One-dimensional nickel–cobalt bimetallic phosphide nanostructures for the oxygen evolution reaction†
Yue
Wang
,
Xin
Chang
 ,
Zexing
Huang
,
Jiahui
Fan
,
Lu
Li
* and
Mingyi
Zhang
,
Zexing
Huang
,
Jiahui
Fan
,
Lu
Li
* and
Mingyi
Zhang
 *
*
Key Laboratory for Photonic and Electronic Bandgap Materials, Ministry of Education, School of Physics and Electronic Engineering, Harbin Normal University, Harbin 150025, PR China. E-mail: zhangmingyi@hrbnu.edu.cn
First published on 22nd November 2023
Abstract
Nickel–cobalt bimetallic phosphides have widely been used in the oxygen evolution reaction (OER). However, finding effective strategies for fabricating one-dimensional nanostructures of bimetallic phosphides with enhanced electrocatalytic OER properties is still challenging. Herein, novel catalysts based on NiCoP nanofibers with exceptional electrocatalytic performances were synthesized for efficient OER. The resulting NiCoP nanofibers required only a minimum overpotential of 325 mV in 1.0 M KOH solution to drive a current density of 50 mA−2 for oxygen production. After 5000 OER cycles, the activity of NiCoP nanofibers remained practically unaltered, showcasing exceptional stability. The proposed strategy for designing and implementing nanostructured electrocatalysts looks promising for enhancing the performance of catalysts toward the OER for better energy production devices.
1. Introduction
The excessive exploitation of conventional energy sources has resulted in environmental pollution, energy resource depletion, and other significant negative impacts.1 As a result, developing efficient and environmentally friendly alternative energy sources has become an urgent matter. Electrocatalytic hydrolysis combines the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) as promising energy conversion pathways. More importantly, electrocatalytic hydrolysis is also promising for large-scale hydrogen production, thereby being useful for overcoming current energy problems. However, the anodic oxygen evolutionary reaction (OER) is characterized by slow kinetics and a high thermodynamic energy barrier, seriously hindering the efficiency of electrochemical hydrolysis.2 Currently used electrocatalysts with better activity for electrocatalytic oxygen evolution reaction (OER) consist of precious metals, such as IrO2 and RuO2. Nevertheless, the exorbitant cost and limited availability of these precious metal-based catalysts severely restrict their applications in water electrolysis. Therefore, exploring efficient and stable non-precious metal electrocatalysts is highly desirable for better energy outcomes.3,4Materials, such as transition metal-based oxides, bimetallic alloys, selenides, sulfides, and phosphates have attracted significant attention in electrochemical hydrolysis due to their diverse range of candidate material components with different catalytic efficiencies.5,6 Among these, transition metal phosphides (TMPs) are abundant on Earth and possess exceptional catalytic activity attributed to their favorable electrical conductivity,7,8 electron orbital properties of phosphorus atoms,9,10 and moderate hydrogen (H) binding energy.11 As a result, the catalytic properties of various forms of TMPs toward the HER, OER, and total hydrolysis were thoroughly investigated. These encompass Ni/Ni2 hollow nanoparticles,12 Ni5P4 discoides,13 WP nanowire arrays,14 CoP@CoMoO4 nanotubes,15 Ce–Co nanosheets,16 hollow urchin-like FeP,17 and MoP2 hollow nanospheres.18 However, monometallic phosphides require enhancement in terms of electrocatalytic activity and stability. By comparison, bimetallic phosphides exhibit better electrocatalytic activity than monometallic phosphides. However, finding effective strategies for fabricating one-dimensional nanostructures and bimetallic phosphides for enhanced electrocatalytic properties of transition metal phosphides is still challenging.
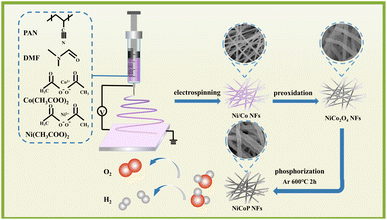
Herein, a novel approach synergistically integrating one-dimensional nanostructures with bimetallic phosphides was developed to significantly enhance the electrocatalytic performance of transition metal phosphides. The precursor nanofibers were obtained through an electrospinning process. Subsequently, NiCo2O4 nanofibers were obtained by thermal annealing and finally converted into bimetallic phosphides (NiCoP nanofibers) through a phosphorylation process. The introduction of bimetal enhanced the electrocatalytic activity of transition metal phosphides toward the OER. The obtained NiCoP nanofibers outperformed monometallic phosphides in all aspects. Additionally, the synergistic effect between phosphorus (P) and isocyanate enhanced the OER properties of NiCoP nanofibers, while the formation of P–M bonds effectively inhibited P degradation, greatly improving the catalyst stability toward the OER.19–22
The proposed one-dimensional structure also facilitated the adsorption of numerous compounds.22 The unique fiber network structure allowed electrospinning to significantly enhance stabilization and increase the interfacial area between the catalytic electrode and the electrolyte, making it an ideal technique for catalysis. The ultra-long one-dimensional nanofibers were then treated as self-contained binderless electrodes, and the resulting NiCoP nanofibers showed ample electrocatalytically active sites and enhanced charge conduction capacity owing to their unique one-dimensional structure. Consequently, the as-obtained NiCoP nanofibers required only a minimum overpotential of 325 mV to drive a current density of 50 mA−2 for oxygen production. Furthermore, NiCoP nanofibers exhibited outstanding durability after undergoing 5000 OER cycles, showing outstanding catalyst performances.
2. Experimental
2.1 Chemical reagents
Polyacrylonitrile (PAN) was purchased from Sigma-Aldrich. N,N-Dimethylformamide (DMF), KOH, Ni(CH3COO)2·4H2O, and Co(CH3COO)2·6H2O were all obtained from Zhiyuan Chemical Reagents, Tianjin. Sodium hypophosphite (NaH2PO2·4H2O) was provided by Shanghai Aladdin Biochemical Technology Corporation. All chemicals were of analytical grade and used as received without additional purification.2.2 Synthesis of NiCo2O4 nanofibers
The preparation process of NiCo2O4 nanofibers is shown in Scheme 1. First, 0.5 g of polyacrylonitrile (PAN) powder was added to a 5 mL solution of N,N-dimethylformamide (DMF). The mixture was covered with a plastic wrap to prevent evaporation and stirred to form a uniform solution with a concentration of 10 wt%. Stoichiometric amounts of 0.4 mmol Ni (CH3COO)2·4H2O and 0.4 mmol Co(CH3COO)2·6H2O were then added to the solution by maintaining a molar ratio of Ni/Co at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was magnetically stirred at room temperature for 10 h to guarantee thorough mixing and obtain a uniform clear viscous precursor solution for electrospinning. The PAN fibers were produced by pouring the mixed precursor solution into a syringe fitted with a stainless-steel needle of 40 mm in length. Afterward, high voltage was applied between the aluminum collector and the tip of the needle at an operating voltage of approximately 7 kV while keeping the collector distance at 15 cm under humidity conditions from 30% to 40%. Electrospinning begins when there is weakened balance between pressure at the needle tip and applied voltage. The droplets of the solution gradually decreased in size, and approximately 10 μL of the solution was evenly dispersed onto the collector. Upon approaching the target electrode, electrostatic repulsion acted on the fiber while facilitating solvent evaporation. After 12 h of electrostatic spinning, the resulting fiber membrane was collected and used as original nanofibers, which then were subjected to heating at a rate of 2 °C min−1 until reaching 600 °C followed by calcination in air for 2 h to produce NiCo2O4 nanofibers. The synthesis processes of NiO and Co3O4 followed a similar procedure to that of NiCo2O4.
1. The mixture was magnetically stirred at room temperature for 10 h to guarantee thorough mixing and obtain a uniform clear viscous precursor solution for electrospinning. The PAN fibers were produced by pouring the mixed precursor solution into a syringe fitted with a stainless-steel needle of 40 mm in length. Afterward, high voltage was applied between the aluminum collector and the tip of the needle at an operating voltage of approximately 7 kV while keeping the collector distance at 15 cm under humidity conditions from 30% to 40%. Electrospinning begins when there is weakened balance between pressure at the needle tip and applied voltage. The droplets of the solution gradually decreased in size, and approximately 10 μL of the solution was evenly dispersed onto the collector. Upon approaching the target electrode, electrostatic repulsion acted on the fiber while facilitating solvent evaporation. After 12 h of electrostatic spinning, the resulting fiber membrane was collected and used as original nanofibers, which then were subjected to heating at a rate of 2 °C min−1 until reaching 600 °C followed by calcination in air for 2 h to produce NiCo2O4 nanofibers. The synthesis processes of NiO and Co3O4 followed a similar procedure to that of NiCo2O4.
2.3 Synthesis of NiCoP nanofibers
The process consisted of phosphating NiCo2O4 nanofibers with NaH2PO2 as the phosphorus source to synthesize NiCoP nanofibers. Briefly, 30 mg of NiCo2O4 nanofibers and 600 mg of NaH2PO2·4H2O were loaded into separate ceramic vessels and placed in a tube furnace. The NaH2PO2·4H2O was positioned 10 cm upstream from the NiCo2O4 nanofibers inside the quartz tube. The furnace was then purged with nitrogen (N2, 99.999%) for 1 h to remove air and then kept under a constant flow rate of nitrogen gas at 320 mL min−1 throughout the process. Subsequently, the temperature was increased to 400 °C for 2 h at a heating rate of 2 °C min−1 before allowing natural cooling to room temperature to obtain NiCoP nanofibers.For comparison, NiCo2O4 nanofiber catalysts with different Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co molar ratios were prepared by the same process. The obtained NiCo2O4 NFs and Ni
Co molar ratios were prepared by the same process. The obtained NiCo2O4 NFs and Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co at ratios of 1
Co at ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 2
2, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were denoted as NCO-1
1 were denoted as NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NCO-1
1, NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and NCO-2
2, and NCO-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Next, the same phosphorylation process was used to convert the precursor NiCo2O4 into NCO-1
1, respectively. Next, the same phosphorylation process was used to convert the precursor NiCo2O4 into NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P, NCO-1
1P, NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2P, and NCO-2
2P, and NCO-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P at phosphating temperatures of 400 °C, 500 °C, 600 °C, 700 °C, and 800 °C. The resulting samples were named NCO-1
1P at phosphating temperatures of 400 °C, 500 °C, 600 °C, 700 °C, and 800 °C. The resulting samples were named NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P400(NiCoP-400), NCO-1
1P400(NiCoP-400), NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P500(NiCoP-500), NCO-1
1P500(NiCoP-500), NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P600(NiCoP-600), NCO-1
1P600(NiCoP-600), NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P700(NiCoP-700), and NCO-1
1P700(NiCoP-700), and NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P800(NiCoP-800), respectively. Similarly, NiCoP-1
1P800(NiCoP-800), respectively. Similarly, NiCoP-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and NiCoP-2
2 and NiCoP-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 obtained at phosphating temperatures of 400 °C, 500 °C, 600 °C, 700 °C, and 800 °C were named NCO-1
1 obtained at phosphating temperatures of 400 °C, 500 °C, 600 °C, 700 °C, and 800 °C were named NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2P400, NCO-1
2P400, NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2P500, NCO-1
2P500, NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2P600, NCO-1
2P600, NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2P700, NCO-1
2P700, NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2P800, NCO-2
2P800, NCO-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P400, NCO-2
1P400, NCO-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P500, NCO-2
1P500, NCO-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P600, NCO-2
1P600, NCO-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P700, and NCO-2
1P700, and NCO-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1P800, respectively. For comparison, synthetic Ni2P/Ni5P4 and CoP2 nanofibers were prepared and used as control samples.
1P800, respectively. For comparison, synthetic Ni2P/Ni5P4 and CoP2 nanofibers were prepared and used as control samples.
2.4 Materials characterization
The crystal structures of the as-prepared compounds were analyzed by X-ray diffraction (XRD, D/max 2600, Rigaku, Japan). The morphologies were viewed by scanning electron microscopy (SEM, SU70, Hitachi, Japan). The atomic structures were observed by transmission electron microscopy (TEM, FEI, Tecnai TF20) and high resolution TEM (HRTEM). The surface chemical components of the composites were identified by X-ray photoelectron spectroscopy (XPS, Thermofisher Scientific).2.5 Electrochemical measurements
The working electrodes were prepared by first mixing 15 mg of NiCoP-600 nanofibers, polyvinylidene fluoride (PVDF), and carbon nanotubes at the mass ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with PVDF as the binder and carbon nanotubes as the conductivity agent. Next, a solution of 50 μL N-methylpyrrolidone (NMP) was added and the mixture was ultrasonically treated for 1 h to obtain a homogeneous suspension. The slurry was vigorously mixed and sonicated for an additional 1 h before being uniformly coated onto a 1 cm2 carbon paper substrate. After drying in a vacuum oven at 60 °C for 10 h, the modified substrates with a loading mass of the active substance of approximately 2.5 mg cm−2 were tested as working electrodes. The electrochemical measurements were conducted on a workstation (VMP-300, France) connected to a three-electrode cell. All electrochemical measurements were performed in a solution containing 1.0 M KOH, with the counter electrode made of a platinum sheet, reference electrode consisting of Ag/AgCl, and working electrode comprising the catalyst sample.
1 with PVDF as the binder and carbon nanotubes as the conductivity agent. Next, a solution of 50 μL N-methylpyrrolidone (NMP) was added and the mixture was ultrasonically treated for 1 h to obtain a homogeneous suspension. The slurry was vigorously mixed and sonicated for an additional 1 h before being uniformly coated onto a 1 cm2 carbon paper substrate. After drying in a vacuum oven at 60 °C for 10 h, the modified substrates with a loading mass of the active substance of approximately 2.5 mg cm−2 were tested as working electrodes. The electrochemical measurements were conducted on a workstation (VMP-300, France) connected to a three-electrode cell. All electrochemical measurements were performed in a solution containing 1.0 M KOH, with the counter electrode made of a platinum sheet, reference electrode consisting of Ag/AgCl, and working electrode comprising the catalyst sample.
The reversible hydrogen electrode (RHE) was used as the reference for conversion of all measured potentials according to the formula: ERHE = ESCE + 0.059 pH + 0.196 V (pH = 14), with overpotential calculated by η = ERHE − 1.23 V. To ensure reliable cyclic voltammetry curves, the electrocatalysts were activated for a total of 70 cycles at a scan rate of 20 mV s−1. Linear sweep voltammetry (LSV) was performed at the scan rate of 5 mV−1. The Tafel slopes were obtained using the formula: η = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(j) + a, where η represents the overpotential, b is the Tafel slope, and j denotes the current density. Electrochemical impedance spectroscopy (EIS) measurements were performed at a potential of 500 mV and frequencies ranging from 0.01 Hz to 100 kHz. Cyclic voltammetry (CV) measurements were carried out in a non-Faraday current regime for evaluating the electrochemical surface area (ECSA). The capacitance of the electric double layer (Cdl) was studied at various CV scan rates ranging from 2 to 12 mV s−1. Cycle tests consisting of 5000 cycles at a scan rate of 100 mV s−1 were carried out to test the long-term stability of the electrocatalysts using the time potential method (J = 10 mA cm−2) with stability recorded after 15 h. All potentials were corrected by the 95%-IR compensation rule.
log(j) + a, where η represents the overpotential, b is the Tafel slope, and j denotes the current density. Electrochemical impedance spectroscopy (EIS) measurements were performed at a potential of 500 mV and frequencies ranging from 0.01 Hz to 100 kHz. Cyclic voltammetry (CV) measurements were carried out in a non-Faraday current regime for evaluating the electrochemical surface area (ECSA). The capacitance of the electric double layer (Cdl) was studied at various CV scan rates ranging from 2 to 12 mV s−1. Cycle tests consisting of 5000 cycles at a scan rate of 100 mV s−1 were carried out to test the long-term stability of the electrocatalysts using the time potential method (J = 10 mA cm−2) with stability recorded after 15 h. All potentials were corrected by the 95%-IR compensation rule.
3. Results and discussion
The synthesis route of NiCo2O4 nanofibers and NiCoP-600 nanofibers is illustrated in Scheme 1. The NiCo2O4 nanofibers were first fabricated by electrospinning, followed by the synthesis of NiCoP-600 nanofibers by a low-temperature phosphating process.The SEM images of NiO, Co3O4, NiCo2O4, and their phosphating products Ni2P/Ni5P4, CoP2, and NiCoP-600 nanofibers are provided in Fig. 1. For further clarification, the HRTEM images of NiCo2O4 and NiCoP-600 nanofibers were also included. As shown in Fig. 1a and b, fibrous structures of NiO nanofibers were formed at different magnifications, with uniform diameters of approximately 50–100 nm and slight fractures observed in the fiber structure (Fig. 1b). The SEM images in Fig. 1c and d display Co3O4 nanofibers with adhered fibrous structures and consistent diameters of around 200–250 nm (Fig. 1d). The SEM images in Fig. 2e and f show distinctly rough surface fibrous structures of NiCo2O4 nanofibers with a uniform diameter ranging from approximately 150 to 200 nm (Fig. 1f). Ni2P/Ni5P4 nanofibers in Fig. 2g and h illustrate smoother surfaces than NiO nanofibers (Fig. 2h). The SEM images in Fig. 2i and j display CoP2 nanofibers with a uniform diameter of about 400–500 nm, smooth surfaces, and good adhesion between the fibers. NiCoP-600 nanofibers in Fig. 2k and l exhibit rough surfaces and uniform diameters of about 200–250 nm (Fig. 2l). From the above SEM images, it can be seen that through the electrospinning method, we can prepare a relatively ideal one-dimensional oxide nanofiber, and the phosphide prepared on this basis can better retain the one-dimensional nanostructure. The complete one-dimensional structure of phosphide is conducive to improving its performance in the field of electrochemistry. The HRTEM images of NiCo2O4 nanofibers in Fig. 1m reveal a distinct lattice spacing of 0.47 nm corresponding to the (111) plane of the cubic structure, as indicated by XRD. The HRTEM images of NiCoP-600 nanofibers in Fig. 1n demonstrate a distinct lattice spacing of 0.22 nm, consistent with the XRD pattern and indicating the presence of the (111) plane within the cubic structure. The high chemical reactivity interaction between isocyanate and P formed NiCoP-600 nanofibers with densely packed structures (Fig. 1l). The elemental mappings of P, Ni, and Co in NiCoP-600 nanofibers can be found in Fig. S1 of the ESI.†
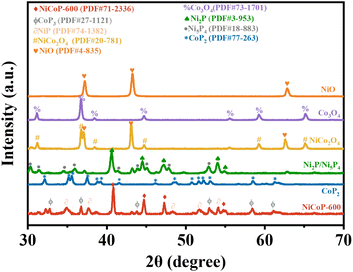 | ||
| Fig. 2 XRD patterns of NiO nanofibers, Co3O4 nanofibers, NiCo2O4 nanofibers, Ni2P/Ni5P4 nanofibers, CoP2 nanofibers, and NiCoP-600 nanofibers. | ||
The crystal structures of NiO, Co3O4, NiCo2O4, Ni2P/Ni5P4, CoP2, and NiCoP-600 nanofibers obtained by XRD are depicted in Fig. 2. The diffraction peaks of NiO nanofibers and Co3O4 nanofibers were consistent with those of NiO (PDF#4-835) and Co3O4 (PDF#73-1701). The diffraction peaks of NiCo2O4 nanofibers can be attributed to the spinel structure of NiCo2O4 (PDF#20-0781). XRD analysis according to the PDF#20-0781 database indicated the presence of peaks at 31.1°, 36.6°, 44.6°, 59.0°, and 64.5°, which can be assigned to the crystal planes (220), (311), (400), (511), and (440) of typical NiCo2O4, respectively. Additionally, NiO was present in the XRD detection profiles. After phosphating treatment, two phosphates namely Ni2P and Ni5P4 were obtained with characteristic diffraction peaks matching those reported for Ni2P(PDF#3-953) and Ni5P4(PDF#18-883), respectively. Phosphating treatment of Co3O4 nanofibers resulted in the formation of CoP2 nanofibers with characteristic diffraction peaks corresponding to those of CoP2(PDF#77-263). NiCoP-600 nanofibers obtained by phosphating NiCo2O4 nanofibers showed minor amounts of NiP and CoP3. The XRD pattern revealed distinct peaks at 41.02°, 44.92°, and 47.6°, attributed to the crystal planes (111), (201), and (210) of NiCoP-600 nanofibers successfully synthesized in this study (PDF#71-2366), respectively. Thus, P and isocyanate were covalently linked in the NiCoP-600 structure through Ni–P and Co–P bonds.
The chemical makeup of NiCoP-600 was analyzed by XPS on both nanofibers of NiCo2O4 and NiCoP-600. The presence of Ni 2p, Co p, and P 2p characteristic peaks in the XPS spectrum of NiCoP-600 nanofibers confirmed the existence of these elements, aligning well with EDS mapping results (Fig. S2a†). The complete XPS spectrum of NiCo2O4 is presented in Fig. S3,† while that of NiCoP-600 is given in Fig. 3a. For comparison, the Ni 2p XPS spectra of NiCo2O4 and NiCoP-600 are presented in Fig. 3b. The spectrum of NCO exhibited six distinct peaks, with two peaks at 853.2 eV and 854.9 eV related to Ni 2p3/2 orbitals. Additionally, twin peaks were observed at 871.8 eV and 875.9 eV, associated with the Ni 2p1/2 orbital, while satellite peaks can be found at approximately 860.2 eV and 878.9 eV. Compared to NiCo2O4, the binding energy of Ni 2p peaks in NiCoP-600 shifted by approximately 2.6 eV toward higher values. Additionally, the Ni 2p spectrum in Fig. 3b depicts a peak of P–Ni originating from NiCoP due to the formation of a P–Ni bond between isocyanate and P under an argon atmosphere.22–24,36 The high-resolution Co 2p XPS spectra of both NiCo2O4 and NiCoP-600 samples in Fig. 3c exhibit similar characteristics to those of Ni 2p that can be deconvoluted into six distinct peaks. The observed peaks at 777.3 eV and 780.9 eV can be attributed to the Co 2p3/2 state, while those at 793.6 eV and 795.2 eV correspond to the Co 2p1/2 state. Hence, Co was predominantly present in a metallic state.33,35 Two distinct peaks were detected at energy levels of 785 eV and 802.2 eV, respectively. The appearance of peaks at 777.1 eV and 792.1 eV can be assigned to the bonding between P and Co atoms.22,25,34 The P 2p spectrum of NiCoP-600 in Fig. 3d exhibits three distinct peaks, indicative of metal phosphide's characteristic metal–P bonds. The P 2p3/2 and P 2p1/2 depicted binding energies of respectively 131.6 eV and 132.5 eV, with an extra peak around 137.1 eV corresponding to the bond between P and Ni/Co.26–28 All these data confirmed the successful synthesis of NiCoP-600 catalysts.
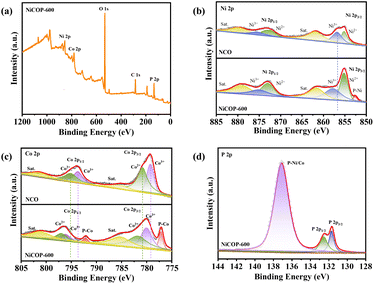 | ||
| Fig. 3 (a) Full spectrum of NiCoP-600 nanofibers. XPS spectra of (b) Ni 2p and (c) Co 2p of NiCo2O4 and NiCoP-600 nanofibers. (d) XPS spectrum of P 2p of NiCoP-600 nanofibers. | ||
The electrocatalytic performances of NiCoP-600 nanofibers toward the OER were assessed by linear sweep voltammetry (LSV) in 1.0 M potassium hydroxide solution in comparison with NiO, Co3O4, NiCo2O4, Ni2P/Ni5P4, and CoP2 nanofibers under the same conditions. The recorded LSV curves of NiO, Co3O4, NiCo2O4, Ni2P/Ni5P4, CoP2, NiCoP-600 nanofibers, and RuO2 are given in Fig. 4a. Before each catalytic test, repeated CV scans were performed at 5 mV−1 and 1.0–1.65 V as pre-activation scans in the potential range of RHE. The required overpotential (η) at a specific current density was used to evaluate the performance of each electrocatalyst. Meanwhile, the overpotential of each catalyst was determined by analyzing the LSV curve at a current density of 50 mA cm−2. Obviously, the overpotential of NiCoP-600 was significantly lower than those of NiO, Co3O4, NiCo2O4, Ni2P/Ni5P4, CoP2, NiCoP-600 nanofibers, and RuO2. Under the same current density (j = 50 mA cm−2), the overpotential of NiCoP-600 was the lowest at 325 mV, a value very close to that of RuO2 (313 mV) while surpassing those of NiO (460 mV), Co3O4 (439 mV), NiCo2O4 (400 mV), Ni2P/Ni5P4 (384 mV), and CoP2 nanofibers (348 mV). Further analysis of LSV curves revealed that NiCoP-600 preserved its superior overpotential (355 mV) under a current density of 100 mA cm−2 when compared to Co3O4 (472 mV), NiCo2O4 (440 mV), Ni2P/Ni5P4 (427 mV), and CoP2 nanofibers (378 mV) (Fig. 4b), as well as outperforming some reported OER catalysts (Fig. 4d). In this paper, the OER characteristics exhibited by bimetallic phosphides surpassed those of monometallic phosphides, which, in turn were superior to those of bimetallic oxides and monometallic oxides. Also, the OER performance of NiCoP-600 nanofibers surpassed that of NiCoP-600 powders (Fig. S4†). Thus, the advantages of one-dimensional nanostructures over nanofibers with irregular shapes were substantial in terms of the OER.29 Overall, the catalytic activity of NiCoP-600 nanofibers toward the OER looked remarkable when compared to other catalysts, demonstrating good performances.
The optimal experimental conditions in terms of nickel–cobalt ratio and corresponding calcination temperature were determined by comparing the OER activities of NiCo2O4 nanofiber catalysts with different Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co molar ratios. The LSV curves of NCO-1
Co molar ratios. The LSV curves of NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NCO-1
1, NCO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and NCO-2
2, and NCO-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are summarized in Fig. S5a.† Subsequently, a sequence of phosphating procedures was conducted at temperatures ranging from 400 °C to 800 °C, and structural and catalytic characterization studies were conducted to investigate the influence of calcination temperature. As shown in the SEM image (Fig. S6†), the calcination at 500 °C and 600 °C resulted in the formation of clear fiber shapes (Fig. S6a, b, e and f†). By comparison, calcination at 700 °C resulted in a semi-melt state (Fig. S6c and g†) while 800 °C caused complete melting and loss of the distinct fiber shape (Fig. S6d and h†). The corresponding LSV curves in Fig. S5b† indicate 600 °C as a suitable phosphating temperature for enhancing the OER activity of NiCoP-600 (Fig. S5b†). The samples calcined at 600 °C exhibited the best OER performances, while those calcined at 800 °C showed the worst OER performances. Therefore, the most favorable temperature for the fabrication of NiCoP nanofibers was identified as 600 °C.
1 are summarized in Fig. S5a.† Subsequently, a sequence of phosphating procedures was conducted at temperatures ranging from 400 °C to 800 °C, and structural and catalytic characterization studies were conducted to investigate the influence of calcination temperature. As shown in the SEM image (Fig. S6†), the calcination at 500 °C and 600 °C resulted in the formation of clear fiber shapes (Fig. S6a, b, e and f†). By comparison, calcination at 700 °C resulted in a semi-melt state (Fig. S6c and g†) while 800 °C caused complete melting and loss of the distinct fiber shape (Fig. S6d and h†). The corresponding LSV curves in Fig. S5b† indicate 600 °C as a suitable phosphating temperature for enhancing the OER activity of NiCoP-600 (Fig. S5b†). The samples calcined at 600 °C exhibited the best OER performances, while those calcined at 800 °C showed the worst OER performances. Therefore, the most favorable temperature for the fabrication of NiCoP nanofibers was identified as 600 °C.
The crystal structures of nanofibers with different compositions, namely NiCoP-400, NiCoP-500, NiCoP-600, NiCoP-700, and NiCoP-800 were determined by XRD analysis (Fig. S7†). At 400 °C, 500 °C, and 600 °C, minor amounts of NiP and CoP3 were also detected. However, only NiCoP was present at 700 °C and 800 °C. The combination of this observation with the OER performance suggested superior OER performance of the non-pure phase of NiCoP when compared to its pure phase counterpart. Thus, the presence of other valence compounds synergistically enhanced the catalytic activity of NiCoP in promoting the oxygen evolution reaction. In sum, NiCoP-600 samples demonstrated the highest OER performances.
The Tafel slopes can be used to reveal the reaction mechanism and determine the inherent activity of catalysts through analysis of the reaction kinetic rate. Thus, Tafel analysis was conducted on all TMP “pre-catalysts” to analyse their OER kinetics. The results suggested a decrease in Tafel slopes in a similar order to the apparent OER activities. The Tafel slope was obtained by fitting the graph to the Tafel equation (η = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j + a), where j denotes the current density and b represents the slope of the Tafel curve. As shown in Fig. 4c, NiCoP-600 presented the lowest Tafel slope at 96.4 mV dec−1, a value lower than those of NiO (158.9 mV dec−1), Co3O4 (99.2 mV dec−1), NiCo2O4 (119.2 mV dec−1), Ni2P/Ni5P4 (113.3 mV dec−1), and CoP2 (99.1 mV dec−1). As a result, the surface of the CoNiP catalyst exhibited a mechanism of rapid adsorption, implying enhanced electron transfer kinetics and an elevated rate of electron transfer.26 The prepared NiCoP-600 nanofibers exhibited excellent properties and their one-dimensional nanostructure enhanced the regional dynamics of the active material, resulting in significant oxygen generation capacity of NiCoP-600.
j + a), where j denotes the current density and b represents the slope of the Tafel curve. As shown in Fig. 4c, NiCoP-600 presented the lowest Tafel slope at 96.4 mV dec−1, a value lower than those of NiO (158.9 mV dec−1), Co3O4 (99.2 mV dec−1), NiCo2O4 (119.2 mV dec−1), Ni2P/Ni5P4 (113.3 mV dec−1), and CoP2 (99.1 mV dec−1). As a result, the surface of the CoNiP catalyst exhibited a mechanism of rapid adsorption, implying enhanced electron transfer kinetics and an elevated rate of electron transfer.26 The prepared NiCoP-600 nanofibers exhibited excellent properties and their one-dimensional nanostructure enhanced the regional dynamics of the active material, resulting in significant oxygen generation capacity of NiCoP-600.
As displayed in Fig. 5a and S8b,† the charge transfer resistance (Rct) of NiCoP-600 was estimated to be 1.8 Ω, a value lower than those of NiO (122.1 Ω), Co3O4 (27.1 Ω), NiCo2O4 (22.3 Ω), Ni2P/Ni5P4(7.6 Ω), and CoP2 (3.8 Ω). Thus, NiCoP-600 exhibited faster electron transfer capability. Additionally, the one-dimensional carbon nanofiber structures effectively reduced the electrical contact resistance between adjacent NiCoP-600 nanoparticles, facilitating efficient electrical contact and rapid mass transfer of reactants and products during the reaction.
The electrochemically active surface area (ECSA) was evaluated using the capacitance of the electrochemical double layer (Cdl). The cyclic voltammetry (CV) activation process demonstrated that the electrode current can be segregated into two components. The first had to do with non-Faraday currents, encompassing charge and discharge currents, and the second dealt with Faraday currents associated with electrochemical reactions.29,30 The difference in current density between charge–discharge reactions would linearly vary with the CV scanning speed. In the region with nonapplicable Faraday potential, the electrochemically accessible surface area (ECSA) of each catalyst was estimated by determining the electrochemical double-layer capacitance (Cdl) by the CV method to provide insights into the number of active sites. Cdl was obtained by performing a linear fit between the current density and scanning rate of cyclic voltammetry (CV) curves, where the slope represents the Cdl value. As shown in Fig. 5b, the Cdl of NiCoP-600 toward the OER reaction under different scanning rates was estimated to be 827 mF cm−2, a value significantly higher than those of NiO (21 mF cm−2), Co3O4 (23 mF cm−2), NiCo2O4 (65 mF cm−2), Ni2P/Ni5P4 (414 mF cm−2), and CoP2 (550 mF cm−2). In the scanning potential range from 1.24 to 1.34 V, the ECSA of NiCoP-600 was the largest, indicating the presence of more active sites for the OER reaction when compared to other materials mentioned above. As displayed in Fig. 5b and S9a,† Cobalt-nickel bimetals contributed to increasing the Cdl values. For example, the Cdl value of NiCoP-600 (827 mF cm−2) was significantly higher than those of Ni2P/Ni5P4 (414 mF cm−2) and CoP2 (550 mF cm−2). The increased ECSA of bimetallic cobalt-nickel phosphating nanofibers resulted in a larger contact area between the catalyst and electrolyte, promoting the kinetics of the OER reaction.32,33
During real-time manufacturing procedures, the durability of the electrocatalyst would also play a pivotal role in assessing the efficacy of catalyst materials since the catalyst material would facilitate the liberation of oxygen from the electrode surface during O2 release. Thus, the long-term stability of NiCoP-600 was investigated in a potassium hydroxide (1.0 M) solution. As demonstrated in Fig. 5c, the current of the NiCoP-600 catalyst remained relatively stable (347 mV) even after 5000 cycles, suggesting excellent electrochemical durability in an alkaline environment. Additionally, long-term electrochemical stability testing at 50 mA cm−2 for 50 h revealed good durability for the NiCoP-600 catalyst (Fig. 5d). The formation of P–M bonds can effectively prevent hydrogen bonding between oxygen atoms bonded to phosphorus and water molecules, thereby significantly enhancing stability. The structural stability of NiCoP-600 during electrocatalytic OER was verified by SEM imaging and EDS mapping before and after electrocatalytic OER. In Fig. S10,† the fibers did not break but the surface roughness increased after the OER. In Fig. S2a and b,† the EDS mapping results indicated a decrease in only the peak value of P, suggesting high structural stability for NiCoP-600.
The anodic process in the electrolytic cell involved a four-electron mechanism OER. By comparison to the hydrogen evolution reaction (HER), the OER would have slower kinetics with three generated adsorption intermediates (M–OOHads, M–Oads, and M–OHads).31 In alkaline electrolytes, the OER was initiated by hydrolysis or OH coordination, followed by consecutive oxidation steps from M–OHads to M–Oads, then to M–OOHads, ultimately producing O2. All suggested mechanisms involved stages of hydroxyl coordination in alkaline environments, with M representing the active site and ads indicating the adsorbed form on the catalyst surface. Accordingly, the appropriate OER mechanism in alkaline electrolytes can be summarized as follows:
| NiCoP + 2OH− → NiCoP(OH)2 ads + 2e− | (1) |
| NiCoP(OH)2 ads + 2OH− → NiCoPO2 ads + 2H2O + 2e− | (2) |
| NiCoPO2 ads → NiCoP + 2O2 | (3) |
| NiCoPO2 ads + 2OH− → NiCoP(OOH)2 ads + e− | (4) |
| NiCoP(OOH)2 ads + 2OH− → NiCoP + 2O2 + 2H2O + 2e− | (5) |
Two primary pathways would influence oxygen production through distinct basic steps. The initial route entailed the direct amalgamation of two M–Oads intermediates, proceeding through steps (1) → (2) → (3). In the following sequential progression of (1) → (2) → (4) → (5), the intermediate M–OOHads initially transformed into M–Oadsvia hydroxide, subsequently merging with another hydroxide to generate O2. Note that reaction (3) always exhibited a higher thermodynamic barrier than reactions (4) and (5). The principal approaches for oxygen generation involved metal phosphide and sulfide following the second pathway.
4. Conclusions
Novel NiCoP-600 bimetallic phosphides were successfully prepared by electrostatic spinning technology, thermal annealing, and phosphatization processes. The catalysts demonstrated exceptional OER efficiency, with only 325 mV overpotential needed to attain a current density of 50 mA cm−2 in 1 M potassium hydroxide solution. After 5000 cycles of OER, the catalysts also exhibited a remarkable overpotential of 347 mV, surpassing the performance of numerous reported transition metal phosphides. The one-dimensional nanostructure of the cross-linked network promoted charge transport, as well as facilitating electrolyte penetration and oxygen bubble release, thereby accelerating the catalytic kinetics of the OER. The introduction of bimetallic components also modulated the central electronic structure of monometallic phosphides. The NiCoP-600 catalysts outperformed their monometallic counterparts (Ni2P/Ni5P4 and CoP2 nanofibers) in all aspects. Overall, the proposed cost-effective nanocomposite catalysts look promising for future reversible electrochemical energy applications.Author contributions
Yue Wang: conceptualization, data curation, writing – original draft. Xin Chang: software, formal analysis. Zexing Huang: software. Jiahui Fan: investigation. Lu Li: writing – review & editing. Mingyi Zhang: conceptualization, supervision, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to be supported in part by the National Natural Science Foundation of China (no. 52102228).References
- L. Rößner, H. Schwarz, I. Veremchuk, R. Zerdoumi, T. Seyller and M. Armbrüster, ACS Appl. Mater. Interfaces, 2021, 13, 23616–23626 CrossRef PubMed.
- G. A. Gebreslase, M. V. Martínez-Huerta and M. J. Lázaro, J. Energy Chem., 2022, 67, 101–137 CrossRef CAS.
- Y.-C. Zhang, C. Han, J. Gao, L. Pan, J. Wu, X.-D. Zhu and J.-J. Zou, ACS Catal., 2021, 20, 12485–12509 CrossRef.
- Q. Pan and L. Wang, J. Energy Chem., 2021, 485, 229335 CAS.
- X. Li, Q. Hu, H. Yang, T. Ma, X. Chai and C. He, Chin. Chem. Lett., 2022, 33, 3657–3671 CrossRef CAS.
- F. Aftab, H. Duran, K. Kirchhoff, M. Zaheer, B. Iqbal, M. Saleem and S. N. Arshad, ChemCatChem, 2020, 12, 932–943 CrossRef CAS.
- Y. Li, R. Tong, W. Zhang and S. Peng, J. Catal., 2022, 410, 22–30 CrossRef CAS.
- G. M. S. Salvador, A. L. Silva, L. P. C. Silva, F. B. Passos and N. M. F. Carvalho, Int. J. Hydrogen Energy, 2021, 46, 26976–26988 CrossRef CAS.
- Sk. Riyajuddin, S. K. Tarik Aziz, S. Kumar, G. D. Nessim and K. Ghosh, ChemCatChem, 2019, 12, 1394–1402 CrossRef.
- W. Yu, Y. Gao, Z. Chen, Y. Zhao, Z. Wu and W. Li, Chin. J. Catal., 2021, 42, 1876–1902 CrossRef CAS.
- L. Yu, J. Zhang, Y. Dang, J. He, Z. Tobin, P. Kerns, Y. Dou, Y. Jiang, Y. He and S. L. Suib, ACS Catal., 2019, 9, 6919–6928 CrossRef CAS.
- X. Li, J. Zhou, C. Liu, L. Xu, C. Lu, J. Yang, H. Pang, W. Hou and W. Hou, Appl. Catal., B, 2021, 298, 120578 CrossRef CAS.
- M. Ledendecker, S. K. Calderón, C. Papp, H.-P. Steinrück, M. Antonietti and M. Shalom, Angew. Chem., Int. Ed., 2015, 54, 12361–12365 CrossRef CAS PubMed.
- C. Lv, J. Liu, P. Lou, X. Wang, L. Gao, S. Wang and Z. Huang, Nanoscale, 2022, 14, 5430–5438 RSC.
- Z. Gao, Z. Zeng, X. Xu, R. Mao, R. Jia and S. Han, Int. J. Hydrogen Energy DOI:10.1016/j.ijhydene.2023.07.179.
- J. Bi, H. Ying, H. Xu, X. Zhao, X. Du, J. Hao and Z. Li, Chem. Commun., 2022, 58, 7817–7820 RSC.
- P. P. Gao, M. Gao, T. Lei, Z. Ren, J. Luo, Z. Huang and A. Wu, Inorg. Chem. Commun., 2023, 156, 111143 CrossRef CAS.
- W. Yang, J. Tian, L. Hou, B. Deng, S. Wang, R. Li, F. Yang and Y. Li, ChemSusChem, 2019, 12, 4662–4670 CrossRef CAS PubMed.
- J. Zheng, X. Peng, Z. Xu, J. Gong and Z. Wang, ACS Catal., 2022, 12, 10245–10254 CrossRef CAS.
- C. Zhang, J. Wang, Y. Liu, W. Li, Y. Wang, G. Qin and Z. Lv, Chem.–Asian J., 2022, 17, e202200377 CrossRef CAS PubMed.
- P. M. Bodhankar, D. S. Dhawale, S. Giddey, R. Kumar and P. B. Sarawade, Sustainable Energy Fuels, 2022, 6, 5491–5502 RSC.
- T. Wu, J. Fan, Q. Li, P. Shi, Q. Xu and Y. Min, Adv. Energy Mater., 2018, 8, 1701799 CrossRef.
- M. A. Ahsan, T. He, K. Eid, A. M Abdullah, M. F. Sanad, A. Aldalbahi, B. Alvarado-Tenorio, A. Du and A. R. P. Santiago, ACS Appl. Mater. Interfaces, 2022, 14, 3919–3929 CrossRef CAS PubMed.
- A. R. P. Santiago, T. He, O. Eraso, M. A. Ahsan, A. N. Nair, V. S. N. Chava, T. Zheng, S. Pilla, O. Fernandez-Delgado, A. Du, S. T. Sreenivasan and L. Echegoyen, J. Am. Chem. Soc., 2020, 142, 17923–17927 CrossRef PubMed.
- H. Wang, C. Li, J. An, Y. Zhuang and S. Tao, J. Mater. Chem. A, 2021, 9, 18421–18430 RSC.
- I. Amorim, J. Xu, N. Zhang, D. Xiong, S. M. Thalluri, R. Thomas, J. P. S. Sousa, A. Araújo, H. Li and L. Liu, Catal. Today, 2020, 358, 196–202 CrossRef CAS.
- J. Yin, J. Jin, M. Lu, B. Huang, H. Zhang, Y. Peng, P. Xi and C.-H. Yan, J. Am. Chem. Soc., 2020, 142, 18378–18386 CrossRef CAS PubMed.
- X. Li, L. Xiao, L. Zhou, Q. Xu, J. Weng, J. Xu and B. Liu, Angew Chem. Int. Ed. Engl., 2020, 59, 21106–21113 CrossRef CAS PubMed.
- B. Yang, X. Chang, X. Ding, X. Ma and M. Zhang, J. Colloid Interface Sci., 2022, 623, 196–204 CrossRef CAS PubMed.
- S. Haschke, D. Pankin, Y. Petrov, S. Bochmann, A. Manshina and J. Bachmann, ChemSusChem, 2017, 10, 3644–3651 CrossRef CAS PubMed.
- H. Shi, H. Liang, F. Ming and Z. Wang, Angew. Chem., 2017, 129, 588–592 CrossRef.
- X. Qiao, H. Kang, Y. Li, K. Cui, X. Jia, X. Wu and W. Qin, Appl. Catal., B, 2022, 305, 121034 CrossRef CAS.
- W. Jiang, Y. Tao, J. Ma, X. Liu, S. Zhu, W. Hao, Q. Xu and J. Fan, ACS Sustainable Chem. Eng., 2023, 11, 6629–6640 CrossRef CAS.
- M. A. Ahsan, T. He, K. Eid, A. M. Abdullah, M. L. Curry, A. Du, A. R. P. Santiago, L. Echegoyen and J. C. Noveron, J. Am. Chem. Soc., 2021, 143, 1203–1215 CrossRef CAS PubMed.
- M. Yu, X. Guo, X. Chang, X. Ma and M. Zhang, Sustainable Energy Fuels, 2022, 6, 5000–5007 RSC.
- H. Liang, A. N. Gandi, D. H. Anjum, X. Wang, U. Schwingenschlögl and H. N. Alshareef, Nano Lett., 2016, 16, 7718–7725 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3se01412f |
| This journal is © The Royal Society of Chemistry 2024 |