Open Access Article
Open Access ArticleUtilization of Musa acuminata blossom peel waste mediated heterogeneous catalyst for biodiesel production from sesame oil†
Manoranjan
Sarkar
a,
Jennifer
Daimari
b,
Sunshri
Basumatary
b,
Kushwaha Jashvant
Kumar
b,
Ranjay
Das
c and
Anamika Kalita
Deka
 *b
*b
aDepartment of Electrical and Mechanical Engineering, Central Institute of Technology Kokrajhar (Deemed to be University, under MoE, Govt. of India), Kokrajhar, Assam 783370, India
bDepartment of Chemistry, Central Institute of Technology Kokrajhar (Deemed to be University, under MoE, Govt. of India), Kokrajhar, Assam 783370, India. E-mail: a.kalita@cit.ac.in
cDepartment of Electrical Engineering, Central Institute of Technology Kokrajhar (Deemed to be University, under MoE, Govt. of India), Kokrajhar, Assam 783370, India
First published on 16th May 2024
Abstract
The peel waste of the Musa acuminata blossom after fruit maturation was used for the preparation of a heterogeneous catalyst. The prepared catalyst was utilised for the transesterification of sesame oil (oil from Sesamum indicum L.) to biodiesel. The catalyst was calcined at 550 °C for 2 h and then characterized by Fourier transform infrared spectrometry (FTIR), powder X-ray diffractometry (XRD), field emission scanning electron microscopy (FESEM), and energy dispersive X-ray spectroscopy (EDX). The EDX studies showed that the peels of Musa acuminata blossom ash had a high potassium content with a good yield of 97% biodiesel efficiency with 9 wt% catalyst load. The biodiesel was characterized by FTIR, and gas chromatography-mass spectroscopy (GC-MS), nuclear magnetic resonance spectroscopy (NMR) analytical methods. The fuel properties were also evaluated and compared with to the International Standards of ASTM and EN.
Sustainability SpotlightIn the present study, peels of banana blossom were used for heterogenous catalyst preparation. The prepared heterogenous catalyst was utilized for the conversion of sesame oil to biodiesel. The present study fulfills the US Sustainability Development Goal 7, for Affordable and Clean Energy. We used the thrown away parts of banana blossom (peels) for the catalyst preparation, which is very cheap and affordable. |
Introduction
In the recent decades, the use of energy and fossil fuel consumption has increased; and simultaneously an increase of CO2 concentration, has led to global warming.1,2 Replacement of fossil fuel with renewable sources is now adapted worldwide. Because of their non-toxicity, economic friendliness and their biodegradable nature, the transesterification of oils (edible and non-edible) with both homogenous/heterogeneous catalysts is of great demand as potential renewable fuel.However, due to their low cost, good catalytic activity, and faster reaction rates, homogenous catalysts were generally used in biodiesel production but because of some disadvantages like the possibility of the presence of water in the product, difficulty in separation of the water, catalyst recycle and reuse and so on, heterogeneous catalysts have replaced them nowadays in the renewable energy sector. Because of its environmentally friendly behaviour, easy separation, and more stability at high temperatures; the heterogeneous catalyst was more efficient.3–6 In addition to this, the heterogeneous catalyst prepared from various agriculture wastes namely, vegetable peels, fruits peels, plants and so on, are far cheaper than the conventional homogenous catalyst. An ash based heterogenous catalyst, for example pineapple leaf etc., have a very high catalytic activity and good recyclable behaviour during the transesterification of oils to biodiesel. This is because of the presence of both alkali, alkaline earth metals and metal oxides in the ash-based heterogeneous catalysts.7–9
Although, an ash-based heterogeneous catalyst from banana plants has been reported from time to time by researchers,10 in the present communication we considered using the peels of the Musa acuminata blossom which remained after the fruit maturation for the preparation of a catalyst and used in the transesterification of sesame oil, was not reported until now. Musa acuminata is a species of banana, mostly available in the Indian subcontinent. In some parts of India, the blossom of Musa acuminata that remained after the fruit maturation are used for making curry, spices and so on. During, this process the peels of the Musa acuminata blossom are thrown away. Herein, we utilised the parts of the thrown away peels for the production of the heterogeneous catalyst.
Experimental
Preparation of catalyst
Approximately 10 g of banana blossom peels (Musa acuminata) were gathered carefully and washed with double-distilled water (500 mL). The water rinsed peels were sun-dried (6.9 g) for 2–3 days and then pulverised to a fine powder with an electric mixer grinder. Thereafter, the powdered banana blossom peels were calcined in a muffle furnace for 2 h at 550 °C. The dark pinkish coloured catalyst was obtained after calcination (6.80 g), and the catalyst was coded as the BBP-catalyst.Transesterification reaction
Three transesterification reactions were carried out under various reaction conditions. The catalyst and sesame oil were combined with methanol in a round-bottomed flask at various temperatures.The first transesterification reaction, was done with a constant amount of methanol (9 mL), at a temperature of 60 °C, and sesame oil content (1 mL) with various amounts of the BBP catalyst (0.031–0.12 g, entries 1–4, Table S2†).
The second transesterification reaction was done with a constant catalyst content (0.09 g), a temperature of 60 °C, sesame oil (1 mL) various amounts methanol (3–12 mL, entries 1–4, Table S3†).
Similarly, the third reaction was carried out with a constant catalyst content (0.09 g), methanol (9 mL), sesame oil (1 mL) at various temperatures (55 °C −70 °C, entries 1–4, Table S4†).
Using a separating funnel, the biodiesel was separated from the glycerol layer once the transesterification reaction had finished, and a maximum yield of 97% was obtained. The extra methanol was then distilled under a vacuum. The catalyst was separated in a separating funnel after collection of the biodiesel and the glycerol layer. By further passing the catalyst over Na2SO4 in a funnel it was dried for future use (about 0.086 g, entry 3 in Tables S2–S4†).
Instrumentation
The FTIR spectrophotometer (Shimadzu IR Affinity-1S Fourier transform infra-red spectrometer, Central Institute of Technology, Kokrajhar, Assam, India), the FESEM (Jeol, JSM-6390 LV, Saif, Tezpur University, Napam, Tezpur, Assam, India) and the X-ray diffraction method (Bruker D8 Advance, IASST, Assam, India), were employed for characterisation of the BBP-catalyst. The biodiesel was characterised by GC-MS (PerkinElmer Clarus 680 GC/600 MS, Guwahati Biotech Park, Guwahati, Assam, India) with helium as the carrier gas at 8 mL min−1 flow rate during the analysis. Nuclear magnetic resonance spectrophotometry (Bruker 400 MHz, SAIF, IISC Bangalore, India) was also used for the characterisation of biodiesel.Results and discussion
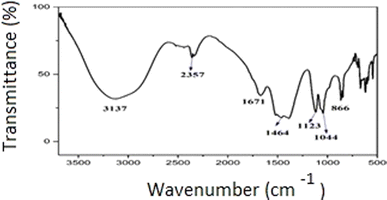
The IR analysis was carried out to identify the functional groups present in the BBP-catalyst. The broad peak at 3137 cm−1 in the spectrum represents the –OH group, which may be due to the adsorbed water molecules on the surface of the catalyst. In Fig. 1, the FTIR peaks at 1671 and 1464 cm−1 denoted the stretching frequencies of C–O bond due to the presence of carbonates, which were attributing to the existence of metal carbonates in the catalyst. The C–O bending frequency of carbonate in the catalyst is represented by the peak at 1123 cm−1.The appearance of a sharp peak at 1044 cm−1 represented the Si–O–Si bond vibration, and showed the presence of SiO2. The weak peaks at 866 cm−1 may be due to the stretching vibrations of the metal–O bonds. These results were consistent with the results found from the XRD analysis. The XRD patterns (Fig. 2) of the catalyst were measured and the crystalline nature of the calcined BBP catalysts were compared to those reported in the literature11 and in the JCPDS statistics from 2003.
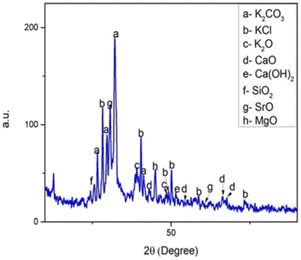
Large amounts of potassium-containing compounds were observed in the BBP catalyst. The abundance of K2CO3 was demonstrated by the values at 26.60, 29.72, 32.20, and 41.36 in the banana blossom catalyst. The 2θ readings at 28.32, 40.44, 50.08, 58.60, and 73.20 in the catalyst confirmed the presence of KCl. The peaks at a 2θ value of 25.60 indicated the presence of SiO2 in the catalysts. The SrO present in the catalysts was confirmed by the peaks at 2θ values at 62.60 and 30.72. The XRD pattern also showed the presence of the numbers of Ca constituents, including CaO, CaCO3, and Ca(OH)2. The presence of K2O in the catalysts was evaluated by the peaks at 2θ values of 39.20, and 48.32 in the catalyst.
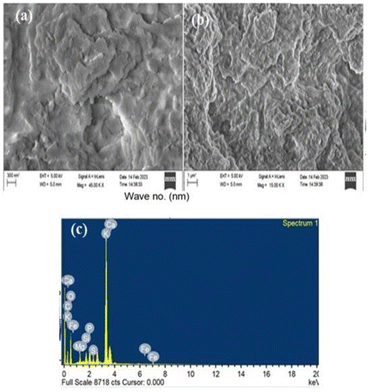
The external morphology of catalyst was examined using FESEM analysis (Fig. 3a and b). The FESEM images revealed the morphology of the catalyst, including the presence of aggregated porous materials and the characteristic appearances of spongy, sheet-like, or plate-like structures. These morphological features aligned with the expected porosity of the catalyst material, indicating its ability to accommodate and facilitate various chemical reactions. Fig. 3c, shows the EDX analysis of the catalyst. The primary elements identified (Table S1†) in significant amounts were oxygen (43.31 wt%), potassium (34.26 wt%), carbon (11.92 wt%), and calcium (3.27 wt%). The presence of such elevated levels of oxygen, potassium, carbon, and calcium, as determined by the EDX analysis, confirmed the existence of compounds like K2CO3, K2O, CaO, CaCO3, and Ca(OH)2, as predicted by the XRD analysis.
It was reported previously that plant derived wastes are characterized by a high potassium content, and exhibit a notable catalytic activity.11,12 Potassium in the form of carbonate and oxide, with other active components, can effectively facilitate the transesterification process of oil into biodiesel. A series of reactions were carried out by changing the catalyst quantity in order to investigate the impact of the catalyst-to-oil molar ratio on the transesterification reaction of sesame oil with methanol. It was observed that 9 wt% catalyst loading resulted in 97% conversion in the shortest amount of time.
Apart from this, no appreciable difference in the conversion of oil to biodiesel was observed. This may be because the base catalysed processes may also promote a saponification side reaction, which might decrease oil conversion, and larger catalyst concentrations may make the three-phase solution more viscous, which could impede the mass transfer between them.
By adjusting the ratio from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 12
1 to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the effects of methanol/oil on biodiesel conversion were also examined. Because there was less contact between the reactants and the catalyst at a 3
1, the effects of methanol/oil on biodiesel conversion were also examined. Because there was less contact between the reactants and the catalyst at a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, it was found that the reaction only produced 95% of the desired product. The conversion of biodiesel should rise with an increase in the methanol to oil ratio, yet with a 12
1 molar ratio, it was found that the reaction only produced 95% of the desired product. The conversion of biodiesel should rise with an increase in the methanol to oil ratio, yet with a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, only 97% conversion was accomplished.
1 ratio, only 97% conversion was accomplished.
The impact of the reaction temperature on biodiesel conversion was also investigated at various temperatures. The conversion was examined between 55 °C and 70 °C. The maximum conversion was attained in 32 min at 65 °C with a catalyst loading of 9 wt% and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ratio of oil to methanol. From Fig. 4, the bar diagrams for biodiesel production at various catalyst loadings, methanol to oil ratios and at various temperatures are shown.
9 ratio of oil to methanol. From Fig. 4, the bar diagrams for biodiesel production at various catalyst loadings, methanol to oil ratios and at various temperatures are shown.
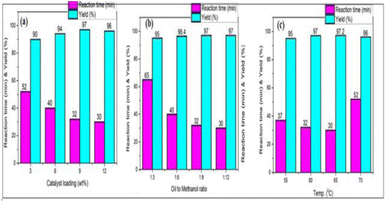 | ||
| Fig. 4 Variations in the production of biodiesel: (a) the effect of catalyst loading, (b) the effect of the methanol/oil ratio, and (c) the effect of the reaction temperature. | ||
The BBP-catalyst was used for twice with same catalyst load with a decrease in production of the biodiesel from 97% to 95.6%.
The purity of the biodiesel prepared in this way was also determined by NMR. The results of the NMR (1H-NMR Fig. S5†) spectroscopic analysis verified that the sesame oil biodiesel showed distinct signals corresponding to different protons across different chemical shifts; most significantly the signal at δ 3.663 ppm indicated the successful esterification of biodiesel in the presence of methoxy protons (–COOCH3).13 It was reported that triglycerides usually contain methine protons (–CH–) at glycerides C2 (–CH–CO2R) and methylene protons (–CH2–) at C1 and C3 of glycerides (–CH2–CO2R), whereas the methoxy protons of the ester groups are absent. The 1H-NMR spectra of biodiesel did not reveal the presence of methine or methylene protons.14
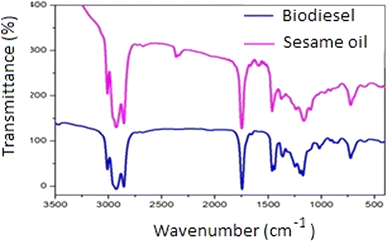
Similarly, the signal that appeared at δ 51.66 ppm (13C-NMR, Fig. S6†) was due to the presence of methoxy carbon (OCH3) which indicated the conversion of biodiesel. The characteristic peaks at 128.2–130.2 ppm indicated the presence of methyl esters in the prepared biodiesel.13,15 Therefore, it was verified that the sesame oil was successfully converted to biodiesel following transesterification and the observation of a singlet signal also indicated the presence of methoxy protons. Fig. 5 shows that the absorption peak resulting from the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the glyceride linkage of the sesame oil shifted from 1745 cm−1 to 1740 cm−1 in biodiesel, indicating the conversion had occurred during the transesterification reaction. Both oil and biodiesel exhibited peaks at 3013 cm−1 and 3015 cm−1, respectively, which corresponded to the stretching frequencies of the –CH
O bond in the glyceride linkage of the sesame oil shifted from 1745 cm−1 to 1740 cm−1 in biodiesel, indicating the conversion had occurred during the transesterification reaction. Both oil and biodiesel exhibited peaks at 3013 cm−1 and 3015 cm−1, respectively, which corresponded to the stretching frequencies of the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) =CH– bonds. The signals at 2927 cm−1 and 2855 cm−1 in oil, as well as those at 2929 cm−1 and 2853 cm−1 in biodiesel, were attributed to the stretching vibrations of the C–H bonds.
=CH– bonds. The signals at 2927 cm−1 and 2855 cm−1 in oil, as well as those at 2929 cm−1 and 2853 cm−1 in biodiesel, were attributed to the stretching vibrations of the C–H bonds.
In oil, the bending vibrations of the CH3 groups were observed at 1460 cm−1 and 1371 cm−1, whereas in biodiesel, these were observed at 1460 cm−1, 1440 cm−1, and 1364 cm−1. The infrared peaks at 1241 cm−1, 1160 cm−1, and 1095 cm−1 in oil, and at 1247 cm−1, 1194 cm−1, and 1168 cm−1 in biodiesel represented the stretching bands of the C–O bonds in the triglyceride and ester molecules, respectively. The –CH2 rocking motion of the fatty acid chains exhibited an infrared signal at 725 cm−1 in oil and at 728 cm−1 in methyl ester molecules, which corresponded to biodiesel (Fig. 6).
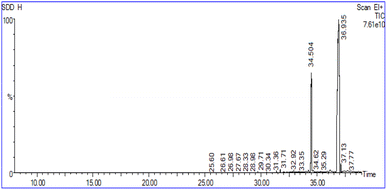
The chemical composition of biodiesel derived from sesame oil was also determined by GC-MS. The library search identified the presence of two distinct fatty acid methyl esters (FAMEs). The predominant methyl ester detected was methyl palmitate (34.504), and a saturated fatty acid ester followed by methyl linoleate (36.935), which is an unsaturated fatty acid ester.13,14 The fuel properties of the produced biodiesel were investigated and compared with other biodiesels and the international standards established by ASTM D6751 and EN 14214 (Table 1). In this study, the density and specific gravity of the biodiesel obtained were at 886 kg m−3 and 0.854 at 25 °C, respectively,16,17 corresponded well with the range of standard values, and were favourable when compared to the other results, shown in Table 1. The kinematic viscosity of the current biodiesel's value at 40 °C is 4.50 mm2 s−1 which was also within the range of international standards.18
| Fuel properties | Units | Sesame biodiesel | Biodiesel standards | |
|---|---|---|---|---|
| ASTM D751 | EN 14214 | |||
| a API – American petroleum index, DI – diesel index, ASTM – American society for testing and materials, EN – European standard. | ||||
| Density at 25 °C | (kg m−3) | 886 | 860–900 | 860–900 |
| Specific gravity at 25 °C | (kg m−3) | 0.854 | 0.86–0.90 | 0.86–0.90 |
| Kinematic viscosity at 40 °C | (mm2 s−1) | 4.50 | 1.9–6.0 | 3.5–5.0 |
| Caloric value | (MJ kg−1) | 40.21 | ≥42.0 | ≥35.0 |
| Iodine value | (gI2/100 g) | 77.40 | ≤120.0 | ≤140.0 |
| Saponification value | (mgKOH g−1) | 196.35 | — | — |
| Cetane number | — | 56.68 | ≥47.0 | ≥51.0 |
| Diesel index (DI) | — | 64.83 | 50.4 | — |
| API | — | 34.19 | 36.95 | — |
| Moisture content | % | 0.026 | ≤0.03 | ≤0.05 |
The determined saponification value (SV) was 196.35 (mgKOH g−1) which expresses saponifiable units per unit weight of sesame oil to biodiesel. The iodine value of biodiesel was estimated to be 77.40 (gI2/100 g), and represents the degree of the unsaturation of the oil. The iodine values were within the stated limits of the ASTM D6751 and EN 14214 standards. The cetane number of the biodiesels was found was to be 56.68 and it was slightly greater than the minimal value specified by the ASTM D6751 and EN 14214 standards. The current biodiesel's energy content was calculated using the caloric value (higher heating value), and the result was 40.21 MJ kg−1, which was in agreement with the fuel characteristic for a good biodiesel.17 The other fuel properties such as diesel index, American petroleum index (API) and moisture content for the biodiesel produced were 64.83, 34.19 and 0.026, respectively, and these compared favourably to the other results, shown in Table 1. The kinematic viscosity of current biodiesel's value at 40 °C is 4.50 mm2 s−1 which was also within the limits of the international standards.
Conclusions
A solid catalyst prepared from banana blossom peel (Musa acuminata) is described for the production of biodiesel from sesame oil. Various heterogenous catalysts prepared from the banana leaves were reported previously, but the heterogenous catalyst prepared from banana blossom peel waste has not been reported until now. In the present research, the maximum conversion of oil to biodiesel is achieved at 65 °C, with a sesame-to-oil molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, a reaction period of 32 minutes, and a catalyst quantity of 9 wt%. Although a more detailed study of the biodiesel produced, and the reusability of the catalyst has to studied in more detail in the future.
9, a reaction period of 32 minutes, and a catalyst quantity of 9 wt%. Although a more detailed study of the biodiesel produced, and the reusability of the catalyst has to studied in more detail in the future.
Abbreviations
| ASTM | American Society for Testing and Materials |
| EN | European standard |
| BBP | Banana blossom peel catalyst |
| FTIR | Fourier-transform infrared spectroscopy |
| FESEM | Flame emission scanning electron microscopy |
| GC-MS | Gas chromatography-mass spectroscopy |
| JCPDS | Joint Committee on Powder Diffraction Standards |
| NMR | Nuclear magnetic resonance spectroscopy |
Data availability
Supporting data for the findings are available in the ESI† of this article.Authors contributions
Manoranjan Sarkar: methodology, investigation, writing original draft. Jennifer Daimari: investigation. Sunshri Basumatary: investigation and writing draft. Kushwaha Jashvant Kumar: investigation. Ranjay Das: writing the draft. Anamika Kalita Deka: conceptualization, methodology, supervision, writing review and editing, resources. Funding this work received no funding from any source.Conflicts of interest
The authors have no conflict of interests for the content.Acknowledgements
The authors are thankful to the Institute of Advanced Study in Science & Technology, Boragaon, Guwahati Assam, India and the Guwahati Biotech Park, Guwahati, Assam, India for the use of their instrumental facilities.References
- K. Malak, C. de la Seiglière and C. Fernández, M, et al., Green Coal: A New Energy Source from Leaves, Energy Procedia, 2016, 100, 484–491, DOI:10.1016/j.Sc.2010.00372.
- H. Meriatna, M. Husin and M. Riza, et al., Proceedings. Biodiesel production using waste banana peel as renewable base catalyst, Mater. Today, 2023, 87, 214–217, DOI:10.1016/j.matpr.2023.02.400.
- R. Mohandass, K. Ashok and A. Selvaraju, et al., Homogeneous Catalysts used in Biodiesel Production: A Review, Int. J. Eng. Res., 2016, 5, 264–268, DOI:10.17577/IJERTV5IS050381.
- D. Yanti, H. Husin and F. T. Yani, et al., Transesterification of pangium edule reinw oil to biodiesel using durian rind ash as heterogeneous catalysts, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 845, 1–5, DOI:10.1088/1757-899X/845/1/012031.
- J. A. Melero, L. F. Bautista and J. Iglesias, et al., Production of biodiesel from waste cooking oil in a continuous packed bed reactor with an agglomerated Zr-SBA-15/bentonite catalyst, Appl. Catal., B, 2014, 145, 197–204, DOI:10.1016/j.apcatb.2013.02.050.
- R. Gabriel, S. H. V. de Carvalho and J. L. da S. Duarte, et al., Mixed metal oxides derived from layered double hydroxide as catalysts for biodiesel production, Appl. Catal., A, 2022, 630, 118470, DOI:10.1016/j.apcata.2021.118470.
- H. Husin, T. M. Asnawi and A. Firdaus, et al., Solid Catalyst Nanoparticles derived from Oil-Palm Empty Fruit Bunches (OP-EFB) as a Renewable Catalyst for Biodiesel Production, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 358, DOI:10.1088/1757-899X/358/1/012008.
- O. T. Iriany, O. Bani and H. L. M. Purba, Potential of papaya seeds as a heterogenous catalyst in biodiesel synthesis, IOP Conf. Ser. Earth Environ. Sci., 2021, 912, 1–5, DOI:10.1088/1755-1315/912/1/012022.
- S. de S Barros, A. G. Wanison and J. Pessoa, et al., Pineapple (Ananás comosus) leaves ash as a solid base catalyst for biodiesel synthesis, Bioresour. Technol., 2020, 312, 123569, DOI:10.1016/j.biortech.2020.123569.
- J. B. Tarigan, S. Perangin-Angin and S. R. Simanungkalit, et al., Utilization of waste banana peels as heterogeneous catalysts in room-temperature biodiesel production using a homogenizer, RSC Adv., 2023, 13, 6217–6224, 10.1039/d3ra00016h.
- S. Basumatary, B. Nath, B. Das, P. Kalita and B. Basumatary, Utilization of renewable and sustainable basic heterogeneous catalyst from Heteropanax fragrans (Kesseru) for effective synthesis of biodiesel from Jatropha curcas Oil, Fuel, 2021, 286, 119357, DOI:10.1016/j.fuel.2020.119357.
- B. Eriola and O. A. Sheriff, Modeling and optimization of Thevetia peruviana (yellow oleander) oil biodiesel synthesis via Musa paradisiacal (plantain) peels as heterogeneous base catalyst: A case of artificial neural network vs. response surface methodology, Ind. Crops Prod., 2014, 53, 314–322, DOI:10.1016/j.indcrop.2013.12.046.
- M. Ahmad, K. Ullah, M. A. Khan, S. Ali, M. Zafar and S. Sultana, Quantitative and Qualitative Analysis of Sesame Oil Biodiesel, Energy Sources, Part A, 2011, 33, 1239–1249, DOI:10.1080/15567036.2010.531510.
- B. Basumatary, A. Atmanli and Md. Azam, et. al., Catalytic Efficacy, Kinetic, and Thermodynamic Studies of Biodiesel Synthesis Using Musa AAA Plant Waste-Based Renewable Catalyst, Int. J. Energy, 2024, 1–27, DOI:10.1155/2024/8837343.
- B. Nath, P. Kalita, B. Das and S. Basumatary, et.al., Highly efficient renewable heterogeneous base catalyst derived from waste Sesamum indicum plant for synthesis of biodiesel, Renewable Energy, 2020, 151, 295–310, DOI:10.1016/j.renene.2019.11.029.
- T. F. Adepoju, B. E. Olatunbosun, O. M. Olatunj and M. A. Ibeh, Brette pearl spar mable (BPSM): A potential recoverable catalyst as a renewable source of biodiesel from Thevetia peruviana seed oil for the benefit of sustainable development in West Africa, Energy, Sustain. Soc., 2018, 8(1), 23 CrossRef.
- E. Betiku and T. F. Adepoju. Metanalysis optimization of sesame (Sesamum indicum) oil to biodiesel and fuel quality characterization, Int. J. Energy Environ. Eng., 2013, 4:9, 1–8http://www.journal-ijeee.com/content/4/1/9 Search PubMed.
- D. Beyene, M. Abdulkadir and A. Befekadu, Production of Biodiesel from Mixed Castor Seed and Microalgae Oils: Optimization of the Production and Fuel Quality Assessment, Int. J. Chem. Eng., 2022, 1–14, DOI:10.1155/2022/1536160.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00428g |
| This journal is © The Royal Society of Chemistry 2024 |