Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution
Xiao-Sai
Hu
,
Rui
Liang
and
Guoxing
Sun
*
Joint Key Laboratory of the Ministry of Education, Institute of Applied Physics and Materials Engineering, University of Macau, Avenida da Universidade, Taipa, Macau, China. E-mail: gxsun@umac.mo
First published on 8th April 2024
Abstract
Correction for ‘Super-adsorbent hydrogel for removal of methylene blue dye from aqueous solution’ by Xiao-Sai Hu et al., J. Mater. Chem. A, 2018, 6, 17612–17624, https://doi.org/10.1039/C8TA04722G.
As outlined in the Comment article, https://doi.org/10.1039/C9TA11420C, several errors were identified in the published article. The authors apologize for the mistakes and outline the corrections below:
1. Adsorption kinetics
Firstly, the issues on the units of rate constants (k1, k2 and kid), and the values of qe and k2 for the pseudo-second-order model can be found corrected in Table 1.| Adsorbent | Dye | Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pseudo-first-order | Pseudo-second-order | Intra-particle diffusion | ||||||||
| q e (mg g−1) | k 1 (h−1) | R 2 | q e (mg g−1) | k 2 × 10−5 (g mg−1 h−1) | R 2 | C (mg g−1) | k id (mg g−1 h−1/2) | R 2 | ||
| NC gel | MB | 1364 | 0.03507 | 0.964 | 1118 | 2.087 | 0.993 | 82.7676 | 68.0952 | 0.949 |
The issues with the units of the KL and KF values are corrected below in Table 3.
Table 2 aimed to compare the adsorption capacity at equilibrium of different adsorbents towards dyes. However, the original Table 2 mistakenly contained the calculated maximum adsorption capacity based on the Langmuir model instead of the experimental equilibrium adsorption capacity. This has been corrected in Table 2 below:
| Adsorbent | Dye | Adsorption capacity (mg g−1) | Reference |
|---|---|---|---|
| Poly(AAc-co-AAm) | Crystal violet | 4 | 12 |
| Basic magenta | 11 | ||
| Poly(AAc) | Basic red 29 | 986 | 13 |
| Methylene blue | 220 | ||
| BPCMC-g-poly (NaAc-co-AM) | Methylene blue | <333 | 2 |
| Poly(AA-co-AMPS)/montmorillonite | Methylene blue | 215 | 15 |
| Poly (AA-co-NaAc-co-AM) | Azure-I | 1169 | 14 |
| β-CD/PAA/GO nanocomposite | Methylene blue | 247 | 27 |
| PAA/CNS | Methylene blue | 2100 | This work |
| Poly(APTMACl)/γ-Fe2O3 | Acid orange 52 | 1428 | 21 |
| Cellulose/chitosan hydrogel | Congo red | 38 | 22 |
| Poly(DEAEMA)/starch | Direct red 81 | <120 | 19 |
| Cellulose nanocrystal-alginate | Methylene blue | 72 | 20 |
| Activated carbon | Methylene blue | 581 | 9 |
| Sodium alginate/PAA/TiO2 | Methyl violet | 728 | 23 |
| Graphene oxide/calcium alginate | Methylene blue | <350 | 44 |
| MgAl-layered double hydroxides | Methylene blue | 121 | 43 |
| Graphene–carbon nanotube | Methylene blue | 68 | 10 |
| Graphene oxide/chitosan sponge | Methylene blue | <300 | 24 |
| Fe3C/Fe3O4/C nanosheets | Methylene blue | 982 | 25 |
| Exfoliated montmorillonite nanosheets (MMTNS) and chitosan (CS) | Methylene blue | <530 | 26 |
| Adsorbent | Dye | Model | Parameter | 298 K | 308 K | 318 K |
|---|---|---|---|---|---|---|
| NC gel | MB | Langmuir isotherm | q max (mg g−1) | 2455 | 1941 | 1504 |
| K L (L mg−1) | 0.0229 | 0.0257 | 0.0377 | |||
| R 2 | 0.9808 | 0.9883 | 0.9941 | |||
| Freundlich isotherm | n | 1.84 | 2.04 | 2.30 | ||
| K F (mg g−1)/(mg L−1)1/n | 127.1 | 130.1 | 143.6 | |||
| R 2 | 0.8955 | 0.8608 | 0.8045 |
After correction, by comparing the experimental equilibrium data, it was found that the NC gel showed a good adsorption capacity for MB. Thus, it can still be claimed that the NC gel is a super-adsorbent.
The statement (on page 17618) “It can be concluded that the pseudo-second-order kinetic mechanism can better describe the adsorption behavior of the NC gel for MB, indicating that the adsorption rate-controlling step was ascribed to the chemical process” should be changed into “Obviously, the pseudo-second-order model is more suitable for describing the adsorption behavior of the NC gel for MB removal.”
2. Adsorption isotherms
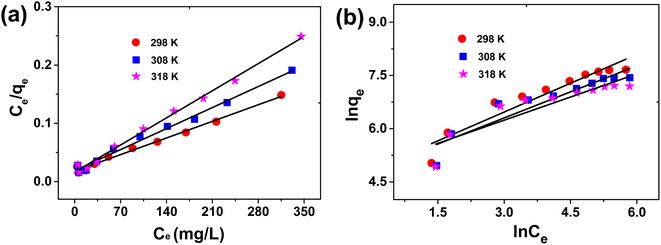
The graph with the qe and the correct Ce values has been provided in Fig. 8. The corresponding qmax value obtained by the Langmuir plot has been provided in Fig. 9a and Table 3.
Fig. 8 Equilibrium isotherms of MB adsorption onto the NC gel adsorbent at different temperatures: pH = 7, t = 7 days, 40 mg L−1 adsorbent.
Fig. 9 Fitting curves of the Langmuir isotherm model (a) and the Freundlich isotherm model (b) of MB adsorption onto the NC gel adsorbent at different temperatures and different initial MB concentrations: pH = 7, t = 7 days, 40 mg L−1 adsorbent.
According to the relevant parameters in Fig. 9 and Table 3, it was clear that the Langmuir model can better match the isotherm data of MB adsorption by the NC gel because the R2 values of the Langmuir model were much higher than those of the Freundlich model at three different temperatures. Based on the Langmuir model, the theoretical maximum adsorption capacity (qmax) of MB by the NC gel was calculated to be 2455 mg g−1 at 298 K, 1941 mg g−1 at 308 K and 1504 mg g−1 at 318 K, which are close to the experimental data (2120 mg g−1, 1720 mg g−1 and 1380 mg g−1). These showed that the Langmuir model can be applied for the adsorption of MB onto the NC gel.
3. Difference between pHpzc and pHIEP
In the “Characterization” section, the authors mentioned “the point of zero charge (pHpzc) of the swollen NC gel was obtained according to the zeta potentials”. “point of zero charge (pHpzc)” should be corrected into “isoelectric point (pHIEP)” in the corrected manuscript.pHpzc is the pH at which the charge of the surface is zero. It is designated the point of zero charge.
pHIEP is the pH at which zeta potential is zero, which can be measured by zeta potential.
When there is no presence of a characteristic adsorption ion, the values of pHpzc and pHIEP are the same.
4. Comment on the energy-dispersive spectroscopy (EDS) data
The EDS spectra in the original published work contains additional elements (Au and Pd), which are from the sputtered gold powder during the EDS test. In the correction, Au and Pd are removed from the EDS spectra.Fig. 4 (c) EDS spectra of the as-obtained Ca3SiO5.
5. Unclear information on the dried mass of adsorbent used in the study of adsorption
The unit of adsorbent dosage in the original published work was g. In the latest correction, the unit of adsorbent dosage should be changed into mg L−1.Fig. 6 (a) Effect of NC gel adsorbent dose on adsorption of MB at room temperature (298 K), pH = 7.0, C0 = 50 mg L−1, t = 7 days.
6. Discussion on the method determining the porosity of the adsorbent
Adobe Photoshop was employed to calculate the porosity of the freeze-dried swollen NC gel. The porosity was defined as the ratio of a pore’s pixel value (A1) relative to the total pixel value (A0) of the SEM image, R(%) = A1/A0 × 100%.7. Other comments
In the section “Dye adsorption experiment” (on page 17614), the authors rewrite the paragraph as the following:The removal ratio (R%) and the adsorption capacity at equilibrium (qe, mg g−1) and at time t (qt, mg g−1) for MB were evaluated using the following eqn (3)–(5):
 | (3) |
 | (4) |
 | (5) |
The adsorbent dosage (8, 24, 40, 56 and 72 mg L−1) on the effect of the NC gel towards MB was firstly studied. Different adsorbent doses were added into 50 mL of MB solution (50 mg L−1) at room temperature (298 K) and at a solution pH of 7.0. The final MB concentration of the solution was determined after a contact time of 7 days.
To investigate the effect of solution pH (1–13) on the removal of MB, NC gel (40 mg L−1) was added into 50 mL of MB solution (50 mg L−1) at room temperature (298 K), in which the initial pH of the solution was adjusted by using HCl or NaOH. The final MB concentration of the solution was analyzed after a contact time of 7 days.
Adsorption kinetics experiments were carried out within different contact times by adding NC gel (40 mg L−1) into 50 mL of MB solution (50 mg L−1) at the optimal solution pH of 7.0 at room temperature (298 K). After designated time periods, the final MB concentration of the solution was determined.
Both adsorption isotherm and thermodynamic experiments were conducted by adding NC gel (40 mg L−1) into 50 mL of MB solution with different initial MB concentrations (10, 20, 50, 70, 100, 150, 200, 250, 300 and 400 mg L−1) at different temperatures (298 K, 308 K and 318 K) with a solution pH of 7.0. The final MB concentration of the solution was determined after a contact time of 7 days.
To investigate the reusability of the adsorbents, 40 mg L−1 adsorbent was added to 50 mL MB solution (50 mg L−1) to achieve saturated adsorption at room temperature (298 K) and pH = 7.0. The MB adsorbed onto the NC gel adsorbent was then eluted with adequate 0.1 M HCl solution and further regenerated in deionized water. The recycled adsorbent was used for the next adsorption cycle.
In the two sentences beginning “This polymerization provided...” (on page 17614 and 17615) that refer to Fig. 1(f), “hydrogen bonds” should be changed into “chelation interactions”.
Fig. 1 Schematic diagram of the NC gel adsorbent. (a) Tricalcium silicate (Ca3SiO5) was dispersed in water at 0 °C and maintained at 0 °C for 3 days; (b) Ca2+ released from the surface of the Ca3SiO5 powder; (c) Ca2+ crystallized to form calcium hydroxide (Ca(OH)2) nano-spherulites (CNSs) with a diameter of around 5 nm; (d) acrylic acid, sodium acrylate and CNSs were mixed uniformly; (e) and (f) formation of the super-adsorbent NC gel using CNSs as cross-linkers.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2024 |