 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in electrochemiluminescence immunosensing
Jing
Yu
a,
Dalibor
Stankovic
 b,
Jasmina
Vidic
c and
Neso
Sojic
b,
Jasmina
Vidic
c and
Neso
Sojic
 *ad
*ad
aCollege of Chemistry and Chemical Engineering, Yantai University, Yantai 264005, China
bFaculty of Chemistry, University of Belgrade, Studentski trg 12-16, 11000 Belgrade, Serbia
cINRAE, AgroParisTech, Micalis Institute, UMR 1319, Université Paris-Saclay, 78350 Jouy-en-Josas, France
dUniversity of Bordeaux, CNRS, Bordeaux INP, Institut des Sciences Moléculaires, UMR 5255, 33607 Pessac, France. E-mail: sojic@u-bordeaux.fr
First published on 23rd October 2024
Abstract
Electrogenerated chemiluminescence, also called electrochemiluminescence (ECL), has attracted much attention in various fields of analysis due to its high sensitivity, extremely wide and dynamic range and excellent control of space and time of the light emission. The great success of ECL for in vitro detection results from the advantages of combining the selectivity of biological recognition elements and the sensitivity and controllability of ECL technology. ECL is widely applied as a powerful analytical technique for ultrasensitive detection of biomolecules. In this review, we summarize the recent developments and applications of ECL for immunosensing. Herein, we present the sensing schemes and their applications in different areas, such as detection of biomarkers, bead-based detection and bacteria and cell analysis and provide future perspectives on new developments in ECL immunosensing. In particular, ECL-based sensing assays for clinical sample analysis and medical diagnostics and the development of immunosensors for these purposes are highlighted.
1. Introduction
Electrochemiluminescence (ECL) is a chemiluminescence (CL) phenomenon triggered by an initial electrochemical process.1–4 Compared to conventional CL, it exhibits several advantages resulting from the use of electrochemical methods, including simplicity, stability and ease of use. In addition, ECL has superior temporal and spatial control on light emission. Also, the absence of excitation light in ECL promises a near-zero background, while fluorescence or phosphorescence suffers from an unselective photoexcitation-inducing background. Since the first ECL studies were published in the mid-1960s,5,6 extensive research efforts have been pouring into the ECL field, and now ECL is a well-established powerful analytical technique and widely used in many applications, such as ECL imaging,7,8 bioanalysis,9–11 DNA probe assays,12 food safety analysis13 and environmental monitoring.14A paramount breakthrough in the development of ECL is the ECL immunoassay, which combines the specific immunoreaction with the intrinsic properties of ECL, enabling the sensitive and specific detection of targets with a fast analysis procedure and simple device. It has been applied commonly in the analysis of trace immunogenic substances, showing the characteristics of good specificity, high sensitivity and selectivity, short time consumption and high trace determination. The working principle of the ECL immunosensor is similar to that of the traditional immunoassay; the antigen or antibody is generally functionalized by ECL luminophores and immobilized on the electrode surface, and the specific recognition of antigens or antibodies can then cause changes in ECL response signal, thereby achieving the detection with quantitative analysis of the target protein and high efficiency of the sample. ECL-based immunoassays are commercialized for clinical diagnostics mainly by two companies (Roche Diagnostics and Meso Scale Discovery), with ≈2 billion assays running world-wide per year.15,16 This review highlights the recent developments in ECL immunosensing, such as new detection schemes, detection of protein biomarkers, bacteria analysis, cell analysis and other bioanalytical applications. Lastly, future prospects for the development of ECL immunosensor has been discussed.
2. Fundamentals of ECL
2.1. ECL mechanisms
Generally, ECL can be generated by either the annihilation pathway or the co-reactant pathway. In the annihilation pathway, radical anion and cation of the luminophore are firstly electrogenerated on the cathode and anode, respectively, or by pulsing the same electrode at sufficient cathodic and anodic potentials. Then, anion and cation react exergonically with each other to generate the excited state of the luminophore. It relaxes to the ground state, followed by light emission. However, this pathway is usually performed in organic solvents because it requires usually quite negative and positive potentials. Meanwhile, in the co-reactant pathway, the luminophore and co-reactant can be electrochemically oxidized or reduced on the electrode surface by pulsing a positive or negative unidirectional step potential. The electrogenerated intermediates undergo homogeneous electron-transfer reactions to form the excited state luminophore, which can relax to the ground state with light emission. The co-reactant pathway is most largely used in analytical and bioanalytical applications because ECL is generated in aqueous media at physiological pH values.3,10,17,182.2. The ECL emitters
The archetypal ECL luminophores are tri(2,2′-bipyridine)ruthenium(II) ([Ru(bpy)3]2+) and luminol.4,8,12,19 Afterward, the pursuit of ECL luminophores with higher ECL efficiency and lower excitation potential has pushed the exploration of new organometallic complexes, mainly based on iridium, and of nanomaterials. The model ECL system composed of the [Ru(bpy)3]2+ luminophore and tri-n-propylamine (TPrA) co-reactant has achieved overwhelming success for immunosensing, because of high sensitivity, specific chemical reactivity, extremely wide dynamic range, rapidness, simplicity and excellent controllability.20 Secondly, other metal complexes, such as transition metal complexes incorporating Ru, Os and rare earth chelates, including Ir, Au and Pt, have been reported as ECL labels enabling color tuning for light-emitting systems.1 Luminol and its analogues (e.g. 8-amino-5-chloro-2,3-dihydro-7-phenyl-pyrido[3,4-d]pyridazine-1,4-dione, L-012) operating with H2O2 constitutes a popular system that is widely used to generate ECL, due to its low cost, low oxidation potentials, easy functionalization and broad bioanalytical applications.21 Finally, nanomaterials, including nanoparticles, porous luminophore-doped nanoparticles, quantum dots (QDs), carbon-based materials, metal composites and even aromatic hydrocarbon nanoparticles have been used in ECL sensing and imaging.22,23 Luminophore-doped silica nanoparticles have also been developed with remarkable performances for efficient ECL emission.24–27 After the initial silicon QDs (Si semiconductor) reported in 2002,28 many QD-based ECL emitters, II–VI, III–V and IV–VI nanocrystals, carbon QDs with different sizes, shapes and compositions have generated great interest for their potential ECL applications on account of their size-controlled photoluminescence and excellent stability against photobleaching. Up to now, a series of III–V and II–VI QDs, such as CdSe, CdTe, PbS, CdS and carbon-based materials, such as carbon dots (CDs), graphene quantum dots (GQDs) and graphene-like C3N4 nanosheets (g-C3N4 NSs) have been exploited as promising ECL nanoemitters.29,30 Significantly, nanoclusters (NCs) or nanoparticles (NPs), particularly, noble metal NCs/NPs, are often introduced into ECL system as labels to improve ECL efficiency, due to their ultra-small size and nontoxicity. For example, Au18, Au21, Au25, and Au38 NCs have appeared, enriching the type of Au NCs and providing new insights into ECL applications.31,32 In addition to the various sizes of Au NCs, their various valence states are found to be important for ECL biosensing applications, especially in the near-infrared region.33–36 In this review, we will summarize the studies related to ECL immunosensing with the emitters mentioned above.3. Analytical applications and strategies
3.1. Bead-based ECL immunoassays
Bead-based ECL assays have been the object of numerous works and are commercialized for the quantification of a large number of biomarkers involved in various pathology studies, such as cardiac and infectious diseases, thyroid and tumor markers, bacteria, and viruses.37–39 Generally, in such assays, magnetic beads with micrometric sizes are functionalized with a specific capture antibody. In the sandwich format, the biorecognition chain is continued with the analytical target (e.g. the antigen), if present in the sample, and a detection antibody is labeled with the ECL luminophore. Classically, [Ru(bpy)3]2+ is used as the label and the co-reactant is the freely-diffusing TPrA. ECL of this efficient ECL system is combined with magnetic beads in a flow cell because it offers several advantages in automated immunoassays for the quantification of biomarkers in body fluids: (i) the 3D structure of the beads increases the surface-to-volume ratio, (ii) easy preprocessing and processing of the samples during separation, enrichment, and washing steps, (iii) the application of an external magnetic field captures the magnetic beads carrying the labeled immuno-complexes on the working electrode, (iv) the precise control over time and position generates the analytical ECL signal in front of the photodetector.16 The capture of the bead on the electrode surface is an essential step because ECL is a surface-confined technique. In other words, the beads carrying the immuno-complexes with the ECL labels have to be very close to the electrode surface where the co-reactant radicals are produced electrochemically due to their limited lifetimes.20,40 The reactivity of the TPrA radicals governs mainly the sensitivity of the assay. The spatial distribution of ECL emission at the surface of the bead can be mapped by employing ECL microscopy (Fig. 1). Several groups investigated the mechanisms operating in such assays by imaging the spatial distribution on beads.40–46 Initially, they reported the ECL detection of streptavidin-modified [Ru(bpy)3]2+-label with TPrA or DBAE in a top-view or side-view configuration, this 3D imaging approach provides insights into the ECL mechanistic route operating in bioassays and on the optical effects.40 Later, the dynamic imaging of the ECL reactivity in space at the single-bead level was further studied to investigate the kinetics of the [Ru(bpy)3]2+/TPrA system.42 Then, the optical effects due to the light propagation through the bead decorated with the ruthenium labels were reported,44 determining mainly the spatial distribution of the recorded 3D ECL signals. The combination of ECL microscopy and optical modelling based on the discrete dipole approximation (DDA) provided global description of the ECL chemical reactivity and the possibility of investigating the chemical mechanism by deconvoluting the ECL patterns from the optics. The factors influencing the kinetics of ECL were found, which could support the optimization of ECL immunoassays. | ||
| Fig. 1 a) Schematic of bead-based ECL imaging in top (A) and side-view (B) configurations.40 b) Simulations of the optical effects by DDA.44 c) Dynamic imaging at the single bead level.42 Reprinted with permission from ref. 40 (copyright 2014 Royal Society of Chemistry), ref. 42 (copyright 2023 American Chemical Society), ref. 44 (copyright 2023 American Chemical Society). | ||
Many strategies were implemented to enhance ECL signals for bead-based immunoassays.41 For example, Paolucci and coworkers reported the insertion of carbon nanotubes to extend and enhance the ECL emission.47 It leads to a remarkable enhancement of the ECL intensity by increasing the efficiency of the “remote” ECL mechanism and the concurrence of an additional ECL-generating mechanism. To extend the ECL-emitting layer, Dai and coworkers demonstrated the use of conductive gold beads.48 In comparison with non-conductive magnetic beads, they obtained a 21.7-fold increase in the turn-over frequency of ECL generation with such gold beads. In addition, in this case, the ECL generation is no longer restricted by the limited lifetime of the electrogenerated TPrA radicals. Finally, the authors reported the development of size-encoded multiplex assays for the simultaneous detection of four kinds of acute myocardial infarction biomarkers, namely, CRP, cardiac troponin I (cTnI), fatty acid-binding protein, and myoglobin. Qi and coworkers also played with the electrode design by developing a gold nanoelectrode ensembles49,50 taken as a disposable ECL platform with immunomagnetic beads.51 Since the electrode material is an essential parameter in ECL,52–56 various materials were tested. For example, Einaga and coworkers exploited boron-doped diamond as an electrode material for ECL immunoassay based on the [Ru(bpy)3]2+/TPrA system and compared it with the approach used in commercial instrumentation (i.e. Pt).57 They obtained an increase of 70% in the resulting ECL and a double signal-to-noise ratio compared to Pt electrodes. The same group reported an original strategy based on the in situ electrochemical generation of H2O2 at a boron-doped diamond electrode. Hydrogen peroxide reacted with the beads labeled with luminol that were deposited on the electrode surface.58 However, the main drawback is that luminol is a sacrificial luminophore that can generate a photon just once per molecule whereas [Ru(bpy)3]2+ is regenerated during the ECL reaction and generates several photons in the presence of a sacrificial co-reactant.
The composition of the solution was also investigated to improve the performances of bead-based assays. Various surfactants have been added to increase (i) the efficiency of the TPrA oxidation since it is a key step to promote ECL emission59,60 and (ii) the solubility of TPrA. Valenti and coworkers exploited the buffer capacity of the solution to modify the rate of the reactions involved in the ECL generation. They demonstrated a “chemical lens effect” that enabled modification of the thickness of the ECL-emitting layer at the bead level.61 Moreover, the same group was able to increase ECL emission by a maximum of 128% through (i) optimization of luminophore distribution by decreasing the bead size and (ii) addition of a branched amine to increase the efficiency of the co-reactant mechanism.20 They reported a highly efficient mechanistic path for ECL generation, very close to the electrode surface. This highlights the importance of investigating the ECL mechanism and the optical effects occurring in the bead-based assays.42,44,62 Francis and coworkers proposed a very original possibility by adding a redox mediator that can act as both an electrocatalyst for TPrA oxidation and a more stable alternative to TPrA˙+ for the chemi-excitation of the luminophore.7,63,64 They tested several water-soluble Ir(III) complexes43 as redox mediators and found that [Ir(sppy)3]3− (where sppy = 5′-sulfo-2-phenylpyridinato-C2,N) elicited a significant improvement (70.9-fold at 0.9 V and 2.9-fold at 1.2 V) of the ECL signal from [Ru(bpy)3]2+ labels immobilized on the surface of the beads. It is due to the combination of (i) high solubility in aqueous solution, (ii) a chemically and electrochemically reversible oxidation (Eox) at a potential close to that of the irreversible oxidation of TPrA, (iii) an Ered beyond the reduction strength of TPrA˙, and (iv) a greater excited state energy than [Ru(bpy)3]2+. However, the Ir(III) complexes also generated ECL light. Therefore, the same group prepared a non-emissive sulfonated tris(1-phenylpyrazolato)-iridium(III) ([Ir(sppz)3]3−) that is an effective enhancer of the ECL reaction of [Ru(bpy)3]2+ and TPrA in aqueous solution, without an inherent interfering emission.65 They showed the potential application of this approach for the development of highly efficient redox-mediated ECL for ultrasensitive biomarker detection and for practical sample analysis.
New Ir(III) complexes acting as ECL luminophores (and not redox mediators as described in the previous paragraph) were designed to improve ECL efficiency for immunosensing and also the multiplexing.53–56 The [Ru(bpy)3]2+-grafted microgels with 3D network (diameter ∼100 nm) were used to decorate micrometric beads, enhancing ECL signals.66 The fraction of [Ru(bpy)3]2+ centers located on the very bottom of the microgels can let them oxidize directly at the GCE surface and react with TPrA efficiently. Thus, stable and efficient ECL emission was obtained. Moreover, a new kind of co-reactant BIS-TRIS can be also introduced into a bead-based ECL system to well balance ECL distance reactivity trade-off and enhance the sensitivity by 236% compared with TPrA for the detection of carcinoembryonic antigen.45
Feng and coworkers followed a different strategy by imaging single molecules at single-photon sensitivity using ECL.67 They applied their detection scheme to single-molecule ECL bioassay in order to detect carcinoembryonic antigen, showing a limit of detection of 67 attomoles, a concentration 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times better than that from conventional ECL bioassays.68
000 times better than that from conventional ECL bioassays.68
3.2. ECL immunosensors for protein biomarker detection
As a subcategory of biomarkers, protein biomarkers have become hot target analytes in ECL immunoassay.69 Protein biomarkers are a kind of substances that are secreted in the process of disease occurrence and even deterioration, or abnormal changes of the body in response to diseases, which reflect the existence and process of diseases, and are recognized as reliable dynamic indexes to diagnose diseases. In some points, protein biomarkers can be classified into three types, enzymes, hormones and other proteins. The common protein biomarkers related to the tumor, include carcinoembryonic antigen (CEA), carbohydrate antigen (CA), alpha fetoprotein, prostate-specific antigen (PSA) and mucin 1 (MUC1). The other proteins, such as cTnI, C-reactive protein (CRP), lactoferrin, β2-microglobulin, are the biomarkers for the detection of other diseases. Here, we summarize the ECL immunosensing for detecting disease biomarkers. | ||
| Fig. 2 Schematic of three kinds of ECL immunosensing system. a) Direct-type ECL assay for the detection of AFP.73 b) Competitive-type ECL assay for the detection of AFP.74 c) Sandwich-type ECL assay for the detection of CEA.75 Reprinted with permission from ref. 73 (copyright 2010 Elsevier), ref. 74 (copyright 2022 American Chemical Society), and ref. 75 (copyright 2022 American Chemical Society). | ||
Ju and co-authors74 designed a competitive immunosensor by using Au NPs coupled with polyaniline as an energy receptor to absorb energy emitted from polyoxomolybdate–ZrO2 (energy donor). The energy and electron transfer occurred at the same time for quenching the ECL intensity of polyoxomolybdate–ZrO2. This dual-mechanism quenching strategy was applied to detect 17β-estradiol. CEA is considered to be one of the tumor markers for the clinical diagnosis of various tumors, such as lung cancer, breast cancer, and colorectal cancer. Yang and co-authors75 proposed a sandwich-type immunosensor by using Pt@SnS2 as a matrix and N, B-doped Eu MOF (N, B-Eu MOF) nanospheres as a signal amplifier for highly sensitive and selective detection of CEA. The dual “antenna” effect of 5-boronoisophthalic acid (5-bop) and 5-nitroisophthalic acid (5-nop) enabled the N, B-Eu MOFs to show a very good ECL signal. Thus, this sandwich-type immunosensor provided specific immune responses for the detection of CEA. Deng et al.76 established a co-reactant-dependent ratiometric ECL sandwich-type immunosensor by using ZnSe@ZnS QD conjugated with poly-(diallyldimethylammonium chloride) (PDDA) reduced graphene oxide (PDDA-rGO) and second antibody to detect CEA in human serum. Furthermore, red-emitting (680 nm) carbon QDs (RCQDs) as luminophores were introduced to construct sandwich-type ECL immunosensors for the detection of CEA.77 The secondary antibody label fabricated by the synthesis of aminated graphene can remarkably quench the ECL of RCQDs modified on the electrode because of the ECL resonance energy transfer; thus, this immunological recognition sensitively quantifies CEA through ECL quenching.
CA is identified from tumor cell lines by monoclonal antibody technology, so it exhibits high accuracy in the diagnosis of specific tumors. Different types of CAs, such as CA125,78 CA153 (ref. 79) and CA 199,51 have been chosen as the model analytes for ECL bioassays, among which CA125 is the primarily used diagnostic biomarker for epithelial ovarian cancer and endometrial cancer. CA153 and CA199 are specific to breast and pancreatic cancers, respectively. A sandwich-type ECL immunosensor was proposed for the sensitive detection of CA 125 on a nanoporous gold (NPG) modified GCE. [Ru(bpy)3]2+–gold nanoparticles (Ru–AuNPs) composite assembled with poly(diallyldimethylammonium chloride) functionalized graphene nanosheets (GR) (Ru–AuNPs/GR) was used as ECL labels, modified on the electrode. This proposed sandwich-type ECL immunocomplex provide a wide linear response range over 0.01–100 U mL−1 with a detection limit of 0.005 U mL−1.78 Yang et al.51 proposed a disposable ECL immunosensor for CA199 detection with a special Au nanoelectrode as the platform, magnetic bead as the capture probe, and [Ru(bpy)3]2+-labeled antibody as the ECL indicator, which brought hopes for the detection of biomarkers at the point-of-care test.
PSA, an oncofetal glycoprotein, is a widely used tumor marker, which is expressed in normal mucosal cells and overexpressed in prostate cancer. Xu et al.80 proposed a dual ECL enhancement strategy on a closed ITO bipolar electrode for PSA detection in human serum. Au NPs catalyzed the anodic ECL reaction between luminol and H2O2, meanwhile, thionine (Th)@SiO2 NPs were introduced in cathodic pole as the recognition probes of PSA and signal amplification indicators. By using various QDs as ECL indicators, Fu et al.81 prepared several sandwich-type ECL immunosensors for PSA detection with a wide detection range, presenting promising application potential in clinical diagnosis. Currently, the label-free detection technique is a new kind of detection strategy without the usage of labels, attracting much attention. It is based on the direct detection of changes in physical parameters, such as impedance, capacitance, optical properties, or mass change during antigen and antibody interactions without the secondary antibody. Liu et al.82 reported a strategy quantified by the ratio of cathodic and anodic signals (ECLcathodic/ECLanodic) to determine PSA in human serum. The measured PSAs were immobilized on the CdS QDs electrode to generate a cathodic working signal and the fixed PSA was modified on the luminol–Au NPs electrode to get the anodic signal. This ECL immunosensor offers reliability for detecting PSA through simple tests.
cTnI is considered the golden standard for myocardial infarction screening. An ECL immunosensor fabricated using luminol–AuNPs as both antibody carriers and sensing platform was described for the detection of myocardial infarction biomarker cTnI in clinical diagnosis, as shown in Fig. 3.83 Xu et al.84 proposed a visual and high-throughput ECL immunosensor with a simple-electrode system, achieving the simple but accurate determination of cTnI via employing the smartphone as the detector. CRP, as the sensitive indicator of infection and inflammation, is also a general biomarker to evaluate a patient's risk for AMI and cardiovascular diseases. Recently, the first lateral flow-based ECL immunosensor was established via the co-reactant pathway. A sandwich immunocomplex generated strong ECL emission with the [Ru(bpy)3]2+/TPrA system, allowing full-range CRP detection with high sensitivity due to the simple detection device and easy operation procedure.85 A simple and effective ECL immunosensor for quantitative detection of CRP was reported on the modified ITO electrode.86 The ITO was prefunctionalized by Ti NTs and Pt NWs and with Nafion as a linker, the CRP antibody was then attached and finally this sensor was constructed by blocking the residual active sites with BSA. The introduction of nano-composite significantly promoted the sensing performance for CRP assay.
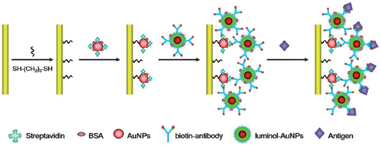 | ||
| Fig. 3 Schematic of the fabrication processes of the ECL immunosensor fabricated using luminol–AuNPs as antibody carriers and sensing platform.83 Reprinted with permission from ref. 83 (copyright 2013 Royal Society of Chemistry). | ||
Alzheimer's disease (AD) is actually a brain disease that seriously influences the daily life of the elderly. It is crucial to develop sensitive and specific ECL immunoassays to realize the early intervention of AD by detecting its corresponding biomarkers. β-Amyloid1–42 (Aβ42) oligomers with strong neurotoxicity are considered well-established and internationally validated model analytes for early diagnosis of AD. Liu et al.87 presented a simple signal-on ECL aptasensor by using the co-reactant mechanism for the detection of amyloid-beta (Aβ) peptide. ROS as a co-reactant was generated via a catalysis reaction between Cu2+-Aβ. It reacted with luminol to exhibit favorable analytical performance for the Aβ16 monomer with a low detection limit of 3.5 × 10−14 mol L−1. Tu et al.88 recently proposed a dual-screening ECL bioassay by employing mesoporous silica membrane as the nano-sieving and specific aptamer as the capturer, which achieved the discrimination of the Aβ monomer from the Aβ oligomer and fiber, and offered a promising opportunity to detect Aβ in complex matrices.
Human epididymis protein 4 (HE4) is a specific biomarker for ovarian cancer. An enhanced ECL immunosensor was designed based on an efficient TiO2 MCs@rGO loading with luminol/DBAE system for amplifying ECL emission, realizing the quantitative analysis of HE4.89 An ECL signal amplification strategy was proposed via employing carbon nano-horns as the photothermal converter to elevate the electrode surface temperature, and a ratiometric ECL immunosensor exhibited excellent sensitivity for HE4 detection.90
Ju and co-authors91 reported a competitive immunosensor constructed by immobilizing meso-2,3-dimercaptosuccinic acid (DMSA)-stabilized CdTe QDs and human IgG (HIgG) as antigen on GCE, shown in Fig. 4. The competitive immuno-recognition of the immobilized HIgG and analyte HIgG to horseradish peroxidase (HRP)-labeled antibody produce on a HRP-immobilized surface, leading to the detection of HIgG with high sensitivity. CdS QDs-carbon nanotubes (CNTs) and Au NPs–chitosan as effective antibody immobilization matrix were presented to detect the target human IgG.92
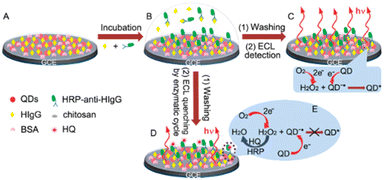 | ||
| Fig. 4 Construction (A) and incubation (B) of the immunosensor, and ECL detection without (C) and with (D and E) the enzymatic amplification by consumption of H2O2 as co-reactant.91 Reprinted with permission from ref. 91 (copyright 2010 American Chemical Society). | ||
A sandwich ECL immunosensing platform based on bifunctional polymer dots (TEA–Tyr dots) and reduced graphene mesoporous silica gold hybrids was designed for ultrasensitive detect cytokine biomarker (IL-6), realizing the quantitative and qualitative dual signal amplification (Fig. 5). The two synergistic effects between TEA and the tyramine group (Tyr) improved the sensitivity remarkably.93 Hormone-based biomarkers and enzyme-based biomarkers have become promising model targets for ECL bioassays. At present, the most frequently investigated hormone-based biomarkers in ECL immunoassays are many kinds, including insulin,94 thyroid stimulating hormone (TSH),95 procalcitonin (PCT)96 and human chorionic gonadotrophin (HCG).97 Insulin is the target analyte for diabetic mellitus, TSH is related to hyperthyroidism, PCT is an indicator of inflammation, and HCG is a biomarker of pregnancy. As for enzyme-based biomarkers, thrombin,98 protein kinase A (PKA),99N-acetyl-β-D-glucosaminidase,100 and apurinic/apyr-imidinic endonuclease 1 (ref. 101) have been widely applied as the target analytes in the construction of ECL biosensing methods.
3.3. ECL immunosensors for bacterial analysis
Pathogenic bacteria pose a significant threat to human health and safety, highlighting the critical need for early detection methods. Rapid detection of pathogens in the initial stages of infection is crucial for selecting appropriate treatment, suppressing pathogen transmission, reducing mortality rates, and minimizing economic burden. While conventional techniques such as polymerase chain reaction and enzyme-linked immunosorbent assays are accurate, their complexity and time-intensive nature often hinder widespread applications. ECL immunosensors offer a promising solution by combining the advantages of both electrochemical and photoluminescence analyses, enabling highly sensitive and simple detection of pathogenic bacteria. In this section, we will address the latest developments in ECL sensors for the detection of pathogenic bacteria.In the work of Wei and co-authors,102 a newly developed dual-mode detection ECL/SERS immunosensor offers a precise method to quantify pathogenic Vibrio vulnificus. This Gram-negative bacterium is widely distributed in seawater and seafood, and there is a need for its sensitive and rapid on-site detection. The biosensor was based on a multifunctional 2D Ti3C2Tx MXene loaded with ECL signal tags ABEI and detection of Ab2 antibodies. Due to numerous surface functional groups of Ti3C2Tx MXene it was also loaded with SERS signal tags rhodamine 6G and closely packed plasmonic gold nanorods to enable SERS measurements. The utilisation of 2-D MXene for electrode functionalization thus allowed the construction of a dual-mode immunosensor with improved sensitivity and accuracy. Under optimal experimental conditions, Vibrio vulnificus was detected with the limit of quantification for ECL and SERS of 1 CFU mL−1 and 102 CFU mL−1, respectively.
Similarly, the advantages of MOFs were exploited to construct an ECL immunobiosensor for Burkholderia pseudomallei detection.103 A new type of versatile catalytic nanomaterial called Co-MOF@AuNP@ABEI has been introduced, comprising cobalt-doped metal–organic frameworks (Co-MOF), gold nanoparticles (AuNP), and N-(4-aminobutyl)-N-(ethylisoluminol) (ABEI). This nanomaterial demonstrates remarkable catalytic properties, displaying high synergistic effects and zero-distance catalysis, which significantly enhances the sensitivity of an ECL biosensor. By integrating with the ECL system and a 3D magnetic walking nanomachine amplification approach, the Co-MOF@AuNP@ABEI achieved an exceptionally sensitive ECL assay for Burkholderia pseudomallei, with a detection limit of as low as 60.3 aM. This LOD surpasses the sensitivity of individual ECL systems without the nanomachine (4.97 fM) and individual walking nanomachine (340 fM) by 2 and 4 orders of magnitude, respectively, outperforming previous pathogenic bacteria analyses. The proposed ECL detection system exhibited an impressively low LOD of 9.0 CFU mL−1 for detecting B. pseudomallei in serum samples. In testing five serum samples spiked with B. pseudomallei, the ECL intensity showed relative standard deviations (RSD) ranging from 0.21% to 4.02%, underlining the precision of the biosensor. The ECL biosensor also demonstrated excellent recovery rates of 93.63–107.83% for detecting B. pseudomallei DNA-spiked serum samples.
Chen and co-authors applied nitrogen-doped graphene quantum dots (N-GQDs) to construct an ECL-based immunosensor for the detection of E. coli O157:H7 bacterium that produces Shiga toxins with polydopamine (PDA) surface imprinted polymer (SIP).104 The synthesis of N-GQDs, possessing a high quantum yield of 43.2%, was achieved. A uniform PDA SIP film specific for E. coli O157:H7 was successfully created using a straightforward method. By directly electropolymerizing dopamine and the target bacterium on the electrode, the PDA SIP designed for E. coli O157:H7 was established effectively. Following the removal of the E. coli O157:H7 template, the developed PDA SIP exhibited selective recognition towards E. coli O157:H7. Subsequently, the E. coli O157:H7 polyclonal antibody (pAb) was labeled with N-GQDs. Combining the SIP-E. coli O157:H7/pAb-N-GQDs bioconjugation led to strong ECL emission under K2S2O8, facilitating the detection of E. coli O157:H7 using the ECL system. This innovative approach demonstrated linear correlations between ECL intensity and E. coli O157:H7 concentration from 101 to 107 colony-forming units (CFU) mL−1, with a detection limit of 8 CFU mL−1 when operating under optimized conditions. The biosensor utilizing this SIP film proved to be successful in detecting E. coli O157:H7 in water samples. A study performed by Zhou and coworkers presents ECL microscopy as a cutting-edge technology offering exceptional spatial and temporal resolution along with a distinctive chemical contrast for visualizing and distinguishing individual bacteria.105 The method showcases precise bacterial quantification and classification accuracy of up to 90.5%. Additionally, they introduced an innovative tunable ECL imaging mode that transitions between negative contrast ECL imaging without labeling to positive contrast ECL imaging by leveraging [Ru(bpy)3]2+ for bacterial visualization. This flexibility enables detailed observation of a single bacterium at the molecular level.
In a study by Wang and co-authors a specialized ECL sensor was developed for both detecting and sterilization Staphylococcus aureus.106 The sensor utilized silver nanoclusters labeled hairpin DNA (H-Ag NCs) as the energy acceptor responsible for receiving ECL emission from CdS quantum dots (CdS QDs), facilitating resonance energy transfer and ECL signal quenching. The ECL signal was amplified through energy transfer from ECL-excited surface plasmon resonance in Au NPs to CdS QDs. This innovative approach displayed effective S. aureus detection over a linear range of 5–108 CFU mL−1. Moreover, this sensor system demonstrated exceptional discriminatory ability and efficacy in eliminating S. aureus in food samples, underscoring its practical reliability and utility.
3.4. ECL biosensors for DNA analysis
ECL immunosensing can be combined with ECL genosensing to improve disease diagnostics. The biorecognition of oligonucleotides by ECL genosensors is a beneficial diagnostic approach because of the high specificity of base-pairing interaction between complementary sequences. The biological sensing element in genosensors is a DNA probe, whose sequence is complementary to the DNA sequence of the target. Due to the specificity of the DNA probe, ECL genosensors can detect DNA nucleotide moieties,107–109 RNA110,111 and micro-RNA112–115 which makes them suitable for a wide range of applications going from clinical diagnostics to food safety and environmental monitoring.The binding of target sequences on the surface carrying the DNA probe generates signals that are further amplified. In recent years, various isothermal gene amplification techniques have been widely used in genosensors to enhance the signal. Moreover, ECL geno- and immunosensing can be combined to improve disease diagnostics. For instance, Zhao et al., coupled a system based on Au nanoclusters (AuNCs)/Au–MnO2 nanoflowers resonant energy transfer (RET) with rolling circle amplification (RCA) for the detection of PSA and Let-7a microRNA, that are both biomarkers of prostate cancer.116 MicroRNAs, small (19–23 nt) noncoding RNAs, are reliable biomarkers not only for cancers but also for inflammatory bowel disease, cardiovascular and cerebrovascular diseases. Au NCs were employed for ECL signal enhancement through the stabilization of 3,4-thiophene dicarboxylic acid (TDA)-modified electrode. A sandwich ECL immunosensor for signal “OFF” detection of PSA was obtained with methionine-Au NCs with intense near-infrared ECL emission as ECL donors and Au–MnO2 nanoflowers with strong light absorption capability as acceptors (Fig. 6). In addition, Let-7a microRNA was targeted via RCA reaction to produce long DNA nanowires, which when labeled with alkaline phosphatase, catalysed the production of ascorbic acid. Accumulation of ascorbic acid in situ reduced MnO2, which inhibited ECL quenching and provided a “ON” detection of Let-7a. Such a dual biomarker detection is proposed for early clinical tumor diagnosis.
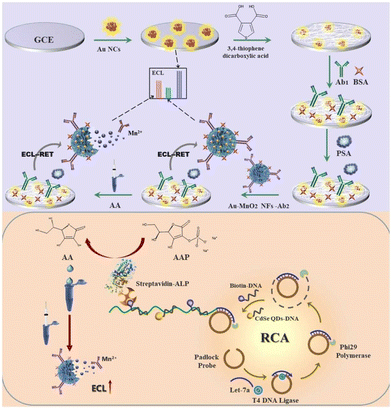 | ||
| Fig. 6 Schematic of the ECL biosensor based on Au nanoclusters/Au–MnO2 nanoflowers ECL-RET (resonant energy transfer) system coupled with RCA (rolling circle amplification) for dual target detection of PSA (prostate-specific antigen) and Let-7a microRNA.116 Reprinted with permission from ref. 116 (copyright 2022 Elsevier). | ||
Recent years witnessed great development of metal–organic frameworks (MOF)-based ECL immuno- and genosensors.117–119 Although MOF composites have poor intrinsic conductivity and low water solubility, they have exceptional physicochemical features, such as large surface area, high porosity, tunable size, tailorable structure and versatile functionality. To overcome their limitations, MOFs are combined with a variety of functional materials or loading guests such as ruthenium, luminol, and quantum dots. Alternatively, MOFs can be doped with transition metal elements to increase their low conductivity.117,120 MOFs in ECL reactions for rapid detection of viral infection are of particular importance. Although viral infections can be detected by immunosensors, during the acute phase of infection specific antibodies are absent, and also immunosensors are unable to discriminate between current and previous infections. In contrast, direct detection of specific viral genes allows for early diagnosis. For instance, a 2D MOF with an excellent ECL performance was obtained by combining a porphyrin-based heterobimetallic MOF and a photosensitizer Zn2+-tetrakis(4-carboxyphenyl)porphine (ZnTCPP) linker with the electroactive Co2+-ions in the presence of 2-methylimidazole (MeIm).121 The ECL sensor detected a low amount of the RdRp gene of SARS-CoV-2 (30 aM) without a need for target gene amplification. Finally, a new era of ECL biosensors for precise DNA analysis based on CRISPR (clustered regularly interspaced short palindromic repeats)–Cas systems are paving the way for the design of advanced diagnostics of infectious diseases, early-stage cancers, detrimental genetic conditions, and mutagenic defects.122–125 Although CRISPR–Cas-based biosensors (especially using CRISPR-12a and CRIPR-13a systems for gene editing) are highly specific, fast, easy to use, and of low cost, they still have some drawbacks including the need for DNA amplification, and low reproducibility.
3.5. Cell analysis
Cancer cells are one of the greatest causes of death in human beings. ECL has been used to be the sensitive method for cancer cell detection. NPs, such as CdS NPs,126–128 Au NPs,129–131 are often utilized to construct the base of the ECL immunosensor for cell-level detection. CdS-coated-ZnO nanorod arrays labeled with 3-aminopropyltriethoxysilane and Au NPs, can not only offer the substrates for the conjugation of antibodies but also effectively enhance the ECL signal, resulting in the production of the high-performance ECL immunosensor. This immunosensor exhibits a sensitive response to HepG2 cells with a detection limit of 256 cells per mL.126 Ding et al.128 reported that CdS NPs were used to be functionalized with Au NPs and aptamer for electrochemical signal amplification, demonstrating excellent sensitivity and selectivity of the Ramos cells. Due to the unique amplification of Au NPs and the excellent selectivity of aptamers, the Ramos cells were rapidly and simply detected with high sensitivity. Later, Ding et al.129 studied a polymerase chain reaction (PCR)-free ECL approach to determine cancer cells with high sensitivity based on aptamers, nanoparticles and magnetic beads. ECL probes consist of Au NPs with [Ru(bpy)3]2+, hybridize with the captured DNA with a magnetic bead to form the magnetic nanocomposite, which directly reflects the amount of cancer cells. Au nanostars (AuNSs) decorated with graphitic carbon nitride nanosheets (g-CN nanosheets) were designed to detect the CD133 peptide as a cancer stem cell membrane biomarker. AuNSs can increase electron transfer and electroreduced S2O82−, effectively enhancing the ECL intensity. Due to the localized surface plasmon resonance (LSPR) effect of AuNSs, AuNSs@g-CN nanosheets exhibited strong and stable cathodic ECL emission, realizing the detection of CD133 peptide in low concentration.1304. Conclusions and perspectives
ECL has become a powerful technique for the ultrasensitive detection of a wide range of analytes, due to its inherent sensitivity, simplicity, well-control, and low background. Herein, we summarized the recent advances that have been made in novel ECL immunoassays, the ECL enhanced strategies, and the applications for the detection of biomarkers, DNA analysis, bacteria and cell analysis. These important topics cover the vast majority of the hotspots in the realm of ECL immunosensing. Clearly, the necessity of developing sensitive immunoassays and understanding fundamental ECL mechanisms have constantly stimulated breakthroughs in medical diagnosis, biological detection and tracking. Particular emphasis is placed on developing bead-based ECL immunoassays by using microscopy that aims to understand the mechanism of ECL of bead-based assays, to further assess at the single molecule level in the future, which could expand to other ECL sensing systems and ECL-based imaging. The commercialized ECL immunoassays are mainly based on the classical ECL system–ruthenium complexes and TPrA. Meanwhile, other materials, such as Au nanomaterials, metal composites, carbon nanomaterials, or organic materials are often introduced into the ECL system to obtain stable and enhanced emission, improving efficiency. Fortunately, some novel immunosensing with new kinds of ECL materials and luminophores may promote the development of ECL technology.Although novel ECL systems are overwhelmingly used to fabricate sensors with high efficiency, there are still great challenges as well as great opportunities to be faced in the future. First, though various signal amplification strategies have been designed, novel efficient ECL methodologies still need to be developed to understand the details of ECL kinetics and mechanisms, in order to meet the need for different applications. Second, despite the commercial immunosensing of [Ru(bpy)3]2+/TPrA system being successfully used, the high toxicity and poor biocompatibility of this system is still a major issue. Thus, more biocompatible and environmentally friendly ECL materials as the alternatives are highly desirable. Third, ECL emission can be observed at high positive or negative voltages, the problem is that the high voltage process unavoidably brings a series of undesired side effects, such as electrical damage to biological samples and hydrogen/oxygen evolution reactions. Therefore, it is urgently required to design and explore the low voltage-driven ECL luminophores,132,133 not only to mitigate the electrode passivation but also to decrease the possibility of ECL change, which results from various physiological activities. Finally, the development of ECL-based point of care (POC) diagnostic devices, possibly with mobile phone powering and detection, constitutes a major direction in the field but it requires achieving full portability, de-laboratory, de-specialization and automation as detailed by several authors.134–136
In conclusion, we believe that ECL can have a brilliant future and fast development due to the great contributions from many researchers, and the affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free ECL technology.
Data availability
The authors confirm that the data presented in this review are available within the cited articles.Author contributions
N. S. defined the scope and J. Y. defined the structure of the review article. All the authors prepared the manuscript by writing the initial draft, drawing or selecting the illustrations, reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is kindly supported by the National Natural Science Foundation of China (Grants No. 22304148), Foundation of Shandong Educational Committee (ZR2023QB130), Shandong Province Scientific Foundation for Excellent Youths (2024HWYQ-073) and the Agence Nationale de la Recherche (ELISE-ANR-21-CE42). The authors gratefully acknowledge the financial support of the European Union (Grant agreement No. 101135402, Mobiles project). DS wishes to acknowledge the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Contract No: 451-03-66/2024-03/200168).Notes and references
- Z. Liu, W. Qi and G. Xu, Chem. Soc. Rev., 2015, 44, 3117–3142 RSC.
- X. Ma, W. Gao, F. Du, F. Yuan, J. Yu, Y. Guan, N. Sojic and G. Xu, Acc. Chem. Res., 2021, 54, 2936–2945 CrossRef CAS PubMed.
- C. Ma, Y. Cao, X. Gou and J. J. Zhu, Anal. Chem., 2020, 92, 431–454 CrossRef CAS PubMed.
- M. Sornambigai, L. Bouffier, N. Sojic and S. S. Kumar, Anal. Bioanal. Chem., 2023, 415, 5875–5898 CrossRef CAS PubMed.
- K. D. Legg and D. M. Hercules, J. Am. Chem. Soc., 1968, 91, 1902–1907 CrossRef.
- L. R. Faulkner and A. J. Bard, J. Am. Chem. Soc., 1968, 90, 6284–6290 CrossRef CAS.
- S. Knezevic, D. Han, B. Liu, D. Jiang and N. Sojic, Angew. Chem., Int. Ed., 2024, 63, e202407588 CrossRef CAS PubMed.
- S. Rebeccani, A. Zanut, C. I. Santo, G. Valenti and F. Paolucci, Anal. Chem., 2022, 94, 336–348 CrossRef CAS PubMed.
- Y. Liu, H. Zhang, B. Li, J. Liu, D. Jiang, B. Liu and N. Sojic, J. Am. Chem. Soc., 2021, 143, 17910–17914 CrossRef CAS PubMed.
- H. Qi and C. Zhang, Anal. Chem., 2020, 92, 524–534 CrossRef CAS PubMed.
- G. Liang, S. Liu, G. Zou and X. Zhang, Anal. Chem., 2012, 84, 10645–10649 CrossRef CAS PubMed.
- P. Zhou, S. Hu, W. Guo and B. Su, Fundam. Res., 2022, 2, 682–687 CrossRef CAS PubMed.
- N. Hao and K. Wang, Anal. Bioanal. Chem., 2016, 408, 7035–7048 CrossRef CAS PubMed.
- Z. Wang, J. Pan, Q. Li, Y. Zhou, S. Yang, J. J. Xu and D. Hua, Adv. Funct. Mater., 2020, 30, 2000220 CrossRef CAS.
- Z. Cao, Y. Shu, H. Qin, B. Su and X. Peng, ACS Cent. Sci., 2020, 6, 1129–1137 CrossRef CAS PubMed.
- E. Faatz, A. Finke, H. P. Josel, G. Prencipe, S. Quint and M. Windfuhr, Analytical Electrogenerated Chemiluminescence, 2019, pp. 443–469, 10.1039/9781788015776-fp001.
- X. Gou, Z. Xing, C. Ma and J. J. Zhu, Chem. Biomed. Imaging, 2023, 1, 414–433 CrossRef CAS.
- C. Mariani, S. Bogialli, F. Paolucci, P. Pastore, A. Zanut and G. Valenti, Electrochim. Acta, 2024, 489, 144256 CrossRef CAS.
- H. Cui, W. Wang, C. F. Duan, Y. P. Dong and J. Z. Guo, Chem. – Eur. J., 2007, 13, 6975–6984 CrossRef CAS PubMed.
- A. Zanut, A. Fiorani, S. Canola, T. Saito, N. Ziebart, S. Rapino, S. Rebeccani, A. Barbon, T. Irie, H. P. Josel, F. Negri, M. Marcaccio, M. Windfuhr, K. Imai, G. Valenti and F. Paolucci, Nat. Commun., 2020, 11, 2668 CrossRef CAS PubMed.
- M. Mayer, S. Takegami, M. Neumeier, S. Rink, A. Jacobi von Wangelin, S. Schulte, M. Vollmer, A. G. Griesbeck, A. Duerkop and A. J. Baeumner, Angew. Chem., Int. Ed., 2018, 57, 408–411 CrossRef CAS PubMed.
- P. Bertoncello, A. J. Stewart and L. Dennany, Anal. Bioanal. Chem., 2014, 406, 5573–5587 CrossRef CAS PubMed.
- A. Barhoum, Z. Altintas, K. S. S. Devi and R. J. Forster, Nano Today, 2023, 50, 101874 CrossRef.
- G. Valenti, E. Rampazzo, S. Kesarkar, D. Genovese, A. Fiorani, A. Zanut, F. Palomba, M. Marcaccio, F. Paolucci and L. Prodi, Coord. Chem. Rev., 2018, 367, 65–81 CrossRef CAS.
- A. Zanut, F. Palomba, M. Rossi Scota, S. Rebeccani, M. Marcaccio, D. Genovese, E. Rampazzo, G. Valenti, F. Paolucci and L. Prodi, Angew. Chem., Int. Ed., 2020, 59, 21858–21863 CrossRef CAS PubMed.
- G. Valenti, E. Rampazzo, S. Bonacchi, L. Petrizza, M. Marcaccio, M. Montalti, L. Prodi and F. Paolucci, J. Am. Chem. Soc., 2016, 138, 15935–15942 CrossRef CAS PubMed.
- E. R. Simone Zanarini, S. Bonacchi, R. Juris, M. Marcaccio, M. Montalti, F. Paolucci and L. Prodi, J. Am. Chem. Soc., 2009, 131, 14208–14209 CrossRef PubMed.
- Z. Ding, B. M. Quinn, S. K. Haram, L. E. Pell, B. A. Korgel and A. J. Bard, Science, 2002, 296, 1293–1297 CrossRef CAS PubMed.
- T. Zhao, Q. Zhou, Y. Lv, D. Han, K. Wu, L. Zhao, Y. Shen, S. Liu and Y. Zhang, Angew. Chem., Int. Ed., 2020, 59, 1139–1143 CrossRef CAS PubMed.
- E. Yang, Y. Zhang and Y. Shen, Anal. Chim. Acta, 2022, 1209, 339140 CrossRef CAS PubMed.
- M. Hesari and Z. Ding, J. Am. Chem. Soc., 2021, 143, 19474–19485 CrossRef CAS PubMed.
- M. Hesari, M. S. Workentin and Z. Ding, ACS Nano, 2014, 8, 8543–8553 CrossRef CAS PubMed.
- J. M. Kim, S. Jeong, J. K. Song and J. Kim, Chem. Commun., 2018, 54, 2838–2841 RSC.
- Y. Kang and J. Kim, ChemElectroChem, 2020, 7, 1092–1096 CrossRef CAS.
- Z. Huang, Z. Li, Y. Chen, L. Xu, Q. Xie, H. Deng, W. Chen and H. Peng, Anal. Chem., 2021, 93, 4635–4640 CrossRef CAS PubMed.
- D. Wang, X. Gao, J. Jia, B. Zhang and G. Zou, ACS Nano, 2023, 17, 355–362 CrossRef CAS PubMed.
- P. Sabhachandani, S. Sarkar, P. C. Zucchi, B. A. Whitfield, J. E. Kirby, E. B. Hirsch and T. Konry, Microchim. Acta, 2017, 184, 4619–4628 CrossRef CAS.
- N. Scholler, M. Crawford, A. Sato, C. W. Drescher, K. C. O'Briant, N. Kiviat, G. L. Anderson and N. Urban, Clin. Cancer Res., 2006, 12, 2117–2124 CrossRef CAS PubMed.
- K. Y. Lien, L. Y. Hung, T. B. Huang, Y. C. Tsai, H. Y. Lei and G. B. Lee, Biosens. Bioelectron., 2011, 26, 3900–3907 CrossRef CAS PubMed.
- M. Sentic, M. Milutinovic, F. Kanoufi, D. Manojlovic, S. Arbault and N. Sojic, Chem. Sci., 2014, 5, 2568–2572 RSC.
- Y. Feng, W. Zhou, X. Wang, J. Zhang, M. Zou, C. Zhang and H. Qi, Chem. Biomed. Imaging, 2023, 1, 648–658 CrossRef CAS.
- D. Han, D. Fang, G. Valenti, F. Paolucci, F. Kanoufi, D. Jiang and N. Sojic, Anal. Chem., 2023, 95, 15700–15706 CrossRef CAS PubMed.
- A. Fracassa, C. I. Santo, E. Kerr, S. Knezevic, D. J. Hayne, P. S. Francis, F. Kanoufi, N. Sojic, F. Paolucci and G. Valenti, Chem. Sci., 2024, 15, 1150–1158 RSC.
- D. Han, D. Jiang, G. Valenti, F. Paolucci, F. Kanoufi, P. C. Chaumet, D. Fang and N. Sojic, ACS Sens., 2023, 8, 4782–4791 CrossRef CAS PubMed.
- Y. Wang, J. Ding, P. Zhou, J. Liu, Z. Qiao, K. Yu, J. Jiang and B. Su, Angew. Chem., Int. Ed., 2023, 62, e202216525 CrossRef CAS PubMed.
- L. Xiao, Y. Chai, R. Yuan, Y. Cao, H. Wang and L. Bai, Talanta, 2013, 115, 577–582 CrossRef CAS PubMed.
- S. Rebeccani, C. Wetzl, V. A. Zamolo, A. Criado, G. Valenti, F. Paolucci and M. Prato, Chem. Commun., 2021, 57, 9672–9675 RSC.
- X. Yang, J. Hang, W. Qu, Y. Wang, L. Wang, P. Zhou, H. Ding, B. Su, J. Lei, W. Guo and Z. Dai, J. Am. Chem. Soc., 2023, 145, 16026–16036 CrossRef CAS PubMed.
- M. Sentic, F. Virgilio, A. Zanut, D. Manojlovic, S. Arbault, M. Tormen, N. Sojic and P. Ugo, Anal. Bioanal. Chem., 2016, 408, 7085–7094 CrossRef CAS PubMed.
- H. B. Habtamu, M. Sentic, M. Silvestrini, L. De Leo, T. Not, S. Arbault, D. Manojlovic, N. Sojic and P. Ugo, Anal. Chem., 2015, 87, 12080–12087 CrossRef CAS PubMed.
- X. Yang, Y. Wei, Y. Du, H. Qi, Q. Gao and C. Zhang, Anal. Chem., 2020, 92, 15837–15844 CrossRef CAS PubMed.
- G. Valenti, A. Fiorani, H. Li, N. Sojic and F. Paolucci, ChemElectroChem, 2016, 3, 1990–1997 CrossRef CAS.
- X. Yang, Y. Xu, X. Huang, J. Hang, W. Guo and Z. Dai, Anal. Chem., 2023, 95, 4543–4549 CrossRef CAS PubMed.
- W. Guo, H. Ding, C. Gu, Y. Liu, X. Jiang, B. Su and Y. Shao, J. Am. Chem. Soc., 2018, 140, 15904–15915 CrossRef CAS PubMed.
- J. M. Fernandez-Hernandez, E. Longhi, R. Cysewski, F. Polo, H. P. Josel and L. De Cola, Anal. Chem., 2016, 88, 4174–4178 CrossRef CAS PubMed.
- M. A. Haghighatbin, S. E. Laird and C. F. Hogan, Curr. Opin. Electrochem., 2018, 7, 216–223 CrossRef CAS.
- K. Sakanoue, A. Fiorani, C. I. Santo, Irkham, G. Valenti, F. Paolucci and Y. Einaga, ACS Sens., 2022, 7, 1145–1155 CrossRef CAS PubMed.
- A. Fiorani, C. I. Santo, K. Sakanoue, D. Calabria, M. Mirasoli, F. Paolucci, G. Valenti and Y. Einaga, Anal. Bioanal. Chem., 2024 DOI:10.1007/s00216-024-05356-z.
- B. M. B. Factor, S. Workman, E. Bolton, J. Bos and M. M. Richter, Anal. Chem., 2001, 73, 4621–4624 CrossRef CAS PubMed.
- S. Kirschbaum-Harriman, A. Duerkop and A. J. Baeumner, Analyst, 2017, 142, 2648–2653 RSC.
- A. Fiorani, D. Han, D. Jiang, D. Fang, F. Paolucci, N. Sojic and G. Valenti, Chem. Sci., 2020, 11, 10496–10500 RSC.
- P. Dutta, D. Han, B. Goudeau, D. Jiang, D. Fang and N. Sojic, Biosens. Bioelectron., 2020, 165, 112372 CrossRef CAS PubMed.
- E. Kerr, S. Knezevic, P. S. Francis, C. F. Hogan, G. Valenti, F. Paolucci, F. Kanoufi and N. Sojic, ACS Sens., 2023, 8, 933–939 CrossRef CAS PubMed.
- S. J. Blom, N. S. Adamson, E. Kerr, E. H. Doeven, O. S. Wenger, R. S. Schaer, D. J. Hayne, F. Paolucci, N. Sojic, G. Valenti and P. S. Francis, Electrochim. Acta, 2024, 484, 143957 CrossRef CAS.
- S. B. N. Adamson, E. Doeven, T. Connell, C. Hadden, S. Knežević, N. Sojic, A. Fracassa, G. Valenti, F. Paolucci, J. Ding, Y. Wang, B. Su, C. Hua and P. Francis, Angew. Chem., Int. Ed., 2024, 63, e202412097 Search PubMed.
- D. Han, B. Goudeau, V. Lapeyre, V. Ravaine, D. Jiang, D. Fang and N. Sojic, Biosens. Bioelectron., 2022, 216, 114640 CrossRef CAS PubMed.
- J. Dong, Y. Lu, Y. Xu, F. Chen, J. Yang, Y. Chen and J. Feng, Nature, 2021, 596, 244–249 CrossRef CAS PubMed.
- W. Zhu, J. Dong, G. Ruan, Y. Zhou and J. Feng, Angew. Chem., Int. Ed., 2023, 62, e202214419 CrossRef CAS PubMed.
- F. Du, Y. Chen, C. Meng, B. Lou, W. Zhang and G. Xu, Curr. Opin. Electrochem., 2021, 28, 100725 CrossRef CAS.
- M. Guo, D. Du, J. Wang, Y. Ma, D. Yang, M. A. Haghighatbin, J. Shu, W. Nie, R. Zhang, Z. Bian, L. Wang, Z. J. Smith and H. Cui, Chem. Biomed. Imaging, 2023, 1, 179–185 CrossRef CAS.
- W. Fu, X. Wang, X. Ying, T. Sun, Y. Wang, J. Wang and B. Su, Adv. Funct. Mater., 2024, 2409632, DOI:10.1002/adfm.202409632.
- C. Y. Huang, F. Y. Lin, C. J. Chang, C. H. Lu and J. K. Chen, Anal. Chem., 2023, 95, 986–993 CAS.
- S. Yuan, R. Yuan, Y. Chai, L. Mao, X. Yang, Y. Yuan and H. Niu, Talanta, 2010, 82, 1468–1471 CrossRef CAS PubMed.
- X. Dong, G. Zhao, Y. Li, Q. Zeng, H. Ma, D. Wu, X. Ren, Q. Wei and H. Ju, Anal. Chem., 2022, 94, 12742–12749 CrossRef CAS PubMed.
- J. Li, H. Yang, R. Cai and W. Tan, ACS Appl. Mater. Interfaces, 2022, 14, 44222–44227 CrossRef CAS PubMed.
- X. Zheng, G. Mo, Y. He, D. Qin, X. Jiang, W. Mo and B. Deng, J. Electroanal. Chem., 2019, 844, 132–141 CrossRef CAS.
- Y. Hu, Y. Chen, Q. Tang and H. Liu, New J. Chem., 2021, 45, 12613–12621 RSC.
- M. Li, M. Zhang, S. Ge, M. Yan, J. Yu, J. Huang and S. Liu, Sens. Actuators, B, 2013, 181, 50–56 CrossRef CAS.
- X. Li, Y. Xu and L. Zhang, Prog. Mol. Biol. Transl. Sci., 2019, 162, 265–276 CAS.
- H. W. Shi, W. Zhao, Z. Liu, X. C. Liu, M. S. Wu, J. J. Xu and H. Y. Chen, Talanta, 2016, 154, 169–174 CrossRef CAS PubMed.
- L. Fu, K. Fu, X. Gao, S. Dong, B. Zhang, S. Fu, H. Y. Hsu and G. Zou, Anal. Chem., 2021, 93, 2160–2165 CrossRef CAS PubMed.
- J. T. Cao, X. M. Liu, Y. Z. Fu, S. W. Ren and Y. M. Liu, Anal. Lett., 2022, 55, 1810–1821 CrossRef CAS.
- F. Li, Y. Yu, H. Cui, D. Yang and Z. Bian, Analyst, 2013, 138, 1844–1850 RSC.
- F. Du, Z. Dong, Y. Guan, A. M. Zeid, D. Ma, J. Feng, D. Yang and G. Xu, Anal. Chem., 2022, 94, 2189–2194 CrossRef CAS PubMed.
- D. Hong, K. Kim, E. J. Jo and M. G. Kim, Anal. Chem., 2021, 93, 7925–7932 CrossRef CAS PubMed.
- R. Zhou, C. Fang, J. Yan and Y. Tu, Talanta, 2019, 205, 120135 CrossRef PubMed.
- H. Qin, X. Gao, X. Yang, W. Cao and S. Liu, Biosens. Bioelectron., 2019, 141, 111438 CrossRef CAS PubMed.
- R. Tan, Y. Wang, X. Mi, H. Li and Y. Tu, Sens. Actuators, B, 2022, 352, 131065 CrossRef CAS.
- D. Fang, M. Pan, H. Yi, H. Dai, Z. Hong, X. Zheng and Y. Lin, Sens. Actuators, B, 2019, 286, 608–615 CrossRef CAS.
- D. Fang, S. Zhang, H. Dai and Y. Lin, Biosens. Bioelectron., 2019, 146, 111768 CrossRef CAS PubMed.
- Y. Z. X. Liu, J. Lei, Y. Xue, L. Cheng and H. Ju, Anal. Chem., 2010, 82, 7351–7356 CrossRef PubMed.
- G. Jie, P. Liu, L. Wang and S. Zhang, Electrochem. Commun., 2010, 12, 22–26 CrossRef CAS.
- J. Liu, X. Liu, H. Chen, L. Yang, A. Cai, H. Ji, Q. Wang, X. Zhou, G. Li, M. Wu, Y. Qin and L. Wu, Anal. Chem., 2022, 94, 7115–7122 CrossRef CAS PubMed.
- Y. Du, X. Li, X. Ren, H. Wang, D. Wu, H. Ma, D. Fan and Q. Wei, Analyst, 2020, 145, 1858–1864 RSC.
- Y. Liu, Q. Zhang, H. Wang, Y. Yuan, Y. Chai and R. Yuan, Biosens. Bioelectron., 2015, 71, 164–170 CrossRef CAS PubMed.
- X. Shao, X. Song, X. Liu, L. Yan, L. Liu, D. Fan, Q. Wei and H. Ju, Microchim. Acta, 2021, 188, 344 CrossRef CAS PubMed.
- N. Liao, Y. Zhuo, Y. Chai, Y. Xiang, Y. Cao, R. Yuan and J. Han, Chem. Commun., 2012, 48, 7610–7612 RSC.
- Y. Q. Yu, H. Y. Zhang, Y. Q. Chai, R. Yuan and Y. Zhuo, Biosens. Bioelectron., 2016, 85, 8–15 CrossRef CAS PubMed.
- G. Y. Zhang, C. Cai, S. Cosnier, H. B. Zeng, X. J. Zhang and D. Shan, Nanoscale, 2016, 8, 11649–11657 RSC.
- H. Wang, Y. Yuan, Y. Zhuo, Y. Chai and R. Yuan, Anal. Chem., 2016, 88, 2258–2265 CrossRef CAS PubMed.
- Y. Zhuo, N. Liao, Y. Q. Chai, G. F. Gui, M. Zhao, J. Han, Y. Xiang and R. Yuan, Anal. Chem., 2014, 86, 1053–1060 CrossRef CAS PubMed.
- W. Wei, H. Lin, T. Hao, X. Su, X. Jiang, S. Wang, Y. Hu and Z. Guo, Sens. Actuators, B, 2021, 332, 129525 CrossRef CAS.
- Y. Wang, R. Chen, B. Shen, C. Li, J. Chen, Y. Wang, S. Tian, X. Li, N. Luo, R. Liu, S. Ding, C. Zhu and Q. Xia, Microchim. Acta, 2022, 189, 355 CrossRef CAS PubMed.
- S. Chen, X. Chen, L. Zhang, J. Gao and Q. Ma, ACS Appl. Mater. Interfaces, 2017, 9, 5430–5436 CrossRef CAS PubMed.
- Y. Zhou, J. Dong, P. Zhao, J. Zhang, M. Zheng and J. Feng, J. Am. Chem. Soc., 2023, 145, 8947–8953 CrossRef CAS.
- C. Wang, T. Wu, X. Miao, P. Wang and Q. Feng, Talanta, 2023, 253, 124074 CrossRef CAS.
- Y. Chen, J. Xu, J. Su, Y. Xiang, R. Yuan and Y. Chai, Anal. Chem., 2012, 84, 7750–7755 CrossRef CAS PubMed.
- P. F. Liu, K. R. Zhao, Z. J. Liu, L. Wang, S. Y. Ye and G. X. Liang, Biosens. Bioelectron., 2021, 176, 112954 CrossRef CAS PubMed.
- Y. Liu, Y. Wei, Y. Cao, D. Zhu, W. Ma, Y. Yu and M. Guo, Biosens. Bioelectron., 2018, 117, 830–837 CrossRef CAS PubMed.
- L. Gutierrez-Galvez, R. Del Cano, I. Menendez-Luque, D. Garcia-Nieto, M. Rodriguez-Pena, M. Luna, T. Pineda, F. Pariente, T. Garcia-Mendiola and E. Lorenzo, Talanta, 2022, 240, 123203 CrossRef CAS PubMed.
- Y. W. Zhang, W. S. Liu, J. S. Chen, H. L. Niu, C. J. Mao and B. K. Jin, Sens. Actuators, B, 2020, 321, 128456 CrossRef CAS.
- J. Zhao, J. Luo, D. Liu, Y. He, Q. Li, S. Chen and R. Yuan, Sens. Actuators, B, 2020, 316, 128139 CrossRef CAS.
- X. Meng, X. Pang, J. Yang, X. Zhang and H. Dong, Small, 2024, 20, e2307701 CrossRef PubMed.
- Z. Ning, E. Yang, Y. Zheng, M. Chen, G. Wu, Y. Zhang and Y. Shen, Anal. Chem., 2021, 93, 8971–8977 CrossRef CAS PubMed.
- J. M. Wang, L. Y. Yao, W. Huang, Y. Yang, W. B. Liang, R. Yuan and D. R. Xiao, ACS Appl. Mater. Interfaces, 2021, 13, 44079–44085 CrossRef CAS PubMed.
- Y. Zhao, R. Wang, Y. Xue and G. Jie, Sens. Actuators, B, 2022, 369, 132397 CrossRef CAS.
- M. Sentic, I. Trajkovic, D. Manojlovic, D. Stankovic, M. V. Nikolic, N. Sojic and J. Vidic, Materials, 2023, 16, 7502 CrossRef CAS PubMed.
- J. Zhou, Y. Li, W. Wang, X. Tan, Z. Lu and H. Han, Biosens. Bioelectron., 2020, 164, 112332 CrossRef CAS.
- W. Li, Z. Liang, P. Wang and Q. Ma, Biosens. Bioelectron., 2024, 249, 116008 CrossRef CAS.
- H. Fu, Z. Xu, H. Hou, R. Luo, H. Ju and J. Lei, Chemosensors, 2023, 11, 422 CrossRef CAS.
- Y. X. Li, J. Li, D. Zhu, J. Z. Wang, G. F. Shu, J. Li, S. L. Zhang, X. J. Zhang, S. Cosnier, H. B. Zeng and D. Shan, Adv. Funct. Mater., 2022, 202209743, DOI:10.1002/adfm.202209743.
- L. Mei-Ling, L. Yi, Z. Mei-Ling, Z. Ying and H. Xiao-Jing, Biosens. Bioelectron., 2022, 214, 114512 CrossRef PubMed.
- Z. H. Xu, Z. Y. Zhao, H. Wang, S. M. Wang, H. Y. Chen and J. J. Xu, Anal. Chim. Acta, 2021, 1188, 339180 CrossRef CAS PubMed.
- K. Zhang, Z. Fan, B. Yao, Y. Ding, J. Zhao, M. Xie and J. Pan, Biosens. Bioelectron., 2021, 178, 113019 CrossRef CAS PubMed.
- S. Y. L. Li, J. Wu and H. Ju, Anal. Chem., 2013, 95, 7396–7402 CrossRef PubMed.
- D. Liu, L. Wang, S. Ma, Z. Jiang, B. Yang, X. Han and S. Liu, Nanoscale, 2015, 7, 3627–3633 RSC.
- E. Han, L. Ding, S. Jin and H. Ju, Biosens. Bioelectron., 2011, 26, 2500–2505 CrossRef CAS PubMed.
- C. Ding, Y. Ge and S. Zhang, Chem, 2010, 16, 10707–10714 CrossRef CAS PubMed.
- C. Ding, S. Wei and H. Liu, Chem, 2012, 18, 7263–7268 CrossRef CAS.
- S. Chenaghlou, A. Khataee, R. Jalili, M. R. Rashidi, B. Khalilzadeh and S. Woo Joo, Bioelectrochemistry, 2021, 137, 107633 CrossRef CAS PubMed.
- A. Zhang, W. Guo, H. Ke, X. Zhang, H. Zhang, C. Huang, D. Yang, N. Jia and D. Cui, Biosens. Bioelectron., 2018, 101, 219–226 CrossRef CAS PubMed.
- Y. Ju, H. J. Park, I. S. Shin, Y. K. Chung and J. Kim, Inorg. Chem. Commun., 2019, 106, 86–90 CrossRef CAS.
- Y. Z. Wang, Y. R. Li, Y. Q. Zhang, Y. M. Xiang, R. R. Bai, Y. Liu, M. L. Li, G. R. Meng, S. L. Pan, F. Zhang, L. Mi and Y. H. Hu, Biosens. Bioelectron., 2024, 261, 116495 CrossRef CAS PubMed.
- M. Jović, D. Prim, O. Righini, D. Tagan, M. Stäuble, M. Pignat, S. Gallay, M. Geiser and M. E. Pfeifer, Sens. Diagn., 2023, 2, 964–975 RSC.
- X. Ying, L. Zhou, W. Fu, Y. Wang and B. Su, Sens. Diagn., 2023, 2, 480–491 RSC.
- J. Totoricaguena-Gorrino, M. Dei, A. F. Alba, N. Perinka, L. R. Rubio, J. L. Vilas-Vilela and F. J. Del Campo, ACS Sens., 2022, 7, 1544–1554 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |

