Unlocking enhanced electrochemical performance through oxygen–nitrogen dual functionalization of iron–nickel–sulfide for efficient energy storage systems†
Lan
Nguyen‡
a,
Roshan Mangal
Bhattarai‡
 a,
Sosiawati
Teke
a,
Kisan
Chhetri
a,
Sosiawati
Teke
a,
Kisan
Chhetri
 b,
Debendra
Acharya
b,
Ragu
Sasikumar
b,
Debendra
Acharya
b,
Ragu
Sasikumar
 cde and
Young Sun
Mok
cde and
Young Sun
Mok
 *a
*a
aDepartment of Chemical Engineering, Jeju National University, Jeju, Republic of Korea. E-mail: smokie@jejunu.ac.kr; Fax: +82-64-755-3670; Tel: +82-64-754-3682
bDepartment of Nano Convergence Engineering, Jeonbuk National University, Jeonju, Republic of Korea
cSchool of Mechatronics Engineering, Korea University of Technology and Education, Cheonan, Republic of Korea
dAdvanced Technology Research Centre, Korea University of Technology and Education, Cheonan, Chungnam, Republic of Korea
eCentre of Molecular Medicine and Diagnostics, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India
First published on 13th June 2024
Abstract
Developing an energy storage electrocatalyst that excels in efficiency, cost-effectiveness, and long-term stability over numerous charge–discharge cycles is paramount for advancing energy storage technologies. In this work, we present a simple and environmentally friendly method to fabricate an asymmetric supercapacitor device (ASCD) as a viable energy storage system. The ASCD features binder-free, oxygen–nitrogen dual functionalized, and sulfurized iron–nickel hydroxysulfide (FNMOS) electrocatalysts, self-grown on nickel foam as a positive electrode, and waste biomass-derived activated carbon (CFAC) as a negative electrode. The FNMOS electrode in a 3-electrode configuration has the highest area-specific capacity of 1.6 mA h cm−2 at 1 mA cm−2, and even at a high current density of 10 mA cm−2, it maintained 0.94 mA h cm−2. The enhanced electrocatalytic activity is due to the synergistic contribution of the sulfurized NiFe composite along with the meticulous oxygen–nitrogen co-functionalization. Additionally, the ASCD with the FNMOS positive electrode and the CFAC negative electrode achieves maximum energy density (ED) and power density (PD) values of 350 μW h cm−2 (825 μW per cm2 PD) and 7960 μW cm−2 (200 μW h per cm2 ED). Furthermore, the device demonstrated exceptional rate capability by maintaining over 96% of its initial capacity even after 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of charge and discharge. The exceptional stability was further characterized by the ex situ post-mortem analysis of the FNMOS electrode after the stability test. These encouraging electrochemical results, paired with some practical use cases, demonstrate the applicability of FNMOS as a next-generation energy storage material.
000 cycles of charge and discharge. The exceptional stability was further characterized by the ex situ post-mortem analysis of the FNMOS electrode after the stability test. These encouraging electrochemical results, paired with some practical use cases, demonstrate the applicability of FNMOS as a next-generation energy storage material.
1 Introduction
In light of energy shortages and the pressing challenge of climate change, there is an urgent need to generate cleaner and more cost-effective energy to keep pace with increasing demand.1,2 Presently, the world relies heavily on non-renewable fossil fuels like coal, wood, natural gas, and oil, which pose significant environmental risks due to their emission of greenhouse gases such as CO2 and NOx.3,4 Consequently, efforts are underway to explore alternative sources such as solar, wind, and tidal energy, aiming to reduce dependence on fossil fuels and mitigate their environmental impact.5–7 In this context, it is worth mentioning that electrochemical energy storage systems such as batteries and supercapacitors (SCs) play a crucial role.8 They offer significant potential in the development of greener energy solutions and in mitigating the reliance on fossil fuels. One of the most promising options for next-generation power devices is electrochemical SCs, which have several desirable qualities such as high power density (tens of times greater than that of batteries), fast charging (within seconds), outstanding cycling stability, small size, and low mass.9–11 SCs outperform fuel cells and rechargeable batteries in many aspects, leading to their widespread adoption in electric vehicles (either solely or in conjunction with batteries), backup power systems, and various other applications.12,13 Generally, SCs are divided into two types, electric double-layer capacitors (EDLCs) and pseudo-capacitors (PCs).14,15 Energy storage in EDLCs occurs through the reversible process of superficial ion adsorption and desorption in the electroactive surface, whereas in PCs, capacitance is obtained from reversible faradaic reactions involving ionic species.16,17 Nowadays, many commercial SCs are constructed using high surface area carbon materials. However, these SCs may not always provide sufficient performance or energy/power density. Consequently, a key area of focus in improving SC capacitance and performance at high current densities is the development of materials that boast a well-organized framework, exhibit high conductivity, and possess numerous active sites.18,19 Consequently, research is focused on the development of electrode materials to enhance energy storage capacity per unit of mass, volume, or surface area.20Fe and Ni are currently employed as electrocatalysts, owing to their affordability and abundance among other transition metals.21–23 To further improve the performance of Fe–Ni SCs, incorporating a non-metallic element has emerged as a viable approach. Among potential candidates, sulfur stands out due to its natural abundance leading to cost-effectiveness. Furthermore, the transition metal chalcogenides have a plethora of attractive electrocatalytic properties such as an abundance of electrocatalytically energetic sites, varied oxidation states, significant electrical conductivity, and reduced bandgap. Owing to these attractive properties, these materials enhance ion transport kinetics, minimizing diffusion paths, reducing electrode/electrolyte interface resistance, and demonstrating outstanding electrochemical performance characterized by high capacity, rate capability, and long-term cycling stability.24–26 Density functional theory modeling also suggests that introducing sulfur, particularly in combination with Fe doping, can increase conductivity, lower the energy barrier, and improve the kinetic responses.27 Consequently, there are studies investigating Fe–Ni–S composites as potential materials for supercapacitor electrode applications. For instance, Li et al. reported the fabrication of an asymmetric supercapacitor device (ASCD) comprising a complex metal–chalcogen–carbon composite cathode that is NiFeSx@CNTs@MnS@D and a graphene anode that delivered a moderate energy density (ED) of 28.9 W h kg−1 at a power density (PD) of 937.5 W kg−1 and 88.03% of capacitance retention after 5000 cycles.28 Likewise, Sun et al. fabricated an Fe5Ni4S8/FeS@N-HPC-800//active carbon ASCD, that delivered commendable ED of 76.8 W h kg−1 at a PD of 395.03 W kg−1 and moderate cycling stability of 93.24% over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles.29 A similar trend can be seen in more complex multi-metal–multi-chalcogen composite cathodes such as FeNiSe2@Ni4.5Co4.5S8 reported by Wan et al. Here the ASCD offered an ED of 69.0 W h kg−1 at PD of 799.2 W kg−1, and moderate cycling stability with 91.2% capacity retention after 10
000 charge–discharge cycles.29 A similar trend can be seen in more complex multi-metal–multi-chalcogen composite cathodes such as FeNiSe2@Ni4.5Co4.5S8 reported by Wan et al. Here the ASCD offered an ED of 69.0 W h kg−1 at PD of 799.2 W kg−1, and moderate cycling stability with 91.2% capacity retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.30
000 cycles.30
Despite various methods reported for preparing Fe–Ni–S electrodes, the challenge remains in designing and synthesizing highly efficient and ‘stable’ Fe–Ni–S electrocatalysts via a ‘simple and cost-effective’ synthesis method. A critical aspect still to be empirically validated is the specific mechanism by which the introduction of sulfur enhances the specific capacity while maintaining substantial stability. This gap in understanding underscores the need for further experimental investigation in this area. Hence, our work proposes a straightforward hydrothermal method for the fabrication of binder-free Fe–Ni–S hetero composite electrode materials on nickel foam (NF). Preceding this synthesis, ammonia, recognized for its cost-effectiveness and suitability for nitrogen doping, is employed to meticulously functionalize conventionally grown nickel–iron layered double hydroxide (NiFe LDH) nanosheets with oxygen vacancies and nitrogen. This process is instrumental in enhancing the heteroatom/defect-induced electrochemical properties of the resulting supercapacitor device. Furthermore, the subsequent step of straightforward air calcination acts as a preparatory phase for the stoichiometric sulfurization process, playing a pivotal role in attaining both high capacity and exceptional stability of the resultant supercapacitor electrode. Consequently, we achieved remarkable supercapacitor performance from an active material synthesized using a seemingly straightforward yet sophisticatedly optimized mechanism. The material when fabricated into an ASCD exhibits a commendable ED of 0.35 mW h cm−2, operating at a PD of 0.825 mW cm−2 along with exceptional long-term stability that registered 96% capacitance retention even after 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles. This establishes the synthesized material at the forefront of supercapacitor research, with the possibility of scale-up facilitated by the straightforward and cost-effective experimental procedures followed.
000 charge–discharge cycles. This establishes the synthesized material at the forefront of supercapacitor research, with the possibility of scale-up facilitated by the straightforward and cost-effective experimental procedures followed.
2 Results and discussion
2.1 Physicochemical characterization
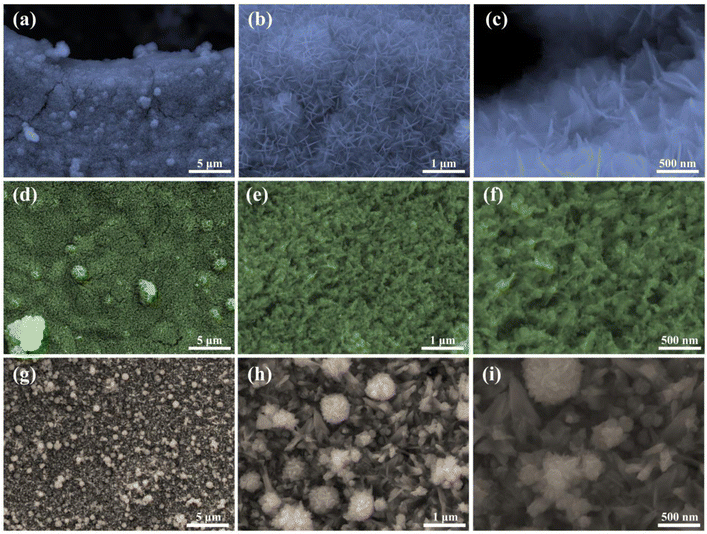
We synthesized FNM (iron–nickel layered double hydroxide calcined under an ammonia atmosphere), FNMS (sulfurized FNM without oxygen treatment), and FNMOS (sulfurized FNM with oxygen treatment) nanoplates on NF by methods as described in the ESI File,† as well as, collectively depicted in Fig. 1. Due to its high electrical conductivity and three-dimensional porous framework, which allows rapid ion diffusion, NF was chosen as the ideal growth substrate in this case.31 Using the hydrothermal method, the pretreated NF was immersed in a precursor solution containing Fe3+ and Ni2+, with the support of CO(NH2)2 and NH4F, to grow FeNi-layered double hydroxide (LDH) nanoarrays. NH4F has a role in activating the substrate, creating more active nucleation sites, and creating suitable metal complexes.32 Additionally, CO(NH2)2 is used as a self-templating agent and precipitant to direct LDH production.32 Subsequently, the prepared sample underwent high-temperature calcination in the reducing environment provided by ammonia and nitrogen mix feed gas. This process aimed to introduce oxygen vacancies as well as doping the nitrogen in the metal composites, which narrows the band gap and enhances both conductivity and the overall electrocatalytic efficiency. Before sulfurization, the sample was again subjected to an oxygen treatment, to ensure the stoichiometric sulfurization as well as the removal of any superficially attached ammonia. Finally, the sample underwent sulfurization through hydrothermal processing at 120 °C for 5 h.Field emission scanning electron microscopy (FE-SEM) was performed at different magnifications to examine the physical morphology of the electrodes. Fig. 2(a–c) depict the evolution of the hydrothermally synthesized FNM samples into nanosheets. These nanosheets are measured to be around 20 nm in thickness with a very homogenous distribution as verified by the FE-SEM images shown in Fig. S1.† Additionally, as seen in Fig. 2(d and e), the FNMS samples underwent a distinct shape shift upon sulfidation, and dense and homogenous nanoparticles covered the nanosheets. The FE-SEM analysis performed on the FNMOS sample can be seen in Fig. 2(g–i), at different magnifications. The formation of metal hydroxy sulfide heterostructures is favored when oxidation precedes sulfurization; this process facilitates the attachment of sulfur to metal nanoparticles, forming a more stoichiometrically precise metal sulfide composite, thereby enhancing their conductivity and overall electrocatalytic effectiveness.33
Energy-dispersive spectroscopy (EDS) was used to examine the elemental composition of the electrode material. FNM confirmed the presence of Fe, Ni, C, O, and N within the sample (Fig. S2(a)†). To verify the incorporation of sulfur, EDS analysis was also performed for the FNMS and FNMOS electrodes. The elemental mapping of the FNMS electrode is shown in Fig. S2(b),† which confirms the even distribution of Fe, Ni, C, O, and S elements in the sample surface. In that, the FNMOS electrode also has Fe, Ni, C, O, and S (Fig. S2(c)†) but it shows a substantially higher atomic weight percentage (Table S1†) of sulfur elements when compared to the FNMS electrode. This is mainly due to the oxygen grafting step employed before the sulfurization for the case of the FNMOS sample. Previous studies have noted a significant disparity in the Gibbs free energy required for sulfurization between pure metals and their corresponding metal oxides. This discrepancy is attributed to the comparatively lower energy demand of the anion exchange process, a phenomenon we have both experimentally and theoretically confirmed in our prior research.33
The X-ray diffraction (XRD) profiles of FNM, FNMS, and FNMOS are shown in Fig. 3(a). It is found that peaks appeared at 18.4, 30.3, 35.7, 37.3, 43.4, 47.5, 53.8, 57.4, 63.0, 66.3, 71.5, 74.6, 75.6, 79.6, and 82.5 2 theta degree corresponding to the lattice planes (111), (220), (311), (222), (400), (331), (422), (511), (440), (531), (620), (533), (622), (444), and (711) respectively belonging to the NiFe2O4 phase (JCPDS No. 00-44-1485).34 This is also verified by the TEM and HR-TEM analysis of the FNMO sample as shown in Fig. S3.† The HR-TEM images analysis reveals distinct lattice planes like (311) and (220) corresponding to interplanar distances of 0.25 nm, and 0.29 nm respectively, of the NiFe2O4 crystal phase. The XRD patterns of FNMS and FNMOS appear reminiscent of that of FNM, yet they display significantly heightened noise signals, undoubtedly attributed to the hydrothermal sulfurization procedure as shown in Fig. 3(a). Hydrothermal sulfurization often results in the formation of amorphous or poorly crystalline phases. 35 These phases do not produce sharp, well-defined XRD peaks but instead contribute to diffuse background noise. The lack of long-range order in the atomic structure scatters X-rays incoherently, leading to increased noise.36 Furthermore, the surface roughness and morphology changes induced by hydrothermal sulfurization can scatter X-rays in various directions, leading to increased noise in the XRD pattern. The irregularities on the surface cause diffuse scattering, which is recorded as noise.37 Moreover, the FNMS and FNMOS XRD profiles did not reveal any other phases, suggesting that any metal sulfide phases present are in the form of an amorphous shell, with an FNM core. Meanwhile, by analyzing the diffraction peak in the range of 34–38 2θ degrees or the (410) plane (Fig. S4†), it is observed that the FNMOS peak slightly shifts towards a smaller angle, undoubtedly verifying the better sulfur incorporation.38
X-ray photoelectron spectroscopy (XPS) was utilized to probe the detailed surface chemical composition of as-fabricated active materials more closely. The XPS survey spectra, as shown in Fig. 3(b), clearly exhibit characteristics of Ni, Fe, and O elements that can be observed in both FNMS and FNMOS samples. However, a prominent peak corresponding to sulfur (S) is observed in FNMS and FNMOS, while it is absent in the FNM sample (Fig. S5(a)†). The presence of the sulfur peak in FNMS and FNMOS samples, but not in the FNM sample, serves as strong evidence of the sulfur incorporation through the sulfurization process.
To precisely determine the distribution and valence states of nickel (Ni), iron (Fe), oxygen (O), and sulfur (S) in the samples, Gaussian fitting was applied to the core level XPS spectrum of the respective elements. In the Fe 2p spectrum (Fig. 3(c)), two distinct peaks were observed at binding energies of 722.7 eV and 712.8 eV, accompanied by a satellite peak at 733.7 eV. These peaks correspond to Fe 2p3/2 and Fe 2p1/2, indicative of the presence of Fe3+.39,40 The peak at 705.9 eV belongs to the metallic Fe (Fe0) and most definitely originated owing to the reducing atmosphere during calcination. In Fig. 3(d), the Ni 2p XPS spectrum reveals two major peaks indicative of Ni2+, which exhibit a spin-energy separation of 17.6 eV. These peaks, located at 855.7 eV (along with a satellite peak at 861.2 eV) and 873.3 eV (accompanied by a satellite peak at 879.7 eV), are assigned to Ni 2p3/2 and Ni 2p1/2 states, respectively. It is important to observe that additional peaks appearing at 852.0 eV, corresponding to the 2p3/2 level, indicate a partial reduction of Ni2+ ions to a metallic state. This metallic nature typically facilitates rapid electron transport, which in turn can significantly improve the electrochemical performance of the material.1 Furthermore, the significant reduction in Fe0 and Ni0 peak intensity for the FNMOS sample clearly verifies the successful oxygen grafting of the FNM sample. In the S 2p spectrum shown in Fig. 3(e), the peak observed at 162.5 eV corresponds to S-metal bonding, aligning consistently with the analyses of Ni 2p and Fe 2p peaks.41 Additionally, the S–O bonding is evidenced by a peak around 168.4 eV.42 Furthermore, the O 1s XPS spectrum, as depicted in Fig. 3(f), shows the peak for the oxygen binding state in FNMOS, with a binding energy of 531.2 eV. This peak corresponds to the O2−, forming oxide with iron and nickel. Furthermore, as shown in Fig. S6,† by comparing the O 1s spectra of FNM and FNMO samples, we observed differences in the oxygen XPS profiles affected by the ammonia and nitrogen mix feed gas as well. The O 1s peaks were deconvoluted into three peaks: O I (lattice oxygen), O II (chemisorbed oxygen indicating defects), and O III (C–O bonds/surface-adsorbed moisture). The higher intensity of the O II peak in the FNM sample compared to FNMO confirms the presence of oxygen vacancies induced by the reducing atmosphere during calcination.43–45 These spectral findings are consistent with XRD and TEM analyses, confirming the successful transformation of FNM into FNMOS.39 In conclusion, the synthesized FNM material is identified as oxygen-vacant Fe–Ni–O, whereas the FNMS and FNMOS samples are characterized as Fe–Ni–S. In general, the peak intensity of pure metal (Fe0 and Ni0) for FNMOS becomes more reduced compared to the FNMS sample. In addition, the rightward shift in the peak of O 1s and S 2p and increased peak intensity suggest an enhanced tendency for sulfur bonding due to the oxidation process. These findings from XPS analysis are consistent with observations from FE-SEM and XRD analyses.
The high-resolution transmission electron microscope (HR-TEM) image in Fig. 3(g) represents the FNMOS sample. Notably, lattice plane spacings of 0.19 nm for (331) and 0.48 nm for (111) closely align with the d-spacing of NiFe2O4. Besides that, it revealed several distinct planes exhibiting a mismatched morphology, indicating the likely presence of a hydroxysulfide mixture. It is thought that the disoriented lattice and defects, resulting from the aqueous sulfurization process, enhance the catalytic properties of the active materials. The transmission electron microscope (TEM) image corresponding to Fig. 3(g) is presented in Fig. 3(h). It is clearly seen that the FNMOS nanostructure is made up of several tiny nanoparticles. Fig. 3(i) shows the high-angle annular dark-field (HAADF) scanning transmission electron microscopy (STEM) image, coupled with the associated elemental mapping images, demonstrating a homogenous distribution of Fe, Ni, C, O, N, and S elements throughout the structure. This uniformity underscores the effective formation of the FNMOS nanostructure.
2.2 Electrochemical characterization
The fabricated electrodes were then electrochemically analyzed for SC applications using both three-electrode and two-electrode configurations. To identify the most electrochemically efficient electrode for the ASCD, a series of tests were conducted on each electrode employing a variety of electrochemical tests.Fig. 4(a) shows the cyclic voltammetry (CV) profiles of different electrodes namely, FNM, FNMS, and FNMOS, measured through a three-electrode system at 10 mV s−1 of scan rate. The FNMS and FNMOS electrodes show a higher CV curve area than the FNM electrodes, which proves that the charge storage capability of the electrode is significantly enhanced after sulfurization. Notably, the nature of the redox peak is different with and without sulfurization, as well as before and after oxygen treatment for different samples. This highlights the change in redox processes for different samples as verified by their respective physical characterization.
Likewise, in Fig. 4(b), discharge curves obtained from the galvanostatic charge–discharge (GCD) analysis for the identical electrodes are presented. The slow discharge time of FNMS and FNMOS electrodes compared to that of the FNM electrode at the same current density suggests that the FNMS and FNMOS electrodes have greater charge storage capacity, which is in line with the CV results. The significance of sulfurization and partial oxidation in the electrode fabrication process is underscored by the results obtained from CV and GCD analyses. The CV analysis reveals that the absence of electrochemically active sites in FNM electrodes, where Fe and Ni predominantly exist in a metallic state, renders them unsuitable for SC applications, as indicated by the minimal magnitude of redox peaks and short discharge times observed in the GCD analysis. On the other hand, hydrothermal sulfurization of the FNM sample notably boosts its electrochemical performance, as indicated by the CV and GCD discharge curves of the FNMS electrode. This enhancement primarily stems from the creation of a metal hydroxysulfide composite with heightened electrochemical activity, stemming from the partial sulfurization of the passivating oxide layer on the metal surface. Moreover, the exceptional performance of the FNMOS electrode underscores the significance of oxygen treatment preceding the sulfurization process. While sulfurizing the FNM sample, the sulfurization happens superficially and non-stoichiometrically. However, sulfurizing the FNMO results in stoichiometrically balanced metal sulfide with a higher degree of sulfurization producing the best electrochemical results.
To gain a deeper insight into the reaction mechanism, the CV behavior of the FNMOS electrode at varying scan rates is illustrated in Fig. 4(c). The redox peaks from the CV curves are visible which is mainly attributed to the faradaic redox processes. In the alkaline electrolyte, the primary electrochemical processes involve the reversible electron transfer associated with Ni3+/Ni2+ and Fe3+/Fe2+ redox couples. This suggests that the capacitance characteristics are predominantly due to faradaic PC, arising from the synergy of the following electrochemical reactions:28,39
| FeS2 + OH− ⇌ FeS2OH + e− | (1) |
| NiS + OH− ⇌ NiSOH + e− | (2) |
| Ni(OH)2 + OH− ⇌ NiOOH + H2O + e− | (3) |
| Fe(OH)2 + OH− ⇌ FeOOH + H2O + e− | (4) |
Fig. 4(d) shows the nonlinear discharge time profiles of the FNMOS electrode, verifying the redox activity-dominated charge storage that matches the CV profile from earlier. These redox reactions are generally reliant on the insertion and extraction of protons from the electrolyte to the active material. At lower scan rates, there is enough time for the diffusion of ions from the electrolyte to permeate nearly all the electroactive area of the electrode.46,47 However, as the scan rate increases, the effective interaction between the ions and the electrode surface reduces, resulting in a decrease in the capacitance. The specific capacity of different electrodes was calculated using eqn (S1)† employing the integral area of the discharge curve from GCD data. The capacitance result is illustrated in Fig. 4(e), alongside the areal capacity and faradaic capacitance of diverse electrodes. The specific capacity demonstrates an increasing trend with the augmented degree of oxygen and sulfur treatment. Particularly, the FNMOS electrode exhibits the highest area-specific capacity, reaching 1.6 mA h cm−2 (14.4 F cm−2) at a current density of 1 mA cm−2 and still maintains it at 0.94 mA h cm−2 (8.4 F cm−2) even at a high current density of 10 mA cm−2. These values are significantly higher than those obtained from the FNM and FNMS samples. The improved specific capacity is largely due to the increased conductivity and enriched porous structure owing to the oxygen treatment promoted sulfurization of the FNMOS sample. The improved capacity of the FNMOS electrode was further studied by interpreting the electrochemical surface area (ECSA) of different samples employing the method reported in ESI Section 1.3.† Briefly, the double-layer capacitance (Cdl) of different electrodes was calculated based on the CV analysis of non-faradaic regions at the scan rates ranging from 10 mV s−1 to 100 mV s−1 as demonstrated in Fig. S7.† As confirmed by the slopes of different samples in Fig. S7(d),† the largest Cdl of 13.0 mF cm−2 was observed for FNMOS, compared to FNMS (9.1 mF cm−2) and FNM (3.4 mF cm−2). The highest Cdl, and subsequently the highest ECSA for FNMOS, is attributed to the stoichiometrically formed iron–nickel hydroxysulfide structures resulting in its superior electrochemical performance.
PCs have primarily two different modes of charge storage namely diffusion-controlled and surface-absorption (capacitive) controlled. Eqn (5), which relates the current (i) response with the scan rate (v) can be used to determine these charge storage contributions qualitatively.48,49
| i = avb | (5) |
Eqn (5) can be rewritten as follows:
log(i) = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(v) + log(a) log(v) + log(a) | (6) |
The gradient of a linear line drawn in the log(i) versus log(v) plot (eqn (6)), depicted in Fig. 4(f), determines the b-value. Here, the logarithm of the oxidation peak current for electrodes FNM, FNMS, and FNMOS was plotted against the logarithm of their corresponding scan rates ranging from 10 to 50 mV s−1. The linear fitting of these points yielded the b-value, detailed in the inset of Fig. 4(g). A process governed by diffusion has a b-value of 0.5, whereas a process dominated by capacitance has a b-value of 1. The computed b values for the anodic peaks of FNMOS, FNMS, and FNM are 0.58, 0.56, and 1, respectively. Hence, FNM shows an ideal surface-absorption-controlled nature of charge storage, whereas FNMS and FNMOS show diffusion-controlled charge storage with FNMOS in slightly greater proportion compared to FNMS. The increased diffusion-controlled charge storage for FNMOS arises from the oxygen-promoted sulfurization process, which expedites the redox processes. Therefore, based on the analysis of the b-value, it can be inferred that effective oxygen treatment is essential for enhancing the pseudocapacitive charge storage. This phenomenon, which prevails in metal oxides/chalcogenides, contributes significantly to improving the electrode's overall charge storage capacity.
For the quantitative capacitive and diffusion-controlled charge storage contribution to the overall capacity of the electrode, eqn (7) is used:50–52
| i(ν) = k1ν + k2ν½ | (7) |
 | (8) |
2.3 Electrochemical characterization of the ASCD
When set up in a three-electrode configuration, the FNMOS electrode shows the best electrochemical performance. Therefore, an ASCD was fabricated to test the application potential of the FNMOS-positive electrode. An NF is coated with activated carbon made from cherry petal biowaste (CFAC) for the negative part of the ASCD. The electrochemical characterization results for CFAC electrodes are presented in Fig. S9† using a three-electrode configuration. The individual CV curves at 10 mV s−1 and GCD at 1 mA cm−2 of the cathode (FNMS) and anode (CFAC) are shown in Fig. S10.† A detailed description of the CFAC electrode preparation process is provided in the ESI† experimental part.Fig. 5(a) presents a schematic diagram of the constructed FNMOS//CFAC device. To identify the operational potential window of the electrode, the cyclic voltammetry (CV) curves of the FNMOS//CFAC ASCD across various potential windows are presented in Fig. 5(b), all carried out at a scan rate of 10 mV s−1. The CV curve indicates that a potential window up to 1.6 V is suitable for further investigation, as there is a minor polarization peak at 1.7 V, likely due to the water oxidation. Consequently, the CV curves of the device at different scan rates were recorded within a 0–1.6 V potential window, ranging from 10 to 50 mV s−1, and are depicted in Fig. 5(c). The CV profiles reveal distinct anodic and cathodic curves, featuring pronounced redox peaks indicating the pseudo-capacitive behavior typical of metal chalcogenide active materials. Also, the CV curves possess a wide main body, especially when compared to their three electrode counterparts, characteristic of the EDLC type of charge storage mechanism, most definitely owing to the CFAC electrode.55 The discharge curves of the ASCD at different current densities within the same potential window of the CV test are shown in Fig. 5(d) validating the ideal pairing of positive and negative electrodes for a commendable capacitance contribution in the FNMOS//CFAC device.
The areal capacity of the ASCD was determined using the galvanostatic discharge data and the formulae outlined in eqn (S2)† as depicted in Fig. 5(e). Encouragingly, the ASCD exhibits an areal capacity of 436.1 μA h cm−2 at a current density of 1 mA cm−2 and a coulombic efficiency of 94.2% at a current density of 10 mA cm−2. Besides that, the electrochemical performance of the ASCD fabricated with the FNMS positive electrode and CFAC negative electrode can also be seen in Fig. S11.† The specific capacity of this device reached 277.7 μA h cm−2 at 2 mA cm−2, which preserved over 59.7% of the starting capacitance at 10 mA cm−2, based on calculations made from the discharge curve. Therefore, it can be inferred that the oxygen treatment procedure enhances the electroactivity of the optimally sulfurized FNMOS electrode, consequently enabling superior electrochemical performance.
The capacitive and diffusive charge storage contribution is calculated using Dunn's method starting from the linear fit of various anodic and cathodic scans as illustrated in Fig. 5(f). Fig. 5(g) depicts a plot contrasting the diffusion and capacitive contributions components with the overall current at a scan rate of 5 mV s−1. The two types of charge storage perfectly complemented each other as one is maximum when the other is minimum and vice versa. Subsequently, Fig. 5(h) displays a bar diagram of capacitive and diffusion-controlled contributions to the FNMOS//CFAC electrode as the scan rate increases. These results suggest efficient diffusion-controlled charge storage at lower scan rates with that being 69% at 5 mV s−1. Interestingly, it can be seen that the capacitive contribution exhibits a consistent increase from 31% to 53% as the scan rate increases from 5 mV s−1 to 9 mV s−1. This phenomenon is attributed to the reduced time available for ion diffusion at higher scan rates.
The durability of FNMOS//CFAC was investigated using a cyclic GCD stability test at a current density of 45 mA cm−2, as depicted in Fig. 5(i). After 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD stability cycles, the ASCD demonstrated a high capacitance retention of 96% and a coulombic efficiency of 90%. The result exhibits the FNMOS//CFAC device's remarkable stability, indicating the benefits of the pre-sulfurization oxygen treatment. The oxygen-promoted sulfurization enables a short and efficient pathway for ion/electron transport within the electrode, facilitating absorption and desorption of the electrolyte ion during the charging and discharging processes thereby enhancing the overall electrochemical activity.33 Furthermore, proper sulfurization owing to the oxygen treatment maintains the electroactive phases of FNMOS for a prolonged duration. The capacity retention exceeding 100% in the initial cycles is due to the introduction of additional electrochemically active spots, primarily resulting from improved electrolyte diffusion across all surfaces following multiple cycles.33
000 GCD stability cycles, the ASCD demonstrated a high capacitance retention of 96% and a coulombic efficiency of 90%. The result exhibits the FNMOS//CFAC device's remarkable stability, indicating the benefits of the pre-sulfurization oxygen treatment. The oxygen-promoted sulfurization enables a short and efficient pathway for ion/electron transport within the electrode, facilitating absorption and desorption of the electrolyte ion during the charging and discharging processes thereby enhancing the overall electrochemical activity.33 Furthermore, proper sulfurization owing to the oxygen treatment maintains the electroactive phases of FNMOS for a prolonged duration. The capacity retention exceeding 100% in the initial cycles is due to the introduction of additional electrochemically active spots, primarily resulting from improved electrolyte diffusion across all surfaces following multiple cycles.33
Fig. 5(j) displays the Ragone plot, illustrating the relationship between ED and PD for the FNMOS//CFAC device, in contrast with findings from prior research. Remarkably, the FNMOS//CFAC ASCD showcases a notable ED of 350 μW h cm−2 at an 825 μW per cm2 PD. Furthermore, it maintains a considerable ED of 200 μW h cm−2 even under significantly elevated power density (PD) conditions, reaching up to 7960 μW cm−2. This performance stands out significantly and is comparable/superior to that of previously reported ASCDs with similar electrochemical properties. For instance, the Fe2O3//NPCTT configuration exhibited an ED of 176 μW h cm−2 at a power density (PD) of 875 μW cm−2.57 Similarly, the NiCo2S4@NiCoP/NF//AC showed an ED of 135 μW h cm−2 at a PD of 755 μW cm−2,58 NA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 LDH-rGO//AC HSC achieved 220 μW h cm−2 at 4500 μW cm−2,59 Cu(Co–Ni)2S4 NTs//P reached 270 μW h cm−2 at 2700 μW cm−2,60 NS-CMS NRAs-12h@NF//AC@NF recorded 147 μW h cm−2 at 600 μW cm−2,61 NC LDH NSs@Ag//AC showed 78.8 μW h cm−2 at 785 μW cm−2,62 and NC DHs@CNTs@Ni2-PFs//AC-CNTs-N2-PFs attained 99.5 μW h cm−2 at 598.7 μW cm−2.63
1 LDH-rGO//AC HSC achieved 220 μW h cm−2 at 4500 μW cm−2,59 Cu(Co–Ni)2S4 NTs//P reached 270 μW h cm−2 at 2700 μW cm−2,60 NS-CMS NRAs-12h@NF//AC@NF recorded 147 μW h cm−2 at 600 μW cm−2,61 NC LDH NSs@Ag//AC showed 78.8 μW h cm−2 at 785 μW cm−2,62 and NC DHs@CNTs@Ni2-PFs//AC-CNTs-N2-PFs attained 99.5 μW h cm−2 at 598.7 μW cm−2.63
The surface morphology of the FNMOS electrode following 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles was studied using FE-SEM and EDS analyses. Fig. 6(a)(i–iii) show the FE-SEM images, and Fig. 6(b and c) show the EDS results for the FNMOS electrode. The observed morphology closely resembles that depicted for the fresh electrode as in Fig. 2(g–i). The EDS analysis reveals the presence of Fe, Ni, S, O, C, and N ions. Notably, a peak corresponding to potassium is also detected. This K+ ion presence is attributed to the residual electrolyte on the electrode. Furthermore, the EIS test was conducted before and after the cyclic stability test to assess the variation in charge transfer resistance of the device as shown in Fig. S12.† The slight increase in series resistance for the ASCD after the stability test suggests the possible evolution of active material towards a less electroactive phase and possible delamination of some active material. Nonetheless, the very small change in the series resistance coupled with the intact physical morphology of the postmortem electrode, highlights the robustness of the as fabricated ASCD.
000 charge–discharge cycles was studied using FE-SEM and EDS analyses. Fig. 6(a)(i–iii) show the FE-SEM images, and Fig. 6(b and c) show the EDS results for the FNMOS electrode. The observed morphology closely resembles that depicted for the fresh electrode as in Fig. 2(g–i). The EDS analysis reveals the presence of Fe, Ni, S, O, C, and N ions. Notably, a peak corresponding to potassium is also detected. This K+ ion presence is attributed to the residual electrolyte on the electrode. Furthermore, the EIS test was conducted before and after the cyclic stability test to assess the variation in charge transfer resistance of the device as shown in Fig. S12.† The slight increase in series resistance for the ASCD after the stability test suggests the possible evolution of active material towards a less electroactive phase and possible delamination of some active material. Nonetheless, the very small change in the series resistance coupled with the intact physical morphology of the postmortem electrode, highlights the robustness of the as fabricated ASCD.
 | ||
| Fig. 6 (a)(i–iii) FE-SEM images of the FNMOS electrode after the electrochemical study. (b) EDS elemental spectrum plot. (c) Corresponding EDS spectrum. | ||
Fig. 7(a) depicts the ASCD connected to the electrochemical workstation. The corresponding CV plot is illustrated in Fig. 7(b). Subsequently, the ASCD was utilized to power low-power electronics such as light-emitting diodes (LEDs) to validate the practicality of the fabricated ASCD. Fig. 7(c) displays the LED connected in parallel with the second serially connected ASCD. Fig. 7(d–f) demonstrate the FNMOS//CFAC ASCD connected in series to illuminate the LED for over 5 min. The LEDs exhibited decent performance, gradually dimming as time progressed beyond 6 minutes. This demonstrates the viability of the FNMOS//CFAC ASCD as a sustainable energy storage unit.
3 Conclusions
In conclusion, our study demonstrates the successful synthesis of binder-free Fe–Ni–S electrocatalysts on NF through a straightforward two-step hydrothermal method. Utilizing an oxidation pre-treatment process significantly enhanced the specific capacitance of the resulting FNMOS electrode to 14.43 F cm−2, leading to an outstanding specific capacity of 1.6 mA h cm−2, surpassing other electrode configurations (FNM and FNMS). This improvement is attributed to the creation of abundant electroactive sites and the facilitation of rapid redox reactions. Moreover, we constructed an ASCD by combining FNMOS with activated carbon derived from CFAC. The FNMOS//CFAC configuration exhibited remarkable electrochemical performance, achieving a high areal capacity of 436.1 μA h cm−2 at 1 mA cm−2. Notably, it demonstrated impressive maximum energy and power density values of 350 μW h cm−2 and 825 μW cm−2, respectively. Furthermore, the device exhibited exceptional charge–discharge stability over 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, retaining over 96% of its initial capacity. To illustrate its practical functionality, the ASCD was successfully employed to power LEDs as a simulated load, underscoring its potential for real-world energy storage applications. Additionally, post-stability test characterization of the electrode revealed the continued integrity of the active material on the NF surface, emphasizing the robustness and feasibility of FNMOS as a promising electrode material for ASCDs. This study underscores the significance of our approach in advancing energy storage technologies toward sustainable and efficient solutions.
000 cycles, retaining over 96% of its initial capacity. To illustrate its practical functionality, the ASCD was successfully employed to power LEDs as a simulated load, underscoring its potential for real-world energy storage applications. Additionally, post-stability test characterization of the electrode revealed the continued integrity of the active material on the NF surface, emphasizing the robustness and feasibility of FNMOS as a promising electrode material for ASCDs. This study underscores the significance of our approach in advancing energy storage technologies toward sustainable and efficient solutions.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
L. N. & R. M. B.: conceptualization, methodology, investigation, visualization, data curation, writing original draft; S. T.: software, formal analysis, characterization, validation; K. C., D. A. & R. S.: validation, writing – review & editing; Y. S. M.: supervision, writing – review & editing, resources, funding acquisition, validation.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This research was supported by “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2023RIS-009).References
- C. L. Li, R. Z. Li and Y. K. Zhou, Energies, 2022, 15, 3877 CrossRef CAS.
- P. O. Agboola, I. Shakir, Z. A. Almutairi, S. S. Shar and M. F. A. Aboud, Phys. B, 2023, 659, 414866 CrossRef CAS.
- S. M. Lee, S. Saud, R. M. Bhattarai, Y. S. Mok, N. Matyakubov, D. B. Nguyen and J. W. Lee, Chem. Eng. J., 2023, 469, 143977 CrossRef CAS.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo, L. A. Hackett, J. P. Hallett, H. J. Herzog, G. Jackson, J. Kemper, S. Krevor, G. C. Maitland, M. Matuszewski, I. S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D. M. Reiner, E. S. Rubin, S. A. Scott, N. Shah, B. Smit, J. P. M. Trusler, P. Webley, J. Wilcox and N. Mac Dowell, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- T. Liu, L. Y. Zhang, B. Cheng and J. G. Yu, Adv. Energy Mater., 2019, 9, 1803900 CrossRef.
- L. Y. Zhang, D. W. Shi, T. Liu, M. Jaroniec and J. G. Yu, Mater. Today, 2019, 25, 35–65 CrossRef CAS.
- H. Liu and H. J. Yu, J. Mater. Sci. Technol., 2019, 35, 674–686 CrossRef CAS.
- Y. Hu, Z. Li, Z. Wang, X. Wang, W. Chen, J. Wang, W. Zhong and R. Ma, Adv. Sci., 2023, 10, 2206995 CrossRef CAS PubMed.
- X. Yan, Y. Y. Tong, X. Z. Wang, F. Hou and J. Liang, Energy Environ. Mater., 2022, 5, 800–822 CrossRef.
- A. M. Zardkhoshoui, B. Ameri and S. S. H. Davarani, Chem. Eng. J., 2022, 435, 135170 CrossRef.
- M. Amiri, A. M. Zardkhoshoui, S. S. H. Davarani, M. Maghsoudi and M. K. Altafi, Sustainable Energy Fuels, 2022, 6, 3626–3642 RSC.
- A. M. Zardkhoshoui, M. M. Ashtiani, M. Sarparast and S. S. H. Davarani, J. Power Sources, 2020, 450, 227691 CrossRef CAS.
- J. L. Sun, X. M. Du, R. Z. Wu, Y. F. Zhang, C. J. Xu and H. Y. Chen, ACS Appl. Energy Mater., 2020, 3, 8026–8037 CrossRef CAS.
- B. Ramulu, S. C. Sekhar, G. Nagaraju, S. J. Arbaz and J. S. Yu, J. Power Sources, 2021, 482, 228944 CrossRef CAS.
- R. M. Bhattarai, S. Moopri Singer Pandiyarajan, S. Saud, S. J. Kim and Y. S. Mok, Dalton Trans., 2020, 49, 14506–14519 RSC.
- M. Alzaid, M. Z. Iqbal, J. Khan, S. Alam, N. M. A. Hadia and W. S. Mohamed, Energy Technol., 2021, 9, 2100110 CrossRef CAS.
- K. Chhetri, A. P. Tiwari, B. Dahal, G. P. Ojha, T. Mukhiya, M. Lee, T. Kim, S. H. Chae, A. Muthurasu and H. Y. Kim, J. Electroanal. Chem., 2020, 856, 113670 CrossRef CAS.
- Z. C. Guo, Y. X. Zhao, J. P. Mu, S. M. Li, F. Li, J. P. Wang, H. Yang, J. B. Mu and M. Y. Zhang, Ceram. Int., 2023, 49, 19652–19663 CrossRef CAS.
- J. P. Mu, Y. X. Zhao, Z. C. Guo, Z. X. Zhang, H. W. Che, Y. M. Wang, X. L. Zhang, G. S. Wang, J. B. Mu and L. Wang, J. Energy Storage, 2023, 61, 106768 CrossRef.
- A. Mohammadi Zardkhoshoui, R. Hayati Monjoghtapeh and S. S. Hosseiny Davarani, Energy Fuels, 2020, 34, 14934–14947 CrossRef CAS.
- M. Batool, A. Hameed and M. A. Nadeem, Coord. Chem. Rev., 2023, 480, 215029 CrossRef CAS.
- D. Acharya, T. H. Ko, R. M. Bhattarai, A. Muthurasu, T. Kim, S. Saidin, J. S. Choi, K. Chhetri and H. Y. Kim, Adv. Compos. Hybrid Mater., 2023, 6, 179 CrossRef CAS.
- Z. Li, R. Ma, Q. Ju, Q. Liu, L. Liu, Y. Zhu, M. Yang and J. Wang, Innovation, 2022, 3, 100268 CAS.
- R. Manikandan, C. J. Raj, G. Nagaraju, R. Velayutham, S. E. Moulton, J. Puigdollers and B. C. Kim, Chem. Eng. J., 2021, 414, 128924 CrossRef CAS.
- M. Cui and X. Meng, Nanoscale Adv., 2020, 2, 5516–5528 RSC.
- R. M. Bhattarai, K. Chhetri, S. Saud, S. Teke, S. J. Kim and Y. S. Mok, ACS Appl. Nano Mater., 2021, 5, 160–175 CrossRef.
- J. Choi, A. Nkhama, A. Kumar, S. R. Mishra, F. Perez and R. K. Gupta, Int. J. Hydrogen Energy, 2022, 47, 7511–7521 CrossRef CAS.
- K. Li, Z. Hu, R. Zhao, J. Zhou, C. Jing, Q. Sun, J. Rao, K. Yao, B. Dong, X. Liu, H. Li, Y. Zhang and J. Ji, J. Colloid Interface Sci., 2021, 603, 799–809 CrossRef CAS PubMed.
- H. D. Sun, L. L. Zheng, Y. R. Xi, S. R. Zhai, Q. D. An and Z. Y. Xiao, Electrochim. Acta, 2023, 466, 143053 CrossRef CAS.
- L. Wan, K. Zhou, Y. Zhang, J. Chen, M. Xie and C. Du, J. Colloid Interface Sci., 2022, 614, 355–366 CrossRef CAS PubMed.
- Y. Wu, X. Liu, D. Han, X. Song, L. Shi, Y. Song, S. Niu, Y. Xie, J. Cai, S. Wu, J. Kang, J. Zhou, Z. Chen, X. Zheng, X. Xiao and G. Wang, Nat. Commun., 2018, 9, 1425 CrossRef PubMed.
- X. Y. Hu, R. J. Wang, P. Sun, Z. Y. Xiang and X. F. Wang, ACS Sustainable Chem. Eng., 2019, 7, 19426–19433 CrossRef CAS.
- R. M. Bhattarai, K. Chhetri, N. Le, D. Acharya, S. Saud, M. C. H. P. L. Nguyen, S. J. Kim and Y. S. Mok, Carbon Energy, 2024, 6, e392 CrossRef CAS.
- X. Wang, Z. H. Chen, M. Hu, Y. F. Tian, X. Y. Jin, S. Ma, T. T. Xu, Z. Q. Hu, S. M. Liu, D. B. Guo and B. Xiao, Chem. Eng. J., 2017, 312, 252–262 CrossRef CAS.
- P. Liu, S. M. Wang, Y. F. Quan, Z. R. Shen and Q. G. Wang, Phys. Status Solidi A, 2021, 218, 2100377 CrossRef CAS.
- H. Lan, J. Wang, L. Cheng, D. Yu, H. Wang and L. Guo, Chem. Soc. Rev., 2024, 53, 684–713 RSC.
- L. Quan, H. Jiang, G. Mei, Y. Sun and B. You, Chem. Rev., 2024, 124, 3694–3812 CrossRef CAS PubMed.
- X. C. Gao, J. Q. Bi, J. Gao, L. J. Meng, L. L. Xie and C. Liu, Electrochim. Acta, 2022, 426, 140739 CrossRef CAS.
- D. Acharya, I. Pathak, A. Muthurasu, R. M. Bhattarai, T. Kim, T. H. Ko, S. Saidin, K. Chhetri and H. Y. Kim, J. Energy Storage, 2023, 63, 106992 CrossRef.
- D. Acharya, A. Muthurasu, T. H. Ko, R. M. Bhattarai, T. Kim, S. H. Chae, S. Saidin, K. Chhetri and H. Y. Kim, ACS Appl. Energy Mater., 2023, 6, 9196–9206 CrossRef CAS.
- R. M. Bhattarai, N. Le, K. Chhetri, D. Acharya, S. M. S. Pandiyarajan, S. Saud, S. J. Kim and Y. S. Mok, Adv. Sci., 2024, 11, e2308160 CrossRef.
- T. Liu, J. H. Liu, L. Y. Zhang, B. Cheng and J. G. Yu, J. Mater. Sci. Technol., 2020, 47, 113–121 CrossRef CAS.
- Z. Xiao, Y.-C. Huang, C.-L. Dong, C. Xie, Z. Liu, S. Du, W. Chen, D. Yan, L. Tao, Z. Shu, G. Zhang, H. Duan, Y. Wang, Y. Zou, R. Chen and S. Wang, J. Am. Chem. Soc., 2020, 142, 12087–12095 CrossRef CAS PubMed.
- Y. Feng, N. Ran, X. Wang, Q. Liu, J. Wang, L. Liu, K. Suenaga, W. Zhong, R. Ma and J. Liu, Adv. Energy Mater., 2023, 13, 2302452 CrossRef CAS.
- H. Du, H. Hu, X. Wang, N. Ran, W. Chen, H. Zhu, Y. Zhou, M. Yang, J. Wang and J. Liu, Small, 2024, 2401053 CrossRef PubMed.
- V. Subramanian, H. Zhu, R. Vajtai, P. M. Ajayan and B. Wei, J. Phys. Chem. B, 2005, 109, 20207–20214 CrossRef CAS.
- Q. Wang, B. Liu, X. Wang, S. Ran, L. Wang, D. Chen and G. Shen, J. Mater. Chem., 2012, 22, 21647–21653 RSC.
- Q. Zong, H. Yang, Q. Q. Wang, Q. L. Zhang, Y. L. Zhu, H. Y. Wang and Q. H. Shen, Chem. Eng. J., 2019, 361, 1–11 CrossRef CAS.
- W. Zhao, X. Ma, X. Wang, H. Zhou, X. He, Y. Yao, Y. Ren, Y. Luo, D. Zheng, S. Sun, Q. Liu, L. Li, W. Chu, Y. Wang and X. Sun, Small, 2024, 2311055 CrossRef PubMed.
- W. Zhao, X. Ma, L. Gao, X. Wang, Y. Luo, Y. Wang, T. Li, B. Ying, D. Zheng, S. Sun, Q. Liu, Y. Zheng, X. Sun and W. Feng, Adv. Mater., 2024, 36, 2305190 CrossRef CAS.
- L. Yue, D. Wang, Z. Wu, W. Zhao, Y. Ren, L. Zhang, B. Zhong, N. Li, B. Tang, Q. Liu, Y. Luo, A. M. Asiri, X. Guo and X. Sun, Chem. Eng. J., 2022, 433, 134477 CrossRef CAS.
- W. Zhao, X. Ma, G. Wang, L. Tan, X. Wang, X. He, Y. Wang, Y. Luo, D. Zheng, S. Sun, Q. Liu, L. Li, W. Chu and X. Sun, SusMat, 2024, e198 CrossRef.
- A. Xu, C. Huang, G. Li, K. Zou, H. Sun, L. Fu, J. Ju, Y. Song, S. Wu, Z. Xu and Y. Yan, J. Mater. Chem. A, 2021, 9, 12169–12178 RSC.
- C. Liu, E. I. Gillette, X. Chen, A. J. Pearse, A. C. Kozen, M. A. Schroeder, K. E. Gregorczyk, S. B. Lee and G. W. Rubloff, Nat. Nanotechnol., 2014, 9, 1031–1039 CrossRef CAS PubMed.
- R. M. Bhattarai, K. Chhetri, S. Natarajan, S. Saud, S. J. Kim and Y. S. Mok, Chemosphere, 2022, 303, 135290 CrossRef CAS PubMed.
- W. Wang, S. Guo, I. Lee, K. Ahmed, J. Zhong, Z. Favors, F. Zaera, M. Ozkan and C. S. Ozkan, Sci. Rep., 2014, 4, 4452 CrossRef PubMed.
- Y. Ding, S. Tang, R. Han, S. Zhang, G. Pan and X. Meng, Sci. Rep., 2020, 10, 11023 CrossRef CAS PubMed.
- X. Chang, W. Li, Y. Liu, M. He, X. Zheng, J. Bai and Z. Ren, J. Colloid Interface Sci., 2019, 538, 34–44 CrossRef CAS PubMed.
- J. L. Chang, Z. Q. Hu, J. Z. Li, D. P. Wu, Z. X. Lian, F. Xu, K. Jiang and Z. Y. Gao, Appl. Surf. Sci., 2021, 538, 148106 CrossRef CAS.
- G. Nagaraju, S. C. Sekhar, B. Ramulu and J. S. Yu, Energy Storage Mater., 2021, 35, 750–760 CrossRef.
- S. C. Sekhar, B. Ramulu, S. J. Arbaz, S. K. Hussain and J. S. Yu, Small Methods, 2021, 5, 2100335 CrossRef CAS PubMed.
- S. C. Sekhar, G. Nagaraju and J. S. Yu, Nano Energy, 2017, 36, 58–67 CrossRef CAS.
- G. Nagaraju, S. C. Sekhar, B. Ramulu, S. J. Arbaz and J. S. Yu, Chem. Eng. J., 2021, 411, 128479 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta02690j |
| ‡ Lan Nguyen and Roshan Mangal Bhattarai contributed equally to this study. |
| This journal is © The Royal Society of Chemistry 2024 |