Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Understanding interactions of plasticisers with a phospholipid monolayer†
Emil
Gustafsson
 *a,
Maja S.
Hellsing‡
*a,
Maja S.
Hellsing‡
 b,
Adrian R.
Rennie
b,
Adrian R.
Rennie
 *ac,
Rebecca J. L.
Welbourn
*ac,
Rebecca J. L.
Welbourn
 d,
Mario
Campana
d,
Arwel
Hughes
d,
Peixun
Li
d,
Mario
Campana
d,
Arwel
Hughes
d,
Peixun
Li
 d and
Tim Melander
Bowden
d and
Tim Melander
Bowden
 a
a
aDepartment of Chemistry, Ångström Laboratory, Uppsala University, Box 538, 752 37 Uppsala, Sweden. E-mail: Gustafsson@kemi.uu.se; Adrian.Rennie@kemi.uu.se
bRISE Research Institutes of Sweden, Box 5604, 114 86 Stockholm, Sweden
cCentre for Neutron Scattering, Ångström Laboratory, Uppsala University, Box 538, 752 37 Uppsala, Sweden
dISIS Pulsed Neutron and Muon Facility, Rutherford Appleton Laboratory, Didcot, Oxfordshire OX11 0QX, UK
First published on 27th February 2024
Abstract
The use of DEHP (diethylhexyl phthalate) is now banned for most applications in Europe; the exception is for blood bags, where its toxicity is overshadowed by its ability to extend the storage life of red blood cells. Another plasticiser, BTHC (butanoyl trihexyl citrate), is used in paediatric blood bags but does not stabilise blood cells as effectively. Interactions between plasticisers and lipids are investigated with a phospholipid, DMPC, to understand the increased stability of blood cells in the presence of DEHP as well as bioaccumulation and identify differences with BTHC. Mixed monolayers of DMPC and DEHP or BTHC were studied on Langmuir troughs where surface pressure/area isotherms can be measured. Neutron reflection measurements were made to determine the composition and structure of these mixed layers. A large amount of plasticiser can be incorporated into a DMPC monolayer but once an upper limit is reached, plasticiser is selectively removed from the interface at high surface pressures. The upper limit is found to occur between 40–60 mol% for DEHP and 20–40 mol% for BTHC. The areas per molecule are also different with DEHP being in the range of 50–100 Å2 and BTHC being 65–120 Å2. Results indicate that BTHC does not fit as well as DEHP in DMPC monolayers which could help explain the differences observed with regards to the stability of blood cells.
Introduction
Plasticisers are commonly used in polymeric materials to make them softer and more flexible. These additives are usually not chemically bound to the polymer and as such may leach into the surrounding environment. Although the use of harmful plasticisers, such as phthalates, has been widely banned1, with diethyl hexyl phthalate (DEHP) in polyvinylchloride (PVC) blood bags as an exception that is permitted. PVC has excellent properties for storage of blood due to its thermal stability and permeability of gases.2,3 For these reasons PVC plasticized with DEHP is the most common material used in blood bags, where DEHP can be as much as 40% by weight.4,5 DEHP has long been known to leach into the blood from the storage bag6 where its presence has been found to stabilise blood cells and as a result significantly extend their shelf life.7–9 Despite environmental and long-term health concerns, it is difficult to replace DEHP in blood bags without risking a shortage in available supply.5,10,11 BTHC (butanoyl trihexyl citrate) is used as a plasticiser in paediatric blood bags to mitigate the hazards of toxicity for patients but is not so effective in prolonging the storage life. Understanding how DEHP interacts with blood cells is key to replace it. The differences between DEHP and BTHC can also provide clues on how to extend the shelf life of blood. To this end there have been several studies aimed at studying the effect of DEHP on blood cells.9,12 While a study by Bider et al.13 using X-ray diffraction showed that DEHP integrates into palmitoyloleylphosphocholine (POPC) bilayers in a lamellar phase and increases the area per lipid, there are few studies on plasticisers with membrane lipids at the molecular level. A more complete overview of the interactions of plasticisers with amphiphiles can be found in Gustafsson et al.14Monolayers of DMPC (dimyristoylphosphatidylcholine) are well studied, with regards to characteristics and phase transitions. It is a convenient, simple model for phospholipids in membranes although much functionality arises with the complex mixtures of many components that are found in living systems. Many studies of DMPC have been made with surface pressure/area isotherms on Langmuir troughs and in combination with reflectometry and surface diffraction.15,16 Additional information regarding the characteristics and measurement of phospholipid monolayers is available in a review by Kaganer et al.17 Binary mixtures with DMPC and other molecules have also been similarly studied. This includes sterols in the case of Sabatini et al.18 and sulfobetaines in the case of Elstone et al.19 These additions change the phase behaviour as well as the area molecules occupy at the interface.
In this study, we compare the behaviour of two plasticisers, BTHC and DEHP, with the lipid DMPC in monolayers spread on the surface of water. Biological membranes such as those of red blood cells are a complex mixture of lipids and other molecules. DMPC is a naturally occurring phospholipid found commonly in nature. Structures of relevant molecules are shown in Fig. S1 in the ESI.† Combining neutron reflectivity and surface tension measurements on these simple model systems, allows one to obtain more detailed molecular information on how these plasticisers interact with DMPC at an interface. Measurement of the surface tension yields surface pressure/area isotherms where the surface pressure, Π, is defined as Π = γ0 − γ where γ0 is the surface tension of the pure subphase and γ is the surface tension of the subphase with added material at the surface.
Neutron reflectivity is a technique to characterise surfaces and interfacial layers in respect of both structure and composition.20,21 Neutrons are sensitive to different isotopes and this allows the use of isotopic labelling for detailed structural information and determination of the composition. Using the isotopic contrast of neutron reflectivity with labelling of the individual components, we can accurately determine the amount of each component at the interface. A mixture of 8% (volume) of D2O and 92% H2O will have no scattering in contrast to air (called null reflecting water), allowing simple analysis of thin layers on such solutions.
The reflectivity depends on the scattering length profile perpendicular to the surface of a sample. For a uniform and thin monolayer on null reflecting water, the fitted parameters are simply the scattering length per unit area and the thickness of the layer. The data are reported as reflectivity profiles versus the amplitude of the momentum transfer vector, q, (=(4π/λ) sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) where λ is the wavelength and θ is the grazing angle of incidence and reflection. The scattering length per area depends on the number of specific atoms or molecules at the interface. The scattering lengths of elements and isotopes are available in the literature.22 The molecular formulae and scattering lengths of the materials used in this study are listed in Table S1 in the ESI.† Neutron reflectivity therefore provides a direct measurement of the amount of material at the interface per unit area also referred to as surface excess, Γ, defined as follows:
θ) where λ is the wavelength and θ is the grazing angle of incidence and reflection. The scattering length per area depends on the number of specific atoms or molecules at the interface. The scattering lengths of elements and isotopes are available in the literature.22 The molecular formulae and scattering lengths of the materials used in this study are listed in Table S1 in the ESI.† Neutron reflectivity therefore provides a direct measurement of the amount of material at the interface per unit area also referred to as surface excess, Γ, defined as follows:
| Γ = N/A | (1) |
The molar ratio, xmol, between two components at the interface is determined through the measured surface excesses as follows:
| xmol = Γ1/Γ2 | (2) |
The surface excess is also related to the inverse of the area occupied by each molecule at the interface, which can be a more convenient description. The surface area, As,n, for each component is given as follows:
| As,n = 1/Γn | (3) |
The surface area occupied by a molecule at the interface, referred to as an average surface area in this work can then be obtained as follows:
| As,avg = 1/(ΣnΓn) | (4) |
Materials and methods
All materials used were obtained from Sigma-Aldrich except when noted and all the materials were used without further purification. Hydrogenous and deuterated DMPC were bought from Avanti Polar Lipids. Hydrogenous BTHC was obtained from Vertellus. The chloroform used contained 0.5–1% ethanol as a stabiliser.Synthesis
Deuterated DEHP was synthesised from deuterated 2-ethylhexanol prepared at the ISIS Deuteration Laboratory23 and phthalic anhydride (purchased from Sigma-Aldrich). The deuterated 2-ethylhexanol (6.8 mmol, 1.0 g) was combined with phthalic anhydride (3.1 mmol, 0.46 g) in a vial with a catalytic amount of p-toluenesulfonic acid hydrate (0.1 g, 0.53 mmol). The vial was then heated to 140 °C on a temperature-controlled heating block and was left for 6 hours. Reaction progress was confirmed with thin layer chromatography. NMR-spectroscopy confirmed the identification of the product. The resulting solution was washed with NaHCO3 solution (30 mL, 0.4 M) to stop the reaction and neutralise and remove the acid. The obtained solution was subsequently washed with saturated brine (2 × 30 mL) and pure water (2 × 30 mL) to remove unreacted and partially reacted reagents. Diethyl ether was used in all washing procedures to extract the organic phase. Excess diethyl ether from the washing was removed using a rotary evaporator before a small amount of solid MgSO4 was added to the resulting solution to remove any residual water from the washing. The resulting slurry was filtered through a syringe packed with silica gel (40–63 μm mesh size, VWR) and washed using a solution of 90% hexane with 10% diethyl ether by volume. Excess solvent was removed using a rotary evaporator and the solution was heated under vacuum (7 mbar, 170 °C) for 70 minutes to remove the volatile material to yield a slightly yellow, clear and viscous liquid which was confirmed by NMR to be deuterated diethyl hexyl phthalate (d-DEHP). NMR data from characterisation are presented in Fig. S2 in the ESI.†To synthesise deuterated BTHC, deuterated hexanol (Sigma-Aldrich) and citric acid (Sigma-Aldrich) were added to a capped vial in a 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with a catalytic amount of p-toluene sulfonic acid. The vial was heated to 140 °C for 4 hours before the product was washed with NaHCO3 and filtered through a syringe packed with silica gel (40–63 μm mesh size, VWR). The resulting product was confirmed by NMR to be trihexyl citrate. Trihexyl citrate was combined with butanoic acid chloride and triethylamine in the presence of catalytic amounts of 4-dimethylaminopyridine amine and acid chloride was added in a 1
1 ratio with a catalytic amount of p-toluene sulfonic acid. The vial was heated to 140 °C for 4 hours before the product was washed with NaHCO3 and filtered through a syringe packed with silica gel (40–63 μm mesh size, VWR). The resulting product was confirmed by NMR to be trihexyl citrate. Trihexyl citrate was combined with butanoic acid chloride and triethylamine in the presence of catalytic amounts of 4-dimethylaminopyridine amine and acid chloride was added in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio but in excess of trihexyl citrate. The acid chloride was added dropwise to the mixture under cooling in an ice bath. The resulting solution was purified using a Kugelrohr distillation apparatus to separate the trihexyl citrate from BTHC. Deuterated butanoyl trihexyl citrate (d-BTHC) was confirmed by NMR; however, the unreacted trihexyl citrate was still present even after distillation at around 15%. NMR data from characterization are presented in Fig. S3 in the ESI.†
1 molar ratio but in excess of trihexyl citrate. The acid chloride was added dropwise to the mixture under cooling in an ice bath. The resulting solution was purified using a Kugelrohr distillation apparatus to separate the trihexyl citrate from BTHC. Deuterated butanoyl trihexyl citrate (d-BTHC) was confirmed by NMR; however, the unreacted trihexyl citrate was still present even after distillation at around 15%. NMR data from characterization are presented in Fig. S3 in the ESI.†
Neutron experiments
Neutron reflectivity measurements were made at the ISIS Neutron and Muon Source with the SURF24 and INTER25 instruments using time of flight to record spectra at either two or three different angles to obtain reflectivity data in the momentum transfer range of 0.01 to 0.3 Å−1. They used a Langmuir trough26 (Nima) of total area about 600 cm2 mounted on the reflectometer in a box with quartz windows to reduce evaporation. The sub-phase used in the Langmuir trough was the mixture of H2O and D2O made up to have a neutron scattering length of zero, matching that of air. Monolayers can then be formed on the surface of the sub-phase by spreading a dilute solution containing lipids and plasticizers in chloroform. Data were reduced by dividing the reflected spectra by the direct beam normalised by the incident beam intensity using standard procedures available using the instruments. Reflectivity data were analysed using custom software, mono and mcatamaran, that allow simultaneous modelling of mixed monolayers of two components in terms of surface excess of each component and thickness.Stock solutions of each component were made in chloroform and mixed in appropriate ratios to obtain the spreading solutions that were used. Spreading solutions were added dropwise to the water surface on the Langmuir trough with a Hamilton-syringe until the surface pressure reached between 4 and 5 mN m−1. The Langmuir trough was kept at a constant temperature of 17 °C throughout the measurements. Neutron measurements were made at 5, 15, 25 and 30 mN m−1 on compression of the layer, and in some cases as described below, on expansion to 25, 15 and 5 mN m−1. When each pressure target was reached, the trough area was held constant until the neutron measurement was finished. The Langmuir trough was kept in a closed container to limit the effect of water evaporation due to the long measurement times. The trough was emptied and cleaned with chloroform after each set of surface pressures were measured.
Results and discussion
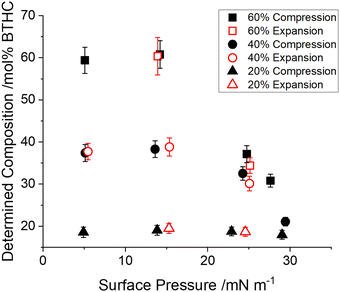
Neutron reflectivity was measured on mixed monolayers containing DMPC and plasticiser (DEHP or BTHC). Each plasticiser was measured at four compositions (0, 20, 40 and 60 mol%). Each composition was prepared at two contrasts with either the lipid or the plasticiser isotopically labelled with deuterium. For both plasticisers a third contrast was also measured at 60 mol% plasticiser content, with both the components labelled with deuterium. The lipid/plasticiser mixtures were measured on a Langmuir trough to control and measure the surface pressure. All the contrasts for a given composition and surface pressure were co-fitted with a single model from which the parameters describing the sample were extracted. Examples of the reflectivity data and the fitted model are shown in Fig. 1 and 2 for DEHP and BTHC at the highest concentration. Reflectivity data for the other samples are shown in Fig. S7 and S8 in the ESI.†The use of contrasts with the different components deuterated means that the amount of each component at the interface can be well-determined. Scattering lengths for the deuterated and normal hydrogenous materials are listed in Table S1 in the ESI.† These values indicate why it is necessary to include the contributions from both components in the overall model when there are small amounts of deuterated material present at the surface. The compositions derived from the fits of data for each measured monolayer are shown in Fig. 3, as a function of the surface pressure. From this data it can be observed that there is a maximum amount of plasticiser that can fit into the DMPC layer. At low surface pressures, the interface composition does not change with the plasticiser content and similarly composition remains constant if the plasticiser content is low. This remains true for both DEHP and BTHC. At higher surface pressures, plasticiser is selectively removed from the interface when the initial plasticiser content is high. Differences between DEHP and BTHC are evident here. DMPC monolayers containing 60 mol% DEHP start to lose a significant amount of DEHP content, while at 40 mol% DEHP the composition remains stable across the measured surface pressures. DMPC monolayers containing BTHC, on the other hand, exhibit significant changes in the interface composition already at an initial BTHC content of 40%. In both cases 20 mol% plasticiser content results in no observed compositional changes.
In the case of monolayers containing DMPC and BTHC, additional measurements were made during the expansion of a previously compressed monolayer. These were measured and fitted in the same way as the compressions and information of changes in the composition could be determined. These data are shown in Fig. 4. The same compression data that are shown in Fig. 3b are compared to the compositions obtained from expansion of the monolayers that were previously compressed. The plasticiser removed from the layer during the initial compression is reintroduced to the interfacial layer when it is allowed to expand. This process appears to be completely reversible with no changes observed in the determined composition between compression and expansion.
In the case of DMPC and DEHP, initial measurements did not include both the compression and expansion of the monolayers as made with BTHC. Limited checks of the reversibility were made: only a single contrast and composition was measured that focused on determining the surface excess of deuterated DEHP in the monolayer with DMPC. Results from this measurement, along with data taken for BTHC under compression and expansion, are shown in Fig. 5. These trends are similar for both DEHP and BTHC in DMPC monolayers. They show a minimum in area per molecule, corresponding to a maximum in surface excess, when plotted against the surface pressure although the area occupied by the different plasticiser molecules is quite different. For a system where the composition at the interface does not change with surface pressure we would expect the area per molecule to decrease with an increase in surface pressure as the surface layer becomes more densely packed. The data represented here indicate the change in composition and its reversibility. DEHP shows the same behaviour as BTHC in this regard. Unfortunately, it was not possible to spread films of either pure DEHP or BTHC on water, although this was attempted, and so direct comparison of surface pressure–area isotherms and structure with those of mixed monolayers is not possible.
Along with composition of the monolayer, the multiple contrasts also allow for an accurate determination of the area per molecule or surface excess. These quantities are the inverse of one another. In Fig. 6, the area per molecule is plotted against the determined interfacial composition. Both of these quantities are obtained from fitting neutron reflectivity data. For DEHP no changes in the area per molecule are seen at the two lowest pressures (5 and 15 mN m−1) but, at higher pressures (25 and 30 mN m−1), the area per molecule decreases with increasing DEHP content. The observed area per molecule for the pure DMPC layer compares well with the results reported previously such as those of Johnson et al.16 who reported 61 and 87 Å2 at surface pressures of 10 and 30 mN m−1, respectively, at a slightly higher temperature of 22 °C. This implies that at lower surface pressures (corresponds to lower density in the layer) a DEHP molecule occupies a similar area to DMPC but at a higher surface pressure (corresponds to a higher density in the layer), it occupies a smaller area. Conversely, BTHC occupies a larger area than DMPC at low surface pressures. As the layer gets denser BTHC occupies a smaller relative area similar to that observed for DEHP. However, this effect is less pronounced in the case of BTHC. Both systems seem to undergo a phase transition of sorts between low pressures and plasticiser content and high pressures and plasticiser content. When the plasticiser content is low and the monolayer has a low density, the plasticisers spread out more across the available space. However, when the monolayer gets increasingly dense, the plasticisers conform to the smaller space available and occupy a smaller area. This remains true for both plasticisers; however, BTHC occupies a larger area at the interface than DEHP and is less capable of reducing the area it occupies. The molecular structure of the individual plasticisers is in line with this observation, as BTHC is bulkier than DEHP and the shape of DEHP is more like that of a lipid. Another consequence of the measurements with multiple contrasts is the confidence in the model of a single uniformly mixed layer. Attempts to add separation within the layer did not provide good fits to the data.
As mentioned, previously the deuterated BTHC was slightly impure. The 15% of the deuterated BTHC missing the butanoyl moiety does not impact the level of deuteration, as the deuteration is entirely located on the hexyl chains. The hydrogenous material had 15% of the corresponding impurity added to ensure comparable results. Scattering lengths used in the fitting procedure considered the impurity in both cases. Finally, the expected change in the molecule size due to the lack of the butanoyl chain is relatively small due to the already bulky nature of BTHC. We estimate that the area per molecule at the surface would be about 2% smaller due to the presence of the impurity in the case of BTHC. The neutron data clearly indicate that BTHC increases the average area per molecule compared to DEHP despite the impurity. Because of this we do not believe that the presence of the impurity has a significant effect on how the results are interpreted.
Another parameter extracted from fitting the monolayer model to neutron reflectivity data is the thickness. However, due to the limited range of momentum transfer available with a significant reflected signal above the background, the fits are less sensitive to the thickness of the layer. No clear trends in layer thickness with composition are observed for the layers with either DEHP or BTHC. The monolayer thicknesses are shown in Fig. S9 in the ESI,† plotted against surface pressure and are in the range of 16 to 22 Å. It should be noted that the fitted value of the thickness has little impact on the fitted value of the surface excess, as they are uncorrelated and independent under the conditions of this experiment.27
Pressure–area isotherms were recorded at the same time as reflectivity data were collected. Example data for DMPC/DEHP and DMPC/BTHC mixtures are shown in Fig. 7a. Each sample had its reflectivity measured with the trough barriers at constant area after compression to the four surface pressures (5, 15, 25 and 30 mN m−1). The drops in surface pressure occur during the long neutron measurement period as shown in Fig. 7b, for example at times of about 4000 and 7000 s. In both cases the shape of the isotherm changes with the initial plasticiser content. The isotherm has a less steep increase with added plasticiser. The appearance of a shoulder at a surface pressure of about 20 mN m−1 in the isotherm becomes increasingly apparent. This is a behaviour commonly associated with a phase transition in the isotherm. In the neutron data we observed changes in both composition and area per molecule and it is reasonable that this is correlated with the ‘transition’ that is observed in the isotherms. DEHP and BTHC data were collected with two different instruments at two different times. Expansion of the trough area was not made for all the DEHP measurements but was studied systematically for BTHC. The comparison between surface pressure and trough area for the plasticisers on compression and expansion is shown in Fig. 7. Other recorded isotherms are provided in the ESI.† Although the compositions as determined by neutron reflectivity are the same within error upon compression and expansion of the trough area, the isotherms are not identical as apparently small amounts of both lipid and plasticiser are lost during measurement. The pressure changed slightly during the time of neutron measurements. For example, the monolayer with 60% d-BTHC that is shown in Fig. 7b showed a reduction in surface pressure from 25.0 to 24.6 mN m−1 (mean 24.7, standard deviation 0.1 mN m−1) during compression measurement. This was typical and the results shown in Fig. 3–5 are plotted against mean values of surface pressure during the measurements of the samples with the same chemical but different isotopic compositions although, for brevity of labelling, the legends in Fig. 2 and 6 show the initial values.
The small overall change in the amount of material at the interface is quite distinct to the composition change that is large. The experiment cannot identify directly where the removed plasticiser is located at high surface pressures. A separate extra interfacial layer was not observed. One could therefore speculate that the plasticisers form small beads or droplets at the surface, where they do not have a significant effect on the scattering or reflection from the surface.
The study by Bider et al. indicates that DEHP molecules enter the hydrophobic tail region of a lipid (POPC) but that the plasticiser molecules are located predominantly close to the headgroups and that the ordering of the lipid tails was found to increase at high DEHP concentrations while the curvature also increased. The present neutron reflectivity study shows that both DEHP and BTHC can be incorporated in DMPC monolayers at concentrations well above the 1 and 9 mol% DEHP reported by Bider et al.13
The complexity of the clinical significance of DEHP leaching from blood storage containers is long established, for example, Sasakawa and Mitomi have reported levels of DEHP as high as 50 μg mL−1 in stored blood and blood products.28 The amount of DEHP in the blood of transfused patients has been reported to be as high as 1 μg mL−1 in that study. It is still of interest to identify how DEHP is transported and incorporated in the body as these mechanisms remain unclear. As PVC plasticized with DEHP is still widely used for whole blood storage as it provides good stability,3 it remains of interest to understand why other plasticizers such as BTHC do not impart that benefit. The present studies indicate that it may give rise to different changes in lipid layers that are subject to stress such as the compression on a trough. Further studies will be needed to investigate interactions in the presence of other blood components such as albumin proteins and cholesterol.
Conclusions
In the case of both DEHP and BTHC, large amounts of the plasticiser can be incorporated in a DMPC monolayer. The content changes when the surface layer is compressed. At a high plasticiser content, plasticiser is selectively removed from the layer as the surface pressure increases. This effect is observed at lower plasticiser content for BTHC than for DEHP, i.e., less BTHC fits into a compact DMPC layer. Furthermore, BTHC molecules occupy a larger area at the interface than DEHP molecules. A phase transition in area per molecule can be observed at the point where plasticiser content starts changing. As the surface film is compressed, the average area per molecule decreases for both the DEHP-lipid and BTHC-lipid monolayers. The same phase transition can also be observed in the pressure–area isotherms that correspond to the change in plasticiser content and area per molecule. The changes in plasticiser content and area per molecule are completely reversible as both the material and the area per molecule is recovered upon expanding the layer.This study has identified that much more plasticiser can be incorporated with lipids than has been known previously.13 The differences between DEHP and BTHC are significant and provide clues as to how different plasticisers may affect lipid layers. Further studies will be needed to make progress in clinical systems where the interest is likely to centre on why DEHP favourably changes the storage life of blood and whether an appropriate substitute with that advantage can be found.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank SSF for the funding for EG in the SwedNESS graduate school, grant number GSn15-0008. The ISIS Pulsed Neutron and Muon Facility is thanked for the allocations of beam time under RB1810574 (SURF) and RB1820418 (INTER). The original neutron reflection data are available at https://doi.org/10.5286/ISIS.E.RB1810574 and https://doi.org/10.5286/ISIS.E.RB1820418.Notes and references
- Regulation 2017/745 of the European Parliament and of the Council of 5 April 2017.
- M. Rahman and C. S. Brazel, Prog. Polym. Sci., 2004, 29, 1223–1248 CrossRef CAS.
- C. V. Prowse, D. de Korte, J. R. Hess, P. F. van der Meer and the Biomedical Excellence for Safer Transfusion Collaborative, Vox Sang., 2014, 106, 1–13 CrossRef CAS PubMed.
- J. A. Tickner, T. Schettler, T. Guidotti, M. McCally and M. Rossi, Am. J. Ind. Med., 2001, 39, 100–111 CrossRef CAS PubMed.
- K. M. Shea, Pediatrics, 2003, 111, 1467–1474 CrossRef PubMed.
- P. R. Graham, Environ. Health Perspect., 1973, 3, 3–12 CAS.
- B. Horowitz, M. H. Stryker, A. A. Waldman, K. R. Woods, J. D. Gass and J. Drago, Vox Sang., 1985, 48, 150–155 CAS.
- J. P. AuBuchon, T. N. Estep and R. J. Davey, Blood, 1988, 71, 448–452 CrossRef CAS PubMed.
- T. N. Estep, R. A. Pedersen, T. J. Miller and K. R. Stupar, Blood, 1984, 64, 1270–1276 CrossRef CAS PubMed.
- G. Latini, A. Verrotti and C. De Felice, Curr. Drug Targets: Immune, Endocr. Metab. Disord., 2004, 4, 37–40 CAS.
- M. Wormuth, M. Scheringer, M. Vollenweider and K. Hungerbühler, Risk Anal., 2006, 26, 803–824 CrossRef PubMed.
- B. Bicalho, K. Serrano, A. dos Santos Pereira, D. V. Devine and J. P. Acker, Transfus. Medi. Hemother., 2016, 43, 19–26 CrossRef PubMed.
- R.-C. Bider, T. Lluka, S. Himbert, A. Khondker, S. M. Qadri, W. P. Sheffield and M. C. Rheinstädter, Langmuir, 2020, 36, 11899–11907 CrossRef CAS PubMed.
- E. Gustafsson, T. Melander Bowden and A. R. Rennie, Adv. Colloid Interface Sci., 2020, 277, 102109 CrossRef CAS PubMed.
- L. K. Nielsen, T. Bjørnholm and O. G. Mouritsen, Langmuir, 2007, 23, 11684–11692 CrossRef CAS PubMed.
- S. J. Johnson, T. M. Bayerl, W. Weihan, H. Noack, J. Penfold, R. K. Thomas, D. Kanellas, A. R. Rennie and E. Sackmann, Biophys. J., 1991, 60, 1017–1025 CrossRef CAS PubMed.
- V. M. Kaganer, H. Möhwald and P. Dutta, Rev. Mod. Phys., 1999, 71, 779–819 CrossRef CAS.
- K. Sabatini, J.-P. Mattila and P. K. J. Kinnunen, Biophys. J., 2008, 95, 2340–2355 CrossRef CAS PubMed.
- N. Elstone, T. Arnold, M. W. A. Skoda, S. E. Lewis, P. Li, G. Hazell and K. J. Edler, Phys. Chem. Chem. Phys., 2022, 24, 22679–22690 RSC.
- R. K. Thomas and J. Penfold, Curr. Opin. Colloid Interface Sci., 1996, 1, 23–33 CrossRef CAS.
- R. K. Thomas, Annu. Rev. Phys. Chem., 2004, 55, 391–426 CrossRef CAS PubMed.
- V. F. Sears, Neutron News, 1992, 3(3), 26–37 CrossRef.
- Z. X. Li, J. R. Lu, R. K. Thomas and J. Penfold, J. Phys. Chem. B, 1997, 101, 1615–1620 CrossRef CAS.
- J. Penfold, R. M. Richardson, A. Zarbakhsh, J. R. P. Webster, D. G. Bucknall, A. R. Rennie, R. A. L. Jones, T. Cosgrove, R. K. Thomas, J. S. Higgins, P. D. I. Fletcher, E. Dickinson, S. J. Roser, I. A. McLure, A. R. Hillman, R. W. Richards, E. J. Staples, A. N. Burgess, E. A. Simister and J. W. White, J. Chem. Soc., Faraday Trans., 1997, 93, 3899–3917 RSC.
- J. Webster, S. Holt and R. Dalgliesh, Phys. B, 2006, 385–386, 1164–1166 CrossRef CAS.
- G. T. Barnes and I. R. Gentle, Interfacial Science: An introduction, Oxford University Press, Oxford, 1st edn, 2005 Search PubMed.
- T. L. Crowley, Phys. A, 1993, 195, 354–374 CrossRef.
- S. Sasakawa and Y. Mitomi, Vox Sang., 1978, 34, 81–86 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sm01611k |
| ‡ Present address: Swedish Research Council, Sweden. |
| This journal is © The Royal Society of Chemistry 2024 |