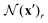Open Access Article
Open Access ArticleDeep-learning optical flow for measuring velocity fields from experimental data†‡
Phu N.
Tran
 a,
Sattvic
Ray
c,
Linnea
Lemma
ac,
Yunrui
Li
a,
Sattvic
Ray
c,
Linnea
Lemma
ac,
Yunrui
Li
 b,
Reef
Sweeney
c,
Aparna
Baskaran
b,
Reef
Sweeney
c,
Aparna
Baskaran
 a,
Zvonimir
Dogic
a,
Zvonimir
Dogic
 acd,
Pengyu
Hong
acd,
Pengyu
Hong
 *b and
Michael F.
Hagan
*b and
Michael F.
Hagan
 *a
*a
aDepartment of Physics, Brandeis University, Waltham, MA 02453, USA. E-mail: hagan@brandeis.edu
bDepartment of Computer Science, Brandeis University, Waltham, MA 02453, USA. E-mail: hongpeng@brandeis.edu
cDepartment of Physics, University of California at Santa Barbara, Santa Barbara, CA 93106, USA
dBiomolecular and Engineering Science, University of California at Santa Barbara, Santa Barbara, CA 93106, USA
First published on 23rd August 2024
Abstract
Deep learning-based optical flow (DLOF) extracts features in adjacent video frames with deep convolutional neural networks. It uses those features to estimate the inter-frame motions of objects. We evaluate the ability of optical flow to quantify the spontaneous flows of microtubule (MT)-based active nematics under different labeling conditions, and compare its performance to particle image velocimetry (PIV). We obtain flow velocity ground truths either by performing semi-automated particle tracking on samples with sparsely labeled filaments, or from passive tracer beads. DLOF produces more accurate velocity fields than PIV for densely labeled samples. PIV cannot reliably distinguish contrast variations at high densities, particularly along the nematic director. DLOF overcomes this limitation. For sparsely labeled samples, DLOF and PIV produce comparable results, but DLOF gives higher-resolution fields. Our work establishes DLOF as a versatile tool for measuring fluid flows in a broad class of active, soft, and biophysical systems.
1 Introduction
Accurate measurement of flow fields is a cornerstone for modeling diverse phenomena ranging from fluid dynamics1 and active matter2 to biology.3 A conventional approach to estimating flow fields is particle image velocimetry (PIV), where flow velocities are computed by correlating features of two consequent images.4–6 However, PIV has limitations. One arises from the dependence of the interrogation window size on seeding particle speed. Consequently, PIV cannot estimate turbulent flows smaller than the interrogation window, leading to potential errors in the velocity field.7 Furthermore, significant Brownian motion can introduce uncertainty into PIV measurements.8 Another limitation is that tracer particles must be within an optimal range of density and size.7 This requirement can be impractical in biological systems using fluorescent proteins as markers, preventing the use of smaller window sizes as a workaround for issues related to Brownian motion or smaller turbulent flows.9 To overcome these limitations we explore a deep learning-based optical flow (DLOF) algorithm for the estimation of the flow fields.In computer vision, optical flow describes the apparent motions of objects in a sequence of images.10 Various rule-based techniques for optical flow estimation have been developed, including differential methods,11–14 variational methods,15–18 and feature-based methods.19–22 Implementations of rule-based optical flow algorithms can be advantageous over PIV for applications in biological images.23–28 Rapid advancements in machine learning resulted in deep learning optical flow (DLOF) algorithms, where the automatic feature extraction offered by deep convolutional neural networks has significantly improved the algorithm accuracy.29–55
Although recent efforts used DLOF to estimate velocity fields in applications that would otherwise rely on PIV,56–61 these works trained and evaluated DLOF with synthetic data from fluid dynamics simulations or computer-generated and augmented PIV datasets that mimic noisy data in real-world experiments. Obtaining ground-truth velocities required for training machine learning models has been challenging with real-world data. We overcome this limitation by investigating the performance of DLOF on experimental data from extensively studied active nematic liquid crystals.62–72 We image microtubule (MT)-based active nematics under conditions that are beyond the limitations of PIV and present a significant challenge to its performance. We then develop a computational framework to apply DLOF to quantify the microtubule velocity fields. We test the framework with ground truth velocity fields obtained by particle tracking methods. We compare the velocity fields obtained by PIV and DLOF against this ground-truth data. Importantly, this data is characteristic of flow fields from diverse soft matter and biophysical systems, suggesting that our conclusions are broadly applicable.
Microtubule (MT)-based active nematics are powered by ATP-consuming kinesin molecular motors. In such materials the extensile MT bundles generate internal active stresses, which in turn give rise to motile topological defects and associated autonomous flows.64 Active nematics are described by two continuous fields, the director field, which describes the average orientation of the anisotropic MT filaments, and the velocity field, which describes their motions. Accurate measurement of the director field requires samples in which all the filaments are labeled. However, such samples yield low variations in spatial intensity, which makes application of PIV techniques challenging.73 In fully labeled active nematics PIV underestimates the velocity component along the nematic director,68,74–76 which can be attributed to the nematic anisotropy; the intensity of MT bundles is fairly uniform along the nematic directors, which presents challenges for implementation of PIV. Alternatively, obtaining accurate PIV fields requires samples with a low volume fraction of labeled MTs, which creates highly speckled patterns suitable for PIV application, but from which the director field cannot be extracted. Overcoming these competing challenges requires active nematics containing high-concentration MTs in one color and dilute tracer MTs in a different wavelength.76 The former are suitable for director field measurement while the latter allow for accurate application of PIV techniques. However, these samples are cumbersome to prepare, and sequential imaging can introduce a time lag between the measurement of the two fields.
We show that DLOF produces an accurate measurement of the flow field irrespective of the fraction of labeled filaments. Thus, DLOF techniques can fully characterize the instantaneous state of an active nematic from one set of images. Furthermore, the DLOF results are higher resolution and less noisy than those from PIV. Importantly, while we use the MT-based active nematic system to test optical flow, the implications of our results are more general. The velocity fields from the MT-based active nematic system closely resemble the chaotic flow fields that arise in different soft matter and biophysical systems, such as unstable elastic polymer solutions,77 bacterial suspensions,78 interface dynamics of confined active droplets,79 tissues dynamics driving biological morphogenesis,80,81 and flows generated by biological swimmers.82 Thus, our results suggest that DLOF models can be used for more accurate and robust measurements of the velocity fields across this wide range of active, soft, and biological systems.
2 Deep learning optical flow (DLOF)
DLOF uses convolutional neural networks for the automatic extraction of relevant features from two adjacent frames in a video and uses the extracted features to estimate the movements of objects between the two video frames.29,32,38,83,84 DLOF models are typically trained using supervised learning algorithms, in which training data are synthetic videos that include the true motions of all the objects in the videos across the video frames.85–91 Synthetic data are required by this approach because obtaining the true displacements of objects in real-world videos is highly challenging. Thus, the ability of the models to properly adapt to unseen data from a different domain becomes crucial for the trained models to be useful in real-world scenarios. A recent study suggested that a model called RAFT (Recurrent all-pairs field transforms for optical flow), which was originally trained using synthetic data, could generalize well to unseen fluid dynamics videos.58,84 However, this study evaluated the model's performance on simulation-generated videos and did not evaluate the performance on challenging videos obtained in experiments, such as the active nematics described above.2.1 Architecture of the RAFT model
RAFT estimates the optical flow from a pair of images (I1, I2) in three main stages: (1) extract features of the input images using a convolutional neural network, (2) use those extracted features to construct a correlation volume that computes the visual similarity of the images, and (3) compute the final flow through an iterative process. , where H0 and W0 are the height and width of the images, C the number of color channels (C = 3 for RGB and C = 1 for grayscale images), and D the number of desired feature maps to be extracted. The encoder gradually reduces the resolution of the output feature maps; i.e., it successively outputs feature maps at 1/2, 1/4, and finally 1/8 resolution. For each of these steps, the resolution reduction is performed by convolutional residual neural network blocks (Fig. 2A). In general, feature maps produced at lower resolutions extract spatial correlations at higher levels with a wider receptive field, and it has been shown empirically that learning features at the aforementioned resolutions offers a balance between the model's performance and complexity.84
, where H0 and W0 are the height and width of the images, C the number of color channels (C = 3 for RGB and C = 1 for grayscale images), and D the number of desired feature maps to be extracted. The encoder gradually reduces the resolution of the output feature maps; i.e., it successively outputs feature maps at 1/2, 1/4, and finally 1/8 resolution. For each of these steps, the resolution reduction is performed by convolutional residual neural network blocks (Fig. 2A). In general, feature maps produced at lower resolutions extract spatial correlations at higher levels with a wider receptive field, and it has been shown empirically that learning features at the aforementioned resolutions offers a balance between the model's performance and complexity.84
![[Doublestruck R]](https://www.rsc.org/images/entities/char_e175.gif) H×W×D, and then that of the second image gθ(I2) ∈
H×W×D, and then that of the second image gθ(I2) ∈ ![[Doublestruck R]](https://www.rsc.org/images/entities/char_e175.gif) H×W×D (right part of Fig. 2A). The elements of a correlation volume C(gθ(I1), gθ(I2) ∈
H×W×D (right part of Fig. 2A). The elements of a correlation volume C(gθ(I1), gθ(I2) ∈ ![[Doublestruck R]](https://www.rsc.org/images/entities/char_e175.gif) H×W×H×W) are given by
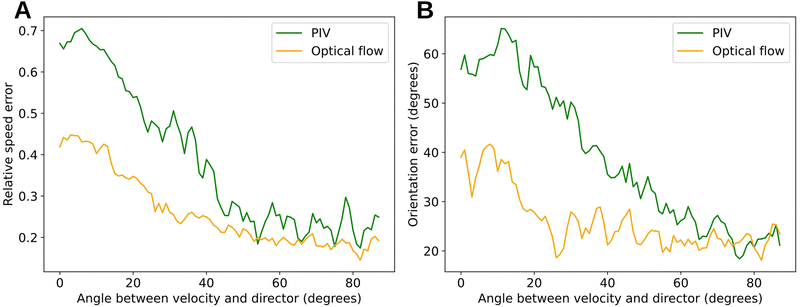
H×W×H×W) are given by 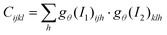 . Correlations are further computed as a 4-layer pyramid {C1,C2,C3,C4}, where Ck has dimensions H × W × H/2k × W/2k (Fig. 2B). Here, the reduction of the last two dimensions of the correlation volume C by a factor of 2k is achieved by pooling the last two dimensions of C with kernel size k and equivalent stride. Having correlations at multiple levels through {C1,C2,C3,C4} allows the model to handle both small and large displacements. The first two dimensions (that belong to I1) are maintained to preserve high-resolution information, enabling the model to detect motions of small fast-moving objects.
. Correlations are further computed as a 4-layer pyramid {C1,C2,C3,C4}, where Ck has dimensions H × W × H/2k × W/2k (Fig. 2B). Here, the reduction of the last two dimensions of the correlation volume C by a factor of 2k is achieved by pooling the last two dimensions of C with kernel size k and equivalent stride. Having correlations at multiple levels through {C1,C2,C3,C4} allows the model to handle both small and large displacements. The first two dimensions (that belong to I1) are maintained to preserve high-resolution information, enabling the model to detect motions of small fast-moving objects.
The link between an object in I1 and its estimated correspondence in I2 is determined through correlation lookup using the correlation pyramid, as described in Fig. 2C. The correspondence x′ ∈ I2 of a pixel x = (u, v) ∈ I1 is estimated by x′ = (u + f1(u), v + f2(v)), where (f1, f2) is the current estimate of DLOF between I1 and I2. A local grid around x′ is then defined as  , a set of integer offsets that are within a radius of r of x′ (using
, a set of integer offsets that are within a radius of r of x′ (using ![[small script l]](https://www.rsc.org/images/entities/i_char_e146.gif) 1 distance). The local neighborhood
1 distance). The local neighborhood  is used to index from all levels of the correlation pyramid using bilinear sampling, such that the grid
is used to index from all levels of the correlation pyramid using bilinear sampling, such that the grid 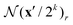 is used to index the correlations Ck. At a constant searching radius r across all levels, a local neighborhood on a lower level implies a larger context; for example, at k = 4, a neighborhood of r = 4 effectively includes a range of 256 pixels at the video's resolution. The interpolated correlation scores at all levels are concatenated to form a single feature map, which serves as an input for iterative flow refinement described below.
is used to index the correlations Ck. At a constant searching radius r across all levels, a local neighborhood on a lower level implies a larger context; for example, at k = 4, a neighborhood of r = 4 effectively includes a range of 256 pixels at the video's resolution. The interpolated correlation scores at all levels are concatenated to form a single feature map, which serves as an input for iterative flow refinement described below.
| zt = σ(Conv3×3([ht−1,xt],Wz)) | (1) |
| rt = σ(Conv3×3([ht−1,xt],Wr)) | (2) |
![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) t = tanh(Conv3×3([rt ⊙ ht−1,xt],Wh)) t = tanh(Conv3×3([rt ⊙ ht−1,xt],Wh)) | (3) |
ht = (1 − zt)⊙ ht−1 + zt ⊙ ![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) t t | (4) |
![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) t, ht are the input, update gate, reset gate, internal memory state, and hidden state at time t, respectively; σ(·) is the sigmoid function, tanh(·) the hyperbolic tangent, and Conv3×3(·, W) the convolution operator with kernel size 3 × 3 and bias W. Here, the hidden state ht is further processed by two convolutions to produce the flow update Δf at time t.
t, ht are the input, update gate, reset gate, internal memory state, and hidden state at time t, respectively; σ(·) is the sigmoid function, tanh(·) the hyperbolic tangent, and Conv3×3(·, W) the convolution operator with kernel size 3 × 3 and bias W. Here, the hidden state ht is further processed by two convolutions to produce the flow update Δf at time t.
In the above set of equations, at a current time t, the input xt is the concatenation of the current flow estimate, correlation, and context features. The update gate zt, which is calculated using the last hidden state ht−1 and the current input xt, controls how much past knowledge should be considered in the computation of the current hidden state ht. The reset signal rt is a function of the current input xt and the last hidden state ht−1, and determines how much of the past knowledge to forget. The internal memory ![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) t of the GRU cell is calculated using the current input xt and the last hidden state ht−1 weighted by the reset gate rt. Finally, the hidden state is updated by the weighted sum of the last hidden state ht−1 and the current cell memory
t of the GRU cell is calculated using the current input xt and the last hidden state ht−1 weighted by the reset gate rt. Finally, the hidden state is updated by the weighted sum of the last hidden state ht−1 and the current cell memory ![[h with combining tilde]](https://www.rsc.org/images/entities/i_char_0068_0303.gif) t, with the update gate zt controlling the weights distribution.
t, with the update gate zt controlling the weights distribution.
2.2 Training DLOF
Most DLOF models are trained by supervised learning using synthetic data, where flow ground truths can be obtained straightforwardly during data generation. The supervised loss![[script L]](https://www.rsc.org/images/entities/char_e144.gif) s used to optimize RAFT's parameters compares the sequence of predictions {f1,…,fN} with the flow ground truth fgt, with exponentially increasing weights:
s used to optimize RAFT's parameters compares the sequence of predictions {f1,…,fN} with the flow ground truth fgt, with exponentially increasing weights: | (5) |
![[script L]](https://www.rsc.org/images/entities/char_e144.gif) u = wphoto· u = wphoto·![[script L]](https://www.rsc.org/images/entities/char_e144.gif) photo + wsmooth· photo + wsmooth·![[script L]](https://www.rsc.org/images/entities/char_e144.gif) smooth smooth | (6) |
![[script L]](https://www.rsc.org/images/entities/char_e144.gif) photo denotes the photometric loss between I2 and Ĩ2,
photo denotes the photometric loss between I2 and Ĩ2, ![[script L]](https://www.rsc.org/images/entities/char_e144.gif) smooth flow smoothness regularization, and wphoto, wsmooth are the weights. The photometric loss quantifies the structural and visual differences between I2 and Ĩ2, being aware of occluded regions in which pixels in I1 do not have their correspondences in I2. A common metric used for photometric loss is the occlusion-aware structural similarity index (SSIM).25,93 A major challenge in unsupervised training of DLOF models is to obtain an accurate estimate of occlusions,83 which cannot be directly measured when dealing with real-world data. The unsupervised loss above also has a second term to encourage the smoothness of the resultant velocity fields. For example, the k-th order smoothness is defined as83
smooth flow smoothness regularization, and wphoto, wsmooth are the weights. The photometric loss quantifies the structural and visual differences between I2 and Ĩ2, being aware of occluded regions in which pixels in I1 do not have their correspondences in I2. A common metric used for photometric loss is the occlusion-aware structural similarity index (SSIM).25,93 A major challenge in unsupervised training of DLOF models is to obtain an accurate estimate of occlusions,83 which cannot be directly measured when dealing with real-world data. The unsupervised loss above also has a second term to encourage the smoothness of the resultant velocity fields. For example, the k-th order smoothness is defined as83 | (7) |
We obtained the results in the benchmarks of this work using a RAFT model that was trained with the FlyingThings synthetic datasets,91 which yielded the highest performance in our investigation. During velocity computation, we empirically set the number of iterations for flow refinement to 24.
3 Active nematics samples
We tested the performance of the DLOF framework using a MT-based active nematic liquid crystal.64 An active nematic is a quasi-2D liquid crystal comprised of locally aligned filamentous MTs. When powered by kinesin molecular motors, extensile MTs spontaneously generate a chaotic flow field that varies over space and time, and in turn reorients the nematic texture (Fig. 1A and B). Typically, the velocity field is computed by performing PIV on images of active nematics comprised of fluorescently labeled MTs. However, this method can be inaccurate when all the MTs are labeled, as these samples have poor contrast variations in fluorescence intensity, especially in the direction of the MT alignment.73We performed two distinct experiments, each containing a different type of tracer that we used to estimate the ground truth. In both experiments, a large fraction of MTs were labeled with a fluorescent dye that emits 647 nm wavelength photons. In Experiment 1, samples contained a very low concentration of 488 nm labeled MTs. They were dilute enough so that individual filaments could be distinguished (Fig. 1C, bottom panel). However, accurately linking the detected MTs into time trajectories was only possible for a small fraction of the dilute population. In Experiment 2, instead of relying on dilute labeling, we mixed passivated 488 nm fluorescent microbeads into the active nematic (Fig. 1D, bottom panel). Although not directly incorporated into the quasi-2D active nematic, these beads were located right above the nematic layer and followed the same flow field. Compared to the sparsely-labeled MTs, the beads could be reliably tracked across several frames with an automated algorithm [ESI‡], thus providing a larger set of velocity values that served as the ground truth.
4 Results and discussion
4.1 Experiment I: ground truth provided by sparsely labeled MTs
We first studied active nematics containing both densely and sparsely labeled MTs with different fluorophores. The sample was imaged sequentially in the dense and sparse channel. Using these samples we first performed PIV and DLOF on densely labeled samples. This data was compared to particle tracking of sparsely-labeled active nematics, which served as ground truth (Fig. 3). The velocities estimated by PIV for densely labeled systems are inaccurate. DLOF overcomes this limitation providing more accurate estimates of both the velocity magnitude and direction. We hypothesize that the breakdown of the PIV for densely labeled systems arises because the algorithm cannot reliably distinguish contrast variations at high densities. As we show below, the breakdown is strongest in directions parallel to the director field. PIV significantly underestimates the velocity tangent to the MT bundles because the contrast is more uniform in that direction, as was previously reported.68,73–76To quantify the above-described observations, we used PIV and DLOF to estimate the velocity fields from the dense and dilute channels. We compared these to the ground truth based on single-particle tracking. PIV and DLOF estimate the flow field everywhere while single particle tracking yields velocities only at the location of tracked points. The velocity magnitude error is calculated by |||v|| − ||v*|||/||v*|| where v* is the true displacement vector obtained from particle tracking at a particular position and v is the velocity obtained at the same position from either the PIV or DLOF. The orientation error θ is calculated using the cosine similarity, where cos(θ) = v·v*/(||v||·||v*||). By repeating the procedure for all tracked particles we obtained the distribution of measurement errors (Fig. 4). PIV and DLOF have comparable errors for sparse labels (Fig. 4B). However, with dense labels, PIV results were more unreliable. In contrast, the DLOF estimates are nearly as good as those with sparse labels (Fig. 4A). Similarly, the mean orientation errors of PIV and DLOF are also comparable when using sparse labels, 14 and 17 degrees, respectively (Fig. 4D). The discrepancy between orientation errors produced by PIV and DLOF becomes significant when using dense labels, where the mean orientation error of PIV increases to 44 degrees while that of DLOF is 29 degrees (Fig. 4C).
Previous studies68,74–76 had shown that uniform contrast along densely labeled MT bundles poses a major challenge to PIV, resulting in significantly underestimated velocity component tangent to the MT bundles. We therefore evaluated the contribution of this effect to our observed breakdown of PIV as follows. We extracted the director, i.e., the local orientation, of the MT bundles using the dense labels and computed average errors of velocities obtained by PIV and DLOF as functions of the angle between ground truth velocity and director (Fig. 5). We find that when the MTs are moving in directions with significant components along the director, PIV produces high relative speed errors (Fig. 5A) and orientation errors (Fig. 5B). DLOF strongly improves the estimation of velocities in these directions. In particular, the improvement of DLOF over PIV uniformly increases as the velocity direction approaches the director field. When the velocities are parallel to the directors (i.e., angles between velocity and director are less than 1 degree), the average relative speed error is reduced by 37% with DLOF (compared to PIV), and average orientation error reduced by 31%. This analysis shows that DLOF can resolve this well-known limitation of PIV, and thus establishes DLOF as an alternative method capable of obtaining accurate velocity fields with dense labels.
We compared the flow speeds obtained from PIV and DLOF averaged over the entire field (Fig. 7). Consistent with the previous analysis above, the PIV and DLOF estimates are nearly identical for sparsely labeled samples. The DLOF estimates for dense labels fall within the 95% confidence interval. In contrast, PIV significantly underestimates the velocities for dense labels.
For a final comparison, we define the normalized zero-lag cross-correlation between an estimated velocity and the ground truth as
 | (8) |
 are the estimated and the ground truth velocities of the traced label i, and
are the estimated and the ground truth velocities of the traced label i, and  sums over all the traced labels in the current frame. A perfect velocity estimation would yield
sums over all the traced labels in the current frame. A perfect velocity estimation would yield 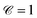 , while
, while  indicates that, on average, flow speeds are overestimated and
indicates that, on average, flow speeds are overestimated and 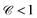 underestimated. PIV and DLOF perform similarly for sparse labels (Fig. 8). The performance discrepancy between PIV and DLOF becomes significant for dense labels, where velocities produced by DLOF are still highly correlated with the ground truths. In contrast, velocities estimated by PIV result in significantly lower correlations.
underestimated. PIV and DLOF perform similarly for sparse labels (Fig. 8). The performance discrepancy between PIV and DLOF becomes significant for dense labels, where velocities produced by DLOF are still highly correlated with the ground truths. In contrast, velocities estimated by PIV result in significantly lower correlations.
 | ||
| Fig. 8 Normalized zero-lag cross-correlation between velocity estimates and ground truth. The normalized spatial correlation (eqn (8)) is shown for optical flow on sparsely and densely labeled systems, as well as PIV on densely labeled systems, as a function of time. | ||
4.2 Experiment 2: ground truth provided by passive beads
We also compared DLOF and PIV against tracked passive beads, which served as the ground truth. This measurement assumes that passive beads within active nematic samples provide good estimates of MT velocities, as previously shown by Tayar et al. (Fig. 12).73 We include it here for two reasons. First, it provides an alternative means to compare the relative accuracy of DLOF and PIV, without relying on the PIV measurement itself. Second, the fact that (as shown next) we observe nearly identical results from both methods is a significant observation. Active nematics are hierarchical materials, and the velocity field can depend on the length scale on which it is characterized. At the microscopic scale, clusters of molecular motors induce relative extensile sliding of adjacent MTs. In principle, tracking individual filaments could at least partially include this microscopic dynamics, whereas micron-sized beads will not. Hydrodynamic theories coarse-grain over such jittery microscopic motions, suggesting that micron-sized beads could be a better tracer for measuring such coarse-grained velocity fields. The fact that we observe an indistinguishable velocity field between the two approaches means that both methods provide a good description of the coarse-grained velocity field.In each frame, we compared the instantaneous velocity of each bead to the velocities at the same position generated by PIV and DLOF. Since we computed PIV on a sparse grid, we interpolated its values as necessary to correspond to bead positions. As in Experiment 1, the comparison shows that DLOF is more accurate than PIV (Fig. 9). In particular, the difference in speeds between the beads and the DLOF velocities was significantly smaller than that between the beads and PIV (Fig. 9A). Similarly, the angular orientations of DLOF velocities were also closer to the bead velocities (Fig. 9B). At each time point, the spatially averaged mean speed of the DLOF field was closer to that of the beads, while the mean speed of PIV was systematically lower (Fig. 9C). This result is consistent with the notion that PIV systematically underestimates the motion of MTs when their motion is locally parallel, rather than perpendicular, to intensity gradients in the image on a length scale larger than the size of PIV's interrogation region.6,7 Lastly, the zero-lag cross-correlation eqn (8) between the DLOF and bead velocities was consistently higher than the correlation between PIV and bead velocities (Fig. 9D).
 | ||
| Fig. 9 Comparison of PIV and DLOF where passive tracer beads generate the ground-truth velocities. (A) Histograms of speed differences between PIV and bead velocities, and between optical flow and bead velocities. (B) Histograms of angular orientation differences between PIV and bead velocities, and between optical flow and bead velocities. (C) Mean speed of the beads, PIV, and optical flow over time. The speed is averaged over all available points for the given field (note that there are far more optical flow points than PIV points, and far more PIV points than beads, in each frame). Error bars indicate the standard deviation. (D) Zero-lag cross-correlation between PIV and bead velocities, and between PIV and optical flow velocities over time (eqn (8)). | ||
Our benchmarks demonstrate the accuracy of DLOF for extracting velocities from active nematics, surpassing the limitations of traditional PIV methods. Although we have trained and demonstrated the model on 2D active nematics samples captured with a 60× magnification objective, we note that it appears to generalize well to other magnifications and situations, such as 2D slices from a 3D isotropic active MT system64 captured at lower magnification (10×), provided that: there is sufficient contrast between labeled MTs and the background, the illumination of MTs does not change significantly between the two input frames, and the movements between two input frames are smaller than the algorithm's search window and the scale of the moving textures in the images.
5 Conclusions
We compared DLOF and PIV for estimating the velocity fields of active nematics, by generating ground truth velocity fields that enabled quantitative comparison of the two techniques. DLOF produces spatially smoother velocity fields. It also generates more accurate flows than PIV for high densities of fluorescent filaments. The high performance of DLOF arises because it determines displacements between frames by finding maximum cross-correlations in the rich feature space extracted by deep neural networks across multiple scales of spatial resolution. Furthermore, unlike PIV, DLOF eliminates the need to manually tune and readjust the model's parameters when working with data that have high contrast variances across the entire data. This is essential for analyzing large amounts of data, or for real-time control applications where it is impractical to manually tune parameters of algorithms such as PIV.Importantly, these results have implications that extend beyond the field of active nematics. Active nematics velocity fields resemble those of a wide variety of soft matter and biophysical systems,77–79 suggesting that DLOF might be more accurate than PIV in these systems as well. In particular, it is likely that DLOF will significantly outperform PIV in other systems with anisotropic constituents, based on our observation that PIV is especially inaccurate in estimating velocities along the long direction of particles. However, as noted above, PIV may be more accurate than DLOF when there is highly nonuniform illumination on the sample or the resolution is insufficient to visually represent the moving textures in the data between successive frames.
There is growing interest in applying data-driven and machine-learning approaches to physics and materials discovery,94–102 but these approaches are limited by the availability of training data. The ability of DLOF to autonomously generate high-quality velocity fields is a crucial step for advancing these applications.
Data availability
The data that support the findings of this study are openly available at https://github.com/tranngocphu/opticalflow-activenematics.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the Department of Energy (DOE) DE-SC0022291. Computing resources were provided by the NSF XSEDE allocation TG-MCB090163 and the Brandeis HPCC which is partially supported by the NSF through DMR-MRSEC 2011846 and OAC-1920147. The authors thank Michael M. Norton (Physics Deparment, Brandeis University) for insightful feedback on the manuscript.Notes and references
- T. Corpetti, E. Memin and P. Perez, IEEE Trans. Pattern Anal. Mach. Intell., 2002, 24, 365–380 CrossRef.
- M. C. Marchetti, J. F. Joanny, S. Ramaswamy, T. B. Liverpool, J. Prost, M. Rao, R. A. Simha and M. Curie, Rev. Mod. Phys., 2013, 85, 1143–1189 CrossRef CAS.
- S. Vogel, Life in moving fluids: the physical biology of flow-revised and expanded, Princeton University Press, 2nd edn, 2020 Search PubMed.
- W. Thielicke and R. Sonntag, J. Open Res. Softw., 2021, 9, 12 CrossRef.
- L. Sarno, A. Carravetta, Y. C. Tai, R. Martino, M. N. Papa and C. Y. Kuo, Adv. Powder Technol., 2018, 29, 3107–3123 CrossRef.
- M. Raffel, C. E. Willert, F. Scarano, C. J. Kähler, S. T. Wereley and J. Kompenhans, Particle image velocimetry: a practical guide, Springer, 2018 Search PubMed.
- S. Scharnowski and C. J. Kähler, Opt. Laser Eng., 2020, 135, 106185 CrossRef.
- M. G. Olsen and C. J. Bourdon, Meas. Sci. Technol., 2007, 18, 1963 CrossRef CAS.
- C. J. Kähler, S. Scharnowski and C. Cierpka, Exp. Fluids, 2012, 52, 1629–1639 CrossRef.
- J. L. Barron, D. J. Fleet and S. S. Beauchemin, Int. J. Comput. Vis., 1994, 12, 43–77 CrossRef.
- A. Verri, F. Girosi and V. Torre, JOSA A, 1990, 7, 912–922 CrossRef.
- A. Bainbridge-Smith and R. G. Lane, Image Vis. Comput., 1997, 15, 11–22 CrossRef.
- P. Baraldi, A. Sarti, C. Lamberti, A. Prandini and F. Sgallari, IEEE Trans. Biomed. Eng., 1996, 43, 259–272 CrossRef CAS PubMed.
- A. Bruhn, J. Weickert and C. Schnörr, Int. J. Comput. Vis., 2005, 61, 211–231 CrossRef.
- A. Bruhn, J. Weickert, C. Feddern, T. Kohlberger and C. Schnorr, IEEE Trans. Image Process., 2005, 14, 608–615 Search PubMed.
- I. Cohen, Proceedings of the Scandinavian conference on image analysis, 1993.
- A. Bruhn, J. Weickert, C. Feddern, T. Kohlberger and C. Schnörr, Computer Analysis of Images and Patterns, Berlin, Heidelberg, 2003, pp. 222–229 Search PubMed.
- Z. Tu, W. Xie, D. Zhang, R. Poppe, R. C. Veltkamp, B. Li and J. Yuan, Signal Process. Image Commun., 2019, 72, 9–24 CrossRef.
- C.-C. Cheng, K.-H. Ho, H.-T. Li and G.-L. Lin, Proceedings of the IEEE Internatinal Symposium on Intelligent Control, 2002, pp. 350–355.
- C.-C. Cheng and H.-T. Li, Int. J. Inf. Technol., 2006, 12, 82–90 Search PubMed.
- S. S. Beauchemin and J. L. Barron, ACM Comput. Surv., 1995, 27, 433–466 CrossRef.
- A. Becciu, H. van Assen, L. Florack, S. Kozerke, V. Roode and B. M. ter Haar Romeny, Scale Space and Variational Methods in Computer Vision, Berlin, Heidelberg, 2009, pp. 588–599 Search PubMed.
- J.-Y. Bouguet, et al., Intel corporation, 2001, vol. 5, p. 4 Search PubMed.
- G. Farnebäck, Image Analysis, Berlin, Heidelberg, 2003, pp. 363–370 Search PubMed.
- T. Brox, A. Bruhn, N. Papenberg and J. Weickert, Computer Vision-ECCV 2004: 8th European Conference on Computer Vision, Prague, Czech Republic, May 11–14, 2004, Proceedings, Part IV 8, 2004, pp. 25–36.
- C. Zach, T. Pock and H. Bischof, Pattern Recognition, Berlin, Heidelberg, 2007, pp. 214–223 Search PubMed.
- X. Yong, C.-K. Huang and C. T. Lim, J. R. Soc., Interface, 2021, 18, 20210248 CrossRef PubMed.
- D. K. Vig, A. E. Hamby and C. W. Wolgemuth, Biophys. J., 2016, 110, 1469–1475 CrossRef CAS PubMed.
- A. Ranjan and M. J. Black, Proceedings of the IEEE conference on computer vision and pattern recognition, 2017, pp. 4161–4170.
- P. Fischer, A. Dosovitskiy, E. Ilg, P. Häusser, C. Hazrbas, V. Golkov, P. Van der Smagt, D. Cremers and T. Brox, IEEE International Conference on Computer Vision (ICCV), 2015, pp. 2758–2766, DOI:10.1109/ICCV.2015.316.
- Z. Ren, J. Yan, B. Ni, B. Liu, X. Yang and H. Zha, Proceedings of the AAAI conference on artificial intelligence, 2017.
- C. Yu, X. Bi and Y. Fan, Ocean Eng., 2023, 271, 113693 CrossRef.
- S. Bai, Z. Geng, Y. Savani and J. Z. Kolter, Deep Equilibrium Optical Flow Estimation, Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), 2022, pp. 620–630, DOI:10.1109/CVPR52688.2022.00070.
- A. Bar-Haim and L. Wolf, Proceedings of IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), 2020, pp. 7995–8004, DOI:10.1109/CVPR42600.2020.00802.
- Y. Han, K. Luo, A. Luo, J. Liu, H. Fan, G. Luo and S. Liu, European conference on computer vision, 2022, DOI:10.1007/978-3-031-19800-7_17.
- Z. Huang, X. Shi, C. Zhang, Q. Wang, K. C. Cheung, H. Qin, J. Dai and H. Li, European conference on computer vision, 2022, pp. 668–685, DOI:10.1007/978-3-031-19790-1_40.
- T.-W. Hui, X. Tang and C. C. Loy, IEEE Transactions on Pattern Analysis and Machine Intelligence, 2021, pp. 2555–2569, DOI:10.1109/TPAMI.2020.2976928.
- E. Ilg, N. Mayer, T. Saikia, M. Keuper, A. Dosovitskiy and T. Brox, Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, 2017, pp. 2462–2470.
- J. Jeong, H. Cai, R. Garrepalli and F. Porikli, Proceedings of IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), 2023, pp. 13691–13700, DOI:10.1109/CVPR52729.2023.01316.
- S. Jiang, D. Campbell, Y. Lu, H. Li and R. Hartley, Proceedings of IEEE/CVF International Conference on Computer Vision (ICCV), 2021, pp. 9752–9761, DOI:10.1109/ICCV48922.2021.00963.
- S. Jiang, Y. Lu, H. Li and R. Hartley, Proceedings of IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), 2021, pp. 16587–16595, DOI:10.1109/CVPR46437.2021.01632.
- L. Liu, J. Zhang, R. He, Y. Liu, Y. Wang, Y. Tai, D. Luo, C. Wang, J. Li and F. Huang, 2020 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Seattle, WA, USA, 2020, pp. 6488–6497.
- S. Liu, K. Luo, N. Ye, C. Wang, J. Wang and B. Zeng, IEEE Trans. Image Process., 2021, 30, 6420–6433 Search PubMed.
- K. Luo, C. Wang, S. Liu, H. Fan, J. Wang and J. Sun, 2021 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Nashville, TN, USA, 2021, pp. 1045–1054.
- A. Luo, F. Yang, X. Li and S. Liu, 2022 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), New Orleans, LA, USA, 2022, pp. 8896–8905.
- C. Min, T. Kim and J. Lim, 2023 IEEE/CVF Winter Conference on Applications of Computer Vision (WACV), pp. 2144–2153.
- A. Nebisoy and S. Malekzadeh, arXiv, 2021, preprint, arXiv:2103.05101, DOI:10.48550/arXiv.2103.05101.
- Z. Pan, D. Geng and A. Owens, Proceedings of the 37th International Conference on Neural Information Processing Systems, 2023, pp. 253–273, DOI:10.5555/3666122.3666135.
- A. Stone, D. Maurer, A. Ayvaci, A. Angelova and R. Jonschkowski, Proceedings of IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), 2021, pp. 3886–3895, DOI:10.1109/CVPR46437.2021.00388.
- D. Sun, X. Yang, M.-Y. Liu and J. Kautz, PWC-Net: CNNs for Optical Flow Using Pyramid, Warping, and Cost Volume, 2018 Search PubMed.
- D. Sun, C. Herrmann, F. Reda, M. Rubinstein, D. Fleet and W. T. Freeman, European Conference on Computer Vision, 2022, pp. 165–182, DOI:10.1007/978-3-031-20047-2_10.
- A. Ullah, K. Muhammad, J. Del Ser, S. W. Baik and V. H. C. de Albuquerque, IEEE Trans. Ind. Electron., 2019, 66, 9692–9702 Search PubMed.
- H. Xu, J. Yang, J. Cai, J. Zhang and X. Tong, Proceedings of IEEE/CVF International Conference on Computer Vision (ICCV), 2021, pp. 10478–10487, DOI:10.1109/ICCV48922.2021.01033.
- H. Xu, J. Zhang, J. Cai, H. Rezatofighi and D. Tao, Proceedings of IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), 2022, pp. 8111–8120, DOI:10.1109/CVPR52688.2022.00795.
- S. Zhao, Y. Sheng, Y. Dong, E. I.-C. Chang and Y. Xu, 2020 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), 2020, pp. 6277–6286.
- S. Cai, S. Zhou, C. Xu and Q. Gao, Exp. Fluids, 2019, 60, 73 CrossRef.
- S. Discetti and Y. Liu, Meas. Sci. Technol., 2022, 34, 021001 CrossRef.
- C. Lagemann, K. Lagemann, S. Mukherjee and W. Schröder, Nat. Mach. Intell., 2021, 3, 641–651 CrossRef.
- C. Yu, X. Bi, Y. Fan, Y. Han and Y. Kuai, IEEE Trans. Instrum. Meas., 2021, 70, 1–15 Search PubMed.
- C. Yu, X. Bi and Y. Fan, Ocean Eng., 2023, 271, 113693 CrossRef.
- W. Zhang, X. Dong, Z. Sun and S. Xu, Phys. Fluids, 2023, 35, 077108 CrossRef CAS.
- R. Aditi Simha and S. Ramaswamy, Phys. Rev. Lett., 2002, 89, 058101 CrossRef CAS PubMed.
- V. Narayan, S. Ramaswamy and N. Menon, Science, 2007, 317, 105–108 CrossRef CAS PubMed.
- T. Sanchez, D. T. N. Chen, S. J. DeCamp, M. Heymann and Z. Dogic, Nature, 2012, 491, 431–434 CrossRef CAS PubMed.
- P. Guillamat, J. Ignés-Mullol, S. Shankar, M. C. Marchetti and F. Sagués, Phys. Rev. E, 2016, 94, 1–5 CrossRef PubMed.
- N. Kumar, R. Zhang, J. J. de Pablo and M. L. Gardel, Sci. Adv., 2018, 4, eaat7779 CrossRef CAS PubMed.
- C. Blanch-Mercader, V. Yashunsky, S. Garcia, G. Duclos, L. Giomi and P. Silberzan, Phys. Rev. Lett., 2018, 120, 208101 CrossRef CAS PubMed.
- A. J. Tan, E. Roberts, S. A. Smith, U. A. Olvera, J. Arteaga, S. Fortini, K. A. Mitchell and L. S. Hirst, Nat. Phys., 2019, 15, 1033–1039 Search PubMed.
- L. Giomi, L. Mahadevan, B. Chakraborty and M. F. Hagan, Phys. Rev. Lett., 2011, 106, 2–5 CrossRef PubMed.
- L. Giomi, L. Mahadevan, B. Chakraborty and M. F. Hagan, Nonlinearity, 2012, 25, 2245–2269 CrossRef.
- S. P. Thampi, R. Golestanian and J. M. Yeomans, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2014, 90, 1–5 CrossRef PubMed.
- T. N. Shendruk, A. Doostmohammadi, K. Thijssen and J. M. Yeomans, Soft Matter, 2017, 13, 3853–3862 RSC.
- A. M. Tayar, L. M. Lemma and Z. Dogic, Microtubules: Methods and Protocols, Springer, 2022, pp. 151–183 Search PubMed.
- A. Opathalage, M. M. Norton, M. P. N. Juniper, B. Langeslay, S. A. Aghvami, S. Fraden and Z. Dogic, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 4788–4797 CrossRef CAS PubMed.
- F. L. Memarian, D. Hammar, M. M. H. Sabbir, M. Elias, K. A. Mitchell and L. Hirst, Phys. Rev. Lett., 2024, 228301 CrossRef PubMed.
- M. Serra, L. Lemma, L. Giomi, Z. Dogic and L. Mahadevan, Nat. Phys., 2023, 1–7 Search PubMed.
- C. A. Browne and S. S. Datta, Sci. Adv., 2021, 7, eabj2619 Search PubMed.
- X. Wen, Y. Sang, Y. Zhang, F. Ge, G. Jing and Y. He, ACS Nano, 2023, 17, 10104–10112 CrossRef CAS PubMed.
- P. Ramesh, B. V. Hokmabad, D. O. Pushkin, A. J. T. M. Mathijssen and C. C. Maass, J. Fluid Mech., 2023, 966, A29 CrossRef CAS.
- N. P. Mitchell, D. J. Cislo, S. Shankar, Y. Lin, B. I. Shraiman and S. J. Streichan, eLife, 2022, 11, e77355 CrossRef PubMed.
- S. J. Streichan, M. F. Lefebvre, N. Noll, E. F. Wieschaus and B. I. Shraiman, eLife, 2018, 7, e27454 CrossRef PubMed.
- D. Mondal, R. Adhikari and P. Sharma, Sci. Adv., 2020, 6, eabb0503 CrossRef CAS PubMed.
- R. Jonschkowski, A. Stone, J. T. Barron, A. Gordon, K. Konolige and A. Angelova, Computer Vision-ECCV 2020: 16th European Conference, Glasgow, UK, August 23–28, 2020, Proceedings, Part II 16, 2020, pp. 557–572.
- Z. Teed and J. Deng, Computer Vision-ECCV 2020: 16th European Conference, Glasgow, UK, August 23–28, 2020, Proceedings, Part II 16, 2020, pp. 402–419.
- D. J. Butler, J. Wulff, G. B. Stanley and M. J. Black, European Conf. on Computer Vision (ECCV), 2012, pp. 611–625.
- M. Menze and A. Geiger, Conference on Computer Vision and Pattern Recognition (CVPR), 2015.
- A. Geiger, P. Lenz and R. Urtasun, Conference on Computer Vision and Pattern Recognition (CVPR), 2012.
- A. Geiger, P. Lenz, C. Stiller and R. Urtasun, Int. J. Robot. Res., 2013, 1231–1237 CrossRef.
- J. Fritsch, T. Kuehnl and A. Geiger, International Conference on Intelligent Transportation Systems (ITSC), 2013.
- A. Dosovitskiy, P. Fischer, E. Ilg, P. Hausser, C. Hazirbas, V. Golkov, P. van der Smagt, D. Cremers and T. Brox, Proceedings of the IEEE International Conference on Computer Vision (ICCV), 2015.
- N. Mayer, E. Ilg, P. Häusser, P. Fischer, D. Cremers, A. Dosovitskiy and T. Brox, IEEE International Conference on Computer Vision and Pattern Recognition (CVPR), 2016.
- R. Dey and F. M. Salem, 2017 IEEE 60th international midwest symposium on circuits and systems (MWSCAS), 2017, pp. 1597–1600.
- A. Gordon, H. Li, R. Jonschkowski and A. Angelova, Proceedings of the IEEE/CVF International Conference on Computer Vision, 2019, pp. 8977–8986.
- B. W. Brunton, L. A. Johnson, J. G. Ojemann and J. N. Kutz, J. Neurosci. Meth., 2016, 258, 1–15 CrossRef PubMed.
- E. de Bézenac, A. Pajot and P. Gallinari, J. Stat. Mech.: Theory Exp., 2019, 2019, 124009 CrossRef.
- F. Cichos, K. Gustavsson, B. Mehlig and G. Volpe, Nat. Mach. Intell., 2020, 2, 94–103 CrossRef.
- J. Colen, M. Han, R. Zhang, S. A. Redford, L. M. Lemma, L. Morgan, P. V. Ruijgrok, R. Adkins, Z. Bryant, Z. Dogic, M. L. Gardel, J. J. de Pablo and V. Vitelli, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, 10 CrossRef PubMed.
- C. Joshi, S. Ray, L. M. Lemma, M. Varghese, G. Sharp, Z. Dogic, A. Baskaran and M. F. Hagan, Phys. Rev. Lett., 2022, 129, 258001 CrossRef CAS PubMed.
- Z. Zhou, C. Joshi, R. Liu, M. M. Norton, L. Lemma, Z. Dogic, M. F. Hagan, S. Fraden and P. Hong, Soft Matter, 2021, 17, 738–747 RSC.
- S. L. Brunton and J. N. Kutz, Data-Driven Science and Engineering: Machine Learning, Dynamical Systems, and Control, Cambridge University Press, 2022 Search PubMed.
- M. Golden, R. O. Grigoriev, J. Nambisan and A. Fernandez-Nieves, Sci. Adv., 2023, 9, eabq6120 CrossRef CAS PubMed.
- Y. Li, Z. Zarei, P. N. Tran, Y. Wang, A. Baskaran, S. Fraden, M. F. Hagan and P. Hong, Soft Matter, 2024, 20, 1869–1883 RSC.
Footnotes |
| † Data and code available: https://github.com/tranngocphu/opticalflow-activenematics. |
| ‡ Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sm00483c |
| This journal is © The Royal Society of Chemistry 2024 |