Open Access Article
Open Access ArticleA ciprofloxacin derived task specific ionic liquid as a highly selective extractant of thorium versus uranium†‡
David
Lledó
a,
Guillermo
Grindlay
 b,
H. Q. Nimal
Gunaratne
b,
H. Q. Nimal
Gunaratne
 c,
Abel
de Cózar
c,
Abel
de Cózar
 de,
Ana
Sirvent
de,
Ana
Sirvent
 f and
José M.
Sansano
f and
José M.
Sansano
 *f
*f
aMedalchemy, S. L. Ancha de Castelar, 46-48, entlo. A. San Vicente del Raspeig, 03690-Alicante, Spain
bDepartment of Analytical Chemistry, Nutrition and Food Sciences, University of Alicante, PO Box 99, 03080 Alicante, Spain
cThe QUILL Research Centre, School of Chemistry and Chemical Engineering, The Queen's University of Belfast, Stranmillis Road, Belfast, Northern Ireland, BT9 5AG, UK
dDepartamento de Química Orgánica I/Kimika Organikoa I Saila, Facultad de Química/Kimika Fakultatea, Universidad del País Vasco/Euskal Herriko Unibertsitatea (UPV/EHU), Donostia International Physics Center (DIPC), Centro de Innovación en Química Avanzada (ORFEO-CINQA), P. K, 1072, 20018 San Sebastián-Donostia, Spain
eIkerbasque, Basque Foundation for Science, Plaza Euskadi 5, 48009, Bilbao, Spain
fDepartamento de Química Orgánica, Centro de Innovación en Química Avanzada (ORFEO-CINQA), Institute of Organic Synthesis, Universidad de Alicante, Ctra. Alicante-San Vicente s/n, 03080-Alicante, Spain. E-mail: jmsansano@ua.es
First published on 23rd October 2024
Abstract
A selective extraction system of thorium(IV), formed with a TSIL derived from ciprofloxacin and [BMIM][NTf2] as a co-solvent, is described. Under acidic conditions, this IL solution was able to complex selectively this metal versus the rest of the other metals of the periodic table. The synthesis of the TSIL was detailed using known procedures and it was isolated in good overall yield. The extraction coefficients (K) were determined by a microextractive survey at 25 °C. Spectroscopic analyses of thorium(IV) and uranium(VI) complexes were performed in order to demonstrate the strength of the metal–TSIL interaction, which was supported by DFT-based computational optimization of the formation of the experimentally observed complexes. Kinetic analysis, loading tests for the extractive protocol, and the optimization for recycling were also performed to justify the conditions of a laboratory prototype for this selective extraction of thorium(IV) salts.
Introduction
Stimulated by the world's demand for energy, nuclear reactors are expected to play a key role as a power source in the future due to the low emission of carbon dioxide or other greenhouse gases and the abundant fuel reservoirs.1,2 However, regarding the fuel sources, uranium and thorium (the actinide elements that are predominantly present in nature) are very dispersed in ores and require selective extraction methods. Additionally, the waste that is generated as a result of the use of this energy source poses a great threat to the environment and human health. In this regard, one of the most important processes in the management of nuclear waste is the intra- and inter-group separation of lanthanides and actinides.Thorium is rather diluted in rock thus requiring more mining, milling and treatment local to where it is recovered than is the case for uranium.3 Apparently, thorium is much more abundant than uranium in nature3 and has become one of the important potential energy sources for the future. In fact, thorium is occasionally heralded as a ‘safe’ alternative to uranium and plutonium and it has often been integrated into reactor system designs via accelerator-driven systems.4 This is because a thorium-232 (232Th) nucleus has the ability to produce a uranium-233 (233U) nucleus by absorbing a neutron. Thorium itself is known as fertile5 because it is not fissile but provides a fissile uranium isotope 233U. In the long term, consumption of thorium could increase substantially if its use as a nuclear fuel becomes commercialized. Thorium is anticipated to produce lower quantities of minor actinides and no plutonium, considerations often perceived as key advantages in terms of long-term radioactive waste management.
In addition, [Th(IV)] and its compounds are highly toxic and cause severe damage to bone and kidney through very long-term exposures. Conventional methods of Th(IV) detection are complicated and costly. However, the daughters of Th-229 (Ac-225 and Bi-213) and other α-emitting radioisotopes are rapidly becoming of great interest for short-range and site-specific therapy of cancers and micrometastatic diseases.6
The separation of thorium(IV) is very important in countries such as India and China. However, the process is quite challenging and co-extraction of uranium and thorium is frequently reported.7–11
For this purpose, the most common traditional methods are liquid–liquid extraction, ion exchange high performance liquid chromatography12 and the use of hollow fiber renewal liquid membranes (HFRLM, designed for reusing the system).13–16 However, a significant disadvantage of these processes is the waste chemicals generated.17
Examples of liquid–liquid extraction for specific and selective Th(IV) separation have been described in the literature using as extraction systems, for instance, phosphate buffer solutions,18 hydrophobic deep eutectic solvents using thorin as a chelating agent,19 or fatty acids.20 Separation processes that employ extractant systems based on 2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy] acetic acid (POAA),21 functionalized graphene oxide with phenanthroline diamide (GO-PDA),22 or 1,5-bis[о-(dioxyphosphoryl)-π-ethylphenoxy]-3-oxapentane and trioctylamine have been also used to selectively recover Th(IV) from solutions.23,24 Other ligands that have been found to be specific for the selective extraction of thorium(IV) are N-n-heptylaniline,25,26 2,3-dihydroxynaphthalene,27 tri-n-butylphosphate (TBP),28N,N-di-n-hexyloctanamide (DHOA),29 tri-n-octylphosphineoxide (Cyanex 923)30 and bis(2,4,4-trimethyl)pentyl phosphinic acid (Cyanex 272)31 were found to be specific Th(IV) ligands for extractive processes.32,33 Selective extraction of thorium from uranium and rare earth elements using a sulfonated covalent organic framework and its membrane derivate was also reported.34 Metal organic frameworks (MOFs) have been employed as selective extractants.35
If we focus our attention on the favorable properties of ionic liquids (ILs) as extractants,36–38 room temperature ionic liquids (RTILs) are considered as a replacement of molecular diluents in the extraction of metal ions from an aqueous phase. Thus, for example, dialkylsulfosuccinate-based ionic-liquids,39 and N,N-dialkyl-malonamic acid (MA)-based ionic liquid anions were synthesized and applied for the extraction of thorium ions using a nitric acid solution. The solid samples were obtained by simultaneous extraction and solidification of Th(IV) based on a self-assembly process. Dioctadecyldimethylammonium ([DC DMA]) was pioneered as the preferred organic cation of the ionic liquids for the extraction process.40,41 IL based solvent systems are found to be radio resistant compared to molecular diluent based solvent systems. They act as a sink for the radiation damage to protect the ligand functionality. Along these lines, TSILs have been used for selective extraction of uranium (phosphoramidate-containing ILs)42 and for excellent separation of thorium(IV) from other metals (including uranium);43 a TSIL formed by CMPO-functionalized ionic liquid 1-[3-[[(diphenylphosphinyl)acetyl]amino]propyl]-3-tetradecyl-1H-imidazol-3-ium hexafluorophosphate (CMPO-FIL) was used for general Ln(III), Th(IV) and U(VI) extraction.44 TSILs are frequently used for the extraction of other actinides.45
In general, stripping procedures are performed employing inorganic acids at different pHs. In most cases, the recovery of the TSIL is not considered but only the amount of actinide ion stripped. Employing ILs together with extractive ligands is very well documented, for example amines,46 1,3-diketones (stripping with SC-CO2),47N,N-dialkylamides (stripping with SC-CO2),48 diglycolamides,49–51 phosphine oxides (stripping with oxalic acid),52 using phosphane derived ligands (stripping with acidic solutions),53 methyl imidazoles,54,55N,N′-dimethyl-N,N′-dioctyl-4-oxaheptanediamide (DMDOHA),56 carbamoylmethylphosphine oxides (CMPO),57,58 polymeric CMPO,59N,N,N,N-tetraoctyldiglycolamide (TODGA),60 other tridentate and tetradentate amides,61 and N,N-di(2-ethylhexyl)-diglycolamic acid.62 In most cases, the ionic liquid based systems stripping was found to be the most challenging compared to molecular diluents and required strong aqueous complexing agents.
Concerning the coordination of thorium(IV) and uranium(VI) (uranyl cation in aqueous solution) the hexacoordination is the most common feature,63,64 although there are uncommon environments published in the literature.65,66 In addition, one of the most promising areas in f-element research in ILs is computer-aided simulations that aim to elucidate the solvating power of ILs towards actinides, lanthanides, and even fission products, all of which are important components of nuclear wastes and processing streams.67
According to all these precedents, we envisage that the design and computational simulation of a TSIL considering the preferential coordination of Th(IV) would be crucial for efficient modulation of the selectivity of separation of this metal and for the implementation of a very easy and environmentally benign method for the recovery of the metal and the TSIL/IL system.68 Herein, we report a concise synthesis of a TSIL system based on antibiotic ciprofloxacin for highly selective extraction of thorium(IV).
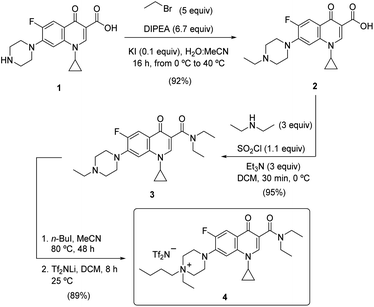
Results and discussion
Synthesis of the TSIL 4 and preliminary microextractions
1,3-Dicarbonyl compounds are known as excellent ligands for stabilizing metal complexes,69 specially in the case of highly coordinative rare earth metals.70 Previous results demonstrated that an IL tethered to a 1,2-diamide motif exhibited a potent donor effect extracting all lanthanide series without any selectivity.62 The basic and rapid in silico analysis of the electronic structure/interactions of the β-dicarbonylic moiety, combining an aromatic ketone and an amido array (in order to modulate the affinity by the cationic metals) and including a hydrophobic area, able to interact with the solvent of the extractive system, turned our attention to the ciprofloxacin structure 1. The inexpensive commercially available compound 1 was N-alkylated with ethyl bromide, in an acetonitrile/water mixture, and catalyzed by potassium iodide. The resulting compound 2 (isolated in 92% yield) underwent the amidation reaction using N,N-diethylamine, after the in situ generation of the chloride of the corresponding acid with thionyl chloride in dichloromethane, at 0 °C for 30 min. Amide 3 (obtained in 95% yield) was transformed on its quaternary ammonium salt, in the presence of n-butyl iodide, followed by ion exchange using lithium bis(trifluorosulfonyl)amide in dichloromethane at room temperature for 8 h. These two steps afforded the final TSIL molecule 4 in 89% (78% overall yield from ciprofloxacin 1) (Scheme 1). It is remarkable that the association of the bis-triflimide anion (NTf2) would ameliorate the extraction of such metals from an aqueous phase to a hydrophobic environment.Next, the micro-extractive multimetallic survey of the extraction system, composed of a 0.005 M solution of TSIL 4 in [BMIM][NTf2] (100 μL), was performed (at 25 °C) from a multimetal sample (5 mL) (Li, B, Al, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, As, Se, Zr, Mo, Ru, Pd, Ag, Cd, In, Sn, Sb, Te, Pt, Au, Hg, Tl, Pb and Bi) using ICP-MS as an instrumental analytical technique (employing rhodium as an internal standard). For all the elements, the extraction coefficient, defined as the ratio between the concentration of the metal in the organic phase and the aqueous phase after the extraction procedure (eqn (1)), was calculated at different pHs (1–6).
| K = final [M]org/final [M]aq | (1) |
Table 1 shows the results obtained under optimum experimental conditions for each element (Kmax). After analysing the pH range 1–6, in the third column of Table 1 the pHs in which the maximum K was obtained using the ICP technique are depicted. The same conditions were used for the extraction of all elements of Table 1, where K values registered at pH = 1 were also included (fourth column of Table 1) in order to compare the extraction of all metals. With the later value, it was observed that thorium (Table 1, entry 1) was selectively extracted in comparison with other different rare earth metals, and with the rest of the series s, d and p metals (Table 1). Only scandium, mercury and bismuth exhibited a significant (but low) extraction coefficient (K) at the end of the process (Table 1, entries 3, 46 and 49). But the paramount result (and the main goal) of this work was the large extraction difference observed between thorium and uranium (Table 1, entries 1 and 12).
| Entry | Metal | pH/Kmax | K at pH = 1 |
|---|---|---|---|
| 1 | Th | 1/197 | 197 |
| 2 | Nd | 4/136 | 8 |
| 3 | Sc | 2/129 | 30 |
| 4 | Sm | 6/82 | 3 |
| 5 | Eu | 6/77 | 3 |
| 6 | Tb | 6/59 | 3 |
| 7 | Gd | 6/52 | 1 |
| 8 | Pr | 6/50 | 4 |
| 9 | Dy | 6/50 | 2 |
| 10 | Ho | 6/38 | 3 |
| 11 | Ce | 6/37 | 0 |
| 12 | U | 6/34 | 7 |
| 13 | Er | 6/30 | 1 |
| 14 | Tm | 6/26 | 1 |
| 15 | Y | 4/25 | 2 |
| 16 | Yb | 6/21 | 1 |
| 17 | La | 6/20 | 0 |
| 18 | Lu | 6/18 | 2 |
| 19 | Li | 6/2 | 0 |
| 20 | B | 6/2 | 0 |
| 21 | Al | 6/8 | 0 |
| 22 | Ti | 6/107 | 3 |
| 23 | V | 6/4 | 1 |
| 24 | Cr | 6/4 | 0 |
| 25 | Mn | 6/2 | 0 |
| 26 | Fe | 6/4 | 1 |
| 27 | Co | 6/1 | 0 |
| 28 | Ni | 6/1 | 0 |
| 29 | Cu | 6/21 | 0 |
| 30 | Zn | 6/32 | 0 |
| 31 | Ga | 6/8 | 1 |
| 32 | As | 6/3 | 0 |
| 33 | Se | 6/0 | 0 |
| 34 | Zr | 1/3 | 3 |
| 35 | Mo | 1/2 | 2 |
| 36 | Ru | 6/4 | 0 |
| 37 | Pd | 1/4 | 4 |
| 38 | Ag | 1/6 | 6 |
| 39 | Cd | 6/4 | 0 |
| 40 | In | 1/4 | 4 |
| 41 | Sn | 6/4 | 0 |
| 42 | Sb | 6/1 | 0 |
| 43 | Te | 6/3 | 0 |
| 44 | Pt | 6/0 | 0 |
| 45 | Au | 6/0 | 0 |
| 46 | Hg | 1/23 | 23 |
| 47 | Tl | 6/0 | 0 |
| 48 | Pb | 6/3 | 0 |
| 49 | Bi | 2/28 | 10 |
The amazing selectivity of the extraction of thorium(IV) versus uranyl cations or even the rest of rare earth metals controlling the pH at 1 can be observed in the histogram depicted in Fig. 1. Thorium exists as a Th4+ cation, whereas uranium remains in acidic solutions as the UO22+ cation, so these cations are chemically very different and consequently, no parallelism is established in the next tests. We will lay emphasis in demonstrating the high selectivity observed during the extraction process of these two cations.
 | ||
| Fig. 1 Plot of the K values at pH = 1 of the extraction of rare earth metals by TSIL 4 in [BMIM][NTf2]. | ||
Spectroscopic analyses of thorium(IV) and uranium(IV) complexes
The metal-cation complexation to the ionic liquid 4 was studied by spectroscopic methods (1H NMR, 13C NMR and FTIR) under analogous conditions. The maximum coordination number of the TSIL (4) ligands per cation was determined by the signal chemical shifting of the singlet that appeared at 7.95 ppm and the doublet located at 7.73 ppm. The additional doublet at 7.42 ppm was hardly affected by the ligand–metal cation interaction. Thus, uranyl nitrate was combined with different proportions of ligand 4 in DMSO-d6 at room temperature. Although the chemical shift variation was not notable, the sequence of the spectra clearly revealed that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of uranyl nitrate
1 ratio of uranyl nitrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TSIL 4 is the coordination pattern in this system (stack plot of Fig. 2A). The difference of ppm in 13C NMR was 0.6 ppm for the two carbonyl groups of the ciprofloxacin scaffold (Fig. 2B). However, a stronger interaction between the thorium(IV) cation and TSIL 4 was observed in DMSO. The increment of the chemical shifts was very important and a 1
TSIL 4 is the coordination pattern in this system (stack plot of Fig. 2A). The difference of ppm in 13C NMR was 0.6 ppm for the two carbonyl groups of the ciprofloxacin scaffold (Fig. 2B). However, a stronger interaction between the thorium(IV) cation and TSIL 4 was observed in DMSO. The increment of the chemical shifts was very important and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of thorium(IV) nitrate was the coordination pattern (stack plot of Fig. 2C). The separation of the carbonyl group signals between the free ligand 4 and Th(IV)·4313C NMR spectra showed a difference of 2 ppm (Fig. 2D) that justifies also a powerful metal-4 interaction.
3 ratio of thorium(IV) nitrate was the coordination pattern (stack plot of Fig. 2C). The separation of the carbonyl group signals between the free ligand 4 and Th(IV)·4313C NMR spectra showed a difference of 2 ppm (Fig. 2D) that justifies also a powerful metal-4 interaction.
The complementary plots depicted in Fig. 3 and 4, showing the variation of Δδ (difference in the chemical shift = δ4·M − δ4) against the concentration 4:Mx+ (mmol mL−1), provide the best visual behavior of the chelating process. So, this quantitative analysis revealed that, independently of the number of coordinating molecules of DMSO solvating both cations, the preference towards three ligands 4 by the thorium(IV) species contrasted with the affinity of the uranium(VI) units by only one 1,3-dicarbonyl ligand 4. Fig. 3 and 4 illustrate all these results. The uranyl cation only exhibited a weak interaction with one ligand 4 due to the low increment of the chemical shift of the 7.95 ppm singlet observed versus the analogous chemical shift of pure molecule 4 (Δδ ∼ 0.005 ppm) (Fig. 3). The analysis of the same effect in the formation of the [Th]·43 indicated a stronger metal–ligand interaction due to a larger Δδ ∼ 0.009 ppm (Fig. 4A). However, the highest signal displacement (almost 0.011 ppm) was detected for the doublet placed at 7.73 ppm (Fig. 4B).
 | ||
| Fig. 3 Plot of the variation of the chemical shift (in ppm) versus concentration of the TSIL 4 during the titration of uranyl nitrate monitored by 1H NMR spectroscopy. | ||
An ATR-FTIR study was employed to confirm the strongest interaction detected between 4 and thorium(IV) rather than the TSIL 4 with uranium(VI) species. The samples were prepared in a solution in the smallest amount of DMSO and methanol and, after mixing the components and refluxing for 1 h, the solvent was evaporated under vacuum. The remaining DMSO, the ciprofloxacin fingerprint signals, and the absorption peaks of the corresponding cations offered a small window of the infrared region (1450–1790 cm−1) in which band shifting could be detected. Peaks around 800–1000 and 1000–1100 cm−1, which correspond to the UO22+ cation, could not be observed due to the presence of an intense and wide absorption band. Thus, mixtures of pure 4, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [TSIL (4)]
1 [TSIL (4)]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Th4+] and 1
[Th4+] and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [TSIL (4)]·[U6+] were separately suspended in DMSO/methanol and then refluxed for 1 h. After evaporation of the solvent the sticky powders were analysed. Fig. 5 reveals a significant downward shift to lower wave numbers for the carbonyl group stretching frequency from 1736 cm−1 to 1716 cm−1 and from 1681 cm−1 to 1663 cm−1. The metal–O interactions forced a reduction of the bond order of the 1,3-dicarbonylic moiety. Unfortunately, crystalline structures could not be isolated to determine the real coordination environment of the metal.
1 [TSIL (4)]·[U6+] were separately suspended in DMSO/methanol and then refluxed for 1 h. After evaporation of the solvent the sticky powders were analysed. Fig. 5 reveals a significant downward shift to lower wave numbers for the carbonyl group stretching frequency from 1736 cm−1 to 1716 cm−1 and from 1681 cm−1 to 1663 cm−1. The metal–O interactions forced a reduction of the bond order of the 1,3-dicarbonylic moiety. Unfortunately, crystalline structures could not be isolated to determine the real coordination environment of the metal.
DFT-based computational optimization of the complexes
The X-ray diffraction analyses of solid samples of the corresponding metal–cyprofloxacin aggregates could not be successfully completed. This fact moved us to analyze the observed coordinative preferences of both metals with the ligand 4. Hexacoordination of the uranyl cation is very well documented71,72 and this strict profile contrasts with the variable coordination of the thorium(IV). The ability of thorium to coordinate in different geometries and a wide range of coordination numbers confirm the difficulty to predict its preferred geometry.73 Within this complex scenario, we decided to perform DFT calculations trying to shed light on the different behavior observed for uranyl and thorium(IV) complexes (see the ESI‡ for computational details).All the microextractions and other tests, such as loading of the extractive mixtures, as well as the solution stability studies, were conducted with the corresponding metal nitrates. The acidic pH for the selective extraction was achieved using nitric acid, so we focused our attention on these species whose internal spheres were surrounded by nitrate anions and water molecules. In order to simplify the model of calculations the energies corresponding to the substitution of molecules of water by ligand 4 units (Scheme 2) in an aqueous environment were obtained.
We hypothesize that the extraction coefficients are related with the water–TSIL(4) exchange equilibrium where 4·metal complexes will be extracted in the IL phase, whereas water complexes would remain in the aqueous phase. In that sense, enhanced extraction coefficients would be related to the exergonic character of the exchange reaction. In contrast, low extraction coefficients would be related to endergonic reactions [note that in the proposed reaction, two molecules of water are exchanged by one molecule of 4].
Given the plethora of possible coordination patterns and metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand ratio combinations, especially in the case of thorium, the coordination number for the uranyl cation was fixed at six and the selected coordination numbers for thorium(IV) were ten and eight because they are the most frequent coordination numbers reported for this cation.72 In addition, we took advantage of the spectroscopic results, so compounds with a 1
ligand ratio combinations, especially in the case of thorium, the coordination number for the uranyl cation was fixed at six and the selected coordination numbers for thorium(IV) were ten and eight because they are the most frequent coordination numbers reported for this cation.72 In addition, we took advantage of the spectroscopic results, so compounds with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for the uranyl nitrate
1 ratio for the uranyl nitrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TSIL 4 complex and 1
TSIL 4 complex and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 thorium(IV) nitrate
3 thorium(IV) nitrate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TSIL 4 complex were considered respectively (vide supra). The obtained results indicated that the water–ligand exchange is not thermodynamically favored for uranyl nitrate as shown by the Gibbs reaction energy value of +1.8 kcal mol−1. As a consequence, only residual extraction of uranyl nitrate is expected (Fig. 6A).
TSIL 4 complex were considered respectively (vide supra). The obtained results indicated that the water–ligand exchange is not thermodynamically favored for uranyl nitrate as shown by the Gibbs reaction energy value of +1.8 kcal mol−1. As a consequence, only residual extraction of uranyl nitrate is expected (Fig. 6A).
The Gibbs free energies of the thorium(IV) complexes with the different most abundant coordination numbers (eight and ten)72 were calculated independently (Fig. 6B and C). In this case, our calculations indicated that the water–ligand exchange process is strongly favored for thorium(IV) nitrate both considering 10 and 8 coordinated complexes (Gibbs reaction energy of −31.8 kcal mol−1 or –99.8 kcal mol−1, respectively). Therefore, complete extraction of thorium(IV) is theoretically assessed, in good agreement with the experimental evidence (see the ESI‡ for further information about the equilibrium reaction of other complexes presumably involved). Moreover, the computed data revealed that these two possible complexes of thorium bearing three units of ligand 4 and different number of nitrate anions (apart from others in equilibrium) can exist in the solution making it very difficult to obtain a pure monocrystal.
Kinetic analysis and loading tests
The kinetics of the affinity of the TSIL compound 4 towards the thorium(IV) cation at pH = 1 was next evaluated using ICP as a detection technique. In this first experiment, an aqueous 0.1 M solution of thorium(IV) nitrate (5 mL) was extracted with a 0.1 M solution of 4 in [BMIM][Tf2N] (1 mL). Using an excess of thorium cations in the aqueous solution to be extracted (Fig. 7a), the amount of the extracted thorium(IV) remained constant at 10 min. The maximum amount of metal cation reached 0.033 mmol approximately, which is in agreement with the already mentioned most stable geometry of ThIV·43.Under the same conditions, a second experiment was run using an aqueous 6.7 × 10−3 M solution of thorium(IV) nitrate (5 mL), which was extracted with a 0.1 M solution of 4 in [BMIM][Tf2N] (1 mL). Here, the final 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 metal
3 metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TSIL 4 ratio was again confirmed and the kinetics were very similar to the previous test, completing the overall extraction in 10–11 min (Fig. 7b).
TSIL 4 ratio was again confirmed and the kinetics were very similar to the previous test, completing the overall extraction in 10–11 min (Fig. 7b).
The kinetics of the extractive system (0.1 M solution of 4 in [BMIM][Tf2N], 1 mL) versus an original solution (5 mL) containing 0.1 M solution of both thorium(IV) and uranyl nitrates was assessed (Fig. 8). The extraction profile of the thorium cation was identical to the plot observed in Fig. 7A. However, the uranyl cation was slightly extracted at the earlier stages of the extractive sequence but finally, after 3 min, the complexation of ligand 4 with uranium(VI) was extremely low.
 | ||
| Fig. 8 Competitive extraction of 0.1 M of both thorium(IV) and uranyl nitrate with 0.1 M solution of 4 in [BMIM][Tf2N]. | ||
The plot fragment between 0 and 3 min in Fig. 8 suggests that thorium captured the ligand initially bound to the uranyl cation. Thus, a solution of uranyl nitrate (0.2 M, 1 mL) at pH = 1, and a solution of 4 in [BMIM][Tf2N] (0.1 M, 1 mL) were stirred vigorously for 1 h at 25 °C. After separation of both phases, the aqueous one was discarded and the IL phase was analyzed by ICP (after the digestion and the appropriate preparation of the sample). The final amount of the uranium(VI) species revealed that 0.090 mmol of them were ligated to the IL system. Next, this organic solution was mixed with a thorium nitrate solution (0.035 M, 1 mL) at pH = 1 and the liquid was vigorously stirred for 10 min at 25 °C. The two phases were separated and analyzed by ICP obtaining dd(VI) in the aqueous portion, whilst all the thorium(IV) was concentrated in the IL medium (eqn (2)).
| 3[UO22+·4] + Th4+ → Th4+·43 + 3[UO22+] | (2) |
A ternary combination of metals composed of thorium(IV), scandium(III) and uranyl nitrates was analogously assessed under the identical experimental pathway showing the same behavior (see the Experimental section). Therefore, the selective extraction of thorium(IV) versus other different metal cations can be extrapolated. However, the main aim of this article is to highlight the selectivity of the extraction of thorium versus uranium.
Optimal loading of the extractive solution and recycling studies
Several protocols were designed to determine the optimum loading of the extractive mixture and its stability to run several cycles for an efficient recovery. The extraction of solution A was performed by stirring the mixed phases (A + B) for 10 min followed by the separation of the aqueous phase (A) containing the uranium and the IL phase (B) with the thorium(IV) cation (Fig. 9).The stripping process recovering the IL phase (B) without the thorium(IV) cation was carried out according to Fig. 10. For this purpose, a phosphate buffered aqueous solution at pH = 6 (solution C) was added to solution B and the resulting mixture stirred for 6 min (see below). Thorium(IV) was dissolved in the C solution whilst the B solution was reused in another extractive process. The yield of the extraction was 98% (according to ICP analysis).
Following these two procedures, the optimal concentration of 4 in [BMIM][Tf2N] was assessed, determining the most appropriate one ensuring a total recovery of the IL solution B. Three different concentrations of 4 in [BMIM][Tf2N] were tested, 0.5 M, 0.4 M and 0.3 M. To our surprise, the solution with the highest concentration seemed unstable once the metal is fully coordinated by the ligand (Fig. 11). 0.4 M solution was appropriate at the beginning but, after the 4th extractive cycle, the yield of the recovery was reduced to 88%. The optimal concentration was 0.3 M, which can be used for more than seven cycles, maintaining stripping yields higher than 96% (Fig. 11).
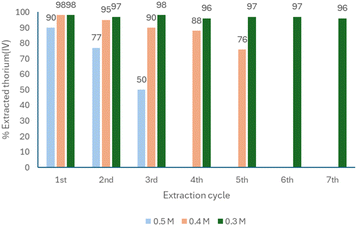 | ||
| Fig. 11 Stability of the concentration of 4 in [BMIM][Tf2N] after extraction of thorium(IV) and its recovery. | ||
Finally, the kinetics of the stripping sequence were studied following the protocol of Fig. 10 using a 0.3 M solution of 4 in [BMIM][Tf2N]. In the plot of Fig. 12 the constant amount of the stripped thorium(IV) was found after 6 min.
Conclusions
The solution of 4, a TSIL derived from ciprofloxacin, in [BMIM][Tf2N] (as an IL solvent) was extremely efficient for the extraction of thorium(IV) cations versus uranium(VI) at pH = 1. The spectroscopic tests confirmed that three ligands 4 coordinated strongly to a single thorium(IV) cation whilst only one ligand 4 was very weakly coordinated with the uranyl cation. The simplified aqueous model, established for DFT calculations, confirmed the high affinity of 4 with thorium(IV) in several coordination (8 and 10) modes. The stability of the optimal solution of 4 in the IL was 0.3 M for the extraction and the mixing time for extraction was 11 min. The recycling of the resulting IL mixture was very satisfactory up to the 7th batch using a stripping process at pH = 6 for 6 min. The designed kinetic models could be considered as a very important tool for predicting the appropriate extraction processes. This prototype is ready to be implemented in mining selective extraction or even in the separation of metals from the radioactive waste mixtures in nuclear power plants.In comparison with the three more representative examples reported in the literature (to the best of our knowledge) where excellent extractions of thorium(IV) vs. uranium(VI) were demonstrated, here we reported the most comprehensive environmental/sustainable system. The separation by employing the recyclable sulfonated membrane34 showed excellent selectivity for the separation of thorium vs. uranium(VI) and two rare earth elements. Here, the recovery of thorium(IV) is very high but with lower K than the analogous ones obtained in this study. Similar conclusions can be deduced comparing the MOF-LIC-1 system.35 In addition, in the titled method a complete multimetallic test confirmed its preference for thorium(IV). Finally, the phosphoramididate-based RTIL method43 showed comparable extractive results with high efficiency of the recovery step. However, the procedure involved chloroform as a co-solvent, which is not desirable in a high scale procedure.
Experimental section
General remarks
Reagents and solvents were purchased from commercial suppliers and used as received. Low-resolution mass spectra (EI) were obtained with an Agilent GC/MS5973N spectrometer at 70 eV, with fragment ions in m/z and relative intensities (%) in parentheses. NMR spectra were recorded at 300 or 400 MHz for 1H NMR, at 75 or 100 MHz for 13C NMR and at 282 or 376 MHz for 19F NMR with a Bruker AV300 Oxford or a Bruker AV400 spectrometer, respectively, using CDCl3 as solvent, and TMS as an internal standard (0.00 ppm). The data are reported as: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet or unresolved, br s = broad signal, coupling constant(s) in Hz, integration. 13C NMR spectra were recorded with 1H-decoupling at 100 MHz and referenced to CDCl3 at 77.16 ppm. 19F NMR spectra were recorded with 1H-decoupling and referenced to CF3CO2H at −76.6 ppm. ICP and microanalyses were performed using a PerkinElmer Optima 7300 DV and a LECO Micro TruSpec, respectively.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 30 mL mmol−1, 240 mL), bromoethane (5.0 equiv., 3.0 mL, 4.4 g, 40 mmol) was added dropwise at 0 °C. Potassium iodide (10 mol%, 132 mg, 0.8 mmol) was then added at the same temperature. The mixture was stirred at 0 °C for 1 h and then it was heated to 40 °C and it was stirred at this temperature for 16 h. The solvent was removed under reduced pressure and the residue was dissolved in DCM (40 mL), washed with a phosphate buffer solution at pH = 6.5 (30 mL) and dried with MgSO4. The solvent was removed under reduced pressure and pure compound 2 was obtained without further purification (2.6 g, 7.4 mmol, 92%) as a white solid; 1H NMR (300 MHz, CDCl3) δ 8.75 (s, 1H), 7.99 (d, J = 13.1 Hz, 1H), 7.36 (d, J = 7.1 Hz, 1H), 3.58–3.52 (m, 1H), 3.39–3.34 (m, 4H), 2.73–2.65 (m, 4H), 2.52 (q, J = 7.2 Hz, 2H), 1.42–1.32 (m, 2H), 1.22–1.18 (m, 2H), 1.15 (t, J = 7.2 Hz, 3H), *Proton from the carboxylic acid was not observed; 13C NMR (101 MHz, CDCl3) δ 177.2, 167.8, 153.8 (d, J = 252.5 Hz), 147.5, 146.1 (d, J = 10.1 Hz), 139.2, 119.8 (d, J = 8.1 Hz), 112.4 (d, J = 24.2 Hz), 108.2, 104.9, 52.5 (2C), 52.4, 49.9 (2C), 35.4, 12.0, 8.3 (2C); 19F NMR (282 MHz, CDCl3) δ −120.6 (s, 1F); IR (neat) νmax 3420–3395, 1707–1675 cm−1; LRMS (EI) m/z 360 (M+ + 1, 16%), 359 (M+, 69%), 344 (24), 316 (21), 315 (100), 287 (13), 257 (13), 230 (12), 150 (10), 84 (24), 70 (12), 57 (32), 56 (11), 44 (27), 42 (19). Elemental analysis calcd for C19H22FN3O3: C, 63.50; H, 6.17; F, 5.29; N, 11.69; O, 13.35. Found: C, 63.3; H, 6.5; N, 11.5.
1, 30 mL mmol−1, 240 mL), bromoethane (5.0 equiv., 3.0 mL, 4.4 g, 40 mmol) was added dropwise at 0 °C. Potassium iodide (10 mol%, 132 mg, 0.8 mmol) was then added at the same temperature. The mixture was stirred at 0 °C for 1 h and then it was heated to 40 °C and it was stirred at this temperature for 16 h. The solvent was removed under reduced pressure and the residue was dissolved in DCM (40 mL), washed with a phosphate buffer solution at pH = 6.5 (30 mL) and dried with MgSO4. The solvent was removed under reduced pressure and pure compound 2 was obtained without further purification (2.6 g, 7.4 mmol, 92%) as a white solid; 1H NMR (300 MHz, CDCl3) δ 8.75 (s, 1H), 7.99 (d, J = 13.1 Hz, 1H), 7.36 (d, J = 7.1 Hz, 1H), 3.58–3.52 (m, 1H), 3.39–3.34 (m, 4H), 2.73–2.65 (m, 4H), 2.52 (q, J = 7.2 Hz, 2H), 1.42–1.32 (m, 2H), 1.22–1.18 (m, 2H), 1.15 (t, J = 7.2 Hz, 3H), *Proton from the carboxylic acid was not observed; 13C NMR (101 MHz, CDCl3) δ 177.2, 167.8, 153.8 (d, J = 252.5 Hz), 147.5, 146.1 (d, J = 10.1 Hz), 139.2, 119.8 (d, J = 8.1 Hz), 112.4 (d, J = 24.2 Hz), 108.2, 104.9, 52.5 (2C), 52.4, 49.9 (2C), 35.4, 12.0, 8.3 (2C); 19F NMR (282 MHz, CDCl3) δ −120.6 (s, 1F); IR (neat) νmax 3420–3395, 1707–1675 cm−1; LRMS (EI) m/z 360 (M+ + 1, 16%), 359 (M+, 69%), 344 (24), 316 (21), 315 (100), 287 (13), 257 (13), 230 (12), 150 (10), 84 (24), 70 (12), 57 (32), 56 (11), 44 (27), 42 (19). Elemental analysis calcd for C19H22FN3O3: C, 63.50; H, 6.17; F, 5.29; N, 11.69; O, 13.35. Found: C, 63.3; H, 6.5; N, 11.5.
General procedure for the selective extraction of thorium versus uranium
To extract the analytes (thorium and uranium) from an aqueous sample A (5 mL of both 0.1 M thorium nitrate and 0.1 M uranyl nitrate), the pH of this sample was adjusted to 1.0 with 1 M aqueous solution of HNO3. Then, the extractant [5 mL of a 0.3 M solution of compound 4 in [BMIM][NTf2] (5)] was added and the solution was stirred for 11 minutes using a vortex mixer. The mixture was then allowed to rest and the two phases were separated. Thorium was extracted in solution B (IL), while uranium remains in the aqueous sample A. Fig. 9 shows a detailed scheme of the selective extraction process of thorium versus uranium.Yield of extracted thorium(IV) >99.5% (from ICP-MS data analysis). Uranium(VI) content in solution A >99.5% (from ICP-MS).
General procedure (stripping) for the liberation of the extracted metal and recuperation of the extraction system (4 in 5)
To remove the thorium from solution B and recover the extraction system (4 in 5), a dihydrogen–monohydrogen phosphate buffer solution at pH = 6.0 (solution C, 5 mL) was added to solution B (4 in 5 saturated in thorium, 5 mL). The mixture was stirred in a vortex mixer for 7 min. The mixture was then allowed to rest and the two phases were separated. Thorium was extracted in aqueous phase C, and the extraction system 4 + 5 was recovered (B). Fig. 10 shows a detailed scheme of the recovery of the extraction system. The thorium recovery was higher than 98% (from ICP-MS data analysis) and the recovered extraction system could be used in subsequent extraction cycles.Data availability
NMR spectra of all new compounds, kinetics of a ternary component extraction survey and DFT models with their Cartesian coordinates are included in the ESI.‡ “This material is available free of charge via the Internet at https://pubs.acs.org”.Author contributions
Dr D. L. and A. S. performed the experimental work and the revision of the article (investigation and methodology). A. d. C. performed all the computational work. H. Q. N. G. supervised the international collaboration and conceptualization. G. G. and J. M. S. directed and supervised all the studies, experiments, conceptualization, funding acquisition, project administration, and writing the original manuscript and the corresponding revisions and editions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the continued financial support from the Spanish Ministerio de Economía y Competitividad (CTQ2017-85093-P) and Ministerio de Ciencia, Innovación y Universidades (RED2018-102387-T, RED2022-134287-T, PID2019-104772GB-I00 and PID2019-107268GB-100) funded by MCIN/AEI/10.13039/501100011033 and the Grant IT1553-22, funded by the Gobierno Vasco/Eusko Jaurlaritza. Generalitat Valenciana- Conselleria de Educación, Cultura, Universidades y Empleo (IDIFEDER/2021/013, GVA-COVID19/2021/079), Medalchemy S. L. (Medalchemy-22T) and the University of Alicante (VIGROB-050; UADIF17-42 UAUSTI16-02, VIGROB-068 and UAUSTI21-05). The authors also thank the SGI/IZO-SGIker of the UPV/EHU and DIPC for the generous allocation of analytical and computational resources. David Lledó thanks the Spanish Ministerio de Ciencia, Innovación y Universidades for the fellowship (FPU15/00097).Notes and references
- Nuclear Energy, ed. R. L. Murray and K. E. Holbert, Elsevier Inc., Oxford, UK, 5th edn, 2020 Search PubMed.
- Encyclopedia of Nuclear Energy, E. Greenspan, Elsevier Inc., Oxford, UK, 1st edn, 2021 Search PubMed.
- C. Degueldre and M. J. Joyce, Evidence and uncertainty for uranium and thorium abundance: A review, Prog. Nucl. Energy, 2020, 124, 103299 Search PubMed.
- V. K. Manchanda, Thorium as an abundant source of nuclear energy and challenges in separation science, Radiochim. Acta, 2023, 111, 243–263 Search PubMed.
- M. J. Joyce, in Nuclear Engineering, Elsevier, Oxford, UK, 2018, ch. 5, pp. 87–110 Search PubMed.
- M. Makvandi, E. Dupis, J. W. Englen, F. M. Nortier, M. E. Fassbender, S. Simon, E. R. Birnbaum, R. W. Atcher, K. D. John, O. Rixe and J. P. Norenberg, Alpha-emitters and targeted alpha therapy in oncology: from basic science to clinical investigations, Targeted Oncol., 2018, 13, 189–203 Search PubMed.
- V. K. Manchanda and P. N. Pathak, Amides and diamides as promising extractants in the back end of the nuclear fuel cycle: an overview, Sep. Purif. Technol., 2004, 35, 85–103 Search PubMed.
- Z. Li, Z. Zhang, S. Qi, L. Zhao, J. Pan, Z. Feng and X. Huang, Stripping and recovery of U and Th from EHEHPA in rare earth separation plant, Sep. Purif. Technol., 2024, 331, 125423 Search PubMed.
- C. Jin, X. Yang, D. Fang, S. Ni, S. Wang, A. Ding, P. Cen and C. Xiao, Selective separation of radioactive thorium and uranium from scandium using N-heterocyclic carboxamide ligands, Sep. Purif. Technol., 2024, 328, 125028 Search PubMed.
- F. Aydin, E. Yilmaz, G. Demirkiran, Z. Erbas, M. Vurucuel and M. Soylak, TiO2@ZnO nanocomposite: bifunctional material for solid phase extraction of U(VI) and Th(IV) and photocatalytic degradation of organic contaminant, J. Radioanal. Nucl. Chem., 2023, 332, 3879–3892 Search PubMed.
- S. Wang, X. Yang, L. Xu, Y. Miao, X. Yang and C. Xiao, Selective Extraction of Uranium(VI) from Thorium(IV) Using New Unsymmetrical Acidic Phenanthroline Carboxamide Ligands, Ind. Eng. Chem. Res., 2023, 62, 15613–15624 Search PubMed.
- V. M. Telmore, P. Kumar, P. G. Jaison, A. Mhatre and H. Naik, High performance liquid chromatographic separation of 228,229Th and 232,233U and their estimation by α- and γ-ray spectrometry, J. Radioanal. Nucl. Chem., 2017, 313, 319–326 Search PubMed.
- S. A. Allahyari, S. J. Ahmadi, A. Minuchehra and A. Charkhi, Th(IV) recovery from aqueous waste via hollow fiber renewal liquid membrane (HFRLM) in recycling mode: modelling and experimental validation, RSC Adv., 2017, 7, 7413–7423 Search PubMed.
- A. K. Dinkar, S. K. Singh, S. C. Tripathi, R. Verma and A. V. R. Reddy, Carrier mediated transport of thorium from nitric acid medium using 2-ethyl hexyl hydrogen 2-ethyl hexyl phosphonate (PC88A)/N-dodecane as carrier, Sep. Sci. Technol., 2013, 48, 728–735 Search PubMed.
- C. S. Kedari, S. S. Pandit and P. M. Gandhi, Separation by competitive transport of uranium(VI) and thorium(IV) nitrates across supported renewable liquid membrane containing trioctyl phosphineoxide as metal carrier, J. Membr. Sci., 2013, 430, 188–195 Search PubMed.
- S. Panja, P. K. Mohapatra, S. C. Tripathi and V. K. Manchanda, Transport of thorium(IV) across a supported liquid membrane containing N,N,N0,N0-Tetraoctyl-3-oxapentanediamide (TODGA) as the Extractant, Sep. Sci. Technol., 2010, 45, 1112–1120 Search PubMed.
- T. P. Rao, P. Metilda and J. M. Gladis, Preconcentration techniques for uranium(VI) and thorium(IV) prior to analytical determination-an overview, Talanta, 2006, 68, 1047–1064 Search PubMed.
- R. G. Silva, C. A. Morais and E. D. Oliveira, Selective precipitation of rare earth from non-purified and purified sulfate liquors using sodium sulfate and disodium hydrogen phosphate, Miner. Eng., 2019, 134, 402–416 Search PubMed.
- S. Sadeghi and A. Davami, A rapid dispersive liquid-liquid microextraction based on hydrophobic deep eutectic solvent for selective and sensitive preconcentration of thorium in water and rock samples: A multivariate study, J. Mol. Liq., 2019, 291, 111242 Search PubMed.
- M. A. Didi, D. Villemin, O. Abderrahim and A. Azzouz, Liquid-liquid extraction of thorium(IV) by fatty acids: a comparative study, J. Radioanal. Nucl. Chem., 2014, 299, 1191–1198 Search PubMed.
- J. Su, R. Xu, S. Ni, F. Li and X. Sun, A cost-effective process for recovering thorium and rare earths from radioactive residues, J. Cleaner Prod., 2020, 254, 119931 Search PubMed.
- F. Li, Z. Yang, H. Weng, G. Chen, M. Lin and C. Zhao, High efficient separation of U(VI) and Th(IV) from rare earth elements in strong acidic solution by selective sorption on phenanthroline diamide functionalized graphene oxide, Chem. Eng. J., 2018, 332, 340–350 Search PubMed.
- A. M. Safiulina, D. V. Ivanets, E. M. Kudryavtsev, D. V. Baulin, V. E. Baulin and A. Y. Tsivadze, Extraction of f-elements by binary extractants based on 1,5-bis[o-(dioxyphosphoryl)phenoxy]-3-oxapentane derivatives and trioctylamine, Russ. J. Inorg. Chem., 2018, 63, 1679–1683 Search PubMed.
- A. H. Orabi, B. T. Mohamed, D. A. Ismaiel and S. S. Elyan, Sequential separation and selective extraction of uranium and thorium from monazite sulfate leach liquor using dipropylamine extractant, Miner. Eng., 2021, 172, 107151 Search PubMed.
- R. R. Pawar, V. J. Suryavanshi, S. T. Salunkhe, S. S. Patil and G. N. Mulik, Liquid–liquid extraction of thorium(IV) with N-n-heptylaniline from acid media, J. Radioanal. Nucl. Chem., 2017, 311, 419–426 Search PubMed.
- For the employment of 4-methyl-N-n-octylaniline as an extracting agent, see: P. S. More, U. B. Barache, S. H. Gaikwad and L. V. Gavali, Extraction of Th(IV) and U(VI) with 4-methyl-N-n-octylaniline as an extracting agent, J. Radioanal. Nucl. Chem., 2022, 331, 4149–4158 Search PubMed.
- P. K. Tarafder, S. K. Pradhan and R. K. Mondal, Separation of thorium by facile liquid–liquid extraction, and its rapid spectrophotometric and ICP-AES determination in rocks and minerals, J. Radioanal. Nucl. Chem., 2016, 309, 1021–1028 Search PubMed.
- V. G. Maiorov and A. I. Nikolaev, Extraction of Th(IV) with tributyl phosphate from aqueous HCl solutions containing sodium, calcium, or aluminum chloride, Russ. J. Appl. Chem., 2006, 79, 1196–1199 Search PubMed.
- D. R. Prabhu, A. Sengupta, M. S. Murali and P. N. Pathak, Role of diluents in the comparative extraction of Th(IV), U(VI) and other relevant metal ions by DHOA and TBP from nitric acid media and simulated wastes: Reprocessing of U–Th based fuel in perspective, Hydrometallurgy, 2015, 158, 132–138 Search PubMed.
- B. Gupta, P. Malik and A. Deep, Extraction of uranium, thorium and lanthanides using Cyanex-923: Their separations and recovery from monazite, J. Radioanal. Nucl. Chem., 2002, 251, 451–456 Search PubMed.
- P. S. Mansingh, V. Chakravortty and K. C. Dash, Solvent Extraction of Thorium(IV) by Cyanex 272/Cyanex 302/Cyanex 301/PC-88A and their Binary Mixtures with TBP/DOSO from Aq. HNO3 and H2SO4 Media, Radiochim. Acta, 1996, 73, 139–144 Search PubMed.
- Very recently, novel mesoporous hybrid bio-adsorbents were found as very selective materials for Th(IV): H. Gomaa, M. A. Shenashen, M. F. Cheira, K. Sueki, T. A. Seaf El-Nasr, M. M. Selim and S. A. El-Safty, A novel, spongy mesoporous hybrid bio-adsorbents derived from agricultural waste for highly selective thorium recovery, J. Cleaner Prod., 2023, 402, 136819 Search PubMed.
- For a comprehensive analysis of the efficiency of phosphorus ligands, see: E. Kukkonen, E. J. Virtanen and J. O. Moilanen, α-Aminophosphonates, -Phosphinates, and -Phosphine Oxides as Extraction and Precipitation Agents for Rare Earth Metals, Thorium, and Uranium: A Review, Molecules, 2022, 27, 3465–3493 Search PubMed.
- X. H. Xiong, Y. Tao, Z. W. Yu, L. X. Yang, L. J. Sun, Y. L. Fan and F. Luo, Selective extraction of thorium from uranium and rare earth elements using sulfonated covalent organic framework and its membrane derivate, Chem. Eng. J., 2020, 384, 123240 Search PubMed.
- V. Venkata-Sravani, S. Sengupta, B. Sreenivasulu, G. Gopakumar, S. Tripathi, M. Chandra, C. V. S. Brahmmananda-Rao, A. Suresh and S. Nagarajan, Highly efficient functionalized MOF-LIC-1 for extraction of U(VI) and Th(IV) from aqueous solution: experimental and theoretical studies, Dalton Trans., 2022, 51, 3557–3571 Search PubMed.
- S. K. Singh and A. W. Savoy, Ionic liquids synthesis and applications: An overview, J. Mol. Liq., 2020, 297, 112038 Search PubMed.
- K. Binnemans, Lanthanides and Actinides in Ionic Liquids, Chem. Rev., 2007, 107, 2592–2614 Search PubMed.
- I. Billard, A. Ouadi and C. Gaillard, Liquid–liquid extraction of actinides, lanthanides, and fission products by use of ionic liquids: from discovery to understanding, Anal. Bioanal. Chem., 2011, 400, 1555–1566 Search PubMed.
- A. N. Turanov, V. K. Karandashev, Zh. P. Burmii and A. N. Yarkevich, Effect of Dialkylsulfosuccinate-Based Ionic-Liquids on the Extraction of Lanthanides(III), Uranium(VI), and Thorium (IV) with Diphenyl(dibutylcarbamoylmethyl)phosphine Oxide from Nitric Acid Solutions, Russ. J. Gen. Chem., 2022, 92, 418–423 Search PubMed.
- (a) Q. Wu, F. Zhang, Q.-G. Huang, X. Fu, Y. Li, X.-X. Li, Y.-N. Niu and Z.-Y. Yan, A novel one-step strategy for extraction and solidification of Th(IV) based on self-assembly driven by malonamide-based [DC18DMA]+ ionic liquids, Chem. Eng. J., 2022, 430(Part_1), 132717 Search PubMed; (b) F. Zhang, Q. Wu, J.-X. Yan, Q.-G. Huang, Y. Li, X. Fu, X.-X. Li and Z.-Y. Yan, An integrated strategy for the extraction and solidification of Th(IV) ions from aqueous HNO solution based on self-assembly triggered by [DODMA] [DGA] ionic liquids, Sep. Purif. Technol., 2022, 282(Part_B), 120111 Search PubMed.
- For an effective process for the separation of U(VI) and Th(IV) from rare earth elements, see: J. Lu, K. He, Y. Wang, G. Chen, H. Weng and M. Lin, An effective process for the separation of U(VI), Th(IV) from rare earth elements by using ionic liquid Cyphos IL 104, Chin. Chem. Lett., 2022, 33, 3422–3428 Search PubMed.
- X. Xie, Z. Qin, Y. He, P. Xiong, Z. Huang, Y. Mao, H. Wei and L. Zhuo, Significant enhanced uranyl ions extraction efficiency with phosphoramidate-functionalized ionic liquids via synergistic effect of coordination and hydrogen bond, Sci. Rep., 2017, 7, 15735–15746 Search PubMed.
- A. Saha, K. Kumari, S. Sharma, R. Kumar, M. Sahu, R. M. R. Dumpala, M. Shafeeq PP, S. B. Deb and M. K. Saxena, Selective Extraction of Thorium(IV) from Uranium and Rare Earth Elements Using Tetraphenylethane-1,2-diylbis(phosphoramidate), Inorg. Chem., 2023, 62, 9391–9399 Search PubMed.
- A. N. Turanov, V. K. Karandashev, O. I. Artyushin and E. V. Sharova, Extraction of U(VI), Th(IV), and Lanthanides(III) from nitric acid solutions with CMPO-Functionalized ionic liquid in molecular diluents, Solvent Extr. Ion Exch., 2015, 33, 540–553 Search PubMed.
- (a) A. Sengupta, P. K. Mohapatra, M. Iqbal, J. Huskens and W. Verboom, A diglycolamide-functionalized task specific ionic liquid (TSIL) for actinide extraction: Solvent extraction, thermodynamics and radiolytic stability studies, Sep. Purif. Technol., 2013, 118, 264–270 Search PubMed; (b) D. Ternova, A. Ouadi, V. Mazan, S. Georg, M. Boltoeva, V. Kalchenko, S. Miroshnichenko, I. Billard and C. Gaillard, New ionic liquid based on the CMPO pattern for the sequential extraction of U(VI), Am(III) and Eu(III), J. Solution Chem., 2018, 47, 1309–1325 Search PubMed; (c) M. Paramanik, D. R. Raut, A. Sengupta, S. K. Ghosh and P. K. Mohapatra, A trialkyl phosphine oxide functionalized task specific ionic liquid for actinide ion complexation: extraction and spectroscopic studies, RSC Adv., 2016, 6, 19763–19767 Search PubMed; (d) A. Saha, N. Tiwari, S. B. Deb and M. K. Saxena, Selective liquid-liquid extraction of uranium by phosphoramidate-group bearing ionic liquid, ChemistrySelect, 2019, 4, 7691–7697 Search PubMed; (e) L. María, A. Cruz, J. M. Carretas, B. Monteiro, C. Galinha, S. S. Gomes, M. F. Araújo, I. Paiva, J. Marçalo and J. P. Leal, Improving the selective extraction of lanthanides by using functionalized ionic liquids, Sep. Purif. Technol., 2020, 237, 116354 Search PubMed.
- Y. Zuo, J. Chena and D. Li, Reversed micellar solubilization extraction and separation of thorium(IV) from rare earth(III) by primary amine N1923 in ionic liquid, Sep. Purif. Technol., 2008, 63, 684–690 Search PubMed.
- J. Fu, Q. Chen, T. Sun and X. Shen, Extraction of Th(IV) from aqueous solution by room-temperature ionic liquids and coupled with supercritical carbon dioxide stripping, Sep. Purif. Technol., 2013, 119, 66–71 Search PubMed.
- A. Rao and B. S. Tomar, Extraction of thorium employing N,N-dialkyl amide into room temperature ionic liquid followed by supercritical carbon dioxide stripping, Sep. Purif. Technol., 2016, 161, 159–164 Search PubMed.
- Y. Shen, S. Wang, L. Zhu, J. Wang and W. Wu, Extraction of Th(IV) from an HNO3 solution by diglycolamide in ionic liquids, Ind. Eng. Chem. Res., 2011, 50, 13990–13996 Search PubMed.
- H. M. Luo, R. A. Boll, J. R. Bell and S. Dai, Facile solvent extraction separation of Th-227 and Ac-225 based on room-temperature ionic liquids, Radiochim. Acta, 2012, 100, 771–777 Search PubMed . New separation methodologies for radioisotopes are crucial to many medical applications..
- A. N. Turanov, V. K. Karandashev, V. A. Khvostikov, K. V. Tcarkova, E. V. Sharova, O. I. Artyushin and N. A. Bondarenko, Extraction of REE(III), U(VI), and Th(IV) with Bis[N-alkyl-N-(2-diphenylphosphinylethyl)]diglycolamides from Nitric Acid Solutions, Russ. J. Inorg. Chem., 2022, 67, 2045–2049 Search PubMed.
- A. Sengupta, M. Singh, M. Sundarajan, L. Yuan, Y. Fang, X. Yuan and W. Feng, Understanding the extraction and complexation of thorium using structurally modified CMPO functionalized pillar[5]arenes in ionic liquid: Experimental and theoretical investigations, Inorg. Chem. Commun., 2017, 75, 33–36 Search PubMed.
- Z. Karamzadeh, M. R. Yaftian, Z. Shiri-Yekta, A. Nilchi and L. Dolatyari, Extraction-separation of Eu(III)/Th(IV) Ions with a Phosphorylated Ligand in an Ionic Liquid, Iran. J. Chem. Chem. Eng., 2016, 35, 89–95 Search PubMed.
- W. Li, S. Yang, H. Lv, Z. Liu, J. Wu, S. Li and Y. Shen, Solvent extraction of Th(IV) from aqueous solution with methylimidazole in ionic liquid, Radiochim. Acta, 2016, 104, 681–690 Search PubMed.
- Z. Shiri-Yekta, M. R. Yaftian and A. Nilchi, Extraction-separation of Eu(III) and Th(IV) ions from nitrate media into a room-temperature ionic liquid, J. Iran. Chem. Soc., 2013, 10, 221–227 Search PubMed.
- P. Ren, Y. Li, Z. Wang, Y. Geng, T. Yu and R. Hua, Extraction and separation of thorium(IV) and uranium(VI) with 4-oxaheptanediamide into ionic liquid system from aqueous solution, Chem. Pap., 2020, 74, 2049–2057 Search PubMed.
- A. N. Turanov, V. K. Karandashev, A. N. Yarkevich and V. A. Khvostikov, Extraction of REE(III), U(VI), and Th(IV) from nitric acid solutions with diphenyl(dibutylcarbamoylmethyl)phosphine oxide in the presence of quaternary ammonium bis[(trifluoromethyl)sulfonyl]imides, Radiochemistry, 2019, 61, 694–699 Search PubMed.
- A. N. Turanovа, V. K. Karandashevb and A. N. Yarkevichc, Extraction of REEs(III), U(VI), and Th(IV) from nitric acid solutions with carbamoylmethylphosphine oxides in the presence of an ionic liquid, Radiochemistry, 2013, 55, 382–387 Search PubMed.
- S. Annam, C. C. S. B. Rao, N. Sivaraman, A. Sivaramakrishna and K. Vijayakrishna, Carbamoylmethylphosphine oxide functionalised porous crosslinked polymers towards sequential separation of uranium (VI) and thorium (IV), React. Funct. Polym., 2018, 131, 203–210 Search PubMed.
- Y. Zhang, Z. Liu, F. Fan, L. Zhu and Y. Shen, Extraction of Uranium and Thorium from nitric acid solution by TODGA in Ionic Liquids, Sep. Sci. Technol., 2014, 49, 1895–1902 Search PubMed.
- M. Y. Alyapyshev, V. A. Babain and L. I. Tkachenko, Amides of heterocyclic carboxylic acids as novel extractants for high-level waste treatment, Radiochemistry, 2014, 56, 565–574 Search PubMed.
- S. M. Ibrahim, Y. Zhang, Y. Xue, S. Yang, F. Ma and G. Tian, Extraction of Lanthanides(III) along with Thorium(IV) from chloride solutions by N,N-di(2-Ethylhexyl)-diglycolamic acid, Solvent Extr. Ion Exch., 2020, 38, 417–429 Search PubMed.
- R. Boyd, L. Jin, P. Nockemann, P. K. J. Robertson, L. Stella, R. Ruhela, K. R. Seddon and H. Q. N. Gunaratne, Ionic liquids tethered to a preorganized 1,2-diamide motif for extraction of lanthanides, Green Chem., 2019, 21, 2583–2588 Search PubMed.
- Y. Li, Z. Weng, Y. Wang, L. Chen, D. Sheng, Y. Liu, J. Diwu, Z. Chai, T. E. Albrecht-Schmittd and S. Wang, Centrosymmetric and chiral porous thorium organic frameworks exhibiting uncommon thorium coordination environments, Dalton Trans., 2015, 44, 20867–20873 Search PubMed.
- M. W. Rosenzweig, J. Hümmer, A. Scheurer, C. Álvarez-Lamsfus, F. W. Heinemann, L. Maron, M. Mazzanti and K. Meyer, A complete series of uranium(IV) complexes with terminal hydrochalcogenido (EH) and chalcogenido (E) ligands E = O, S, Se, Te, Dalton Trans., 2019, 48, 10853 Search PubMed.
- P. Sharma, D. R. Pahls, B. L. Ramírez, C. C. Lu and L. Gagliardi, Multiple Bonds in Uranium-Transition Metal Complexes, Inorg. Chem., 2019, 58, 10139–10147 Search PubMed.
- V. A. Cocalia, K. E. Gutowski and R. D. Rogers, The coordination chemistry of actinides in ionic liquids: A review of experiment and simulation, Coord. Chem. Rev., 2006, 250, 755–764 Search PubMed.
- J. M. Sansano, D. Lledó, G. Grindlay, M. G. Retamosa and H. Q. N. Gunaratne, International pat., PCT/ES2023/070664, 2023 Search PubMed.
- V. I. Saloutin, Y. O. Edilova, Y. S. Kudyakova, Y. V. Burgart and D. N. Bazhin, Heterometallic Molecular Architectures Based on Fluorinated β-Diketone Ligands, Molecules, 2022, 27, 7894–7937 Search PubMed.
- S. Benedini, Y. Zheng, A. Nitti, M. M. A. Mazza, D. Dondi, F. M. Raymo and D. Pasini, Large polarization of push–pull “Cruciforms” via coordination with lanthanide ions, New J. Chem., 2022, 46, 221–227 Search PubMed.
- J. J. Woods, R. Unnerstall, A. Hasson, D. S. Abou, V. Radchenko, D. L. J. Thorek and J. J. Wilson, Stable Chelation of the Uranyl Ion by Acyclic Hexadentate Ligands: Potential Applications for 230U Targeted α-Therapy, Inorg. Chem., 2022, 61, 3337–3350 Search PubMed.
- A. C. Sather, O. B. Berryman and J. Rebek Jr, Selective Recognition and Extraction of the Uranyl Ion, J. Am. Chem. Soc., 2010, 132, 13572–13574 Search PubMed.
- C. D. Tutson and A. E. V. Gorden, Thorium coordination: A comprehensive review based on coordination number, Coord. Chem. Rev., 2017, 333, 27–43 Search PubMed.
Footnotes |
| † Dedicated to Professors Carmen Nájera and Miguel Yus for their outstanding contribution to Organic and Organometallic Chemistry. |
| ‡ Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta06496h |
| This journal is © The Royal Society of Chemistry 2024 |