | 0.019 | |
| BODIPY-Mito-I-4 | PBS | 514 (2.28), 545 (2.74) | 540 | 588b | <0.001 |
| CH3CN | 532 (8.18) | 535 | 554 | 0.017 | |
| BODIPY-Mito-I-5 | PBS | 517 (2.33), 539 (2.54) | 534 | 575b | 0.002 |
| CH3CN | 532 (6.85) | 535 | 552 | 0.020 | |
| BODIPY-Mito-I-6 | PBS | 515 (2.92), 537 (4.06) | 534 | 557b | 0.004 |
| CH3CN | 532 (7.39) | 536 | 553 | 0.016 | |
| Previous work32 | |||||
| BODIPY-Mito-4 | PBS | 497 (5.92) | 497 | 511 | 0.59 |
| CH3CN | 499c | 500 | 512 | 0.57 | |
| BODIPY-Mito-6 | PBS | 498 (6.49) | 500 | 512 | 0.72 |
| CH3CN | 499c | 504 | 513 | 0.60 | |
In aqueous media, BODIPY-Mito-I-n compounds showed considerably broader absorption spectra (Fig. 1) when compared to acetonitrile (Fig. S1, ESI†). Additionally, BODIPY-Mito-I-n compounds displayed low fluorescence quantum yields (Φem = 0.016–0.020), due to efficient ISC from the singlet (S1) to triplet (T1) excited state following di-iodination (Table 1). A weaker emission intensity was displayed in aqueous environments across the series when compared to acetonitrile (Fig. 3B and Fig. S2, ESI†). Therefore, a further reduction in the fluorescence quantum yields was displayed (Φem ≤ 0.004), and is likely attributed to the increased aggregation of BODIPY-Mito-I-n analogues in aqueous media.
The introduction of DEG (BODIPY-Mito-I-5) and PEG (BODIPY-Mito-I-6) moieties resulted in a blue shift in the absorption (Fig. 3A) and emission wavelength maximum (Fig. S2, ESI†), as well as a small increase in the fluorescence quantum yield (Table 1). However, due to the similarity of photophysical properties displayed for BODIPY-Mito-I-n analogues in acetonitrile, it is expected that such characteristics are as a result of reduced aggregation following the installation of hydrophilic DEG or PEG moieties.
Singlet oxygen generation of BODIPY-Mito-I-n analogues
The 1O2 quantum yields (ΦΔ) of BODIPY-Mito-I-n analogues were determined via the indirect method (Table 2), using 1,3-diphenylisobenzofuran (DPBF) as the 1O2 scavenger.36 Methanol was chosen as a solvent due to enhanced solubility of molecular oxygen.37 The ΦΔ values were also recorded in acetonitrile, to compliment the photophysical characterisation of our BODIPY-Mito-I-n analogues.| Compound | Medium | Φ Δ |
|---|---|---|
| a [BODIPY] = 0.5 μM, [DPBF] = 50 μM, 298 K. b Φ Δ were measured using Rose Bengal in aerated methanol (ΦΔ = 0.79) as the standard.38 c Φ Δ were measured using Rose Bengal in aerated acetonitrile (ΦΔ = 0.53) as the standard.39 | ||
| BODIPY-Mito-I-1 | CH3OH | 0.64b |
| CH3CN | 0.63c | |
| BODIPY-Mito-I-2 | CH3OH | 0.61b |
| CH3CN | 0.55c | |
| BODIPY-Mito-I-3 | CH3OH | 0.60b |
| CH3CN | 0.56c | |
| BODIPY-Mito-I-4 | CH3OH | 0.65b |
| CH3CN | 0.60c | |
| BODIPY-Mito-I-5 | CH3OH | 0.60b |
| CH3CN | 0.63c | |
| BODIPY-Mito-I-6 | CH3OH | 0.61b |
| CH3CN | 0.62c | |
Following irradiation at 525 nm for 10 s intervals, a decrease in the DPBF absorbance at 411 nm was observed in both methanol (Fig. S3 and S4, ESI†) and acetonitrile, (Fig. S5 and S6, ESI†), confirming the generation of 1O2. All BODIPY-Mito-I-n analogues displayed high 1O2 photosensitisation efficiencies between 0.60–0.65 in methanol and 0.55–0.63 in acetonitrile (Table 2), through the promotion of ISC from the S1 to the T1 excited state due to the heavy atom effect. It has previously been reported that ΦΔ decreases following PEGylation (PEG average Mw = 5000 Da).36 However, in our case, following the introduction of three PEG moieties with a lower molecular weight (PEG average Mw = 350 Da), no decrease in ΦΔ was observed.
Furthermore, no significant changes in the absorption at 525 nm were observed for BODIPY-Mito-I-n analogues under the same irradiation conditions. Additionally, the 1O2 generation efficiency of BODIPY-Mito-n analogues without di-iodination, was 4.3- and 4.6-fold lower for previously synthesised32BODIPY-Mito-1 (ΦΔ = 0.15) and BODIPY-Mito-6 (ΦΔ = 0.13), respectively, in methanol (Fig. S7, ESI†). Therefore, di-iodination is essential in facilitating efficient 1O2 generation.
(Photo)cytotoxicity of BODIPY-Mito-I-n analogues
The (photo)cytotoxicity of BODIPY-Mito-I-n analogues towards HeLa cells was examined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red uptake (NRU) assays. The MTT assay measures cellular metabolic activity as an indicator of cell viability, while the NRU assay measures the ability of viable cells to incorporate and bind neutral red in lysosomes. The use of two different cell viability assays can help avoid possible artefacts caused by the mitochondrial localisation of the PSs, which might disrupt mitochondrial activity and cellular metabolism.Upon incubation in the dark for 24 h, the BODIPY-Mito-I-n analogues displayed moderate cytotoxic activity towards HeLa cells (Table 3 and Fig. S8, S9, ESI†). The results obtained from the MTT and NRU assays were very similar, revealing IC50,dark values ranging from 0.59 to 7.25 μM and 0.58 to 6.68 μM, respectively (Table 3). Notably, the IC50,dark values of BODIPY-Mito-I-5 (1.02–1.09 μM) and BODIPY-Mito-I-6 (6.68–7.25 μM) were higher than those of analogues BODIPY-Mito-I-1 to BODIPY-Mito-I-4 (0.58–0.81 μM). The lower dark cytotoxicity of BODIPY-Mito-I-5 and BODIPY-Mito-I-6 is attributed to the DEG and PEG pendants on the TPP+ moiety, respectively, which can reduce non-specific interactions of the compounds with mitochondrial proteins and membrane structures, enhancing their biocompatibility. The lower cellular uptake of these compounds should also be accounted for their reduced dark cytotoxic activity (Fig. S10, ESI†). Previous studies by us32,40–43 and others44–46 have also demonstrated that PEGylation could lower the cellular uptake efficiencies of the compounds due to reduced lipophilicity.
| Compound | MTT | NRU | ||||
|---|---|---|---|---|---|---|
| IC50,dark/μM | IC50,light/nM | PI | IC50,dark/μM | IC50,light/nM | PI | |
| BODIPY-Mito-I-1 | 0.81 ± 0.018 | 1.72 ± 0.055 | 471 | 0.72 ± 0.022 | 1.71 ± 0.060 | 421 |
| BODIPY-Mito-I-2 | 0.59 ± 0.074 | 1.30 ± 0.050 | 454 | 0.58 ± 0.072 | 1.41 ± 0.095 | 411 |
| BODIPY-Mito-I-3 | 0.78 ± 0.040 | 1.46 ± 0.093 | 534 | 0.73 ± 0.071 | 1.66 ± 0.113 | 440 |
| BODIPY-Mito-I-4 | 0.66 ± 0.045 | 1.53 ± 0.064 | 431 | 0.62 ± 0.059 | 1.60 ± 0.073 | 388 |
| BODIPY-Mito-I-5 | 1.02 ± 0.093 | 6.93 ± 0.487 | 147 | 1.09 ± 0.083 | 6.63 ± 0.562 | 164 |
| BODIPY-Mito-I-6 | 7.25 ± 0.571 | 4.22 ± 1.090 | 1718 | 6.68 ± 1.009 | 3.15 ± 0.141 | 2120 |
Upon irradiation of the treated cells at 525 nm (10 mW cm−2) for 10 min, the cytotoxic activity of the BODIPY-Mito-I-n analogues was significantly enhanced (IC50,light = 1.30–6.93 nM), resulting in PI values of 147 to 2120 (Table 3). The high photocytotoxicity of the compounds is ascribed to their high 1O2 generation efficiencies (ΦΔ = 0.60–0.65 in methanol; Table 2). Among these compounds, BODIPY-Mito-I-6 exhibited the largest PI value (2120) due to its low dark cytotoxicity (IC50,dark = 6.68–7.25 μM) and high photocytotoxicity (IC50,light = 3.15–4.22 nM). To the best of our knowledge, PI values of 1718 and 2120 in HeLa cells for BODIPY-Mito-I-6, determined by the MTT and NRU assay, respectively, are the highest reported for a TPP+-functionalised BODIPY-based compound.22,29–31 Such a result highlights the importance of using multiple PEG moieties to enhance the phototherapeutic efficacy of BODIPY-based PSs in cells.12,13
Mechanism of cell death induced by BODIPY-Mito-I-n analogues
Although the intracellular localisation of the BODIPY-Mito-I-n compounds could not be accurately determined by confocal microscopy due to their very low fluorescence quantum yields (≤0.004; Table 1), they should presumably exhibit similar localisation properties as their non-iodinated counterparts and localise in the mitochondria because iodination of the BODIPY scaffold should not pose a significant impact on their cellular localisation.12,27,31 Therefore, it is likely that the effective photoinduced generation of 1O2 by these compounds in the mitochondria induced mitochondrial dysfunction and triggered cell death, leading to the very high photocytotoxicity. To validate that the BODIPY-Mito-I-n analogues can generate ROS inside cells, we used CM-H2DCFDA, a cell-permeable, non-fluorescent probe that generates a strongly fluorescent 2′,7′-dichlorofluorescein derivative upon oxidation by ROS, to examine intracellular ROS generation. Treatment of HeLa cells with BODIPY-Mito-I-6 in the dark resulted in extremely weak fluorescence (Fig. 4, top). However, strong fluorescence was detected upon exposure of the BODIPY-Mito-I-6-loaded cells to irradiation, indicating efficient ROS production by BODIPY-Mito-I-6 inside cells upon irradiation. We also utilised rhodamine 123 (R123), a fluorescent mitochondrial stain that localises in the mitochondria in an MMP-dependant manner, to monitor changes in MMP upon the treatment. The bright green fluorescence and intact mitochondrial morphology observed in the cells treated with BODIPY-Mito-I-6 in the dark (Fig. 4, bottom) indicate that the MMP remained stable. However, upon irradiation, the fluorescence intensity of R123 in BODIPY-Mito-I-6-loaded cells decreased substantially, suggesting a significant reduction in MMP. These results collectively illustrate that BODIPY-Mito-I-6 triggered ROS overproduction and mitochondrial depolarisation in cells upon irradiation, leading to mitochondrial dysfunction and significant cell death. We further studied the mechanism of cell death in BODIPY-Mito-I-6-treated cells using the Annexin V/propidium iodide (PI) staining assay. Incubation of the cells with BODIPY-Mito-I-6 in the dark for 24 h results in a high live cell population (Annexin V−/PI−; 94.3%) (Fig. 5). However, upon irradiation, the population of early apoptotic cells (Annexin V+/PI−) and late apoptotic/necrotic cells (Annexin V+/PI+) significantly increased from 5.48 and 0.01% to 45.65 and 45.86%, respectively, indicating that the cell death pathways mediated by BODIPY-Mito-I-6 under light conditions involved apoptosis and necrosis. Similar cell death pathways were also observed in cells treated with BODIPY-Mito-I-1 to BODIPY-Mito-I-5 followed by light irradiation (Fig. S11, ESI†).Conclusions
In conclusion, a series of BODIPY-Mito-I-n analogues were designed, synthesised and characterised as mitochondria-targeting PSs for PDT. All six compounds were synthesised in good yields, under mild conditions, and demonstrated fast reaction times of between 30 and 60 min. Additionally, the incorporation of DEG (BODIPY-Mito-I-5) and PEG (BODIPY-Mito-I-6) functionalities significantly enhanced water solubility.The di-iodination of the BODIPY scaffold enabled highly efficient population of the T1 state, resulting in the high 1O2 photosensitisation efficiencies of the compounds of between 0.60–0.65 and 0.55–0.63 in methanol and acetonitrile, respectively. Furthermore, as expected, BODIPY-Mito-I-n analogues had significantly lower fluorescence quantum yields in both PBS (pH = 7.4) and acetonitrile, compared to the non-iodinated BODIPY-Mito-n series.
Notably, BODIPY-Mito-I-6 showed relatively low dark cytotoxicity due to the PEG pendants on the TPP+ moiety but displayed very high photocytotoxic activity upon irradiation, due to 1O2 generation, leading to a PI value of up to 2120 in HeLa cells. Further studies revealed that BODIPY-Mito-I-6 induced ROS overproduction and mitochondrial dysfunction in cells upon irradiation, resulting in significant cell death through a combination of both apoptosis and necrosis.
It is anticipated that the use of aryl-PEGylated phosphine precursors will provide a significant improvement on the widely used TPP+ moiety for developing new mitochondria-targeting PSs for PDT, due to the notable reduction in dark cytotoxicity without sacrificing the 1O2 photosensitisation efficiency of the PSs and mitochondria-targeting ability of the TPP+ unit. Therefore, we believe that this work will contribute to the development of more effective PDT strategies, providing valuable insights into the design and optimisation of mitochondria-targeting PSs for enhanced cancer treatment.
Author contributions
E. R. H. W., P. K.-K. L., L. C.-C. L., K. K.-W. L. and N. J. L. designed the project. E. R. H. W carried out the synthesis, characterisation, UV-Vis, fluorescence spectroscopy and 1O2 quantum yield measurements of all the compounds. P. K.-K. L. and L. C-.C. L. carried out the biological characterisation and (photo)cytotoxicity studies. E. R. H. W., P. K.-K. L., L. C.-C. L., K. K.-W. L. and N. J. L. analysed data. E. R. H. W., P. K.-K. L., L. C.-C. L., K. K.-W. L. and N. J. L. wrote the manuscript.Data availability
We confirm that all the relevant research data is contained with the manuscript and electronic supporting information. No databases have been used and no references to such databases are contained in the manuscript or ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Laboratory for Synthetic Chemistry and Chemical Biology (LSCCB) under the Health@InnoHK Programme launched by Innovation and Technology Commission, The Government of Hong Kong SAR, P. R. China. The authors also thank Professor Matthew J. Fuchter and his research group at Imperial College London, UK, for use of their 525 nm LED for the determination of 1O2 quantum yields.References
- L. D. Zorova, V. A. Popkov, E. Y. Plotnikov, D. N. Silachev, I. B. Pevzner, S. S. Jankauskas, V. A. Babenko, S. D. Zorov, A. V. Balakireva, M. Juhaszova, S. J. Sollott and D. B. Zorov, Anal. Biochem., 2018, 552, 50–59 CrossRef CAS PubMed.
- N. Sun, R. J. Youle and T. Finkel, Mol. Cell, 2016, 61, 654–666 CrossRef CAS PubMed.
- M. P. Murphy, Biochim. Biophys. Acta, Bioenerg., 2008, 1777, 1028–1031 CrossRef CAS PubMed.
- J. Zielonka, J. Joseph, A. Sikora, M. Hardy, O. Ouari, J. Vasquez-Vivar, G. Cheng, M. Lopez and B. Kalyanaraman, Chem. Rev., 2017, 117, 10043–10120 CrossRef CAS PubMed.
- M. P. Murphy, Trends Biotechnol., 1997, 15, 326–330 CrossRef CAS PubMed.
- H. M. Begum and K. Shen, WIREs Mech. Dis., 2023, 15, e1595 CrossRef CAS PubMed.
- B. E. Osborne, T. T. C. Yue, E. C. T. Waters, F. Baark, R. Southworth and N. J. Long, Dalton Trans., 2021, 50, 14695–14705 RSC.
- S. P. McCluskey, A. Haslop, C. Coello, R. N. Gunn, E. W. Tate, R. Southworth, C. Plisson, N. J. Long and L. A. Wells, J. Nucl. Med., 2019, 60, 1750–1756 CrossRef CAS PubMed.
- M. P. Murphy and R. C. Hartley, Nat. Rev. Drug Discovery, 2018, 17, 865–886 CrossRef CAS PubMed.
- J. Usuda, H. Kato, T. Okunaka, K. Furukawa, H. Tsutsui, K. Yamada, Y. Suga, H. Honda, Y. Nagatsuka, T. Ohira, M. Tsuboi and T. Hirano, J. Thorac. Oncol., 2006, 1, 489–493 CrossRef PubMed.
- J. Karges, Angew. Chem., Int. Ed., 2022, 61, e202112236 CrossRef CAS PubMed.
- I. W. Badon, C. Kim, J. M. Lim, D. K. Mai, T. P. Vales, D. Kang, S. Cho, J. Lee, H.-J. Kim and J. Yang, J. Mater. Chem. B, 2022, 10, 1196–1209 RSC.
- Q. Guan, L.-L. Zhou, Y.-A. Li and Y.-B. Dong, Inorg. Chem., 2018, 57, 10137–10145 CrossRef CAS PubMed.
- A. P. Thomas, L. Palanikumar, M. T. Jeena, K. Kim and J.-H. Ryu, Chem. Sci., 2017, 8, 8351–8356 RSC.
- Z. Cheng, S. Benson, L. Mendive-Tapia, E. Nestoros, C. Lochenie, D. Seah, K. Y. Chang, Y. Feng and M. Vendrell, Angew. Chem., Int. Ed., 2024, 63, e202404587 CrossRef CAS PubMed.
- Satrialdi, Y. Takano, E. Hirata, N. Ushijima, H. Harashima and Y. Yamada, Nanoscale Adv., 2021, 3, 5919–5927 RSC.
- J.-H. Zhu, G.-X. Xu, J. Shum, L. C.-C. Lee and K. K.-W. Lo, Chem. Commun., 2021, 57, 12008–12011 RSC.
- L. Huang, P. K.-K. Leung, L. C.-C. Lee, G.-X. Xu, Y.-W. Lam and K. K.-W. Lo, Chem. Commun., 2022, 58, 10162–10165 RSC.
- L. Qiao, J. Liu, S. Kuang, X. Liao, J. Kou, L. Ji and H. Chao, Dalton Trans., 2021, 50, 14332–14341 RSC.
- T. Yogo, Y. Urano, Y. Ishitsuka, F. Maniwa and T. Nagano, J. Am. Chem. Soc., 2005, 127, 12162–12163 CrossRef CAS PubMed.
- E. Bassan, A. Gualandi, P. G. Cozzi and P. Ceroni, Chem. Sci., 2021, 12, 6607–6628 RSC.
- S. Wang, L. Gai, Y. Chen, X. Ji, H. Lu and Z. Guo, Chem. Soc. Rev., 2024, 53, 3976–4019 RSC.
- B. Sui, S. Tang, A. W. Woodward, B. Kim and K. D. Belfield, Eur. J. Org. Chem., 2016, 2016, 2851–2857 CrossRef CAS.
- A. Kumar, A. Dixit, S. Banerjee, A. Bhattacharyya, A. Garai, A. A. Karande and A. R. Chakravarty, Med. Chem. Commun., 2016, 7, 1398–1404 RSC.
- K. Mitra, S. Gautam, P. Kondaiah and A. R. Chakravarty, ChemMedChem, 2016, 11, 1956–1967 CrossRef CAS PubMed.
- U. Bhattacharyya, B. Kumar, A. Garai, A. Bhattacharyya, A. Kumar, S. Banerjee, P. Kondaiah and A. R. Chakravarty, Inorg. Chem., 2017, 56, 12457–12468 CrossRef CAS PubMed.
- Q. Wang, D. K. P. Ng and P.-C. Lo, J. Mater. Chem. B, 2018, 6, 3285–3296 RSC.
- D. Chen, J. Zhang, Y. Tang, X. Huang, J. Shao, W. Si, J. Ji, Q. Zhang, W. Huang and X. Dong, J. Mater. Chem. B, 2018, 6, 4522–4530 RSC.
- M. A. Masood, Y. Wu, Y. Chen, H. Yuan, N. Sher, F. Faiz, S. Yao, F. Qi, M. I. Khan, M. Ahmed, N. Mushtaq, W. He and Z. Guo, Dyes Pigm., 2022, 202, 110255 Search PubMed.
- P. Hu, X.-L. Weng, C.-H. Zhu, D.-C. Yang, J.-Y. Liu, Z. Chen and M. Huang, ChemPlusChem, 2022, 87, e202200158 CrossRef CAS PubMed.
- T. P. Vales, S. Cho, J. Lee, H. T. Bui, D. K. Mai, I. W. Badon, H. Lim, W. Jeong, J.-L. Kim, H.-K. Kim and H.-J. Kim, J. Mol. Struct., 2021, 1246, 131284 Search PubMed.
- E. R. H. Walter, L. C.-C. Lee, P. K.-K. Leung, K. K.-W. Lo and N. J. Long, Chem. Sci., 2024, 15, 4846–4852 Search PubMed.
- J. Wu, J. Tian, L. Rui and W. Zhang, Chem. Commun., 2018, 54, 7629–7632 Search PubMed.
- M. Fischer and J. Georges, Chem. Phys. Lett., 1996, 260, 115–118 CrossRef CAS.
- B. Yuan, H. Wu, H. Wang, B. Tang, J. F. Xu and X. Zhang, Angew. Chem., Int. Ed., 2021, 60, 706–710 CrossRef CAS PubMed.
- G.-X. Xu, L. C.-C. Lee, P. K.-K. Leung, E. C.-L. Mak, J. Shum, K. Y. Zhang, Q. Zhao and K. K.-W. Lo, Chem. Sci., 2023, 14, 13508–13517 RSC.
- D. Dvoranová, Z. Barbieriková and V. Brezová, Molecules, 2014, 19, 17279–17304 CrossRef PubMed.
- R. W. Redmond and J. N. Gamlin, Photochem. Photobiol., 1999, 70, 391–475 Search PubMed.
- N. Epelde-Elezcano, V. Martínez-Martínez, E. Peña-Cabrera, C. F. A. Gómez-Durán, I. L. Arbeloa and S. Lacombe, RSC Adv., 2016, 6, 41991–41998 Search PubMed.
- S. P.-Y. Li, T. S.-M. Tang, K. S.-M. Yiu and K. K.-W. Lo, Chem. – Eur. J., 2012, 18, 13342–13354 Search PubMed.
- A. W.-T. Choi, M.-W. Louie, S. P.-Y. Li, H.-W. Liu, B. T.-N. Chan, T. C.-Y. Lam, A. C.-C. Lin, S.-H. Cheng and K. K.-W. Lo, Inorg. Chem., 2012, 51, 13289–13302 CrossRef CAS PubMed.
- S. P.-Y. Li, C. T.-S. Lau, M.-W. Louie, Y.-W. Lam, S. H. Cheng and K. K.-W. Lo, Biomaterials, 2013, 34, 7519–7532 Search PubMed.
- K. K.-S. Tso, K.-K. Leung, H.-W. Liu and K. K.-W. Lo, Chem. Commun., 2016, 52, 4557–4560 RSC.
- J. Xie, C. Xu, N. Kohler, Y. Hou and S. Sun, Adv. Mater., 2007, 19, 3163–3166 CrossRef CAS.
- Y. Guo, H. Yuan, W. L. Rice, A. T. N. Kumar, C. J. Goergen, K. Jokivarsi and L. Josephson, J. Am. Chem. Soc., 2012, 134, 19338–19341 Search PubMed.
- L. Sanchez, Y. Yi and Y. Yu, Nanoscale, 2017, 9, 288–297 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of synthesis, and photophysical and biological characterisation of all compounds used in the study. See DOI: https://doi.org/10.1039/d4tb01609b |
| ‡ Equal contribution from both authors. |
| This journal is © The Royal Society of Chemistry 2024 |





