Open Access Article
Open Access ArticleDevelopment and evaluation of 3D composite scaffolds with piezoelectricity and biofactor synergy for enhanced articular cartilage regeneration†
Bowen
Xie‡
 ab,
Hebin
Ma‡
ab,
Hebin
Ma‡
 cd,
Fengyuan
Yang‡
cd,
Fengyuan
Yang‡
 e,
Hongguang
Chen‡
e,
Hongguang
Chen‡
 d,
Ya’nan
Guo
d,
Hongxing
Zhang
a,
Tengfei
Li
a,
Xiaogang
Huang
a,
Yantao
Zhao
*d,
Xiaojie
Li
*a and
Junjie
Du
*abe
d,
Ya’nan
Guo
d,
Hongxing
Zhang
a,
Tengfei
Li
a,
Xiaogang
Huang
a,
Yantao
Zhao
*d,
Xiaojie
Li
*a and
Junjie
Du
*abe
aDepartment of Orthopedics, Air Force Medical Center, Beijing 100142, China. E-mail: albertlxj@126.com
bAir Force Clinical College, The Fifth School of Clinical Medicine, Anhui Medical University, Hefei 230032, China. E-mail: dujunjie205@hotmail.com
cMedical School of the PLA General Hospital, Beijing 100853, China
dSenior Department of Orthopedics, The Fourth Medical Center of the PLA General Hospital, Beijing 100048, China. E-mail: biodoctor1981@163.com
eGraduate School of Medicine, China Medical University, Shenyang 110122, China
First published on 18th September 2024
Abstract
The inability of articular cartilage to self-repair following injuries frequently precipitates osteoarthritis, profoundly affecting patients' quality of life. Given the limitations inherent in current clinical interventions, an urgent need exists for more effective cartilage regeneration methodologies. Previous studies have underscored the potential of electrical stimulation in cartilage repair, thus motivating the investigation of innovative strategies. The present study introduces a three-dimensional scaffold fabricated through a composite technique that leverages the synergy between piezoelectricity and biofactors to enhance cartilage repair. This scaffold is composed of polylactic acid (PLLA) and barium titanate (BT) for piezoelectric stimulation and at the bottom with a collagen-coated layer infused with fibroblast growth factor-18 (FGF-18) for biofactor delivery. Designed to emulate the properties of natural cartilage, the scaffold enables controlled generation of piezoelectric charges and the sustained release of biofactors. In vitro tests confirm that the scaffold promotes chondrocyte proliferation, matrix hyperplasia, cellular migration, and the expression of genes associated with cartilage formation. Moreover, in vivo studies on rabbits have illustrated its efficacy in catalyzing the in situ regeneration of articular cartilage defects and remodeling the extracellular matrix. This innovative approach offers significant potential for enhancing cartilage repair and holds profound implications for regenerative medicine.
1 Introduction
Articular cartilage is essential for smooth joint motion and protecting bones within synovial joints.1 Due to its lack of blood vessels, nerves, and lymphatic tissues, the repair of cartilage presents significant challenges.2,3 If not addressed promptly and effectively, cartilage damage may progress to osteoarthritis (OA), a degenerative disease. Current treatment modalities, including medication and surgical interventions, primarily aim to mitigate symptoms yet fail to cure the underlying conditions.4 This limitation highlights the imperative for pioneering strategies, such as cartilage tissue engineering.5 This innovative approach employs a combination of seed cells, suitable materials, and physical cues embedded within scaffolds that emulate the natural extracellular matrix (ECM). These scaffolds provide not only a structural framework but also metabolic sites essential for tissue regeneration.6–8Decades of research studies have underscored the stimulatory effects of piezoelectricity on cartilage.9 It has been observed that cartilage generates bioelectrical signals during joint movement or when subjected to stress.10,11 In response to these signals, an increase in chondrocyte proliferation and matrix production occurs, thereby facilitating cartilage repair. This indicates that electrical stimulation might replicate this natural environment to enhance repair processes.12–14 Research has shown that electrical fields can promote proteoglycan synthesis, which strengthens the cartilage further.15,16 However, traditional methods of delivering electrical fields are fraught with limitations, which has led to the development of innovative implantable biological scaffolds possessing intrinsic electrical activity.14,17,18 Piezoelectric materials, which respond to mechanical stress autonomously, are deemed ideal for this purpose in tissue engineering.
Commonly utilized piezoelectric materials comprise barium titanate (BT), sodium potassium niobate (KNN), zinc oxide (ZnO), polyvinylidene fluoride (PVDF), and poly L-lactic acid (PLLA). Among these, BT is distinguished due to its superior piezoelectric performance and biocompatibility.19 Though not readily absorbable, it is capable of being retained in the body harmlessly for prolonged periods.20 Furthermore, poly L-lactic acid (PLLA) is recognized for its mechanical properties and biodegradability.21 To enhance cartilage regeneration under joint loading, researchers have developed PLLA-collagen scaffolds, thereby introducing a novel therapeutic approach.
Biofactors, such as fibroblast growth factor-18 (FGF-18), play a critical role in the regeneration of cartilage. Being predominantly found in organs including the lungs and kidneys, FGF-18 is essential for promoting the proliferation, differentiation, and maturation of chondrocytes, all of which are vital for maintaining the integrity of cartilage.22–25 In preclinical models, FGF-18 has demonstrated the potential to enhance cartilage repair, particularly in cases of osteoarthritis. This is achieved through the stimulation of key ECM component production and the alleviation of symptoms.26 Consequently, the application of FGF-18 could be significant in cartilage repair promoted by piezoelectricity.
Prior research has primarily focused on either the piezoelectric stimulation for cartilage regeneration11,27–30 or the release of active factors to facilitate cartilage repair.31–34 However, there has been no integration of piezoelectricity with biofactors that would synergize the electrical properties of PLLA and BT with the biological effects of FGF-18. The development of PLLA–BT composite nanofiber membranes through electrostatic spinning, which are designed to produce a high electrical output and simultaneously release FGF-18, presents a novel therapeutic strategy to enhance cartilage regeneration (Scheme 1).
2 Materials and methods
2.1 Materials preparation
A layer of pure collagen solution was applied to the surface of the collagen/FGF-18 composite membrane. It was then laminated with the PLLA/BT piezoelectric nanofiber membrane to form a PLLA/BT/collagen/FGF-18 (PBCF) composite membrane. The composite was finally lyophilized at −80 °C.
2.2 Materials characterization
The mechanical properties of the PBCF composite supports were evaluated using a uniaxial tensile tester (Shandong Wanchen Testing Machine Co., Ltd, China). Samples, each measuring 5.0 × 1.0 cm2 with an approximate thickness of 0.3 mm (n = 3), were subjected to a stretching rate of 5 mm per minute until fracture.35 The Young's modulus and tensile strength were derived precisely from the stress–strain curves. Mechanical properties were recorded for at least three specimens and the results were averaged.
2.3 In vitro studies of PBCF composite scaffolds
Localized electrical signals were induced by subjecting the culture plates to ultrasonic homogenization, with the ultrasonic parameters set to 200 W for 20 minutes daily, divided into two sessions of 10 minutes each in the morning and afternoon. This 200 W setting was chosen based on prior investigations that demonstrated a stable piezoelectric output and enhanced proliferation of chondrocytes at this intensity level.
Further details on chondrocyte extraction methods and experimental setups are provided in our previous study.37
Live/dead assays were executed in accordance with established protocols on 48-well plates. Each set of piezoelectric composite scaffolds was cultured with chondrocytes at a concentration of 1 × 104 cells mL−1 and maintained at 37 °C in an atmosphere of 5.0% CO2 humidity until day 3. The cells were subjected to ultrasonic vibrations for 20 minutes daily. Afterward, the samples were washed three times with PBS and double-stained with calcein acetoxy methylester/propidium iodide for 30 minutes at room temperature, protected from light exposure. The adhesion and cellular activity of the articular chondrocytes on the various materials were subsequently observed using a fluorescence microscope.
2.4 In vivo studies of PBCF composite scaffolds
2.5 Statistical analysis
A one-way analysis of variance (ANOVA) was utilized to compare multiple groups, with the LSD test serving as the post hoc comparison. Quantitative data were presented as mean ± standard deviation (n = 3). P values of < 0.05 (*), < 0.01 (**), and < 0.001 (***) were deemed statistically significant.3 Results and discussion
3.1 Physical characterization of PLLA/BT/collagen/FGF-18 (PBCF) composite scaffolds
The morphology and elemental distribution of the PLLA/BT (PB) samples were analyzed using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Fig. 1a illustrates that the surface of the PLLA/BT nanofibrous membrane was devoid of beads, with the nanofibers being uniform in diameter, continuous, and aligned along the fiber direction. This uniformity suggests that BT nanoparticles (NPs) were evenly dispersed within the fiber matrix, and the arrangement of the crystalline particles was depicted. Energy-dispersive X-ray spectroscopy (EDS) confirmed the presence of BT NPs, showing a uniform distribution of carbon (C), oxygen (O), barium (Ba), and titanium (Ti) within the fiber matrix. Elemental mapping images further verified the uniform distribution of these elements throughout the fiber, as depicted in Fig. 1c. Additionally, SEM images revealed that the PLLA/BT nanofibrous membranes exhibited a porous structure reminiscent of a natural ECM, which is essential for facilitating nutrient transport and waste metabolism.46 As shown in Fig. 1b, a 3D scaffold was engineered by combining the PB membrane with collagen, using hydrolyzed collagen as a binding agent. The PB membrane measured approximately 120 μm in thickness, whereas the collagen hydrogel layer was about 1600 μm thick, forming a tightly bonded, non-detachable composite. The collagen foam scaffolds displayed increased porosity, enhancing the diffusion of nutrients and oxygen. This increased porosity is crucial for promoting inward cell growth and tissue remodeling.47 Therefore, we posit that the composite scaffolds not only provide a piezoelectric physiological environment conducive to cell growth but also support favorable 3D structures that enhance cell proliferation and matrix synthesis.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, while the peaks at 1179 cm−1 and 1127 cm−1 are indicative of the C–O stretching vibrations, confirming the presence of ester groups. Additionally, the characteristic peak at 1383 cm−1 is identified as belonging to a methyl group, which substantiates the structural composition of PLLA. A strong absorption band at 577 cm−1 further corroborates the stretching vibrations of the BT nanoparticles (Ti–O).28 Analysis conducted through Fourier transform infrared spectroscopy (FTIR) indicates that neither the electrostatic spinning process nor the introduction of BT alters the chain conformation or the crystal morphology of the PLLA substrate.
O stretching vibration, while the peaks at 1179 cm−1 and 1127 cm−1 are indicative of the C–O stretching vibrations, confirming the presence of ester groups. Additionally, the characteristic peak at 1383 cm−1 is identified as belonging to a methyl group, which substantiates the structural composition of PLLA. A strong absorption band at 577 cm−1 further corroborates the stretching vibrations of the BT nanoparticles (Ti–O).28 Analysis conducted through Fourier transform infrared spectroscopy (FTIR) indicates that neither the electrostatic spinning process nor the introduction of BT alters the chain conformation or the crystal morphology of the PLLA substrate.
Fig. 2b presents the XRD spectrum of the PBC nanofiber membrane. The diffraction peak of BT nanoparticles, observed as a split peak at around 45°, confirms the tetragonal phase structure of the BT particles, which is known for its excellent piezoelectric properties.48 Additionally, a distinctive peak at 16.2° was detected, indicative of a β-type PLLA phase possessing piezoelectric characteristics.49
The mechanical properties of the PBC nanofiber membrane were evaluated using a mechanical testing machine, which revealed exceptional mechanical strength. As shown in Fig. 2c, the initial fracture of the PBC scaffold, occurring at 19.98 MPa, resulted from collagen failure. The second fracture, recorded at 24.02 MPa, was due to the failure of the PB nanofibrous membrane, marking the maximum load the composite could withstand. The superior mechanical properties of the PBC composites can be ascribed to several factors. Primarily, PLLA, recognized for its piezoelectric and mechanical qualities, enhances the strength of nanofiber membranes aligned along the fiber orientation compared to those with the random orientation.35 This alignment is crucial for cartilage defect repair, which requires resilience against significant pressure and shear forces. Additionally, dehydrated collagen, known for its increased mechanical strength, contributes to this robustness due to tighter molecular packing.50 After freeze-drying, the PBCF scaffolds exhibited increased brittleness and significantly enhanced the mechanical properties, outperforming those of the PB nanofiber membranes, as shown in Fig. S4a (ESI†). Collectively, the PBC scaffolds not only demonstrate excellent mechanical properties but also meet the biomechanical demands of natural cartilage tissues, with physical tensile properties ranging from 0.3 to 10 MPa.51
3.2 In vitro study of PBCF composite scaffolds
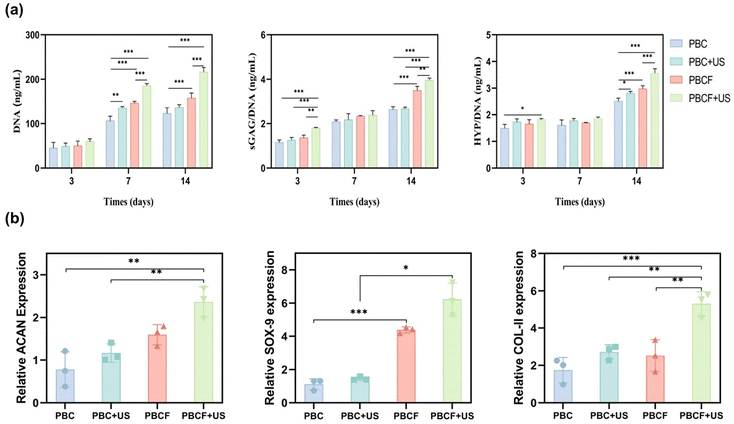
To further explore the cytocompatibility of the materials, live–dead cell staining was utilized to assess the cell viability. Articular chondrocytes were cultured on PBC and PBCF composite scaffolds and subjected to 200 W ultrasonic stimulation for three days. As depicted in Fig. 4c, the live–dead staining experiments showed that articular chondrocytes adhered to and proliferated on both types of scaffolds. Both the PBC and PBCF groups, regardless of ultrasonic stimulation, demonstrated excellent biocompatibility with minimal cell death (indicated by red staining) and preserved the cell morphology and structure. The survival rates were significantly higher in the PBCF and PBCF + US groups compared to the PBC group without piezoelectricity and the PBC + US group. A significantly greater number of proliferating chondrocytes was observed in the PBCF + US group relative to the PBC + US group, suggesting that the release of the FGF-18 promoted chondrocyte proliferation, corroborating the cell counting results described previously. The ratio of live cell area to dead cell area, illustrated in Fig. 4b, showed a lower proportion of dead cells across all groups, with the PBCF + US group exhibiting a notably smaller dead cell area compared to the PBC group.
The chondrocyte growth morphology, characterized by actin (green) and nuclei (blue), was assessed through 4′,6-diamidino-2-phenylindole (DAPI) and F-actin staining in PBC, PBCF, and their respective US stimulation groups. Fig. 4d illustrates that chondrocytes were uniformly dispersed and well-aligned on the scaffold surfaces across all groups, exhibiting a good growth status. Specifically, the actin skeletons were aligned parallel to the nanofiber axes on the surfaces of the PB membranes, and cells appeared elongated, mimicking the orientation of the nanofibers. This alignment was consistent with findings from previous studies,8 which have demonstrated that nanofiber topology significantly influences cell orientation, proliferation, and the augmentation of the cartilage matrix content.59 The favorable condition of all cells across the scaffold groups underscores their excellent biocompatibility and potential as piezoelectric materials for cartilage repair.
The PBCF + US group exhibited a superior generation of the cartilage ECM, a result attributed to multiple factors. Initially, ECM secretion in the PBC group consistently increased over time, illustrating the scaffolds’ effectiveness in facilitating cartilage ECM secretion.60,61 The morphological characteristics of the material, including its chemical composition, wettability, and microstructure, significantly influenced cellular ECM secretion. Surface pores within the fibrous scaffolds were deemed essential for enhancing GAG and HYP formation. By day 14, the PBC + US group demonstrated increased HYP generation relative to the PBC group, signifying enhanced ECM production. US stimulation has been shown to activate genes or receptors that mediate the aggregation of mesenchymal stem cells (MSCs) and cartilage formation, while also promoting chondrogenic differentiation through cascade signaling.62–64 Moreover, by day 14, the PBCF group displayed superior pro-chondrogenic ECM generation compared to the PBC group. Studies by Muller et al.65 reported a significant increase in the chondrocyte count and GAG content following in vitro FGF-18 treatment using bovine chondrocytes. Similarly, Ellsworth et al. confirmed that FGF-18 effectively stimulated chondrocyte proliferation and enhanced GAG expression. Additionally, it has been established that FGF-18 selectively activates fibroblast growth factor receptor 3 (FGFR3) in cartilage and chondrocytes; this activation plays a critical role in the development of cartilage and bone, promoting both chondrocyte proliferation and extracellular matrix deposition in monolayer cell cultures.66
In summary, the superior performance of the PBCF + US group in promoting chondrocyte cytoplasmic matrix deposition can be attributed to the scaffold material's excellent biocompatibility and the dual promotional effects of piezoelectricity combined with the active FGF-18 on chondrocyte proliferation and matrix generation.
In summary, significantly higher levels of ACAN, SOX-9, and COL-II gene expression were exhibited by the PBCF + US group compared to the PBC group. This disparity is attributable to two main factors. Firstly, studies by Gigout et al. demonstrated that FGF-18 in vitro enhances the type II collagen content and the extracellular matrix volume, whereas Shimoaka et al. found that FGF-18 promotes cartilage matrix formation by mitigating chondrocyte differentiation.68,70 These studies suggest a superior ability of FGF-18 to promote chondrogenic matrix formation and chondrocyte proliferation in vitro. Secondly, piezoelectric stimulation has been shown to be crucial in enhancing the expression of cartilage-related genes and ECM synthesis. Electrical stimulation effectively promotes the expression of the bone morphogenetic protein (BMP), initiating cartilage formation and subsequently driving mRNA expression for COL-II, ACAN, and SOX-9.13 McCullen et al.71 demonstrated that piezoelectric stimulation decreases type I collagen levels while increasing the expression of cartilage-forming markers such as COL-II, ACAN, and SOX-9, thus facilitating cartilage matrix formation. Compared with either the FGF-18 alone or the piezoelectric stimulation alone, the synergistic effect of combining FGF-18 with piezoelectric stimulation plays a significant role in promoting chondrocyte proliferation and matrix formation.71
3.3 In vivo study of PBCF composite scaffold-induced cartilage defect repair in rabbits
Materials characterization and in vitro cellular experiments were followed by the use of a rabbit model to assess the reparative effects of PBCF scaffolds on critical-size osteochondral defects. Motor stimulation-induced piezoelectric generation was utilized. Osteochondral defects were surgically created in the femoral condyles of the rabbits using a mill drill. Before the surgical intervention, all rabbits underwent one month of treadmill exercise training to acclimate them to the activity. Thirty-six rabbits were randomly assigned into six groups: defect only, PBC, PBCF, defect + exercise, PBC + exercise, and PBCF + exercise, with three legs per group (n = 3). Following surgery, the rabbits were administered daily antibiotics for three days, with no adverse reactions observed until euthanasia. A postoperative rest period of two weeks was allowed for organic recovery before resuming exercise training. It has been demonstrated in previous studies that exercise training within two weeks post-cartilage injury exacerbates the damage.72 The complete animal experimental procedure is depicted in Fig. 6a.Treadmill training was conducted for 20 minutes per day, five days a week, for durations of either one or two months (Video S1, ESI†). This training regimen was designed to induce joint loading during movement, thereby promoting piezoelectric effects within the scaffold. The chosen duration is supported by prior studies, which suggest that 15 minutes of daily exercise in conjunction with poly(lactic-co-glycolic acid) (PLGA) scaffolds enhances cartilage regeneration.73 Furthermore, Liu et al.11 reported that extended durations of exercise lead to increased fatigue in rabbits.
Fig. 6d presents the International Cartilage Repair Society (ICRS) system scores for specimens at 1 and 2 months, offering a quantitative evaluation of cartilage defect repair across the groups. The PBCF + exercise group consistently recorded significantly higher scores than the control group, the PBC group, and its exercise counterpart at both evaluated time points, indicating enhanced cartilage regeneration. Furthermore, scores at 2 months for the PBCF + exercise group were significantly higher than those at 1 month, demonstrating marked improvement over time, with a statistically significant difference (P < 0.01).
After eight weeks, the cartilage healing observed in the PBC + exercise group was notably superior compared to both the PBCF + exercise and blank groups. The PBCF + exercise group exhibited a smoother, curved, and continuous cartilage surface on MRI, in contrast to the PBCF, PBC, control, and their respective exercise groups. However, the PBC + exercise group displayed a slightly concave surface. According to the Henderson score, the PBCF + exercise group scored significantly lower than the control, PBC, and its exercise group (P < 0.01), indicating improved cartilage regeneration compared to the control group. Remarkably, there was no significant difference between the PBCF + exercise and the PBC + exercise groups, suggesting limitations in the precision of the Henderson scoring system for the detailed assessment of cartilage repair.
The distribution of glycosaminoglycans (GAGs) within the newly formed cartilage tissue was assessed using safranin O-fast green (Saf O-Fg) staining, while toluidine blue staining, known for its affinity for sulfate groups, was employed to detect proteoglycans in the cartilage (Fig. 7b and 8a). At 1 month postoperatively, negligible new cartilage formation was observed in the control, PBC, and PBC + exercise groups, with a lower GAG content compared to the defect center in the PBCF + exercise group. By 2 months postoperatively, the PBCF + exercise group exhibited a significantly narrower boundary with the surrounding normal cartilage, indicative of hyaline cartilage formation. In contrast, the PBC group and its exercise counterpart displayed some new cartilage formation, yet their subchondral bone formation remained inadequate. Meanwhile, both the control group and its exercise counterpart continued to exhibit noticeable cartilage defects. Additionally, immunohistochemical staining for collagen type II (COL-II) was utilized to specifically assess cartilage tissue regeneration, as depicted in Fig. 8b. High positivity for type II collagen staining in the PBCF + exercise group suggested that the defects were rich in type II collagen and displayed a collagen fiber structure similar to that of the natural tissues. In comparison, the other groups showed minimal or no type II collagen production. Slight cartilage improvement was also observed in the PBCF and PBC scaffold groups not subjected to exercise, possibly attributable to the free movement of the rabbits, which may activate piezoelectricity to some extent, corroborating the findings of Liu et al.11
As depicted in Fig. 8c, the histological scores demonstrated significantly superior cartilage defect repair in the PBCF + exercise group compared to the control, PBCF, PBC, and PBC + exercise groups, with notable differences observed at both one and two months (p < 0.05 at 1 month; p < 0.001 at 2 months). Furthermore, histological scores for cartilage repair in the PBCF + exercise group were significantly higher at 2 months than at 1 month (p < 0.001). Although limited subchondral bone and chondrocyte production occurred at the defect sites in the PBC + exercise and PBCF groups, these components exhibited poor integration. The combination of PBCF composite scaffolds with exercise-stimulated synergistic factor release led to smoother defect planes, enhanced regeneration of the hyaline cartilage matrix, increased cell viability, and improved cellular distribution, culminating in enhanced histologic scores.
Liu et al.11 were pioneers in implanting PLLA nanofiber piezoelectric scaffolds into cartilage defects in rabbits, proposing for the first time the combination of biomechanical activity with piezoelectric materials to foster cartilage repair. Their study required a one-month rest period for rabbits post-surgery before initiating running exercises. In contrast, our study found that rabbits could begin running exercises independently after only two weeks of the post-surgical test, based on two primary considerations: firstly, prior research suggests that exercising within two weeks post-surgery may exacerbate cartilage damage;72 secondly, during the initial two weeks, rabbits generally lack the capability to exercise or generate piezoelectricity. Nonetheless, the slow release of FGF-18 in vivo compensates for the absence of piezoelectricity in facilitating cartilage repair during this period. Vinikoor et al.27 further advanced the field by implanting a novel piezoelectric hydrogel into rabbits, opting for direct ultrasound stimulation in vitro to promote cartilage repair. Conversely, our approach leveraged the natural piezoelectricity generated through the biomechanical activities of the rabbits. This decision was influenced by several factors: firstly, ultrasound stimulation is labor-intensive and its effects cannot be standardized accurately; secondly, exercise has been shown to exert an anti-inflammatory effect by creating an anti-inflammatory microenvironment during loading and unloading exercises, whereas a lack of physical activity increases the risk of inflammation.75–77
In conclusion, the activation of critical size defects in osteochondral cartilage by ultrasound led to enhanced subchondral bone formation and significant improvement in the structure of hyaline cartilage, which closely resembled natural cartilage. These studies collectively suggest that the in vivo application of piezoelectric scaffolds for cartilage defect repair, whether through self-motion or ultrasound stimulation, significantly enhances cartilage repair by inducing the piezoelectric stimulation of the scaffolds. Furthermore, in the in vivo repair of articular cartilage defects, the active factor FGF-18 has proven effective in promoting chondrocyte proliferation and in facilitating the production of a clearer and more transparent extracellular matrix. Additionally, FGF-18 influences the metabolism of articular cartilage by enhancing the metabolic activity and promoting the production of type II collagen.78 FGF-18 also contributes to the increased cartilage thickness while minimizing cartilage loss.24 The combination of piezoelectric stimulation with active biological factors synergistically enhances cartilage repair, leading to improved outcomes in the repair of cartilage defects. Moreover, this approach significantly reduces the overall time required for cartilage repair, thereby facilitating more effective and rapid cartilage regeneration.
4 Conclusions
The development of a piezoelectric composite scaffold, composed of polylactic acid (PLLA), barium titanate (BT), collagen, and fibroblast growth factor-18 (FGF-18), has yielded a material with exceptional mechanical and piezoelectric properties. This PLLA/BT/collagen/FGF-18 (PBCF) composite scaffold, characterized by a high mechanical strength of 24 kPa and a piezoelectric output of up to approximately 5 V, demonstrates significant potential for cartilage repair. In vitro studies have confirmed its excellent biocompatibility, which facilitates chondrocyte proliferation, enhances glycosaminoglycan production, and promotes regeneration of the ECM. Additionally, these studies have observed a notable upregulation in the expression of cartilage-specific genes. And, in vivo experiments focusing on the repair of cartilage defects have shown that the PBCF composite scaffold accelerates cartilage tissue repair, especially when combined with exercise-induced stimulation, thereby highlighting its viability as a treatment option for patients with superficial cartilage defects.Author contributions
Bowen Xie: data curation, formal analysis, investigation, methodology, visualization, writing – original draft, and writing – review and editing. Hebin Ma: data curation, formal analysis, investigation, methodology, visualization, writing – original draft, and writing – review and editing. Fengyuan Yang: data curation, formal analysis, investigation, methodology, visualization, writing – original draft, and writing – review and editing. Hongguang Chen: data curation, formal analysis, investigation, methodology, visualization, writing – original draft, and writing – review and editing. Ya’nan Guo: methodology, supervision, validation, and writing – review and editing. Hongxing Zhang: methodology, supervision, validation, and writing – review and editing. Tengfei Li: formal analysis, validation, and writing – review and editing. Xiaogang Huang: formal analysis, validation, and writing – review and editing. Yantao Zhao: conceptualization, funding acquisition, methodology, project administration, supervision, and writing – review and editing. Xiaojie Li: conceptualization, funding acquisition, methodology, project administration, supervision, and writing – review and editing. Junjie Du: conceptualization, funding acquisition, methodology, project administration, supervision, and writing – review and editing. All authors read and approved the final manuscript.Data availability
The data that support the findings of this study are available upon request from the corresponding author, [Junjie Du].Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (82072451), the Research and Translational Application of Clinical Characteristic Diagnosis and Treatment Techniques in the Capital (Z221100007422014), the Natural Science Foundation of Beijing (7202199), the Research Projects of Army Logistics Priority (BKJ20J004), and the Top Young Talent Program (22BJQN006).Notes and references
- A. J. Sophia Fox, A. Bedi and S. A. Rodeo, Sports Health, 2009, 1, 461–468 CrossRef PubMed.
- J. A. Buckwalter and H. J. Mankin, Arthritis Rheum., 1998, 41, 1331–1342 CrossRef CAS.
- S. L. Vega, M. Y. Kwon and J. A. Burdick, Eur. Cells Mater., 2017, 33, 59–75 CrossRef CAS PubMed.
- B. R. da Costa, T. V. Pereira, P. Saadat, M. Rudnicki, S. M. Iskander, N. S. Bodmer, P. Bobos, L. Gao, H. D. Kiyomoto, T. Montezuma, M. O. Almeida, P. S. Cheng, C. A. Hincapié, R. Hari, A. J. Sutton, P. Tugwell, G. A. Hawker and P. Jüni, BMJ, 2021, 375, n2321 CrossRef PubMed.
- E. B. Hunziker, K. Lippuner, M. J. Keel and N. Shintani, Osteoarthritis Cartilage, 2015, 23, 334–350 CrossRef CAS PubMed.
- R. S. Tuan, A. F. Chen and B. A. Klatt, J. Am. Acad. Orthop. Surg., 2013, 21, 303–311 CrossRef PubMed.
- D. Kai, M. P. Prabhakaran, G. Jin and S. Ramakrishna, J. Biomed. Mater. Res., Part B, 2011, 98, 379–386 CrossRef PubMed.
- B. Xie, F. Yang, H. Chen, H. Zhang, H. Ma, T. Li, Z. Chen, J. Li, X. Li and J. Du, Front. Mater., 2023, 10 Search PubMed.
- L. Massari, F. Benazzo, M. De Mattei, S. Setti and M. Fini, J. Bone Jt. Surg., Am., 2007, 89(3), 152–161 Search PubMed.
- B. Hiemer, M. Krogull, T. Bender, J. Ziebart, S. Krueger, R. Bader and A. Jonitz-Heincke, Mol. Med. Rep., 2018, 18, 2133–2141 CAS.
- Y. Liu, G. Dzidotor, T. T. Le, T. Vinikoor, K. Morgan, E. J. Curry, R. Das, A. McClinton, E. Eisenberg, L. N. Apuzzo, K. T. M. Tran, P. Prasad, T. J. Flanagan, S. W. Lee, H. M. Kan, M. T. Chorsi, K. W. H. Lo, C. T. Laurencin and T. D. Nguyen, Sci. Transl. Med., 2022, 14, eabi7282 CrossRef CAS PubMed.
- S. D. Cook, S. L. Salkeld, L. S. Popich-Patron, J. P. Ryaby, D. G. Jones and R. L. Barrack, Clin. Orthop. Relat. Res., 2001, S231–S243, DOI:10.1097/00003086-200110001-00022.
- H. J. Kwon, G. S. Lee and H. Chun, Sci. Rep., 2016, 6, 39302 CrossRef CAS PubMed.
- Z. Liu, X. Wan, Z. L. Wang and L. Li, Adv. Mater., 2021, 33, e2007429 CrossRef PubMed.
- C. T. Brighton, W. Wang, R. Seldes, G. Zhang and S. R. Pollack, J. Bone Jt. Surg., Am., 2001, 83, 1514–1523 CrossRef CAS PubMed.
- R. K. Aaron and D. M. Ciombor, J. Cell. Biochem., 1993, 52, 42–46 CrossRef CAS PubMed.
- L. P. da Silva, S. C. Kundu, R. L. Reis and V. M. Correlo, Trends Biotechnol., 2020, 38, 24–49 CrossRef CAS PubMed.
- R. Nuccitelli, Radiat. Prot. Dosim., 2003, 106, 375–383 CrossRef CAS PubMed.
- T. Zheng, H. Wu, Y. Yuan, X. Lv, Q. Li, T.-L. Men, C. Zhao, D.-Q. Xiao, J. Wu, K. Wang, J. Li, Y. Gu, J. Zhu and S. J. Pennycook, Energy Environ. Sci., 2017, 10, 528–537 RSC.
- Y. Tang, C. Wu, Z. Wu, L. Hu, W. Zhang and K. Zhao, Sci. Rep., 2017, 7, 43360 CrossRef PubMed.
- X. Dai, X. Yao, W. Zhang, H. Cui, Y. Ren, J. Deng and X. Zhang, Int. J. Nanomed., 2022, 17, 4339–4353 CrossRef PubMed.
- D. Davidson, A. Blanc, D. Filion, H. Wang, P. Plut, G. Pfeffer, M. D. Buschmann and J. E. Henderson, J. Biol. Chem., 2005, 280, 20509–20515 CrossRef CAS PubMed.
- Y. Wang, T. Yang, Y. Liu, W. Zhao, Z. Zhang, M. Lu and W. Zhang, Int. J. Mol. Sci., 2017, 18 Search PubMed.
- J. L. Ellsworth, J. Berry, T. Bukowski, J. Claus, A. Feldhaus, S. Holderman, M. S. Holdren, K. D. Lum, E. E. Moore, F. Raymond, H. Ren, P. Shea, C. Sprecher, H. Storey, D. L. Thompson, K. Waggie, L. Yao, R. J. Fernandes, D. R. Eyre and S. D. Hughes, Osteoarthritis Cartilage, 2002, 10, 308–320 CrossRef CAS PubMed.
- Y. R. Yun, J. E. Won, E. Jeon, S. Lee, W. Kang, H. Jo, J. H. Jang, U. S. Shin and H. W. Kim, J. Tissue Eng., 2010, 2010, 218142 CrossRef PubMed.
- E. E. Moore, A. M. Bendele, D. L. Thompson, A. Littau, K. S. Waggie, B. Reardon and J. L. Ellsworth, Osteoarthritis Cartilage, 2005, 13, 623–631 CrossRef CAS PubMed.
- T. Vinikoor, G. K. Dzidotor, T. T. Le, Y. Liu, H. M. Kan, S. Barui, M. T. Chorsi, E. J. Curry, E. Reinhardt, H. Wang, P. Singh, M. A. Merriman, E. D'Orio, J. Park, S. Xiao, J. H. Chapman, F. Lin, C. S. Truong, S. Prasadh, L. Chuba, S. Killoh, S. W. Lee, Q. Wu, R. M. Chidambaram, K. W. H. Lo, C. T. Laurencin and T. D. Nguyen, Nat. Commun., 2023, 14, 6257 CrossRef CAS PubMed.
- N. Peidavosi, M. Azami, N. Beheshtizadeh and A. Ramazani Saadatabadi, Sci. Rep., 2022, 12, 20828 CrossRef PubMed.
- N. More, A. Srivastava and G. Kapusetti, ACS Appl. Bio Mater., 2020, 3, 6823–6835 CrossRef CAS PubMed.
- F. Barbosa, F. C. Ferreira and J. C. Silva, Int. J. Mol. Sci., 2022, 23 Search PubMed.
- J. M. Patel, K. S. Saleh, J. A. Burdick and R. L. Mauck, Acta Biomater., 2019, 93, 222–238 CrossRef CAS PubMed.
- Z. H. Deng, Y. S. Li, X. Gao, G. H. Lei and J. Huard, Osteoarthritis Cartilage, 2018, 26, 1153–1161 CrossRef CAS PubMed.
- M. A. Szychlinska, U. D'Amora, S. Ravalli, L. Ambrosio, M. Di Rosa and G. Musumeci, Curr. Pharm. Biotechnol., 2019, 20, 32–46 CAS.
- L. A. Fortier, J. U. Barker, E. J. Strauss, T. M. McCarrel and B. J. Cole, Clin. Orthop. Relat. Res., 2011, 469, 2706–2715 CrossRef PubMed.
- Y. Yang, X. Yin, H. Wang, W. Qiu, L. Li, F. Li, Y. Shan, Z. Zhao, Z. Li, J. Guo, J. Zhang and Y. Zhao, Nano Energy, 2023, 107, 108145 CrossRef CAS.
- C. E. Kilmer, C. M. Battistoni, A. Cox, G. J. Breur, A. Panitch and J. C. Liu, ACS Biomater. Sci. Eng., 2020, 6, 3464–3476 CrossRef CAS PubMed.
- B. Xie, F. Yang, H. Chen, H. Zhang, H. Ma, T. Li, Z. Chen, J. Li, X. Li and J. Du, Front. Mater., 2023, 10, 1292098 CrossRef.
- Y. Li, X. Dai, Y. Bai, Y. Liu, Y. Wang, O. Liu, F. Yan, Z. Tang, X. Zhang and X. Deng, Int. J. Nanomed., 2017, 12, 4007–4018 CrossRef CAS PubMed.
- K. Imamura, K. Tachi, T. Takayama, R. Shohara, H. Kasai, J. Dai and S. Yamano, J. Biomater. Appl., 2018, 32, 1382–1391 CrossRef CAS PubMed.
- K. L. Riedler, A. Shokrani, A. Markarian, L. M. Fisher and J. P. Pepper, Laryngoscope, 2017, 127, E399–e407 CrossRef CAS PubMed.
- T. M. Campbell, K. Reilly, O. Laneuville, H. Uhthoff and G. Trudel, Bone, 2018, 106, 42–51 CrossRef CAS PubMed.
- M. P. van den Borne, N. J. Raijmakers, J. Vanlauwe, J. Victor, S. N. de Jong, J. Bellemans and D. B. Saris, Osteoarthritis Cartilage, 2007, 15, 1397–1402 CrossRef CAS PubMed.
- P. Mainil-Varlet, T. Aigner, M. Brittberg, P. Bullough, A. Hollander, E. Hunziker, R. Kandel, S. Nehrer, K. Pritzker, S. Roberts and E. Stauffer, J. Bone Jt. Surg., Am., 2003, 85(2), 45–57 CrossRef.
- J. S. Wayne, C. L. McDowell, K. J. Shields and R. S. Tuan, Tissue Eng., 2005, 11, 953–963 CrossRef CAS PubMed.
- E. J. Curry, T. T. Le, R. Das, K. Ke, E. M. Santorella, D. Paul, M. T. Chorsi, K. T. M. Tran, J. Baroody, E. R. Borges, B. Ko, A. Golabchi, X. Xin, D. Rowe, L. Yue, J. Feng, M. D. Morales-Acosta, Q. Wu, I. P. Chen, X. T. Cui, J. Pachter and T. D. Nguyen, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 214–220 CrossRef CAS PubMed.
- Z. Li, P. Liu, T. Yang, Y. Sun, Q. You, J. Li, Z. Wang and B. Han, J. Biomater. Appl., 2016, 30, 1552–1565 CrossRef CAS PubMed.
- S. J. Hollister, Nat. Mater., 2005, 4, 518–524 CrossRef CAS PubMed.
- C. Shuai, G. Liu, Y. Yang, W. Yang, C. He, G. Wang, Z. Liu, F. Qi and S. Peng, Colloids Surf., B, 2020, 185, 110587 CrossRef CAS PubMed.
- E. J. Curry, K. Ke, M. T. Chorsi, K. S. Wrobel, A. N. Miller, 3rd, A. Patel, I. Kim, J. Feng, L. Yue, Q. Wu, C. L. Kuo, K. W. Lo, C. T. Laurencin, H. Ilies, P. K. Purohit and T. D. Nguyen, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 909–914 CrossRef CAS PubMed.
- A. Gautieri, S. Vesentini, A. Redaelli and M. J. Buehler, Nano Lett., 2011, 11, 757–766 CrossRef CAS PubMed.
- A. Ode, G. N. Duda, S. Geissler, S. Pauly, J. E. Ode, C. Perka and P. Strube, PLoS One, 2014, 9, e106462 CrossRef PubMed.
- D. Khare, B. Basu and A. K. Dubey, Biomaterials, 2020, 258, 120280 CAS.
- S. M. Damaraju, Y. Shen, E. Elele, B. Khusid, A. Eshghinejad, J. Li, M. Jaffe and T. L. Arinzeh, Biomaterials, 2017, 149, 51–62 CrossRef CAS PubMed.
- A. Wang, Z. Liu, M. Hu, C. Wang, X. Zhang, B. Shi, Y. Fan, Y. Cui, Z. Li and K. Ren, Nano Energy, 2018, 43, 63–71 CrossRef CAS.
- L. Vannozzi, L. Ricotti, C. Filippeschi, S. Sartini, V. Coviello, V. Piazza, P. Pingue, C. La Motta, P. Dario and A. Menciassi, Int. J. Nanomed., 2016, 11, 69–91 CAS.
- Y. Mori, T. Saito, S. H. Chang, H. Kobayashi, C. H. Ladel, H. Guehring, U.-I. Chung and H. Kawaguchi, J. Biol. Chem., 2014, 289, 10192–10200 CrossRef CAS PubMed.
- T. Shimoaka, T. Ogasawara, A. Yonamine, D. Chikazu, H. Kawano, K. Nakamura, N. Itoh and H. Kawaguchi, J. Biol. Chem., 2002, 277, 7493–7500 CrossRef CAS PubMed.
- Z. Zhou, J. Zheng, X. Meng and F. Wang, Int. J. Mol. Sci., 2023, 24 Search PubMed.
- Y. Zhang, F. Yang, K. Liu, H. Shen, Y. Zhu, W. Zhang, W. Liu, S. Wang, Y. Cao and G. Zhou, Biomaterials, 2012, 33, 2926–2935 CrossRef CAS PubMed.
- J. B. Jonnalagadda, I. V. Rivero and J. S. Dertien, J. Biomater. Sci., Polym. Ed., 2015, 26, 401–419 CrossRef CAS PubMed.
- C. Y. Ko, K. L. Ku, S. R. Yang, T. Y. Lin, S. Peng, Y. S. Peng, M. H. Cheng and I. M. Chu, J. Tissue Eng. Regener. Med., 2016, 10, E485–e496 CrossRef CAS PubMed.
- D. Schumann, R. Kujat, J. Zellner, M. K. Angele, M. Nerlich, E. Mayr and P. Angele, Biorheology, 2006, 43, 431–443 CAS.
- P. Xia, Y. Shi, X. Wang and X. Li, Stem Cell Res. Ther., 2022, 13, 214 CrossRef PubMed.
- C. H. Lai, S. C. Chen, L. H. Chiu, C. B. Yang, Y. H. Tsai, C. S. Zuo, W. H. Chang and W. F. Lai, Ultrasound Med. Biol., 2010, 36, 1022–1033 CrossRef PubMed.
- S. Müller, S. Lindemann and A. Gigout, J. Orthop. Res., 2020, 38, 653–662 CrossRef PubMed.
- D. H. Sohn, L. M. Lottman, L. Y. Lum, S. G. Kim, R. A. Pedowitz, R. D. Coutts and R. L. Sah, Clin. Orthop. Relat. Res., 2002, 254–262, DOI:10.1097/00003086-200201000-00030.
- E. M. Darling and K. A. Athanasiou, J. Orthop. Res., 2005, 23, 425–432 CrossRef CAS PubMed.
- A. Gigout, H. Guehring, D. Froemel, A. Meurer, C. Ladel, D. Reker, A. C. Bay-Jensen, M. A. Karsdal and S. Lindemann, Osteoarthritis Cartilage, 2017, 25, 1858–1867 CrossRef CAS PubMed.
- J. Jacob, N. More, C. Mounika, P. Gondaliya, K. Kalia and G. Kapusetti, ACS Appl. Bio Mater., 2019, 2, 4922–4931 CrossRef CAS PubMed.
- T. Shimoaka, T. Ogasawara, A. Yonamine, D. Chikazu, H. Kawano, K. Nakamura, N. Itoh and H. Kawaguchi, J. Biol. Chem., 2002, 277, 7493–7500 CrossRef CAS PubMed.
- S. D. McCullen, J. P. McQuilling, R. M. Grossfeld, J. L. Lubischer, L. I. Clarke and E. G. Loboa, Tissue Eng., Part C, 2010, 16, 1377–1386 CrossRef CAS PubMed.
- N. J. Chang, M. Y. Shie, K. W. Lee, P. H. Chou, C. C. Lin and C. J. Chu, Int. J. Mol. Sci., 2017, 18 Search PubMed.
- N. J. Chang, C. C. Lin, M. Y. Shie, M. L. Yeh, C. F. Li, P. I. Liang, K. W. Lee, P. H. Shen and C. J. Chu, Acta Biomater., 2015, 28, 128–137 CrossRef CAS PubMed.
- T. G. Morgan, A. D. Rowan, S. C. Dickinson, D. Jones, A. P. Hollander, D. Deehan and T. E. Cawston, Ann. Rheum. Dis., 2006, 65, 184–190 CrossRef CAS PubMed.
- D. M. Knapik, J. D. Harris, G. Pangrazzi, M. J. Griesser, R. A. Siston, S. Agarwal and D. C. Flanigan, Arthroscopy, 2013, 29, 1722–1731 CrossRef PubMed.
- N. J. Chang, C. C. Lin, C. F. Li, D. A. Wang, N. Issariyaku and M. L. Yeh, Biomaterials, 2012, 33, 3153–3163 CrossRef CAS PubMed.
- M. Kapoor, J. Martel-Pelletier, D. Lajeunesse, J. P. Pelletier and H. Fahmi, Nat. Rev. Rheumatol., 2011, 7, 33–42 CrossRef CAS PubMed.
- D. Reker, C. F. Kjelgaard-Petersen, A. S. Siebuhr, M. Michaelis, A. Gigout, M. A. Karsdal, C. Ladel and A. C. Bay-Jensen, J. Transl. Med., 2017, 15, 250 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01319k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |