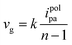Conjugated microporous polymers: their synthesis and potential applications in flexible electrodes
Dun
Zhou†
a,
Kongqing
Zhang†
a,
Shuqi
Zou
a,
Xiaobai
Li
*ab and
Hongwei
Ma
 *ab
*ab
aCollege of Chemistry Chemical Engineering and Resource Utilization, Northeast Forestry University, Harbin 150040, P. R. China. E-mail: lixiaobai2008@126.com; mahw@nefu.edu.cn
bCenter for Innovative Research in Synthetic Chemistry and Resource Utilization, Northeast Forestry University, Harbin 150040, P. R. China
First published on 4th June 2024
Abstract
Conjugated microporous polymers (CMPs) are a unique class of porous materials with π conjugated systems and permanent intrinsic porosity, and have a wide range of applications. With the rapid development of intelligent flexible electronic devices, the application of CMPs as a new electrode material in batteries and supercapacitors has attracted more and more attention due to their high redox activity, good physical and chemical stability, rich porous structure and large surface area. Although CMPs are widely used as electrode materials, they usually exist in an amorphous or semi-crystalline powder state with poor processing properties, thus limiting their application as flexible electrode materials. It is particularly important to develop CMPs as flexible electrode materials. In this paper, first, the synthesis of CMPs is reviewed. Second, the application of CMPs in flexible electronics is emphatically introduced. Finally, the problems and challenges of CMPs in flexible electrode materials are discussed. This review provides a comprehensive understanding of CMPs, which will facilitate the application of CMPs as high-performance flexible electrodes.
1 Introduction
In response to the imperatives of sustainable societal development, a variety of renewable energy production technologies have been developed in the past few decades. The development of smart flexible electronic devices, including flexible supercapacitors, flexible sensors and new mobile electronic devices,1–7 is gradually becoming the trend of the future. Intelligent flexible electronic devices require low-cost, well-cycled, high-energy and high-power density energy storage materials as energy storage systems. Therefore, the exploration and development of functional flexible energy storage materials (flexible electrode materials) have emerged as one of the most prominent areas of current research.8,9 The introduction of organic materials will directly affect the structure and electrochemical properties of the electrodes of electronic devices.10–12 As a novel material category, flexible porous organic functional materials are regarded as candidates for next-generation electronic devices.13,14 In comparison with traditional ones, organic porous materials offer the following advantages: (a) they are inexpensive and possess excellent photoelectric properties. (b) These materials demonstrate stable electrochemical properties and offer adjustable band gaps. For instance, the redox potential or theoretical charge storage capacity of the material can be tailored through structural adjustments. (c) When porous organic materials are utilized as organic electrode materials, their large number of micropores provides ample contact points, thus enhancing electron and ion transport between the electrode and the electrolyte.15–17 (d) Porous organic functional materials are characterized by high specific surface area, cross-linked polymer networks, intrinsic porosity and multiple functionalities with the incorporation of various functional groups.Conjugated microporous polymers (CMPs) are emerging as a representative class of porous organic materials that allow precise control of pore size, chemical characteristics, and the overall structure. These polymers have a purely covalently bonded π-conjugated backbone and a highly interconnected network structure, with pore diameters typically measuring 2 nm or less.18–22 CMPs can be constructed in various forms, including 2D23,24 and 3D25 networks, dendritic polymers26,27 and branched polymers.27 Because of these fascinating properties, CMPs have been developed for a range of corresponding applications, encompassing heterogeneous catalysis,28–30 gas storage,31 light-harvesting materials,32,33 chemical sensing,34 chemiluminescence,35 photovoltaic materials36 and electrode materials.37,38 Although CMPs are widely used as electrode materials, the insolubility and non-processability of CMPs are recognized as serious bottlenecks which hinder their application as flexible electrode materials. This problem arises from their inherent rigid structure and existence as semi-crystalline powders.39–41 Much of the prior research on CMPs has concentrated on devising novel constructs and polymerization strategies. These efforts typically involve adjusting the size of the constructs to modulate the specific surface area and pore size distribution of the resulting network, without strict morphological control.19 However, relatively few methods have been reported for the preparation of CMPs of flexible nanostructures.
In recent years, researchers have provided extensive summaries of the synthesis strategies of CMPs and their diverse ranges of applications, offering valuable insights into enhancing the performance of CMPs. However, there is a paucity of comprehensive and in-depth reviews on the utilization of CMPs as flexible electrodes for electronic devices. Therefore, this review provides a comprehensive summary of the research on CMPs in flexible electronic electrodes. First, we provide a comprehensive introductory overview of the synthesis of CMPs. Subsequently, we provide a comprehensive overview of the potential flexible electronics applications of CMPs. The emphasis is primarily on their utilization as anode materials for batteries, cathode materials for batteries, and electrodes for supercapacitors. Additionally, other applications such as ion exchange membranes and luminescent materials are also discussed. Finally, reasonable conclusions and prospects on CMP research in flexible electrode materials are given, aiming to enhance contributions to this field (Fig. 1).
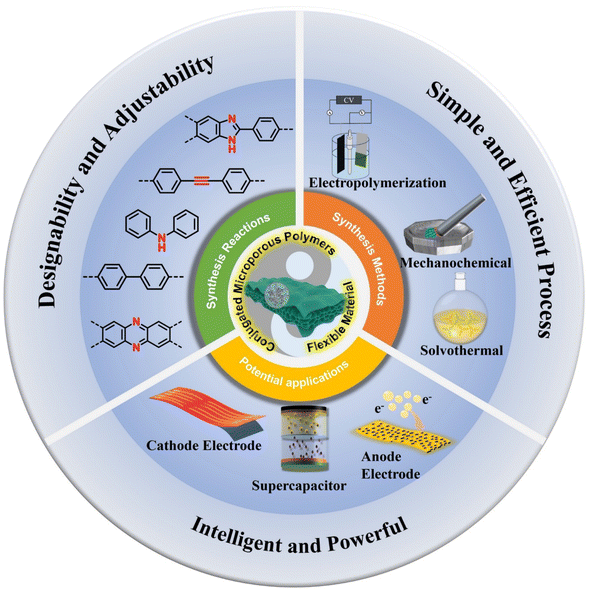 | ||
| Fig. 1 General content overview: ynthetic reactions and methods for CMPs and application of CMPs in flexible electrodes. | ||
2 Synthetic reactions and methods of CMPs
The network structure of CMPs can be commonly formed through a chemical reaction involving two or more different monomer molecules or via the coupling of a single monomer.17 As a result, the inherent diversity of monomer molecules and reaction groups leads to an extensive range of network structures and molecular functions for CMPs.42 Designing an efficient and straightforward synthesis route to precisely control the spatial network structure and photoelectric properties of CMPs involves significant challenges for researchers. Currently, some researchers have overcome this challenge by developing a variety of reaction routes and strategies for the synthesis of CMPs. Using these reaction routes and strategies, researchers can design electrode materials with greater electrical conductivity, larger surface area, and higher electrochemical stability, thereby advancing the development of high-performance energy storage and conversion devices such as batteries, supercapacitors, and electrochemical sensors. This section will delve into the synthesis (including synthetic reactions and methods) of CMPs in detail.2.1 Synthetic reactions of CMPs
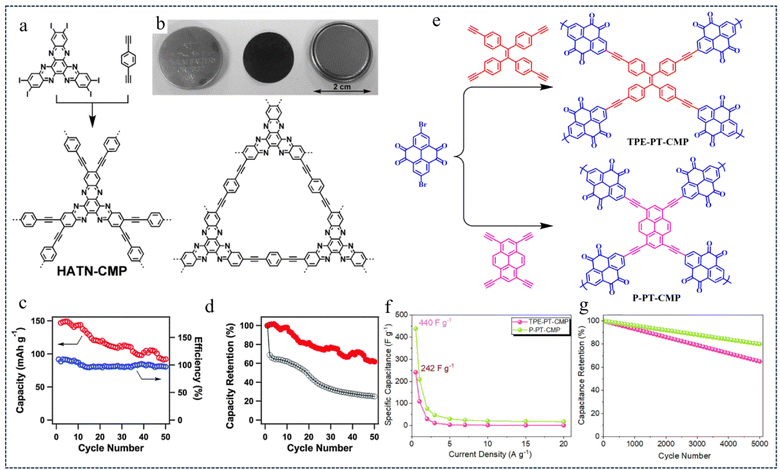 | ||
| Fig. 2 (a) Synthesis of HATN-CMP networks. (b) The photos of HATN-CMP electrodes. (c) Cycle stability and (d) capacity retention ratio of HATN-CMP.44 Copyright 2014, Royal Society of Chemistry. (e) Synthetic pathway of TPE-PT-CMP and P-PT-CMP. (f) Capacitance retention and (g) Ragone curve.45 Copyright 2022, American Chemical Society. | ||
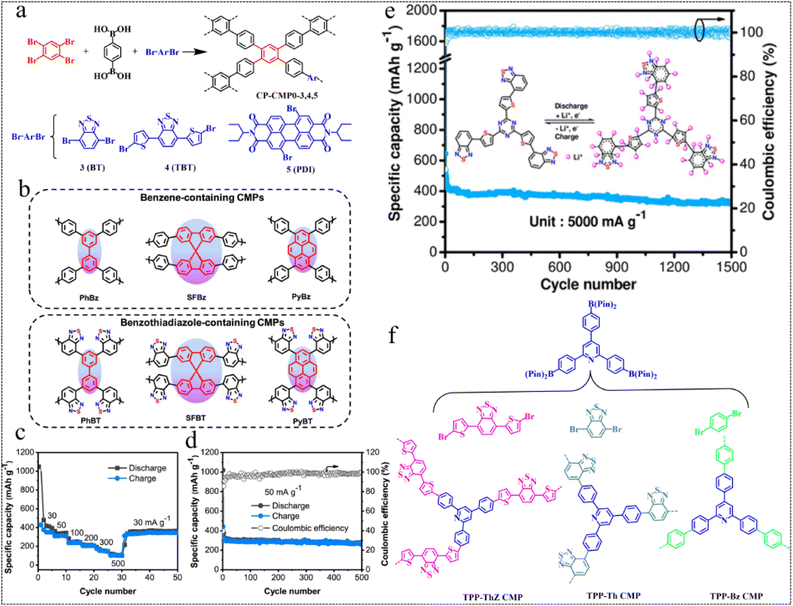 | ||
| Fig. 3 Suzuki–Miyaura coupling reaction routes. (a) Synthesis of CP-CMP0, 3, 4, 5.48 Copyright 2016, American Chemical Society. (b) Polymer structures. (c) Rate performance of PyBT. (d) Cycling stability and coulombic efficiency of PyBT.49 Copyright 2019, American Chemical Society. (e) Electrochemical performance graph of the TzThBT polymer.50 Copyright 2020, Wiley. (f) Synthesis and chemical structures of the TPP-based CMPs.51 Copyright 2023, Royal Society of Chemistry. | ||
In 2020, Jiang et al.50 prepared a novel CMP, TzThBT, incorporating redox-active substances of triazine (Tz), thiophene (Th) and benzothiadiazole (BT), using Pd-catalyzed Suzuki–Miyaura cross-coupling polycondensation. They discovered its potential as an anode material for LIBs and observed remarkable storage performance for lithium-ions. The polymer anode exhibited a high Li+ storage capacity of up to 1599 mA h g−1 at a current rate of 50 mA g−1. With excellent rate behavior (363 mA h g−1 at 5 A g−1) and long-term cycling capability of 326 mA h g−1 in more than 1500 cycles at 5 A g−1, this polymer anode offers considerable potential for next-generation LIBs (Fig. 3e). In 2023, Kuo et al.51 prepared a series of CMPs, namely TPP-ThZ, TPP-Th, and TPP-Bz CMPs, characterized by high thermal stability, smooth structure and tunable surface area by using TPP as a constant linker and incorporating three monomers (ThZ, Th, and Bz). It was found that the ability of CMPs for Cr(VI) reduction increased with the increase of thiophene content, owing to different thiophene content, topology and molecular structure (Fig. 3f). Exploiting their redox properties, the development of these CMPs into an electrode for LIBs would result in excellent electrochemical properties.
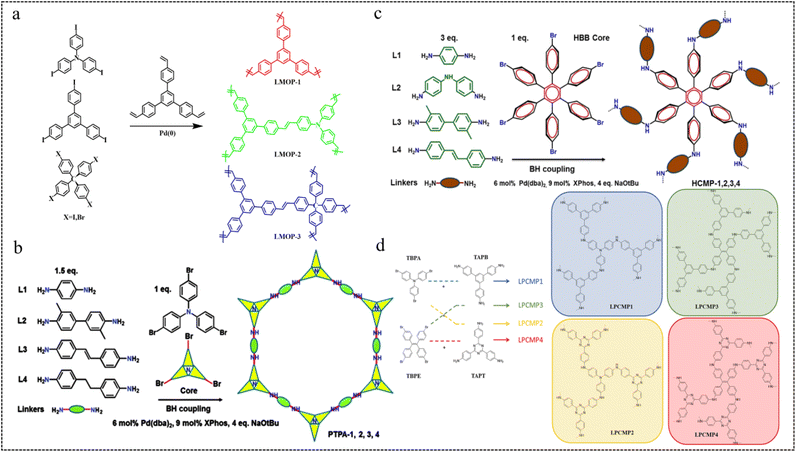 | ||
| Fig. 4 (a) Syntheses of LMOPs by the Heck reaction.53 Copyright 2013, Royal Society of Chemistry. (b) Synthetic route to CMP-PTPAs.55 Copyright 2014, Royal Society of Chemistry. (c) Synthetic route to HCMP-1,2,3,4.56 Copyright 2016, American Chemical Society. (d) Synthetic route of LPCMP1-4.57 Copyright 2019, American Chemical Society. | ||
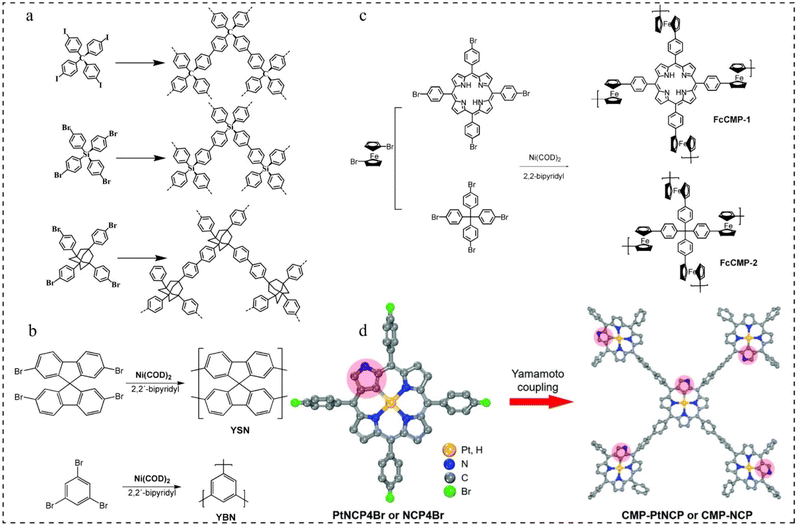 | ||
| Fig. 5 Yamamoto reaction routes. (a) Synthesis routes to polymer networks 1–3.60 Copyright 2010, American Chemical Society. (b) Polymerization of YSN and YBN.61 Copyright 2009, American Chemical Society. (c) Synthesis routes of FcCMP-1 and FcCMP-2.62 Copyright 2020, MDPI. (d) Preparation of CMP-PtNCP and CMP-NCP.63 Copyright 2022, Royal Society of Chemistry. | ||
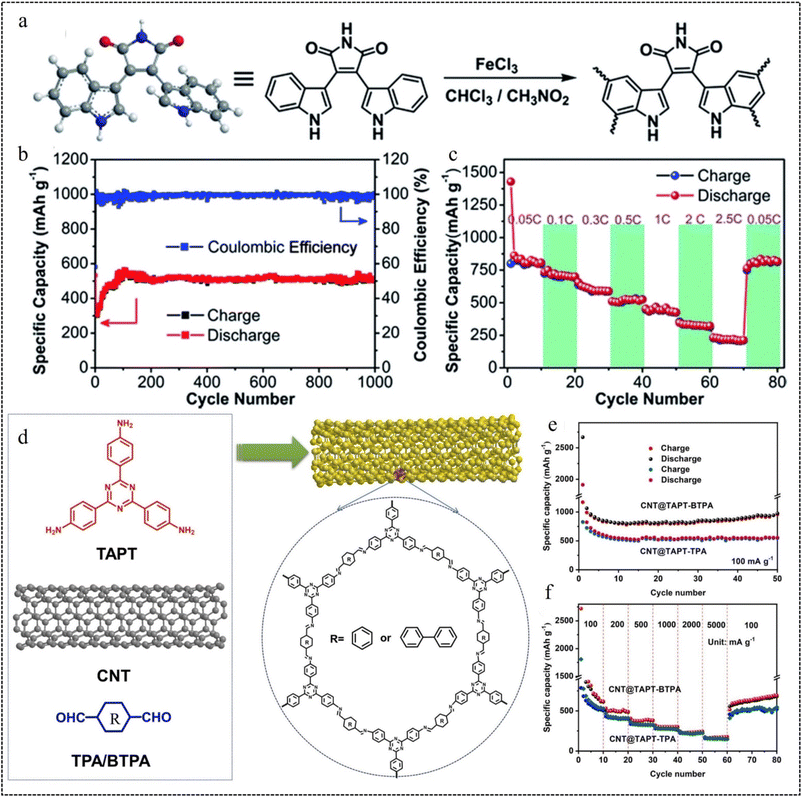 | ||
| Fig. 6 Oxidative coupling polymerization routes. (a) Synthesis of PBIM. (b) Cycle performance of CMP-PBIM. (c) The rate performance at different current densities from 0.05C to 2.5C.67 Copyright 2018, Royal Society of Chemistry. Schiff-base reaction routes. (d) Synthetic route of CNT@TAPT-TPA/BTPA. (e) Cycling performance of CNT@TAPT-TPA and CNT@TAPT-BTPA. (f) Rate performance of CNT@TAPT-TPA and CNT@TAPT-BTPA.68 Copyright 2023, Elsevier. | ||
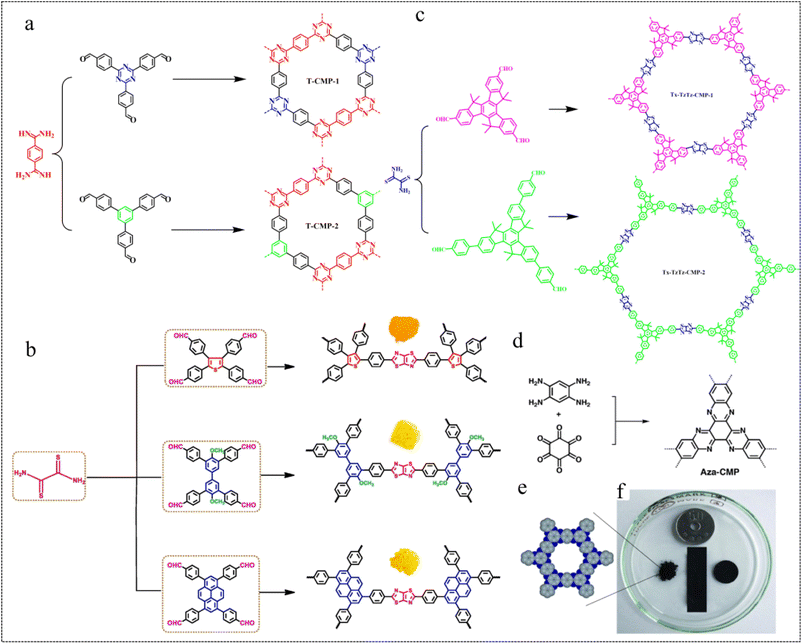 | ||
| Fig. 7 Heterocycle linkages. (a) Synthesis of T-CMP-1 and TCMP-2.69 Copyright 2021 American Chemical Society. (b) Synthesis of Ts-ThTh-CMP, Bi-ThTh-CMP, and Py-ThTh-CMP.70 Copyright 2022, Elsevier. (c) Synthesis of Tx-TzTz-CMP-1 and TxTzTz-CMP-2.71 Copyright 2023, American Chemical Society. (d) Synthesis of Aza-CMP. (e) The elementary pore structure of Aza-CMP. (f) Photographic image of different shapes of Aza-CMPs.72 Copyright 2019, Royal Society of Chemistry. | ||
In 2022, Mohamed et al.70 designed a highly efficient and inexpensive, donoracceptor (D–A) system based on CMPs featuring thiazole[5,4-D]thiazole (ThTh) linkages (Fig. 7b). This system separates hydrogen from H2O under visible light without adding any catalyst and uses ascorbic acid (AA) as a sacrificial electron donor. The results show that Py-ThTh-CMP has the highest photocatalytic activity attributed to its high planarity of the Py cell, highly extended conjugation (electron-rich), the strongest absorption in the visible region, narrowest band gap, and strong interaction between the π orbitals of Py (donor) and ThTh (acceptor) units. This results in a hydrogen evolution rate (HER) of 1874 μmol g−1 h−1. This study provides a new method for the preparation of efficient photocatalysts and promotes the practical development of efficient hydrogen evolution.
In 2023, Bai et al.71 designed and synthesized two redox-active truxene-based CMPs (Tx-TzTz-CMP-1 and Tx-TzTz-CMP-2) linked by thiazolo[5,4-d]thiazole for the photocatalytic reduction of CO2 to CH4 (Fig. 7c). The polymer Tx-TzTz-CMP-2, featuring an extended π-conjugated system, exhibited a remarkable CH4 reduction yield of up to 300.6 μmol g−1 h−1 and achieved 71.2% selectivity, without the need for any metal cocatalyst and photosensitizer. We note that the use of high-density π-conjugated aromatic heterocyclic linked CMPs for battery electrodes can effectively improve the electrochemical energy storage performance of materials.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. 7e and f). Based on the results of this research, the great potential of para-CMPs as high-energy storage devices has been demonstrated.
000 cycles (Fig. 7e and f). Based on the results of this research, the great potential of para-CMPs as high-energy storage devices has been demonstrated.
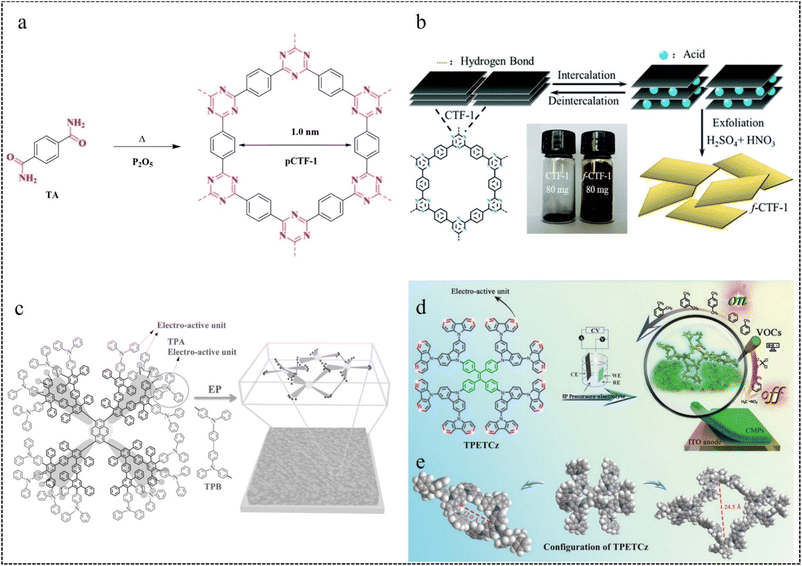 | ||
| Fig. 8 (a) Synthesis of pCTF-1.75 Copyright 2018, Wiley. (b) Preparation process of f-CTF-1 nanosheets.76 Copyright 2019, Royal Society of Chemistry. (c) Dimerisation of TPA with TPB and schematic representation of the crosslinked network microstructure of EP membranes.80 Copyright 2016, Wiley. (d) EP preparation of TPETCz-CMP films. (e) Structural model of TPETCz-CMP.26 Copyright 2020, Wiley. | ||
2.2 Synthetic methods of CMPs
In the EP process, sites with higher electron cloud density in the organic building unit are readily oxidized to form cationic radicals. These cationic radicals exhibit heightened activity, facilitating their interaction with the monomer or other cationic radicals. Furthermore, in the reaction between two cationic radicals, the formation of an ion-pair occurs as the cationic radical bond with the anions in solution. This reduction in electrostatic repulsion promotes the polymerization coupling of the two cationic radicals. Following the coupling reaction, the dimer intermediate undergoes deprotonation and reoxidation to form an aromatic system. Throughout the reaction, a chain-like process persists, involving electron transfer and chemical reaction steps, until the growth of this chain ceases. Furthermore, unlike conventional chemical polymerization, the activity of EP diminishes notably as the reaction progresses, likely attributed to the elongation of polymer chains. Moreover, the deprotonation reaction subsequent to the coupling reaction typically occurs rapidly, propelled by the regeneration of the aromatic system. If the growth rate of the EP membrane is linear, the relative growth rate of the membrane per cycle can be calculated from the following equation:
In 2016, we80 developed the dendritic macromolecule PYTPAG2 via the EP method. We utilized this material as an electroactive precursor to fabricate CMP films with favorable fluorescence properties. By adjusting the EP parameters, they could control the thickness of the fluorescent film and achieve the film as a freestanding membrane on the substrate (Fig. 8c). This study provides a new way to construct fluorescent thin-film sensors from dendrimers.
In 2020, our group26 employed a straightforward in situ electropolymerization method to prepare a film. They utilized a novel dendritic macromolecular material (TPETCz) as the polymerization monomer, notable for its high specific surface area and facilitation of analyte diffusion. The obtained CMP sensor film has a microporous structure with a BET specific surface area as high as 1042.5 m2 g−1. And the selective detection of 18 VOCs was achieved by linear discriminant analysis (LDA) (Fig. 8d and e).
In 2022, our group81 synthesized two EP films (TCz-CMP and TCzP-CMP) via the EP method, employing molecules (TCz and TCzP) with hybridized localized and charge transfer (HLCT) excited state properties as polymerization monomers. In addition, TCzP-CMP had a larger BET surface area (407 m2 g−1) and total pore volume (1.29 cm3 g−1) compared to TCz-CMP, corresponding to pore size distributions of 0.81–1.0 nm and 0.71–0.74 nm, respectively.
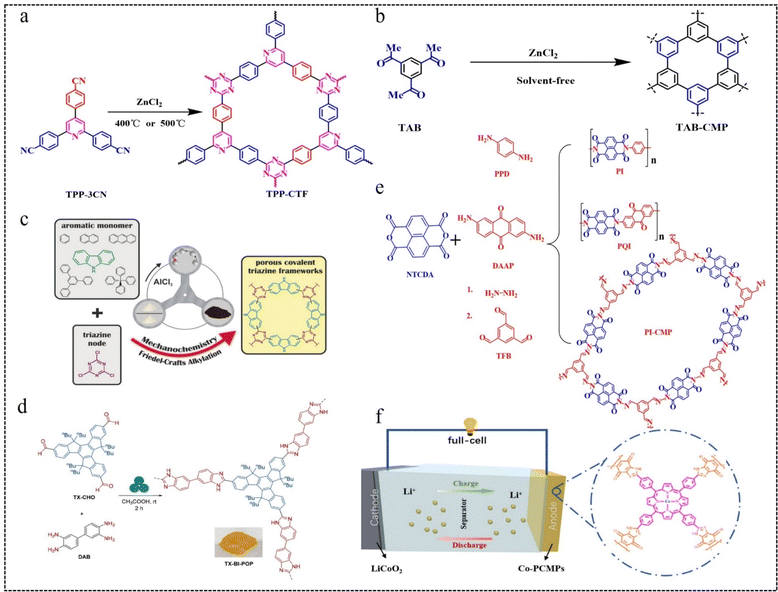 | ||
| Fig. 9 (a) Synthetic routes of TPP-CTFs.83 Copyright 2022, Elsevier. (b) Synthesis method of TAB-CMP.84 Copyright 2021, American Chemical Society. (c) Construction of a porous CTF.85 Copyright 2017, Wiley. (d) Mechanochemical route to the synthesis of benzimidazole-bridged conjugated porous polymers (TX-BI-POP).86 Copyright 2023, American Chemical Society. (e) Synthesis routes for 1D PI and PQI polymers and 2D PI-CMP polymers.87 Copyright 2019, Royal Society of Chemistry. (f) Theoretical cell modelling of Co-PCMPs.88 Copyright 2022, Elsevier. | ||
3 Potential applications of CMPs
The previous section summarized the synthetic reaction routes and synthetic methods for CMPs, and many researchers have put forward various visions for the preparation of CMPs. Based on these synthetic methods, the chemical structure and macroscopic morphology of CMPs can be largely controlled, and scientific researchers have successively proposed the application concepts of CMPs, which has broadened the application scope of CMPs. In this section, we will mainly summarize the representative applications of CMPs in flexible electrode materials.3.1 CMPs as anode materials for batteries
Currently, battery anode materials are predominantly categorized into two groups: inorganic and organic materials. When employing inorganic metal materials as anode materials, the formation of dendrites and corrosion on the metal anode is common, resulting in reduced utilization efficiency and poor cycle performance of the metal anodes.89,90 Organic electrode materials have the advantages of designable structure, low cost and high theoretical capacity,91–93 but need to overcome the problems of poor conductivity and dissolution in the electrolyte.94,95CMPs have emerged as a novel class of organic electrode materials owing to their high redox activity, exceptional physicochemical stability, highly crosslinked polymer network, abundant porous structure, and expansive surface area.16,44,96–98 Polythiophene is an n-doped conjugated polymer, but its low redox activity and poor multiplicity performance limit its further development as an anode material for LIBs. In 2018, Xu et al.99 reported a structural design strategy for thiophene-based CMPs with excellent electrochemical performance as anode materials for LIBs. It was found that the thiophene content, crosslinked porous structure, and surface area play a decisive role in improving the electrochemical performance. The poly(3,3-dithiophene) (P33DT) all-thiophene-based polymer has a crosslinked structure and a high surface area of 696 m2 g−1, whereas polythiophene (PT) has 13 m2 g−1 (Fig. 10a). The pore size distribution (PSD) of the polymers was determined using nonlocal density-functional theory (NL-DFT). P33DT displayed a median micropore diameter of 0.92 nm, while linear PT predominantly showcased graded mesopores spanning from 2 to 20 nm (Fig. 10b). The P33DT exhibits a discharge capacity of up to 1215 mA h g−1 at 45 mA g−1, excellent multiplicative capacity, and excellent cycling stability, with a capacity retention of 663 mA h g−1 at 500 mA g−1 after 1000 cycles, which is 79.9% of the initial reversible capacity (Fig. 10c and d). This work mainly reveals the structure–performance relationship, which further provides a basis for the rational design of CMP anode materials for high-performance LIBs.
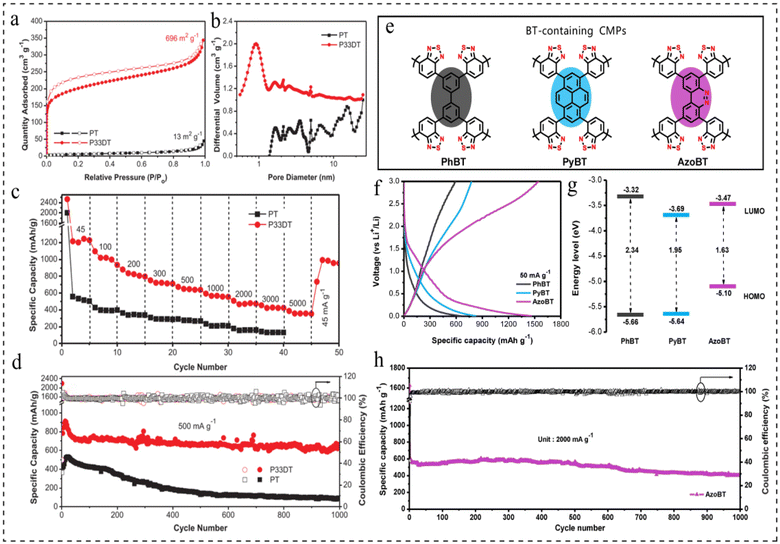 | ||
| Fig. 10 (a) Nitrogen adsorption–desorption isotherms. (b) PSD calculated by NL-DFT for PT and P33DT. (c) The rate performance at different current densities from 45 to 5000 mA g−1. (d) The cyclability at 500 mA g−1 of PT and P33DT.99 Copyright 2017, Wilye. (e) Conceptual polymer structure of BT-containing CMPs. (f) GCD curves of PhBT, PyBT and AzoBT at 50 mA g−1. (g) The energy level band gap for CMPs containing BT. (h) Cycling stability and coulombic efficiency of AzoBT at 2000 mA g−1.98 Copyright 2020, Elsevier. | ||
In 2020, Zhang et al.98 reported a class of azo-fused CMPs with unique electronic structures as anode materials for rechargeable LIBs. The effect of electronic structure on the redox activity of azo-thickened CMPs was further investigated, and three materials containing benzothiadiazole (BT) groups (PhBT, PyBT, and AzoBT) were prepared separately (Fig. 10e). The galvanostatic charge–discharge (GCD) testing of the three materials revealed that AzoBT obtained a higher reversible capacity (1534 mA h g−1), followed by PyBT (776 mA h g−1), and PhBT the lowest (591 mA h g−1) (Fig. 10f). Theoretical calculations show that the energy levels of AzoBT, PyBT and PhBT are reduced by 2.34 ev, 1.95 ev and 1.63 ev, respectively (Fig. 10g). The above results demonstrate the significant influence of the electronic structure on the Li ion storage capacity of these CMP electrodes, and that the band gap plays a more important role than the LUMO energy level in the redox activity of the resulting CMPs. In conclusion, AzoBT exhibits higher redox activity due to the presence of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N redox active centres, which in turn provides more redox activation sites for Li ion storage. The resulting LIBs exhibited ultra-high reversible capacity, excellent multiplicity performance and stable cycling performance (1000 cycles at 2 A g−1) (Fig. 10h).
N redox active centres, which in turn provides more redox activation sites for Li ion storage. The resulting LIBs exhibited ultra-high reversible capacity, excellent multiplicity performance and stable cycling performance (1000 cycles at 2 A g−1) (Fig. 10h).
In 2021, Yu et al.100 developed a new redox donor–acceptor CMP (AQ-CMP) utilizing anthraquinone and benzene as linkers via C–C bonding and used AQ-CMP as an ultra-long-life anode for rechargeable air batteries (RABs) (Fig. 11a). AQ-CMP features an interconnected octapole network, which facilitates a favorable electronic structure for enhanced electron transfer efficiency and N-doping activity. Additionally, it boasts a high density of active sites, maximizing the redox capacity based on the weight of the formulation. The AQ-CMP anode yielded near theoretical capacity (202 mA h g−1 at 2 A g−1) (Fig. 11b), with excellent multiplicative performance (58% capacity retention at 20 A g−1), and excellent cycling stability (more than 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 20 A g−1), which was far superior to that of its linear counterpart (AQ-Lin, 73% retention after 250 cycles) (Fig. 11c–g). When combining an AQ-CMP anode with a commercial Pt/C@Ir/C catalyst-based cathode, the resulting CMP air cell demonstrates a consistent specific capacity of 181 mA h g−1 at 3 A g−1. Notably, full capacity recovery is solely achieved by refreshing the cathode. Furthermore, by decoupling the electrolyte and cathode design, both the output voltage and voltage efficiency can be enhanced to −1 V and 87.5%, respectively, surpassing those of existing polymer air batteries (Fig. 11h). This research not only enriches the family of redox CMPs and broadens their applications but also offers novel insights into the design of CMP-based electrodes for diverse energy storage systems and other applications.
000 cycles at 20 A g−1), which was far superior to that of its linear counterpart (AQ-Lin, 73% retention after 250 cycles) (Fig. 11c–g). When combining an AQ-CMP anode with a commercial Pt/C@Ir/C catalyst-based cathode, the resulting CMP air cell demonstrates a consistent specific capacity of 181 mA h g−1 at 3 A g−1. Notably, full capacity recovery is solely achieved by refreshing the cathode. Furthermore, by decoupling the electrolyte and cathode design, both the output voltage and voltage efficiency can be enhanced to −1 V and 87.5%, respectively, surpassing those of existing polymer air batteries (Fig. 11h). This research not only enriches the family of redox CMPs and broadens their applications but also offers novel insights into the design of CMP-based electrodes for diverse energy storage systems and other applications.
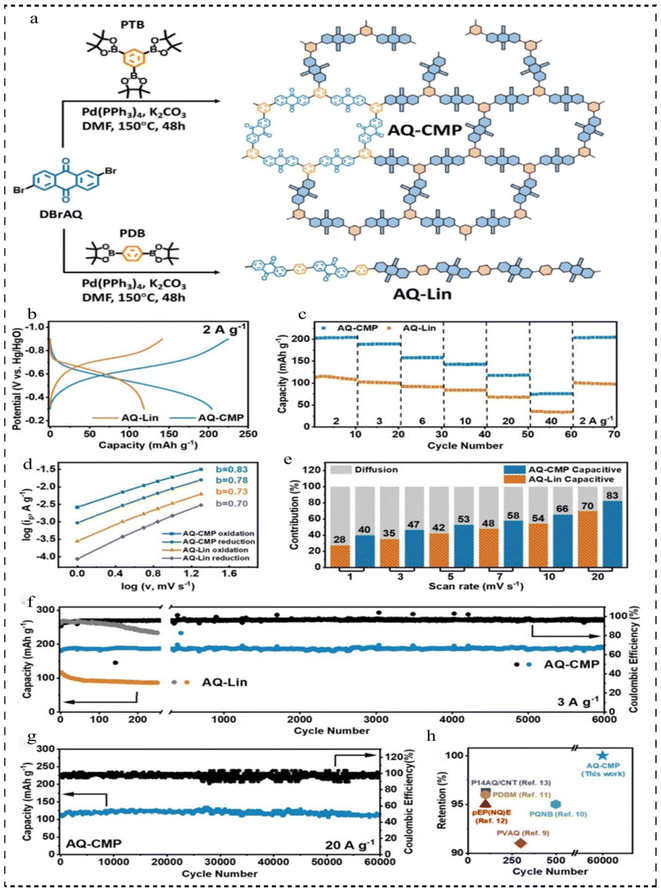 | ||
| Fig. 11 (a) Synthetic route of AQ-CMP and AQ-Lin. (b) Comparison between AQ-CMP and AQ-Lin based on GCD curves at 2 A g−1. (c) Rate capability at different current densities. (d) b-value analysis. (e) Normalized percentage capacitive contribution at different scan rates. (f) The long-term GCD stability tests of AQ-CMP and AQ-Lin at 3 A g−1. (g) The long-term GCD stability tests of AQ-CMP and AQ-Lin at 20 A g−1. (h) Capacity retention comparison of various polymer anodes for RABs.100 Copyright 2021, Wiley. | ||
Porphyrin-based molecules have large π-conjugated aromatic structures, active sites of multi-electron redox activity, and narrow HOMO–LUMO gaps, and are often used as anodes for LIBs.101,102 In 2022, Zhai et al.103 prepared porphyrin CMPs by Sonogashira–Hagihara cross-coupling of Br-functionalized porphyrin monomers and alkyne-based monomers (Fig. 12a). The CMPs exhibit a notable surface area of 419 m2 g−1 (Fig. 12b) and a narrow bandgap, with optical and calculated values of 1.31 eV and 0.73 eV, respectively. The valence band (VB) spectrum indicates a VB energy (EVB) of 3.81 eV (Fig. 12c–e). Additionally, it achieves a high specific capacity of 1200 mA h g−1 at 1 A g−1 and maintains a capacity retention rate of 242% after 5000 cycles at 3 A g−1 (Fig. 12f and g). These findings present an effective approach for designing and fabricating organic electrode materials characterized by high specific capacity and long-term cycle life.
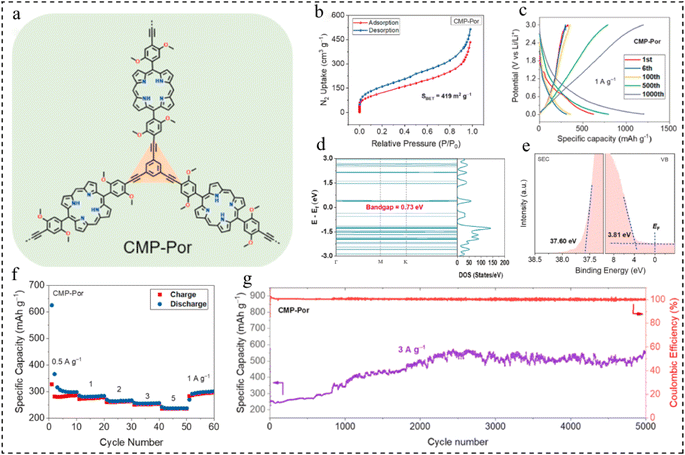 | ||
| Fig. 12 (a) Structure of CMP-Por. (b) BET test for CMP-Por. (c) GCD profiles at 1 A g−1. (d) Density of states of CMP-Por. (e) Ultraviolet photoelectron spectroscopy of CMP-Por. (f) Rate capability. (g) Long-term cycle life at 3 A g−1.103 Copyright 2022, Elsevier. | ||
Alkaline rechargeable batteries (ARBs) are an attractive solution for large-scale electrochemical energy storage applications. However, their development has been greatly hindered by the lack of high-performance and sustainable anodes capable of stable operation with low corrosivity and low electrolyte concentration. However, redox-active CMPs with their robust and mechanically stable 3D microporous structure can provide even better long-life ARBs.104 In 2022, Patil et al.105 developed high-performance ARBs with anthraquinone-based CMPs (IEP-11)/single-walled CNTs as the anode and commercial Ni(OH)2 as the cathode (Fig. 13a and c). In 1 M KOH electrolyte, when IEP-11 served as the anode, a comprehensive comparison with PAQS showed that IEP-11 had a high specific surface area and total pore volume (738 m2 g−1 and 0.7 cm3 g−1) (Fig. 13b), high cell voltage (0.98 V) (Fig. 13d), long cycle life (up to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730 cycles/960 h, 75% capacity at 20C) (Fig. 13e and f), high specific capacity (150 mA h g−1 at 1C) (Fig. 13g), excellent rate performance (90 mA h g−1 at 50C) (Fig. 13h) and low-temperature operability (Fig. 13i). The ideas presented in this research not only pave the way for the design of high-performance and advanced ARBs, but also the possibility of developing safe, environmentally friendly and practical organic batteries by utilizing nickel-based cathodes and less corrosive aqueous electrolytes.
730 cycles/960 h, 75% capacity at 20C) (Fig. 13e and f), high specific capacity (150 mA h g−1 at 1C) (Fig. 13g), excellent rate performance (90 mA h g−1 at 50C) (Fig. 13h) and low-temperature operability (Fig. 13i). The ideas presented in this research not only pave the way for the design of high-performance and advanced ARBs, but also the possibility of developing safe, environmentally friendly and practical organic batteries by utilizing nickel-based cathodes and less corrosive aqueous electrolytes.
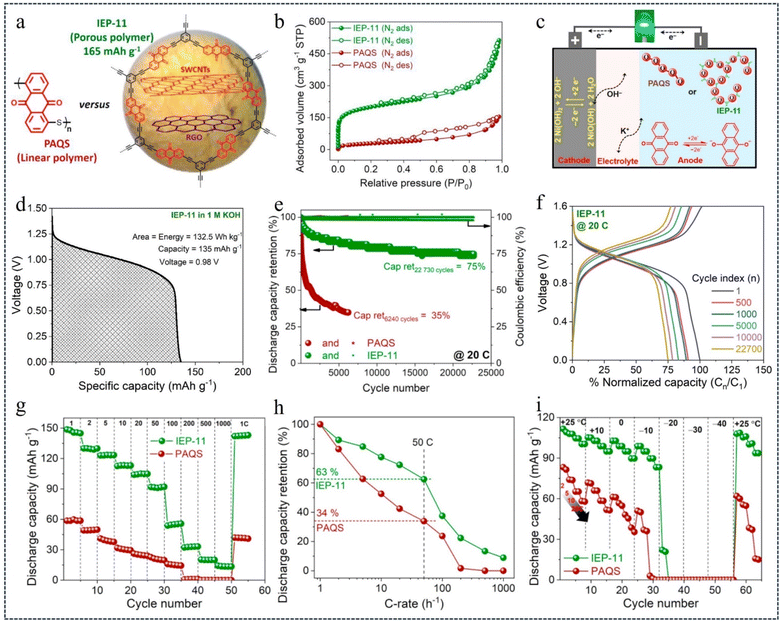 | ||
| Fig. 13 (a) Chemical structure of PAQS and IEP-11. (b) Nitrogen adsorption–desorption isotherm profiles of PAQS and IEP-11. (c) Schematic of the full cell and its working mechanism. (d) Specific capacity–voltage profile of IEP-11 in 1 M KOH. (e) Cycle stability plot: discharge capacitance retention and coulombic efficiency measured at 20C. (f) Cycle stability plot of IEP-11. (g) and (h) Discharge capacity retention at different C-rates. (i) Low-temperature operativity.105 Copyright 2022, Elsevier. | ||
3.2 CMPs as cathode materials for batteries
CMP materials have some unique properties due to their large π-electron conjugated structure: (1) good electron transport properties, (2) most CMP materials are structurally stable and rigid, making them difficult to dissolve in conventional electrolytes, and (3) large specific surface area and microporous structure. These properties make CMPs a good choice for building battery cathode materials.In recent years, some researchers have discovered that conductive polymers with good electronic conductivity and rich redox functional groups are promising candidates for the construction of high-energy aqueous zinc batteries. In 2021, Liu et al.106 prepared a multi-hollow poly(triphenylamine)-CMPs cathode material (m-PTPA) via a solvothermal synthesis strategy (Fig. 14). The cathode material has a more regular porous structure similar to the covalent organic frameworks (COF) material, facilitating the accommodation of Cl− in a pseudocapacitive-dominated manner for energy storage purposes. Moreover, its specific 3D organic framework-shaped conjugated porous network can significantly increase the N activity (up to 83.2% at 0.5 A g−1) and stability (87.6% capacity retention after 1000 cycles) of the material. This electrode material also enabled the zinc ion battery device to have a high energy density of 236 W h kg−1 and a maximum power density of 6.8 kw kg−1, which is a significant advantage over organic zinc ion batteries reported at the same time. This study provides new ideas for the rational design of CMP organic cathode materials.
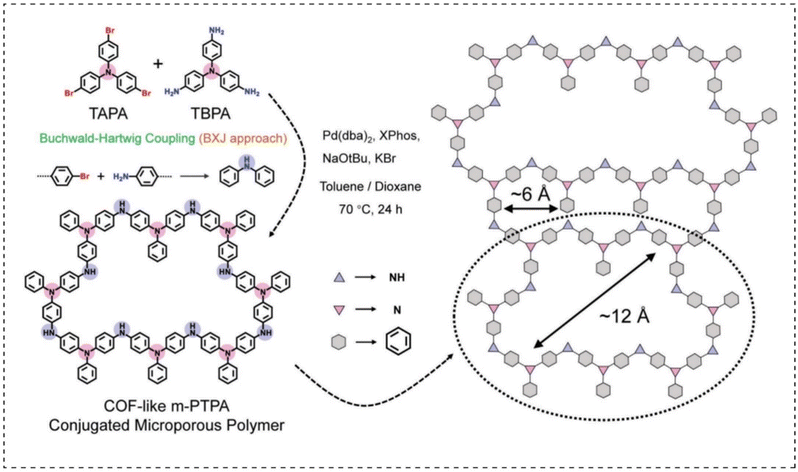 | ||
| Fig. 14 The synthesis route of COF-like m-PTPA.106 Copyright 2021, Wiley. | ||
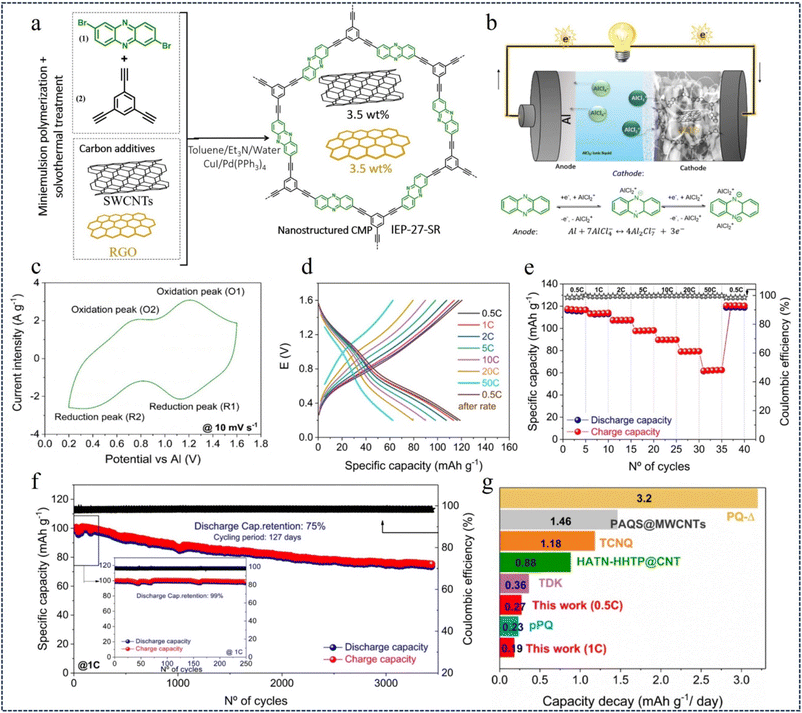
Rechargeable aluminum batteries (RABs) use redox active polymers as cathodes to address the problems of slow inorganic cathode kinetics, low capacity, and poor integrity.94 Recent studies have found that n-type phenozines have high capacity, reversibility and rapid redox kinetics in aqueous electrolytes due to the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds. In 2023, Bitenc et al.107 reported for the first time a phenazine-based hybrid microporous polymer (IEP-27-SR) for use in an organic cathode in aluminum batteries with AlCl3-EMIMCl ionic liquid electrolytes (Fig. 15a and b). The cyclic voltammetry (CV) curve of the Al//IEP-27-SR cell shows two pairs of reversible redox peaks at about 1.1 V (peak O1 and R1) and about 0.6 V (peak O2 and R2), which are believed to be due to the reversible reduction/oxidation of the phenazine portion of the polymer structure (Fig. 15c). The electrochemical performance of the Al//IEP-27-SR battery was evaluated by GCD at different C-rates, and the specific capacity decreased as the current density gradually increased (Fig. 15d). At a minimum current density of 0.5C, the highest specific capacity value of 116 mA h g−1 was obtained, with a high coulombic efficiency (CE) of 99% (Fig. 15e). By evaluating the cycle life of the battery, in the first 250 cycles, the battery showed a capacity retention rate of 99% and a capacity of 98 mA h g−1. 75% of the initial capacity was maintained after 3440 charge–discharge cycles (127 days of continuous cycling) at 1C (Fig. 15f and g). This study improves battery performance and paves the way for the development of advanced multivalent batteries by providing an efficient pathway for the movement of ions and electrons.
N double bonds. In 2023, Bitenc et al.107 reported for the first time a phenazine-based hybrid microporous polymer (IEP-27-SR) for use in an organic cathode in aluminum batteries with AlCl3-EMIMCl ionic liquid electrolytes (Fig. 15a and b). The cyclic voltammetry (CV) curve of the Al//IEP-27-SR cell shows two pairs of reversible redox peaks at about 1.1 V (peak O1 and R1) and about 0.6 V (peak O2 and R2), which are believed to be due to the reversible reduction/oxidation of the phenazine portion of the polymer structure (Fig. 15c). The electrochemical performance of the Al//IEP-27-SR battery was evaluated by GCD at different C-rates, and the specific capacity decreased as the current density gradually increased (Fig. 15d). At a minimum current density of 0.5C, the highest specific capacity value of 116 mA h g−1 was obtained, with a high coulombic efficiency (CE) of 99% (Fig. 15e). By evaluating the cycle life of the battery, in the first 250 cycles, the battery showed a capacity retention rate of 99% and a capacity of 98 mA h g−1. 75% of the initial capacity was maintained after 3440 charge–discharge cycles (127 days of continuous cycling) at 1C (Fig. 15f and g). This study improves battery performance and paves the way for the development of advanced multivalent batteries by providing an efficient pathway for the movement of ions and electrons.
 | ||
| Fig. 15 (a) Synthesis strategy for IEP-27-SR mixtures. (b) Scheme of the aluminium–phenazine battery. (c) CV recorded at 10 mV s−1. (d) GCD profiles of the Al//IEP-27-SR battery. (e) Battery rate performance. (f) Long-term cycle stability of an Al//IEP-27-SR battery. (g) Capacity fade per day.107 Copyright 2023, Royal Society of Chemistry. | ||
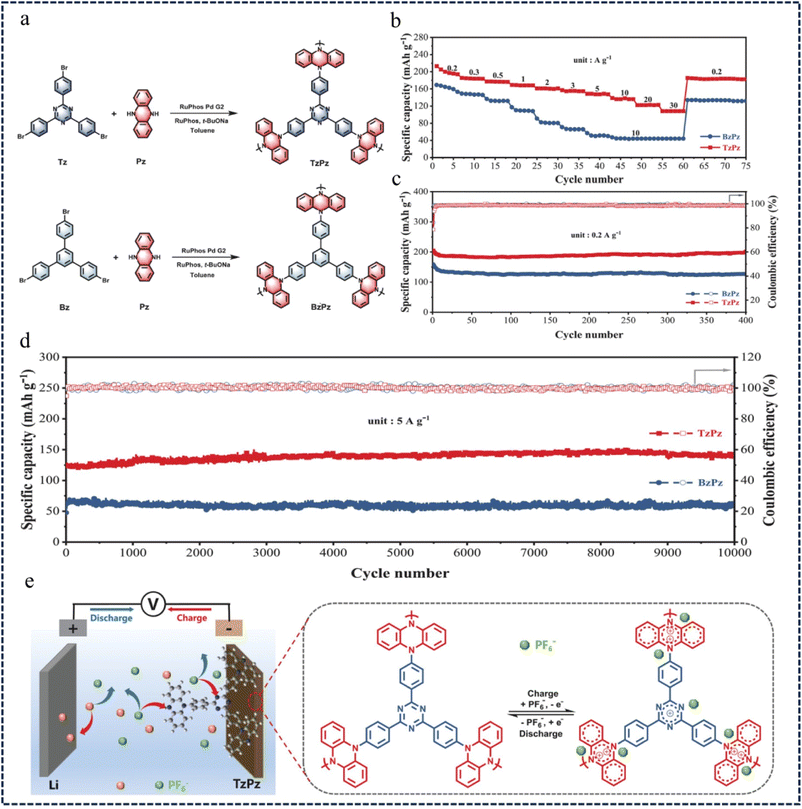
In the construction of dual-ion batteries (DIBs) with redox-active building block polymer cathodes, p-type dihydrophenazine (Pz) unit modules have attracted extensive attention due to their high reversible redox reaction and high theoretical charge storage capacity.108–111 However, most of the reported Pz-based polymer cathodes still have problems such as low redox activity, slow kinetics and short cycle life. To address these issues, in 2021, Zhang et al.112 developed a CMP (TzPz) cathode material based on donor–acceptor (D–A) dihydrophenazine, by assembling the electron-donating Pz unit and the electron-withdrawing 2,4,6-triphenyl-1,3,5-triazine (Tz) units into the polymer chain (Fig. 16a). This D–A structure enhances the conjugation of the polymer, reduces the band gap of TzPz, and promotes the transport of electrons along the polymer backbone. The rate performance of BzPz and TzPz was evaluated at different current rates, and the specific capacity gradually decreased with the increase of current rates (Fig. 16b). At current rates of 0.2 A g−1 and 5 A g−1, BzPz and TzPz exhibit excellent stability (more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) and high specific capacities (TzPz 192 mA h g−1, BzPz 148 mA h g−1) (Fig. 16c and d). Ex situ characterization showed that the charge storage of PF6− in the TzPz cathode was due to the p-type doping reaction of dihydrophenazine and triazine units (Fig. 16e). These studies suggest that D–A structure design is an effective strategy for the development of high-performance polymer cathodes for DIBs.
000 cycles) and high specific capacities (TzPz 192 mA h g−1, BzPz 148 mA h g−1) (Fig. 16c and d). Ex situ characterization showed that the charge storage of PF6− in the TzPz cathode was due to the p-type doping reaction of dihydrophenazine and triazine units (Fig. 16e). These studies suggest that D–A structure design is an effective strategy for the development of high-performance polymer cathodes for DIBs.
 | ||
| Fig. 16 (a) The synthetic reaction route of TzPz and BzPz. (b) The rate performance at current densities from 0.2 to 30 A g−1. (c) Cycling performance at current densities of 0.2 A g−1 and (d) 5 A g−1. (e) Schematic diagram of the electrochemical reaction mechanism of the TzPz-based cathode.112 Copyright 2021, Wiley. | ||
In 2023, Liu et al.113 designed and synthesized two CMP materials (CMPs-B and CMPs-By) with spiro-difluorenyl and phenyl groups connected by different bonds (Fig. 17a). However, the CMPs-B materials with monomers linked by C–C bonds have a larger specific surface area of 45 m2 g−1 (CMPs-By: 17 m2 g−1), smaller microporous size of 1.58 nm (CMPs-By: 2.03 nm), and higher stability in comparison (Fig. 17b and c). This structural feature provides a larger specific surface area in contact with the electrolyte, improves the rapid transfer of Li+ during charging and discharging, retains a certain amount of capacity after multiple cycles (the capacity retention after 100 cycles is 36.6%) and has better multiplicative performance (34.44% for 300 mA g−1) (Fig. 17d and e). This work suggests that increasing the specific surface area of the CMPs is an effective design strategy to improve the electrochemical performance of highly efficient organic electrode materials for LIBs.
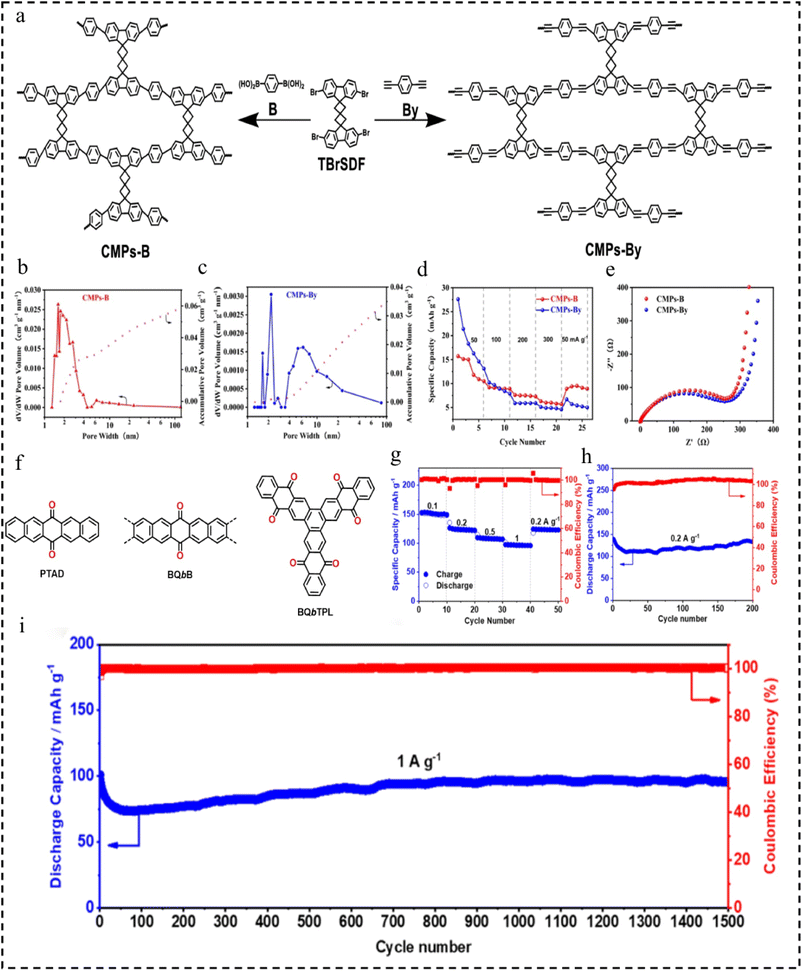 | ||
| Fig. 17 (a) Synthesis of CMPs-B and CMPs-By. (b) Pore size distribution curve (red) and cumulative pore volume curve (pink) of CMPs-B. (c) Pore size distribution curve (blue) and cumulative pore volume curve (pink) of CMPs-By. (d) Rate performance of the CMP-based electrode at different current densities. (e) Nyquist plots of the pristine and CMP-based electrode.113 Copyright 2023, Springer. (f) The structure of PTAD, BQbB, and BQbTPL. (g) Rate capability at different current densities and cycle performance at (h) 0.2 A g−1 and (i) 1.0 A g−1.114 Copyright 2021, American Chemical Society. | ||
Among various organic cathode materials, structures enriched with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups have garnered significant attention globally. Particularly, π-CMPs featuring enriched C
O groups have garnered significant attention globally. Particularly, π-CMPs featuring enriched C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and stable backbones have emerged as a focal point for research efforts. In 2021, Zhai's team114 synthesized novel CMPs enriched by the bond of C
O groups and stable backbones have emerged as a focal point for research efforts. In 2021, Zhai's team114 synthesized novel CMPs enriched by the bond of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O utilizing the highly efficient Diels–Alder reaction (Fig. 17f). The resulting CMPs showcase a fused carbon skeleton structure and semiconducting properties, characterized by a band gap of 1.4 eV. Due to their structural properties, the CMPs exhibit good stability (96.1% capacity retention at 0.2 A g−1 after 200 cycles and 94.8% capacity retention at 1 A g−1 after 1500 cycles), superior lithium-ion diffusion coefficient (5.30 × 10−11 cm2 s−1) and excellent multiplication capacity (95.8 mA h g−1 at 1 A g−1) (Fig. 17g–i). This study broadens the way for the design strategy of CMPs as a cathode material for LIBs.
O utilizing the highly efficient Diels–Alder reaction (Fig. 17f). The resulting CMPs showcase a fused carbon skeleton structure and semiconducting properties, characterized by a band gap of 1.4 eV. Due to their structural properties, the CMPs exhibit good stability (96.1% capacity retention at 0.2 A g−1 after 200 cycles and 94.8% capacity retention at 1 A g−1 after 1500 cycles), superior lithium-ion diffusion coefficient (5.30 × 10−11 cm2 s−1) and excellent multiplication capacity (95.8 mA h g−1 at 1 A g−1) (Fig. 17g–i). This study broadens the way for the design strategy of CMPs as a cathode material for LIBs.
3.3 CMPs as electrode materials for supercapacitors
Supercapacitors have evolved into prominent energy storage systems for electronic devices due to their high charge/discharge rate, exceptional power density, long cycle life, balanced rate performance, superior cycle efficiency, and environmental friendliness. These properties have garnered significant research interest among scientists and researchers.115–117 Recently, both organic and inorganic substances have been increasingly utilized as electrodes in supercapacitors. CMPs, in particular, have emerged as attractive electrode materials for capacitors owing to their affordability, chemical stability, flexibility, and widespread availability. They play a pivotal role in enhancing capacitor efficiency.118–123In 2017, Lai et al.124 designed and synthesized the CMPs (TAT-CMP-1, and TAT-CMP-2) with redox activity based on nitrogen-rich and highly conductive triazatriene building blocks as efficient and stable electrode materials for high-performance supercapacitors (Fig. 18a). Due to their favorable porous structure and high nitrogen content, TAT-CMP-1 and TAT-CMP-2 demonstrate high capacitances of 141 F g−1 and 183 F g−1, respectively, at a current density of 1 A g−1. Remarkably, they exhibit an ultra-high capacitance per unit surface area (>160 μF cm−2) (Fig. 18b and c). In addition, TAT-CMP also exhibits excellent reversibility, with cycle efficiencies of 95% and 83% at a high current density of 10 A g−1 and after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, respectively (Fig. 18d). The results show that selectively adjusting nitrogen content by chemical doping is an effective method to improve electrochemical performance.
000 cycles, respectively (Fig. 18d). The results show that selectively adjusting nitrogen content by chemical doping is an effective method to improve electrochemical performance.
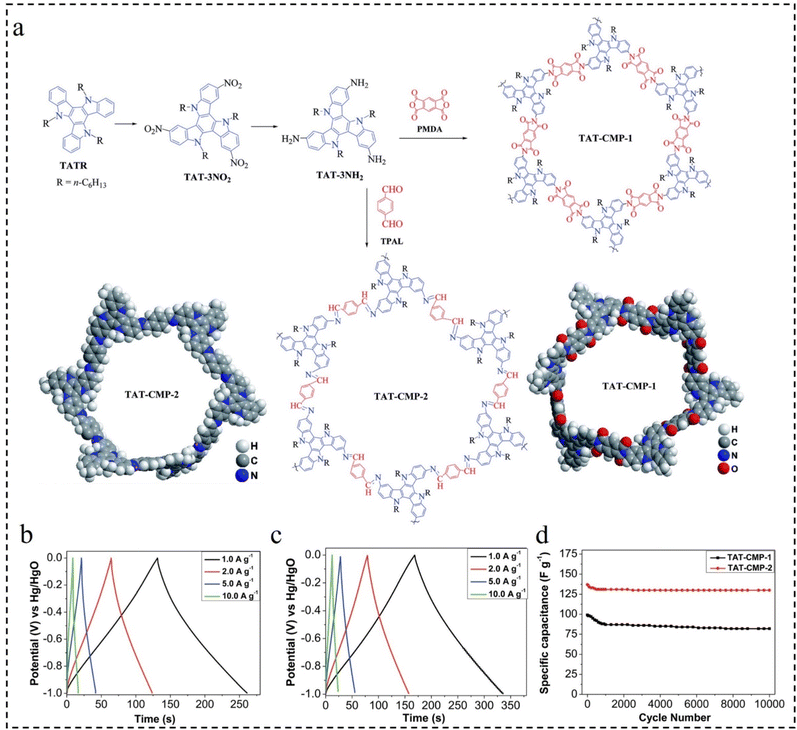 | ||
Fig. 18 (a) The structure of TAT-CMP-1 and TAT-CMP-2. GCD curves of (b) TAT-CMP-1 and (c) TAT-CMP-2 at different current densities. (d) The relationship of the specific capacitance with cycling number for TAT-CMP-1 and TAT-CMP-2 at a current density of 10 A g−1 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.124 Copyright 2017, Royal Society of Chemistry. 000 cycles.124 Copyright 2017, Royal Society of Chemistry. | ||
In 2022, Lai et al.125 developed a set of novel CMPs based on n-type perylene diimide (PDI), namely CMP-1, CMP-2, and CMP-3, as electrode materials for organic capacitors used for flexible energy storage. The morphological characterization showed that they have an irregularly stacked and highly cross-linked morphology, and they have micro- and mesoporous structures (Fig. 19a–c). Given the electron-accepting redox active sites, the hierarchical porous structure, and the amide-linked network, the PDI-CMP electrodes show n-type pseudocapacitive behaviour with high capacity (139–205 F g−1 at 0.5 A g−1), wide and negative bias (−1.0–0 V vs. Ag/AgCl), and long cycling stability (Fig. 19d and e). Compared to the corresponding CMP-1, CMP-3 features a rigid backbone comprising tetraphenylmethane three-dimensional (3D) structural units and PDI units. This structure promotes ion transfer, increases ionic association, and enhances surface exposure, leading to high specific capacitance, good thermal stability, and excellent reversibility. Notably, CMP-3 exhibits a cycling efficiency of 96% after 5000 cycles at a high current density of 10 A g−1 (Fig. 19f). CMP-3 and CMP-2 exhibit faster diffusion kinetics than CMP-1, which can be attributed to the 3D rigid structure of CMP-3 and the twisted molecular structure of CMP-2. Asymmetric supercapacitors employing CMP-3 and poly (3.4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT/PSS) exhibited a wider potential window (1.8 V) and increased capacity (17.4 mF cm−2) compared to symmetric supercapacitors utilizing PEDOT/PSS electrodes. In addition, CMP-3 demonstrates attractive potential as an anode for rechargeable LIBs. This study elucidates a fundamental understanding of the key structural parameters that determine its electrochemical and transport properties, thus opening new doors for the rational design of efficient and stable n-type organic electrode materials for flexible energy storage applications.
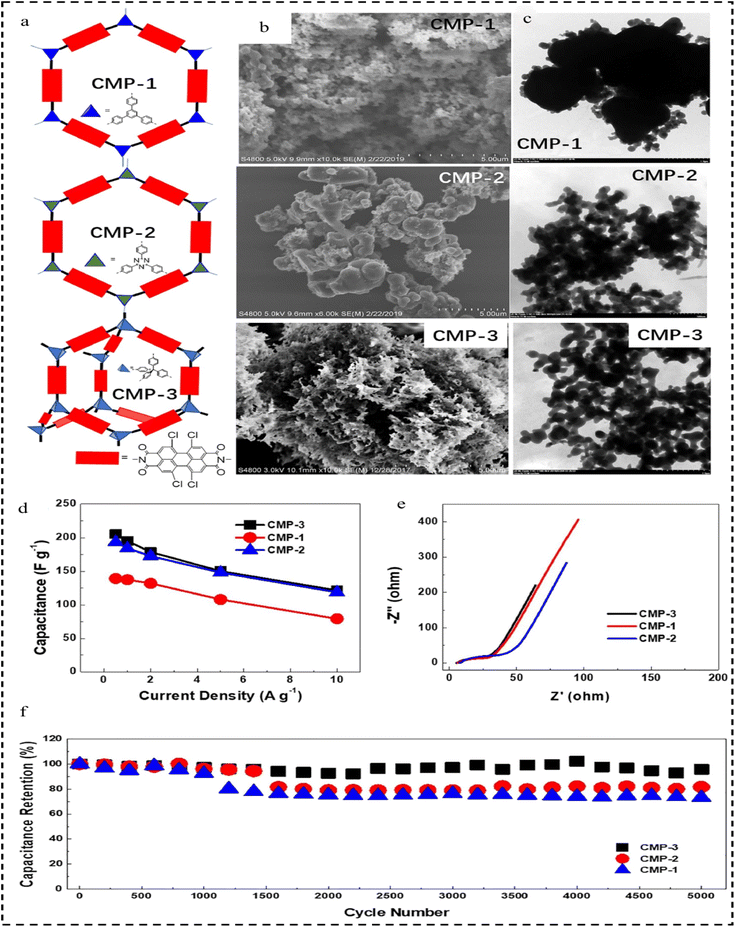 | ||
| Fig. 19 (a) Graphic representation of CMP-1, CMP-2 and CMP-3. (b) SEM images of CMP-1, CMP-2 and CMP-3. (c) TEM images of CMP-1, CMP-2 and CMP-3. (d) Specific capacitance versus current density curve of CMP-1, CMP-2 and CMP-3. (e) Nyquist plot of CMP-1, CMP-2 and CMP-3. (f) Cycling stability of CMP-1, CMP-2 and CMP-3 at a current density of 10 A g−1.125 Copyright 2022, Springer. | ||
In 2018, Liao et al.126 proposed a novel CMP network for energy storage in supercapacitors, a three-dimensional polyaminoanthraquinone (PAQ) network synthesised via B–H coupling between 2,6-diaminoanthraquinone (DAQ) and aryl bromides (PAQTA, PAQTB, PAQCB, PAQSF, and PAQTM) (Fig. 20a). PAQ possesses a surface area of up to 600 m2 g−1 and exhibits excellent dispersion in polar solvents, making it suitable for processing into flexible electrodes. Notably, PAQTA demonstrated a specific capacitance of 576 F g−1 (three-electrode configuration) in 0.5 M H2SO4 at a current density of 1 A g−1. Furthermore, it maintained 80–85% capacitance over 6000 cycles at a current density of 2 A g−1, with a nearly 100% coulombic efficiency (95–98%) (Fig. 20b–e). Asymmetric two-electrode supercapacitors assembled from PAQs show a total electrode material capacitance of 168 F g−1, an energy density of 60 W h kg−1 at a power density of 1300 W kg−1, and a wide operating potential window (0–1.6 V). The asymmetric supercapacitor has a coulombic efficiency of 97% and maintains 95.5% of its initial capacitance under 2000 cycles (Fig. 20f). This work presents new promising CMP networks for charge storage.
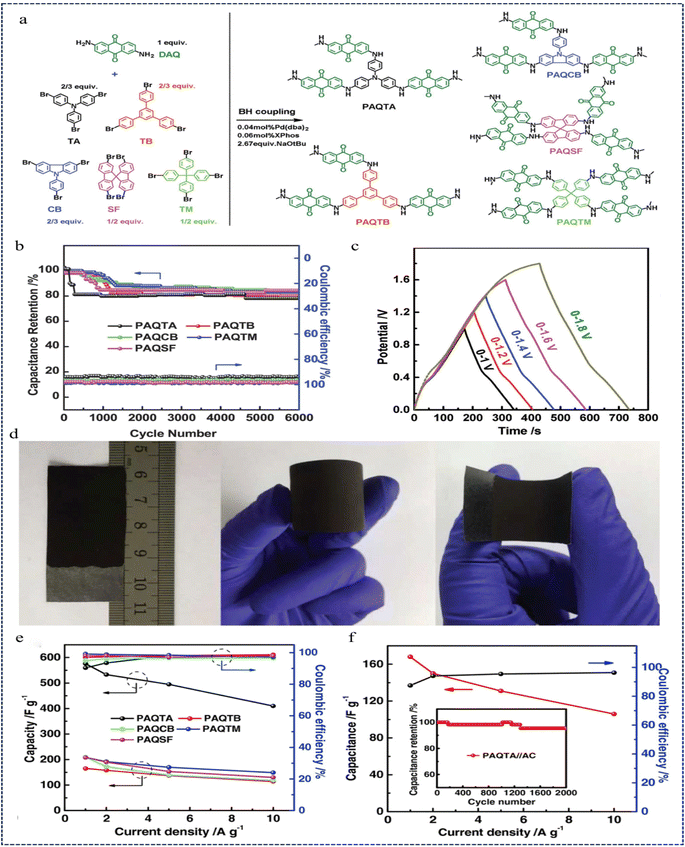 | ||
| Fig. 20 (a) Synthetic routes of PAQTA, PAQTB, PAQCB, PAQSF, and PAQTM. (b) Cyclability tests and coulombic efficiencies of PAQs. (c) GCD curves at a current density of 1.0 A g−1. (d) Photographs of flexible PAQTA electrodes and electrochemical performance of PAQs obtained in 0.5 M H2SO4. (e) Specific capacitance and coulombic efficiency of PAQs obtained at different current densities. (f) Specific capacitance and its retention as well as the coulombic efficiency based on the total mass of the two electrodes versus current density with the inset showing the cycle durability of the device at a current of 2.0 A g−1.126 Copyright 2018, Wiley. | ||
Fiber-based supercapacitors (FSCs) are a promising energy storage device to meet the growing demand for miniaturization, flexibility and compatibility in wearable electronic devices.127,128 However, low energy density compared to batteries remains a major limitation for practical applications.129,130 In 2020, Liao et al.131 utilized various amine monomers (aniline, pyridine, and anthraquinone) along with 1,3,5-tris(3-bromo)-benzene (TPBA) monomers, which were polymerized on the surface of a carbon nanotube fiber (CNF) through a B–H cross-coupling reaction. This process yielded a CMP network (CNF@CMP) characterized by a tailorable porous structure and reversible redox chemistry (Fig. 21a), exhibiting highly efficient capacitive properties. Due to the flexibility and high conductivity of CNFs, along with the porosity and strong redox properties of the polytriphenylamine network structure (PTPA), as well as the enhanced synergistic interaction between the CNF core and PTPA shell, it is used to prepare high-performance wearable supercapacitors (671.9 mF cm−2 at a current density of 1 mA cm−2). All-solid-state symmetrically twisted CNF@PTPA FSCs prepared with PVA/H3PO4 as the gel electrolyte exhibited a high specific surface area capacitance of 398 mF cm−2 (0.28 mA cm−2) (Fig. 21b and c), a maximum operating voltage of 1.4 V, and an energy density of 18.33 μWh cm−2 (Fig. 21d). In addition, they exhibit excellent flexibility and mechanical stability, maintaining 84.5% of the initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bending cycles (Fig. 21e). These materials provide a new avenue for high-performance wearable supercapacitors (HPWS) with a wide range of potential applications in wearable electronics.
000 bending cycles (Fig. 21e). These materials provide a new avenue for high-performance wearable supercapacitors (HPWS) with a wide range of potential applications in wearable electronics.
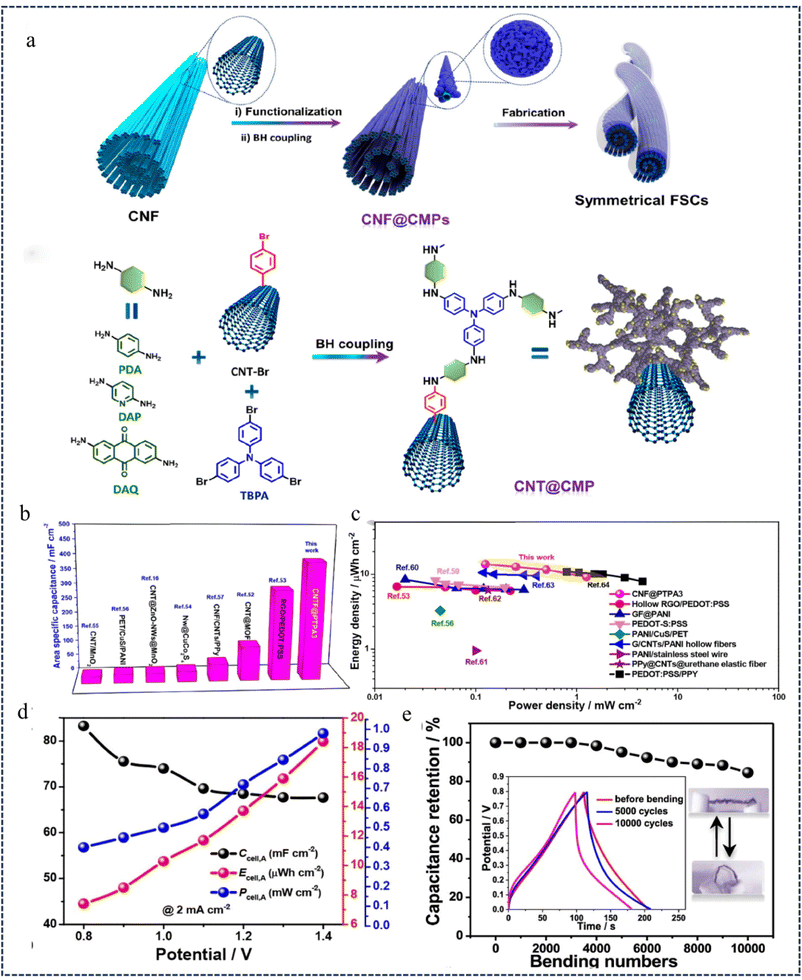 | ||
| Fig. 21 (a) Schematic illustration of the fabrication process for symmetrical fiber-shaped supercapacitors (FSCs). (b) Areal Ragone plots of the CNF@PTPA-based FSCs. (c) Comparison of electrochemical performance of CNF@PTPA FSCs with other FSCs in terms of specific capacitance. (d) GCD curves of CNF@CMP fibers. (e) Relationship between capacitance retention and bending times.131 Copyright 2020, American Chemical Society. | ||
CMPs with active functional groups have received increasing attention in energy conversion systems.132,133 However, their low conductivity results in low capacitance, which limits their practical applications.134,135 In 2021, Duan et al.136 designed and synthesised CMPs comprising trityl aldehydes linked to metal phthalocyanines (MNC, Co, and Fe). Composite membranes were prepared by combining them with highly conductive CNTs in different ratios (MNC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CNT = 1
CNT = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) using a microwave method and vacuum filtration strategy. These composite membranes are denoted as MNCCs-x, where x = 1, 2, 3, 5 (Fig. 22a). Due to the highly active metallic properties of CoNC, it can be compounded with highly conductive CNTs through π–π interactions without any covalent bonding into CoNCCs. These CoNCCs are flexible and can serve as self-supporting, binder-free flexible electrodes for supercapacitors. The optimized CoNCCs-3 flexible electrodes exhibit a high specific capacitance of 213.4 F g−1 at a current of 0.5 A g−1 (Fig. 22b). In addition, a capacity retention of 85.3% was achieved after 1750 cycles at 20 A g−1 (Fig. 22c). The good electrochemical performance can be attributed to the synergistic effect and strong biphasic interaction between MNC and CNTs. This work opens the way for the development of high-performance organic supercapacitor electrode materials with a low environmental footprint.
5) using a microwave method and vacuum filtration strategy. These composite membranes are denoted as MNCCs-x, where x = 1, 2, 3, 5 (Fig. 22a). Due to the highly active metallic properties of CoNC, it can be compounded with highly conductive CNTs through π–π interactions without any covalent bonding into CoNCCs. These CoNCCs are flexible and can serve as self-supporting, binder-free flexible electrodes for supercapacitors. The optimized CoNCCs-3 flexible electrodes exhibit a high specific capacitance of 213.4 F g−1 at a current of 0.5 A g−1 (Fig. 22b). In addition, a capacity retention of 85.3% was achieved after 1750 cycles at 20 A g−1 (Fig. 22c). The good electrochemical performance can be attributed to the synergistic effect and strong biphasic interaction between MNC and CNTs. This work opens the way for the development of high-performance organic supercapacitor electrode materials with a low environmental footprint.
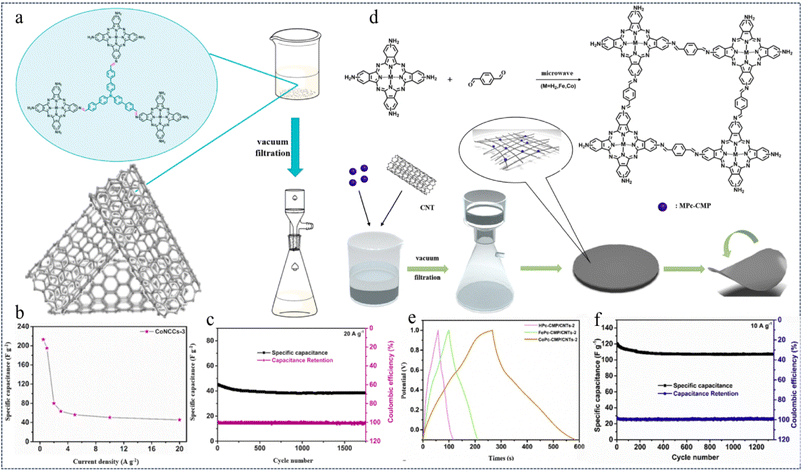 | ||
| Fig. 22 (a) Schematic preparation process for MNCCs (M = Co, Fe). (b) Specific capacitances of CoNCCs-3 at different current densities. (c) Cycling performance and coulombic efficiency of CoNCCs-3 at 20 A g−1.136 Copyright 2021, Elsevier. (d) Synthetic process of MPc-CMP/CNTs. (e) GCD curves of MPc-CMP/CNTs-2 (M = H2, Co, Fe) obtained at 1 A g−1. (f) Cycling performance and coulombic efficiency of CoPc-CMP/CNTs-2 at 10 A g−1.142 Copyright 2020, Elsevier. | ||
Metal phthalocyanines (MPcs), an important functional π-conjugated structural unit, are two-dimensional aromatic molecules containing a metal at the centre of the inner ring, and MPcs exhibit excellent electron-transfer ability due to the interaction between the phthalocyanine ring and the metal centre.137–140 MPc-based CMPs may have potential applications in energy conversion and storage systems.141 In 2020, Duan et al.142 fabricated three MPc-based CMPs (M = Co, Fe, and H2) using a rapid microwave method. Subsequently, they hybridized MPc-CMPs with CNTs to produce free-standing and flexible composite membranes through non-covalent bonding. These composite membranes serve as flexible electrodes for supercapacitors without the need for additives or binders (Fig. 22d). Due to the synergistic effect and strong π–π interaction between the MPc-CMP and CNTs, the flexible electrodes CoPc-CMP/CNTs-2, HPcCMP/CNTs-2 and FePc-CMP/CNTs-2 showed high specific capacitances of 289.1 F g−1, 53.6 F g−1 and 100.7 F g−1 at a current density of 1 A g−1, respectively (Fig. 22e). Even at a current density of 10 A g−1, CoPc CMP/CNTs-2 can show a high specific capacitance of 107.2 F g−1 and a high specific capacitance retention rate of 89.2% after 1350 cycles (Fig. 22f). The study shows that the synergistic effect and powerful functions of metal phthalocyanine-linked CMPs and highly conductive CNTs open up the possibility of developing high-performance supercapacitors.
Porphyrin-based CMPs have great potential for applications in energy storage systems, but their low conductivity limits their practical applications.143,144 To address this issue, in 2021, Duan et al.145 introduced a solution by designing and synthesizing copper porphyrin-CMPs (CuTAPP-CMPs). They further created CuTAPP-CMP/CNTs-3 by integrating CuTAPP-CMPs with highly conductive CNTs through a straightforward vacuum filtration method (Fig. 23a). These materials serve as non-adhesive, standalone flexible electrodes for supercapacitors. In the absence of any covalent bonding, the active CuTAPP-CMP can bind to the conducting CNTs in view of the π–π interactions. The flexible electrode of CuTAPP-CMP/CNTs-3 exhibits a specific capacitance of 207.8 F g−1 at 1 A g−1 (Fig. 23b) and a long cycling performance of more than 3700 cycles at 20 A g−1 (Fig. 23c). The impressive electrochemical performance observed can be attributed to the synergistic effect of the high electrical conductivity of CNTs combined with the high pseudocapacitance of CuTAPP-CMP. This study paves the way for the exploration of high-performance organic active materials for supercapacitors.
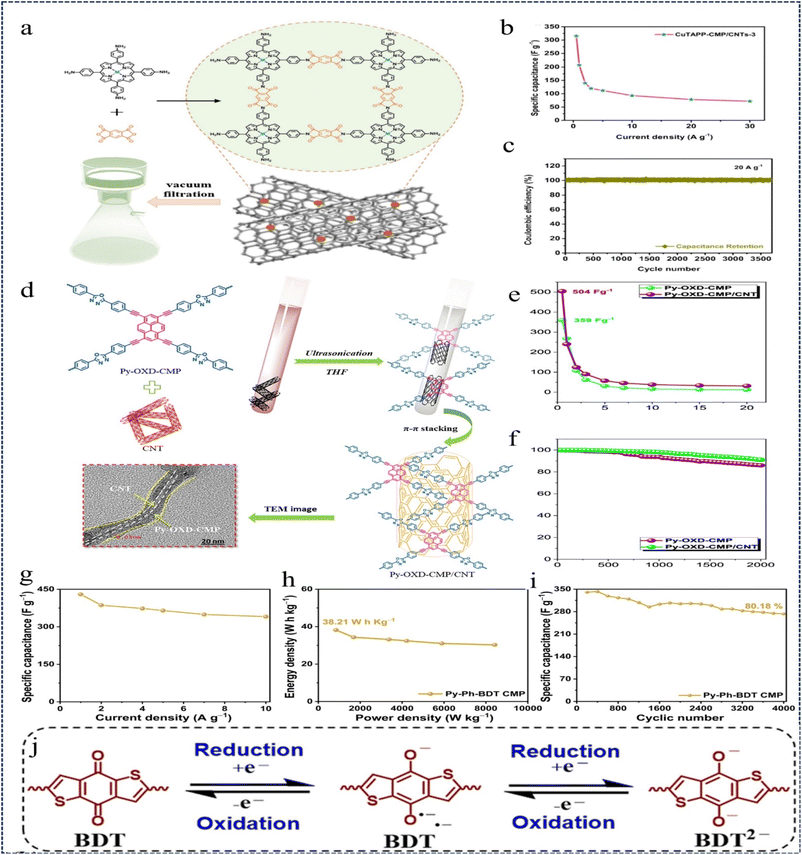 | ||
| Fig. 23 (a) Schematic preparation process for MTAPP-CMP/CNTs (M = Cu and H2). (b) Specific capacitances of CuTAPP-CMP/CNTs-3 at different current densities. (c) Coulombic efficiency of CuTAPPCMP/CNTs-3 at 20 A g−1.145 Copyright 2021, Springer. (d) Schematic synthesis of the Py-OXD-CMP/CNT nanocomposite. (e) Specific capacitances of the Py-OXD-CMP and Py-OXD-CMP/CNT nanocomposites. (f) Stability profiles of the Py-OXD-CMP and Py-OXD-CMP/CNT nanocomposites.123 Copyright 2022, American Chemical Society. (g) Calculated specific capacitances of the Py-Ph-BDT CMP tethered supercapacitor. (h) Ragone plot of energy density versus power density for the Py-Ph-BDT CMP-tethered supercapacitor. (i) Cycling performance of the Py-PhBDT CMP-tethered supercapacitor at a current density of 5 A g−1. (j) Redox mechanism of the BDT unit.146 Copyright 2023, Royal Society of Chemistry. | ||
In 2022, Mohamed et al.123 constructed three 1,3,4-oxadiazole-CMPs (OXD-CMPs: TPA-OXD-CMP, Py-OXD-CMP, and TPE-OXD-CMP) by simple Sonogashira coupling. CNTs were combined with OXD-CMPs using non-covalent bonding π–π interactions to provide OXD-CMPs/CNTs composites (Fig. 23d). When used as electrodes for supercapacitors, they have greatly enhanced capacitance and cycling stability, where the capacity of the Py-OXD-CMP/CNT composites is 504 F g−1 at a current density of 0.5 A g−1, which is much higher than that of other OXD-CMP-based samples. The Py-OXD-CMP/CNT composite exhibits excellent cycling stability (91.1% capacitance retention) as measured by GCD for 2000 cycles at a current density of 10 A g−1 (Fig. 23e and f). The results show that OXD-CMPs/CNTs nanocomposites are promising for high-performance charge storage.
In 2023, Gaber et al.146 synthesised unique redox-active CMPs, Py-BDT and Py-Ph-BDT-CMPs. Such CMPs are based on pyrene (Py) and redox-active benzo[1,2-b:4,5-b′] dithiophene-4-one (BDT) units, and act as highly efficient and stable electrodes for supercapacitor energy storage devices. CMPs exhibit excellent thermal stability (Td10: ∼564 °C; coke yield: ∼70.5%) and surface area (∼427 m2 g−1). At a current density of 0.5 A g−1, Py-BDT and Py-Ph-BDT CMPs exhibit excellent three-electrode capacitance of 636 and 712 F g−1, respectively, which is better than the previously reported specific capacitance of conventional CMPs. Symmetric two-electrode supercapacitors constructed with Py-Ph BDT CMPs showed an effective capacitance of 429 F g−1 and an energy density of 38.21 W h kg−1 at a potential of 0.8 V and maintained 80% of their initial capacitance over 4000 cycles (Fig. 23g–i). It was found that the redox process of such CMPs mainly depends on the BDT monomer, which is reduced using two electrons during discharge, whereas the reversible oxidation of the BDT2− anion occurs continuously during the charging phase, and thus the integration of the pyrene and BDT monomers into CMP cores could lead to fast charge transfer, excellent Faraday energy storage, and remarkable electrical conductivity (Fig. 23j). This work presents a new strategy for the preparation of high-capacity supercapacitors from reducible CMPs.
In 2022, Jang et al.147 prepared a conjugated microporous anthraquinone amide polymer network (CMAP@AG) with electron-transporting properties by a simple one-step B–H coupling of anthraquinone amide and tris-aniline as the basic polymerization units on an activated graphene substrate (Fig. 24a). The structural properties of the CMAP@AG composites were optimized by varying the etching time of the AG substrate material (Fig. 24b), exhibiting up to 751 F g−1 (Fig. 24c) at a current density of 1.0 A g−1 and maintaining 97% of the initial capacitance after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 effective cycles after increasing the current density to 10.0 A g−1 (Fig. 24d). In addition, the asymmetric supercapacitors assembled from CMAP@A4G exhibit a high energy density of 76.6 W h kg−1 and a high-power density of 27
000 effective cycles after increasing the current density to 10.0 A g−1 (Fig. 24d). In addition, the asymmetric supercapacitors assembled from CMAP@A4G exhibit a high energy density of 76.6 W h kg−1 and a high-power density of 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 634 W kg−1 in the voltage range of 0–1.5 V. Moreover, the composite CMP network is capable of bending and has certain mechanical strength, which is a better flexible CMP composite material. This study demonstrated through extensive experiments that CMAP@A4G has high redox activity, good electrochemical properties and stable cyclability, and the surface area of the composite can reach up to 498 m2 g−1. This study provides insights into the rational design and effective regulation of CMP-based active graphene composites for energy storage devices.
634 W kg−1 in the voltage range of 0–1.5 V. Moreover, the composite CMP network is capable of bending and has certain mechanical strength, which is a better flexible CMP composite material. This study demonstrated through extensive experiments that CMAP@A4G has high redox activity, good electrochemical properties and stable cyclability, and the surface area of the composite can reach up to 498 m2 g−1. This study provides insights into the rational design and effective regulation of CMP-based active graphene composites for energy storage devices.
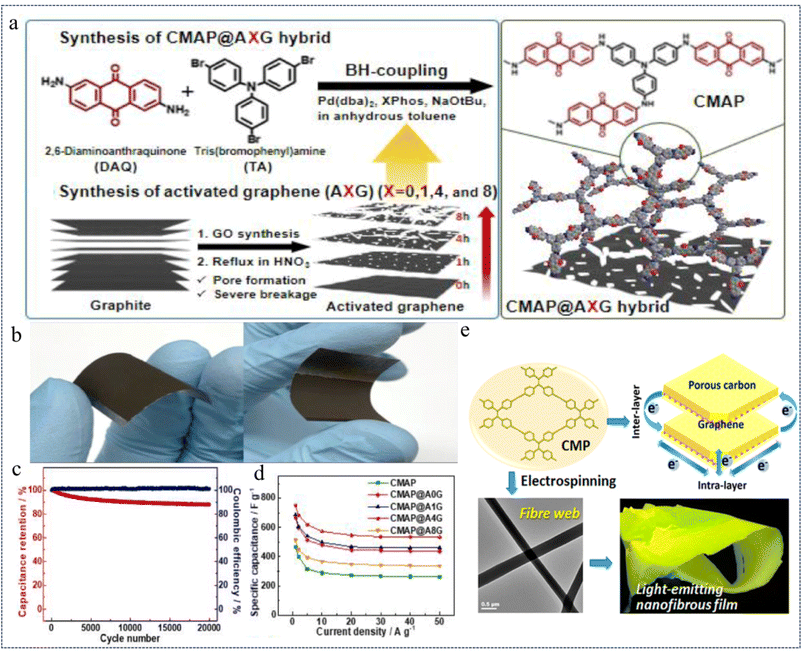 | ||
| Fig. 24 (a) Synthesis of the CMAP@XG hybrid. (b) Photographs of flexible CMAP@A4G electrodes. (c) Specific capacitance obtained at different scan rates from 1 to 50 A g−1. (d) Capacitance retention and coulombic efficiency test charts at a current density of 10 A g−1.147 Copyright 2022, Elsevier. (e) Schematic diagram of electron transfer in graphene templated CMPs.41 Copyright 2015, American Chemical Society. | ||
CMPs are usually obtained as amorphous or semi-crystalline powders, taking into account the fact that electrostatic spinning is a well-established and versatile technique for the fabrication of nanofibres with diameters ranging from tens of nanometers to a few micrometres, which are usually highly flexible and have a high surface area-to-volume ratio, which is conducive to the fabrication of chemosensor devices with a fast response and a high sensitivity.39,148,149 In 2015, Chen and his team41 prepared CMP materials by the Sonogashira coupling reaction using tetraaryl ethylene as the polymerization unit, and prepared a sensitive sensor with high flexibility, high porosity and surface area-to-volume ratio, and suitable for the detection of nitroaromatics and benzoquinone vapours, as well as oxidising metal ions, by efficiently mixing the CMPs with PLA by means of electrostatic spinning (Fig. 24e). Furthermore, sandwich structures comprising graphene-based CMPs (G-CMPs) boast high surface area and aspect ratio properties. These structures can be synthesized via pyrolysis at 800 °C to yield hierarchical porous carbon-rich 2D carbon nanosheets. Remarkably, these carbon nanomaterials demonstrate exceptional supercapacitor performance, showcasing a 48% increase in capacitance compared to porous carbon without graphene templates. The introduction of graphene scaffolds facilitates efficient inter/intralayer electron transport, which improves ion injection/extraction from or to the electrodes and enhances energy storage capacity.
3.4 CMPs as other flexible materials
CMP materials are excellent across various fields owing to their unique structure and large specific surface area, among other advantages. However, the formation of rigid π-conjugated networks renders them insoluble in most organic solvents, resulting in poor processability and limitations in certain practical applications. To address these scientific challenges and expand the application scope of CMP materials, researchers have shown considerable interest in developing CMP flexible membranes.Membranes with fast and selective ion transport have great potential for water and energy-related applications, and the structural and material design of membranes plays a key role in improving their performance.150 In 2021, Lai et al.151 prepared two flexible ionic CMP films (i-CMP and n-CMP) with adjustable thickness by co-electro polymerization (Fig. 25a). Unlike conventional CMP film materials, the Young's modulus of the two films averages 4 GPa (Fig. 25b), and the specific surface areas of i-CMP and n-CMP are 821 m2 g−1 and 773 m2 g−1, respectively. Due to the introduction of soft carbon chains into the CMP monomer, the thin film structure has dual flexibility and functional control. The selectivity of the two CMP films for H+/Mg2+ was 9.3 (i-CMP) and 2.5 (n-CMP), respectively, during the electrically driven process. In the concentration-driven process, the selectivity of i-CMP and n-CMP for K+/Mg2+ is 7.1 and 40.9 (Fig. 25c), respectively, which is better than that of the commercially available Nafion 117 in both driving modes. This study provides a new idea for the fabrication of flexible CMP films.
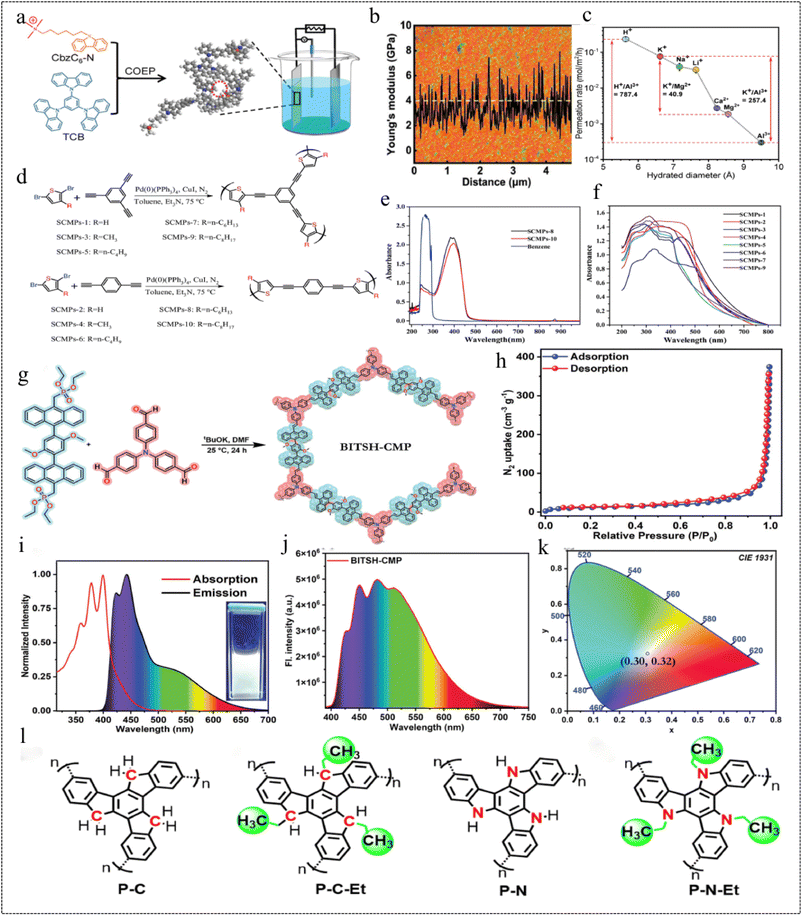 | ||
| Fig. 25 (a) Schematic diagram of the i-CMP membrane prepared by co-electrolytic polymerization. (b) AFM image of CMP membranes. (c) Selective ion permeation of the i-CMP membrane.151 Copyright 2021, Wiley. (d) Representative molecular structure of the repeat units for SCMPs. (e) Ultraviolet-visible absorption spectra of SCMPs-8 and SCMPs-10 in chloroform solution. (f) Solid-state ultraviolet-visible absorption spectra of SCMPs.152 Copyright 2018, Wiley. (g) Synthetic method for BITSH-CMP. (h) N2-isothermal adsorption curve at 77 K. (i) Normalised absorption and fluorescence spectra of BITSH-CMP. (j) Fluorescence spectra of BITSH-CMP blended with PVA. (k) CIE plot.153 Copyright 2023, Wiley. (l) The molecular structures of the CMPs.154 Copyright 2021, Elsevier. | ||
In 2018, Ren et al.152 prepared a series of soluble flexible CMP materials (SCMPs). A significant improvement in the solubility of SCMPs was achieved by adjusting the length of the alkyl chains in the alkyl-substituted dibromothiophene monomers (Fig. 25d). The BET specific surface areas of SCMPs-1, SCMPs-2, SCMPs-3, and SCMPs-4 were determined to be 182.18 m2 g−1, 32.12 m2 g−1, 1.86 m2 g−1, and 4.70 m2 g−1, respectively. Notably, the pore size distribution (PSD) for SCMPs-1 exhibited a narrower distribution, with a pore size of only 12.79 nm, this suggests that the introduction of alkyl chains in the monomer leads to a decrease in the BET specific surface area. The SCMP samples have a strong UV absorption and fluorescence effect. At optimal excitation wavelengths, these polymers, characterized by short carbon branches (no more than 4 carbon atoms) or no alkyl substitutions, emit blue and green fluorescence for SCMPs-1 and SCMPs-6, respectively. Conversely, samples featuring longer carbon branches (nC = 6 or 8), spanning from SCMPs-7 to SCMPs-10, emit blue fluorescence (Fig. 25e). Functional composite flexible films of SCMPs were formed on a number of different soft and hard substrates using spin-coating, substrate casting and dip-coating methods, and these soluble SCMP samples can be used as functional coatings with some UV protection due to their strong UV absorption at 250 and 380 nm (Fig. 25f). The results revealed that both SCMP-coated PDMS films exhibited outstanding optical transparency in the visible region (>75% from 450 to 780 nm). However, the UV transmittance of the as-prepared SCMP-coated PDMS films at 357 nm was observed to be only 39.26% (SCMPs-8-PDMS) and 26.70% (SCMPs-10-PDMS), respectively. In conclusion, it was found that the structure and composition of the monomers can be fine-tuned to provide new possibilities for the rational design of novel CMP materials with desired functionality and porosity.
In 2023, Prusti et al.153 quantitatively obtained a newly designed CMP (BITSH-CMP) by the Horner–Wadsworth–Emmons reaction (Fig. 25g). The CMP was characterized by conformational distortions and electron-rich two-state white light emission, and had a specific surface area of up to 248 m2 g−1 with a pore size of 1.76 nm (Fig. 25h). This CMP emits an intense white light in solution, produces yellow emission in the solid state, and when mixed with polyvinyl alcohol, produces a flexible, intense white light emission in the film. BITSH-CMP uses the electron-rich TPA nucleus (TPA2A) as a donor and the anthracene nucleus (An2P2) as an acceptor, allowing the intramolecular charge transfer process to proceed smoothly. The tuning of the white emission can be scrutinised by varying the concentration of the CMP (Fig. 25i and j), and the solution white emission can be tuned by adjusting the concentration in dimethylsulphoxide [CIE: 0.29, 0.32], and the flexible film produces white emission (CIE: 0.30, 0.32) (Fig. 25k). This CMP is currently under investigation for solid-state device fabrication, involving its blending with commercially available, electron-rich polyvinyl alcohol to enhance its intramolecular charge transfer (ICT) process. This results in the formation of a flexible white light emitter (WLE) with significant strength. This robust and accessible flexible device emits strong white light, expanding the practical applications of solid-state white light emitting materials.
CMPs with rigid π-conjugated backbones and tunable microporous structures show great potential as gas storage and sequestration materials.18 In 2021, Zhu et al.154 prepared flexible alkyl@rigid containing backbone CMPs (P-C(-Et) and P-N(-Et)) based on truxene and triazatruxene, which are highly gas-absorbent (Fig. 25l). The results suggest that incorporating rational flexible moieties with additional rigid backbone structures can induce a transformation of the 2D C3 symmetric structural core into a 3D stereoscopic configuration. Gas adsorption measurements further indicate that alkyl@rigid incorporated CMPs are likely to exhibit higher surface areas (ranging from 339 to 1031 m2 g−1 for truxene and from 324 to 1150 m2 g−1 for triazatruxene) and narrower pore size distributions. The optimized molecular structures exhibit a high CO2 uptake capacity, ranging from 5.8 to 11.5 wt% for turquoise and 7.8–17.6 wt% for triazapatite at 273 K and 1.0 bar. Furthermore, these optimized structures demonstrate superior selectivity for CO2 even in the presence of N2 or CH4 in the system. In conclusion, this work provides a fundamental understanding of the design of CMPs with high CO2 adsorption and separation performance.
4 Conclusions and perspectives
CMPs, as an important part of the porous organic polymer family, have a unique microporous π-conjugated backbone and also represent a future multifunctional platform. CMPs have numerous applications for electrode energy storage due to their high redox activity, excellent physicochemical stability, highly crosslinked polymer network, rich porous structure and large surface area. As smart and flexible electronic devices have gradually become the trend of the future, CMP flexible electrodes are becoming a new area of scientific interest. It is possible to adjust the composition, internal morphological structure and performance of the organic modules by optimising the molecular design to meet the requirements of a specific application, and the research on CMP flexible electrodes is moving towards the direction of low-cost, high-energy density, high power density, and long cyclic life.In this review, we systematically demonstrate a variety of CMP construction methods and applications in flexible electrodes, which mainly have the following problems: (1) many coupling reactions require the introduction of transition metal catalysts, which may have unclean catalyst treatment, blocking the pores of CMPs and affecting the electrochemical performance of CMPs. (2) In the metal-free catalytic system, CMPs with complex structures are difficult to prepare and cannot be produced on a large scale. (3) In the natural state, CMPs exist in a powder or semi-crystalline state, which is difficult to process.
Therefore, based on the above problems, researchers should consider in-depth research on the development and application of flexible electrodes based on CMPs in the future, mainly in the following aspects: (1) development of green and environmentally friendly synthetic methods to reduce the dependence on transition metal catalysts, e.g., synthesis of CMPs using light energy, or formation of desired CMPs by self-assembly of precursor molecules of CMPs. (2) Since nanomaterials such as nanowires, nanorods, nanotubes, and nanofibres have the characteristics of flexible materials, these materials are further combined with CMPs to develop flexible electrodes with good mechanical and electrochemical properties. (3) Integration of CMPs with flexible materials such as MXenes, carbon materials, and layered metal oxides is anticipated to augment the redox activity, theoretical capacity, and conductivity of electrode materials. (4) CMPs feature a highly crosslinked polymer network. Incorporating hyperbranching and alkylation techniques into the construction of CMP polymer networks, particularly on the linker and core, can enhance compatibility with the electrolyte and facilitate electrolyte penetration. This refinement promotes optimal activation of the active substances within the electrode. (5) Introduction of conjugated microporous polymer precursor molecules with flexible alkyl chains into the molecular design of CMPs can increase the flexibility of the precursor molecules. (6) In the design and synthesis of conjugated microporous polymers, precursor molecules such as quinoxaline-based aromatic heterocycles, aryl quinones and ketones, polyimides, and benzothiazoles should be included as much as possible to improve their electron transfer efficiency. (7) In order to improve the chemical and physical stability of CMPs, the degree of cross-linking of CMPs can be increased by introducing more stable chemical groups (sulfone, aryl groups, etc.) through chemical modification, which can make CMPs more reliable under extreme conditions (strong acids, bases, high temperatures, etc.).
In conclusion, although there are still some pressing issues regarding the synthesis methods and applications of CMPs, this does not detract from the fact that CMPs are an interesting “Möbius ring” with unlimited technological possibilities. We look forward to a bright future for CMP materials in various industrial applications.
Author contributions
D. Zhou and K. Q. Zhang co-authored the paper for this review. S. Q. Zou collected papers related to the topic of this review. X. B. Li and H. W. Ma: project administration, supervision, funding acquisition, writing. The paper was revised by all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22374017 and 62205052) and Fundamental Research Funds for the Central Universities (2572023CT12).Notes and references
- H. Cha, J. Kim, Y. Lee, J. Cho and M. Park, Small, 2017, 14, 1702989 CrossRef PubMed.
- T. M. Gür, Energy Environ. Sci., 2018, 11, 2696–2767 RSC.
- S. Tao, R. Momen, Z. Luo, Y. Zhu, X. Xiao, Z. Cao, D. Xiong, W. Deng, Y. Liu, H. Hou, G. Zou and X. Ji, Small, 2023, 19, 2207975 CrossRef CAS PubMed.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- J. Wang, Z. Wang, J. Ni and L. Li, Energy Storage Mater., 2022, 45, 704–719 CrossRef.
- M. G. Mohamed, M. H. Elsayed, C. J. Li, A. E. Hassan, I. M. A. Mekhemer, A. F. Musa, M. K. Hussien, L. C. Chen, K. H. Chen, H. H. Chou and S. W. Kuo, J. Mater. Chem. A, 2024, 12, 7693–7710 RSC.
- Y. Zhao and J. Guo, InfoMat, 2020, 2, 866–878 CrossRef CAS.
- P. N. Singh, M. G. Mohamed and S. W. Kuo, ACS Appl. Energy Mater., 2023, 6, 11342–11351 CrossRef CAS.
- Y. H. Zhu, X. Y. Yang, T. Liu and X. B. Zhang, Adv. Mater., 2019, 32, 1901961 CrossRef PubMed.
- H. Cha, Y. Lee, J. Kim, M. Park and J. Cho, Adv. Energy Mater., 2018, 8, 1801917 CrossRef.
- Z. Fang, J. Wang, H. Wu, Q. Li, S. Fan and J. Wang, J. Power Sources, 2020, 454, 227932 CrossRef CAS.
- S. B. Huang, Y. Y. Hsieh, K. T. Chen and H. Y. Tuan, Chem. Eng. J., 2021, 416, 127697 CrossRef CAS.
- T. B. Schon, B. T. McAllister, P. F. Li and D. S. Seferos, Chem. Soc. Rev., 2016, 45, 6405–6406 RSC.
- T. Yokoji, H. Matsubara and M. Satoh, J. Mater. Chem. A, 2014, 2, 19347–19354 RSC.
- C. Su, H. He, L. Xu, K. Zhao, C. Zheng and C. Zhang, J. Mater. Chem. A, 2017, 5, 2701–2709 RSC.
- C. Zhang, X. Yang, W. Ren, Y. Wang, F. Su and J. X. Jiang, J. Power Sources, 2016, 317, 49–56 CrossRef CAS.
- S. Luo, Z. Zeng, H. Wang, W. Xiong, B. Song, C. Zhou, A. Duan, X. Tan, Q. He, G. Zeng, Z. Liu and R. Xiao, Prog. Polym. Sci., 2021, 115, 101374 CrossRef CAS.
- M. G. Mohamed, S. V. Chaganti, M. S. Li, M. M. Samy, S. U. Sharma, J. T. Lee, M. H. Elsayed, H. H. Chou and S. W. Kuo, ACS Appl. Energy Mater., 2022, 5, 6442–6452 CrossRef CAS.
- Y. Xu, S. Jin, H. Xu, A. Nagai and D. Jiang, Chem. Soc. Rev., 2013, 42, 8012–8031 RSC.
- J. Chen, W. Yan, E. J. Townsend, J. Feng, L. Pan, V. Del Angel Hernandez and C. F. J. Faul, Angew. Chem., Int. Ed., 2019, 58, 11715–11719 CrossRef CAS PubMed.
- J. Chen, T. Qiu, W. Yan and C. F. J. Faul, J. Mater. Chem. A, 2020, 8, 22657–22665 RSC.
- K. Amin, N. Ashraf, L. Mao, C. F. J. Faul and Z. Wei, Nano Energy, 2021, 85, 105958 CrossRef CAS.
- Z. Liu, Y. Yin, F. Xiu, X. Wang, S. Ju, M. Song, Q. Chang, J. Chen, J. Liu and W. Huang, J. Mater. Chem. C, 2018, 6, 7295–7301 RSC.
- H. T. Le, C. G. Wang and A. Goto, Nat. Commun., 2023, 14, 171 CrossRef CAS PubMed.
- T. H. Weng, M. G. Mohamed, S. U. Sharma, I. M. A. Mekhemer, H. H. Chou and S. W. Kuo, ACS Appl. Energy Mater., 2023, 6, 9012–9024 CrossRef CAS.
- H. Liu, Y. Wang, W. Mo, H. Tang, Z. Cheng, Y. Chen, S. Zhang, H. Ma, B. Li and X. Li, Adv. Funct. Mater., 2020, 30, 1910275 CrossRef CAS.
- Z. Q. Chen, T. Chen, J. X. Liu, G. F. Zhang, C. Li, W. L. Gong, Z. J. Xiong, N. H. Xie, B. Z. Tang and M. Q. Zhu, Macromolecules, 2015, 48, 7823–7835 CrossRef CAS.
- M. Liu, B. Zhou, L. Zhou, Z. Xie, S. Li and L. Chen, J. Mater. Chem. A, 2018, 6, 9860–9865 RSC.
- Y. Zang, S. Gao, B. Jing, H. Sun, J. Wang, J. Liu, F. Miao and L. Xu, J. Mater. Sci., 2022, 58, 170–185 CrossRef.
- X. Cheng, R. Guan, Z. Wu, Y. Sun, W. Che and Q. Shang, InfoMat, 2024, e12535, DOI:10.1002/inf2.12535.
- C. J. Sun, X. Q. Zhao, P. F. Wang, H. Wang and B. H. Han, Sci. China: Chem., 2017, 60, 1067–1074 CrossRef CAS.
- M. G. Mohamed, W. C. Chang, S. V. Chaganti, S. U. Sharma, J. T. Lee and S. W. Kuo, Polym. Chem., 2023, 14, 4589–4601 RSC.
- I. Ullah, T. A. Taha, A. M. Alenad, I. Uddin, A. Hayat, A. Hayat, M. Sohail, A. Irfan, J. Khan and A. Palamanit, Surf. Interfaces, 2021, 25, 101227 CrossRef CAS.
- Q. Zhang, S. Yu, Q. Wang, Q. Xiao, Y. Yue and S. Ren, Macromol. Rapid Commun., 2017, 38, 1700445 CrossRef PubMed.
- C. Hu, Y. C. Gao, C. Zhang, M. Liu and T. M. Geng, RSC Adv., 2020, 10, 5108–5115 RSC.
- I. M. A. Mekhemer, M. M. Elsenety, A. M. Elewa, K. D. G. Huynh, M. M. Samy, M. G. Mohamed, D. M. Dorrah, D. C. K. Hoang, A. F. Musa, S. W. Kuo and H. H. Chou, J. Mater. Chem. A, 2024, 12, 10790–10798 RSC.
- C. W. Kang, Y. J. Ko and H. Jin Kim, J. Mater. Chem. A, 2021, 9, 17978–17984 RSC.
- P. N. Singh, M. G. Mohamed, S. V. Chaganti, S. U. Sharma, M. Ejaz, J. T. Lee and S. W. Kuo, ACS Appl. Energy Mater., 2023, 6, 8277–8287 CrossRef CAS.
- C. Gu, N. Huang, J. Gao, F. Xu, Y. Xu and D. Jiang, Angew. Chem., Int. Ed., 2014, 53, 4850–4855 CrossRef CAS PubMed.
- M. Ejaz, M. G. Mohamed, W. C. Huang and S. W. Kuo, J. Mater. Chem. A, 2023, 11, 22868–22883 RSC.
- K. Yuan, P. G. Wang, T. Hu, L. Shi, R. Zeng, M. Forster, T. Pichler, Y. Chen and U. Scherf, Chem. Mater., 2015, 27, 7403–7411 CrossRef CAS.
- J. M. Lee and A. I. Cooper, Chem. Rev., 2020, 120, 2171–2214 CrossRef CAS PubMed.
- F. S. J. X. Jiang, A. Trewin, C. D. Wood, H. J. Niu, J. T. A. Jones, Y. Z. Khimyak and A. I. Cooper, J. Am. Chem. Soc., 2008, 130, 7710–7720 CrossRef PubMed.
- F. Xu, X. Chen, Z. Tang, D. Wu, R. Fu and D. Jiang, Chem. Commun., 2014, 50, 4788–4790 RSC.
- M. G. Mohamed, S. V. Chaganti, S. U. Sharma, M. M. Samy, M. Ejaz, J. T. Lee, K. Zhang and S. W. Kuo, ACS Appl. Energy Mater., 2022, 5, 10130–10140 CrossRef CAS.
- N. Miyaura and A. Suzuki, J. Chem. Soc., Chem. Commun., 1979, 866–867 RSC.
- K. Y. Norio Miyaura and A. Suzuki, Tetrahedron Lett., 1979, 36, 3437–3440 CrossRef.
- B. Bonillo, R. S. Sprick and A. I. Cooper, Chem. Mater., 2016, 28, 3469–3480 CrossRef CAS.
- C. Zhang, Y. Qiao, P. Xiong, W. Ma, P. Bai, X. Wang, Q. Li, J. Zhao, Y. Xu, Y. Chen, J. H. Zeng, F. Wang, Y. Xu and J. X. Jiang, ACS Nano, 2019, 13, 745–754 CrossRef CAS PubMed.
- S. B. Ren, W. Ma, C. Zhang, L. Chen, K. Wang, R. R. Li, M. Shen, D. M. Han, Y. Chen and J. X. Jiang, ChemSusChem, 2020, 13, 2295–2302 CrossRef CAS PubMed.
- M. G. Kotp, N. L. Torad, H. Nara, W. Chaikittisilp, J. You, Y. Yamauchi, A. F. M. El-Mahdy and S. W. Kuo, J. Mater. Chem. A, 2023, 11, 15022–15032 RSC.
- R. F. Heck, J. Am. Chem. Soc., 1968, 90, 5518–5526 CrossRef CAS.
- L. Sun, Z. Liang, J. Yu and R. Xu, Polym. Chem., 2013, 4, 1932–1938 RSC.
- A. S. Guram and S. L. Buchwald, J. Am. Chem. Soc., 1994, 116, 7901–7902 CrossRef CAS.
- Y. Liao, J. Weber and C. F. J. Faul, Chem. Commun., 2014, 50, 8002–8005 RSC.
- Y. Liao, J. Weber, B. M. Mills, Z. Ren and C. F. J. Faul, Macromolecules, 2016, 49, 6322–6333 CrossRef CAS.
- L. Pan, Z. Liu, M. Tian, B. C. Schroeder, A. E. Aliev and C. F. J. Faul, ACS Appl. Mater. Interfaces, 2019, 11, 48352–48362 CrossRef CAS PubMed.
- H. Y. Yamamoto Takakazu and Y. Akio, Bull. Chem. Soc. Jpn., 1978, 51, 2091–2097 CrossRef.
- Z. H. Zhou and T. Yamamoto, J. Organomet. Chem., 1991, 414, 119–127 CrossRef CAS.
- J. R. Holst, E. Stöckel, D. J. Adams and A. I. Cooper, Macromolecules, 2010, 43, 8531–8538 CrossRef CAS.
- J. Schmidt, M. Werner and A. Thomas, Macromolecules, 2009, 42, 4426–4429 CrossRef CAS.
- Z. Tan, H. Su, Y. Guo, H. Liu, B. Liao, A. M. Amin and Q. Liu, Polymers, 2020, 12(3), 719 CrossRef CAS PubMed.
- Q. He, J. Kang, J. Zhu, S. Huang, C. Lu, H. Liang, Y. Su and X. Zhuang, Chem. Commun., 2022, 58, 2339–2342 RSC.
- M. Grzybowski, K. Skonieczny, H. Butenschön and D. T. Gryko, Angew. Chem., Int. Ed., 2013, 52, 9900–9930 CrossRef CAS PubMed.
- T. A. Mitsuru Ueda and H. Awano, Macromolecules, 1992, 25, 5125–5130 CrossRef.
- Q. Chen, D. P. Liu, M. Luo, L. J. Feng, Y. C. Zhao and B. H. Han, Small, 2014, 10, 308–315 CrossRef CAS PubMed.
- W. Wei, G. Chang, Y. Xu and L. Yang, J. Mater. Chem. A, 2018, 6, 18794–18798 RSC.
- L. Lian, K. Li, L. Ren, D. Han, X. Lv and H. G. Wang, Colloids Surf., A, 2023, 657, 130496 CrossRef CAS.
- W. Hao, R. Chen, Y. Zhang, Y. Wang and Y. Zhao, ACS Omega, 2021, 6, 23782–23787 CrossRef CAS PubMed.
- M. Mohamed Samy, I. M. A. Mekhemer, M. G. Mohamed, M. Hammad Elsayed, K. H. Lin, Y. K. Chen, T. L. Wu, H. H. Chou and S. W. Kuo, Chem. Eng. J., 2022, 446, 137158 CrossRef CAS.
- J. W. F. Y. Meng, M. H. Chen, Z. G. Wang, G. Y. Bai and X. W. Lan, ACS Catal., 2023, 13, 12142–12152 CrossRef.
- Y. Kou, Y. Xu, Z. Guo and D. Jiang, Angew. Chem., Int. Ed., 2011, 50, 8753–8757 CrossRef CAS PubMed.
- O. Buyukcakir, R. Yuksel, Y. Jiang, S. H. Lee, W. K. Seong, X. Chen and R. S. Ruoff, Angew. Chem., Int. Ed., 2018, 58, 872–876 CrossRef PubMed.
- J. Kim, M. H. Le, M. C. Spicer, C. M. Moisanu, S. M. Pugh and P. J. Milner, J. Mater. Chem. A, 2023, 11, 17159–17166 RSC.
- S. Y. Yu, J. Mahmood, H. J. Noh, J. M. Seo, S. M. Jung, S. H. Shin, Y. K. Im, I. Y. Jeon and J. B. Baek, Angew. Chem., Int. Ed., 2018, 57, 8438–8442 CrossRef CAS PubMed.
- Y. Zhu, X. Chen, Y. Cao, W. Peng, Y. Li, G. Zhang, F. Zhang and X. Fan, Chem. Commun., 2019, 55, 1434–1437 RSC.
- E. T. Seo, J. M. Fritsch, L. S. Marcoux, D. W. Leedy and R. N. Adams, J. Am. Chem. Soc., 1966, 88, 3498–3503 CrossRef CAS.
- R. F. Nelson and J. F. Ambrose, J. Electrochem. Soc., 1968, 115, 1159–1164 CrossRef.
- H. Ma, Y. Chen, X. Li and B. Li, Adv. Funct. Mater., 2021, 31, 2101861 CrossRef CAS.
- H. Ma, F. Li, P. Li, H. Wang, M. Zhang, G. Zhang, M. Baumgarten and K. Müllen, Adv. Funct. Mater., 2016, 26, 2025–2031 CrossRef CAS.
- W. Mo, Z. Zhu, F. Kong, X. Li, Y. Chen, H. Liu, Z. Cheng, H. Ma and B. Li, Nat. Commun., 2022, 13, 5189 CrossRef CAS PubMed.
- A. Hayat, M. Sohail, A. El Jery, K. M. Al-Zaydi, S. Raza, H. Ali, Y. Al-Hadeethi, T. A. Taha, I. Ud Din, M. Ali Khan, M. A. Amin, E. Ghasali, Y. Orooji, Z. Ajmal and M. Zahid Ansari, Mater. Today, 2023, 64, 180–208 CrossRef CAS.
- K. Y. Lin and A. F. M. El-Mahdy, Mater. Chem. Phys., 2022, 281, 125850 CrossRef CAS.
- J. Kim, C. M. Moisanu, C. N. Gannett, A. Halder, J. J. Fuentes-Rivera, S. H. Majer, K. M. Lancaster, A. C. Forse, H. D. Abruña and P. J. Milner, Chem. Mater., 2021, 33, 8334–8342 CrossRef CAS.
- E. Troschke, S. Grätz, T. Lübken and L. Borchardt, Angew. Chem., Int. Ed., 2017, 56, 6859–6863 CrossRef CAS PubMed.
- Y. Q. Hu, C. Zhou, X. Huang, B. An, R. Wang, F. Lan and X. Zhang, ACS Sustainable Chem. Eng., 2023, 11, 10225–10232 CrossRef CAS.
- B. Tian, J. Zheng, C. Zhao, C. Liu, C. Su, W. Tang, X. Li and G. H. Ning, J. Mater. Chem. A, 2019, 7, 9997–10003 RSC.
- L. Shu, J. Yu, Y. Cui, Y. Ma, Y. Li, B. Gao and H. G. Wang, Int. J. Hydrogen Energy, 2022, 47, 10902–10910 CrossRef CAS.
- F. Cheng and J. Chen, Chem. Soc. Rev., 2012, 41, 1111–1129 RSC.
- Y. G. Li and J. Lu, ACS Energy Lett., 2017, 2, 1370–1377 CrossRef CAS.
- Y. Liang, Z. Tao and J. Chen, Adv. Energy Mater., 2012, 2, 742–769 CrossRef CAS.
- C. Luo, X. Ji, S. Hou, N. Eidson, X. Fan, Y. Liang, T. Deng, J. Jiang and C. Wang, Adv. Mater., 2018, 30, 1706498 CrossRef PubMed.
- J. Wang, C. S. Chen and Y. Zhang, ACS Sustain. Chem. Eng., 2017, 6, 1772–1779 CrossRef.
- D. J. Kim, D. J. Yoo, M. T. Otley, A. Prokofjevs, C. Pezzato, M. Owczarek, S. J. Lee, J. W. Choi and J. F. Stoddart, Nat. Energy, 2018, 4, 51–59 CrossRef.
- C. Peng, G. H. Ning, J. Su, G. Zhong, W. Tang, B. Tian, C. Su, D. Yu, L. Zu, J. Yang, M. F. Ng, Y. S. Hu, Y. Yang, M. Armand and K. P. Loh, Nat. Energy, 2017, 2, 17074 CrossRef CAS.
- S. Zhang, W. Huang, P. Hu, C. Huang, C. Shang, C. Zhang, R. Yang and G. Cui, J. Mater. Chem. A, 2015, 3, 1896–1901 RSC.
- S. Xu, G. Wang, B. P. Biswal, M. Addicoat, S. Paasch, W. Sheng, X. Zhuang, E. Brunner, T. Heine, R. Berger and X. Feng, Angew. Chem., Int. Ed., 2018, 58, 849–853 CrossRef PubMed.
- W. Ma, C. Zhang, X. Gao, C. Shu, C. Yan, F. Wang, Y. Chen, J. H. Zeng and J. X. Jiang, J. Power Sources, 2020, 453, 227868 CrossRef CAS.
- C. Zhang, Y. He, P. Mu, X. Wang, Q. He, Y. Chen, J. Zeng, F. Wang, Y. Xu and J. X. Jiang, Adv. Funct. Mater., 2018, 28, 1705432 CrossRef.
- L. Zhong, Z. Fang, C. Shu, C. Mo, X. Chen and D. Yu, Angew. Chem., Int. Ed., 2021, 60, 10164–10171 CrossRef CAS PubMed.
- J. Min Park, J. H. Lee and W. D. Jang, Coord. Chem. Rev., 2020, 407, 213157 CrossRef.
- P. Gao, Z. Chen, Z. Zhao Karger, J. E. Mueller, C. Jung, S. Klyatskaya, T. Diemant, O. Fuhr, T. Jacob, R. J. Behm, M. Ruben and M. Fichtner, Angew. Chem., Int. Ed., 2017, 56, 10341–10346 CrossRef CAS PubMed.
- Y. Yang, J. Yuan, S. Huang, Z. Chen, C. Lu, C. Yang, G. Zhai, J. Zhu and X. Zhuang, J. Power Sources, 2022, 531, 231340 CrossRef CAS.
- A. Molina, N. Patil, E. Ventosa, M. Liras, J. Palma and R. Marcilla, Adv. Funct. Mater., 2019, 30, 1908074 CrossRef.
- R. Grieco, A. Molina, J. S. Sanchez, N. Patil, M. Liras and R. Marcilla, Mater. Today Energy, 2022, 27, 101014 CrossRef CAS.
- H. Zhang, L. Zhong, J. Xie, F. Yang, X. Liu and X. Lu, Adv. Mater., 2021, 33, 2101857 CrossRef CAS PubMed.
- R. Grieco, O. Luzanin, D. Alván, M. Liras, R. Dominko, N. Patil, J. Bitenc and R. Marcilla, Faraday Discuss., 2024, 250, 110–128 RSC.
- G. Dai, Y. He, Z. Niu, P. He, C. Zhang, Y. Zhao, X. Zhang and H. Zhou, Angew. Chem., Int. Ed., 2019, 58, 9902–9906 CrossRef CAS PubMed.
- C. N. Gannett, B. M. Peterson, L. Melecio-Zambrano, C. Q. Trainor, B. P. Fors and H. D. Abruña, J. Mater. Chem. A, 2021, 9, 5657–5663 RSC.
- G. Dai, Y. Liu, Z. Niu, P. He, Y. Zhao, X. Zhang and H. Zhou, Matter, 2019, 1, 945–958 CrossRef.
- W. Ma, L. W. Luo, X. Huang, P. Dong, Y. Chen, C. Zhang, F. Huang, J. X. Jiang and Y. Cao, Adv. Energy Mater., 2022, 13, 2203253 CrossRef.
- W. Ma, L. W. Luo, P. Dong, P. Zheng, X. Huang, C. Zhang, J. X. Jiang and Y. Cao, Adv. Funct. Mater., 2021, 31, 2105027 CrossRef CAS.
- R. Liu, C. Wei, X. Liu, Y. Qu, L. Yan, H. Zhao, Y. Wu, H. Cao, J. Li and H. Wang, J. Mater. Sci.: Mater. Electron., 2023, 34, 159 CrossRef CAS.
- Z. Ouyang, D. Tranca, Y. Zhao, Z. Chen, X. Fu, J. Zhu, G. Zhai, C. Ke, E. Kymakis and X. Zhuang, ACS Appl. Mater. Interfaces, 2021, 13, 9064–9073 CrossRef CAS PubMed.
- S. Liu, L. Kang, J. Zhang, E. Jung, S. Lee and S. C. Jun, Energy Storage Mater., 2020, 32, 167–177 CrossRef.
- Z. Xu, S. Sun, Y. Han, Z. Wei, Y. Cheng, S. Yin and W. Cui, ACS Appl. Energy Mater., 2020, 3, 5393–5404 CrossRef CAS.
- M. Ahmed, M. G. Kotp, T. H. Mansoure, R. H. Lee, S. W. Kuo and A. F. M. El-Mahdy, Microporous Mesoporous Mater., 2022, 333, 111766 CrossRef CAS.
- J. Kim, J. H. Kim and K. Ariga, Joule, 2017, 1, 739–768 CrossRef CAS.
- H. Zhao, J. Wang, Y. Zheng, J. Li, X. Han, G. He and Y. Du, Angew. Chem., Int. Ed., 2017, 56, 15334–15338 CrossRef CAS PubMed.
- Y. Wang, W. Li, L. Zhang, X. Zhang, B. Tan, J. Hao, Z. Jian, X. Wang, Q. Hu and X. Lu, J. Power Sources, 2020, 449, 227487 CrossRef CAS.
- M. G. Mohamed, C. C. Lee, A. F. M. El-Mahdy, J. Lüder, M.-H. Yu, Z. Li, Z. Zhu, C. C. Chueh and S.-W. Kuo, J. Mater. Chem. A, 2020, 8, 11448–11459 RSC.
- D. Kim, J. Kang, B. Yan, K. d. Seong and Y. Piao, ACS Sustain. Chem. Eng., 2020, 8, 2843–2853 CrossRef CAS.
- M. G. Mohamed, M. M. Samy, T. H. Mansoure, S. U. Sharma, M. S. Tsai, J. H. Chen, J. T. Lee and S. W. Kuo, ACS Appl. Energy Mater., 2022, 5, 3677–3688 CrossRef CAS.
- X. C. Li, Y. Zhang, C. Y. Wang, Y. Wan, W. Y. Lai, H. Pang and W. Huang, Chem. Sci., 2017, 8, 2959–2965 RSC.
- X. Liu, G. Sun, Y. Gong, C. F. Liu, S. Wang, S. Xu, X. Yang, L. Yang and W. Y. Lai, Sci. China: Chem., 2022, 65, 1767–1774 CrossRef CAS.
- Y. Liao, H. Wang, M. Zhu and A. Thomas, Adv. Mater., 2018, 30, 1705710 CrossRef PubMed.
- D. P. Dubal, N. R. Chodankar, D. H. Kim and P. Gomez-Romero, Chem. Soc. Rev., 2018, 47, 2065–2129 RSC.
- L. Wang, X. Fu, J. He, X. Shi, T. Chen, P. Chen, B. Wang and H. Peng, Adv. Mater., 2019, 32, 1901971 CrossRef PubMed.
- Y. Li, X. Yan, X. Zheng, H. Si, M. Li, Y. Liu, Y. Sun, Y. Jiang and Y. Zhang, J. Mater. Chem. A, 2016, 4, 17704–17710 RSC.
- Z. Pan, J. Yang, Q. Zhang, M. Liu, Y. Hu, Z. Kou, N. Liu, X. Yang, X. Ding, H. Chen, J. Li, K. Zhang, Y. Qiu, Q. Li, J. Wang and Y. Zhang, Adv. Energy Mater., 2019, 9, 1802753 CrossRef.
- W. Lyu, W. Zhang, H. Liu, Y. Liu, H. Zuo, C. Yan, C. F. J. Faul, A. Thomas, M. Zhu and Y. Liao, Chem. Mater., 2020, 32, 8276–8285 CrossRef.
- M. Khalid and H. Varela, J. Mater. Chem. A, 2018, 6, 3141–3150 RSC.
- J. Cao, Y. Zhao, Y. Xu, Y. Zhang, B. Zhang and H. Peng, J. Mater. Chem. A, 2018, 6, 3355–3360 RSC.
- X. Zhuang, F. Zhang, D. Wu and X. Feng, Adv. Mater., 2014, 26, 3081–3086 CrossRef CAS PubMed.
- Z. S. Wu, Y. Zheng, S. Zheng, S. Wang, C. Sun, K. Parvez, T. Ikeda, X. Bao, K. Müllen and X. Feng, Adv. Mater., 2016, 29, 1602960 CrossRef PubMed.
- L. Mei, X. Cui, J. Wei, Q. Duan and Y. Li, Dyes Pigm., 2021, 190, 109299 CrossRef CAS.
- R. K. Pavel, A. Troshin, A. S. Peregudov, S. M. Peregudova, M. Egginger, R. N. Lyubovskaya and N. Serdar Sariciftci, Chem. Mater., 2007, 19, 5363–5372 CrossRef.
- J. Mu, C. Shao, Z. Guo, M. Zhang, Z. Zhang, P. Zhang, B. Chen and Y. Liu, Nanoscale, 2011, 3, 5126–5131 RSC.
- Z. Cabrane, M. Ouassaid and M. Maaroufi, Int. J. Hydrogen Energy, 2016, 41, 20897–20907 CrossRef CAS.
- H. Ahn, Y. C. Huang, C. W. Lin, Y. L. Chiu, E. C. Lin, Y. Y. Lai and Y. H. Lee, ACS Appl. Mater. Interfaces, 2018, 10, 29145–29152 CrossRef CAS PubMed.
- K. I. O. Alfred and T. Chidembo, Electroanalysis, 2010, 22, 2529–2535 CrossRef.
- X. C. Lan Mei, Q. Duan, Y. Li, X. Lv and H. Wang, Int. J. Hydrogen Energy, 2020, 45, 22950–22958 CrossRef.
- A. Modak, M. Nandi, J. Mondal and A. Bhaumik, Chem. Commun., 2012, 48, 248–250 RSC.
- E. L. Spitler, J. W. Colson, F. J. Uribe-Romo, A. R. Woll, M. R. Giovino, A. Saldivar and W. R. Dichtel, Angew. Chem., Int. Ed., 2012, 51, 2623–2627 CrossRef CAS PubMed.
- L. Mei, J. C. Wei and Q. Duan, J. Mater. Sci.: Mater. Electron., 2021, 32, 24953–24963 CrossRef CAS.
- T. A. Gaber, L. R. Ahmed and A. F. M. El-Mahdy, J. Mater. Chem. A, 2023, 11, 19408–19417 RSC.
- M. Jang, Y. Cho, Y. Kim, M. Hahn, D. Jung, S. Y. Park, W. Lee and Y. Piao, Electrochim. Acta, 2022, 434, 141315 CrossRef CAS.
- C. D. X. Wang, S.-H. Lee, K. J. Senecal, J. Kumar and L. A. Samuelson, Nano Lett., 2002, 2, 1273–1275 CrossRef.
- Y. Wang, A. La, Y. Ding, Y. Liu and Y. Lei, Adv. Funct. Mater., 2012, 22, 3547–3555 CrossRef CAS.
- R. M. DuChanois, C. J. Porter, C. Violet, R. Verduzco and M. Elimelech, Adv. Mater., 2021, 33, 2101312 CrossRef CAS PubMed.
- Z. Zhou, D. B. Shinde, D. Guo, L. Cao, R. A. Nuaimi, Y. Zhang, L. R. Enakonda and Z. Lai, Adv. Funct. Mater., 2021, 32, 2108672 CrossRef.
- F. Ren, F. Wang, Y. Pan, H. Sun, Z. Zhu, C. Ma, C. Xiao, W. Liang, L. Chen and A. Li, Macromol. Mater. Eng., 2018, 303, 1700619 CrossRef.
- B. Prusti, S. Tripathi, P. K. Samanta and M. Chakravarty, Adv. Opt. Mater., 2024, 12, 2301746 CrossRef CAS.
- Z. Li, W. Wang, Y. Xu, Y. Zhu and X. Guo, J. CO2 Util., 2021, 49, 101550 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |