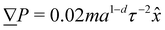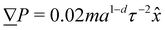Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lock-key microfluidics: simulating nematic colloid advection along wavy-walled channels
Karolina
Wamsler
,
Louise C.
Head
 and
Tyler N.
Shendruk
and
Tyler N.
Shendruk
 *
*
School of Physics and Astronomy, The University of Edinburgh, Peter Guthrie Tait Road, Edinburgh, EH9 3FD, UK. E-mail: t.shendruk@ed.ac.uk
First published on 16th April 2024
Abstract
Liquid crystalline media mediate interactions between suspended particles and confining geometries, which not only has potential to guide patterning and bottom-up colloidal assembly, but can also control colloidal migration in microfluidic devices. However, simulating such dynamics is challenging because nemato-elasticity, diffusivity and hydrodynamic interactions must all be accounted for within complex boundaries. We model the advection of colloids dispersed in flowing and fluctuating nematic fluids confined within 2D wavy channels. A lock-key mechanism between homeotropic colloids and troughs is found to be stronger for planar anchoring on the wavy walls compared to homeotropic anchoring on the wavy walls due to the relative location of the colloid-associated defects. Sufficiently large amplitudes result in stick–slip trajectories and even permanent locking of colloids in place. These results demonstrate that wavy walls not only have potential to direct colloids to specific docking sites but also to control site-specific resting duration and intermittent elution.
1 Introduction
Manipulating particle trajectories is an important feature of many microfluidic applications, including drug delivery,1 cell sorting2 and medical diagnoses.3 Accordingly, a variety of approaches have been developed to control the trajectories of microparticles.4–7 One means of sculpting particle trajectories is by suspending them in complex carrier fluids, as in topological microfluidics,8 in which particles are guided by liquid crystalline carrier fluids.9 Liquid crystalline materials are alluring for microfluidic transport because they are highly responsive to flows,10–12 suspended inclusions13,14 and confining surfaces.15–17 When colloidal particles are dispersed in liquid crystalline fluids, the anisotropic nature of liquid crystals gives rise to emergent properties18–21 and imposed anchoring at colloidal surfaces results in topological defects in the vicinity of the colloids to ensure topological charge neutrality. Strong homeotropic or planar anchoring endows the surface with a topological charge of +1 and necessitates the existence of an accompanying −1 charge in the bulk fluid, either as two −1/2 point defects in 2D, or in 3D as defect loops (Saturn rings), −1 point defect (hyperbolic hedgehog), or surface defects (boojum defects).Such defects mediate particle–particle22–24 and particle–wall interactions.25,26 Particle–wall elastic interactions allow micropatterned structures on walls to have significant, long-ranged effects on colloids.27,28 For example, indentations in walls can govern colloid position: when the width of an indentation is comparable to colloid size there can be a strong attraction,29 whereas this attraction is weak when the width is much greater or smaller than the diameter of the colloid.27,30,31 These ideas were advanced by investigating periodic wavy boundaries, in which periodic nematic deformations near the wavy walls causes colloids with Saturn ring defects to be attracted to the troughs, and dock via a ‘lock-and-key’ mechanism, while docking location depends on the orientation of the defect for colloids with hedgehog defects.32 Variations of this setup have included imposed twist,33 saw-tooth or crenellated substrates,34 and ellipsoidal35 or four-armed colloids.36 In addition to setting stable docking sites, structured boundaries can control dynamic trajectories. Trajectories can arise due to diffusion or external fields,37 with docking or continued motion controlled by a balance between nemato-elasticity and driving force. Periodic structures, in particular, allow precise control of colloidal transport.38 In these cases, nematic colloids must respond to both elastic deformations through free energy minimization and advection through drag.
In this manuscript, we model discoidal colloids advecting in fluctuating 2D nematics flowing through microfluidic wavy channels (Fig. 1) and vary the amplitude of the undulations. We investigate this using a multi-particle collision dynamics (MPCD) algorithm to simulate thermalized nematodynamics and the inclusion of colloidal particles, and we provide a method for implementing wavy boundaries. This approach includes colloidal diffusion, nemato-elasticity and hydrodynamics within complex boundaries. Inspired by experimental studies in which docking sites are of comparable size to colloids,32,33,35 we focus on the effect of the amplitude of the wavy boundaries on the colloidal trajectories and velocities, for different anchoring conditions, and demonstrate that these systems have the potential to trap colloids at given points or allow colloidal conveyance. For intermediate amplitudes, elution follows stick–slip dynamics, with the “sticking” duration increasing with amplitude until the colloid locks in place. The amplitude at which the transition from stick–slip to locking occurs decreases with nematic elasticity for a given pressure gradient. We consider colloids with strong homeotropic anchoring but allow the anchoring on the channel walls to vary. Our results show simple microfluidic systems can temporarily lock particles in place by combining surface structure and advective flow, allowing precise control over elution dynamics and the ability to lock colloids in place for specific times.
2 Methods
To simulate colloids in a flowing and thermally fluctuating nematic liquid crystal confined within a complex channel, a multi-particle collision dynamics (MPCD) algorithm is used.39–42 MPCD has been employed to simulate reaction–diffusion dynamics,43,44 electrophoresis,45,46 thermophoresis,47 swimmers,48–50 polymers,51–54 colloidal suspensions,55,56 binary mixtures,57 viscoelastic fluids,58 ferrofluids,59,60 and dense stellar systems.61–63 Most relevantly, MPCD has been used to simulate nematic liquid crystals.64 In this context, MPCD has simulated nematohydrodynamics,65 suspended colloids,66,67 magnetic colloids,68,69 living liquid crystals,70 and active nematics.71–73 For the present study, MPCD is chosen because it is ideal for moderate Péclet numbers, mobile solutes and complex boundaries.The nematic MPCD (N-MPCD) algorithm discretizes the continuous fluid into N point particles. Each such point particle i is given a set mass mi = m ∀ i, possesses an instantaneous velocity ![[v with combining low line]](https://www.rsc.org/images/entities/i_char_0076_0332.gif) i(t) and unit-length orientation
i(t) and unit-length orientation ![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) i(t) to model the direction of the nematogens at each instant t. The MPCD algorithm consists of two steps:74,75 (i) streaming and (ii) collision. The streaming step (see Appendix A) updates each particle position
i(t) to model the direction of the nematogens at each instant t. The MPCD algorithm consists of two steps:74,75 (i) streaming and (ii) collision. The streaming step (see Appendix A) updates each particle position ![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif) i assuming ballistic motion over a time step δt. The collision step (see Appendix A) simulates the interactions between fluid particles via a coarse-grained collision operator. The collision operator stochastically updates the particles' velocities and orientations, while constrained to respect conservation laws. To do this, the simulation domain is split into cubic cells of size a and index c containing Nc(t) particles at any instant t. A random grid shift ensures Galilean invariance.76 Only particles in the same cell interact and the interactions involve all particles in the same cell. The average number of particles per cell sets the fluid density ρ = m〈Nc〉/ad, where 〈·〉 is the average over all cells and d = 2 for a two-dimensional fluid. The collision event can itself be broken into three stages (see Appendix A):
i assuming ballistic motion over a time step δt. The collision step (see Appendix A) simulates the interactions between fluid particles via a coarse-grained collision operator. The collision operator stochastically updates the particles' velocities and orientations, while constrained to respect conservation laws. To do this, the simulation domain is split into cubic cells of size a and index c containing Nc(t) particles at any instant t. A random grid shift ensures Galilean invariance.76 Only particles in the same cell interact and the interactions involve all particles in the same cell. The average number of particles per cell sets the fluid density ρ = m〈Nc〉/ad, where 〈·〉 is the average over all cells and d = 2 for a two-dimensional fluid. The collision event can itself be broken into three stages (see Appendix A):
1. Stochastic momentum exchange: particle velocities are updated by a collision operator Ξi,c(t) for particle i in cell c at time t. An Andersen-thermostatted collision operator randomly generates velocities from a Boltzmann distribution with energy kBT.77,78 This operator conserves momentum and thermostats the energy.
2. External forces: a pressure gradient is applied as 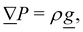 where the effective external acceleration
where the effective external acceleration ![[g with combining low line]](https://www.rsc.org/images/entities/i_char_0067_0332.gif) is included in the collision operator.
is included in the collision operator.
3. Orientation exchange: in N-MPCD, each particle's orientation ![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) i is stochastically drawn from the Maier–Saupe distribution about each cell's director
i is stochastically drawn from the Maier–Saupe distribution about each cell's director ![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif) c as pc(
c as pc(![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) i) ∝ exp(USc(
i) ∝ exp(USc(![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif) c·
c·![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) i)2/kBT), where Sc is the scalar order parameter of cell c and U is the mean field potential, which controls how strongly the orientations align. Additionally, MPCD particle orientation responds to velocity gradients through Jeffery's equation, which is parameterized by a bare tumbling parameter ξ and heuristic shear coupling coefficient χ. The χ parameter acts as a relaxation parameter, effectively allowing Jeffrey's equation to be averaged over a small number of time steps of the fluctuating hydrodynamic field. Finally, director dynamics are coupled back to the velocity field through local torques parameterized by a rotational mobility coefficient γR (see Appendix A for details).64,69 A small value is chosen to keep backflow effects to a minimum. Numerical analysis has shown that N-MPCD describes a linearized nematohydrodynamic model in which viscosity and elastic effects are isotropic.79
i)2/kBT), where Sc is the scalar order parameter of cell c and U is the mean field potential, which controls how strongly the orientations align. Additionally, MPCD particle orientation responds to velocity gradients through Jeffery's equation, which is parameterized by a bare tumbling parameter ξ and heuristic shear coupling coefficient χ. The χ parameter acts as a relaxation parameter, effectively allowing Jeffrey's equation to be averaged over a small number of time steps of the fluctuating hydrodynamic field. Finally, director dynamics are coupled back to the velocity field through local torques parameterized by a rotational mobility coefficient γR (see Appendix A for details).64,69 A small value is chosen to keep backflow effects to a minimum. Numerical analysis has shown that N-MPCD describes a linearized nematohydrodynamic model in which viscosity and elastic effects are isotropic.79
The wavy 2D channel is modeled with plane-wavy solid boundaries (see Appendix B). Each surface (both bounding walls and colloidal) is labelled by index b and represented as a surface on which 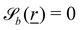 . A baseline surface is defined for planes and, to account for wavy boundaries, a second term is included, which allows waves with amplitude Bb,0 and frequency Bb,1. In this study, we focus on 2D; however, this framework allows simulations of egg-carton and corrugated walls (Appendix B). Both the top and bottom wavy walls of the channel have the same amplitude Bb,0 = B0 and wavelength λ given by
. A baseline surface is defined for planes and, to account for wavy boundaries, a second term is included, which allows waves with amplitude Bb,0 and frequency Bb,1. In this study, we focus on 2D; however, this framework allows simulations of egg-carton and corrugated walls (Appendix B). Both the top and bottom wavy walls of the channel have the same amplitude Bb,0 = B0 and wavelength λ given by 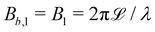 for all wall boundaries, where
for all wall boundaries, where 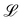 is the system size in that direction. The boundaries defining the discoidal colloids are smooth and so Bb,0 = Bb,1 = 0 for all colloids, though wavy colloids and cylinders are possible in our framework (Appendix C). Bounce-back boundary conditions with phantom particles ensure no-slip.80–82 Periodic boundary conditions are plane surfaces at the channel extremities.
is the system size in that direction. The boundaries defining the discoidal colloids are smooth and so Bb,0 = Bb,1 = 0 for all colloids, though wavy colloids and cylinders are possible in our framework (Appendix C). Bounce-back boundary conditions with phantom particles ensure no-slip.80–82 Periodic boundary conditions are plane surfaces at the channel extremities.
2.1 Simulation parameters
The simulation units are particle mass m, thermal energy kBT and cell size a. From these, the time unit is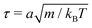 . Additional derived units include dynamic viscosity μ0 ≡ kBTτ/ad,78 stress kBTa−d and Frank elasticity kBTa2−d. In these two dimensional (d = 2) simulations, the time step is set to δt = 0.1τ and density is set to ρ = 20m/a2. The mean field potential is U = 10kBT (unless otherwise stated), placing the fluid deep in the nematic phase.64 A mean field potential of U = 5kBT is relatively close to the nematic–isotropic transition point, with a system-wide (averaged over all cells c) scalar parameter S ≈ 0.5, while U = 10kBT and 20kBT have scalar parameters much closer to S ≈ 1.64 These large values are an idealization that ensures the system is deep in the nematic phase and that elastic constants are large enough to ensure small Ericksen numbers (Appendix D). The Frank elastic constants were previously found to obey a one constant approximation and be linear with the mean field potential as K = (113 ± 6)U for ρ = 20m/a2.64 The nematic is in the flow aligning regime with ξ = 2, shear susceptibility χ = 0.5 and rotational friction γR = 0.01τkBT. The choice of small rotational friction minimizes the amount of backflow and limits any backflow-related effects in this study. The gravitational acceleration is set to
. Additional derived units include dynamic viscosity μ0 ≡ kBTτ/ad,78 stress kBTa−d and Frank elasticity kBTa2−d. In these two dimensional (d = 2) simulations, the time step is set to δt = 0.1τ and density is set to ρ = 20m/a2. The mean field potential is U = 10kBT (unless otherwise stated), placing the fluid deep in the nematic phase.64 A mean field potential of U = 5kBT is relatively close to the nematic–isotropic transition point, with a system-wide (averaged over all cells c) scalar parameter S ≈ 0.5, while U = 10kBT and 20kBT have scalar parameters much closer to S ≈ 1.64 These large values are an idealization that ensures the system is deep in the nematic phase and that elastic constants are large enough to ensure small Ericksen numbers (Appendix D). The Frank elastic constants were previously found to obey a one constant approximation and be linear with the mean field potential as K = (113 ± 6)U for ρ = 20m/a2.64 The nematic is in the flow aligning regime with ξ = 2, shear susceptibility χ = 0.5 and rotational friction γR = 0.01τkBT. The choice of small rotational friction minimizes the amount of backflow and limits any backflow-related effects in this study. The gravitational acceleration is set to ![[g with combining low line]](https://www.rsc.org/images/entities/i_char_0067_0332.gif) = 0.001a/τ2
= 0.001a/τ2![[x with combining circumflex]](https://www.rsc.org/images/entities/i_char_0078_0302.gif) to generate a pressure gradient
to generate a pressure gradient 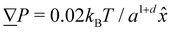 . Ten repeats (n = 10) of each configuration are run for 1.5 × 104τ.
. Ten repeats (n = 10) of each configuration are run for 1.5 × 104τ.
The channel is modeled with plane-wavy solid boundaries along ![[x with combining circumflex]](https://www.rsc.org/images/entities/i_char_0078_0302.gif) . Both walls have the same amplitude B0 and frequency B1. Planes set 60a apart define the channel walls with average normal ±ŷ. The domain size is 60a × (20a + 2B0) and the frequency of the wavy walls, B1, is set to π, so that 3 full periods fit within the length of the channel, which gives a wavelength λ = 20a. Positions of x = λ/4 and x = 3λ/4 correspond to the center of the trough and crest, respectively. The amplitude of the waves B0 is varied between 0 and 4.5a.
. Both walls have the same amplitude B0 and frequency B1. Planes set 60a apart define the channel walls with average normal ±ŷ. The domain size is 60a × (20a + 2B0) and the frequency of the wavy walls, B1, is set to π, so that 3 full periods fit within the length of the channel, which gives a wavelength λ = 20a. Positions of x = λ/4 and x = 3λ/4 correspond to the center of the trough and crest, respectively. The amplitude of the waves B0 is varied between 0 and 4.5a.
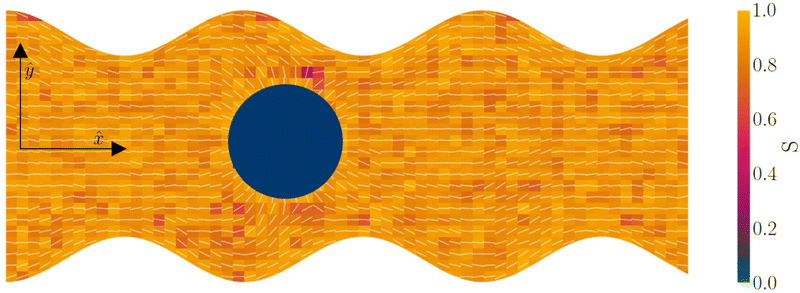
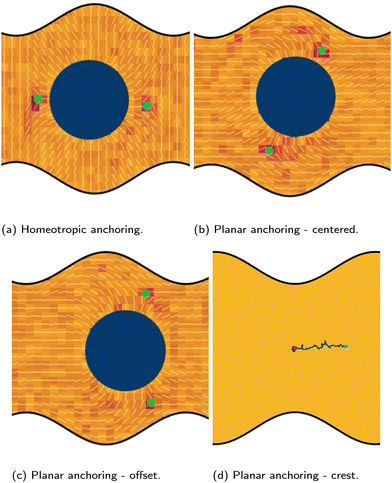
The colloid is simulated as a moving circular boundary with a radius R = 5a and mass ρπr2. The presence of the −1/2 companion defect pair (Fig. 1) indicates that the anchoring is strong (Appendix B) and that the characteristic anchoring length is small compared to the colloid size.83 Colloids are initialized with their center at ![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif) c = (x, y) = (25a, 10a + B0), which is between the wavy walls and offset to the left between a crest and a trough on the walls (Fig. 1), unless otherwise stated. Immediately after initialization, colloids are free to move in response to nematic and hydrodynamic forces. The colloid position
c = (x, y) = (25a, 10a + B0), which is between the wavy walls and offset to the left between a crest and a trough on the walls (Fig. 1), unless otherwise stated. Immediately after initialization, colloids are free to move in response to nematic and hydrodynamic forces. The colloid position ![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif) c is a continuous variable that is not constrained to the MPCD collision lattice. These choices define a characteristic length scale L for the geometry of our system. Here, L = 10a coincides with the diameter of the colloid 2R, the average distance from the centerline to the wall 〈h〉/2, and the distance from trough to crest λ/2. This length scale results in characteristic velocities. The velocity scale of the nematoelastic response is Ṽ = K/μLd−1 for viscosity μ and the scale due to pressure gradients is V = L2ρgx/μ. For the parameters used here, the nematoelastic speed Ṽ ≈ 12a/τ is much larger than the advective speed V ≈ 0.2a/τ (Appendix D), which we identify to be the characteristic speed of interest in our lock-key dynamics. In this study, all colloids have homeotropic anchoring but the anchoring on the channel walls varies.
c is a continuous variable that is not constrained to the MPCD collision lattice. These choices define a characteristic length scale L for the geometry of our system. Here, L = 10a coincides with the diameter of the colloid 2R, the average distance from the centerline to the wall 〈h〉/2, and the distance from trough to crest λ/2. This length scale results in characteristic velocities. The velocity scale of the nematoelastic response is Ṽ = K/μLd−1 for viscosity μ and the scale due to pressure gradients is V = L2ρgx/μ. For the parameters used here, the nematoelastic speed Ṽ ≈ 12a/τ is much larger than the advective speed V ≈ 0.2a/τ (Appendix D), which we identify to be the characteristic speed of interest in our lock-key dynamics. In this study, all colloids have homeotropic anchoring but the anchoring on the channel walls varies.
3 Results
Lock-key microfluidics result from two competing processes: nematoelastic relaxation pushes the colloids to locations that minimize the distortion free energy, while the minimum dissipation theorem leads colloids to advect with a velocity that matches the fluid flow. For this reason, we begin by simulating homeotropic colloid kinetics in the absence of externally driven flows (Section 3.1), then proceed to consider nematohydrodynamic flows in wavy channels in the absence of the colloid (Section 3.2) before putting the two aspects together to study lock-key microfluidics (Section 3.3). In all cases, each colloid has a topological charge of +1 and so necessitates the existence of companion defects in the vicinity of the colloid. In 3D, both Saturn rings and hedgehog defects are possible84 with rings more likely in simulations.85,86 In 2D only the quadrapolar state is expected to be stable87,88 and the results reported here are consistent with this since they exhibit a pair of −1/2 companion defects.3.1 Colloid trajectories with no pressure gradient
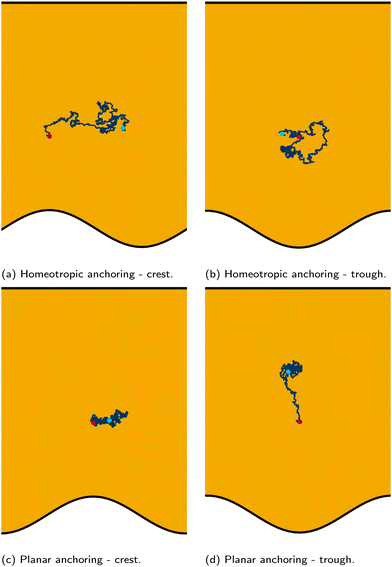
We place colloids into channels with one plane boundary wall and one wavy wall, with an amplitude of B0 = 2.5a. The nematic anchoring on the colloid is always homeotropic but both planar and homeotropic anchoring on the channel walls is considered. No pressure gradient is applied. The colloid is initially positioned either directly above a crest or trough (Fig. 2; top row) for homeotropic anchoring on the boundary walls. The observed numerical trajectories are consistent with Luo et al.37 For homeotropic anchoring on the boundary walls, the colloid is attracted to the trough, traversing away from the crest where it settles (Fig. 2a). The principle motion is in the![[x with combining circumflex]](https://www.rsc.org/images/entities/i_char_0078_0302.gif) direction with primarily stochastic motion in the ŷ direction. Although the colloid does not move substantially in the ŷ direction, careful inspection shows that it does drift upward since the repulsive forces from the different walls are not expected to balance at the same height for each x-position. When the colloid is initiated above the trough (x = λ/4), it still diffuses, but remains within the trough in the vicinity of the free energy minimum, exhibiting no deterministic forcing away from this point (Fig. 2b).
direction with primarily stochastic motion in the ŷ direction. Although the colloid does not move substantially in the ŷ direction, careful inspection shows that it does drift upward since the repulsive forces from the different walls are not expected to balance at the same height for each x-position. When the colloid is initiated above the trough (x = λ/4), it still diffuses, but remains within the trough in the vicinity of the free energy minimum, exhibiting no deterministic forcing away from this point (Fig. 2b).
Homeotropic anchoring on the colloid and planar anchoring on the walls was not investigated by Luo et al.,37 but shows interesting features (Fig. 2; bottom row). In the case of planar anchoring on the walls, the colloid is stable above the crest (Fig. 2c), rather than the trough. This is consistent with experimental images of the analogous case of a planar colloid being attracted to the crests of a homeotropic wall37 and with the trapping of colloidal particles at the tips of sharp protrusions whenever the colloid and surface have opposing anchoring conditions.89 In this case, the colloid simply diffuses as though it is in a well within the free energy landscape. Likewise, a colloid initiated above the trough does not move left or right; but rather, moves directly away from the wavy wall towards the upper plane wall (Fig. 2d). While the wall above the colloid is also repulsive, the repulsion is smaller than from the wavy wall. Thus, the homeotropic colloid's equilibrium position is closer to the plane wall than the wavy wall.
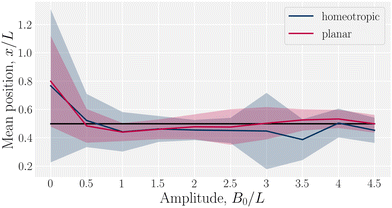
Having considered the dynamics of a colloid confined by one wavy wall, we further consider how homeotropic colloids move in response to wavy boundaries on both the lower and upper wall. The colloid is initialized in the center of the channel, and at the inflection point halfway between a trough and a crest. For both homeotropic and planar anchoring on the walls, colloids are observed to move towards the troughs (Fig. 3) and generally remains centered in the trough for all amplitudes.
While the colloid's equilibrium position is centered on the troughs in all cases, the locations of the colloid-associated defects differ (Fig. 4). When the colloid is confined between two wavy walls with strong homeotropic anchoring, the two accompanying −1/2 defects are found immediately to the left and right of the colloid along the centerline (Fig. 4a). However, for the same colloid initialized in the trough of a wavy channel with planar anchoring, the defects take a diagonal configuration (Fig. 4b), rotating off the centerline to the points where the walls are closest to the colloid. By residing in the smallest space between the planar walls and the homeotropic-anchored colloid, the defect pair can reduce the nematoelastic free energy. Since there are four equal points for which the distance between surfaces are minimal, the orientation of the diagonal configuration is spontaneous. When the colloid is initialized off center from at the crest between planar-anchored walls (Fig. 4c), the diagonal pairing of defects is no longer the preferred configuration. Instead the system prefers to have the two defects shifted towards the closest boundary. This configuration lowers the instantaneous deformation free energy by placing the pair of defects at the two minimal separation points between surfaces but the state is not stable. In this state, the colloid moves away from the crests towards the center of the trough. In this non-diagonal configuration, the defect separation is not as large as when they are found at polar opposite locations. Once the colloid approaches the center of the troughs one of the defects is able to escape the deformation barrier cost of crossing to the opposite side. Unlike the case of one wavy and one plane wall (Fig. 2), the crest is not a stable point (Fig. 4d).
3.2 Flowing nematodynamics
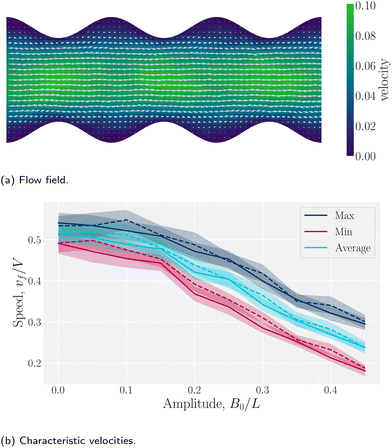
The previous section investigated the diffusive dynamics of colloids through the distortion free energy landscape of the nematic in the absence of externally driven flows (Section 3.1). We now consider pressure-driven flow of a nematic liquid crystal through a wavy microfluidic channel without colloids.Wavy channel walls complicate the hydrodynamics compared to plane walls (Appendix D), since the flow field ![[v with combining low line]](https://www.rsc.org/images/entities/i_char_0076_0332.gif) f has both
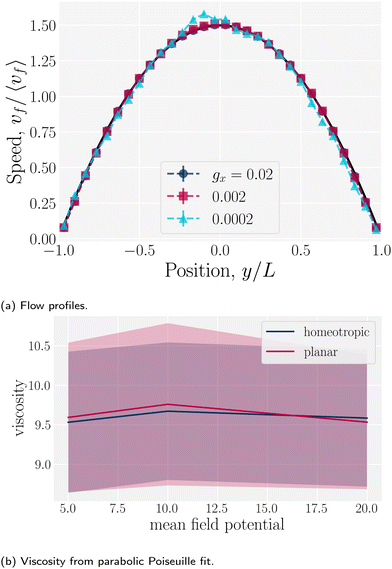
f has both ![[x with combining circumflex]](https://www.rsc.org/images/entities/i_char_0078_0302.gif) - and ŷ-components due to the changing channel height h(x) (Fig. 5a). Increasing the amplitude increases the surface area of the no-slip walls, which in turn increases hydrodynamic resistance. For this reason, the elution of fluid is expected to decrease compared to plane channels for constant pressure gradient. To quantify this, we compared the average, maximum and minimum velocities along the centerline of wavy channels for different amplitudes B0. Consistent with Appendix D, there is nearly no difference between the flow velocities of isotropic or nematic fluids through the channels (Fig. 5b). The average flow through a channel decreases monotonically with increasing amplitude for both nematic and isotropic fluids (Fig. 5b), suggesting that the hydrodynamic resistance increases proportionally in a manner that is not strongly dependent on nematicity. This is analogous to rough walls in plane channels, for which the flow through channels has been found to decrease non-linearly.90
- and ŷ-components due to the changing channel height h(x) (Fig. 5a). Increasing the amplitude increases the surface area of the no-slip walls, which in turn increases hydrodynamic resistance. For this reason, the elution of fluid is expected to decrease compared to plane channels for constant pressure gradient. To quantify this, we compared the average, maximum and minimum velocities along the centerline of wavy channels for different amplitudes B0. Consistent with Appendix D, there is nearly no difference between the flow velocities of isotropic or nematic fluids through the channels (Fig. 5b). The average flow through a channel decreases monotonically with increasing amplitude for both nematic and isotropic fluids (Fig. 5b), suggesting that the hydrodynamic resistance increases proportionally in a manner that is not strongly dependent on nematicity. This is analogous to rough walls in plane channels, for which the flow through channels has been found to decrease non-linearly.90
The difference between the maximum and minimum flow rates generally increases with the amplitude (Fig. 5b). Because N-MPCD is a coarse-grained algorithm for fluctuating hydrodynamics, a difference exists even for B0 = 0. The maximum velocity occurs between the crests where the channel is narrowest and the minimum between troughs (Fig. 5a). This is consistent with previous theoretical and numerical work on flow of isotropic fluids through wavy channels.91 Since the maximum velocity occurs between the crests and the minimum between troughs (Fig. 5a), an advected colloid will speed up and slow down with the fluid at these points, in the absence of nematoelastic forces, Since the nematic forces also tend to drive the colloid towards the center of the troughs, these dynamics can be expected to be further emphasized.
3.3 Lock-key microfluidics
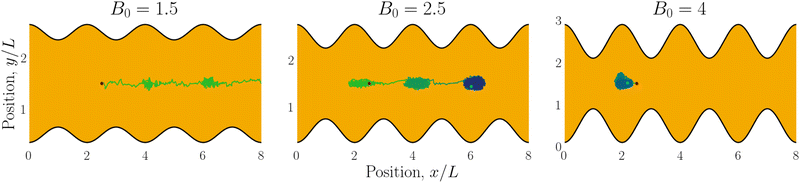
Having explored diffusive lock-key dynamics in wavy channels in the absence of advection (Section 3.1) and flows through wavy channels without colloids (Section 3.2), we now turn our attention to lock-key microfluidics by studying the advective dynamics of nematic colloids with homeotropic anchoring confined within wavy channels with planar anchoring. To investigate the colloidal transport rate through the channel, we track the position of a colloid through the channel (Fig. 6).We begin by considering individual trajectories of colloids. Three example trajectories are shown in Fig. 6 for small (B0 = 1.5; left), intermediate (B0 = 2.5; center) and large (B0 = 4; right) amplitudes. In the small amplitude case (Fig. 6; left), the stick slip dynamics are apparent in the ![[x with combining circumflex]](https://www.rsc.org/images/entities/i_char_0078_0302.gif) -component of the trajectories (Fig. 7a). Individual trajectories are seen to have periods within a trough (sticking events) punctuated by abrupt hops over the crest (slipping events). As the amplitude is increased (Fig. 6; center), the sticking duration increases substantially and slipping events become less frequent (Fig. 7a). Indeed by large amplitudes (Fig. 6; right), the colloids lock into their initial trough and are never observed to slip forward over the crest into the next trough (Fig. 7a).
-component of the trajectories (Fig. 7a). Individual trajectories are seen to have periods within a trough (sticking events) punctuated by abrupt hops over the crest (slipping events). As the amplitude is increased (Fig. 6; center), the sticking duration increases substantially and slipping events become less frequent (Fig. 7a). Indeed by large amplitudes (Fig. 6; right), the colloids lock into their initial trough and are never observed to slip forward over the crest into the next trough (Fig. 7a).
 | ||
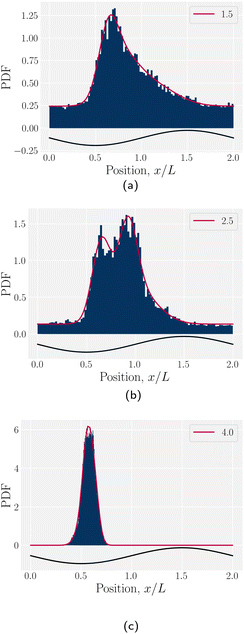
| Fig. 7 Colloid trajectories for different wall amplitudes B0 with homeotropic anchoring on the colloid and planar anchoring on the walls. Colors specify the amplitude B0. Black sinusoids on the right and left present undulations of the wavy walls. (a) Ten (n = 10) individual trajectories for each B0 (thin lines), along with cumulative probability density function t(x) (eqn (1); Fig. 7c). (b) Ensemble averaged x-positions of colloids as a function of time. (c) Ensemble averaged cumulative probability density function of position scaled by time (eqn (1)). | ||
To better quantify the elution rate, we next consider the ensemble average x-position of the colloid over time changes with the amplitude B0 (Fig. 7b). For B0 = 0, the transport occurs at a constant rate of advection. As B0 increases, the average rate decreases. By 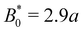 , the colloids are no longer advected through the channel. By averaging over many simulation runs, the elution appears to be rather smooth for all B0. However, we recognize that the average does not fully capture the dynamics seen in the individual runs, which exhibit clear a stick–slip dynamics.
, the colloids are no longer advected through the channel. By averaging over many simulation runs, the elution appears to be rather smooth for all B0. However, we recognize that the average does not fully capture the dynamics seen in the individual runs, which exhibit clear a stick–slip dynamics.
To better measure the stick–slip dynamics of the colloids, we consider the probability density function (PDF) p(x) of finding a colloid at a given position x. The PDF can be integrated to give a cumulative probability, which gives the average time taken to arrive at a point
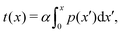 | (1) |
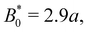 the colloid moves along the channel in a stick–slip manner (Fig. 7c). Stick–slip dynamics are only observed in nematic fluids and not in flowing isotropic fluids (see Appendix E). We can see that the time the colloid spends “sticking” to the docking site of the trough center increases with amplitude, until the repulsive forces from the crests become large enough to fully stop the colloid from advancing. In the case of B0 = 2.8a, the colloid often locks in place for the entire simulation and other times hops one or two troughs over the course of the entire simulation (Fig. 7a). The colloid permanently sticks at an amplitude of
the colloid moves along the channel in a stick–slip manner (Fig. 7c). Stick–slip dynamics are only observed in nematic fluids and not in flowing isotropic fluids (see Appendix E). We can see that the time the colloid spends “sticking” to the docking site of the trough center increases with amplitude, until the repulsive forces from the crests become large enough to fully stop the colloid from advancing. In the case of B0 = 2.8a, the colloid often locks in place for the entire simulation and other times hops one or two troughs over the course of the entire simulation (Fig. 7a). The colloid permanently sticks at an amplitude of 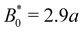 and the colloids are never observed to advance (Fig. 7c).
and the colloids are never observed to advance (Fig. 7c).
We have discussed colloids moving through wavy channels with planar anchoring, but colloids eluting through channels with homeotropic walls exhibit comparable stick–slip dynamics (data not shown). The homeotropic cases do not show qualitative differences from Fig. 7. Indeed, permanent locking occurs at 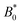 = 3.0a—only slightly above
= 3.0a—only slightly above 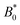 = 2.9a, the point where it occurred for planar anchoring. Later, we will explicitly compare measures of colloid velocity for planar and homeotropic wavy walls and see that they are qualitatively similar.
= 2.9a, the point where it occurred for planar anchoring. Later, we will explicitly compare measures of colloid velocity for planar and homeotropic wavy walls and see that they are qualitatively similar.
The probability density function of finding a colloid at a given position x along the channel explains the trajectories observed for planar anchored wavy walls. The PDFs for three different amplitudes in planar anchored channels demonstrates the three different behaviours (Fig. 8). Fig. 8a shows the typical shape for the low amplitudes, where the peak is slightly offset from the center of the trough at x = λ/4. The PDF is highly biased and skewed downstream of the trough center. In this case, the colloid continuously advects through the channel. This changes as the amplitude increases. At intermediate amplitudes, the probability densities look close to the point where colloids mostly stop moving (stick) but still can hop over crests from time to time (slip) (Fig. 8b). Both the height and location of the peak changes with the amplitude. The distribution becomes bi-modal, with both PDF peaks offset from the trough's center. At the largest amplitudes (Fig. 8c), the probability density is a single peak, demonstrating that the colloid has completely “locked” into the deep free energy minimum at the trough location of x = λ/4. In this case, the probability densities are single peaks, with narrow variance but with a mean position that is slightly offset downstream of x = λ/4, due to the pressure gradient acting on the locked colloids. The biggest difference from smaller amplitude systems is that the probability density is zero everywhere away from the peak.
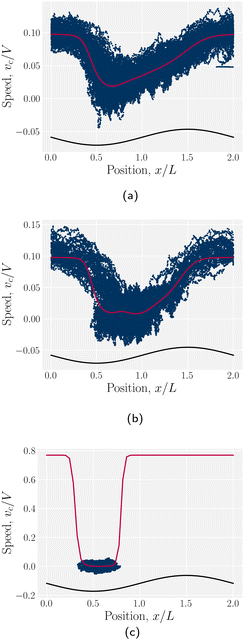
The cumulative probabilities can be used to measure the average axial velocities of colloids vc(x) at each position x. The cumulative probability gives the time as a function of position, and so the velocity along x is found by
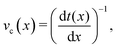 | (2) |
| vc(x) = [αp(x)]−1, | (3) |
The stick–slip dynamics exhibit three different scenarios (Fig. 9). Either the colloid advects smoothly with the flow (Fig. 9a) or the dynamics are stick–slip (Fig. 9b) or the colloids locks into a trough (Fig. 9c). For the continuous advection case (Fig. 9a), the maximum velocity occurs as the channel widens. This coincides with the region where the nematoelastic forces act in the same direction as the flow. The minimum occurs slightly offset from the bottom of the trough (Fig. 8). For the locked scenario (Fig. 9c), the colloid stays in the trough and the velocity is essentially zero. In the locked case, the measured probability p(x) is zero away from the trough, with the sticking time appearing to have diverged; however, longer simulations may eventually find rare crossing events. Any such rare crossing events would necessarily be accompanied by an unlikely thermal fluctuation in colloid speed to overcome the crest (eqn (3)).
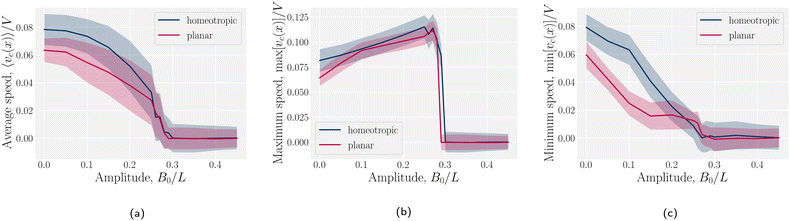
The speed profiles lead to interesting average colloid dynamics, which demonstrate that homeotropic or planar wall anchoring only change quantitative details and not the qualitative behaviour. To quantify the stick–slip translation of the colloid, we track the average speed of the colloid 〈vc(x)〉, the maximum max[vc(x)] and the minimum min[vc(x)] (Fig. 10). Colloids in channels with homeotropic anchoring elute more quickly than colloids in channels with planar walls (Fig. 10a). The average speed 〈vc(x)〉 of both decreases as amplitude increases until hitting zero at 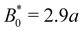 . In general, the average speed of the colloid decreases with an increasing amplitude until it reaches zero (Fig. 10a). This occurs for two reasons. Firstly, as the amplitude increases the flow through the channel decreases even in the absence of the colloid (Fig. 5b). Secondly, the strength of the elastic forces increases with amplitude and so the time spent crossing the free energy barrier increases.
. In general, the average speed of the colloid decreases with an increasing amplitude until it reaches zero (Fig. 10a). This occurs for two reasons. Firstly, as the amplitude increases the flow through the channel decreases even in the absence of the colloid (Fig. 5b). Secondly, the strength of the elastic forces increases with amplitude and so the time spent crossing the free energy barrier increases.
The average elution rate is different for planar and homeotropic anchoring on the walls. While the general shapes are the same, suggesting similar behaviours, the elastic forces from the wavy boundaries appear to be weaker for homeotropic anchoring than for planar. This is likely due to the different positioning of the defects. In the planar case, when the colloid is situated directly above the troughs, there is more space for the defects, compared to when it is situated above the crest where they are shifted closer to the colloid. However, for the homeotropic case, the position of the colloid has no effect on the defect positions—they always reside directly up- and downstream (Fig. 4a). The shape of the colloid matches much better with the walls when situated above the trough, meaning it is a more stable point. Thus, the mechanisms responsible for the motions of the colloid are different in the different anchoring cases.
The maximum speed of the colloids behaves qualitatively differently from the average. It suddenly decreases to max[vc(x)] = 0 (Fig. 10b). This sudden drop occurs at the same 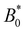 as the average going to zero and signals the transition to a locked state that no longer moves along the channel. This once again illustrates the two distinct states of stick–slip and locked, with a discontinuous transition between them at
as the average going to zero and signals the transition to a locked state that no longer moves along the channel. This once again illustrates the two distinct states of stick–slip and locked, with a discontinuous transition between them at 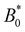 . Prior to the sudden stop, max[vc(x)] increases with the amplitude, which is a consequence of Bernoulli's principle. The situation is nearly identical for homeotropic and planar anchoring (Fig. 10b). Similarly, we can consider the minimum colloidal speed (Fig. 10c), which likewise shows similar behaviour for homeotropic and planar anchoring on the wall. These results indicate that homeotropic and planar anchoring result in very similar average elution rates and as well as similar maximum and minimum colloid velocities.
. Prior to the sudden stop, max[vc(x)] increases with the amplitude, which is a consequence of Bernoulli's principle. The situation is nearly identical for homeotropic and planar anchoring (Fig. 10b). Similarly, we can consider the minimum colloidal speed (Fig. 10c), which likewise shows similar behaviour for homeotropic and planar anchoring on the wall. These results indicate that homeotropic and planar anchoring result in very similar average elution rates and as well as similar maximum and minimum colloid velocities.
However, the minimum speed decreases with the amplitude. The minimum speed occurs at different points along the channel for different anchoring conditions. For homeotropic anchoring, the colloid has its minimum-velocity position much higher up on the side of the trough compared to the planar anchoring. Thus, it is nematic interactions with the wavy wall that set the position of the minimum-speed. Through no-slip boundary conditions, the colloid also impacts the average speed of the fluid. As the nemoelastic forces push the colloid towards a free energy minimum, the colloid applies a drag force to the fluid. When the colloid sticks or slows between the trough and the crest, the nematic interactions work against the pressure gradient and the fluid slows (Appendix F). On the other hand, the colloid speeds up once it crosses the barrier due to the nematic force pushing it towards the next free energy minimum. During this slip phase of motion, the colloid drags the fluid forward and increases the fluid flow (Appendix F).
In addition to comparing planar and homeotropic anchoring on the walls, we also compared different mean field potentials U for planar anchoring on the walls. The maximum colloid speed has the same general shape as we previously found (Fig. 11). As the flow through the channel is independent of the mean field potential, this is expected. However, stronger mean field potentials increase the nematoelastic interactions and so the velocities must be scaled by U, which does not collapse the curves for small amplitudes because the fluid flow is independent of U. Most clearly, the transition amplitude 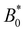 from stick–slip to locked moves to smaller values as the mean field potential U increases (Fig. 11). This suggests a mechanism by which fine control of the stick–slip transition to locking can be controlled.
from stick–slip to locked moves to smaller values as the mean field potential U increases (Fig. 11). This suggests a mechanism by which fine control of the stick–slip transition to locking can be controlled.
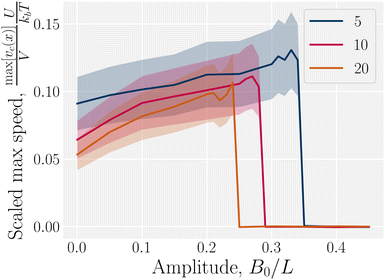 | ||
| Fig. 11 Maximum homeotropic colloid speed max[vc(x)]/V in wavy channels with planar anchoring. Sudden transition from stick–slip dynamics to locking. Same as Fig. 10b but for various mean field potentials U. The velocity has been multiplied by the mean field potential, U, to collapse the curves. | ||
4 Conclusion
In this work, we have considered a simple lock-key microfluidic system. We chose a colloid size commensurate with the walls' wavelength, creating periodically repeating barriers and docking sites, which exhibit interesting dynamics arising from the competition between nematoelastic free energy minimization and nematohydrodynamic advection. To tackle these complex dynamics, we first neglected flow (Section 3.1). In wavy channels, colloids with homeotropic anchoring migrate to the widest point in the channel (the troughs), regardless of whether the anchoring conditions on the wall are homeotropic or planar (Fig. 3). However, the pair of defects associated with the colloids take a different configurations for the different anchoring (Fig. 4). Our results are not comprehensive and indicate that future work is required to fully understand how wall structure and defect configuration lead to equilibrium colloid positions. We then considered flows of nematics within wavy channels in the absence of the colloids (Section 3.2). Since the transport coefficient is constant, the flow rate through the channel is observed to decrease as the the surface area of the boundary surface increased with increasing amplitude of the wavy channel (Fig. 5).Both these results suggest the qualitative dynamics of colloid transport slows as the amplitude to the wavy walls increases, which is indeed demonstrated in simulations (Fig. 7). We find the elution slows because of stick–slip dynamics, with colloids “sticking” to the docking sites for longer durations as a function of boundary amplitude (Fig. 7 and 8). Despite this net decrease in the transport rate, the maximum velocity, of when the colloid hops over the crests from docking site to docking site, increases with amplitude until the colloid suddenly locks into the troughs and no longer moves (Fig. 9). Modifying the material parameters of the nematic fluid can shift this lock transition point (Fig. 11), with nematoelastic forces increasing with the mean field potential to nonlinearly shift the locking transition to smaller amplitudes. The transition between locking and slipping is essentially a type of elastic ‘snap-through’ event,92,93 which would suggest a critical slowing and may allow simple-but-accurate models of the stick–slip dynamics. Such considerations might lead to the development of quantitative methods to classify different trajectories, as well as techniques to accurately measure the fraction of time a colloid spends sticking or slipping. We hope these results will motivate analytical work on the nematic interactions between mobile colloids and complex confining walls that go beyond interactions between colloids and plane walls.94
This study has considered the effect of wavy wall amplitude on the stick–slip dynamics of eluting colloids in 2D. In addition to amplitude there are many more parameters that are expected to impact elution rate. The colloid size, channel height and wall wavelength are all length scales whose combinations will modify elution rate. The geometry could be further complicated by allowing the phase of the top and bottom wall to differ. Additionally, the strength of the wall/colloid interactions could also be tuned by modifying the Frank elastic constant of the nematic or the anchoring strength on the walls or colloids, just as the driving force could be changed by varying the pressure gradient. State diagrams for stick–slip or locking behaviors could be constructed for each pairing of such parameters. Future studies can exploit Appendix B to simulate three dimensional systems confined by wavy walls or study the role of colloid surface structure (Appendix C).
Our results show that wavy walls not only have potential to direct colloids to specific docking sites but also to control site-specific resting duration and intermittent elution, allowing autonomic temporal control. They suggest how experimental systems can be engineered to hold particles in place for a given time by combining surface structure and advective flow. By coupling site-specific resting duration with position-dependent pressure gradients, future studies could explore feedback loops to reinforce or diminish stick-and-slip motion. More complex surface structures could be designed such that particles stick for longer or shorter periods at different docking sites. Such site-specific resting times could be used to illuminate or irradiate particles for a given time without switching the light source. By combining these lock-key microfluidics with different microreactors at each docking site, efficient series chemical reactions could be designed to occur for autonomous-but-responsive durations.
Conflicts of interest
There are no conflicts to declare.Appendix
A Numerical methods
This study utilizes a nematic multi-particle collision dynamics approach, which is chosen for its ability to simulate moderate Péclet numbers, mobile colloids, complex boundaries and fluctuating nematohydrodynamics.A.1.1 Streaming step. The streaming step updates each particle position assuming ballistic motion
![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif) i(t + δt) = i(t + δt) = ![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif) i(t) + i(t) + ![[v with combining low line]](https://www.rsc.org/images/entities/i_char_0076_0332.gif) i(t)δt, i(t)δt, | (4) |
![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif) i is the particle position, t is the current time and δt is the time step.
i is the particle position, t is the current time and δt is the time step.
A.1.2 Collision step.
A.1.2.1 Momentum exchange. The particle velocities are updated by the collision operator Ξi,c(t) for particle i in cell c at time t, which is used to update the velocities
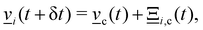 | (5) |
![[v with combining low line]](https://www.rsc.org/images/entities/i_char_0076_0332.gif) c = 〈
c = 〈![[v with combining low line]](https://www.rsc.org/images/entities/i_char_0076_0332.gif) i〉c is the center of mass velocity of the cell and 〈·〉c is the average within cell c. A modified Andersen-thermostatted collision operator77,78 is employed
i〉c is the center of mass velocity of the cell and 〈·〉c is the average within cell c. A modified Andersen-thermostatted collision operator77,78 is employed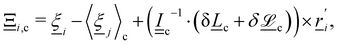 | (6) |
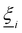 is a randomly generated velocity with magnitude drawn from a Boltzmann distribution with energy kBT. To conserve momentum and keep the average velocity of the cell constant, the average of the random velocities
is a randomly generated velocity with magnitude drawn from a Boltzmann distribution with energy kBT. To conserve momentum and keep the average velocity of the cell constant, the average of the random velocities 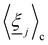 is subtracted. The last term ensures that angular momentum is conserved by removing any spurious angular velocity generated by the collision operation. The moment of inertia of the cell is
is subtracted. The last term ensures that angular momentum is conserved by removing any spurious angular velocity generated by the collision operation. The moment of inertia of the cell is 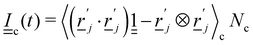 , where
, where  is the identity matrix,
is the identity matrix, ![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif) c = 〈
c = 〈![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif) j〉c is the center of mass position and
j〉c is the center of mass position and 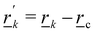 . The spurious angular momentum can arise from the randomly generated velocities
. The spurious angular momentum can arise from the randomly generated velocities 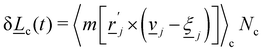 or changes in orientation
or changes in orientation 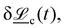 which will be discussed below.
which will be discussed below.
A.1.2.2 Acceleration. Pressure gradients can be written as an effective external acceleration
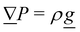 and the external acceleration
and the external acceleration ![[g with combining low line]](https://www.rsc.org/images/entities/i_char_0067_0332.gif) can be included in the collision operator95 as an impulse
can be included in the collision operator95 as an impulse 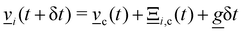 . This approach has been employed to simulate flows around obstacles,80,96 colloid/polymer transport,4,97,98 and rheotaxis of microswimmers.99
. This approach has been employed to simulate flows around obstacles,80,96 colloid/polymer transport,4,97,98 and rheotaxis of microswimmers.99
A.1.2.3 Orientation exchange. Particle orientations are updated according to a nematic collision operator Ψi,c(t) for particle i in cell c.64 After the collision, the updated orientation is
![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) i(t + δt) = Ψi,c i(t + δt) = Ψi,c![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) i(t). i(t). | (7) |
![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) i over the time step δt. The collision operator and resulting re-rotation can be decomposed into two contributions
i over the time step δt. The collision operator and resulting re-rotation can be decomposed into two contributions 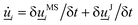 from the thermostat (δ
from the thermostat (δ![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) MSi/δt) and from the flows (δ
MSi/δt) and from the flows (δ![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) Ji/δt).
Ji/δt).
1. A local thermostat: the local thermostat draws random orientations from the Maier–Saupe distribution centered about ![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif) c
c
pc(![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) MS) ∝ exp(USc( MS) ∝ exp(USc(![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif) c· c·![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) MS)2/kBT), MS)2/kBT), | (8) |
![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif) c, the tensor
c, the tensor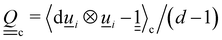 | (9) |
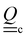 is the scalar order parameter Sc and the corresponding eigenvector is parallel to the local director
is the scalar order parameter Sc and the corresponding eigenvector is parallel to the local director ![[n with combining low line]](https://www.rsc.org/images/entities/i_char_006e_0332.gif) c for the cell. The change in orientation is δ
c for the cell. The change in orientation is δ![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) MSi =
MSi = ![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) i(t) −
i(t) − ![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) MSi.
MSi.
2. Rotations due to flows: the nematodynamic response models the nematogens' response to velocity gradients through Jeffery's equation
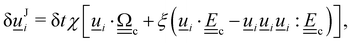 | (10) |
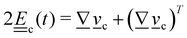 and vorticity tensor
and vorticity tensor 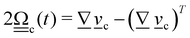 . The director dynamics are coupled back to the velocity field through the change in angular momentum
. The director dynamics are coupled back to the velocity field through the change in angular momentum 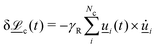 , where γR is a rotational friction coefficient.64,67,69 By choosing a sufficiently small value of γR backflow effects can be kept small, even in the limit of small Ericksen numbers.
, where γR is a rotational friction coefficient.64,67,69 By choosing a sufficiently small value of γR backflow effects can be kept small, even in the limit of small Ericksen numbers.
B Boundary conditions
The main text considers simulations performed in 2D. In this section, we present the boundary conditions generalized to 3D and state the simplifying condition for the 2D surfaces used in the simulation results. To create the wavy channel, particles are subjected to boundary conditions, each of which is composed of two parts: the boundary surface and boundary rules.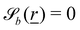 . The baseline form employed for
. The baseline form employed for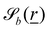 can represent planes, ellipsoids and squircles
can represent planes, ellipsoids and squircles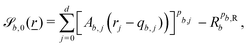 | (11) |
![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif) = (x, y, z) is the position on the surface,
= (x, y, z) is the position on the surface, ![[q with combining low line]](https://www.rsc.org/images/entities/i_char_0071_0332.gif) b = (qb,x, qb,y, qb,z) defines the position of the bth boundary, and
b = (qb,x, qb,y, qb,z) defines the position of the bth boundary, and ![[A with combining low line]](https://www.rsc.org/images/entities/i_char_0041_0332.gif) b = (Ab,x, Ab,y, Ab,z),
b = (Ab,x, Ab,y, Ab,z), ![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif) b = (pb,x, pb,y, pb,z, pb,R) and Rb define the shape.
b = (pb,x, pb,y, pb,z, pb,R) and Rb define the shape.
In this study, the surface has been extended to allow for wavy boundaries by adding a second term, which allows for waves along two perpendicular directions with amplitude Bb,0 and frequencies Bb,1 and Bb,2. We represent these surfaces as
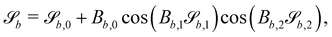 | (12) |
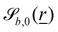 defines the basic surface given by eqn (11), while
defines the basic surface given by eqn (11), while 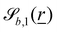 and
and 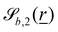 define the orientations of the waves, and thus depend on
define the orientations of the waves, and thus depend on 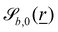 . For 2D simulations, Bb,2 = 0.
. For 2D simulations, Bb,2 = 0.
B.1.1 Wavy planes. For planes,
![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif) b =
b = ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) ,
, ![[A with combining low line]](https://www.rsc.org/images/entities/i_char_0041_0332.gif) b is the normal vector and Rb = 0. For wavy planes, the functions
b is the normal vector and Rb = 0. For wavy planes, the functions 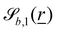 and
and 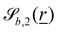 can define (i) an egg carton surface (Fig. 12a) or (ii) a corrugation (Fig. 12b). To get the egg carton-like surface,
can define (i) an egg carton surface (Fig. 12a) or (ii) a corrugation (Fig. 12b). To get the egg carton-like surface, 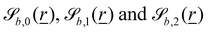 must be mutually perpendicular. A decision has to be made to make
must be mutually perpendicular. A decision has to be made to make 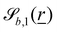 independent of z and take the form
independent of z and take the form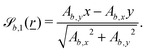 | (13) |
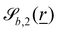 to be
to be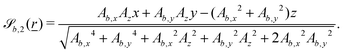 | (14) |
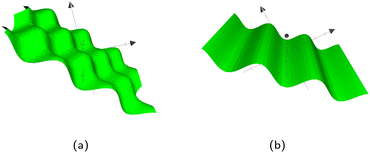 | ||
| Fig. 12 The types of wavy plane walls that can be implemented. (a) Egg carton plane wall. (b) Corrugated plane wall. | ||
While the wavy surfaces are generalized to work in 3D, they work without modification in 2D. For simplicity, it is assumed that 2D simulations occur in the xy-plane, such that Ab,z = 0 for all boundaries b. Additionally, only one cosine wave is needed and so we set Bb,2 = 0 for all cases. Thus, Bb,2 = 0 is not discussed in the main text.
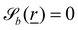 , thus a particle is defined as being within the simulation domain if
, thus a particle is defined as being within the simulation domain if 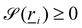 for particle position
for particle position ![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif) i. If a particle is found to be outside the boundary, a set of boundary rules define how the generalized coordinates (position
i. If a particle is found to be outside the boundary, a set of boundary rules define how the generalized coordinates (position ![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif) i(t), orientation
i(t), orientation ![[u with combining low line]](https://www.rsc.org/images/entities/i_char_0075_0332.gif) i(t) and velocity
i(t) and velocity ![[v with combining low line]](https://www.rsc.org/images/entities/i_char_0076_0332.gif) i(t)) are transformed. The boundary rules are applied to the components normal
i(t)) are transformed. The boundary rules are applied to the components normal ![[v with combining low line]](https://www.rsc.org/images/entities/i_char_0076_0332.gif) b and perpendicular
b and perpendicular ![[t with combining low line]](https://www.rsc.org/images/entities/i_char_0074_0332.gif) b to the boundary (Appendix C.3).
b to the boundary (Appendix C.3).
Periodic boundary conditions can be achieved by shifting the position ![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif) i(t) in the normal direction by
i(t) in the normal direction by 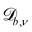 and/or in the tangential direction by
and/or in the tangential direction by 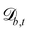 . After crossing the surface b, particle i's position is transformed as
. After crossing the surface b, particle i's position is transformed as 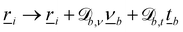 . Likewise, the particle's velocity may be transformed. The velocity is altered by multiplicative parameters
. Likewise, the particle's velocity may be transformed. The velocity is altered by multiplicative parameters 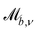 and
and 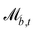 , such that upon colliding with the surface the velocity becomes
, such that upon colliding with the surface the velocity becomes 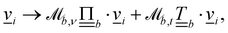 where
where 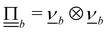 and
and 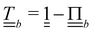 are the normal and tangent projection operators. Lastly, the orientation is also multiplicatively transformed
are the normal and tangent projection operators. Lastly, the orientation is also multiplicatively transformed 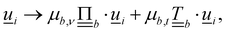 which is then rescaled back to a unit vector.
which is then rescaled back to a unit vector.
The simplest boundaries employed in this study are periodic boundary conditions. Particles that cross a periodic boundary are simply shifted by the length of the simulation domain in the direction normal to the boundary, without change to their velocity or orientation. So 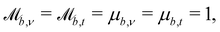 while
while 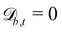 and
and 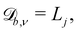 where Lj is the system size in the direction j.
where Lj is the system size in the direction j.
Solid walls are boundaries that do not apply a shift 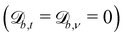 but do transform the direction of the velocity. For perfect-slip boundaries, the particles are reflected and
but do transform the direction of the velocity. For perfect-slip boundaries, the particles are reflected and 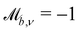 and
and 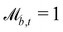 ; whereas, for no-slip conditions bounce-back rules are applied with
; whereas, for no-slip conditions bounce-back rules are applied with 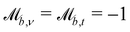 . In order for the particle to not violate the boundary, the particle is rewound to the location where it crossed the boundary, and from there it streams for the rest of the time step using its new velocity.
. In order for the particle to not violate the boundary, the particle is rewound to the location where it crossed the boundary, and from there it streams for the rest of the time step using its new velocity.
Controlling the orientation when a particle crosses a boundary allows for different nematic anchoring conditions for solid boundaries. If both μb,ν and μb,t are set to 1 the particle's orientation does not change and no anchoring conditions are enforced. However, by setting the tangential component to μb,t = 0 homeotropic anchoring is achieved. Similarly, if μb,ν = 0 planar anchoring is enforced on the boundary.
In a similar fashion, the boundary rules for anchoring generate weak anchoring because only particles that have crossed the boundary have the anchoring condition applied, and are then mixed with particles that have not.83 In order to generate strong anchoring conditions, all the particles in cells that intersect 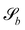 are subjected to the boundary rules described in Section 6.2. Because the entire cell now essentially has the anchoring condition applied, this generates stronger anchoring.
are subjected to the boundary rules described in Section 6.2. Because the entire cell now essentially has the anchoring condition applied, this generates stronger anchoring.
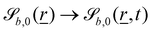 . Firstly, when a particle collides with the boundary, the impulse of the collision must be applied to the moving boundary to conserve both linear and angular momentum.4 Additionally, when anchoring is used, the change in particle orientation applies a torque on the colloid. In this study, the anchoring condition on the colloids are always strong homeotropic anchoring. Moving boundaries also needs to be able to collide with other boundaries, both moving and fixed. For spherical or discoidal colloids of radius Rb, the algorithm checks if it has collided with another solid boundary and applies the same rules as when colliding with an MPCD particle.
. Firstly, when a particle collides with the boundary, the impulse of the collision must be applied to the moving boundary to conserve both linear and angular momentum.4 Additionally, when anchoring is used, the change in particle orientation applies a torque on the colloid. In this study, the anchoring condition on the colloids are always strong homeotropic anchoring. Moving boundaries also needs to be able to collide with other boundaries, both moving and fixed. For spherical or discoidal colloids of radius Rb, the algorithm checks if it has collided with another solid boundary and applies the same rules as when colliding with an MPCD particle.
C Wavy spheres, ellipsoids and cylinders
In addition to corrugated and egg-shell structured planar walls, wavy spheres, ellipsoids and cylinders are possible viaeqn (11)–(14).![[p with combining low line]](https://www.rsc.org/images/entities/i_char_0070_0332.gif) b =
b = ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) ,
, ![[A with combining low line]](https://www.rsc.org/images/entities/i_char_0041_0332.gif) b =
b = ![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) and Rb is the radius of the bth sphere. For ellipsoids,
and Rb is the radius of the bth sphere. For ellipsoids, ![[A with combining low line]](https://www.rsc.org/images/entities/i_char_0041_0332.gif) b is the inverse of the bth ellipsoid's focii. There are two different directions in which a sphere can be wavy: longitudinal and latitudinal (Fig. 13a and b). These can also be combined to create a sphere with egg carton waves on it (Fig. 13c). The longitudinal and the latitudinal waves correspond to
b is the inverse of the bth ellipsoid's focii. There are two different directions in which a sphere can be wavy: longitudinal and latitudinal (Fig. 13a and b). These can also be combined to create a sphere with egg carton waves on it (Fig. 13c). The longitudinal and the latitudinal waves correspond to 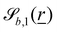 and
and 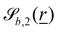 respectively, which take the forms
respectively, which take the forms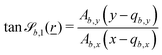 | (15) |
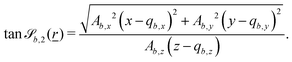 | (16) |
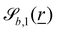 and
and 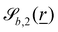 for cylinders have a cyclic property so if Ab,x = 0, then (i, j, k) = (x, y, z), if Ab,y = 0: (i, j, k) = (y, z, x); and if Ab,z = 0 then (i, j, k) = (z, x, y). So then for any choice Ab,i = 0 the wavy surfaces are given by
for cylinders have a cyclic property so if Ab,x = 0, then (i, j, k) = (x, y, z), if Ab,y = 0: (i, j, k) = (y, z, x); and if Ab,z = 0 then (i, j, k) = (z, x, y). So then for any choice Ab,i = 0 the wavy surfaces are given by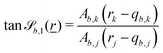 | (17) |
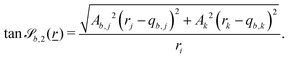 | (18) |
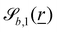 defines the waves around the cylinder (Fig. 14b) and
defines the waves around the cylinder (Fig. 14b) and 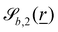 defines waves along the direction of the cylinder (Fig. 14c). By setting the opposite Bb,i to 0, these can be achieved. If both Bb,i ≠ 0, the egg carton pattern emerges on the cylinder (Fig. 14a).
defines waves along the direction of the cylinder (Fig. 14c). By setting the opposite Bb,i to 0, these can be achieved. If both Bb,i ≠ 0, the egg carton pattern emerges on the cylinder (Fig. 14a).
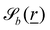 is just the vector minus the normal component, only the normal direction
is just the vector minus the normal component, only the normal direction ![[v with combining low line]](https://www.rsc.org/images/entities/i_char_0076_0332.gif) b(
b(![[r with combining low line]](https://www.rsc.org/images/entities/i_char_0072_0332.gif) ) needs to be calculated as
) needs to be calculated as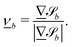 | (19) |
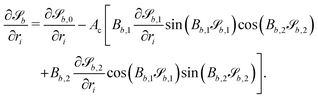 | (20) |
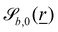 is given by
is given by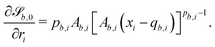 | (21) |
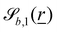 and
and 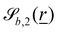 vary depending on the surface type.
vary depending on the surface type.
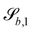
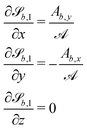 | (22) |
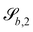
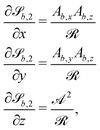 | (23) |
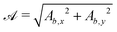 | (24a) |
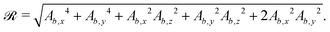 | (24b) |
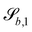
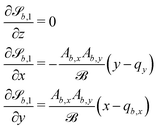 | (25) |
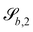
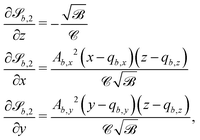 | (26) |
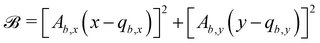 | (27a) |
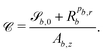 | (27b) |
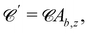 the derivatives are
the derivatives are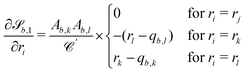 | (28) |
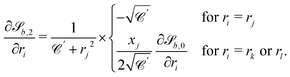 | (29) |
D Flow through plane channels
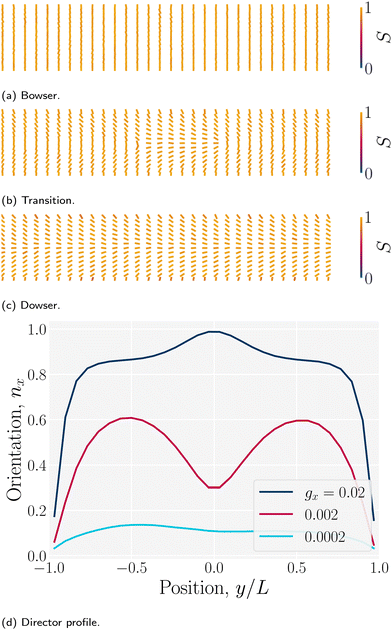
Flowing isotropic fluids at low Reynolds number obey plane Poiseuille flow with parabolic flow profiles,100 which is a direct consequence of the the no-slip condition at the walls of the channel. Though the coupling between flow profile, local director and anisotropic dissipation can induce complications,101 N-MPCD simulates linearized fluctuating nematodynamics with isotropic viscous dissipation.79The director can discontinuously transition from a homogenous alignment to deformed states.102 For strong homeotropic anchoring and sufficiently small pressure gradients, the director remains uniformly homeotropic across the channel; however, for sufficient flow the director field bows into the Bowser state,102 in which the field curves slightly in the direction of the flow (Fig. 15a). The Bowser state transitions (Fig. 15b) to the the Dowser state (Fig. 15c) at sufficiently strong flows, for which the director fully aligns with the flow at the center (Fig. 15d). The Bowser and Dowser states arise from the competition between nematoelastic free energy and flow strength.103 For the pressure gradient and channel height used in this study, the director is found to be in the bowed Bowser state. The planar anchoring case is not as interesting since the boundary conditions and strain rate coalign, though at sufficiently stronger flow the near-wall director may tilt inward as expected.104
Since the flow profiles remain parabolic (Fig. 16a), the viscosity can be calculated directly from the average velocity of the nematic fluid 〈vf〉 and the plane Hagen–Poiseuille equation to be
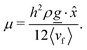 | (30) |
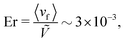 | (31) |
E Absence of stick–slip in isotropic fluid flows
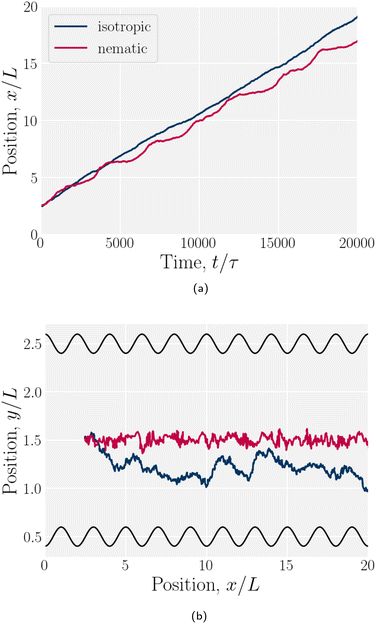
Simulations of a colloid in an isotropic fluid advecting through a wavy channel do not exhibit stick–slip dynamics (Fig. 17). While the nematic case exhibits clear periods of movement (slip) and cessation (stick), the isotropic case exhibits a steady elution rate (Fig. 17a). Furthermore, nematic repulsion between the colloids and the walls keeps the colloid close to the channel centerline (Fig. 17b). On the other hand, the colloid in an isotropic fluid is free to diffuse away from the centerline, sampling different local velocities and exhibiting greater Taylor dispersion. In this way, the lock-key dynamics investigated here differ from the Brownian dynamics of a driven diffusive colloid moving through a periodic landscape.F Impact of colloid dynamics on fluid flow
The colloid elutes due to the drag force that the fluid exerts on it, but it also moves in response to the nematoelastic forces. Therefore, at any instance the colloid may resist or assist the fluid flow. The spatially averaged flow of the fluid is not constant with time during the stick–slip motion. When the colloid sticks, the combination of colloid drag and nematoelastic forces slow the fluid. When the colloid slips, the nematoelastic forces drive fluid flow. We measure the total average fluid velocity 〈vf〉 as a function of the colloid position (Fig. 18). A minimum average fluid velocity occurs just past the trough, where defects oppose elution. Here, nematic interactions work against the pressure gradient and the fluid slows. During the slip phase, the colloid speeds up due to nematic forcing and the fluid flow reaches a maximum as the colloid is forced over the crest by elastic interactions with the walls. As the wall amplitude is increased, crossing events become rarer and faster, causing the statistics to worsen for x/L > 1.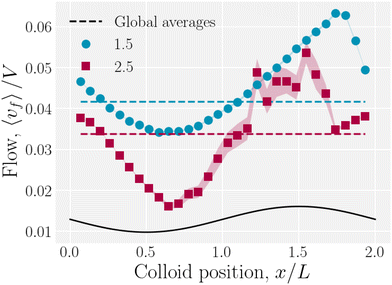 | ||
| Fig. 18 Average fluid velocity 〈vf〉 as a function of instantaneous colloid position x for wall amplitudes B0 = {1.5, 2.5}a. | ||
Acknowledgements
This work was supported by the European Research Council (ERC) under the European Unions Horizon 2020 research and innovation program (grant agreement no. 851196). For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising from this submission.Notes and references
- Y. Liu, G. Yang, Y. Hui, S. Ranaweera and C.-X. Zhao, Small, 2022, 18, 2106580 CrossRef CAS PubMed.
- M. Sivaramakrishnan, R. Kothandan, D. K. Govindarajan, Y. Meganathan and K. Kandaswamy, Curr. Opin. Biomed. Eng., 2020, 13, 60–68 CrossRef.
- A. Burklund, A. Tadimety, Y. Nie, N. Hao and J. X. Zhang, Advances in Clinical Chemistry, Elsevier, 2020, vol. 95, pp. 1–72 Search PubMed.
- T. N. Shendruk, R. Tahvildari, N. M. Catafard, L. Andrzejewski, C. Gigault, A. Todd, L. Gagne-Dumais, G. W. Slater and M. Godin, Anal. Chem., 2013, 85, 5981–5988 CrossRef CAS PubMed.
- J. Zhang, S. Yan, D. Yuan, G. Alici, N.-T. Nguyen, M. Ebrahimi Warkiani and W. Li, Lab Chip, 2016, 16, 10–34 RSC.
- H. Zhang, D. Freitas, H. S. Kim, K. Fabijanic, Z. Li, H. Chen, M. T. Mark, H. Molina, A. B. Martin and L. Bojmar, et al. , Nat. Cell Biol., 2018, 20, 332–343 CrossRef CAS PubMed.
- P. Zhang, H. Bachman, A. Ozcelik and T. J. Huang, Annu. Rev. Anal. Chem., 2020, 13, 17–43 CrossRef CAS PubMed.
- A. Sengupta, C. Bahr and S. Herminghaus, Soft Matter, 2013, 9, 7251–7260 RSC.
- O. D. Lavrentovich, Liquid Crystals Rev., 2020, 8, 59–129 CrossRef CAS PubMed.
- K. Fedorowicz, R. Prosser and A. Sengupta, Soft Matter, 2023, 19(37), 7084–7092 RSC.
- J. Dalby, Y. Han, A. Majumdar and L. Mrad, SIAM J. Appl. Math., 2023, 83(6), 2284–2309 CrossRef.
- A. Sengupta and M. G. Mazza, Liquid Crystals at Interfaces and Under Flow: Recent Advances and Trends, 2020, ch. 6, pp. 183–226 Search PubMed.
- I. I. Smalyukh, Ann. Rev. Condens. Matter Phys., 2018, 9, 207–226 CrossRef.
- H. Stark, Phys. Rep., 2001, 351, 387–474 CrossRef CAS.
- Y. Han and A. Majumdar, J. Nonlinear Sci., 2023, 33, 24 CrossRef.
- Y. Han, B. Shi, L. Zhang and A. Majumdar, IMA J. Appl. Math., 2023, 88(5), 645–676 CrossRef.
- H.-Q. Chen, X.-Y. Wang, H. K. Bisoyi, L.-J. Chen and Q. Li, Langmuir, 2021, 37, 3789–3807 CrossRef CAS PubMed.
- Z. Dogic, P. Sharma and M. J. Zakhary, Ann. Rev. Condens. Matter Phys., 2014, 5, 137–157 CrossRef CAS.
- I. I. Smalyukh, Rep. Prog. Phys., 2020, 83, 106601 CrossRef PubMed.
- H. Mundoor, S. Park, B. Senyuk, H. H. Wensink and I. I. Smalyukh, Science, 2018, 360, 768–771 CrossRef CAS PubMed.
- J. Paget, M. G. Mazza, A. J. Archer and T. N. Shendruk, Nat. Commun., 2023, 14, 1048 CrossRef CAS PubMed.
- Y. Yuan, Q. Liu, B. Senyuk and I. I. Smalyukh, Nature, 2019, 570, 214–218 CrossRef CAS PubMed.
- B. Senyuk, Q. Liu, P. D. Nystrom and I. I. Smalyukh, Soft Matter, 2017, 13, 7398–7405 RSC.
- I. Muševič, Eur. Phys. J. Special Topics, 2019, 227, 2455–2485 CrossRef.
- H. Yoshida, K. Asakura, J.-I. Fukuda and M. Ozaki, Nat. Commun., 2015, 6, 7180 CrossRef CAS PubMed.
- M. Nikkhou, M. Škarabot, S. Čopar, M. Ravnik, S. Žumer and I. Muševič, Nat. Phys., 2015, 11, 183–187 Search PubMed.
- F. R. Hung, B. T. Gettelfinger, G. M. Koenig, N. L. Abbott and J. J. de Pablo, J. Chem. Phys., 2007, 127(12), 124702 CrossRef PubMed.
- C. P. Lapointe, D. H. Reich and R. L. Leheny, Langmuir, 2008, 24, 11175–11181 CrossRef CAS PubMed.
- N. M. Silvestre, P. Patrício and M. M. Telo da Gama, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2004, 69, 6 CrossRef PubMed.
- D. L. Cheung and M. P. Allen, J. Chem. Phys., 2008, 129(11), 114706 CrossRef PubMed.
- Z. Eskandari, N. M. Silvestre, M. M. Telo da Gama and M. R. Ejtehadi, Soft Matter, 2014, 10, 9681–9687 RSC.
- Y. Luo, F. Serra and K. J. Stebe, Soft Matter, 2016, 12, 6027–6032 RSC.
- G. Boniello, Y. Luo, D. A. Beller, F. Serra and K. J. Stebe, Soft Matter, 2019, 15, 5220–5226 RSC.
- Óscar A. Rojas-Gómez, J. M. Romero-Enrique, N. M. Silvestre and M. M. T. da Gama, J. Phys.: Condens. Matter, 2016, 29, 064002 CrossRef.
- Y. Luo, T. Yao, D. Beller, F. Serra and K. Stebe, Langmuir, 2019, 35, 9274–9285 CrossRef CAS PubMed.
- T. Yao, v Kos, Q. X. Zhang, Y. Luo, F. Serra, E. B. Steager, M. Ravnik and K. J. Stebe, Adv. Funct. Mater., 2022, 32, 2205546 CrossRef CAS.
- Y. Luo, D. A. Beller, G. Boniello, F. Serra and K. J. Stebe, Nat. Commun., 2018, 9, 1–11 CrossRef PubMed.
- K. Chen, O. J. Gebhardt, R. Devendra, G. Drazer, R. D. Kamien, D. H. Reich and R. L. Leheny, Soft Matter, 2018, 14, 83–91 RSC.
- R. Kapral, Adv. Chem. Phys., 2008, 140, 89 CrossRef CAS.
- G. Gompper, T. Ihle, D. M. Kroll and R. G. Winkler, in Multi-Particle Collision Dynamics: A Particle-Based Mesoscale Simulation Approach to the Hydrodynamics of Complex Fluids, ed. C. Holm and K. Kremer, Springer Berlin Heidelberg, Berlin, Heidelberg, 2009, pp. 1–87 Search PubMed.
- G. W. Slater, C. Holm, M. V. Chubynsky, H. W. de Haan, A. Dubé, K. Grass, O. A. Hickey, C. Kingsburry, D. Sean, T. N. Shendruk and L. Zhan, Electrophoresis, 2009, 30, 792–818 CrossRef CAS PubMed.
- M. P. Howard, A. Nikoubashman and J. C. Palmer, Curr. Opin. Chem. Eng., 2019, 23, 34–43 CrossRef.
- A. Sayyidmousavi and K. Rohlf, Phys. Biol., 2018, 15, 046007 CrossRef PubMed.
- S. Y. Reigh, M.-J. Huang, H. Löwen, E. Lauga and R. Kapral, Soft Matter, 2020, 16, 1236–1245 RSC.
- O. A. Hickey, T. N. Shendruk, J. L. Harden and G. W. Slater, Phys. Rev. Lett., 2012, 109, 098302 CrossRef PubMed.
- T. N. Shendruk, M. Bertrand and G. W. Slater, ACS Macro Lett., 2015, 4, 472–476 CrossRef CAS PubMed.
- J. Burelbach, D. B. Brückner, D. Frenkel and E. Eiser, Soft Matter, 2018, 14, 7446–7454 RSC.
- A. Zöttl and H. Stark, Eur. Phys. J. E: Soft Matter Biol. Phys., 2018, 41, 1–11 CrossRef PubMed.
- J.-T. Kuhr, F. Rühle and H. Stark, Soft Matter, 2019, 15, 5685–5694 RSC.
- J. Clopés, G. Gompper and R. G. Winkler, Soft Matter, 2020, 16, 10676–10687 RSC.
- A. Lamura, R. G. Winkler and G. Gompper, J. Chem. Phys., 2021, 154(22), 224901 CrossRef CAS PubMed.
- J. Clopés Llahí, A. Martín-Gómez, G. Gompper and R. G. Winkler, Phys. Rev. E, 2022, 105, 015310 CrossRef PubMed.
- K. Choi, J. Lee, W. J. Choi and J. S. Myung, Polym. Eng. Sci., 2023, 63, 1032–1040 CrossRef CAS.
- Z. Wang, Z.-G. Wang, A.-C. Shi, Y. Lu and L. An, Macromolecules, 2023, 56, 2447–2453 CrossRef CAS.
- Y. M. Wani, P. G. Kovakas, A. Nikoubashman and M. P. Howard, J. Chem. Phys., 2022, 156(2), 024901 CrossRef CAS PubMed.
- R. Chen, R. Poling-Skutvik, M. P. Howard, A. Nikoubashman, S. A. Egorov, J. C. Conrad and J. C. Palmer, Soft Matter, 2019, 15, 1260–1268 RSC.
- T. Eisenstecken, R. Hornung, R. G. Winkler and G. Gompper, Europhys. Lett., 2018, 121, 24003 CrossRef.
- D. Toneian, G. Kahl, G. Gompper and R. G. Winkler, J. Chem. Phys., 2019, 151(19), 194110 CrossRef PubMed.
- P. Ilg, J. Chem. Phys., 2022, 156(14), 144905 CrossRef CAS PubMed.
- P. Ilg, Phys. Rev. E, 2022, 106, 064605 CrossRef CAS PubMed.
- P. Di Cintio, M. Pasquato, L. Barbieri, H. Bufferand, L. Casetti, G. Ciraolo, U. N. di Carlo, P. Ghendrih, J. P. Gunn and S. Gupta, et al. , Proc. Int. Astron. Union, 2020, 16, 134–140 CrossRef CAS.
- P. Di Cintio, M. Pasquato, H. Kim and S.-J. Yoon, Astron. Astrophys., 2021, 649, A24 CrossRef.
- P. Di Cintio, M. Pasquato, A. Simon-Petit and S.-J. Yoon, Astron. Astrophys., 2022, 659, A19 CrossRef.
- T. N. Shendruk and J. M. Yeomans, Soft Matter, 2015, 11(25), 5101–5110 RSC.
- S. Mandal and M. G. Mazza, Phys. Rev. E, 2019, 99, 063319 CrossRef CAS PubMed.
- D. Reyes-Arango, J. Quintana-H., J. C. Armas-Pérez and H. Híjar, Phys. A, 2020, 547, 123862 CrossRef CAS.
- H. Híjar, Phys. Rev. E, 2020, 102, 062705 CrossRef PubMed.
- J. Armendáriz and H. Híjar, Int. J. Modern Phys. B, 2021, 35, 2150124 CrossRef.
- J. Armendáriz and H. Híjar, Materials, 2021, 14(11), 2886 CrossRef PubMed.
- S. Mandal and M. G. Mazza, Eur. Phys. J. E: Soft Matter Biol. Phys., 2021, 44, 64 CrossRef CAS PubMed.
- T. Kozhukhov and T. N. Shendruk, Sci. Adv., 2022, 8, eabo5788 CrossRef PubMed.
- T. Kozhukhov, B. Loewe and T. N. Shendruk, arXiv, 2024, preprint, arXiv:2401.17777 DOI:10.48550/arXiv.2401.17777.
- R. R. Keogh, T. Kozhukhov, K. Thijssen and T. N. Shendruk, Phys. Rev. Lett., 2024 Search PubMed , https://journals.aps.org/prl/accepted/35078Y2eR2c1468de9065e368120c71a35d9fdb0b, accepted.
- A. Malevanets and R. Kapral, J. Chem. Phys., 1999, 110, 8605–8613 CrossRef CAS.
- A. Malevanets and R. Kapral, J. Chem. Phys., 2000, 112, 7260–7269 CrossRef CAS.
- T. Ihle and D. M. Kroll, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2001, 63, 020201 CrossRef CAS PubMed.
- H. Noguchi, N. Kikuchi and G. Gompper, Europhys. Lett., 2007, 78, 10005 CrossRef.
- H. Noguchi and G. Gompper, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2008, 78, 016706 CrossRef PubMed.
- H. Híjar, Condens. Matter Phys., 2019, 22, 13601 CrossRef.
- A. Lamura, G. Gompper, T. Ihle and D. M. Kroll, Europhys. Lett., 2001, 56, 319 CrossRef CAS.
- J. K. Whitmer and E. Luijten, J. Phys.: Condens. Matter, 2010, 22, 104106 CrossRef PubMed.
- D. S. Bolintineanu, J. B. Lechman, S. J. Plimpton and G. S. Grest, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2012, 86, 066703 CrossRef PubMed.
- L. C. Head, Y. A. G. Fosado, D. Marenduzzo and T. N. Shendruk, arXiv, 2024, preprint, arXiv:2404.09368 DOI:10.48550/arXiv.2404.09368.
- S. Grollau, N. L. Abbott and J. J. de Pablo, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2003, 67, 011702 CrossRef CAS PubMed.
- D. Andrienko, G. Germano and M. P. Allen, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2001, 63, 041701 CrossRef CAS PubMed.
- D. M. Sussman and D. A. Beller, Front. Phys., 2019, 7 DOI:10.3389/fphy.2019.00204.
- J. Fukuda and H. Yokoyama, Eur. Phys. J. E: Soft Matter Biol. Phys., 2001, 4, 389–396 CrossRef CAS.
- M. Tasinkevych, N. Silvestre, P. Patricio and M. Telo da Gama, Eur. Phys. J. E: Soft Matter Biol. Phys., 2002, 9, 341–347 CrossRef CAS PubMed.
- N. M. Silvestre, Q. Liu, B. Senyuk, I. I. Smalyukh and M. Tasinkevych, Phys. Rev. Lett., 2014, 112, 225501 CrossRef PubMed.
- Y. Liu, J. Li and A. J. Smits, J. Fluid Mech., 2019, 876, 1129–1145 CrossRef CAS.
- N. F. Okechi and S. Asghar, Chin. J. Phys., 2021, 74, 144–156 CrossRef CAS.
- M. Gomez, D. E. Moulton and D. Vella, Nat. Phys., 2017, 13, 142–145 Search PubMed.
- B. Radisson and E. Kanso, Phys. Rev. Lett., 2023, 130, 236102 CrossRef CAS PubMed.
- S. B. Chernyshuk and B. I. Lev, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 011707 CrossRef CAS PubMed.
- E. Allahyarov and G. Gompper, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2002, 66, 036702 CrossRef CAS PubMed.
- A. Lamura and G. Gompper, Eur. Phys. J. E: Soft Matter Biol. Phys., 2002, 9, 477–485 CrossRef CAS PubMed.
- C. Prohm, M. Gierlak and H. Stark, Eur. Phys. J. E: Soft Matter Biol. Phys., 2012, 35, 1–10 CrossRef PubMed.
- A. Liu, Z. Yang, L. Liu, J. Chen and L. An, Macromolecules, 2020, 53, 9993–10004 CrossRef CAS.
- K. Qi, H. Annepu, G. Gompper and R. G. Winkler, Phys. Rev. Res., 2020, 2, 033275 CrossRef CAS.
- H. Bruus, Theoretical microfluidics, Oxford University Press, 2007, vol. 18 Search PubMed.
- F. Brochard, Mol. Cryst. Liq. Cryst., 1973, 23, 51–58 CrossRef CAS.
- S. Čopar, V. Kos, T. Emeršič and U. Tkalec, Nat. Commun., 2020, 11, 59 CrossRef PubMed.
- T. G. Anderson, E. Mema, L. Kondic and L. J. Cummings, Int. J. Non Linear Mech., 2015, 75, 15–21 CrossRef.
- V. M. O. Batista, M. L. Blow and M. M. Telo Da Gama, Soft Matter, 2015, 11, 4674–4685 RSC.
| This journal is © The Royal Society of Chemistry 2024 |