 Open Access Article
Open Access ArticleReply to the ‘Comment on “Dynamics and rheology of vesicles under confined Poiseuille flow”’ by G. Coupier and T. Podgorski, Soft Matter, 2024, 20, DOI: 10.1039/D3SM01679J†
Zhe
Gou‡
 a,
Hengdi
Zhang‡
a,
Abdessamad
Nait-Ouhra
a,
Hengdi
Zhang‡
a,
Abdessamad
Nait-Ouhra
 abc,
Mehdi
Abbasi
a,
Alexander
Farutin
a and
Chaouqi
Misbah
abc,
Mehdi
Abbasi
a,
Alexander
Farutin
a and
Chaouqi
Misbah
 *a
*a
aUniversité Grenoble Alpes, CNRS, LIPhy, F-38000 Grenoble, France. E-mail: chaouqi.misbah@univ-grenoble-alpes.fr
bLaboratoire de Matière Condensée et Sciences Interdisciplinaires, Faculty of Sciences, Mohammed V University of Rabat, Rabat 1014, Morocco
cUniversité de Lorraine, CNRS, GeoRessources, Nancy 54000, France
First published on 9th August 2024
Abstract
In this answer, we provide our arguments in support of the possibility to observe the single file-organization of red blood cells in microvessels and the resulting unexpectedly weak increase of blood viscosity with increasing hematocrit, the physiological relevance of which was questioned in the comment. The key element is that the equivalent diameter in 3D for the maximal hematocrit corresponding to a single file of red blood cells is about 10 µm and not 20 µm, as in 2D. In addition, the viscosity contrast (ratio between the cell internal and external viscosities) value must be chosen in our 2D simulation in a such a way that the effective viscosity (a linear combination of the internal, external and membrane viscosities) be close to that of a real RBC. Taking these two facts into account, we find a reasonable agreement between our 2D viscosity simulations data and experimental data, despite the crude 2D assumption.
The original study1 highlights a peculiar phenomenon (already discovered in ref. 2 for shear flow) in micro-confined flow of red blood cells: at low enough hematocrit (volume fraction of red blood cells, RBCs), the RBCs form a single file in the center of a channel, which leads to an unexpected dependence of the blood viscosity on hematocrit. Namely, as long as the single-file solution persists, increasing the hematocrit does not increase significantly the effective viscosity of blood. In their Comment,3 G. Coupier and T. Podgorski review several experimental and numerical studies of rheology of micro-confined blood flow. They note that while quasi-constant effective viscosity of red blood cell suspensions for low hematocrit is indeed observed in a diverse set of conditions, its presence and the range of red blood cell concentrations for which it is observed depend strongly on the flow geometry (2D vs. 3D pipe flow vs. 3D slit flow) and the visco-elastic properties of RBCs. The Authors of the Comment3 then estimate the range of hematocrit for which a quasi-constant effective viscosity would be observed in a channel of diameter 20 µm (corresponding to channel width the original numerical study in 2D1) as 0 to 3%. This is also confirmed by empirical law derived in ref. 4 by fitting experimental data, which shows a strong increase of blood viscosity with red blood cell concentration for channels of diameter 20 µm. On this basis, G. Coupier and T. Podgorski conclude3 that the phenomenon of reduced dependence of blood viscosity on hematocrit has no physiological relevance.
While we agree with the Authors of the Comment3 that flow geometry and visco-elastic properties of RBCs may strongly affect the hematocrit range at which the single-file solution is observed, we argue here that the proposed phenomenon should be observed in blood flow under physiological conditions, if vessels with diameter about 10 µm are considered.
As observed in the original studies,1,2 the unexpectedly weak increase of the blood viscosity with increasing hematocrit is observed when RBCs form a single file in the center of the channel. Two prerequisites are necessary for this organization to occur: strong enough hydrodynamic lift that would push the red blood cells to the channel center and low enough concentration to prevent the hydrodynamic interactions between red blood cell from destabilizing the single-file arrangement. The Comment3 gives a simple formula for a maximum hematocrit at which a single-file solution is possible:
| ϕ2D = πR2/(WL), ϕ3D = 16R3/(W2L), | (1) |
Decreasing channel diameter also leads to enhanced stability of centered solutions regardless of the visco-elastic properties of the red blood cells. Indeed, it has been observed in many works that increasing the viscosity of the hemoglobin solution inside the red blood cell or decreasing the viscosity of the fluid plasma, or decreasing the flow rate, can lead to stationary off-centered solutions in Poiseuille flow, or can decrease the lift velocity, or can even reverse its direction. The same tendencies have been observed for migration of soft particles from a rigid planar wall. As observed in the Comment, this indicates that a precise model of visco-elastic properties of red blood cells is essential for quantitative analysis of the stability of the single-file solution in a pipe flow. However, as the channel diameter is decreased, the hydrodynamic interactions between the red blood cells and the channel walls approach the lubrication limit, in which soft objects experience repulsion from rigid boundaries regardless of the details of the visco-elastic properties of the soft object. Therefore, we can expect that for vessels of diameter 10 µm or less, since the gap between the cell and the wall is small enough compared to the cell size, the cell should be pushed towards the center of the channel, where it assumes a parachute or a quasi-centered slipper shape. Furthermore, since the tank-treading motion of the membrane is either completely absent (for parachute shape) or very low (for quasi-centered slippers) neither the viscosity of the fluid nor the membrane viscosity affect the dynamics for these solutions.
Using eqn (1), we can derive an equivalent diameter D of a 3D tube that gives the maximal hematocrit for a single file as a 2D channel of width W:
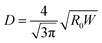 | (2) |
Fig. 1 compares our simulation data in 2D for W = 20 µm to experimental data in 3D for D ≃ 10 µm (extracted from ref. 4), where we see that the difference between our simulation results (symbols) is within less than 7% (for a viscosity contrast λ = 5, a value which is widely adopted for RBCs) consistent with 3D data (dashed line).
 | ||
| Fig. 1 Symbols show the relative viscosity – effective suspension viscosity over that of the suspending fluid – for a viscosity ratio λ = 1 and λ = 5 (ratio between viscosity of the suspending fluid over that of the fluid within cells) obtained from our 2D simulations.1 These data correspond to our Fig. 10 in ref. 1. The dashed line represents a fit of experimental data for D = 9 µm as reported in ref. 4, with a conversion from discharge hematocrit (used in experiments4), into tube hematocrit (as done in our simulations1). The dotted line is a fit of 3D experimental data for D = 21 µm as reported in ref. 4 LBM-2: lattice Boltzmann method with a certain random initial configuration. | ||
Note that in our original article1 we refrained from making any quantitative comparison with experimental data in microcirculation. However, if an attempt has to be made, a certain caution is necessary. Our simplistic 2D model did not take into account the membrane viscosity. In our simulation all cells are centered, but some are symmetric (parachute) and other are non-symmetric (slipper). For parachutes there is no tank-treading, implying that neither membrane viscosity nor that of the internal fluid plays a role. This is, however not the case for slippers, where all the three viscosities (internal, external and the membrane viscosities) should play a role. It has been shown6,7 (see also ref. 8) that the three viscosities combine in an additive way leading to a global effective viscosity
 | (3) |
Data availability
The data of Fig. 1 are provided as a ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank CNES (Centre National d’Etudes Spatiales) and the French-German University Programme “Living Fluids” (Grant CFDA-Q1-14) for financial support.Notes and references
- Z. Gou, H. Zhang, A. Nait-Ouhra, M. Abbasi, A. Farutin and C. Misbah, Soft Matter, 2023, 19, 9101–9114 RSC.
- M. Thiébaud, Z. Shen, J. Harting and C. Misbah, Phys. Rev. Lett., 2014, 112, 238304 CrossRef PubMed.
- G. Coupier and T. Podgorski, Soft Matter, 2024, 20 10.1039/D3SM01679J.
- A. Pries, D. Neuhaus and P. Gaehtgens, Am. J. Physiol.: Heart Circ. Physiol., 1992, 263, H1770–H1778 CrossRef CAS PubMed.
- Y.-C. Fung, Biomechanics: circulation, Springer Science & Business Media, 2013 Search PubMed.
- V. Lebedev, K. Turitsyn and S. Vergeles, Phys. Rev. Lett., 2007, 99, 218101 CrossRef CAS PubMed.
- T. W. Secomb, B. Styp-Rekowska and A. R. Pries, Ann. Biomed. Eng., 2007, 35, 755–765 CrossRef PubMed.
- G. Prado, A. Farutin, C. Misbah and L. Bureau, Biophys. J., 2015, 108, 2126–2136 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sm00367e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |
