Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High performance alkyl dialkoxyalkanoate bioderived transportation fuels accessed using a mild and scalable synthetic protocol†
Nicholas R.
Myllenbeck
 *a,
Eric
Monroe‡
b,
Mysha
Sarwar
b,
Teresa
Alleman‡
c,
Cameron
Hays
c,
Jon
Luecke
c,
Junqing
Zhu‡
d,
Charles
McEnally
*a,
Eric
Monroe‡
b,
Mysha
Sarwar
b,
Teresa
Alleman‡
c,
Cameron
Hays
c,
Jon
Luecke
c,
Junqing
Zhu‡
d,
Charles
McEnally
 d,
Lisa
Pfefferle
d,
Anthe
George
b and
Ryan W.
Davis
d,
Lisa
Pfefferle
d,
Anthe
George
b and
Ryan W.
Davis
 b
b
aMaterials Chemistry Department, Sandia National Laboratories, Livermore, CA 94551, USA. E-mail: nrmylle@sandia.gov
bBioresource and Environmental Security Department, Sandia National Laboratories, Livermore, CA 94551, USA
cNational Renewable Energy Laboratory, Golden, CO 80401, USA
dDepartment of Chemical and Environmental Engineering, Yale University, New Haven, CT 06511, USA
First published on 2nd February 2024
Abstract
Replacement of conventional petroleum fuels with renewable fuels reduces net emissions of carbon and greenhouse gases, and affords opportunities for increased domestic energy security. Here, we present alkyl dialkoxyalkanoates (or DAOAs) as a family of synthetic diesel and marine fuel candidates that feature ester and ether functionality. These compounds employ pyruvic acid and fusel alcohols as precursors, which are widely available as metabolic intermediates at high titer and yield. DAOA synthesis proceeds in high yield using a simple, mild chemical transformation performed under air that employs bioderived and/or easily recovered reagents and solvent. The scalability of the synthetic protocol was proven in continuous flow with in situ azeotropic water removal, yielding 375 g of isolated product. Chemical stability of DAOAs against aqueous 0.01 M H2SO4 and accelerated oxidative conditions is demonstrated. The isolated DAOAs were shown to meet or exceed widely accepted technical criteria for sustainable diesel fuels. In particular, butyl 2,2-dibutoxypropanoate (DAOA-2) has indicated cetane number 64, yield soot index 256 YSI per kg, lower heating value 30.9 MJ kg−1 and cloud point < −60 °C and compares favorably to corresponding values for renewable diesel, biodiesel and petroleum diesel.
Introduction
Establishment of a sustainable, low-net carbon emission transportation infrastructure is attractive for increasing national energy independence. By using renewable fuels, the anthropogenic net emission of carbon dioxide can be reduced, ameliorating climate impact.1 In the United States, the transportation sector consumed 4.85 billion barrels of petroleum-based fuels in 2021, accounting for 67% of the overall petroleum usage.2 Electrification is underway for light duty vehicles, but commercial transportation often involves long distance travel with heavy loads, for which liquid fuels are currently more practical for space and time considerations. This is especially true for marine and air travel, where options to refuel or recharge are more limited.3A range of alternative liquid fuels have been investigated for use in compression ignition (e.g. diesel) engines, but limitations to more widespread usage exist. For example, despite energy content and cetane number (CN) comparable to petroleum diesel, the high cloud point temperature of biodiesel fatty acid esters (FAE) precludes blend ratios > 20% with conventional diesel, especially in cold environments.4,5 Renewable diesel can serve as a drop-in replacement for petroleum diesel in some markets, but similar to biodiesel competes with food usage for lipid feedstocks. More recent renewable fuel concepts, such as branched alkyl ethers and poly(oxymethylene)ethers (POMEs), appear to alleviate low temperature operability concerns while maintaining combustion performance. However, these classes have relatively low energy densities and their production requires multiple operationally complex synthesis and purification steps from bioavailable feedstock, reducing the overall yield and increasing the fuel selling price and/or net greenhouse gas (GHG) emissions.6–9
Our group initially investigated lactate ester–ethers (alkyl alkoxyalkanotes or AOAs) that were synthesized in two steps from lactic acid.10 (Scheme 1) while derived cetane number 43.6, YSI per kg 324, cloud point < −50 °C and LHV 32.1 of isopentyl 2-(isopentyloxy)propanoate was promising, the etherification requires hazardous and toxic reagents, dry solvent and inert atmosphere. A milder route featuring reductive etherification of the corresponding pyruvate ester–ketal was proposed.9,11 While this direct approach to AOAs was not successful using hydrogen at ∼1 atmosphere, it was recognized that the pyruvate ester–ketal (“DAOA”) intermediate could be a diesel fuel candidate itself, based on the promising results obtained for AOAs.4,5
 | ||
| Scheme 1 Realized and envisioned routes to AOA diesel fuel candidates, beginning with biologically available carboxylic acids. | ||
This report details a new class of molecules that combine the high synthesis yield and bioavailability associated with ester functional groups with the strong combustion performance of alkyl ethers. The compounds can be accessed using continuous flow synthesis, and an average of 1.96 g h−1 (2.13 mL h−1) of DAOA-2 was isolated over 8 days of operation. Indicated cetane number (ICN), lower heat of combustion (LHV), yield soot index (YSI), cloud point and other physical properties of several synthesized compounds were found to meet or exceed applicable target values for sustainable diesel fuel candidates, demonstrating their potential as fuels. Together, these findings provide: (1) identification of a new class of drop-in diesel fuels that combines the low temperature performance of renewable diesel with the low sooting of biodiesel, (2) chemically benign production without the need for lipid feedstocks, which are anticipated to be increasingly limiting in light of demand for hydrocarbons for scale-up of sustainable aviation fuels,12 (3) new chemical structure–property relationships for mixed moiety oxygenate diesel fuels, and (4) a path to at least 60% reduction in the CO2-intensity of renewable fuels compared to petroleum diesel.5
Results and discussion
Design
Key properties of a fuel candidate include fluid and combustion properties, operational temperature window, boiling range, emissions produced, production scalability and chemical stability.4,5,13 Leveraging recent reports of alkyl ethers as promising diesel fuel components to meet many of these properties,6,14 we aimed to identify a simple, scalable, sustainable synthetic route that provided access to geminal diethers as high-performance diesel fuels at 100% carbon atom economy.Pyruvate and fusel alcohols were chosen as reactants for their availability from well-studied biochemical transformations. Pyruvate is produced by living organisms during glucose metabolism. Several groups have recently reported biological routes to pyruvate in at hectogram scale from sugars or woody biomass using microbial digestion.15–17 Aliphatic alcohols are also produced biochemically, through fermentation. In particular, the range of C2–C6 has been heavily studied with commercial processes developed for production of fusel alcohols and 1-butanol.18–21 While alkyl-C6DAOAs were identified as the useful limit with respect to purification by common laboratory equipment (normal boiling point ∼345 °C), information from prior work on AOAs indicated that diesel fuel performance increased with increasing alkyl chain length, so C4–C6DAOAs were of most practical interest.
Batch synthesis of DAOAs
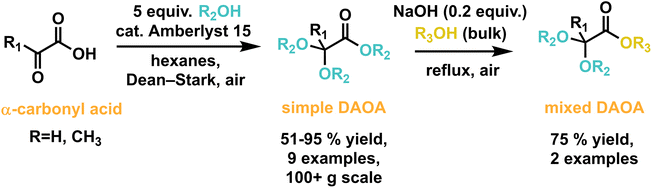
A previous report detailed the synthesis of several DAOAs in 61–72% yield by combining pyruvic acid and geminal dialkoxypropanes in refluxing alcohol solution containing 2 mol% p-TsOH.22 This method requires prior synthesis of dialkoxypropanes from commercially available 2,2-dimethoxypropane, which functions as an auxiliary. We sought a simplified and sustainable method to access the same family of compounds using alcohols directly as starting materials (Scheme 2).Amberlyst 15 was selected as a commercially available, recyclable, solid-supported strong acid catalyst, previously shown to be effective in ester and ketal synthesis.23–25 Aqueous washes of the reaction mixtures after filtration had neutral pH, indicating the possibilities of (1) catalyst recyclability between batches and (2) simplified reaction workup. Alkane solvents were used to induce aqueous-organic azeotrope layer separation, which was not as successful with toluene or bulk alcohols.26
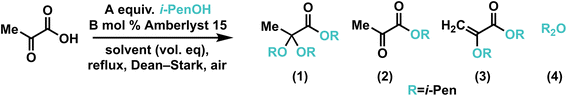
Optimization was carried out for the synthesis of isopentyl 2,2-bis(isopentyloxy)propanoate (DAOA-5) by reacting pyruvic acid and isopentyl alcohol (Table 1). Variables included reaction temperature, alcohol equivalents, solvent, and catalyst loading. Equilibrium position of DAOA synthesis is inversely related to reflux temperature, which is consistent with negative entropy of reaction for acetal formation.27 Additionally, higher temperatures led to increased prevalence of side reactions, including etherification and elimination of alcohol from DAOA. 5 equiv. alcohol and hexane solvent led to effective azeotrope layer separation and 75–80 °C reflux temperature, which balanced reaction rate with equilibrium position and product selectivity and led to 90+% conversion of alkyl pyruvate to DAOA after 18–24 hours. Furthermore, Amberlyst 15 at 5 mol% could be reused at least ten times without degradation to 1H NMR yield (see ESI†). A 1H NMR time series revealed that at 75–80 °C, the rapidly formed pyruvate ester intermediate is steadily converted to DAOA.
| Entry | A | B | Alkane solventa | Temp. (°C) | Time (h) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4b 4b |
|---|---|---|---|---|---|---|
| a Volume equiv. relative to i-PenOH. b Mole ratio calculated by 1H NMR integration of reaction mixture after time specified. c Product distribution did not further favour (1) after 6 hours. | ||||||
| 1 | 6 | 10 | None | 133 | 6c | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
| 2 | 4 | 10 | C7 (0.5) | 120 | 6c | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 3 | 4 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v C6/C7 (0.5) 1 v/v C6/C7 (0.5) |
100 | 6c | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 4 | 4 | 10 | C6 (0.5) | 93 | 25 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 5 | 4 | 10 | C6 (1) | 85 | 25 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 5 | 10 | C6 (1) | 78 | 19 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 7 | 6 | 10 | C6 (1) | 75 | 20 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 8 | 5 | 5 | C 6 (1) | 80 | 24 |
94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 0 0
|
| 9 | 5 | 2.5 | C6 (1) | 78 | 24 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
With the general aspects of the synthetic procedure optimized, the substrate scope was investigated with respect to alcohol and carbonyl compounds (Table 2). 51–95% yield of DAOA compounds was achieved by combining pyruvic or glyoxylic acid and C2–C6 primary alcohols (DAOAs 1–7, 11), but only trace conversion was observed for 2° or 3° alcohol reactants. Combination of glyoxylic acid and 2-butanol furnished DAOA-10 in good yield, but 3° alcohols were still unsuccessful. Base-catalyzed transesterification using catalytic sodium hydroxide enabled chemoselective exchange of the ester functionality (DAOAs 8 and 9).
| DAOA or entry # | R1, R2, R3 | Chemical formula | Synthesis yield | ICN | LHV (MJ kg−1) | YSI per kg | Cloud point (°C) | H2O sol. (g L−1) | ν (cSt) | Density (g cm−3) | Normal BP (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Average of 11 samples from distinct batches. b Transesterification yield. c Reference compound is methyl oleate. d Reference compound is n-hexadecane. e 60% the value of petroleum diesel. | |||||||||||
| 1 | Me, Et, Et | C9H18O4 | 55 g, 51% | <33 | 27.3 | 145 | <−60 | 16.06 | 2.11 | 0.984 | 198 |
| 2 | Me, n-Bu, n-Bu | C15H30O4 | 101 g, 81% | 63.7 | 30.9 | 256 | <−60 | 4.57 | 4.83 | 0.920 | 281 |
| 3 | Me, i-Bu, i-Bu | C15H30O4 | 168 g, 79% | 38.6 | 28.5 | 319 | <−60 | 3.38 | 5.71 | 0.916 | 242 |
| 4 | Me, n-Pen, n-Pen | C18H36O4 | 105 g, 74% | 74.6 | 32.5 | 284 | <−60 | 3.3 | 7.52 | 0.909 | 310 |
| 5 | Me, i-Pen, i-Pen | C18H36O4 | 116 g, 81% | 54.0a | 32.5a | 375 | <−60 | 3.62 | 8.25 | 0.912 | 288 |
| 6 | Me, neo-Pen, neo-Pen | C18H36O4 | 62 g, 69% | 39.2 | 32.4 | 404 | −15 | 1.47 | 15.33 | 0.882 | 270 |
| 7 | Me, n-Hex, n-Hex | C21H42O4 | 135 g, 82% | 80.1 | 33.7 | 288 | <−60 | 2.52 | 10.17 | 0.914 | 346 |
| 8 | Me, i-Pen, Et | C15H30O4 | 83 g, 75 %b | 48 | 28.5 | 298 | <−60 | 2.41 | n.m. | 0.914 | 243 |
| 9 | Me, Et, i-Pen | C12H24O4 | 13 g, 73 %b | <33 | 31.0 | 246 | <−60 | 8.43 | 3.25 | 0.927 | 210 |
| 10 | H, s-Bu, s-Bu | C14H28O4 | 60 g, 85% | <33 | 30.1 | 247 | <−60 | 5.57 | 4.29 | 0.952 | 258 |
| 11 | H, i-Pen, i-Pen | C17H34O4 | 156 g, 95% | 63.3 | 34.4 | 354 | <−60 | 3.1 | 6.07 | 0.902 | 306 |
| 12 |
 R = n-Bu R = n-Bu |
C16H32O4 | 41 g, 23% | 61.6 | 31.4 | 323 | −24 | 2.85 | 5.72 | 0.946 | 288 |
| 13 |
 R = i-Pen R = i-Pen |
C19H38O4 | 15 g, 28% | n.m. | n.m. | 382 | <−60 | 2.21 | 10.01 | 0.904 | 302 |
| 14 |
|
C13H24O6 | 6.5 g, 13% | n.m. | n.m. | 123 | <−60 | 14.57 | n.m. | 1.050 | 276 |
| 15 |
 R = i-Pen R = i-Pen |
C12H26O2 | 74 g, 81% | >64 | 38.3 | 374 | <−60 | 2.16 | 1.82 | 0.832 | n.m. |
| 16 |
 R = i-Pen R = i-Pen |
C15H26O3 | 64 g, 81% | 36.6 | 32.9 | 396 | <−60 | 1.39 | 5.08 | 0.932 | 269 |
| FAME biodiesel4 | C19H36O2 | — | >47 | 37.5 | 461c | −5 to 15 | <0.1 | 1.9–6.0 (40 °C) | 0.88 (15 °C) | 360 | |
| B*POME1–6 (ref. 4, 5 and 34) | C9H20O3 | — | 70 | 34 | 259 | −58 | 7.3 | — | 1.066 | 179 | |
| Renewable diesel5 | C16H34 | — | >70 | 44 | 390d | −5 to −34 | <0.1 | 2–4 (40 °C) | 0.78 | <330 | |
| Petroleum diesel | C12H24 | — | 47 | 42 | 1462 | −9.7 | <0.1 | 0.86 | 335 | ||
| Target values4 | >45 | >30 | <880 e | <0 | <20 | 1.9–4.1 | n/a | <340 | |||
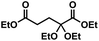
While α-carbonyl acids were shown to be successful substrates for DAOA production, poorer conversion and isolated yield was encountered with acetoacetate (DAOAs 12 and 13). This was thought to result from the decreased inductive influence at the β-carbonyl position and/or stabilization of the hemiacetal intermediate via 6-membered intramolecular hydrogen bonding. Similarly, application of the reaction conditions to ordinary ketones such as 3-pentanone resulted in only trace ketal formation by GC-MS. Glutaric acid (α-ketopentanedioic acid) was a productive substrate in forming a diether–diester (DAOA-14), but as for DAOA-1, this synthesis encountered slow reaction rate and difficult separation from the α-ketodiester intermediate. Finally, the reaction conditions were applied to bioderived acetaldehyde and furfural (compounds 15 and 16), leading to isolation of the corresponding diisoamyl acetals in 81% yield.
Combustion properties of DAOAs
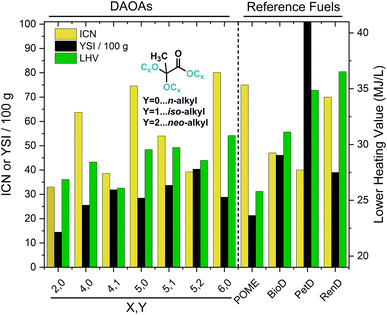
Sustainable diesel fuel candidates are commonly evaluated for combustion performance and for similarity to physical properties of petroleum diesel to minimize changes to existing infrastructure and engine design. Several important measures of diesel fuel performance include ICN, LHV and YSI. Results for DAOA compounds are presented in Table 2.CN is inversely related to ignition delay in compression ignition engines. Higher cetane numbers are associated with improved ignition quality, complete combustion, lower hydrocarbon emissions and increased fuel efficiency.28,29 Values ranging between 38.6 and 80.1 were recorded for DAOAs. Increasing carbon number with constant branching pattern (i-Bu vs. i-Pen and Et → n-Hex) or decreasing branching (neo-Pen vs. i-Pen vs. n-Pen) led to increased ICNs, in agreement with previous studies (Fig. 1).29,30 For DAOA-5, crude product obtained by removal of solvent, alcohol and catalyst from reaction mixtures yielded fuel with increased ICN without detriment to LHV, suggesting that rigorous purification is not required for DAOAs applied as fuels (see ESI†).
LHV is the heat of combustion, corrected for vaporization of water. This property correlates directly to energy density and fuel efficiency (e.g. miles per gallon). Most LHVs for DAOAs were above the 30 MJ kg−1 criterion, and the LHV correlates well with C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio in the series of pyruvate DAOAs.
O ratio in the series of pyruvate DAOAs.
YSI measures the tendency of a specific fuel to form soot under similar conditions.31,32 It is quantified by measuring soot concentrations in methane-air non-premixed laminar flames doped with a small amount of test fuel. In agreement with previous studies, YSI measurements of DAOAs indicate that more sooting is encountered for (1) longer, unbranched alkyl groups (Et3 < n-Bu3 < n-Pen3 < n-Hex3) (2) more branched carbon chain isomers (n-Pen3vs. i-Pen3vs. neo-Pen3) (3) increased C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio (Fig. 1).31 All neat DAOAs investigated have substantially lower YSI than petroleum diesel, which contains appreciable amounts of high-sooting aromatic hydrocarbons (representative YSI per kg = 1462).32 YSI is not currently available for heavy fuel oil used in marine applications, but PM2.5 (particulate matter < 2.5 μm) and polycyclic aromatic hydrocarbon (PAH) emissions exceed those of diesel, per unit marine fuel.33
O ratio (Fig. 1).31 All neat DAOAs investigated have substantially lower YSI than petroleum diesel, which contains appreciable amounts of high-sooting aromatic hydrocarbons (representative YSI per kg = 1462).32 YSI is not currently available for heavy fuel oil used in marine applications, but PM2.5 (particulate matter < 2.5 μm) and polycyclic aromatic hydrocarbon (PAH) emissions exceed those of diesel, per unit marine fuel.33
ICN, LHV and YSI of compounds 12–14 were generally consistent with the structural trends found for DAOAs. Substitution of the ester functionality of DAOA-5 with H in DAO15 led to increased ICN, LHV and similar YSI. The aromatic furan moiety of compound 16 was expected to decrease ICN and LHV, and increase YSI relative to saturated analogues.
Chemical reactivity of DAOAs
An important consideration for fuel candidates is the stability of their chemical composition, combustion performance, and hazard profile are over time. For DAOA-2, no obvious change in composition was observed by 1H NMR and GC-MS for after storage at 22 °C under air for over one year after isolation. In a more rigorous aging study, neat DAOAs were exposed to air in open containers at 43 °C for up to 8 weeks, with aliquots periodically analyzed for chemical composition. While peroxide formation was encountered at the 100–5000 ppm level, gross changes by 1H NMR and GC-MS were only observed for DAOAs 1 and 11 (see ESI†). Dissolution of 100–500 ppm BHT or blending with petroleum diesel at 20% vol DAOA, a typical blend ratio for sustainable diesel, suppressed DAOA-5 oxidative susceptibility under accelerated conditions per ASTM D7545 to meet the >60 minute target for oxidation induction period.35To determine acid stability, 50.0 g DAOA-5 was combined with rapidly stirring 0.01 M H2SO4 aqueous solution at 45 °C for 4 weeks. Significant chemical changes could not be discerned by 1H NMR or GC-MS. 48.9 g (97.8%) DAOA-5 was recovered, indicating stability to a worst-case storage scenario. Elimination of one equivalent of alcohol from the ketal functionality occurs under dynamic vacuum at 80 °C in the presence of catalytic acid, including during isolation if residual acid is not quenched (Scheme 3).
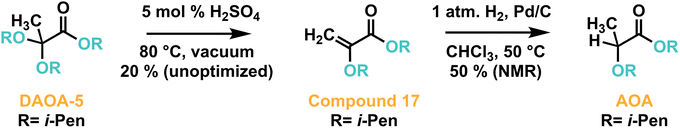 | ||
| Scheme 3 Elimination of one equivalent of alcohol from DAOA-5 to yield compound 17, and subsequent reduction to form AOA. | ||
Scale up in continuous flow
With the realization of recyclable, chemically stable reagents and catalyst in DAOA synthesis, a continuous flow reaction with in situ water removal was pursued for synthesis of DAOA-2.36 The assembled reactor consists of a three-neck round-bottomed flask equipped with a Dean–Stark apparatus, and with inlet and outlet connections to a peristaltic pump (Fig. 2). Perfluorinated elastomeric tubing was selected for maximum chemical compatibility and exhibited no loss of performance after multiple days of continuous operation.Using optimized conditions identified for batch synthesis, 138 g L−1 pyruvic acid in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v hexane/1-butanol solution was introduced at 0.17 mL per minute to the reaction vessel, which initially contained 1.50 L of 1
1 v/v hexane/1-butanol solution was introduced at 0.17 mL per minute to the reaction vessel, which initially contained 1.50 L of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v hexane/1-butanol solution and 54 g Amberlyst 15, resulting in a theoretical average residence time of 6.1 days. Reaction mixture was removed at 0.17 mL per minute at maintain reaction volume. This led to 2.78 g h−1 of theoretical productivity of DAOA-2 at steady state. In practice, 1H NMR in-line and of isolated aliquots revealed 80+% steady-state conversion of intermediate pyruvate ester to DAOA-2, and 376 g (70.4% yield, 1.96 g h−1) was isolated by vacuum distillation as the result of an 8 day run. 1.61 kg (81.9%) of hexanes and 1-butanol at approximately 1
1 v/v hexane/1-butanol solution and 54 g Amberlyst 15, resulting in a theoretical average residence time of 6.1 days. Reaction mixture was removed at 0.17 mL per minute at maintain reaction volume. This led to 2.78 g h−1 of theoretical productivity of DAOA-2 at steady state. In practice, 1H NMR in-line and of isolated aliquots revealed 80+% steady-state conversion of intermediate pyruvate ester to DAOA-2, and 376 g (70.4% yield, 1.96 g h−1) was isolated by vacuum distillation as the result of an 8 day run. 1.61 kg (81.9%) of hexanes and 1-butanol at approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v was recovered during isolation.
1 v/v was recovered during isolation.
This experiment demonstrates the ease of scaling DAOA synthesis in continuous flow, while minimizing hazard scope and process intensity. Adding additional continuous stirred vessels in series with the same total reaction volume should increase throughput while maintaining percent conversion. Further applicability of the method toward other continuous lab-scale reactive distillation and water removal processes is under investigation.
Physical properties of DAOAs
Boiling point, cloud point, density, viscosity and water solubility were measured for DAOAs in alignment with tiered fuel screening criteria (see ESI†).4,5 Calorimetric normal boiling point values for most DAOAs synthesized were between 200–300 °C and were correlated with molecular weight and degree of alkyl branching. Mass densities were in the range 0.882–0.984 g cm−3 and were inversely correlated with steric bulk. Viscosities measured at 23 °C were in the range 2.11–15.33 cSt and were also predictable based on alkyl fragment length and degree of branching. Finally, water solubilities of water saturated, neat DAOAs were measured using Karl Fischer titration. All neat compounds studied phase separated from water at room temperature and met the <20 g H2O per L fuel screening criterion.Cloud point measurements were performed in an environmental chamber using a custom-built optical transmission measurement system that houses a 1 cm cuvette of sample. While cloud point temperatures of neat DAOAs were generally below −60 °C, cloud points of 20 and 50% blends with several commercially available diesel fuels were minimally changed from the neat blendstocks.
Qualitative lifecycle assessment (LCA) of DAOAs
DAOA lifecycle assessment was performed by analogy to the rigorous analysis performed for AOAs, which utilize similar biological feedstocks and chemical processes.10 For AOAs, lignocellulosic biomass is fermented to produce lactic acid and fusel alcohols, which are in turn combined in sequential esterification and etherification units to generate AOAs. A key result of the analysis is that a reduction from 92 to 31 g CO2e per MJ (65%) is anticipated for the AOA lifecycle relative to conventional petroleum diesel, which is one of the largest reductions among recent sustainable diesel concepts and exceeds biofuel emission targets of 60% lifecycle CO2 reduction.4,5 Interestingly, the primary source of GHG production during the AOA lifecycle comes indirectly from NaOH usage in the deacetylation and mechanical refining (DMR) of biomass to aid in enzymatic hydrolysis, which presents opportunities for future optimization. Applied in the maritime industry, reduced sooting potential, and reduced nitrogen oxide, metal, PAH and sulfur content of AOA/DAOA combustion emissions relative to bunker fuel present additional environmental incentives.33,37Since the proposed DAOA production process uses similar chemical, and thermodynamic and process inputs as for AOAs, and since LHV is similar, DAOA synthesis is also anticipated to result in a similar 60–70% overall GHG reduction relative to petroleum diesel. Further minor reductions from AOAs in g CO2e could result from the process intensification of the combined ester- and etherification steps for DAOAs. Two sources of uncertainty in this analogy are (1) the overall efficiency at scale and process integration of the glycolysis unit to generate pyruvic acid, which is less mature than the corresponding lactic acid process and (2) the efficiency of hexane recovery at scale, which represents a positive g CO2e contribution through addition of fresh hexane.
Conclusions
We detail the synthesis, reactivity, and physical property characterization of a family of bioderived DAOA sustainable diesel and marine fuel candidates. The synthetic protocol used exemplifies many principles of green chemistry, chiefly that mild reactants and reaction conditions are used, while high mass yield and atom economy are achieved simultaneously. Continuous flow synthesis with in situ water removal was demonstrated, generating 376 g of DAOA-2 over 8 days. DAOA-5 exhibited adequate oxidative stability when blended with hydrocarbon diesel or 500 ppm BHT, and was not adversely affected by extended exposure to aqueous pH 2 at 45 °C.Fuel performance was assessed by ICN, LHV and YSI. Most DAOAs met or exceeded the threshold values for renewable diesel fuels in each of these categories and are competitive with existing candidates. Use of pyruvate as a tripodal molecular scaffold limited individual carbon chain lengths, benefitting YSI and gravimetric density, while overall hydrocarbon content was sufficient to provide competitive cetane number and specific energy values. Other physical properties such as boiling point, cloud point and water solubility were predictable functions of chemical structure and inside threshold values. Overall, DAOAs 2 and 5 were the most promising in terms of meeting or exceeding the most fuel screening criteria, while leveraging widely available, bioderived starting materials. Given the hesitance of the transportation sector to rapidly deviate from hydrocarbons as primary fuel components, the expected initial implementations of DAOAs are as blends with hydrocarbon diesel and/or biodiesel. However, the reduced cloud point of DAOAs relative to biodiesel affords opportunities to exceed 20 percent v/v blend ratios.
Ongoing work includes (1) establishing rigorous technoeconomic and lifecycle assessments for DAOAs 2 and 5, (2) further developing the lab-scale concept for azeotropic water removal in continuous flow reactions (3) evaluating related molecules in other applications such as bioderived plastics,38 lubricants, green solvents and multi-arm ionic surfactants.
Experimental
General procedure for batch synthesis of DAOAs
To a round-bottomed flask equipped with a magnetic stir bar was added carbonyl compound (1 equiv.), 0.10 eq. Amberlyst 15, alcohol (5 equiv.), and hexanes (1 mL per mL alcohol). The flask was adapted with a Dean–Stark apparatus and a reflux condenser and heated to reflux under air at atmospheric pressure using a hotplate and Al heating block. Conversion was monitored by NMR and GC-MS. Upon completion, the reaction vessel was cooled to rt, and quenched with NaHCO3 (sat., aq.). Drying agents were not used. The organic layer was directly concentrated in vacuo at 75 °C, ∼20 torr to reclaim unreacted alcohol and hexanes. The crude product was vacuum distilled at using an 8′′ Vigreux column yielding clear, colorless oils.Synthesis of DAOA-2 in continuous flow
A 2 liter, three-neck round-bottomed flask was equipped with a magnetic stir bar, Dean–Stark apparatus, reflux condenser and heating mantle. 1.5 L of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v 1-butanol
1 v/v 1-butanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane and 54 g wet Amberlyst 15 were added to the flask. The flask was then sealed with rubber septa modified with inlet and outlet tubing ports. Fluid routing was achieved with an Ismatec ISM4206 cassette-based peristaltic pump equipped with Chem-Durance® Bio elastomeric tubing at pump heads and fluorinated ethylene propylene (FEP) tubing at the reactor. Stirring and refluxing was established in the flask for 12 h whereupon ∼50% water was released from Amberlyst. Then a 138 g L−1 solution of pyruvic acid in 1
hexane and 54 g wet Amberlyst 15 were added to the flask. The flask was then sealed with rubber septa modified with inlet and outlet tubing ports. Fluid routing was achieved with an Ismatec ISM4206 cassette-based peristaltic pump equipped with Chem-Durance® Bio elastomeric tubing at pump heads and fluorinated ethylene propylene (FEP) tubing at the reactor. Stirring and refluxing was established in the flask for 12 h whereupon ∼50% water was released from Amberlyst. Then a 138 g L−1 solution of pyruvic acid in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v 1-butanol
1 v/v 1-butanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane solution was introduced at 0.17 mL per minute. The outlet flow rate was matched to maintain steady-state reaction volume. In-line 1H NMR analysis was performed with a Magritek SpinSolve 80 MHz instrument, equipped with a 5 mm path length glass flow cell. Upon exhausting the pyruvic acid solution, heating was terminated. The combined contents of the reaction flask and outlet reservoir were quenched, worked up and purified offline, as for batch synthesis.
hexane solution was introduced at 0.17 mL per minute. The outlet flow rate was matched to maintain steady-state reaction volume. In-line 1H NMR analysis was performed with a Magritek SpinSolve 80 MHz instrument, equipped with a 5 mm path length glass flow cell. Upon exhausting the pyruvic acid solution, heating was terminated. The combined contents of the reaction flask and outlet reservoir were quenched, worked up and purified offline, as for batch synthesis.
 | (1) |
The subscripts TC, TOL, and HEP refer to the test compound (DAOA), toluene, and n-heptane respectively. This rescaling method removes sources of systematic uncertainty such as errors in the gas-phase reactant flow rates. Furthermore, it allows the new results to be quantitatively compared with a database that contains measured YSIs for hundreds of organic compounds. The parameters YSITOL and YSIHEP are constants that define the YSI scale; their values—170.9 and 36.0—were taken from the database41 so that the newly measured YSIs would be on the same scale. Measured YSI are per mole of compound, and the values presented here are per kg or per liter.
| LHV = HHV − (0.2122 × % H) | (2) |
Author contributions
NRM, EM and RWD conceived the study. NRM performed synthesis and spectroscopy. MS measured physical properties. TA, CH, JL performed ICN, LHV measurements, and performed the oxidative stability study. JZ and CM performed YSI testing. LP and AG edited the manuscript.Conflicts of interest
NRM, EM, RWD disclose authorship of United States patent number 11,492,565, “Alkyl dialkoxyalkanoates as bioderived, high cetane diesel fuels”.Acknowledgements
The authors thank Nicholas Sizemore for helpful discussions. This research was conducted as part of the Co-optimization of Fuels & Engines (Co-Optima) project sponsored by the U.S. Department of Energy (DOE) Office of Energy Efficiency and Renewable Energy (EERE), Bioenergy Technologies and Vehicle Technologies Offices. Sandia National Laboratories is a multimission laboratory managed and operated by National Technology and Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International, Inc., for the U.S. Department of Energy's National Nuclear Security Administration under contract DE-NA0003525. “This article has been authored by an employee of National Technology & Engineering Solutions of Sandia, LLC under Contract No. DE-NA0003525 with the U.S. Department of Energy (DOE). The employee owns all right, title and interest in and to the article and is solely responsible for its contents. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this article or allow others to do so, for United States Government purposes. The DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan https://www.energy.gov/downloads/doe-public-access-plan.”References
- P. R. Shukla, J. Skea, R. Slade, A. A. Khourdajie, R. v. Diemen, D. McCollum, M. Pathak, S. Some, P. Vyas, R. Fradera, M. Belkacemi, A. Hasija, G. Lisboa, S. Luz and J. Malley, Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, The Intergovernmental Panel on Climate Change, Cambridge, UK and New York, NY, USA, 2022 Search PubMed.
- Monthly Energy Review, April 2021, U.S. Energy Information Administration, Washington, DC, 2021 Search PubMed.
- S. S. Doliente, A. Narayan, J. F. D. Tapia, N. J. Samsatli, Y. R. Zhao and S. Samsatli, Front. Energy Res., 2020, 8, 110 CrossRef.
- D. J. Gaspar, C. J. Mueller, R. L. McCormick, J. Martin, S. Som, G. M. Magnotti, J. Burton, D. Vardon, V. Dagle, T. L. Alleman, N. Huq, D. A. Ruddy, M. Arellano-Trevino, A. Landera, A. George, G. Fioroni, E. R. Sundstrom, E. Oksen, M. R. Thorson, R. T. Hallen, A. J. Schmidt, E. Polikarpov, E. Monroe, J. Carlson, R. W. Davis, A. Sutton, C. M. Moore, L. Cosimbescu, K. K. Ramasamy, M. D. Kass, T. R. Hawkins, A. Singh, A. Bartling, P. T. Benavides, S. D. Phillips, H. Cai, Y. Jiang, L. Ou, M. Talmadge, N. Carlson, G. Zaimes, M. Wiatrowski, Y. Zhu and L. J. Snowden-Swan, Top 13 Blendstocks Derived from Biomass for Mixing-Controlled Compression-Ignition (Diesel) Engines: Bioblendstocks with Potential for Decreased Emissions and Improved Operability, United States, 2021 Search PubMed.
- A. W. Bartling, P. T. Benavides, S. D. Phillips, T. Hawkins, A. Singh, M. Wiatrowski, E. C. D. Tan, C. Kinchin, L. Ou, H. Cai, M. Biddy, L. Tao, A. Young, K. Brown, S. Li, Y. Zhu, L. J. Snowden-Swan, C. R. Mevawala and D. J. Gaspar, ACS Sustain. Chem. Eng., 2022, 10, 6699–6712 CrossRef CAS.
- N. A. Huq, X. C. Huo, G. R. Hafenstine, S. M. Tifft, J. Stunkel, E. D. Christensen, G. M. Fioroni, L. Fouts, R. L. McCormick, P. A. Cherry, C. S. McEnally, L. D. Pfefferle, M. R. Wiatrowski, P. T. Benavides, M. J. Biddy, R. M. Connatser, M. D. Kass, T. L. Alleman, P. C. John, S. Kim and D. R. Vardon, Proc. Natl. Acad. Sci. U.S.A., 2019, 116, 26421–26430 CrossRef CAS PubMed.
- B. W. Li, Y. F. Li, H. Y. Liu, F. Liu, Z. Wang and J. X. Wang, Appl. Energy, 2017, 206, 425–431 CrossRef CAS.
- H. Y. Liu, Z. Wang, J. Zhang, J. X. Wang and S. J. Shuai, Appl. Energy, 2017, 185, 1393–1402 CrossRef CAS.
- S. Bruniaux, D. Luart and C. Len, Sci. Synth., 2018, 50, 1849–1856 CrossRef CAS.
- E. Monroe, J. S. Carlson, R. Dhaoui, M. Sarwar, P. T. Benavides, J. Zhu, C. S. McEnally, L. Pfefferle, A. George, N. Sizemore and R. W. Davis, Energy Fuels, 2023, 37(3), 2091–2099 CrossRef CAS.
- D. Jadhav, A. M. Grippo, S. Shylesh, A. A. Gokhale, J. Redshaw and A. T. Bell, ChemSusChem, 2017, 10, 2527–2533 CrossRef CAS PubMed.
- Clean Skies for Tomorrow: Sustainable Aviation Fuels as a Pathway to Net-Zero Aviation, World Economic Forum Geneva, Switzerland, 2020 Search PubMed.
- M. I. Arbab, H. H. Masjuki, M. Varman, M. A. Kalam, S. Imtenan and H. Sajjad, Renewable Sustainable Energy Rev., 2013, 22, 133–147 CrossRef CAS.
- J. S. Carlson, E. A. Monroe, R. Dhaoui, J. Zhu, C. S. McEnally, S. Shinde, L. D. Pfefferle, A. George and R. W. Davis, Energy Fuels, 2020, 34, 12646–12653 CrossRef CAS.
- Y. Li, J. Chen and S. Y. Lun, Appl. Microbiol. Biotechnol., 2001, 57, 451–459 CrossRef CAS PubMed.
- H. Rajpurohit and M. A. Eiteman, Appl. Biochem. Biotechnol., 2020, 192, 243–256 CrossRef CAS PubMed.
- J. Becker, A. Lange, J. Fabarius and C. Wittmann, Curr. Opin. Biotechnol., 2015, 36, 168–175 CrossRef CAS PubMed.
- F. Liu, P. Lane, J. C. Hewson, V. Stavila, M. B. Tran-Gyamfi, M. Hamel, T. W. Lane and R. W. Davis, Bioresour. Technol., 2019, 283, 350–357 CrossRef CAS PubMed.
- T. J. Tse, D. J. Wiens, F. Chicilo, S. K. Purdy and M. J. T. Reaney, Fermentation, 2021, 7, 267 CrossRef CAS.
- T. C. Ezeji, N. Qureshi and H. P. Blaschek, Curr. Opin. Biotechnol., 2007, 18, 220–227 CrossRef CAS PubMed.
- B. Kolesinska, J. Fraczyk, M. Binczarski, M. Modelska, J. Berlowska, P. Dziugan, H. Antolak, Z. J. Kaminski, I. A. Witonska and D. Kregiel, Materials, 2019, 12(3), 350 CrossRef CAS PubMed.
- N. E. Hoffman and T. V. Kandathil, J. Org. Chem., 1967, 32, 1615–1617 CrossRef CAS.
- C. S. M. Pereira, V. Silva and A. E. Rodrigues, Ind. Eng. Chem. Res., 2012, 51, 8928–8938 CrossRef CAS.
- N. S. Graca, L. S. Pais, V. Silva and A. E. Rodrigues, Ind. Eng. Chem. Res., 2010, 49, 6763–6771 CrossRef CAS.
- N. Boz, N. Degirmenbasi and D. M. Kalyon, Appl. Catal., B, 2015, 165, 723–730 CrossRef CAS.
- Y. C. Yan, U. T. Bornscheuer and R. D. Schmid, Biotechnol. Bioeng., 2002, 78, 31–34 CrossRef CAS PubMed.
- G. Daw, A. C. Regan, C. I. F. Watt and E. Wood, J. Phys. Org. Chem., 2013, 26, 1048–1057 CrossRef CAS.
- Y. Kidoguchi, C. Yang, R. Kato and K. Miwa, JSAE Rev., 2000, 21, 469–475 CrossRef CAS.
- O. Chukwuezie, N. Nwakuba, S. Asoegwu and K. Nwaigwe, Am. J. Eng. Res., 2017, 06, 56–67 Search PubMed.
- W. J. Pitz and C. J. Mueller, Prog. Energy Combust. Sci., 2011, 37, 330–350 CrossRef CAS.
- C. S. McEnally and L. D. Pfefferle, Environ. Sci. Technol., 2011, 45, 2498–2503 CrossRef CAS PubMed.
- D. L. Bartholet, M. A. Arellano-Trevino, F. L. Chan, S. Lucas, J. Q. Zhu, P. C. St John, T. L. Alleman, C. S. McEnally, L. D. Pfefferle, D. A. Ruddy, B. Windom, T. D. Foust and K. F. Reardon, Fuel, 2021, 295, 120509 CrossRef CAS.
- O. Sippula, B. Stengel, M. Sklorz, T. Streibel, R. Rabe, J. Orasche, J. Lintelmann, B. Michalke, G. Abbaszade, C. Radischat, T. Gröger, J. Schnelle-Kreis, H. Harndorf and R. Zimmermann, Environ. Sci. Technol., 2014, 48, 11721–11729 CrossRef CAS PubMed.
- M. A. Arellano-Treviño, D. Bartholet, A. T. To, A. W. Bartling, F. G. Baddour, T. L. Alleman, E. D. Christensen, G. M. Fioroni, C. Hays, J. Luecke, J. Zhu, C. S. McEnally, L. D. Pfefferle, K. F. Reardon, T. D. Foust and D. A. Ruddy, ACS Sustain. Chem. Eng., 2021, 9, 6266–6273 CrossRef.
- Standard Test Method for Oxidation Stability of Middle Distillate Fuels—Rapid Small Scale Oxidation Test (RSSOT), 2019, DOI:10.1520/D7545-14R19E01.
- C. Grosjean, J. Parker, C. Thirsk and A. R. Wright, Org. Process Res. Dev., 2012, 16, 781–787 CrossRef CAS.
- J. C. Corbin, A. A. Mensah, S. M. Pieber, J. Orasche, B. Michalke, M. Zanatta, H. Czech, D. Massabò, F. Buatier de Mongeot, C. Mennucci, I. El Haddad, N. K. Kumar, B. Stengel, Y. Huang, R. Zimmermann, A. S. H. Prévôt and M. Gysel, Environ. Sci. Technol., 2018, 52, 6714–6722 CrossRef CAS PubMed.
- M. Niwa, H. Fujii and H. Tanaka, Des. Monomers Polym., 2014, 17, 647–653 CrossRef CAS.
- C. S. McEnally and L. D. Pfefferle, Combust. Flame, 2007, 148, 210–222 CrossRef CAS.
- C. S. McEnally, Y. Xuan, P. C. S. John, D. D. Das, A. Jain, S. Kim, T. A. Kwan, L. K. Tan, J. Zhu and L. D. Pfefferle, Proc. Combust. Inst., 2019, 37, 961–968 CrossRef CAS.
- C. S. McEnally, D. D. Das and L. D. Pfefferle, Yield Sooting Index Database Volume 2: Sooting Tendencies of a Wide Range of Fuel Compounds on a Unified Scale, 2017, DOI:10.7910/DVN/7HGFT8.
- ASTM, Standard Test Method for Determination of Indicated Cetane Number (ICN) of Diesel Fuel Oils Using a Constant Volume Combustion Chamber—Reference Fuels Calibration Method, 2022, DOI:10.1520/D8183-22.
- Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter, 2019, DOI:10.1520/D0240-19.
- W. G. Lloyd and D. A. Davenport, J. Chem. Educ., 1980, 57, 56 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, spectral characterization, physical property measurement. See DOI: https://doi.org/10.1039/d3se00804e |
| ‡ E. M. is now with Indigo Ag. T. A. is now with Holly Energy. J. Z. is now with ExxonMobil. |
| This journal is © The Royal Society of Chemistry 2024 |