 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A highly sensitive self-powered photodetector based on pinhole-free PEA0.2FA0.8SnI3 films with aminopyrimidine†
Meiyue
Liu
 a,
Yuanchuang
Li
a,
Xiang
Yao
*b,
Shengjun
Li
a,
Yuanchuang
Li
a,
Xiang
Yao
*b,
Shengjun
Li
 a and
Hin-Lap
Yip
a and
Hin-Lap
Yip
 *cde
*cde
aKey Laboratory of Photovoltaic Materials, Henan University, Kaifeng, Henan 475004, China. E-mail: liumy@vip.henu.edu.cn
bInstitute of Molecular Plus and Tianjin Key Laboratory of Molecular Optoelectronic Science, Tianjin University, Tianjin 300072, China. E-mail: yaoxiang@tju.edu.cn
cDepartment of Materials Science and Engineering, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong
dSchool of Energy and Environment, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong
eHong Kong Institute for Clean Energy, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong. E-mail: a.yip@cityu.edu.hk
First published on 6th February 2024
Abstract
Tin-based perovskite halides have emerged as promising lead-free perovskite photodetectors. However, achieving high performance is hampered by poor stability and a high density of intrinsic defects. In this study, we developed an efficient tin-based perovskite photodetector that is sensitive to visible and near-infrared light through the incorporation of 2-aminopyrimidine as an additive. Our results demonstrated that the inclusion of 2-aminopyrimidine significantly improves film morphology, reduces roughness, passivates defect states, and suppresses the oxidation of Sn2+. The PEA0.2FA0.8SnI3 films with 2-aminopyrimidine exhibited a high responsivity of 0.4 A W−1 and a detectivity of 1.16 × 1012 Jones, a fast response time of 0.93 ms, and excellent operation stability after 104 on/off cycles. The findings highlight the significant potential of tin-based perovskite in the development of advanced photodetectors.
Introduction
Photodetectors (PDs) are capable of sensing optical signals over a wide range of wavelengths, including ultraviolet, visible light, and near-infrared (NIR) light. NIR detection is particularly important for various optical–electrical applications, such as medical imaging, biometric identification, and image sensing.1–3 However, current commercial NIR PDs based on inorganic compounds (silicon and III–V group materials) are costly and require high-temperature and vacuum processes.4,5 The development of new materials, such as conjugated molecules, two-dimensional materials, and halide perovskites, has led to the creation of lighter and lower-cost PDs.6–9 Organic–inorganic halide perovskites, in particular, are promising options as next-generation PDs due to their low cost, ease of fabrication, and excellent optoelectronic properties.10–18 Although Pb-based perovskite PDs have demonstrated high responsivity, detectivity, and fast response time,19–21 the toxicity of Pb limits their commercialization.22,23 Substitution of Pb with a less toxic alternative is therefore necessary to ensure commercial viability.Tin (Sn)-based perovskites have emerged as a possible solution that addresses the issue of toxicity in Pb-based perovskite PDs, offering the advantages of a narrow bandgap, high charge-carrier mobility, and low exciton binding energy.24–26 Sn-based perovskites facilitate optical detection ranging from visible to NIR.27,28 However, most of the previously reported Sn-based PDs are based on nanowire structures, all inorganic perovskites, or 2D perovskites with a device architecture of photoconductors or transistors.29–36 Unfortunately, these Sn-based PDs exhibit unsatisfactory response times, where FASnI3-based PDs have delivered a rise/fall response time of 8.7/50 s and BA2FASn2I7-based PDs showed a rise/fall response time of 5.78/0.96 s.27,36 Recently, Seong and co-workers reported PDs based on 2D PEA2SnI4 with a fast response speed of 0.56 ms, but the associated wavelength range was limited to 300–650 nm.37 In order to achieve faster response times, devices with short charge transport channels are needed, such as a vertical photodiode with a transparent electrode on one side.38,39 However, there are only a few reports of Sn-based photodiodes, and even fewer dealing with 3D Sn-based PDs.29,30,40,41 This is mainly due to the challenge of fabricating high quality and dense Sn-based perovskite thin films. Moreover, Sn-based perovskite thin films undergo Sn2+ oxidation to Sn4+ and rapid crystallization,42–45 making it critical to achieve high quality and dense active films with low defect density to realize Sn-based perovskite PDs. Additive engineering to passivate defects and suppress Sn2+ oxidation is an effective way to obtain high quality Sn-based perovskite films.46
In this study, we have fabricated Pb-free perovskite PDs using a PEA0.2FA0.8SnI3 thin film that incorporated 2-aminopyrimidine. The addition of 2-aminopyrimidine enabled controlled crystal growth, improved film morphology, passivated defect states, and prevented Sn2+ oxidation. The Sn-based PDs with 2-aminopyrimidine have exhibited a broad response range from 300 to 900 nm, a fast rise/fall response time of 0.93/1.74 ms, a high responsivity of 0.4 A W−1 and a detectivity of 1.16 × 1012 Jones, and a linear dynamic range (LDR) of 94.6 dB. Moreover, the PEA0.2FA0.8SnI3-based PDs with 2-aminopyrimidine exhibited excellent operational stability after 104 on/off cycles.
Results and discussion
Sn-based perovskite films were prepared by the antisolvent-assisted spin-coating of a PEA0.2FA0.8SnI3 precursor solution with varying amounts of 2-aminopyrimidine dissolved in a mixture of N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) solvents, following the methodology outlined in the Experimental section. The impact of 2-aminopyrimidine concentration on the crystallinity and optical properties of PEA0.2FA0.8SnI3 films was evaluated using X-ray diffraction (XRD) and absorption spectroscopy. The amount of 2-aminopyrimidine was adjusted from 0 to 2 mg to investigate the impact on the optoelectronic properties of PEA0.2FA0.8SnI3 films. The XRD patterns, shown in Fig. 1a, are consistent with the reported orthorhombic structure of the perovskite film, with peaks at 14.1°, 24.5°, 28.4°and 43.0° attributed to the (100), (102), (200) and (213) planes, respectively.47 The peaks located at 24.5° and 43.0° are too weak to be visible, and the enlarged patterns are shown in Fig. S1.† Increasing the amount of 2-aminopyrimidine (0–1 mg) led to an increase in the intensity of the (100) and (200) peaks indicating that the addition of 2-aminopyrimidine could regulate the crystalline process of the PEA0.2FA0.8SnI3 films. However, the intensity of the (100) plane decreased when the PEA0.2FA0.8SnI3 film was accompanied by 2 mg of 2-aminopyrimidine, suggesting that an excessive amount of additive hampers the growth of high-quality crystals in the PEA0.2FA0.8SnI3 film. The full width at half maximum (FWHM) of the (100) diffraction peak is presented as a function of 2-aminopyrimidine concentration in Fig. 1b. As the amount of 2-aminopyrimidine additive increased from 0 to 1.0 mg, the FWHM of the (100) peak decreased from 0.0824° to 0.0807°. However, it increased to 0.089° with the addition of 2 mg. The PEA0.2FA0.8SnI3 films treated with 1 mg 2-aminopyrimidine exhibited the smallest FWHM, indicating that the well-growth and high crystallinity of the PEA0.2FA0.8SnI3 film could be attributed to the optimized amount of 2-aminopyrimidine.The absorption spectra of the PEA0.2FA0.8SnI3 films with varying 2-aminopyrimidine concentrations are presented in Fig. 1c. All the films exhibited an absorption edge at approximately 900 nm, which remained unaffected by the increasing amount of 2-aminopyrimidine (inset of Fig. 1c). This suggests that the additive had no significant effect on the absorption properties of the film. Steady-state photoluminescence (PL) measurements were performed to evaluate the optical properties of the PEA0.2FA0.8SnI3 films as a function of 2-aminopyrimidine concentration. As shown in Fig. 1d, the films exhibited an emission peak at approximately 880 nm. The PEA0.2FA0.8SnI3 film with 1 mg 2-aminopyrimidine exhibited the highest PL intensity, suggesting that the additive effectively passivated defect states and reduced non-radiative recombination losses.
To investigate the effect of the amounts of 2-aminopyrimidine on the surface morphology and roughness of PEA0.2FA0.8SnI3 films, we employed scanning electron microscopy (SEM) and atomic force microscopy (AFM). In Fig. 2a, the PEA0.2FA0.8SnI3 film without 2-aminopyrimidine showed poor uniformity and numerous cracks, which can act as charge recombination centers and are detrimental to dark current. Furthermore, the pristine film exhibited a rough surface with a high roughness value of 31 nm (Fig. S2†). However, as the amount of 2-aminopyrimidine was increased from 0 to 1 mg (Fig. 2b and c), the film became denser and smoother. A more uniform morphology is crucial for efficient charge transport during photodetection. Additionally, the PEA0.2FA0.8SnI3 film with 1 mg 2-aminopyrimidine displayed the lowest roughness value (13.2 nm). On the other hand, increasing the additive to 2 mg resulted in the appearance of numerous pinholes at the grain boundaries, leading to an increase in the roughness value (32 nm) (Fig. 2d and S2†). These results indicate that the use of excessive additives has a negative effect on the crystallization and leads to poor surface coverage. Therefore, adding 1 mg of 2-aminopyrimidine was found to be optimal, and the subsequent discussion will focus on the PEA0.2FA0.8SnI3 film with 1 mg 2-aminopyrimidine and the pristine PEA0.2FA0.8SnI3 film.
 | ||
| Fig. 2 Representative SEM images of PEA0.2FA0.8SnI3 films with (a) 0 mg, (b) 0.5 mg, (c) 1 mg and (d) 2 mg 2-aminopyrimidine. | ||
To explore the chemical interaction between the 2-aminopyrimidine additive and the PEA0.2FA0.8SnI3 film, Fourier transform infrared (FTIR) spectroscopy was conducted. As shown in Fig. S3,† the FTIR spectra of neat 2-aminopyrimidine and the perovskite with 1 mg of 2-aminopyrimidine were compared. In neat 2-aminopyrimidine, the peak corresponding to the N–H bond in 2-aminopyrimidine was observed at 3400.2 cm−1. However, in the perovskite with 2-aminopyrimidine, the peak corresponding to the N–H bond shifted to 3384.9 cm−1, indicating an interaction between 2-aminopyrimidine and the perovskite. Furthermore, X-ray photoelectron spectroscopy (XPS) measurements were performed to examine the valence state of Sn and to assess the effect of the additive on the stability of the Sn-based perovskite. The Sn 3d core-level XPS spectra of the PEA0.2FA0.8SnI3 films without and with 2-aminopyrimidine are presented in Fig. 3a and b, respectively. The peaks at 486.1 and 494.6 eV correspond to the energy levels of Sn 3d5/2 and 3d3/2 for Sn2+, while the peaks at 486.8 and 495.0 eV correspond to Sn4+.48 Fitting analysis has revealed that the peak composition associated with Sn4+ decreased from 36.2 to 26.1% area following the introduction of 2-aminopyrimidine, indicating an effective inhibition of Sn2+ oxidation. Therefore, the introduction of the 2-aminopyrimidine additive can significantly enhance film quality through the N–H bond and improve stability by suppressing the oxidation of Sn2+ to Sn4+.
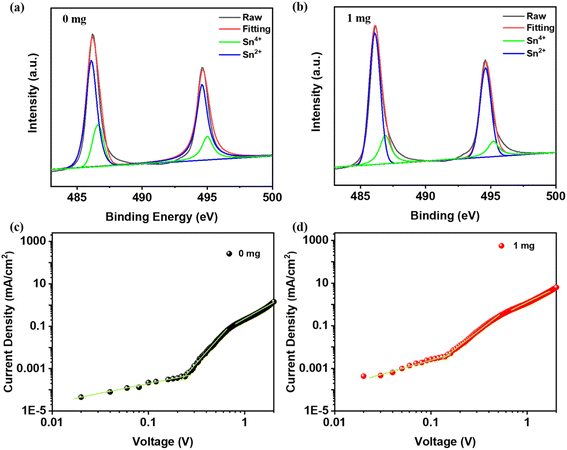 | ||
| Fig. 3 (a and b) XPS spectra, and (c and d) J–V curves for the electron-only devices based on the PEA0.2FA0.8SnI3 films without and with 1 mg 2-aminopyrimidine. | ||
To validate the possible effect of 2-aminopyrimidine in reducing trap densities in PEA0.2FA0.8SnI3 films, we fabricated electron-only devices with the structure of ITO/SnO2/PEA0.2FA0.8SnI3/PCBM/Ag. The corresponding current–voltage curves are plotted in Fig. 3c and d using the space charge limited current (SCLC) method. The trap density (Nt) can be calculated using the equation, Nt = 2ε0εrVTFL/eL2, where εr represents the relative dielectric constant, ε0 is the vacuum permittivity, VTFL denotes the trap-filled voltage, e is the elementary charge, and L is the thickness of the PEA0.2FA0.8SnI3 layer.49 The VTFL value of PEA0.2FA0.8SnI3-based devices with and without 2-aminopyrimidine was 0.24 and 0.16 V, respectively. The incorporation of 2-aminopyrimidine resulted in a lower trap density (5.8 × 1015 cm−3) compared to the film without 2-aminopyrimidine (3.9 × 1015 cm−3), confirming that the additive can effectively reduce trap densities. This reduction in trap density can be attributed to the improved film quality and suppressed Sn2+ oxidation, leading to a decrease in dark current and an enhancement in PL intensity, which are beneficial features for high-performance photodetectors.
The schematic diagram in Fig. 4a illustrates the structural arrangement of the PEA0.2FA0.8SnI3-based PDs. The PDs were fabricated with the structure of ITO/poly(3,4-ethylene dioxythiophene):polystyrene sulfonate (PEDOT:PSS)/PEA0.2FA0.8SnI3/[6,6]-phenyl C61-butyric acid methyl ester (PCBM)/Bathocuproine (BCP)/Ag. The effect of the 2-aminopyrimidine additive on the performance of the PDs was evaluated using current density–voltage (J–V) curves in the dark and under light illumination. The J–V curves of the PEA0.2FA0.8SnI3-based perovskite PDs with and without 2-aminopyrimidine are shown in Fig. 4b. The current density of the PEA0.2FA0.8SnI3-based PDs with 1 mg 2-aminopyrimidine was 3.69 × 10−7 A cm−2 at −0.1 V, lower than that of the PDs without 2-aminopyrimidine (4.6 × 10−6 A cm−2 at −0.1 V). The reduction in dark current can be attributed to the improved uniformity of the films and the lower trap densities resulting from the introduction of 2-aminopyrimidine. The dense and smooth morphology with enhanced crystallinity led to an increase in the photocurrent density for the PEA0.2FA0.8SnI3-based PDs containing 2-aminopyrimidine, establishing efficient carrier transport in the device.
The external quantum efficiency (EQE) spectra of PDs with varying concentrations of 2-aminopyrimidine were measured, where all PDs exhibited a broadband light response from 300 to 900 nm (Fig. S4†). Notably, the PD with 1 mg 2-aminopyrimidine achieved a high EQE of over 60% at 400–810 nm (Fig. 4c), demonstrating high charge-carrier collection efficiency. Responsivity (R) is a key parameter for evaluating the performance of a PD, representing the response efficiency of the PD to the optical signal. The value of R can be calculated from the equation, R = EQE × e/hν, where e represents the elementary charge, h is Planck's constant, and ν denotes the frequency of the optical signal.50 The dependence of responsivity on wavelength at zero voltage is presented in Fig. 4d. The PEA0.2FA0.8SnI3-based PD with 1 mg 2-aminopyrimidine generated higher R values over the 300–900 nm range. The maximum R value was 0.4 A W−1 at 810 nm, demonstrating the suitability of the PEA0.2FA0.8SnI3-based PDs for broadband detection from visible to NIR. The enhanced photoresponse performance of the PEA0.2FA0.8SnI3-based PDs incorporating 1 mg of 2-aminopyrimidine was superior to that of the PDs without 2-aminopyrimidine (Table 1).
| J d (A cm−2) | R (A W−1) | D* (Jones) | T rise/fall (ms) | LDR (dB) | |
|---|---|---|---|---|---|
| Control | 4.60 × 10−6 | 0.35 | 2.90 × 1011 | 0.99/1.78 | 71.4 |
| 2-Aminopyrimidine | 3.69 × 10−7 | 0.40 | 1.16 × 1012 | 0.93/1.74 | 94.6 |
The specific detectivity (D*) is a figure of merit used to quantify the capability of a photodetector to measure weak optical signals. The D* can be calculated using the formula, D* = R/(2qeJd)1/2, where Jd represents the dark current density. The detectivity profiles with respect to spectral wavelength are shown in Fig. 4e. The D* values for the PEA0.2FA0.8SnI3-based PDs with 1 mg of 2-aminopyrimidine were appreciably higher, with a maximum detectivity of 1.16 × 1012 Jones at 810 nm under a bias voltage of −0.1 V, four times higher than that of the pristine PEA0.2FA0.8SnI3-based PDs (2.9 × 1011 Jones). The results indicate that the lower trap density, which suppressed carrier recombination, resulted in the highest R and D* associated with the PDs containing 1 mg 2-aminopyrimidine. The range of D* values reported for Sn-based PDs with different structures and active layers is summarized in Fig. 4f and Table 2. Previously reported Sn-based perovskite PDs with high D* mostly employed two-dimensional or all-inorganic perovskites as photoactive layers, which resulted in a narrow device detection range. It should be noted that our fabricated PEA0.2FA0.8SnI3-based PDs delivered the highest D* when compared with previously reported Sn-based PDs based on a photodiode device architecture.
| Materials | D* (Jones) | R (A W−1) | Response time | Structure type | Ref. |
|---|---|---|---|---|---|
| FASnI3 | 1.9 × 1012 | 1.1 × 105 @ 0.5 V | 8.7/57 s | Photoconductor | 27 |
| Cs2SnBr6 | 2.71 × 1010 | 9.52/4.34 ms | Photoconductor | 31 | |
| Cs2SnI6 | 100/100 ms | Photoconductor | 32 | ||
| (PEA)2SnI4 | 1.92 × 1011 | 16 | 0.63/3.6 s | Photoconductor | 33 |
| BA2SnI4 | 1.46 × 1013 | 2.65 @ 4 V | 2.62/0.3 s | Photoconductor | 34 |
| BA2FASn2I7 | 6.3 × 1012 | 1.55 @ 4 V | 5.78/0.96 s | Photoconductor | 34 |
| CsSnBr3 | 4.27 × 1011 | 0.0623 @ 3 V | 50/51 ms | Photoconductor | 35 |
| (PEA)2SnI4 | 2.06 × 1011 | 3.29 × 103 | 0.37/3.05 s | Photoconductor | 36 |
| PEA2SnI4 | 6.32 × 1013 | 509 | 0.56/10.7 ms | Photoconductor | 37 |
| (PEA)2SnI4 | 8.09 × 109 | 0.121 | 34/38 ms | Photoconductor | 42 |
| MA0.7FA0.3SnBr3 | 6.7 × 1010 | 45/420 ms | Photoconductor | 44 | |
| (PEA)2SnI4 | 1.74 × 1012 | 14.57 @ 10 V | 50 ms/1.5 s | Photo transistor | 43 |
| MASnI3 | 8.8 × 1010 | 0.47 | 1500/400 ms | Photodiode | 29 |
| Nanowire array | |||||
| CsSnI3 | 1.5 × 1011 | 0.257 | 0.35/1.6 ms | Photodiode | 30 |
| PEA0.15FA0.85SnI3 | 8.29 × 1011 | 0.39 | 0.78/1.31 μs | Photodiode | 40 |
| FA0.8PEA0.2SnI3 | 2.3 × 1011 | 0.262 | 27.7/20.4 μs | Photodiode | 41 |
| PEA0.2FA0.8SnI3 | 1.16 × 1012 | 0.4 | 0.93/1.74 ms | Photodiode | This work |
The linear dynamic range (LDR) is a crucial parameter that characterizes the range of linear dependence on light intensity and is particularly significant in practical photodetection applications, specifically in imaging. It can be quantified using the formula, LDR = 20 log(Imax/Imin), where Imax represents the highest light intensity and Imin indicates the lowest light intensity within the linear range. As illustrated in Fig. 5a, the Imin of the PEA0.2FA0.8SnI3-based PDs with 1 mg 2-aminopyrimidine is one order of magnitude lower than that of the reference PDs (Fig. 5b). Both the reference and Sn-based PEA0.2FA0.8SnI3 PDs with 1 mg 2-aminopyrimidine exhibited comparable upper limits. The LDR of the PEA0.2FA0.8SnI3-based PDs with 1 mg 2-aminopyrimidine was 94.6 dB, significantly larger than the LDR of the PD without 2-aminopyrimidine (71.4 dB). This finding demonstrates that the addition of 2-aminopyrimidine can promote more effective charge separation and collection, resulting in an enhanced response and detection over a wide range of light intensities, ranging from weak to intense.
The response time of PEA0.2FA0.8SnI3-based PDs with 1 mg of 2-aminopyrimidine is shown in Fig. 5c, measured using an adjustable light source with current amplification. The rise and fall times were defined as the time required for the photocurrent to reach 10% and 90% of the maximum value, respectively. The PDs with 1 mg 2-aminopyrimidine exhibited a fast response speed, with a rise/fall time of 0.93/1.74 ms at 533 nm light illumination, appreciably faster than the pristine PDs (Fig. S5†). The fast response time of the PDs with 2-aminopyrimidine indicates that this additive effectively suppressed carrier recombination and minimized the dark current. Fig. 5d compares the response times of PDs using different Sn-based compounds, and it is evident that the PDs developed in this study showed a faster response time than other Sn-based perovskite PDs. Furthermore, when comparing response in terms of device structure (photodiode or photoconductor), our study delivers a similar response speed to the fastest response time Sn-based PDs reported previously. Additionally, the stability of the PEA0.2FA0.8SnI3-based PDs with 1 mg 2-aminopyrimidine was superior to that of pristine PDs. Fig. 5 shows the device stability under green light with a pulse width of 50 Hz. The photocurrent did not exhibit any appreciable degradation after 104 on/off cycles, demonstrating excellent operation stability and repeatability. The stability was further illustrated by the enlarged I–t curve during testing in air that is presented in Fig. 5f. In contrast, the PEA0.2FA0.8SnI3-based PD without 2-aminopyrimidine displayed noticeable degradation, as shown in Fig. S6,† indicating that the addition of 2-aminopyrimidine effectively improved the stability of Sn-based PDs.
Conclusions
In summary, we developed lead-free and environmentally friendly PEA0.2FA0.8SnI3-based PDs by incorporating 2-aminopyrimidine as an additive. The addition of 2-aminopyrimidine not only regulated crystal quality, improved surface morphology, and decreased roughness of the PEA0.2FA0.8SnI3 thin film, but also suppressed oxidation of Sn2+ and passivated defects. As a direct consequence, devices based on PDs with the 2-aminopyrimidine additive exhibited a lower defect density and dark current. The PEA0.2FA0.8SnI3-based PDs achieved a high responsivity (0.4 A W−1) and detectivity (1.16 × 1012 Jones) and fast response speed (0.93 ms). Additionally, the unencapsulated PDs exhibited excellent operation stability and repeatability after 104 on/off cycles. Our study demonstrates that the use of 2-aminopyrimidine as an additive is an effective means of producing high-performance Sn-based perovskite PDs.Experimental section
Materials
Tin iodide (SnI2, 99.999%), tin fluoride (SnF2, 99.8%), dimethyl sulfoxide (DMSO, 99.8%), N,N-dimethylformamide (DMF, 99.8%), chlorobenzene (CB, 99.8%), and bathocuproine (BCP, 99.5%) were purchased from Sigma-Aldrich. Phenyl-ethylammonium iodide (PEAI, 99.5%) and formamidinium iodide (FAI, 99.5%) were purchased from Xi'an P-OLED Co. All materials and reagents were used as received without further purification.Fabrication of perovskite photodetectors
The glass substrate with ITO was ultrasonically cleaned using acetone, ethyl alcohol, and deionized water and then treated with UV-ozone. The PEDOT:PSS (Clevios P VP Al 4083) layer was spin-coated onto the substrates at 4000 rpm for 30 s, followed by thermal annealing at 150 °C for 15 min in air. The Sn-based perovskite precursors were prepared by dissolving FAI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEAI
PEAI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnI2
SnI2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnF2 with a molar ratio of 0.8
SnF2 with a molar ratio of 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 in a 1 mL solution of DMF and DMSO (4
0.1 in a 1 mL solution of DMF and DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume). In the case of the perovskite films with additives, a known amount of 2-aminopyridine was added to the precursor solution. In the fabrication of the Sn-based perovskite film, the precursor solution was filtered using a 0.22 μm PTFE filter and subjected to spin-coating at 1000 rpm for 10 s and then at 5000 rpm for 30 s. The antisolvent (CB) was dripped for 12 s in a high speed step. Then, the film was annealed at 70 °C for 10 min. PCBM (20 mg mL−1 in CB) was spun on the perovskite film at 1000 rpm for 20 s, followed by BCP (0.5 mg mL−1 in isopropyl alcohol) which was spun at 3000 rpm for 30 s. Finally, 100 nm thick Ag electrodes were deposited by thermal evaporation under a pressure of 5 × 10−4 Pa.
1 volume). In the case of the perovskite films with additives, a known amount of 2-aminopyridine was added to the precursor solution. In the fabrication of the Sn-based perovskite film, the precursor solution was filtered using a 0.22 μm PTFE filter and subjected to spin-coating at 1000 rpm for 10 s and then at 5000 rpm for 30 s. The antisolvent (CB) was dripped for 12 s in a high speed step. Then, the film was annealed at 70 °C for 10 min. PCBM (20 mg mL−1 in CB) was spun on the perovskite film at 1000 rpm for 20 s, followed by BCP (0.5 mg mL−1 in isopropyl alcohol) which was spun at 3000 rpm for 30 s. Finally, 100 nm thick Ag electrodes were deposited by thermal evaporation under a pressure of 5 × 10−4 Pa.
Film characterization
SEM images were obtained using a JSM-7001F scanning electron microscope. Sample roughness was determined with a Bruker Multimode 8 instrument. XRD analysis was conducted using Cu Kα radiation (Bruker D8 Advance). UV-visible absorption spectroscopy was performed using a UH4150 spectrophotometer. The PL spectra were measured using a fluorescence spectrometer (PerkinElmer LS 55), and XPS spectra were collected using a K-ALPHA+ XPS.Device characterization
Current density as a function of voltage was recorded using a Keithley 2400 instrument under dark conditions and simulated AM1.5G sunlight generated using a solar simulator (SS-F5-3A, Enli Technology Co., Ltd). The light intensity was calibrated using a certified reference silicon cell. EQE measurements were conducted with a QE tester (Enlitech, QE-R 3018). A linear dynamic range test was performed at different white light intensities using ThorLabs metallic-coated neutral density filters. The on/off PD response was measured using a galvanostatic instrument (Metrohm Autolab PGSTAT204) with a light emitting diode (Ocean Optics (LSM)) at a wavelength of 533 nm. The LED pulse was generated using an LED controller (Ocean Optics, LDC-1).Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that influenced the work reported in this paper.Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (22205158) for financial support. H.-L. Yip thanks the Innovation Technology Fund (MRP/040/21X) and the Green Technology Fund (GTF202020164) for their financial support.References
- F. Teng, K. Hu, W. Ouyang and X. Fang, Adv. Mater., 2018, 30, 1706262 CrossRef PubMed.
- S. Chen, C. Teng, M. Zhang, Y. Li, D. Xie and G. Shi, Adv. Mater., 2016, 28, 5969–5974 CrossRef CAS PubMed.
- A. Armin, R. D. Jansen-van Vuuren, N. Kopidakis, P. L. Burn and P. Meredith, Nat. Commun., 2015, 6, 6343 CrossRef CAS PubMed.
- H. Chen, H. Liu, Z. Zhang, K. Hu and X. Fang, Adv. Mater., 2016, 28, 403–433 CrossRef CAS PubMed.
- F. P. García de Arquer, A. Armin, P. Meredith and E. H. Sargent, Nat. Rev. Mater., 2017, 2, 1 Search PubMed.
- P. C. Y. Chow and T. Someya, Adv. Mater., 2020, 32, 1902045 CrossRef CAS PubMed.
- H. Qiao, Z. Huang, X. Ren, S. Liu, Y. Zhang, X. Qi and H. Zhang, Adv. Opt. Mater., 2020, 8, 1900765 CrossRef CAS.
- C. Xie, C.-K. Liu, H.-L. Loi and F. Yan, Adv. Funct. Mater., 2020, 30, 1903907 CrossRef CAS.
- P. Bhatnagar, M. Patel, K. Lee and J. Kim, InfoMat, 2023, 5, e12408 CrossRef.
- Q. Jiang, J. Tong, R. A. Scheidt, X. Wang, A. E. Louks, Y. Xian, R. Tirawat, A. F. Palmstrom, M. P. Hautzinger, S. P. Harvey, S. Johnston, L. T. Schelhas, B. W. Larson, E. L. Warren, M. C. Beard, J. J. Berry, Y. Yan and K. Zhu, Science, 2022, 378, 1295–1300 CrossRef CAS PubMed.
- C. Li, X. Wang, E. Bi, F. Jiang, S. M. Park, Y. Li, L. Chen, Z. Wang, L. Zeng, H. Chen, Y. Liu, C. R. Grice, A. Abudulimu, J. Chung, Y. Xian, T. Zhu, H. Lai, B. Chen, R. J. Ellingson, F. Fu, D. S. Ginger, Z. Song, E. H. Sargent and Y. Yan, Science, 2023, 379, 690–694 CrossRef CAS PubMed.
- Y. Jiang, C. Sun, J. Xu, S. Li, M. Cui, X. Fu, Y. Liu, Y. Liu, H. Wan, K. Wei, T. Zhou, W. Zhang, Y. Yang, J. Yang, C. Qin, S. Gao, J. Pan, Y. Liu, S. Hoogland, E. H. Sargent, J. Chen and M. Yuan, Nature, 2022, 612, 679–684 CrossRef CAS PubMed.
- Z. Chu, Q. Ye, Y. Zhao, F. Ma, Z. Yin, X. Zhang and J. You, Adv. Mater., 2021, 33, 2007169 CrossRef CAS PubMed.
- Y. Fang, Q. Dong, Y. Shao, Y. Yuan and J. Huang, Nat. Photonics, 2015, 9, 679–686 CrossRef CAS.
- L. Li, H. Chen, Z. Fang, X. Meng, C. Zuo, M. Lv, Y. Tian, Y. Fang, Z. Xiao, C. Shan, Z. Xiao, Z. Jin, G. Shen, L. Shen and L. Ding, Adv. Mater., 2020, 32, 1907257 CrossRef CAS PubMed.
- C. Chen, Y. Zhu, D. Gao, M. Li, Z. Zhang, H. Chen, Y. Feng, C. Wang, J. Sun, J. Chen, H. Tian, L. Ding and C. Chen, Small, 2023, 19, 2303200 CrossRef CAS PubMed.
- Y. Zhou, C. Fei, M. A. Uddin, L. Zhao, Z. Ni and J. Huang, Nature, 2023, 616, 712–718 CrossRef CAS PubMed.
- H. Liu, H. L. Zhu, Z. Wang, X. Wu, Z. Huang, M. R. Huqe, J. A. Zapien, X. Lu and W. C. H. Choy, Adv. Funct. Mater., 2021, 31, 2010532 CrossRef CAS.
- Y. Fang and J. Huang, Adv. Mater., 2015, 27, 2804–2810 CrossRef CAS PubMed.
- J. Wang, S. Xiao, W. Qian, K. Zhang, J. Yu, X. Xu, G. Wang, S. Zheng and S. Yang, Adv. Mater., 2021, 33, 2005557 CrossRef CAS.
- Q. Song, Y. Wang, F. Vogelbacher, Y. Zhan, D. Zhu, Y. Lan, W. Fang, Z. Zhang, L. Jiang, Y. Song and M. Li, Adv. Energy Mater., 2021, 11, 2100742 CrossRef CAS.
- A. Abate, Joule, 2017, 1, 659 CrossRef CAS.
- X. Li, F. Zhang, H. He, J. J. Berry, K. Zhu and T. Xu, Nature, 2020, 578, 555–558 CrossRef CAS PubMed.
- J. Cao and F. Yan, Energy Environ. Sci., 2021, 14, 1286–1325 RSC.
- Y. Zhang, Y. Ma, Y. Wang, X. Zhang, C. Zuo, L. Shen and L. Ding, Adv. Mater., 2021, 33, 2006691 CrossRef CAS PubMed.
- M. M. Byranvand, W. Zuo, R. Imani, M. Pazoki and M. Saliba, Chem. Sci., 2022, 13, 6766–6781 RSC.
- C.-K. Liu, Q. Tai, N. Wang, G. Tang, H.-L. Loi and F. Yan, Adv. Sci., 2019, 6, 1900751 CrossRef PubMed.
- M. Han, J. Sun, M. Peng, N. Han, Z. Chen, D. Liu, Y. Guo, S. Zhao, C. Shan, T. Xu, X. Hao, W. Hu and Z. Yang, J. Phys. Chem. C, 2019, 123, 17566–17573 CrossRef CAS.
- A. Waleed, M. M. Tavakoli, L. Gu, Z. Wang, D. Zhang, A. Manikandan, Q. Zhang, R. Zhang, Y.-L. Chueh and Z. Fan, Nano Lett., 2017, 17, 523–530 CrossRef CAS.
- F. Cao, W. Tian, M. Wang, M. Wang and L. Li, InfoMat, 2020, 2, 577–584 CrossRef CAS.
- J. Zhou, J. Luo, X. Rong, P. Wei, M. S. Molokeev, Y. Huang, J. Zhao, Q. Liu, X. Zhang, J. Tang and Z. Xia, Adv. Opt. Mater., 2019, 7, 1900139 CrossRef.
- X. Han, J. Liang, J.-H. Yang, K. Soni, Q. Fang, W. Wang, J. Zhang, S. Jia, A. A. Martí, Y. Zhao and J. Lou, Small, 2019, 15, 1901650 CrossRef PubMed.
- L. Qian, Y. Sun, M. Wu, C. Li, D. Xie, L. Ding and G. Shi, Nanoscale, 2018, 10, 6837–6843 RSC.
- Y. Yang, H. Zhang, S. Hou, T. Wang, W. Chen, S. Xian, Z. Zhang and Y. Mao, Appl. Phys. Lett., 2021, 119, 161106 CrossRef CAS.
- X. Ma, Y. Xu, S. Li, T. W. Lo, B. Zhang, A. L. Rogach and D. Lei, Nano Lett., 2021, 21, 9195–9202 CrossRef CAS PubMed.
- L. Qian, Y. Sun, M. Sun, Z. Fang, L. Li, D. Xie, C. Li and L. Ding, J. Mater. Chem. C, 2019, 7, 5353–5358 RSC.
- K.-R. Yun, T.-J. Lee, S.-K. Kim, J.-H. Kim and T.-Y. Seong, Adv. Opt. Mater., 2023, 11, 2201974 CrossRef CAS.
- M. Ahmadi, T. Wu and B. Hu, Adv. Mater., 2017, 29, 1605242 CrossRef PubMed.
- W. Jang, B. G. Kim, S. Seo, A. Shawky, M. S. Kim, K. Kim, B. Mikladal, E. I. Kauppinen, S. Maruyama and I. Jeon, Nano Today, 2021, 37, 101081 CrossRef CAS.
- W. Jang, K. Kim, B. G. Kim, J.-S. Nam, I. Jeon and D. H. Wang, Adv. Funct. Mater., 2022, 32, 2207713 CrossRef CAS.
- M. He, Z. Xu, C. Zhao, Y. Gao, K. Ke, N. Liu, X. Yao, F. Kang, Y. Shen, L. Lin and G. Wei, Adv. Funct. Mater., 2023, 33, 2300282 CrossRef CAS.
- C. Fang, H. Wang, Z. Shen, H. Shen, S. Wang, J. Ma, J. Wang, H. Luo and D. Li, ACS Appl. Mater. Interfaces, 2019, 11, 8419–8427 CrossRef CAS PubMed.
- H. Wang, Y. Chen, E. Lim, X. Wang, S. Yuan, X. Zhang, H. Lu, J. Wang, G. Wu, T. Lin, S. Sun, J. Wang, Y. Zhan, H. Shen, X. Meng and J. Chu, J. Mater. Chem. C, 2018, 6, 12714–12720 RSC.
- Y. Zhu, G. Lai, J. Yi, T. Zhang, Y. Zhang, S. Wen and H. Zhou, J. Alloys Compd., 2021, 866, 158990 CrossRef CAS.
- J. Liu, H. Yao, S. Wang, C. Wu, L. Ding and F. Hao, Adv. Energy Mater., 2023, 13, 2300696 CrossRef CAS.
- H. Zhang, L. Pfeifer, S. M. Zakeeruddin, J. Chu and M. Grätzel, Nat. Rev. Chem, 2023, 7, 632–652 CrossRef CAS PubMed.
- B.-B. Yu, Z. Chen, Y. Zhu, Y. Wang, B. Han, G. Chen, X. Zhang, Z. Du and Z. He, Adv. Mater., 2021, 33, 2102055 CrossRef CAS PubMed.
- T.-B. Song, T. Yokoyama, C. C. Stoumpos, J. Logsdon, D. H. Cao, M. R. Wasielewski, S. Aramaki and M. G. Kanatzidis, J. Am. Chem. Soc., 2017, 139, 836–842 CrossRef CAS PubMed.
- M. Liu, Z. Chen, Y. Yang, H.-L. Yip and Y. Cao, J. Mater. Chem. A, 2019, 7, 17324–17333 RSC.
- L. Dou, Y. Yang, J. You, Z. Hong, W.-H. Chang, G. Li and Y. Yang, Nat. Commun., 2014, 5, 5404 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ta07499d |
| This journal is © The Royal Society of Chemistry 2024 |



