Open Access Article
Open Access ArticleSodium lanthanide tungstate-based nanoparticles as bimodal contrast agents for in vivo high-field MRI and CT imaging†
Elisabet
Gómez-González‡
 *a,
Carlos
Caro‡
*a,
Carlos
Caro‡
 bc,
Nuria O.
Núñez
bc,
Nuria O.
Núñez
 a,
Daniel
González-Mancebo
a,
Jesús D.
Urbano-Gámez
bc,
Maria L.
García-Martín
a,
Daniel
González-Mancebo
a,
Jesús D.
Urbano-Gámez
bc,
Maria L.
García-Martín
 bcd and
Manuel
Ocaña
bcd and
Manuel
Ocaña
 *a
*a
aInstituto de Ciencia de Materiales de Sevilla (CSIC-US), c/Américo Vespucio, 49, 41092 Sevilla, Spain. E-mail: elisabet.gomez@icmse.csic.es; mjurado@icmse.csic.es
bBiomedical Magnetic Resonance Laboratory-BMRL, Andalusian Public Foundation Progress and Health-FPS, Seville, Spain
cInstituto de Investigación Biomédica de Málaga y Plataforma en Nanomedicina–IBIMA Plataforma Bionand, C/Severo Ochoa, 35, 29590 Malaga, Spain
dBiomedical Research Networking Center in Bioengineering, Biomaterials & Nanomedicine (CIBER-BBN), 28029 Madrid, Spain
First published on 4th September 2024
Abstract
Research on high-field magnetic resonance imaging (HF-MRI) has been increased in recent years, aiming to improve diagnosis accuracy by increasing the signal-to-noise ratio and hence image quality. Conventional contrast agents (CAs) have important limitations for HF-MRI, with the consequent need for the development of new CAs. Among them, the most promising alternatives are those based on Dy3+ or Ho3+ compounds. Notably, the high atomic number of lanthanide cations would bestow a high capability for X-ray attenuation to such Dy or Ho-based compounds, which would also allow them to be employed as CAs for X-ray computed tomography (CT). In this work, we have prepared uniform NaDy(WO4)2 and NaHo(WO4)2 nanoparticles (NPs), which were dispersible under conditions that mimic the physiological media and were nontoxic for cells, meeting the main requirements for their use in vivo. Both NPs exhibited satisfactory magnetic relaxivities at 9.4 T, thus making them a promising alternative to clinical CAs for HF-MRI. Furthermore, after their intravenous administration in tumor-bearing mice, both NPs exhibited significant accumulation inside the tumor at 24 h, attributable to passive targeting by the enhanced permeability and retention (EPR) effect. Therefore, our NPs are suitable for the detection of tumors through HF-MRI. Finally, NaDy(WO4)2 NPs showed a superior X-ray attenuation capability than iohexol (commercial CT CA), which, along with their high r2 value, makes them suitable as the dual-probe for both HF-MRI and CT imaging, as demonstrated by in vivo experiments conducted using healthy mice.
1. Introduction
Imaging-based clinical diagnosis is pivotal in patient care.1 Among the various imaging modalities available, magnetic resonance imaging (MRI) and computed tomography (CT) stand out for their ability to provide high-quality 3D anatomical and functional images, serving as invaluable tools for the non-invasive diagnosis of a large variety of diseases, being particularly relevant to their role in the battle against cancer through early diagnosis.2The contrast in MRI images is due to the differences in the longitudinal (T1) and transverse (T2) relaxation times of the protons present in different tissues as well as in the proton content. T1 shortening typically produces bright regions, while T2 shortening typically produces dark regions. In some cases, contrast agents (CAs) that shorten the longitudinal (T1) and transverse (T2) relaxation times of the protons3,4 are required to increase the contrast. The capability of a CA to shorten T1 and T2 is known as longitudinal (r1) and transverse (r2) relaxivities, respectively, which can be obtained as the slope of the line resulting from the plot of 1/T1,2vs. CA concentration.
Although the conventional MRI technique is very well established in clinics, high-field MRI (HF-MRI) is being increasingly used in the field of preclinical imaging to improve the quality of the images since, as the magnetic field becomes stronger, the signal-to-noise ratio increases, leading to a higher resolution required to image small animals with sufficient details.5 Additionally, high-field MRI systems are already a reality in clinical practice, with the approval of the first 7 T system by the Food and Drug Administration (FDA) in 2017.6 Thus, while clinical scanners using 1.5 and 3 T magnets may achieve image resolutions of up to 1 mm, those using 7 T magnets have been shown to achieve resolutions as fine as 0.5 mm.5,7 Another important advantage of using high fields is the decrease in image acquisition times.8,9
Unfortunately, most of the T1-CAs, based on Gd3+ complexes or Gd-containing nanoparticles (NPs), and T2-CAs, consisting of or superparamagnetic iron oxides (SPIONS), used for conventional MRI are less suitable for HF-MRI. In the first case, this is due to the decrease of the value of r1 of Gd3+-based CAs with the increasing applied magnetic field as a result of increasing electron spin longitudinal relaxation times with increasing field strength.10,11 In the case of T2-CAs, in agreement with the quantum mechanical theory of the outer sphere,12–14 the value of r2 should increase as increasing magnetization, and, hence, as the increasing magnetic field. For SPIONS, a saturation of their magnetization takes place at a low field (1 T), which is an important drawback for the use of these CAs in HF-MRI.15,16 Therefore, the development of new CAs for HF-MRI is required; the best candidates being those based on lanthanide (Ln3+) ions other than Gd3+, such as Dy3+ and Ho3+. These cations have short electronic relaxation times and the highest magnetic moments among all Ln3+ cations that prevent their magnetization from saturating as the magnetic field increases.17 It is noteworthy that, because of the high atomic number of Ln3+ cations, the Dy or Ho-based MRI CAs should show a high capability for X-ray attenuation,18 which would also allow them to be employed as CAs for X-ray computed tomography (CT).19 The interest in such multifunctional probes has increased during the last few years because the combined use of more than one imaging technique is very convenient to obtain a more reliable diagnosis.20
Several reports can be found in the literature regarding Dy3+ or Ho3+ containing NPs for bimodal HF-MRI and CT imaging, most of them based on fluoride matrices,21–24 which tend to dissolve in aqueous media,25,26 leading to the release of fluoride ions that are potentially toxic,27 although this drawback can be mitigated by surface functionalization.28 As an alternative to fluorides, Dy3+ NPs based on other matrices, such as oxide,29,30 phosphate,31,32 vanadate,33 or molybdate,34 have been proposed. However, the potentiality of double sodium-dysprosium or holmium tungstate (NaDy(WO4)2 and NaHo(WO4)2) NPs as CAs for HF-MRI has not yet been addressed, despite the high atomic number of tungsten when compared with fluorine, vanadium, or phosphor, which would confer a higher X-ray attenuation capability to these probes.
Finally, it is widely accepted that, for in vivo applications, the NPs must be uniform with a size of ≤100 nm.35 In addition, they must be colloidally stable in physiological media36 and, obviously, must be biocompatible.37 The later requirements are affected by several factors, such as the size, shape, and composition of the NPs,38 which must be adjusted to optimize the performance of CAs.
It should also be noticed that intravenously administered nanoparticles preferentially accumulate in tumors due to their intrinsic physiology,39 a phenomenon known as the enhanced permeability and retention (EPR) effect.40 Extensively studied in nanotechnology, the EPR effect is widely regarded as the most efficient mechanism for targeting nanoparticles to solid tumors,41,42 despite some existing controversies.43,44
In this work, we have developed a procedure for the synthesis of uniform and well-dispersed sodium dysprosium tungstate (NaDy(WO4)2) NPs with a quasi-spherical shape and more anisometric sodium holmium tungstate (NaHo(WO4)2) NPs, and both of them functionalized with polyacrylic acid (PAA). The colloidal behavior in several physiological media and the in vitro cytotoxicity of such NPs have been evaluated to assess their suitability for in vivo applications. Finally, the magnetic relaxivity at high field (9.4 T) and the X-ray attenuation properties of the developed CAs were comparatively analyzed, and their in vivo behavior (pharmacokinetic and biodistribution) after intravenous injection in tumor-bearing mice was evaluated by using HF-MRI and CT to explore their potentiality as dual contrast agents for both bioimaging techniques.
2. Experimental section
2.1. Reagents
Dysprosium nitrate pentahydrate (Dy(NO3)3·5H2O, Sigma Aldrich, 99.99%), holmium nitrate pentahydrate (Ho(NO3)3·5H2O, Sigma Aldrich, 99.99%), sodium tungstate dihydrate (Na2WO4, Sigma Aldrich, ≥99%), ethylene glycol (EG, Sigma Aldrich, 99.8%), and polyacrylic acid (PAA, Sigma Aldrich, Mw 1800) were used as received. A phosphate-buffered saline (PBS) solution (137 mM NaCl, 2.7 mM KCl and 10 mM phosphate, with pH = 7.4) was prepared by dissolving a PBS tablet (Sigma-Aldrich) in 200 mL of Milli-Q water. 2-(N-Morpholino)ethanesulfonic acid (MES) solution was prepared following the next protocol: 1.06 g of MES were dissolved in Milli-Q water, to obtain a 50 mM buffer, pH 6.5. Saline solution was used as received.2.2. Nanoparticle synthesis
For purification, the resulting dispersions were cooled down and centrifuged to remove the supernatants. The NPs were then washed twice with ethanol and once with double distilled water.
Finally, the particles were stored dispersed in double-distilled water or dried at 50 °C, when required.
2.3. Characterization techniques
A JEOL 2100 Plus (200 kV) transmission electron microscope (TEM) was employed to obtain micrographs of the NPs, from which size distribution histograms were obtained by counting about one hundred of particles on the TEM micrographs, using the free software ImageJ.A Panalytical, X’Pert Pro diffractometer (CuKα) with an X-Celerator detector was used to record the X-ray diffraction (XRD) patterns (10° < 2θ < 90°, a 2θ step width of 0.03°, and 10 s counting time).
The infrared spectra (FTIR) of the NPs dispersed in KBr pellets were recorded with a Jasco FT/IR-6200 Fourier transform spectrometer. A TA Instruments-TGA apparatus (SDT Q600) was employed to conduct the thermogravimetric analysis (TGA) in an air atmosphere at a heating rate of 10 °C min−1.
The hydrodynamic diameter of the NPs in water, MES, PBS or saline solution dispersions (NPs content = 0.5 mg mL−1) was evaluated by dynamic light scattering (DLS) using a Malvern Zetasizer Nano-ZS90 apparatus.
The transverse relaxation rate (R2 = 1/T2) was measured for aqueous suspensions of NPs with different concentrations of Ln3+ (0.062 to 0.5 mM) using a Bruker Biospec MRI system at 9.4 T, employing a Carl–Purcell–Meiboom–Gill (CPMG) imaging sequence. The transverse relaxivity, r2, values were determined from the slope of the linear fit of the transverse relaxation rate (R2 = 1/T2) vs. Ln3+ concentration (mM).
The measurements of the magnetization behavior were performed at 300 K using a vibrating sample magnetometer (8600 Series VSM, Lake Shore Cryotronics, USA) and magnetic fields ranging from −19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 19
000 to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe, with a sample concentration of 1 mg mL−1 of Ln3+.
000 Oe, with a sample concentration of 1 mg mL−1 of Ln3+.
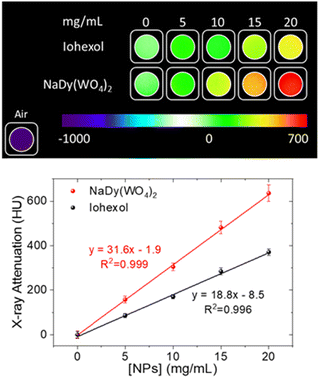
X-ray attenuation of aqueous NP dispersions or iohexol solutions having different CA concentrations (0, 5, 10, 15, and 20 mg mL−1, total volume = 1.0 mL) were measured with a Zeiss Xradia 610 Versa 3D X-ray microscope (XRM) with 0.4× objective lens without any filter, achieving a pixel size of 213 μm. The fixed acquisition parameters were 123 μA current, 70 kVp voltage and 0.1 s of exposure time. The software called Reconstructor Scout and Scan 16.1.13.038 was utilized to reconstruct the final images from 801 projections. Finally, the resulting images were analyzed with ImageJ (a free-license software) using a spherical volume of 0.5 cm in radius. For image calibration, the intensity value of water X-ray attenuation was considered to be 0 Hounsfield units (HU), and the air attenuation was −1000 HU.
The NP concentration in all prepared dispersions was determined by ICP analyses performed using an iCAP 7200 ICP-OES Duo (ThermoFisher Scientific) equipment. The NPs were previously digested in concentrated hydrochloric acid at room temperature overnight.
2.4. Cell experiments
2.5. In vivo experiments
2.6. Statistical analysis
The statistical analysis was performed using the Jamovi software 2.3.21. The Mann–Whitney U test was selected for cell viability, in vivo T2, and ICP values, which are displayed as mean ± standard deviation (SD).2.7. Histology
After in vivo experiments, animals were euthanized, and tissue samples from the liver and tumor were harvested. These samples were processed and stained with hematoxylin and eosin (H&E) before their histological examination by light microscopy. Detailed protocols can be found in the ESI.†3. Results and discussion
3.1. Nanoparticle synthesis and characterization
The NPs obtained for the Dy3+-based system showed an almost equiaxed shape (Fig. 1a) and a mean size of 17 nm with a rather narrow size distribution (standard deviation, σ = 4) (Fig. 1b), whereas those corresponding to the Ho-based system were more anisometric (Fig. 1c) and slightly smaller (19 nm (σ = 4) × 8 nm (σ = 1)) (Fig. 1d). These morphological differences may be explained based on the differences in the precursor concentration used for the synthesis of both systems and by the probable small different reactivity of both lanthanide cations, since, according to the well-known LaMer and Dinegar model,49 these factors determine the precipitation kinetics and hence the particle size and shape. | ||
| Fig. 1 TEM micrographs and particle size histograms of the dysprosium (a) and (b) and holmium (c) and (d) tungstate-based systems. | ||
According to XRD, both types of NPs (Fig. 2a) crystallized into the tetragonal structure since their corresponding pattern was very similar, exhibiting a set of reflections compatible with the PDF file for tetragonal NaDy(WO4)2 (PDF: 96-222-4801). The FTIR spectra of these samples (Fig. 2b) were also very similar and displayed the lattice vibrations modes (<1000 cm−1) expected for the tetragonal NaLn(WO4)2 phases,50 along with two bands at 3400 and 1625 cm−1, respectively, due to adsorbed water. More interestingly, some absorptions between 1400 and 1550 cm−1 were also observed, which correspond to the symmetric and asymmetric stretching vibrational modes of the PAA carboxylate anions, respectively,51 indicating the incorporation of PAA molecules onto the NP surface. The amount of such molecules was quantified from the TG curve (Fig. 2c) obtained for these NPs, which showed a first weight loss (3.5% for NaDy(WO4)2 and 2.5% for NaHo(WO4)2) below 300 °C, due to the release of adsorbed water, and a second one from 300 to 600 °C of ∼4.5% for both samples that can be related to the PAA decomposition.
3.2. Dispersibility in physiological media
The hydrodynamic diameter (HD) in aqueous suspensions obtained by DLS for both systems (Fig. 3) was similar and was kept within the nanometer size range (60 nm for NaDy(WO4)2 NPs and 57 nm for NaHo(WO4)2 NPs). This finding indicates that the NPs were not significantly aggregated in water. More interestingly, also in both cases, the HDs for the NPs dispersed in different media simulating physiological conditions, such as MES, PBS, and saline solution, were below 100 nm with PDI values of <0.2 in all cases, which correspond to moderately monodispersed particles. This finding indicates that our NPs meet the dispersibility requirement for their in vivo applications. It should be noticed that the HD in PBS of NPs synthesized using a similar procedure but in the absence of PAA was much higher (>700 nm) (Fig. S1, ESI†), which revealed the important role of PAA functionalization on the colloidal stability of our NPs required for in vivo applications. | ||
| Fig. 3 DLS curves obtained for the NaDy(WO4)2 (a) and NaHo(WO4)2 (b) NPs dispersed in water, MES, PBS and saline solution. | ||
3.3. Cell viability
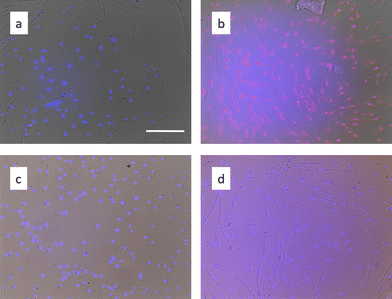
The cell viability analyses were performed using the HFF-1 cell line as a working model, aiming to evaluate the potential cytotoxicity of the NaDy(WO4)2 and NaHo(WO4)2 NPs. This study relied on two procedures: (i) the live–dead assay, which provides information about changes in the cell morphology and the induction of necrotic and apoptotic processes, and (ii) the MTT assay, which assesses the mitochondrial activity of the cells.By merging bright field microscopy images with fluorescence images of DAPI (blue, live cells) and TO-PRO-3 (red, dead cells) staining (Fig. 4a–d), it was observed that when the cells were exposed to NP concentrations of up to 100 μg mL−1, referred to Dy3+ (Fig. 4c) or Ho3+ cations (Fig. 4d), no remarkable morphological changes with respect to death negative control were detected (Fig. 4a). We also found that there was no statistically significant (p < 0.05) decrease for any of the assayed concentrations (≤100 μg mL−1) when counting the total number of cells per well for both the NaDy(WO4)2 (Fig. 5a) and the NaHo(WO4)2 samples (Fig. 5d). This behavior is indicative of the absence of cell necrosis. On the other hand, no statistically significant differences (p < 0.05) were observed between the percentage of dead cells when exposed to the Ln concentrations up to ≤100 μg mL−1 and that corresponding to the negative control, indicating the absence of apoptosis for both samples (Fig. 5b and e).
Finally, the MTT assay showed no statistically significant effect on mitochondrial activity for both NaDy(WO4)2 and NaHo(WO4)2 samples (Fig. 5c and f), as the cell survival was above the limit below which (70%) NPs are considered to be potentially cytotoxic (UNE-EN ISO 10993-5:2009 standard).52 Therefore, it can be concluded that the cell viability of the developed NaDy(WO4)2 and NaHo(WO4)2 NPs functionalized with PAA was very high and, therefore, they would be suitable for use as probes in bioimaging applications.
3.4. High-field magnetic resonance imaging
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) or
or![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2versus lanthanide concentrations. As shown in these figures, for both systems, the r2 value was much higher (105.6 and 58.6 mM−1 s−1, for NaDy(WO4)2 and NaHo(WO4)2, respectively) than the r1 value (0.29 and 0.27 mM−1 s−1, for NaDy(WO4)2 and NaHo(WO4)2, respectively), which results in r2/r1 ratios (364 and 217, respectively) exceedingly above the lower limit (∼10) for a system to be considered as T2 CA.3 It is also outstanding that the r2 value was higher for the Dy-based NPs (105.6 mM−1 s−1) than that for the Ho-based ones (58.6 mM−1 s−1), which manifests the better performance of the former as a CA for HF-MRI. To explain this behavior, it must be considered that the value of the transverse relaxivity, r2, of paramagnetic NPs is mainly due to the contribution of the Curie effect to the transverse relaxivity in the outer-sphere regime according to the quantum-mechanical theory.12 Different factors influence the magnetization of paramagnetic Dy3+- and Ho3+-containing samples, such as the magnetic moment of such cations. Specifically, the r2 value increases with the magnetization of the paramagnetic ion, which is related to the magnetic susceptibility and thus to the magnetic moment of the ion according to Curie's law, which predicts that the magnetization increases quadratically with the magnetic moment.21 Therefore, since the value of the magnetic moment is higher for Dy3+ (10.63μB) than for Ho3+ (10.6μB),53 the magnetization of the Dy-based system is also higher than that of the Ho-based one (Fig. S3, ESI†), thus explaining the higher r2 value of the former. Another factor contributing to such a higher r2 value is the higher particle size of the Dy-based sample since the quantum-mechanical theory12 also predicts that r2 increases with the particle size. Finally, it is important to mention that, as observed in in Table S1 (ESI†), the r2 values of our samples are within the range reported for most of the other Dy or Ho based systems.22–24,29–34,54,55 Only those based on LnF3,22 LnVO4,33 and LnPO4,31,32 (Ln = Ho or Dy) previously reported by us showed clearly higher r2 values due to their higher particle size. However, in these cases, the suitability of developed NPs as CAs for in vivo dual MRI-CT imaging was not investigated.
2versus lanthanide concentrations. As shown in these figures, for both systems, the r2 value was much higher (105.6 and 58.6 mM−1 s−1, for NaDy(WO4)2 and NaHo(WO4)2, respectively) than the r1 value (0.29 and 0.27 mM−1 s−1, for NaDy(WO4)2 and NaHo(WO4)2, respectively), which results in r2/r1 ratios (364 and 217, respectively) exceedingly above the lower limit (∼10) for a system to be considered as T2 CA.3 It is also outstanding that the r2 value was higher for the Dy-based NPs (105.6 mM−1 s−1) than that for the Ho-based ones (58.6 mM−1 s−1), which manifests the better performance of the former as a CA for HF-MRI. To explain this behavior, it must be considered that the value of the transverse relaxivity, r2, of paramagnetic NPs is mainly due to the contribution of the Curie effect to the transverse relaxivity in the outer-sphere regime according to the quantum-mechanical theory.12 Different factors influence the magnetization of paramagnetic Dy3+- and Ho3+-containing samples, such as the magnetic moment of such cations. Specifically, the r2 value increases with the magnetization of the paramagnetic ion, which is related to the magnetic susceptibility and thus to the magnetic moment of the ion according to Curie's law, which predicts that the magnetization increases quadratically with the magnetic moment.21 Therefore, since the value of the magnetic moment is higher for Dy3+ (10.63μB) than for Ho3+ (10.6μB),53 the magnetization of the Dy-based system is also higher than that of the Ho-based one (Fig. S3, ESI†), thus explaining the higher r2 value of the former. Another factor contributing to such a higher r2 value is the higher particle size of the Dy-based sample since the quantum-mechanical theory12 also predicts that r2 increases with the particle size. Finally, it is important to mention that, as observed in in Table S1 (ESI†), the r2 values of our samples are within the range reported for most of the other Dy or Ho based systems.22–24,29–34,54,55 Only those based on LnF3,22 LnVO4,33 and LnPO4,31,32 (Ln = Ho or Dy) previously reported by us showed clearly higher r2 values due to their higher particle size. However, in these cases, the suitability of developed NPs as CAs for in vivo dual MRI-CT imaging was not investigated.
3.5. Dual HF-MRI-CT bioimaging
To explore the dual character of the developed NPs for HF-MRI and CT bioimaging, the NaDy(WO4)2 system was chosen, as a proof of concept, owing to their superior r2 values when compared with the Ho-based system. For such a purpose, the X-ray attenuation properties of the selected sample were first evaluated.4. Conclusions
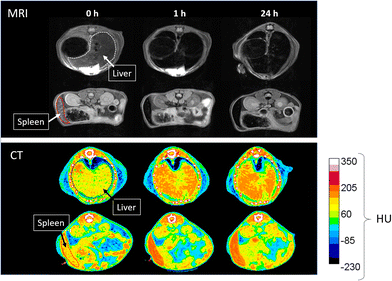
NaDy(WO4)2 and NaHo(WO4)2 NPs functionalized with PAA have been synthesized using a homogeneous precipitation method in polyol media at 220 °C from lanthanide nitrate and sodium tungstate precursors in a microwave oven. The NPs showed good dispersibility in water, MES, PBS and saline solution suspensions. Cell viability studies revealed that such NPs exhibited negligible toxicity effects for the human fibroblast HFF-1 cell line. Transversal relaxivity values (r2) measured at 9.4 T were found to be higher for the NaDy(WO4)2 NPs (105.6 mM−1 s−1) than for the NaHo(WO4)2 ones (58.6 mM−1 s−1), which is mainly ascribed to the higher size of the former and to the higher magnetic moment of Dy3+ when compared with that of Ho3+. Furthermore, pharmacokinetic assays carried out using tumor-bearing mice indicated that for both kinds of NPs a fast uptake by the liver and spleen took place. More importantly, certain NP accumulation in the tumor was observed 24 h after administration, indicating NPs uptake by the EPR effect, which manifests the suitability of both kinds of NPs for tumor detection through HF-MRI. On the other hand, no tissue damage was detected in the analyzed organs, which provides further support to the biocompatibility of NaDy(WO4)2 and NaHo(WO4)2 NPs. Finally, the NaDy(WO4)2 NPs, selected as a proof of concept, showed a superior X-ray attenuation capability than that of a commercial CT contrast agent (iohexol), which along with their high r2 value make them suitable as the dual-probe for dual HF-MRI and CT imaging, as demonstrated by in vivo experiments conducted using healthy mice.Author contributions
M. Ocaña: conceptualization, supervision, formal analysis, funding acquisition and writing – original draft; E. González-Gomez: methodology, data curation, formal analysis, and writing – original draft; C. Caro: data curation, formal analysis, and writing – review and editing; N. O. Nuñez: supervision and writing – review and editing; D. González-Mancebo: data curation and formal analysis; J. D. Urbano-Gámez: data curation and formal analysis; M. L. Garcia: funding acquisition, formal analysis, and writing – review and editing.Data availability
Data for this article are available at DIGITAL CSIC at [URL – format https://doi.org/10.20350/digitalCSIC/16522].Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication is a part of the I + D + I Grants PID2021-122328OB-I00 and PID2020-118448RB-C21, funded by MCIN/AEI/10.13039/501100011033 and “ERDF A way of making Europe”. This work was supported as well by the Junta de Andalucía under grant no. P20_00182, co-financed by EU FEDER funds. Grant PRE2019-090170 funded by the MCIN/AEI/10.13039/501100011033 and the “ESF Investing in your future” is also acknowledged. Relaxivity measurements were performed at the ICTS “NANBIOSIS”, specifically in Unit 28 at the “Instituto de Investigación Biomédica de Málaga y Plataforma en Nanomedicina (IBIMA Plataforma BIONAND)”.References
- H. Otsuka, J. Med. Invest., 2019, 66, 31–34 CrossRef PubMed.
- R. J. Gillies and M. B. Schabath, Cancer Epidemiol., Biomarkers Prev., 2020, 29, 2556–2567 CrossRef CAS PubMed.
- E. Peng, F. H. Wang and J. M. Xue, J. Mater. Chem. B, 2015, 3, 2241–2276 RSC.
- B. Borresen, A. E. Hansen, F. P. Fliedner, J. R. Henriksen, D. R. Elema, M. Brandt-Larsen, L. K. Kristensen, A. T. Kristensen, T. L. Andresen and A. Kjaer, Int. J. Nanomed., 2020, 15, 8571–8581 CrossRef CAS PubMed.
- A. Nowogrodzki, Nature, 2018, 563, 24–26 CrossRef CAS PubMed.
- T. Okada, T. Akasaka, D. H. D. Thuy and T. Isa, Magn. Reson. Med. Sci., 2022, 21, 531–537 CrossRef PubMed.
- A. Gnach and A. Bednarkiewicz, Nano Today, 2012, 7, 532–563 CrossRef CAS.
- M. Norek, E. Kampert, U. Zeitler and J. A. Peters, J. Am. Chem. Soc., 2008, 130, 5335–5340 CrossRef CAS PubMed.
- G. K. Das, Y. Zhang, L. D'Silva, P. Padmanabhan, B. C. Heng, J. S. C. Loo, S. T. Selvan, K. K. Bhakoo and T. T. Y. Tan, Chem. Mater., 2011, 23, 2439–2446 CrossRef CAS.
- M. Rohrer, H. Bauer, J. Mintorovitch, M. Requardt and H. J. Weinmann, Invest. Radiol., 2005, 40, 715–724 CrossRef PubMed.
- I. M. Noebauer-Huhmann, P. Szomolanyi, V. Juras, O. K. Dipl, M. E. Ladd and S. Trattnig, Invest. Radiol., 2010, 45, 554–558 CrossRef CAS PubMed.
- H. Du, Q. Y. Wang, Z. Y. Liang, Q. L. Li, F. Y. Li and D. S. Ling, Nanoscale, 2022, 14, 17483–17499 RSC.
- S. Tong, S. J. Hou, Z. L. Zheng, J. Zhou and G. Bao, Nano Lett., 2010, 10, 4607–4613 CrossRef CAS PubMed.
- S. K. Wan, F. Z. Cui, B. Li, K. L. Zhao, H. N. He, Y. Zhang, J. H. Liu, L. Zhang and K. Liu, ACS Appl. Nano Mater., 2020, 3, 9433–9439 CrossRef CAS.
- H. Dong, S. R. Du, X. Y. Zheng, G. M. Lyu, L. D. Sun, L. D. Li, P. Z. Zhang, C. Zhang and C. H. Yan, Chem. Rev., 2015, 115, 10725–10815 CrossRef CAS PubMed.
- L. Helm, Future Med. Chem., 2010, 2, 385–396 CrossRef CAS PubMed.
- B. M. Alsaadi, F. J. C. Rossotti and R. J. P. Williams, J. Chem. Soc., Dalton Trans., 1980, 2147–2150 RSC.
- D. F. Jackson and D. J. Hawkes, Phys. Rep., 1981, 70, 169–233 CrossRef CAS.
- N. O. Nunez, F. Cusso, E. Cantelar, B. Martin-Gracia, J. M. de la Fuente, A. Corral, M. Balcerzyk and M. Ocana, Nanomaterials, 2020, 10, 149 CrossRef CAS PubMed.
- P. A. Jarzyna, A. Gianella, T. Skajaa, G. Knudsen, L. H. Deddens, D. P. Cormode, Z. A. Fayad and W. J. M. Mulder, Phys. Rep., 2010, 2, 138–150 CAS.
- Y. Zhang, V. Vijayaragavan, G. K. Das, K. K. Bhakoo and T. T. Y. Tan, Eur. J. Inorg. Chem., 2012, 2044–2048 CrossRef CAS.
- D. Gonzalez-Mancebo, A. I. Becerro, T. C. Rojas, M. L. Garcia-Martin, J. M. de la Fuente and M. Ocana, Part. Part. Syst. Charact., 2017, 34, 1700116 CrossRef.
- X. H. Zhang, B. Blasiak, A. J. Marenco, S. Trudel, B. Tomanek and F. van Veggel, Chem. Mater., 2016, 28, 3060–3072 CrossRef CAS.
- G. K. Das, N. J. J. Johnson, J. Cramen, B. Blasiak, P. Latta, B. Tomanek and F. van Veggel, J. Phys. Chem. Lett., 2012, 3, 524–529 CrossRef CAS PubMed.
- D. Lisjak, O. Plohl, J. Vidmar, B. Majaron and M. Ponikvar-Svet, Langmuir, 2016, 32, 8222–8229 CrossRef CAS PubMed.
- M. I. Saleh, B. Ruhle, S. Wang, J. Radnik, Y. You and U. Resch-Genger, Sci. Rep., 2020, 10, 191318 Search PubMed.
- S. Guth, S. Hueser, A. Roth, G. Degen, P. Diel, K. Edlund, G. Eisenbrand, K.-H. Engel, B. Epe, T. Grune, V. Heinz, T. Henle, H.-U. Humpf, H. Jaeger, H.-G. Joost, S. E. Kulling, A. Lampen, A. Mally, R. Marchan, D. Marko, E. Muehle, M. A. Nitsche, E. Roehrdanz, R. Stadler, C. van Thriel, S. Vieths, R. F. Vogel, E. Wascher, C. Watzl, U. Noethlings and J. G. Hengstler, Arch. Toxicol., 2020, 94, 1375–1415 CrossRef CAS PubMed.
- M. Vozlic, T. Cernic, S. Gyergyek, B. Majaron, M. Ponikvar-Svet, U. Kostiv, D. Horak and D. Lisjak, Dalton Trans., 2021, 50, 6588–6597 RSC.
- S. Marasini, H. Yue, S. L. Ho, H. Cha, J. A. Park, K. H. Jung, A. Ghazanfari, M. Y. Ahmad, S. Liu, K. S. Chae, Y. Chang and G. H. Lee, Bull. Korean Chem. Soc., 2020, 41, 829–836 CrossRef CAS.
- S. Marasini, H. Yue, S. L. Ho, J. A. Park, S. Kim, K.-H. Jung, H. Cha, S. Liu, T. Tegafaw, M. Y. Ahmad, A. Ghazanfari, K.-S. Chae, Y. Chang and G. H. Lee, Nanomaterials, 2021, 11, 11051355 CrossRef PubMed.
- E. Gomez-Gonzalez, C. Caro, D. Martinez-Gutierrez, M. L. Garcia-Martin, M. Ocana and A. I. Becerro, J. Colloid Interface Sci., 2021, 587, 131–140 CrossRef CAS PubMed.
- E. Gomez-Gonzalez, C. Caro, M. L. Garcia-Martin, A. Isabel Becerro and M. Ocana, Nanoscale, 2022, 14, 11461–11470 RSC.
- E. Gomez-Gonzalez, N. O. Nunez, C. Caro, M. L. Garcia-Martin, Y. Fernandez-Afonso, J. M. de la Fuente, M. Balcerzyk and M. Ocana, Inorg. Chem., 2021, 60, 152–160 CrossRef CAS PubMed.
- E. Gómez-González, N. O. Núñez, C. Caro, M. L. García-Martín and M. Ocaña, J. Colloid Interface Sci., 2023, 629, 310–321 CrossRef PubMed.
- X. P. Duan and Y. P. Li, Small, 2013, 9, 1521–1532 CrossRef CAS PubMed.
- P. C. Hiemenz and R. Rajagopalan, Principles of colloid and surface chemistry, New York, 3rd edn, 1997 Search PubMed.
- D. Klee and H. Hocker, Biomed. Appl. Polym. Blends, 1999, 149, 1–57 CAS.
- P. Rivera-Gil, D. J. De Aberasturi, V. Wulf, B. Pelaz, P. Del Pino, Y. Y. Zhao, J. M. De La Fuente, I. R. De Larramendi, T. Rojo, X. J. Liang and W. J. Parak, Acc. Chem. Res., 2013, 46, 743–749 CrossRef CAS PubMed.
- M. A. Subhan, S. S. K. Yalamarty, N. Filipczak, F. Parveen and V. P. Torchilin, J. Pers. Med., 2021, 11, 571 CrossRef PubMed.
- Y. Matsumura, J. Controlled Release, 2022, 348, 966–969 CrossRef CAS PubMed.
- Y. Zi, K. Yang, J. He, Z. Wu, J. Liu and W. Zhang, Adv. Drug Delivery Rev., 2022, 188, 114449 CrossRef CAS PubMed.
- C. Caro and D. Pozo, Curr. Pharm. Des., 2015, 21, 4822–4836 CrossRef CAS PubMed.
- J. W. Nichols and Y. H. Bae, J. Controlled Release, 2014, 190, 451–464 CrossRef CAS PubMed.
- C. Caro, A. Avasthi, J. M. Paez-Munoz, M. Pernia Leal and M. L. Garcia-Martin, Biomater. Sci., 2021, 9, 7984–7995 RSC.
- C. Caro, C. Guzzi, I. Moral-Sanchez, J. D. Urbano-Gamez, A. M. Beltran and M. L. Garcia-Martin, Adv. Healthcare Mater., 2024, 2304049 Search PubMed.
- E. Gomez-Gonzalez, D. Gonzalez-Mancebo, N. O. Nunez, C. Caro, M. L. Garcia-Martin, A. I. Becerro and M. Ocana, J. Colloid Interface Sci., 2023, 646, 721–731 CrossRef CAS PubMed.
- J. Edelson, D. Shaw and G. Palace, J. Pharm. Sci., 1984, 73, 993–995 CrossRef CAS PubMed.
- C. Caro, J. M. Paez-Munoz, A. M. Beltran, M. P. Leal and M. L. Garcia-Martin, ACS Appl. Nano Mater., 2021, 4, 4199–4207 CrossRef CAS.
- V. K. Lamer and R. H. Dinegar, J. Am. Chem. Soc., 1950, 72, 4847–4854 CrossRef CAS.
- M. Li, J. Wu, H. Jia, M. Wang and Z. Liu, J. Mater. Sci.: Mater. Electron., 2019, 30, 10465–10474 CrossRef CAS.
- L. J. Kirwan, P. D. Fawell and W. van Bronswijk, Langmuir, 2003, 19, 5802–5807 CrossRef CAS.
- UNE-EN ISO 10993-5:2009: Evaluación biológica de productos sanitarios. Parte 5: Ensayos de citotoxicidad in vitro, https://www.aenor.com/.
- S. Viswanathan, Z. Kovacs, K. N. Green, S. J. Ratnakar and A. D. Sherry, Chem. Rev., 2010, 110, 2960–3018 CrossRef CAS PubMed.
- A. Dash, B. Blasiak, B. Tomanek, P. Latta and F. C. J. M. van Veggel, ACS Appl. Mater. Interfaces, 2021, 13, 24345–24355 CrossRef CAS PubMed.
- Y. L. Balachandran, W. Wang, H. Yang, H. Tong, L. Wang, F. Liu, H. Chen, K. Zhong, Y. Liu and X. Jiang, ACS Nano, 2022, 16, 5647–5659 CrossRef CAS PubMed.
- K. M. Tsoi, S. A. MacParland, X.-Z. Ma, V. N. Spetzler, J. Echeverri, B. Ouyang, S. M. Fadel, E. A. Sykes, N. Goldaracena, J. M. Kaths, J. B. Conneely, B. A. Alman, M. Selzner, M. A. Ostrowski, O. A. Adeyi, A. Zilman, I. D. McGilvray and W. C. W. Chan, Nat. Mater., 2016, 15, 1212–1221 CrossRef CAS PubMed.
- A. Karageorgis, S. Dufort, L. Sancey, M. Henry, S. Hirsjaervi, C. Passirani, J.-P. Benoit, J. Gravier, I. Texier, O. Montigon, M. Benmerad, V. Siroux, E. L. Barbier and J.-L. Coll, Sci. Rep., 2016, 6, 21417 CrossRef CAS PubMed.
- G. Song, D. B. Darr, C. M. Santos, M. Ross, A. Valdivia, J. L. Jordan, B. R. Midkiff, S. Cohen, N. Nikolaishvili-Feinberg, C. R. Miller, T. K. Tarrant, A. B. Rogers, A. C. Dudley, C. M. Perou and W. C. Zamboni, Clin. Cancer Res., 2014, 20, 6083–6095 CrossRef CAS PubMed.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak and W. C. W. Chan, Nat. Rev. Mater., 2016, 1, 16014 CrossRef CAS.
- P. Carrillo, M. Bernal, C. Tellez-Quijona, A. D. Marrero, I. Vidal, L. Castilla, C. Caro, A. Dominguez, M. L. Garcia-Martin, A. R. Quesada, M. A. Medina and B. Martinez-Poveda, Biomed. Pharmacother., 2023, 158, 114070 CrossRef CAS PubMed.
- D. Gonzalez Mancebo, A. Isabel Becerro, A. Corral, M. Moros, M. Balcerzyk, J. M. de la Fuente and M. Ocana, ACS Omega, 2019, 4, 765–774 CrossRef CAS.
- M. Longmire, P. L. Choyke and H. Kobayashi, Nanomedicine, 2008, 3, 703–717 CrossRef CAS PubMed.
- F. Alexis, E. Pridgen, L. K. Molnar and O. C. Farokhzad, Mol. Pharmaceutics, 2008, 5, 505–515 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01157k |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |