Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Red light-triggerable nanohybrids of graphene oxide, gold nanoparticles and thermo-responsive polymers for combined photothermia and drug release effects†
Grazia M. L.
Consoli
 ab,
Ludovica
Maugeri
c,
Giuseppe
Forte
ab,
Ludovica
Maugeri
c,
Giuseppe
Forte
 c,
Gianpiero
Buscarino
d,
Antonino
Gulino
c,
Gianpiero
Buscarino
d,
Antonino
Gulino
 e,
Luca
Lanzanò
f,
Paolo
Bonacci
g,
Nicolò
Musso
g and
Salvatore
Petralia
e,
Luca
Lanzanò
f,
Paolo
Bonacci
g,
Nicolò
Musso
g and
Salvatore
Petralia
 *abch
*abch
aCNR-Institute of Biomolecular Chemistry, Via Paolo Gaifami 18, 95126 Catania, Italy. E-mail: salvatore.petralia@unict.it
bCIB-Interuniversity Consortium for Biotechnologies U.O. of Catania, Via Flavia, 23/1, 34148 Trieste, Italy
cDepartment of Drug and Health Sciences, University of Catania, Via Santa Sofia 64, 95125 Catania, Italy
dDepartment of Physics and Chemistry, University of Palermo, Via Archirafi 36, Palermo, Italy
eDepartment of Chemical Science, University of Catania, and I.N.S.T.M. UdR of Catania, Via Santa Sofia 64, 95125 Catania, Italy
fDepartment of Physics and Astronomy “Ettore Majorana”, University of Catania, Via S. Sofia 64, 95123 Catania, Italy
gDepartment of Biomedical and Biotechnological Sciences, University of Catania, Via S. Sofia 97, Catania, Italy
hNANOMED, Research Centre for Nanomedicine and Pharmaceutical Nanotechnology, University of Catania, Viale A. Doria 6, 95124 Catania, Italy
First published on 9th November 2023
Abstract
The development of multifunctional nanohybrid systems for combined photo-induced hyperthermia and drug release is a challenging topic in the research of advanced materials for application in the biomedical field. Here, we report the first example of a three-component red-light-responsive nanosystem consisting of graphene oxide, gold nanoparticles and poly-N-isopropylacrylamide (GO–Au–PNM). The GO–Au–PNM nanostructures were characterized by spectroscopic techniques and atomic force microscopy. They exhibited photothermal conversion effects at various wavelengths, lower critical solution temperature (LCST) behaviour, and curcumin (Curc) loading capacity. The formation of GO–Au–PNM/Curc adducts and photothermally controlled drug release, triggered by red-light excitation (680 nm), were demonstrated using spectroscopic techniques. Drug–polymer interaction and drug–release mechanism were well supported by modelling simulation calculations. The cellular uptake of GO–Au–PNM/Curc was imaged by confocal laser scanning microscopy. In vitro experiments revealed the excellent biocompatibility of the GO–Au–PNM that did not affect the viability of human cells.
Introduction
A nanohybrid system in the nanoscale dimension implies the combination of a variety of organic, inorganic, or bioactive components in a single material with either enhanced or entirely new properties.1 This approach has provided the opportunity to create a huge number of novel materials with well-defined structures and functions.2–4 Combining exclusive features such as optical, electrical and photo-responsive properties, a nanohybrid system offers advantages in different application fields including biomedical, coating, energy storage, catalysis, and sensing.5–7 In the biomedical field, light-responsive photothermal systems, that allow for a controlled release of drugs in specific regions, are appealing for more effective and safer therapy. Indeed, photothermia and drug release restricted to irradiated areas can reduce the dosage and side effects of conventional chemotherapy. In this scenario, nanohybrid systems that combine the photothermal properties of metal nanoparticles (NPs) and graphene oxide (GO)-based nanostructures and the thermo-responsive properties of poly(N-isopropylacrylamide) (PNM) are attracting interest. GO nanosheets have a large surface area and can load nanostructures as support materials. In particular, the oxygenated functional groups of GO can be utilized as nucleation centres to grow or anchor NPs. PNM is a polymer largely investigated for biomedical applications because of its low toxicity, chemical stability, temperature- and pH-responsivity.4 The stimuli-responsivity of the PNM has been exploited to develop temperature-responsive drug delivery systems. It has been reported that at temperatures below the lower critical solution temperature (LCST), PNM adopts a hydrophilic coil-extended-conformation stabilized by favourable interactions with water molecules. Instead, at a temperature above the LCST value (typically 33–35 °C), a transition takes place, and the polymer adopts a hydrophobic globule-like conformation. In this collapsed state, water molecules are expelled from the shell and the amount of hydrogen bonds decreases allowing the release of entrapped drug molecules. Curcumin (Cur) is a natural substance with a wide range of biological properties8,9 and biomedical applications.10 Cur has shown anticancer,11 antimicrobial,12 antioxidant and anti-inflammatory,13 antidiabetic,14 hepato-15 and neuro-protective activity.16 However, poor stability, water solubility, and bioavailability have limited the clinical use of curcumin and stimulated intense research aimed at overcoming these issues through entrapment in nanocarriers.17–20 Since hyperthermia enhances the anticancer activity of Cur,21 photothermal nanosystems are interesting Cur delivery systems.22Two-component nanohybrid composites that integrate the properties of GO with nanoparticles or organic molecules were widely reported in the literature23,24 and investigated for different applications including drug delivery,25 NO photorelease,26 and antibacterial27 activity. Differently, very few examples of three-component GO-based nanosystems are present in the literature.28
In this work, we report the first example of a three-component nanosystem in which GO sheets are decorated with photothermal gold nanoparticles (AuNPs), which are further functionalized with the thermo-responsive PNM polymer. For application as a light-actuated device for remote controlled drug release, Cur was entrapped into the GO–Au–PNM nanocontainer (Scheme 1A). Cur was selected as a hydrophobic drug model whose performance can be improved by entrapment in a photothermal drug delivery system for dual chemo-photothermal therapy outcomes. The PNM covalently linked to the AuNP surface was chosen to induce a red shift of the localized surface plasmon resonance (LSPR) band to higher wavelengths. Consequently, more biocompatible light could trigger the photothermal-induced PNM conformational change and promote the release of Cur from the nanosystem. The morphology, spectroscopic properties and photothermal behaviour of the hybrid nanosystem as well as the red light-triggered hyperthermia and hyperthermia-induced drug release were investigated (Scheme 1B).
Results and discussion
Design, synthesis, and characterization of the GO–Au–PNM nanosystem
Nanohybrid systems composed of photothermal materials and thermo-responsive polymers are fascinating stimuli-responsive drug delivery systems widely developed by our group.29–32 They match dark stability, good biocompatibility, and absorption in the visible and near-infrared (NIR) region suitable to effectively promote the photothermal effect under the exclusive control of light, which in turn causes the coil-to-globule-transition of the polymer structure and the consequent hyperthermia-induced drug release. The choice of gold nanoparticles and PNM for the preparation of a GO–Au–PNM nanosystem was mainly driven by the high photothermal conversion efficiency of the plasmonic gold nanostructures in the visible region and the LCST behaviour of PNM. Furthermore, the easy preparation and chemical modification enable the AuNPs to be tailored in various ways to facilitate their integration into a variety of nanostructured materials. The GO–Au–PNM nanosystem was prepared and tested as depicted in Scheme 1. In detail, graphene was converted to GO nanosheets by Hummer's modified method. Then, the GO nanosheets were decorated with Au nanoparticles as described in the Experimental section. Finally, the linkage of PNM molecules with the Au nanoparticle surface was obtained exploiting the sulphur chemistry. The GO–Au–PNM nanosystem was fully characterized by various techniques. Fig. 1A illustrates the optical absorption spectra of an aqueous dispersion of GO–Au–PNM (black line). The LSPR broad band related to the Au nanoparticles at around 550 nm and the absorption in the region 230–280 nm related to the GO nanosheet were observed. For comparison, optical absorption spectra of AuNPs (cyan line) and GO sheet (blue line) alone were recorded. Notably, the LSPR band for the AuNPs in the GO–Au–PNM nanohybrid is broadened and red-shifted compared to that of GO–Au (red line) and AuNPs (cyan line). This finding not only corroborates the effective linkage of PNM to the AuNPs, but also indicates the electronic interactions between the two species, as reported for other species grafted to GO.33 Moreover, as previously observed for similar Au nanostructured materials,31 the red shifts of the AuNP LSPR band evidence both surface electronic interaction and nanoaggregation phenomena. The successful GO–Au formation was easily evidenced by the colour change of the GO suspension from brown-yellow to red-purple, which is consistent with the presence of the AuNPs. The further functionalization with thiolated PNM to produce GO–Au–PNM is evidenced by the red-shifted LSPR band in the GO–Au–PNM (Fig. 1A). The effective GO–Au–PNM formation was well supported by FTIR spectroscopy (Fig. 1B), showing the following diagnostic peaks for PNM: 3147.2 cm−1 (N–H, stretching), 2969.2 cm−1 (C–H, stretching), 1630.2 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, amide I), 1534.5 cm−1 (C
O stretching, amide I), 1534.5 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, amide II), 1389.5 cm−1 (C–H, bending) and 1122.2 cm−1 (C–C, stretching). Moreover, the following FTIR peaks for GO sheets were recorded: 3422.5 cm−1 (O–H stretching), 2870.5 cm−1 (C–H stretching), 1650.1 cm−1 (C
O stretching, amide II), 1389.5 cm−1 (C–H, bending) and 1122.2 cm−1 (C–C, stretching). Moreover, the following FTIR peaks for GO sheets were recorded: 3422.5 cm−1 (O–H stretching), 2870.5 cm−1 (C–H stretching), 1650.1 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, stretching), 1554.1 cm−1 (CO, stretching) and 1151.2 cm−1 (C
C, stretching), 1554.1 cm−1 (CO, stretching) and 1151.2 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, bending). For comparison, FTIR-ATR spectra of the precursor GO-sheets and PNM were recorded (Fig. S1, ESI†). The morphology of the AuNPs on the GO sheets was investigated by contact-mode AFM. Fig. 1C shows representative scan 500 × 500 nm AFM images of the AuNPs. The investigation revealed the presence of spherical-shaped gold nanoparticles with a size ranging from 3 to 7 nm at the GO-sheet surface. The histogram distribution for the height size of AuNPs, obtained by analysis of different AFM images, is shown in the inset Fig. 1C. Fig. 1D shows the profile section of GO–AuNPs.
O, bending). For comparison, FTIR-ATR spectra of the precursor GO-sheets and PNM were recorded (Fig. S1, ESI†). The morphology of the AuNPs on the GO sheets was investigated by contact-mode AFM. Fig. 1C shows representative scan 500 × 500 nm AFM images of the AuNPs. The investigation revealed the presence of spherical-shaped gold nanoparticles with a size ranging from 3 to 7 nm at the GO-sheet surface. The histogram distribution for the height size of AuNPs, obtained by analysis of different AFM images, is shown in the inset Fig. 1C. Fig. 1D shows the profile section of GO–AuNPs.
The comparison between the full scan 3 × 3 μm AFM images for GO–AuNPs and GO–Au–PNM revealed the effective GO–Au–PNM nanoaggregation, which is consistent with the red-shifted LSPR band observed for the GO–Au–PNM nanohybrid (Fig. 1A and Fig. S2, S3, ESI†).
The electronic structure of the GO–Au–PNM was investigated by X-ray photoelectron spectroscopy. This technique is ideal as it gives information on the oxidation states and on the chemical environment of the studied species and allows estimation of the surface elemental composition once the relevant atomic sensitivity factors have been considered.34,35
Fig. 2A shows the high-resolution XP spectrum of the functionalized GO–Au–PNM in the C 1s, O 1s, N 1s, S 2p and Au 4f energy regions. In detail, for the C 1s binding energy region, two signals are evident at 284.6 and a shoulder centred at 287.2 eV. The first component at 284.6 eV is due to the aromatic and aliphatic backbones of the PNM functionalized graphene oxide. The components centred at 287.2 is due to the –C–OH and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species of both GO and PNM.36 Similarly, the O 1s binding energy region, consisting of a rather symmetrical peak at 530.7 eV, which is mainly due to the oxygen of the graphene oxide as well as the oxygen of the HN–C
O species of both GO and PNM.36 Similarly, the O 1s binding energy region, consisting of a rather symmetrical peak at 530.7 eV, which is mainly due to the oxygen of the graphene oxide as well as the oxygen of the HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the PNM polymer.36 The experimental profile of this N 1s signal shows just one peak at 399.3 eV, ascribed to the N atom of the HN–C
O group of the PNM polymer.36 The experimental profile of this N 1s signal shows just one peak at 399.3 eV, ascribed to the N atom of the HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the PNM polymer.37–41 The high-resolution XP spectrum of the GO–Au–PNM in the S 2p binding energy region is rather noisy due to the low atomic concentration of S (0.4% of the overall XPS signals). Nevertheless, the peak centred at 162.5 eV, clearly shows the shape of the two expected 2p spin–orbit components and is due to the S of the functionalized PNM ligand. Finally, in the Au 4f binding energy region, the Au 4f7/2,5/2 levels were observed at 84.7 and 88.4 eV, respectively, and indicate the presence of Au0 states. The XPS atomic concentration analysis of the investigated system clearly shows a ratio S/Au = 4.0, N/Au = 115, O/Au = 132, and N/S = 29.
O group of the PNM polymer.37–41 The high-resolution XP spectrum of the GO–Au–PNM in the S 2p binding energy region is rather noisy due to the low atomic concentration of S (0.4% of the overall XPS signals). Nevertheless, the peak centred at 162.5 eV, clearly shows the shape of the two expected 2p spin–orbit components and is due to the S of the functionalized PNM ligand. Finally, in the Au 4f binding energy region, the Au 4f7/2,5/2 levels were observed at 84.7 and 88.4 eV, respectively, and indicate the presence of Au0 states. The XPS atomic concentration analysis of the investigated system clearly shows a ratio S/Au = 4.0, N/Au = 115, O/Au = 132, and N/S = 29.
 | ||
| Fig. 2 GO–Au–PNM characterization: (A) Al Kα excited XPS in the C 1s, O 1s, N 1s, S 2p and Au 4f energy regions; (B) 1H NMR spectrum of GO–Au–PNM (400.13 MHz, D2O, 297 K). | ||
The presence of the PNM pendants on the material surface was corroborated by the 1H NMR spectrum of GO–Au–PNM (Fig. 2B). The proton spectrum showed peaks at 1.13 ppm (CH3 of the isopropyl group), 1.57 ppm (CH2– group on the backbone), 1.99 ppm (CH– group on the backbone) and 3.88 ppm (CH– group of the isopropyl),41,42 which define the structure of the PNM polymer. The proton spectrum of GO–Au–PNM was very similar to the one of the PNM–polymer carbon dots that we reported in a previous paper.29 Since the peaks belonging to the polymer normally are wider,43 the best-defined peaks in the proton spectrum of GO–Au–PNM correspond to impurity, while the peak at 3.39 ppm, which is significantly smaller in the spectrum of pure PNM, could be due to possible isomeric structures. The peak at 8.22 ppm is attributable to residual NH proton that normally does not show up because it exchanges easily with D2O. However, the fact that it shows up on the spectrum of the material but not in the spectrum of only PNM polymer can be related to the more rigid structure of the material that hinders the diffusion of water molecules toward the interior.
The effectiveness of GO functionalization was also corroborated by DLS and Z-potential measurements. The DLS investigation showed for GO dispersion in water is a main population of nanostructures with mean hydrodynamic diameters of 829.1 ± 75 nm (93%) and 194.1 ± 5 nm (7%). The further decoration with AuNPs, as expected, induces an increment of GO-nanosheet exfoliation and GO–Au nanostructures with a smaller diameter of 588.8 ± 23 nm (98.5%) were detected. Next, functionalization of the AuNPs with PNM causes an increase in the hydrodynamic size (674.8 ± 22 nm; 93%) due to the PNM chains (Fig. S4, ESI†). Aggregation of the GO–Au–PNM nanostructures (5098 ± 520 nm; 7%) was also observed according to the AFM data (Fig. S2 and S3, ESI†). Z-potential measurements showed a slight decrease in the GO Z-potential value (−55.4 ± 3.1 mV) upon AuNP decoration (GO–Au: −46.7 ± 1.4 mV). As expected, functionalization of the AuNPs with PNM to give the GO–Au–PNM nanohybrid further decreased the Z-potential value (−24.7 ± 0.9 mV). The PNM functionalization was also revealed by the typical LCST behaviour observed for the GO–Au–PNM, with a value of LCST above the human body temperature of 37 °C (Fig. S5, ESI†). The LCST value higher than that of a homogeneous solution of PNM (32–34 °C) was indicative of an effective interaction between PNM and Au nanoparticle surface as widely reported in the literature.44–46 These data enable the use of PNM-based nanomaterials as drug-release systems at human body temperature.
With the aim to evaluate the potential of GO–Au–PNM as a light-responsive drug delivery system, we selected curcumin as a model of a hydrophobic drug and performed molecular dynamics simulations to investigate the Au–PNM/Cur interactions. In detail, five Au–PNM/Cur structures were randomly selected during the preliminary 10 ns of MD simulations, see the Methods section, respectively, at 298 K and 315 K (Fig. 3A and B). Results obtained at higher temperature revealed three structures with PNM collapsed chains at Au–NP surface (S01, S02 and S03) and two with PNM extended structures (S04 and S05). The energy difference for optimized geometries after conducting MD simulations reveals for the PNM collapsed structures (S01: 0.00 kcal mol−1; S02: 5.52 kcal mol−1 and S03: 7.12 kcal mol−1) lower energy values than the PNM extended structures (S04: 29.13 kcal mol−1 and S05: 32.43 kcal mol−1). The main contribution to these values is mainly due to the Coulombic electrostatic interactions and van der Waals forces between PNM and Au nanoparticles.
These data indicate a strong interaction between PNM and the AuNP surface in agreement with the increase of the LCST values (above 37 °C) experimentally observed for the GO–Au–PNM nanostructures (Fig. 1A and Fig. S5, ESI†). Conversely, the results of MD simulations performed at 298 K indicate the exclusive presence of extended PNM structures, with minor differences in Eint observed among the five structures: (S01: 3.27 kcal mol−1; S02: 0.00 kcal mol−1; S03: 6.51 kcal mol−1; S04: 4.86 kcal mol−1; S05: 6.93 kcal mol−1). No collapsed structure was revealed. The root mean square deviation (RMSD) values calculated within a timescale of 250 ns at 315 K confirm again the structure variation due to the LCST behaviour of PNM. The RMSD values increased by more than 2 Å during the simulation for all the structures investigated, a significant increase of the RMSD value from 2 Å to 4.5 Å within 50 ns, indicative of an effective LCST transition, was observed for the extended PNM structures (S04 and S05). Differently, the collapsed PNM structures (S01, S02 and S03) showed a slow increase of the RMSD values from 2.0 to 4.1 Å within 250 ns (Fig. 3C). All simulations revealed a reduced affinity of the curcumin molecules towards the Au–PNM nanoparticles at 315 K. Indeed, as depicted in Fig. 3D, at 315 K, the distance between curcumin and the Au nanoparticle surface increases within 250 ns indicating an effective release of drug from all the Au–PNM structures investigated. In contrast, RMSD values calculated at 298 K (Fig. 3C) exhibit a slight increase, suggesting that no polymer phase transition occurs at a temperature below LCST. Consequently, no significant structural differences have been revealed. In addition, a notable interaction between curcumin and the polymer chain occurs, as evidenced by the unaffected curcumin–PNM distance during the MD simulations, Fig. 3D.
Curcumin loading and photothermal-induced release
To load Cur into the GO–Au–PNM, excess drug (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) was added to the GO–Au–PNM aqueous dispersion, and the mixture was stirred for 48 h. Then, the sample was dialyzed to remove the unentrapped drug. The formation of the GO–Au–PNM/Cur adduct was confirmed by spectroscopic methods and DLS measurements. The UV-Vis absorption spectrum of GO–Au–PNM/Cur (Fig. 4A) showed the Cur absorption band at 433 nm and the typical LSPR band of the AuNPs above 550 nm. To investigate the photothermal properties of the GO–Au–PNM, an aqueous dispersion (200 μL, Abs680nm = 0.9) was continuously exposed to a 680 nm laser (810 mW power). The temperature changes were monitored using a thermal camera (Fig. 4B inset). When the temperature of the system reached the maximum value of about 54.5 °C (temperature difference = Tmax − Tenviroment = 28 °C), the laser was switched off and the temperature change during cooling was monitored. The cycles were repeated many times to confirm the reversible process; a representative photothermal cycle is depicted in Fig. 4B.
1 w/w) was added to the GO–Au–PNM aqueous dispersion, and the mixture was stirred for 48 h. Then, the sample was dialyzed to remove the unentrapped drug. The formation of the GO–Au–PNM/Cur adduct was confirmed by spectroscopic methods and DLS measurements. The UV-Vis absorption spectrum of GO–Au–PNM/Cur (Fig. 4A) showed the Cur absorption band at 433 nm and the typical LSPR band of the AuNPs above 550 nm. To investigate the photothermal properties of the GO–Au–PNM, an aqueous dispersion (200 μL, Abs680nm = 0.9) was continuously exposed to a 680 nm laser (810 mW power). The temperature changes were monitored using a thermal camera (Fig. 4B inset). When the temperature of the system reached the maximum value of about 54.5 °C (temperature difference = Tmax − Tenviroment = 28 °C), the laser was switched off and the temperature change during cooling was monitored. The cycles were repeated many times to confirm the reversible process; a representative photothermal cycle is depicted in Fig. 4B.
A photothermal conversion efficiency (η) value of about 16% was calculated. The photothermal time constants (τs) were calculated upon photoexcitation with 0.5 and 0.8 W laser power (680 nm, Fig. S6, ESI†). To better investigate the correlation between the photothermal activity and the absorbance at 680 nm, experiments were performed using GO–Au–PNM dispersions with different absorbance values. The results reported in Fig. 4C indicate temperature increases of about 2.3, 5.7, 15.9 and 28.5 °C for absorbance values (λ = 680 nm) of 0.13, 0.28, 0.53 and 1.0 unit, respectively. Moreover, the power-dependent behaviour was confirmed by experiments performed with different laser powers of 300, 500, and 810 mW, and the temperature difference values recorded were about 12.2, 18.6 and 28.3 °C, respectively (Fig. 4D). The photothermal properties of the GO–Au–PNM nanohybrid were also confirmed upon excitation with green-light 532 nm (Fig. S7, ESI†) and blue-light 405 nm (Fig. S8, ESI†) to show the photothermal versatility of the nanosystem.
To assess the combined photothermal and drug release effect of GO–Au–PNM/Cur, the dispersion was first incubated for 20 min at 37 °C under dark conditions and the absorbance measurements (at 433 nm) at various incubation times (0, 5, 10, and 20 min) were recorded. Under dark conditions, the absorbance spectra revealed a very slight Cur release (Fig. S9, ESI†). Then, a concurrent increase in temperature and absorbance signals were recorded upon few minutes of red-light irradiation (680 nm, 810 mW) indicative of an effective combined photothermal and drug release effect. Fig. 5A depicts the appearance of the optical absorption band for the released Cur at various irradiation time (0, 5, 10, 15, 20, and 25 min). Fig. 5B shows the combined increase of temperature (blue line) and the release of Cur (green line) overtime, with a drug release rate value of about 147 μg min−1 and a maximum temperature value of 42.5 °C.
In summary, as revealed by experimental data and supported by modelling simulation, the mechanism of the photothermal triggerable curcumin release is ascribable to the PNM conformational transition induced by the heat released from the Au–NPs upon red-light photoexcitation.
Cell viability and cellular uptake experiments
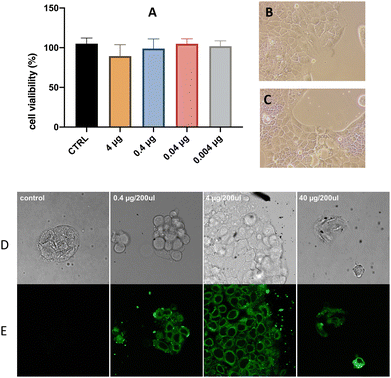
To evaluate the effect of the GO–Au–PNM nanohybrid on cell viability, human colorectal cells were exposed to increasing concentrations of GO–Au–PNM (0.004, 0.04, 0.4, and 4 μg μL−1) for 24 h. As illustrated in Fig. 6A, at the end of the treatment and at any concentration used, the cell viability was comparable to that of untreated controls and no remarkable effects on cell morphology were observed after exposure to GO–Au–PNM (Fig. 6B and C). At the highest concentration, corresponding to 4 μg μL−1, cell growth decrease was about 15.6% compared to the control (p value: 0.1079); at 0.4 μg μL−1, the decrease was about 6.11% (p value: 0.8399); at 0.04 μg μL−1, the difference was about 0.13% (p value: >0.9999), and finally at the lowest concentration of treatment, 0.004 μg μL−1, the percentage difference between the control cells and the treated cells was about 3.07% (p value: 0.9846).To evaluate the potential of GO–Au–PNM as a drug delivery system, cellular uptake experiments were performed on CaCo2 cells. The confocal microscopy analysis (Fig. 6D) and laser scanning microscopy of untreated cells, used as control, showed no significant emission apart from the cell autofluorescence (Fig. 6E). Green staining was instead detected in the cells incubated with the GO–Au–PNM/Cur adduct. This finding evidenced that the luminescent GO–Au–PNM/Cur adducts penetrate the cell and accumulate in the cytoplasm.
Conclusions
A water-dispersible and photothermal-responsive three-component nanohybrid system composed of graphene oxide, gold nanoparticles and a PNM polymer was prepared and characterized for understanding the physicochemical and photothermal properties. The functionalization of the AuNPs anchored on graphene nanosheets with PNM provided a photothermal nanosystem potentially suitable for biomedical applications. Indeed, the GO–Au–PNM nanohybrid showed responsivity to a biocompatible and tissue-penetrating red-light stimulus, biocompatibility, and cellular uptake in human cell lines. The hybrid nanosystem loaded curcumin, selected as a hydrophobic drug model, and upon irradiation-triggered hyperthermia, released the drug by changing the PNM conformation as corroborated by molecular modelling. Microscopy fluorescence investigation confirmed that the GO–Au–PNM/Cur adduct can cross the cell membrane and reach the cytoplasm. The main innovation of the proposed nanohybrid system is the successful combination of good water dispersibility, red-light photoresponsivity, excellent biocompatibility and cellular uptake, drug loading capability and photothermal-controlled drug release. Despite stability and bioaccumulation being limitations, common to most hybrid nanosystems, and requiring further investigation, GO–Au–PNM is one of the first nanohybrid materials activable by a tissue-penetrating biofriendly red light that opens fascinating prospects in the research of novel powerful therapeutic tools based on synergistic chemo-photothermal effects.Materials and methods
Chemicals
All chemicals were obtained from commercial sources in the highest possible purity and were used as received. Milli-Q-grade water was used in all preparations. All solvents used were of spectrophotometric grade.Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) to the GO–Au–PNM colloidal solution (10 mL), and the mixture was stirred for 48 h at room temperature. Then, the sample was dialyzed for 24 h using MilliQ-water–ethanol medium (70
3 w/w) to the GO–Au–PNM colloidal solution (10 mL), and the mixture was stirred for 48 h at room temperature. Then, the sample was dialyzed for 24 h using MilliQ-water–ethanol medium (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) through a dialysis membrane (3.5–5 KDa cut off) to remove the unentrapped drug.
30) through a dialysis membrane (3.5–5 KDa cut off) to remove the unentrapped drug.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) in a standard quartz spectroscopic cuvette. The solution was maintained under stirring during all experiments. The GO–Au–PNM/Cur was exposed to the laser source (680 nm) and the temperature of the GO–Au–PNM/Cur dispersion was measured using a thermal camera. The amount of Cur released was spectrophotometrically measured after each laser exposition.
30) in a standard quartz spectroscopic cuvette. The solution was maintained under stirring during all experiments. The GO–Au–PNM/Cur was exposed to the laser source (680 nm) and the temperature of the GO–Au–PNM/Cur dispersion was measured using a thermal camera. The amount of Cur released was spectrophotometrically measured after each laser exposition.
where N is the total number of atoms, rk(t) are the instantaneous coordinates of atom k and rkinit are the initial coordinates of atom k where N in the total number of atoms, rk(t) are the instantaneous coordinates of atom k and rkinit are the initial coordinates of atom k. For each of the five structures, the interaction energy between AuNP–PNM and curcumin is calculated by optimizing the structure obtained after conducting MD simulations, as
| Eint = EAuNP–PNM–Cur–water − (EAuNP–PNM + Ewater + ECur) |
The total energy comprises contributions from bonded energy terms, out-of-plane energy, cross-interaction terms, Coulombic electrostatic interactions, and the van der Waals force.
Author contributions
The manuscript was written with the contributions from all authors. All authors have given approval for the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been funded by European Union (NextGeneration EU), through the MUR-PNRR project SAMOTHRACE (ECS00000022) and by MUR ex D.M.n.1059 del 09/08/2021 (CUP: E93C22003310001) Project title: L’innovazione delle Biotecnologie nell’Era della Medicina di Precisione, dei Cambiamenti climatici e dell’Economia Circolare.References
- N. Zhao, L. Yan, X. Zhao, X. Chen, A. Li, D. Zheng, X. Zhou, X. Dai and F.-J. Xu, Versatile Types of Organic/Inorganic Nanohybrids: From Strategic Design to Biomedical Applications, Chem. Rev., 2019, 119(3), 1666–1762 CrossRef CAS.
- X. Xue, H. Qu and Y. Li, Stimuli-responsive crosslinked nanomedicine for cancer treatment, Exploration, 2022, 2, 20210134 CrossRef.
- J. Chen, J. Yang and J. Ding, Rational construction of polycystine-based nanoparticles for biomedical applications, J. Mater. Chem. B, 2022, 10, 7173–7182 RSC.
- J. Chen, Z. Jiang, Y. S. Zhang, J. Ding and X. Chen, Smart transformable nanoparticles for enhanced tumor theranostics, Appl. Phys. Rev., 2021, 8, 041321 CAS.
- A. Joy, G. Unnikrishnan, M. Megha, M. Haris, J. Thomas, E. Kolanthai and S. Muthuswamy, Design of biocompatible polycaprolactone-based nanocomposite loaded with graphene oxide/strontium nanohybrid for biomedical applications, Appl. Nanosci., 2023, 13, 4471–4484 CrossRef CAS.
- Q. Li, H. He, S. Wang, H. Zhai, Y. Shen, A. Li and F. Guan, Bis(2-hydroxyethyl) Terephthalate-Modified Ti3C2Tx/Graphene Nanohybrids as Three-Dimensional Functional Chain Extenders for Polyurethane Composite Films with Strain-Sensing and Conductive Properties, ACS Appl. Mater. Interfaces, 2023, 15, 12403–12413 CrossRef CAS PubMed.
- S. Lanzalaco, J. Mingot, J. Torras, C. Alemán and E. Armelin, Recent Advances in Poly(N-isopropylacrylamide) Hydrogelsand Derivatives as Promising Materials for Biomedical and Engineering Emerging Applications, Adv. Eng. Mater., 2023, 25, 2201303 CrossRef CAS.
- B. Joe, M. Vijaykumar and B. R. Lokesh, Biological Properties of Curcumin-Cellular and Molecular Mechanisms of Action, Crit. Rev. Food Sci. Nutr., 2004, 44, 97–111 CrossRef CAS.
- C. Porro and M. A. Panaro, Recent Progress in Understanding the Health Benefits of Curcumin, Molecules, 2023, 28, 2418 CrossRef CAS.
- S.-I. Sohn, A. Priya, B. Balasubramaniam, P. Muthuramalingam, C. Sivasankar, A. Selvaraj, A. Valliammai, R. Jothi and S. Pandian, Biomedical Applications and Bioavailability of Curcumin—An Updated Overview, Pharmaceutics, 2021, 13, 2102 CrossRef CAS PubMed.
- C. de Waure, C. Bertola, G. Baccarini, M. Chiavarini and C. Mancuso, Exploring the Contribution of Curcumin to Cancer Therapy: A Systematic Review of Randomized Controlled Trials, Pharmaceutics, 2023, 15, 1275 CrossRef CAS.
- H. J. Denison, S. L. Schwikkard, M. Khoder and A. F. Kelly, Review: The Chemistry, Toxicity and Antibacterial Activity of Curcumin and Its Analogues, Planta Med., 2023 DOI:10.1055/a-2157-8913.
- M. J. Dehzad, H. Ghalandar, M. Nouri and M. Askarpour, Antioxidant and anti-inflammatory effects of curcumin/turmeric supplementation in adults: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials, Cytokine, 2023, 164, 156144 CrossRef CAS.
- S. Kaushik, N. Masand, M. R. Iyer and V. M. Patil, Preclinical to Clinical profile of Curcuma longa as Antidiabetic Therapeutics, Curr. Top. Med. Chem., 2023, 23, 2267–2276, DOI:10.2174/1568026623666230428101440.
- S. Abolfazli, A. E. Butler, T. Jamialahmadi and A. Sahebkar, A Golden Shield: The Protective Role of Curcumin Against Liver Fibrosis, Curr. Med. Chem., 2023, 31 DOI:10.2174/0929867331666230821095329.
- S. Heidari, S. Mahdiani, M. Hashemi and F. Kalalinia, Recent advances in neurogenic and neuroprotective effects of curcumin through the induction of neural stem cells, Biotechnol. Appl. Biochem., 2020, 67, 430–441 CAS.
- N. A. Torbat, I. Akbarzadeh, N. Rezaei, Z. Moghaddam, S. Bazzazan and E. Mostafavi, Curcumin-Incorporated Biomaterials: In silico and in vitro evaluation of biological potentials, Coord. Chem. Rev., 2023, 492, 215233 CrossRef.
- G. Granata, I. Paterniti, C. Geraci, F. Cunsolo, E. Esposito, M. Cordaro, A. R. Blanco, S. Cuzzocrea and G. M. L. Consoli, Potential Eye Drop based on a Calix[4]arene Nanoassembly for Curcumin Delivery: Enhanced Drug Solubility, Stability and Anti-Inflammatory Effect, Mol. Pharm., 2017, 14, 1610–1622 CrossRef CAS PubMed.
- G. Granata, S. Petralia, G. Forte, S. Conoci and G. M. L. Consoli, Injectable supramolecular nanohydrogel from a micellar self-assembling calix[4]arene derivative and curcumin for a sustained drug release, Mater. Sci. Eng., C, 2020, 111, 110842 CrossRef CAS PubMed.
- V. S. Madamsetty, M. Vazifehdoost, S. H. Alhashemi, H. Davoudi, A. Zarrabi, A. Dehshahri, H. S. Fekri, R. Mohammadinejad and V. K. Thakur, Next-Generation Hydrogels as Biomaterials for Biomedical Applications: Exploring the Role of Curcumin, ACS Omega, 2023, 8, 8960–8976 CrossRef CAS.
- J.-C. Tang, H.-S. Shi, L.-Q. Wan, Y.-S. Wang and Y.-Q. Wei, Enhanced antitumor effect of curcumin liposomes with local hyperthermia in the LL/2 model, Asian Pac. J. Cancer Prev., 2013, 14(4), 2307–2310 CrossRef PubMed.
- Y. Tian, D. Jia, M. Dirican, M. Cui, D. Fang, C. Yan, J. Xie, Y. Liu, C. Li, J. Fu, H. Liu, G. Chen, X. Zhang and J. Tao, Highly Soluble and Stable, High Release Rate Nanocellulose Codrug Delivery System of Curcumin and AuNPs for Dual Chemo-Photothermal Therapy, Biomacromolecules, 2022, 23(3), 960–971 CrossRef CAS PubMed.
- M. N. I. Amir, A. Halilu, N. M. Julkapli and A. Ma’amor, Gold-graphene oxide nanohybrids: A review on their chemical catalysis, J. Ind. Eng. Chem., 2020, 83, 1–13 CrossRef CAS.
- P. Majumdera and R. Gangopadhyay, Evolution of graphene oxide (GO)-based nanohybrid materials with diverse compositions: an overview, RSC Adv., 2022, 12, 5686–5719 RSC.
- E. A. Asl, M. Pooresmaeil and H. Namazi, Chitosan coated MOF/GO nanohybrid as a co-anticancer drug delivery vehicle: Synthesis, characterization, and drug delivery application, Mat. Chem. Phys., 2023, 293, 126933 CrossRef.
- N. Marino, S. Petralia, M. Perez-Loret, J. Mosinger, S. Conoci and S. Sortino, Graphene oxide nanohybrid photoreleasing nitric oxide, J. Mat. Chem. B, 2016, 4, 5763–5948 RSC.
- S. Mohammadi, A. Babaei and Z. Arab-Bafrani, Polyethylene Glycol-decorated GO Nanosheets as a Well-Organized Nanohybrid to Enhance the Performance of Chitosan Biopolymer, J. Polym. Environ., 2022, 30, 5130–5147 CrossRef CAS.
- S. Kakkar, S. Chauhan Bharti, M. Rohit and V. Bhalla, Conformational switching of aptamer biointerfacing graphene-gold nanohybrid for ultrasensitive label-free sensing of cardiac Troponin I, Bioelectrochemistry, 2023, 150, 108348 CrossRef CAS PubMed.
- G. M. L. Consoli, M. L. Giuffrida, C. Satriano, T. Musumeci, G. Forte and S. Petralia, A novel facile one-pot synthesis of photothermally responsive carbon polymer dots as promising drug nanocarriers, Chem. Commun., 2022, 58, 3126–3129 RSC.
- G. Forte, G. Consiglio, C. Satriano, L. Maugeri and S. Petralia, A nanosized photothermal responsive core-shell carbonized polymer dots based on poly(N-isopropylacrylamide) for light-triggered drug release, Colloids Surf., B, 2022, 217, 112628 CrossRef CAS.
- G. M. L. Consoli, G. Forte, L. Maugeri, V. Consoli, V. Sorrenti, L. Vanella, G. Buscarino, S. Agnello, M. Camarda, G. Granata, L. Ferreri and S. Petralia, Near-Infrared-Responsive Choline-Calix[4]arene-Gold Nanostructures for Potential Photothermal Cancer Treatment, ACS Appl. Nano Mater., 2023, 6, 358–369 CrossRef CAS.
- G. M. L. Consoli, M. L. Giuffrida, S. Zimbone, L. Ferreri, L. Maugeri, M. Palmieri, C. Satriano, G. Forte and S. Petralia, Green Light-Triggerable Chemo-Photothermal Activity of Cytarabine-Loaded Polymer Carbon Dots: Mechanism and Preliminary In Vitro Evaluation, ACS Appl. Mater. Interfaces, 2023, 15, 5732–5743 CrossRef CAS.
- N. Karousis, A. S. D. Sandanayaka, T. Hasobe, S. P. Economopoulos, E. Sarantopoulou and N. Tagmatarchis, Graphene oxide with covalently linked porphyrin antennae: Synthesis, characterization and photophysical properties, J. Mater. Chem., 2011, 21, 109–117 RSC.
- A. Gulino, Structural and Electronic Characterization of Self-Assembled Molecular Nanoarchitectures by X-ray Photoelectron Spectroscopy, Anal. Bioanal. Chem., 2013, 405, 1479–1495 CrossRef CAS PubMed.
- A. Gulino, G. G. Condorelli, P. Mineo and I. Fragalà, An x-ray photoelectron spectra and atomic force microscopy characterization of silica substrates engineered with a covalently assembled siloxane monolayer, Nanotechnology, 2005, 16, 2170 CrossRef CAS PubMed.
- H. Ding, S. B. Yu, J. S. Wei and H. M. Xiong, Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism, ACS Nano, 2016, 10, 484–491 CrossRef CAS.
- L. Motiei, M. Altman, T. Gupta, F. Lupo, A. Gulino, G. Evmenenko, P. Dutta and M. E. Van der Boom, Self-Propagating assembly of a molecular-based multilayer, J. Am. Chem. Soc., 2008, 130, 8913–8915 CrossRef CAS PubMed.
- A. Contino, G. Maccarrone, M. E. Fragalà, L. Spitaleri and A. Gulino, Gold–Porphyrin Monolayers Assembled on Inorganic Surfaces, Chem. – Eur. J., 2017, 23, 14937–14943 CrossRef CAS PubMed.
- J. Choudhury, R. Kaminker, L. Motiei, G. de Ruiter, M. Morozov, F. Lupo, A. Gulino and M. E. Van der Boom, Linear vs. Exponential Formation of Molecular-Based Assemblies, J. Am. Chem. Soc., 2010, 132, 9295–9297 CrossRef CAS PubMed.
- L. Spitaleri, G. Nicotra, M. Zimbone, A. Contino, G. Maccarrone Alberti and A. Gulino, Fast and Efficient Sun Light Photocatalytic activity of Au_ZnO Core-Shell Nanoparticles Prepared by a One Pot Synthesis, ACS Omega, 2019, 4, 15061–15066 CrossRef CAS.
- M. V. Deshmukh, A. A. Vaidya, M. G. Kulkarni, P. R. Rajamohanan and S. Ganapathy, LCST in poly(N-isopropylacrylamide) copolymers: high resolution proton NMR investigations, Polymer, 2000, 41, 7951–7960 CrossRef CAS.
- L. Starovoytova, J. Spevaceka and M. Ilavsky, 1H-NMR study of temperature-induced phase transitions in D2O solutions of poly(N-isopropylmethacrylamide)poly(N-isopropylacrylamide) mixtures and random copolymers, Polymer, 2005, 46, 677–683 CrossRef CAS.
- R. A. Stile, W. R. Burghardt and K. E. Healy, Synthesis and characterization of injectable poly(N-isopropylacrylamide)-based hydrogels that support tissue formation in vitro, Macromolecules, 1999, 32, 7370–7379 CrossRef CAS.
- N. M. Huang, N. H. Lim, C. H. Chia, M. A. Yarmo and M. R. Muhamad, Simple room-temperature preparation of high-yield large-area graphene oxide, Int. J. Nanomed., 2011, 6, 3443–3448 CrossRef CAS.
- S. Jo, T. Kim, V. G. Iyer and W. Im, CHARMM-GUI: A Web-based Graphical User Interface for CHARMM, J. Comput. Chem., 2008, 29, 1859–1865 CrossRef CAS.
- G. Consiglio and G. Forte, Molecular dynamics study of coil-to-globule transition in a thermos-responsive oligomer bound to various surfaces: hydrophilic surfaces stabilize the coil form, Phys. Chem. Chem. Phys., 2018, 20, 29754–29763 RSC.
- Material Studio package, BIOVIA, Dassault Systemes, San Diego, USA, 2017.
- G. Consiglio, S. Failla, C. G. Fortuna, L. D’Urso and G. Forte, Aggregation of a Zn(II)-salen complex: Theoretical study of structure and spectra, Comput. Theor. Chem., 2015, 1067, 1–6 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3tb01863f |
| This journal is © The Royal Society of Chemistry 2024 |