A 2D layered semiconducting (LCu3I3)n coordination polymer for energy storage through dual ion intercalation†
Dilip
Pandey‡
a,
Mayank K.
Singh‡
b,
Shivendu
Mishra
a,
Dhirendra K.
Rai
 *b and
Abhinav
Raghuvanshi
*a
*b and
Abhinav
Raghuvanshi
*a
aDepartment of Chemistry, Indian Institute of Technology Indore, Simrol, Indore, Madhya Pradesh 453552, India. E-mail: r.abhinav@iiti.ac.in
bSustainable Energy and Environmental Materials (SEEM) Lab, Department of Metallurgical Engineering and Material Science, Indian Institute of Technology Indore, Simrol, Indore, Madhya Pradesh 453552, India. E-mail: dkrai@iiti.ac.in
First published on 3rd September 2024
Abstract
For an exclusive exploration of renewable energies, it is imperative to have efficient energy storage devices to ensure an uninterrupted energy supply, which mandates the development of new electrode materials with better energy storage performance. This work presents a facile synthesis of a layered two-dimensional semiconducting copper(I) iodide coordination polymer (CuI-CP), using CuI and 1,3,5-trithiane linker, which has been investigated as a cathode material for energy storage applications. The coordination polymer has a hitherto unknown continuous Cu(I) iodide sheet-like structure having a Cu3I3 hexagon repeating unit decorated with the ligand. Electron microscopy suggests the nano-crystalline hexagonal plate morphology of the material. The unique 2D layered structure of semiconducting CuI-CP, originating from the stacking of a redox-active framework, encouraged us to investigate its applicability as an electrode material in electrochemical energy storage devices. The material demonstrates supercapattery behavior, achieving a specific capacity of 498 Cg−1 at 1 Ag−1. Additionally, the symmetric device fabricated with CuI-CP offers high energy and power densities (maxEd = 77 W h kg−1 and maxPd = 3.08 kW kg−1) and maintains excellent cycling stability of 94% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The high efficiency is attributed to the semiconducting nature and facile ion diffusion via the 2D layered structure of the material having redox-active Cu centers. The present work demonstrates the first report of integrating a Cu(I) iodide coordination polymer as an electrode material for energy storage devices.
000 cycles. The high efficiency is attributed to the semiconducting nature and facile ion diffusion via the 2D layered structure of the material having redox-active Cu centers. The present work demonstrates the first report of integrating a Cu(I) iodide coordination polymer as an electrode material for energy storage devices.
Introduction
Renewable energy sources, such as solar, wind, and hydropower, provide an appropriate alternative to traditional energy sources and play a crucial role in addressing climate change, promoting sustainability, ensuring energy security, and fostering economic growth.1–5 To ensure a reliable and uninterrupted supply of energy, it is essential that they are supplemented by efficient energy storage devices.6–10 For energy storage, batteries and supercapacitors (SCs) are the two most promising devices. SCs possess the characteristics of a high power density, quick charging/discharging capabilities, and long cycling stability; however, they suffer from lower energy density.2,11–15 In contrast, batteries have a high energy density, long-term energy storage, and stable voltage supply, but they lack longer cycle life and high power density.16–19 To bridge this gap, there is a pressing demand to explore novel, cost-effective, environmentally friendly electrode materials that can feature high power density and extended cycle life with high energy density.16–19 Recent approaches involve the blending of the positive characteristics of supercapacitors and batteries to develop a hybrid electrochemical energy storage device, termed a supercapattery (supercapacitor + battery).4,20–23 A supercapattery has higher power delivery capability along with higher energy-storage capability.At the forefront of conducting 2D materials lie graphene and MXenes, known for their exceptional electrical conductivity, mechanical strength, and chemical stability. These materials offer distinctive features such as high surface area, tunable electronic properties, and efficient ion diffusion, all of which are critical for addressing the performance limitations of traditional energy storage materials.24–29 Recently, two-dimensional coordination polymers (CPs)/metal–organic frameworks (MOFs) have emerged as noteworthy substitutes of these materials for applications in electrical energy storage. Besides electric double-layer capacitor (EDLC) type charge storage, they also impart faradaic type charge storage through redox-active metal centers.30–32 One major limitation of these CPs/MOFs is their intrinsic poor conductivity, which necessitates the blending of conductive additives and, thus, impacts their specific charge storage capacity. Recent reports have demonstrated the synthesis of semiconducting 2D CPs/MOFs for energy storage materials.7,33–36 For example, Zhang et al. reported a two-dimensional MOF-based material, Ni2[CuPc(NH)8], showing a specific capacitance of 400 Fg−1 at 0.5 Ag−1 with maximum energy and power densities of 51.6 W h kg−1 and 32.1 kW kg−1, respectively, with 90.3% capacitance retention after 5000 cycles.37 The same group synthesized another two-dimensional conjugated MOF Ni2[CuPcS8], which delivered a specific capacitance of 312 Fg−1 at 0.5 Ag−1 current density in 1 M TEABF4/acetonitrile electrolyte with a maximum energy density of 57.4 W h kg−1.26 A 2D MOF (Cu-TBC) was synthesized by Zhao et al., which featured a capacitance of 474.8 Fg−1 at 0.2 Ag−1 current density with 18.89 W h kg−1 and 6.0 kW kg−1 maximum energy and power densities, respectively.38
In the class of 2D organic–inorganic hybrid polymers, copper(I)-iodide CPs, owing to their semiconducting nature, have garnered significant attention for their diverse applications in organic light-emitting diodes, molecular wires, electrically conductive materials, gas sensors, and more.39–45 Notably, despite their semiconducting nature and versatile applications, there is a noticeable absence of reports on the utilization of copper(I)-iodide CPs in electrochemical energy storage devices. Over the recent years, our research endeavors have been directed toward the exploration of MOF/rGO composites7,46 and semiconducting coordination polymers for energy storage applications.47 In continuation of our work on Cu(I) CPs, herein we report a facile synthesis of a Cu(I) iodide-based, hitherto unknown, 2D semiconducting coordination polymer (CuI-CP) using a 1,3,5-trithiane ligand as a linker. The unique layered structure of the Cu–I 2D sheet framework with a Cu3I3 repeating unit of CuI-CP encouraged us to investigate its viability as a cathode material in energy storage devices. The electrochemical investigations reveal that CuI-CP features a supercapattery behavior with a specific capacity of 498 Cg−1 at 1 Ag−1, involving a dual ion intercalation charge storage mechanism. Furthermore, the fabricated symmetrical device without any conductive additive yields maximum energy and power densities of 77 W h kg−1 and 3.08 kW kg−1 with an excellent capacity retention of 94% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The present work highlights the potential of using semiconducting 2D coordination polymers containing earth-abundant redox-active metals for the fabrication of cost-effective energy storage devices for practical applications.
000 cycles. The present work highlights the potential of using semiconducting 2D coordination polymers containing earth-abundant redox-active metals for the fabrication of cost-effective energy storage devices for practical applications.
2. Experimental section
2.1. Synthesis of CuI-CP
To a clear solution of CuI (239.4 mg, 1.26 mmol) in acetonitrile (20 mL), 1,3,5-trithiane was added (30 mg, 0.21 mmol) in an open atmosphere. The mixture was stirred for two hours at room temperature and then refluxed for 5 min. The solution was then allowed to cool down to room temperature. The white precipitate was further washed three times with 5 mL acetonitrile to remove unreacted reactants. It was finally dried under vacuum to obtain a white microcrystalline product. Yield: 130 mg 88%. FT-ATR-IR: 2959, 1360, 1223, 887, and 716 cm−1. Anal. Calc. for CH2CuIS (236.53): C, 5.08; H, 0.85; S, 13.55. Found: C, 5.02; H, 0.83; S, 13.54%.3. Results and discussion
3.1. Structural and morphological analysis
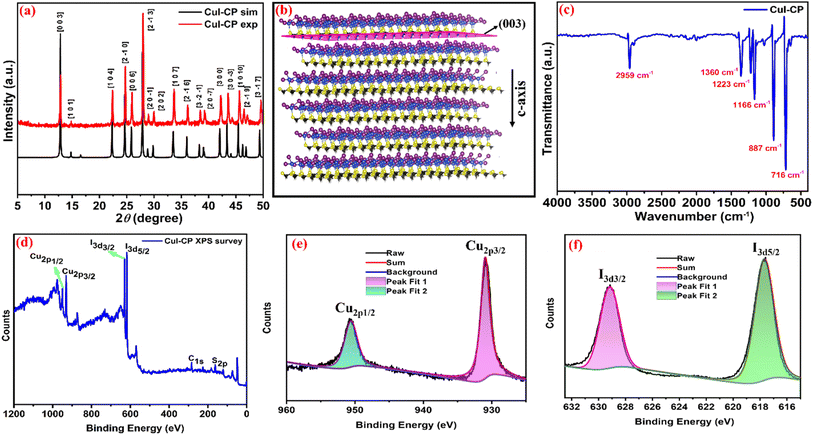
The reaction of 1,3,5-trithiane (L) with CuI in acetonitrile solution resulted in a sparingly soluble white product with composition [Cu(μ3-I)3(μ3-L)]n (Scheme 1). The product was dissolved in boiling acetonitrile and left undisturbed for 24 hours to get suitable crystals for X-ray diffraction analysis. The single crystal X-ray diffraction analysis shows that CuI-CP crystallizes in the trigonal crystal system with an R3 space group, with unit cell parameters of a = b = 7.222(3) Å, c = 20.664(11) Å, α = β = 90°, γ = 120°, and V = 933.50(9) Å3. Tables S1–S3† provide the important crystallographic details, bond lengths, and bond angles of the polymer. The asymmetric unit of CuI-CP contains one-third of the 1,3,5-trithiane ligand, one Cu(I) ion, and three crystallographically independent iodides (Fig. S1a†). The Cu(I) ions display a distorted tetrahedral geometry, which is coordinated by three I− anions and one sulfur atom of the ligand unit. The copper(I) geometry is accurately described using the angular index (τ4) formula (τ4 = [360 − (a + b)]/141), yielding a value of 0.88, confirming the distorted tetrahedral geometry. Each sulfur atom of the ligand is coordinated with one Cu atom, while each iodide atom is coordinated with Cu in μ3-coordination mode. Interestingly, the polymeric structure exhibits a novel continuous copper–iodine (Cu–I) layered 2D sheet-like structure (Fig. S2†) with repeating Cu3I3 hexagonal rings, arranged in a distorted chair conformation (Fig. S4†). This ring is connected to trithiane ligands, as illustrated in Fig. S1(b).† Overall, CuI-CP consists of two layers: one with a continuous Cu–I layer and another containing coordinated sulfur-rich ligands, as shown in Fig. 1 and S3.† The CuI-CP has three different Cu–I bond lengths i.e. Cu1–I1, Cu1–I2 and Cu1–I3, which are 2.655(2), 2.601(3) and 2.719(2) Å, respectively, whereas all Cu–S bond distances are the same (2.306(4) Å). The bond angles between I1–Cu1–I3, I2–Cu1–I1 and I2–Cu1–I3 are 103.98(7)°, 107.58(8)° and 112.93(8)°, respectively. In addition, the bond angles between S1–Cu1–I1, S1–Cu1–I2 and S1–Cu1–I3 are 110.91(13)°, 122.18(13)° and 97.67(12)°, respectively. These bond lengths and bond angles are similar to those of previously reported Cu(I) iodide complexes with thioether ligands.48–51 The most common secondary building units (SBUs) incorporating a (CuI)x unit cluster include the Cu2I2 rhomboid dimer and the Cu4I4 cubane tetramer. However, other noteworthy SBUs, such as the Cu3I3 trimer, Cu4I4 staircase tetramer, Cu6I6 cubane hexamer, Cu6I6 staircase hexamer, Cu7I7 pinwheel heptamer, Cu7I7 heptamer, and Cu8I8 staircase octamer, have also been documented.41,52,53 In our case, a distinctive Cu3I3 hexagonal SBU is observed. The continuous repetition of Cu3I3 generates a sheet-like structure, which, to the best of our knowledge, has not been reported previously.Furthermore, to assess the phase purity of the synthesized CuI-CP, powder X-ray diffraction (PXRD) analyses were conducted at room temperature and the patterns were compared with the simulated PXRD pattern generated from the single crystal. Both PXRD patterns match well, suggesting a single-phase bulk material. Furthermore, the Mercury software package is used for indexing the PXRD peaks, as shown in Fig. 2a. Interestingly, a low angle (2θ = 12.79°) of a very intense peak corresponding to the (0 0 3) plane is observed, which indicates the formation of a layered structure due to the stacking of polymeric 2D sheets stabilized by long-range non-covalent interactions (Fig. 2b). The interlayer separation is calculated to be 3.6 Å. The polymer possesses the unique characteristics of a 2D framework with redox-active metal centers, which stacks to build a layered structure (Fig. 2b). Moreover, the intensity ratio of (0 0 3) and (1 0 4) peaks (I(0 0 3)/I(1 0 4)) is observed to be 1.9, which is much higher than the standard convention of 1.2 for a perfect layered structure.54 Such material characteristics are highly desirable for charge storage applications, particularly involving the insertion mechanism. The ATR-IR spectrum of the CuI-CP shows characteristic C–H symmetric stretching frequencies of the trithiane ring at 2959 cm−1. The peaks corresponding to stretching frequencies of ν(C–S) and δ(–CH2) scissoring frequency of the trithiane ring appear in the range of 1360–716 cm−1, suggesting the presence of the coordinated ligand in the CP (Fig. 2c and S5†). Thermogravimetric analysis (TGA) experiments were performed at a heating rate of 10 °C min−1 under N2 flow in the range of 30–800 °C. The TGA curve (shown in Fig. S6†) demonstrates thermal stability up to 250 °C. Furthermore, CuI-CP was found stable at room temperature and can be stored at room temperature for several months without any degradation.
To know the specific surface area and pore size distribution of the CuI-CP Brunauer–Emmett–Teller (BET) isotherm analysis was performed, which confirms a mesoporous structure of CuI-CP with a surface area of 11.50 m2 g−1 (Fig. S7†). Furthermore, a multimodal pore size distribution with a pore size of 3.44 nm was evaluated by the Barrett–Joyner–Halenda (BJH) method (Fig. S8†). X-ray photoelectron spectroscopy (XPS) was employed to confirm the presence of Cu, I, S, and C elements in CuI-CP (Fig. 2d). The high-resolution spectra show two peaks corresponding to Cu(I) 2p1/2 and Cu(I) 2p3/2 appearing at 949 and 932 eV, respectively. Similarly, two peaks related to iodine, 3d3/2 and 3d5/2, are observed at 630 and 619 eV, respectively (Fig. 2e and f).45,55,56 Moreover, for C1s and S2p, two peaks at 281 and 161 eV, respectively, were also observed (Fig. S9†).
The room temperature electrical conductivity of the CuI-CP was recorded with a pressed pellet (6.0 GPa, 10 min) using the two-probe direct current method by applying voltages from −5 V to +5 V. The observed electrical conductivity of 1.1 × 10−6 S cm−1 suggests the semiconducting nature of the material (Fig. S10†).
To get an insight into the morphology of CuI-CP, field emission scanning electron microscopy (FE-SEM) images were recorded (Fig. 3). The SEM images at different magnification levels reveal CuI-CP as agglomerated nano-crystalline hexagonal plates (Fig. 3a). At higher magnification, these plates show uneven edges, which can create more active sites for electrochemical reactions (inset Fig. 3b). To confirm the elements present in CuI-CP, the energy dispersive spectroscopy (EDS) technique has been used, which shows the presence of copper, iodine, carbon and sulfur in an appropriate proportion (Fig. 3c). Furthermore, elemental mapping analysis confirms the uniform distribution of elements over the surface, suggesting a consistent composition (Fig. 3d1–d4). Transmission electron microscopy (TEM) was also used to get further insight into the morphology, which is in agreement with FE-SEM images. The TEM images at both low and high magnifications reveal well-separated nanocrystalline plates with an average size of 14.4 nm (Fig. 3e and f). Unlike in SEM images, the observation of deagglomerated particles in TEM is due to sample preparation in the latter case, which is carried out in ethanol through ultrasonication. Furthermore, the fast Fourier transform analysis of HR-TEM revealed different planes across the entire crystalline structure of CuI-CP (Fig. 3g). These lattice fringes were found to have a d-spacing of 3.8 Å and 3.2 Å, which corresponded to (0 0 3) and (2 1 0) planes, respectively. Fig. 3h shows the selected area electron diffraction pattern showing concentric rings corresponding to different diffraction planes, indicating the crystalline nature of the material.
3.2. Electrochemical analysis
To assess the energy storage capabilities of the electrode material, an electrochemical workstation was used to conduct cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS). Further details about electrode fabrication and device fabrication for electrochemical analysis are discussed in the ESI.† The investigation into optimizing the performance of the CuI-CP electrode was initiated by determining the optimal working potential window. This entailed the measurement of the electrode within both negative (−0.5 to 0.0 V) and positive potential windows (0.0 to 0.5 V). The electrode material exhibited low current density and discharge time in the negative potential window (see Fig. S11†). Conversely, distinct redox peaks were observed in the positive potential window. Additionally, higher discharge time with minimal IR drop in GCD was also observed (see Fig. S12†). Consequently, all further electrochemical investigations were performed in the positive potential window.The CV of CuI-CP was recorded at different scan rates (10 mV s−1 to 500 mV s−1) in the potential range of 0 to 0.5 V (Fig. 4a). CV profiles reveal distinct anodic and cathodic peaks with increasing peak separation upon increasing the scan rates due to the polarization effect. These peaks are attributed to the Cu redox centers, suggesting the faradaic origin of the current. Except for the peak separation, the CV profiles do not show any alteration in the peak symmetry, even at a rapid scan rate, indicating the quasi-reversible nature of the redox process.
The material's charge storage behavior was evaluated utilizing the power law using the following equation.13
| i = aνb | (1) |
| i(V) = k1ν + k2ν1/2 | (2) |
Fig. 4b and S14† depict the percentage contributions of the two processes to the overall charge storage at different scan rates in a bar chart and the proportion of cyclic voltammograms to the overall cyclic voltammogram recorded at 10 and 50 mV s−1 scan rates. The bar chart shows that the CuI-CP electrode exhibits charge storage mostly dominated by the diffusion-controlled process at low scan rates (90.5% at 10 mV s−1). However, at higher scan rates, surface-controlled charge storage also becomes considerable, but diffusion-controlled processes still dominate overall charge storage (63.2% at 50 mV s−1). The reduction in diffusion capacity at higher scan rates can be attributed to the fact that the electrolyte diffusion within the electrolyte electrode interface fails to keep up with the increase in the scan rate, thus amplifying the dominance of surface-based capacitance. The GCD profiles at different current densities (1.0 to 10 Ag−1, while maintaining a potential window of 0.0 to 0.5 V) were recorded to understand the charge storage mechanism and specific capacity (Fig. 4c). It shows a higher discharge time at a low current density and a gradual reduction in discharge time with increasing current densities. Moreover, the presence of a plateau in the GCD profile suggests the involvement of the faradaic process during charging and discharging.
To understand the charging and discharging mechanism in the material, we performed the PXRD studies of the material in its fully discharged and charged states. The comparison of the low-angle peak corresponding to (0 0 3) planes, originating from stacking of the 2D framework of CuI-CP, reveals a peak shift towards a lower angle in both fully charged and discharged states compared to the as-synthesized state (Fig. 4d). This observation suggests the involvement of the dual ion insertion and de-insertion of electrolyte ions during the charge–discharge cycle. The CV and GCD also reveal the occurrence of the redox process (Cu0/2+ couple) involving concurrent two-electron transfers. Based on the findings of PXRD, CV, and GCD studies, the plausible charge storage mechanism can be proposed using the following redox steps:
In the fully discharged state, the material may be potassiated (Cu(0)LK+, L = 1,3,5-trithiane) through intercalation of K+ between layers of (0 0 3) planes, inducing larger interlayer separation in the plane, which is reflected as a shift in the (0 0 3) peak towards a low angle. After losing two electrons, in the fully charged state, the material is thought to be in the hydroxylated form (Cu(II)LOH−) through the intercalation of OH− between (0 0 3) planes, where the (0 0 3) peak is again observed to be low angle shifted compared to the neat material. The slightly larger shift in the potassiated form compared to the hydroxylated form can be attributed to the relatively larger interplanar separation caused by the intercalation of K+ due to its larger size compared to OH−.
To further provide experimental evidence for the involvement of a dual ion mechanism, we examined the FT-IR spectra of the material in both charged and discharged states. Fig. S15† shows a comparison of the FT-IR spectra for the charged state, discharged state, and the as-synthesized material. In the charged state, a strong broad peak (3150 cm−1), characteristic of the hydroxyl group, appears, indicating the intercalation of hydroxide ions during the charging process. Additionally, a new peak corresponding to Cu–O bond vibrations at 490 cm−1 is also observed.57 In the discharged state, a peak related to KI bond vibration at 3496 cm−1 is observed, suggesting the K+ intercalation.58 The lower intensity peak of the hydroxyl group at 3230 cm−1 in the discharged state is likely due to the surface-adsorbed hydroxide electrolyte.
The electrode material's specific charge storage capacities were assessed by GCD analysis. At a current density of 1 Ag−1, the calculated specific capacity for CuI-CP is 498 Cg−1. Based on the proposed mechanism, the theoretical capacity of CuI-CP has been calculated to be 816 Cg−1, of which 61% has been realized at 1 Ag−1 current density. Fig. 4e presents the specific capacity of CuI-CP with different current densities. A swift decline in specific capacity was observed with an increase in current density up to 3 Ag−1, beyond which only a negligible loss in capacity is detected. This phenomenon is primarily attributed to the dominance of diffusion-controlled processes in charge storage at lower current densities, which diminishes rapidly due to ion diffusion constraints. Beyond 3 Ag−1, surface-controlled processes play a significant role in the overall capacity, and the specific capacity remains stable across different current densities. The material exhibited an excellent capacity retention of 92% after 5000 GCD cycles performed at a current density of 10 Ag−1 (Fig. 4f). Additionally, no evidence of stripping of the electrode material was observed after the prolonged cyclic experiment. Furthermore, the EDS spectrum and elemental mapping display all elements, confirming no compositional alteration of the electrode materials after 5000 cycles (Fig. S16†).
A symmetric device was fabricated to evaluate the practical usability of the electrode material, employing a cellulose membrane as the separator, as depicted in Fig. 5a. This device was examined in a two-electrode cell with an optimized cell voltage of 1.0 V. Cyclic voltammetry studies of the device were conducted across a range of scan rates from 10 to 500 mV s−1, revealing distinct redox peaks at all scan rates (Fig. 5b). Furthermore, to assess the specific capacity of the device, GCD was performed in the current density range from 1.0 to 10.0 Ag−1 (Fig. 5c). Asymmetrical-shaped GCD curves are exhibited, implying that the device's charge storage mechanism involves both EDLC and faradaic reactions. Hence, it should better be termed a supercapattery device having characteristics of both capacitor and battery-type electrodes. The reason for the considerable contribution of EDLC-type charge storage in the device is perhaps due to the restricted diffusion of ions in solid electrolytes compared to liquid electrolytes, which causes a reduction in faradaic charge storage contribution. This also results in the overall low capacity of the device compared to the capacity obtained in three-electrode setups. The decreases in specific capacity with increased current density are similar to the results obtained from the three-electrode system. The device exhibits a specific capacity of 154.7 Cg−1, at a current density of 1 Ag−1, which drops to 44.3 Cg−1 at 10 Ag−1 (Fig. 5d). However, this decrease is more gradual compared to the trend observed in the three-electrode configuration. Additionally, the energy density (Ed) and power density (Pd) for the symmetric device were calculated and displayed on a Ragone chart (Fig. 5e). A peak energy density of 77 W h kg−1 at a power density of 1.96 kW kg−1 and a maximum power density of 3.08 kW kg−1 at an energy density of 25.57 W h kg−1 were observed. We also evaluated the cycling stability of the device over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles at a current density of 10 Ag−1 (Fig. 5f). After 10
000 GCD cycles at a current density of 10 Ag−1 (Fig. 5f). After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, the device retains 93.8% of its initial capacity, demonstrating excellent cycling stability.
000 cycles, the device retains 93.8% of its initial capacity, demonstrating excellent cycling stability.
 | ||
| Fig. 5 (a) Pictorial representation of the device. (b) Variation of the cyclic voltammogram. (c) Galvanostatic charge–discharge profiles. (d) Variation of specific capacities. (e) Ragone plot.11,37,38,59–61 (f) Stability of the device. | ||
In summary, the energy storage device constructed with the CuI-CP coordination polymer demonstrates excellent electrochemical performance. It features high-rate capability and impressive energy and power densities along with outstanding cycling stability. A comparative evaluation of this electrode material's performance against some of the best-reported CP/MOF systems is summarized in Table S4.† The comparison suggests that, in terms of specific capacity, energy density, power density and cycling stability, the CuI-CP 2D polymer electrode material exhibits comparable or better performance than most previously reported MOFs/CPs. Moreover, unlike most of the reported materials, the CuI-CP polymer possesses the additional advantage of being used as an electrode in the absence of any conductive additive, which is a desirable feature to avoid dead mass loading in electrode fabrication.
4. Conclusion
We have presented a straightforward facile synthesis of a unique semiconducting CuI-CP, utilizing a sulfur-rich 1,3,5-trithiane ligand. The CP features a distinctive stacked 2D sheet framework with a continuous Cu3I3 SBU with an interlayer spacing of 3.6 Å. The SEM analysis suggests that CuI-CP forms nano-crystalline hexagonal plates with uneven edges. This work represents the first report on the application of copper(I) iodide coordination polymers as electrode materials for energy storage devices. The results reveal that at a current density of 1 Ag−1, the specific capacity of the CuI-CP electrode can reach up to 498 Cg−1 in a three-electrode system. A symmetrical device (CuI-CP//CuI-CP) has also been fabricated, which shows a maximum energy density of 77 W h kg−1 with a maximum power density of 3.08 kW kg−1 with remarkable cycling stability up to 94% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The excellent performance is attributed to the Cu/Cu2+ redox couple along with the involvement of the dual ion insertion and de-insertion of electrolytes between the 2D sheets during the charge–discharge. The successful integration of CuI-CP opens new avenues for research and development in the quest for more efficient electrode materials in the realm of energy storage devices. Future investigations could explore modifications and optimizations of different Cu(I)-iodide CPs using different ligands, paving the way for new materials with enhanced performance and expanded energy storage applications.
000 cycles. The excellent performance is attributed to the Cu/Cu2+ redox couple along with the involvement of the dual ion insertion and de-insertion of electrolytes between the 2D sheets during the charge–discharge. The successful integration of CuI-CP opens new avenues for research and development in the quest for more efficient electrode materials in the realm of energy storage devices. Future investigations could explore modifications and optimizations of different Cu(I)-iodide CPs using different ligands, paving the way for new materials with enhanced performance and expanded energy storage applications.
Data availability
All the data will be made available on request.Conflicts of interest
The authors declare no conflicts.Acknowledgements
D. P., M. K. S., and S. M. are grateful to IIT Indore for their fellowships. Sophisticated Instrumentation Center (SIC) IIT Indore, the Department of Metallurgical Engineering and Material Science, and the Department of Chemistry IIT Indore are acknowledged for providing characterization facilities. We thank Prof. Preeti A. Bhobe, Department of Physics, IIT Indore, for providing the facility to record electrical conductivity.References
- S.-J. Shin, J. W. Gittins, C. J. Balhatchet, A. Walsh and A. C. Forse, Adv. Funct. Mater., 2023, 2308497 Search PubMed.
- D.-G. Wang, Z. Liang, S. Gao, C. Qu and R. Zou, Coord. Chem. Rev., 2020, 404, 213093 CAS.
- M. Wu, X. Hu, W. Zheng, L. Chen and Q. Zhang, Chem. Eng. J., 2023, 466, 143077 CAS.
- G. Zou, Z. Tian, V. S. Kale, W. Wang, S. Kandembeth, Z. Cao, J. Guo, J. Czaban-Jóźwiak, L. Cavallo, O. Shekhah, M. Eddaoudi and H. N. Alshareef, Adv. Energy Mater., 2023, 13, 2203193 CAS.
- K.-B. Wang, Q. Xun and Q. Zhang, EnergyChem, 2020, 2, 100025 CrossRef.
- Z. Xia, X. Jia, X. Ge, C. Ren, Q. Yang, J. Hu, Z. Chen, J. Han, G. Xie, S. Chen and S. Gao, Angew. Chem., 2021, 133, 10316–10326 CrossRef.
- M. K. Singh, A. K. Gupta, S. Krishnan, N. Guha, S. Marimuthu and D. K. Rai, J. Energy Storage, 2021, 43, 103301 CrossRef.
- H. Rong, P. Song, G. Gao, Q. Jiang, X. Chen, L. Su, W.-L. Liu and Q. Liu, Dalton Trans., 2023, 52, 1962–1969 RSC.
- S. Bi, H. Banda, M. Chen, L. Niu, M. Chen, T. Wu, J. Wang, R. Wang, J. Feng, T. Chen, M. Dincă, A. A. Kornyshev and G. Feng, Nat. Mater., 2020, 19, 552–558 CrossRef CAS PubMed.
- B. Chen, L. Xu, Z. Xie and W.-Y. Wong, EcoMat, 2021, 3, e12106 CrossRef CAS.
- X. Zhang, Z. Liu, X. Jin, F. Liu, X. Ma, N. Qu, W. Lu, Y. Tian and Q. Zhang, Inorg. Chem., 2023, 62, 7360–7365 CrossRef CAS PubMed.
- R. Deka, V. Kumar, R. Rajak and S. M. Mobin, Sustainable Energy Fuels, 2022, 6, 3014–3024 RSC.
- S. Krishnan, A. K. Gupta, M. K. Singh, N. Guha and D. K. Rai, Chem. Eng. J., 2022, 435, 135042 CrossRef CAS.
- X. Liu, C. Shi, C. Zhai, M. Cheng, Q. Liu and G. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 4585–4591 CrossRef CAS PubMed.
- X. Wang, X. Liu, H. Rong, Y. Song, H. Wen and Q. Liu, RSC Adv., 2017, 7, 29611–29617 RSC.
- H. Shi, X. Zhao, Z.-S. Wu, Y. Dong, P. Lu, J. Chen, W. Ren, H.-M. Cheng and X. Bao, Nano Energy, 2019, 60, 743–751 CrossRef CAS.
- Y. Chen, M. Tang, Y. Wu, X. Su, X. Li, S. Xu, S. Zhuo, J. Ma, D. Yuan, C. Wang and W. Hu, Angew. Chem., Int. Ed., 2019, 58, 14731–14739 CrossRef CAS PubMed.
- Y. Chen, Q. Zhu, K. Fan, Y. Gu, M. Sun, Z. Li, C. Zhang, Y. Wu, Q. Wang, S. Xu, J. Ma, C. Wang and W. Hu, Angew. Chem., Int. Ed., 2021, 60, 18769–18776 CrossRef CAS PubMed.
- Y. Wu, Y. Chen, M. Tang, S. Zhu, C. Jiang, S. Zhuo and C. Wang, Chem. Commun., 2019, 55, 10856–10859 RSC.
- Y. Jiao, J. Pei, C. Yan, D. Chen, Y. Hu and G. Chen, J. Mater. Chem. A, 2016, 4, 13344–13351 RSC.
- M. Z. Iqbal and U. Aziz, J. Energy Storage, 2022, 46, 103823 CrossRef.
- K. Karuppasamy, D. Vikraman, S. Hussain, P. Santhoshkumar, R. Bose, P. Sivakumar, A. Alfantazi, J. Jung and H.-S. Kim, Small, 2022, 18, 2107284 CrossRef CAS PubMed.
- B. Huang, H. Wang, S. Liang, H. Qin, Y. Li, Z. Luo, C. Zhao, L. Xie and L. Chen, Energy Storage Mater., 2020, 32, 105–114 Search PubMed.
- S. G. Krishnan, A. Arunachalam, P. Jagadish and M. Khalid, in Adapting 2D Nanomaterials for Advanced Applications, American Chemical Society, 2020, vol. 1353, pp. 33–47 Search PubMed.
- J. Cherusseri, D. Pandey and J. Thomas, Batteries Supercaps, 2020, 3, 860–875 CAS.
- P. Zhang, M. Wang, Y. Liu, Y. Fu, M. Gao, G. Wang, F. Wang, Z. Wang, G. Chen, S. Yang, Y. Liu, R. Dong, M. Yu, X. Lu and X. Feng, J. Am. Chem. Soc., 2023, 145, 6247–6256 CAS.
- S. P. Suman, G. M. R. Dontireddy, T. Chen, J. Wang, J.-H. Dou and H. Banda, ACS Energy Lett., 2024, 9, 1572–1580 CrossRef CAS.
- W. Xu, X. Zhang, X. Xu, J. Chen and Q. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 13042–13051 CrossRef CAS PubMed.
- H. Yao, F. Zhang, G. Zhang, H. Luo, L. Liu, M. Shen and Y. Yang, Chem. Eng. J., 2018, 334, 2547–2557 CrossRef CAS.
- M. Majumder, M. S. Santosh, R. Viswanatha, A. K. Thakur, D. P. Dubal and K. Jayaramulu, Energy Storage Mater., 2021, 37, 396–416 CrossRef.
- M. Z. Iqbal, M. Shaheen, M. W. Khan, S. Siddique, S. Farid, S. Aftab and S. M. Wabaidur, Mater. Today Sustain., 2023, 22, 100331 Search PubMed.
- N. Kumar, S. Ghosh, D. Thakur, C.-P. Lee and P. Kumar Sahoo, Nanoscale Adv., 2023, 5, 3146–3176 RSC.
- R. Mas-Ballesté, C. Gómez-Navarro, J. Gómez-Herrero and F. Zamora, Nanoscale, 2011, 3, 20–30 RSC.
- N. Kumar, N. Bansal, Y. Yamauchi and R. R. Salunkhe, Chem. Mater., 2022, 34, 4946–4954 CrossRef CAS.
- W. Peng, N. Song, Z. Su, J. Wang, K. Chen, S. Li, B. Wei, S. Luo and A. Xie, Microchem. J., 2022, 179, 107506 CrossRef CAS.
- Y. Ehrnst, H. Ahmed, R. Komljenovic, E. Massahud, N. A. Shepelin, P. C. Sherrell, A. V. Ellis, A. R. Rezk and L. Y. Yeo, J. Mater. Chem. A, 2022, 10, 7058–7072 RSC.
- P. Zhang, M. Wang, Y. Liu, S. Yang, F. Wang, Y. Li, G. Chen, Z. Li, G. Wang, M. Zhu, R. Dong, M. Yu, O. G. Schmidt and X. Feng, J. Am. Chem. Soc., 2021, 143, 10168–10176 CrossRef CAS PubMed.
- J. Zhao, T. Zhang, J. Ren, Z. Zhao, X. Su, W. Chen and L. Chen, Chem. Commun., 2023, 59, 2978–2981 RSC.
- J. Troyano, F. Zamora and S. Delgado, Chem. Soc. Rev., 2021, 50, 4606–4628 RSC.
- E. Cariati, E. Lucenti, C. Botta, U. Giovanella, D. Marinotto and S. Righetto, Coord. Chem. Rev., 2016, 306, 566–614 CrossRef CAS.
- A. Schlachter and P. D. Harvey, J. Mater. Chem. C, 2021, 9, 6648–6685 RSC.
- J. Conesa-Egea, F. Zamora and P. Amo-Ochoa, Coord. Chem. Rev., 2019, 381, 65–78 CrossRef CAS.
- M. Murillo, R. Wannemacher, J. Cabanillas-González, U. R. Rodríguez-Mendoza, J. Gonzalez-Platas, A. Liang, R. Turnbull, D. Errandonea, G. Lifante-Pedrola, A. García-Hernán, J. I. Martínez and P. Amo-Ochoa, Inorg. Chem., 2023, 62, 10928–10939 CrossRef CAS PubMed.
- W. Liu, Y. Fang and J. Li, Adv. Funct. Mater., 2018, 28, 1705593 CrossRef.
- S. Mishra, C. Patel, D. Pandey, S. Mukherjee and A. Raghuvanshi, Small, 2024, 20, 2311448 CrossRef CAS PubMed.
- M. K. Singh, S. Krishnan, K. Singh and D. K. Rai, J. Power Sources, 2024, 595, 234060 CrossRef CAS.
- S. Mishra, M. K. Singh, D. Pandey, D. K. Rai and A. Raghuvanshi, J. Mater. Chem. A, 2024, 12, 4534–4543 RSC.
- A. Raghuvanshi, M. Knorr, L. Knauer, C. Strohmann, S. Boullanger, V. Moutarlier and L. Viau, Inorg. Chem., 2019, 58, 5753–5775 CrossRef CAS PubMed.
- A. Raghuvanshi, C. Strohmann, J.-B. Tissot, S. Clément, A. Mehdi, S. Richeter, L. Viau and M. Knorr, Chem.–Eur. J., 2017, 23, 16479–16483 CrossRef CAS PubMed.
- L. Viau, M. Knorr, L. Knauer, L. Brieger and C. Strohmann, Dalton Trans., 2022, 51, 7581–7606 RSC.
- P. D. Harvey and M. Knorr, J. Inorg. Organomet. Polym. Mater., 2016, 26, 1174–1197 CrossRef CAS.
- A. Schlachter, K. Tanner and P. D. Harvey, Coord. Chem. Rev., 2021, 448, 214176 CrossRef CAS.
- P. D. Harvey and M. Knorr, Macromol. Rapid Commun., 2010, 31, 808–826 CrossRef CAS PubMed.
- X. Zhang, W. J. Jiang, A. Mauger, Qilu, F. Gendron and C. M. Julien, J. Power Sources, 2010, 195, 1292–1301 CrossRef CAS.
- Z.-Y. Zhang, Z.-P. Deng, X.-F. Zhang, L.-H. Hou, H. Zhao and S. Gao, CrystEngComm, 2014, 16, 359–368 RSC.
- Z.-F. Wu, C. Wang, X. Liu, K. Tan, Z. Fu, S. J. Teat, Z.-W. Li, X. Hei, X.-Y. Huang, G. Xu and J. Li, J. Am. Chem. Soc., 2023, 145, 19293–19302 CrossRef CAS PubMed.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, John Wiley & Sons, 2009 Search PubMed.
- M. Singh, V. K. Singh, K. Surana, B. Bhattacharya, P. K. Singh and H.-W. Rhee, J. Ind. Eng. Chem., 2013, 19, 819–822 CrossRef CAS.
- Z.-H. Ren, Z.-R. Zhang, L.-J. Ma, C.-Y. Luo, J. Dai and Q.-Y. Zhu, ACS Appl. Mater. Interfaces, 2023, 15, 6621–6630 CrossRef CAS PubMed.
- Y. Pan, Y. Han, Y. Chen, D. Li, Z. Tian, L. Guo and Y. Wang, Electrochim. Acta, 2022, 403, 139679 CrossRef CAS.
- R. Sahoo, S. Ghosh, S. Chand, S. Chand Pal, T. Kuila and M. C. Das, Composites, Part B, 2022, 245, 110174 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC [2289195]. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ta04301d |
| ‡ These authors have equal contributions. |
| This journal is © The Royal Society of Chemistry 2024 |