Open Access Article
Open Access ArticleThe role of optical fiber sensors in the new generation of healthcare devices: a review
Arnaldo
Leal-Junior
 *,
Jussara
Silva
*,
Jussara
Silva
 ,
Leandro
Macedo
,
Arthur
Marchesi
,
Samilly
Morau
,
Janine
Valentino
,
Fabricya
Valentim
and
Magno
Costa
,
Leandro
Macedo
,
Arthur
Marchesi
,
Samilly
Morau
,
Janine
Valentino
,
Fabricya
Valentim
and
Magno
Costa

Federal University of Espírito Santo, Fernando Ferrari Avenue, Vitória, ES 29075-910, Brazil. E-mail: leal-junior.arnaldo@ieee.org
First published on 23rd April 2024
Abstract
This paper presents a review of optical sensor systems for wearable applications aiming at the new demands on healthcare motivated not only by the new paradigms in internet of things, but also in photonics development and artificial intelligence algorithms. In this context, the overview of musculoskeletal disorders and the role of wearable sensor systems in such applications are discussed. In addition, there is a comprehensive discussion of the components of wearable sensor systems with the novel developments and approaches in different wearable applications. Thus, the optical fiber sensor developments, approaches and applications are discussed for their use in smart textiles, biosensors and intrusive applications. Moreover, new developments on power supplies are discussed aiming at self-powered sensor systems. Therefore, this review paper can aid in the development of the new generation of wearable sensor systems in healthcare applications using optical fiber sensors and general optical based sensors, which can overcome or mitigate the shortcomings of conventional sensor technologies.
1 Introduction
The internet of things (IoT) and sensor market have witnessed exponential growth in recent years.1 IoT refers to the interconnected network of physical devices, vehicles, appliances, and even buildings, all embedded with sensors, software, and other technologies, allowing them to collect and exchange data.2 This data-driven ecosystem has found applications across various sectors, including healthcare, agriculture, manufacturing, and smart cities, to name a few.3Sensors play a pivotal role in the IoT scenario, serving as the eyes and ears of this interconnected world.4 These devices can capture a wide array of information, such as temperature, humidity, pressure, motion, and more, providing real-time data that can be analyzed and acted upon.5 The sensor market has expanded significantly to cater to the growing demands of IoT applications. Innovations in sensor technology, including miniaturization, increased sensitivity, and reduced power consumption, have enabled the proliferation of IoT devices and applications.6
As a vital application in IoT context, digital health applications are currently expanding, which comprise developments on novel technologies for healthcare, especially remote healthcare and smart sensors and actuators for monitoring health conditions and mitigating locomotor disorders.7 The population aging throughout the years, where there is an exponential growth in the elderly population (over 65 years), motivates such growth in digital healthcare.8 The global demographic transition presents fresh challenges across various domains. From an economic standpoint, the growing elderly population heightens the strain on pension systems, particularly when coupled with a diminishing ratio of elderly individuals to those in the working-age bracket.9 Additionally, there is a distinct healthcare challenge associated with the aging population, characterized by age-related conditions such as immunosenescence, urological changes, and sensory impairments, encompassing issues like hearing loss, declining visual acuity, and degraded vestibular function.10
The technology of sensors, wireless communications, and data analysis has made significant advancements in recent years. As a result, the assessment of users' health is no longer limited solely to the clinical environment.11 This enables the monitoring of various physical and physiological parameters of patients not only in clinics but also at home, where continuous monitoring of human activities is desirable for remote care, including diagnosis, emergency transportation, and monitoring the patient's health status.12 Concurrently, there is a growing development of advanced materials resulting in 2D materials and heterostructures and metamaterials capable of responding to different optical, electromagnetic, chemical, thermal, and mechanical stimuli.13 In this context, intelligent photonic textiles have been proposed for user monitoring using a fully wearable and portable technology. From a certain perspective, these photonic textiles, when incorporated into clothing, can also be considered multi-purpose devices (or structures) because they can simultaneously act as clothing and sensors.14 It is also important to mention that optical fibers are increasingly employed in the development of biosensors, through which various biomarkers, hormonal parameters, and physiological parameters, in general, can be measured. Thus, the use of artificial intelligence (AI) and cloud computing, combined with optical fiber sensing technology, can result in real-time, accurate, secure, and intelligent decision-making systems within the field of healthcare.15
Considering the wearable technologies for healthcare applications, there are a number of components to be analyzed and designed.16 To that extent, the role of optical fiber sensors in wearable devices as well as in the new generation of healthcare applications and human life improvements is not only limited to telecommunications and local passive optical networks, but also in sensor technologies and their integration into different structures of everyday life, including accessories,17 textiles18 and home devices.19 In this case, the components for a specific application generally involve the transduction element, the signal acquisition and conditioning circuits, the batteries and the system integration for the desired application,20 where the latter can be intrusive applications or unobtrusive ones without the same restriction on biocompatibility. The contribution of this review is the overview and comparison of all components in an optical fiber sensor system, including not only the optical fiber sensing approach, but also the functionalization techniques. In addition, the review includes the discussion of the applications as well as the possibilities of textile integration and novel intrusive applications, including ingestible sensors. As another contribution, this review also includes the energy harvesting approaches with the possibility of achieving a novel self-powered optical fiber sensor system for biomedical applications.
This paper presents a review of the wearable sensor technologies focused on optical fiber sensors for healthcare and physiological parameter assessment. This review includes fundamental aspects on optical sensor technologies, including applications, integration into components, functionalization and energy harvesting. After this introduction in section 1, the remainder of this review paper is divided as follows. Section 2 presents an overview of the population aging and the main musculoskeletal disorders, which lead to the main demands and opportunities in wearable and healthcare sensor technologies. Then, section 3 presents optical fiber sensors with their main technologies, transduction mechanisms and implementation scenarios. Section 4 discusses the functionalization of sensor devices for the assessment of different physiological parameters. Batteries and new energy harvesting technologies are presented in section 5 as a possibility of the development of a new generation of self-powered sensor devices. Thereafter, sensor integration into textiles and components is discussed in section 6 with the latest advances in such areas. In another application scenario, biosensors (including intrusive applications and ingestible sensors) are discussed in section 7. Finally, conclusions and future work suggestions are presented in section 8.
2 Overview of population aging and wearable sensor applications
Aging is a natural process involving changes characterized by physical and cognitive modifications resulting from the passage of time.21 The bodily changes occurring in this process lead to the deterioration of sensory (visual, vestibular, and somatosensory), musculoskeletal, and neuromuscular systems.22 These aspects can result in a decreased capacity to adapt to functional overloads, reflecting in postural control, which may predispose to fall occurrences.23,22The World Health Organization (WHO) states that the elderly population represents 20% of the global population, and the number of elderly individuals (above 65 years old) is projected to reach 1.5 billion by the end of 2050.24 This fact is associated with the progressive advancement of preventive medicine, consequently leading to an extended life expectancy. However, along with increased life expectancy comes a rise in chronic-degenerative diseases, with their metabolic, physical, and emotional consequences, directly impacting the functionality, autonomy, and quality of life of many seniors.25
Therefore, there is an urgent need for the development of public policies and technological resources that directly contribute to the improvement and maintenance of the health of this population segment.26 Geriatric physical therapy is a specialized field that examines movement and functionality in elderly individuals. Through clinical assessments, it is capable of testing and quantifying different types of alterations stemming from kinematic functional disorders.27
However, test protocols are currently carried out manually, relying on the evaluator's expertise. Moreover, the lack of quantitative data diminishes the precision of protocol outcomes.28 As a result, there arises a need for sensory technologies capable of quantifying data and expressing more accurate and reliable results.
Considering that the elderly present greater physical and motor limitations, the risks of domestic accidents also increase. Approximately 35% of individuals (above 65 years old) experience one or more falls per year, which can lead to severe injuries and hospitalization with substantial costs to public health.29 In this context, wearable sensor technology can aid in improving the overall quality of life for the elderly by remotely monitoring physiological signals, seeking to mitigate the consequences and sequelae of falls.30,31
The primary advantage of wearable systems is their ability to collect data outside of laboratory and clinical environments.32 Hence, such systems are viable for analyzing fall detection and other physiological changes like blood pressure, heart rate, oxygen saturation, etc.31,33 In this regard, the IoT presents a promising solution to provide continuous, objective, and holistic monitoring, alleviating the burden not only on human caregivers but also reducing the risk of alterations related to physiological parameters, thus aiding in clinical decision-making.34
The increase in the elderly population and life expectancy, along with the consequent rise in the prevalence of chronic diseases, necessitates improvements in healthcare aimed at the well-being of this population.35 Although there are many assessment protocols aiding in prevention, disease diagnosis, and rehabilitation, almost all of them are conducted manually and based on qualitative data, which diminishes result accuracy.28
One of the leading causes of elderly hospitalization is falls. Falls are considered a serious public health issue, potentially resulting in severe injuries such as fractures, mobility changes, functionality alterations, and fear of falling again.36 One validated approach in the literature to mitigate harm and prevent falls is the assessment of the elderly's fall risk using the timed up and go test experimental protocol.37 Although a simple and widely used test by physiotherapists to assess mobility and fall risk in the elderly, this protocol is executed manually, potentially leading to low analysis precision and the loss of important kinematic parameters.38
Thus, wearable devices capable of conducting quantitative analyses of health assessment protocols, with automatic, portable, remote data collection and temporary data storage, as well as data transmission, can contribute to better physiological monitoring of the elderly and, consequently, improve preventive health measures and aid in clinical diagnosis and rehabilitation. Fig. 1 shows an illustration of the role of wearable sensors in healthcare applications concerning the human being aging. These sensors enable the measurement of vital signs that can be used to automate healthcare protocols and provide data-driven decisions aiming to enhance the efficiency of medical treatments and the patient's recovery.
 | ||
| Fig. 1 The role of wearable sensors in healthcare applications concerning the human being aging illustration. | ||
3 Flexible optical fiber sensor development
A flexible optical fiber sensor uses optical fibers as the sensing element and is designed to be flexible and bendable. These fibers can be easily shaped and molded to fit into tight spaces or conform to curved surfaces.39 The use of these sensors is common in applications where traditional sensors are not suitable for use due to their rigidity or size limitations. Physical and chemical properties can be monitored in harsh, underground, or subsea environments using flexible optical fiber sensors withstanding high temperatures, chemical corrosion, and electromagnetic interference.40In order to manufacture optical fibers, a variety of materials can be used, including silica glass, polymers, multimaterials (combinations of materials, such as silicon and silica, metals and silica, and polymers and chalcogenides, for example),41 and biological materials (spider silk42). Polymers and silica glass are the most common materials.43 There are distinct advantages and limitations to using silica glass fibers and POFs for the development of flexible optical fiber sensors. Due to their low attenuation, high transparency, and chemical inertness, silica glass fibers are ideal for high-performance optical sensing applications and sensor data transmission over long distances. The brittle nature of these materials, however, poses a challenge for applications that require intricate bending or shaping. On the contrary, POFs, such as those made from materials such as poly(methylmethylsiloxane) (PMMA), are highly flexible and biocompatible, making them suitable for applications requiring comfort and conformability, such as wearable health monitoring sensors. Furthermore, polymer fibers are easier to handle and fabricate than silica glass fibers, which facilitates the design of more versatile sensors. The attenuation of polymer fibers may be greater and their thermal stability may be lower than that of silica glass fibers, which may adversely affect their performance in certain sensing applications. Therefore, it is important to balance the trade-off between optical performance, mechanical flexibility, and ease of fabrication for flexible optical fiber sensors when choosing between silica glass and POFs.43–46
A multimaterial approach to optical fiber development offers a pathway for improved performance, increased functionality, and customization. Multimaterial optical fibers can achieve improved transmission efficiency, expanded bandwidth, and reduced signal loss by combining different materials with complementary properties. With these fibers, multiple functionalities can be integrated into a single structure, enabling applications such as fiber amplification and nonlinear optical processes. Designers are able to tailor the properties of multimaterial fibers in order to meet specific application requirements, such as controlling the refractive index and managing dispersion. Multimaterials foster innovation in optical fiber technology, enabling new functionalities in optical communication, sensing, and high-energy lasers by pushing the frontiers of performance and enabling new material combinations.47,48
3.1 Intensity variation sensors
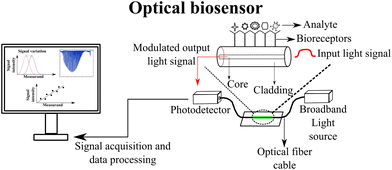
The working principle of optical fiber sensors based on intensity variation consists of the measurement of the intensity of transmitted or reflected light. This is done when the optical fiber cable is subjected to a stimulus such as temperature, strain, or chemical reaction.45 These external parameters modulate the analyzed output signal and correspondences can be established between the monitored parameter and the intensity of the transmitted or reflected light in sensing applications.45 They can be used for different applications, including structural health monitoring,49 biosensor development,50 and healthcare applications.51 Rocha, Matilde, et al. have attached POFs to a handspring, and the sensor's force characterization was evaluated with a tensile tester. The sensor's sensitivity was characterized by force variations caused by decreasing the distance between the two handles of the handspring. At each step, the voltage (correlated to optical power intensity) was collected using an Arduino. The test was performed for the decrease of the distance between the two handles, and the voltage was collected at each step. The respective intensity of the force applied to the handspring handle was recorded, and the voltage was correlated to the optical power intensity variation.52 In another application, plantar pressure sensors were designed using POFs integrated into a commercial insole. The optical power intensity was characterized with respect to load application for monitoring plantar pressure distribution for ground reaction forces monitoring during the gait.53Intensity variation-based optical fibers can also be multiplexed for quasi-distributed sensing applications. The use of time-division multiplexing (TDM) of the sensors in ref. 54 is one possible approach, where the sensors are placed at different distances from the source and detector, so that, upon input, a single pulse of the appropriate duration produces a series of distinct pulses. As the sensors' outputs are interleaved in a time sequence, these pulses represent time samples of their outputs. The required duration of the input pulse is determined by the effective optical delay of the fiber connecting the sensor elements. With the repetitive pulsing of the system, each sensor can be addressed by time-selective gating of the detector output. There is also the possibility of side-coupling the light source in a POF (POF) sensor with a lateral section, which is reported in ref. 55. As a result of this approach, each lateral section is an intensity variation-based sensor, and each sensor has its own source of light. Each end of the fiber is equipped with two photodetectors. Light-emitting diodes (LEDs) are activated by microcontrollers, and signals are acquired when the LEDs are active. In order to avoid simultaneous activation of two or more light sources, the LED array is activated according to a predetermined sequence and frequency. Consequently, each LED is turned on one at a time. When the corresponding LED is active, the signal from each photodetector is acquired. This results in a matrix with P × D vectors, where D is the number of LEDs and P is the number of photodetectors. As a result, it is possible to decouple the effects of different parameters on each lateral section of the fiber. Experimental validation of the sensor has been conducted for three degrees of freedom applications involving simultaneous measurements of temperature, force, and angle. Fig. 2 shows a schematic representation of the working principle of intensity variation-based optical fiber sensors. For healthcare applications, the optical fiber sensor is usually embedded into a flexible matrix responsible for protecting the cable and inducing optical loss when the sensing region is bent or stretched (or coupling LEDs in different regions along the optical fiber cable). These optical power losses modulate the output optical power intensity and they can be characterized according to the desired measurand. This technique can be employed in healthcare applications such as plantar pressure monitoring,51 smart pants for pose classification and movement intensity measurement,56 and smart carpet for gait analysis.19
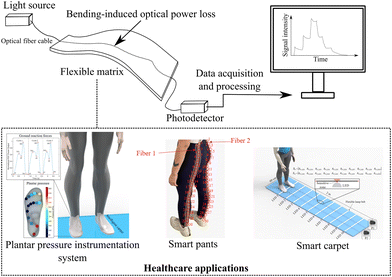 | ||
| Fig. 2 Intensity variation-based optical fiber sensors' working principle schematic representation and health care application examples. Reproduced from ref. 19, 51 and 56 with permission from Optica Publishing Group, copyright 2023, MDPI AG, copyright 2019, MDPI, and MDPI AG, copyright 2019, respectively. | ||
3.2 Fiber Bragg gratings
Uniform fiber Bragg gratings (FBGs) are periodic perturbations of the refractive index along the optical fiber length. They are formed by exposing the fiber core to an intense light interference pattern. The resulting permanent grating acts as a narrowband reflector, reflecting a specific wavelength of light while transmitting all other wavelengths.57 FBGs have found key applications in routing, filtering, control, and amplification of optical signals in high-capacity wavelength division multiplexing telecommunication networks.58 They are also used in fiber optic sensors and quasi-distributed thermophysical measurements.59 FBG sensors can be utilized in the design of flexible optical fiber sensors by encapsulating them with protective materials to ensure their functional integrity and survivability under harsh conditions or by engraving the Bragg gratings in POFs.60,61 These sensors can be used in structural health monitoring of civil infrastructure, such as bridges, tunnels, and buildings,62 mechanical deformation and temperature fluctuation monitoring in asphalt pavement,63–65 temperature and dynamic strain in electrical machine monitoring,66 real-time monitoring of the curing process of resin-based carbon fiber reinforced plastic and aluminum alloys,67 and measurement of deformation accumulation and elastic recovery of materials in asphalt roads.68 The reflected wavelength (known as Bragg wavelength) is proportional to the grating period (distance between subsequent gratings) and the effective refractive index, where these parameters change when the optical fiber is subjected to strain and/or temperature variations where a relationship between the Bragg wavelength and the monitored parameter is established in sensing applications.69In addition to uniform FBGs, there are non-uniform gratings such as long-period fiber Bragg gratings, chirped fiber Bragg gratings, phase-shifted fiber Bragg gratings, and tilted fiber Bragg gratings (TFBGs).70 TFBGs are a type of fiber optic sensor that consists of a fiber Bragg grating with a certain angle between the grating plane and the fiber axis. This angle causes the grating to have a different structural geometry along the radial, azimuth, and axial orientations.71 This makes TFBGs a candidate for multifunctional fiber-optic components with distinct characteristics. In TFBGs, tilting the Bragg grating causes non-uniform spatial compression (or elongation) of the grating pitch. This is manifested as a decrease (or increase) in the tilt angle of the grating plane.72 Due to this non-uniform spatial compression, the spacing of the grating plane changes along the fiber cross-section, with the minimum occurring in the center. Due to the non-uniform tilt plane modulation and the stress-induced refractive index variation along the fiber cross-section, the phase matching condition for each pitch value is partially disturbed.70
TFBGs have a significantly weaker mode coupling between the core and the cladding, and the cladding mode coupling changes significantly, allowing high-sensitivity bending response measurements to be conducted in the cladding mode.73 The resonance wavelengths of TFBG core and cladding modes are sensitive to temperature, while their transmission power is temperature-independent. The transmission power of cladding modes, however, is bending-dependent, while the resonance wavelengths of both the core mode and cladding mode are bending-sensitive. As a result of these unique properties of TFBGs, it is possible to discriminate between bending and temperature effects simultaneously.74 TFBGs can be used as sensors in various applications such as structural health monitoring,75 biomedical sensing,76 and chemical sensing.77 Similar to uniform FBGs, TFBGs can be used as flexible optical fiber sensors encapsulating the TFBG sensors in flexible structures ensuring sensor integrity while the bending resistance is increased.78–80 In these applications, the higher sensitivity to bending compared to uniform FBGs is a key feature in high-sensitivity flexible optical fiber sensors based on TFBGs, and it can be employed for real-time monitoring of human breathing,81 hydrogen sensors,82 and biochemical sensors.83Fig. 3 shows the FBG working principle and some applications. In these sensors, part of a broadband incident light coupled into an optical fiber cable is reflected by the Bragg gratings. The reflected wavelength (called Bragg wavelength) is affected by variations in strain and temperature.84 For sensing purposes, FBG sensors can be embedded in different structures in healthcare applications for gait analysis,85 joint angle measurement,86 pressure plantar monitoring,87 and smart walker instrumentation88 as examples.
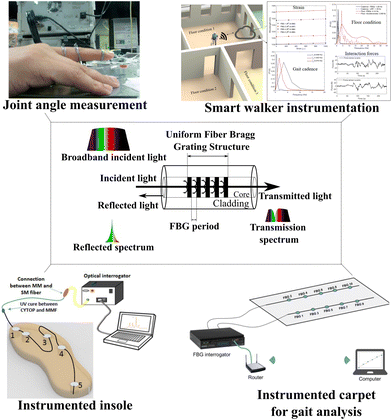 | ||
| Fig. 3 FBG working principle and healthcare applications using FBG examples. Reproduced from ref. 85–88 with permission from IEEE, copyright 2023, MDPI AG, copyright 2014, MDPI AG, copyright 2017, and IEEE, copyright 2019, respectively. | ||
3.3 Interferometers
The most common interferometer techniques in optical fibers include the Michelson interferometer, Mach–Zehnder interferometer, Fabry–Perot interferometer, and Sagnac interferometer.89 In the Michelson interferometer, an input light signal is split into two different paths by a splitter (coupled into two separate optical fibers, for example). These paths contain mirror elements, such as Bragg gratings. The reflected signals of the two paths are recombined, creating an interference pattern. This interference pattern is affected by external perturbations such as temperature and strain variation. It can be characterized according to the interest parameter and used as a sensor.90,91 The working principle of the Mach–Zehnder interferometer based on optical fibers consists of splitting a light signal into two different paths by means of splitters, where one arm of the interferometer serves as a reference and the other is utilized as the sensing element. The split signal is then recombined and an interference pattern is created. This interference pattern is affected by external perturbations applied to the sensor arm. It can be used for sensing applications through the characterization of the interference pattern with respect to the variation of the monitored external parameter.92,93In optical fibers, Fabry–Perot interferometers work by interfering with light waves that are reflected back and forth between two parallel mirrors (such as two Bragg gratings separated by cavities). In the Fabry–Perot cavity, light from a source is transmitted through an optical fiber, where it is reflected between the two mirrors. An optical power detector receives the reflected light through the fiber. During the reflection of light waves, constructive and destructive interference produces the interference pattern. As the cavity length can be altered by environmental factors, such as temperature or strain, the Fabry–Perot interferometer is suitable for use as a fiber sensor.94,95 Sagnac interferometers are based on the Sagnac effect, which is the phase shift of light propagating in a rotating reference frame. This interferometer splits a light beam from a source into two beams that travel in opposite directions around a closed loop, typically a fiber optic coil that maintains polarization. As the two beams recombine at a detector, the phase difference between them produces the interference pattern. The two beams have the same phase when the loop is stationary, but when the loop rotates, the phase of one beam is shifted relative to the other beam due to the Sagnac effect. As the phase shift is proportional to angular velocity, the Sagnac interferometer can be used as a rotation sensor.96 Moreover, external perturbations imply a phase shift of the transmitted optical signal and it can be used for monitoring external parameters in sensing applications.89,97,98 For all aforementioned interferometers, the optical fiber cables can be attached to flexible structures to develop flexible optical fiber sensors for temperature monitoring,99 mechanical vibration sensors,100 and breathing sensors,101 as examples. Fig. 4 shows a schematic representation of the optical circuit of Mach–Zehnder, Michelson, Sagnac, and Fabry–Pérot interferometers in optical fibers. Furthermore, by analyzing the modulation of the interference pattern for external perturbations, these techniques can be used in the monitoring of respiration,102 the arterial pulse wave sensor,103 the monitoring of human physiological activity104 and the detection of thrombus,105 as examples (shown in Fig. 4).
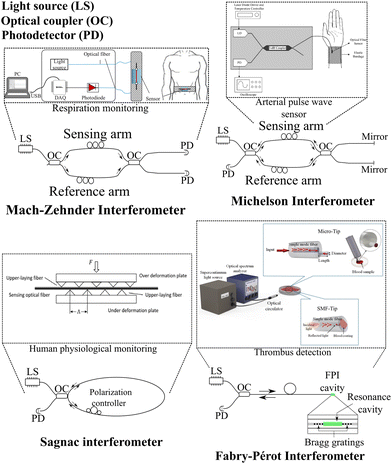 | ||
| Fig. 4 Optical circuit representation of Mach–Zehnder, Michelson, Sagnac, and Fabry–Pérot interferometers in optical fibers and healthcare application examples using these techniques for sensing applications. Reproduced from ref. 102–105 with permission from Optica Publishing Group, copyright 2019, John Wiley and Sons, copyright 2010, IOP publishing group, copyright 2021, and MDPI AG, copyright 2023, respectively. | ||
3.4 Distributed optical fiber sensors
In optical fiber distributed sensing, the most common techniques are based on Rayleigh, Brillouin, and Raman scattering. They use different reflectometry and demodulation schemes, including optical time-domain and optical frequency-domain reflectometry.106 Rayleigh scattering is based on the inhomogeneity of the fiber medium, causing localized variations of density which result in fluctuations of refractive index. It is an elastic scattering process, which means that scattered light has the same frequency as the incident light. The intensity of the backscattered light is proportional to the local refractive index of the fiber, which is affected by external perturbations such as temperature, strain, and pressure. By analyzing the backscattered light, it is possible to determine the location and magnitude of perturbations along the fiber.107 Raman scattering occurs when light interacts with the vibrational modes of a material, resulting in a shift in the frequency of the scattered light. In optical fiber sensing, Raman scattering can be used to determine the location of an event by measuring the arrival time of the scattered light. Raman scattering is affected by temperature and strain perturbations. This results in a temperature and strain-dependent scattering cross-section and spontaneous Raman scattering that can be used for distributed sensing.108 When light interacts with acoustic waves in a material, Brillouin scattering occurs, resulting in a shift in the frequency of the scattered light. In optical fiber sensing, Brillouin scattering can be used to determine the Brillouin frequency shift by measuring the central frequency of the Brillouin scattered light, which is linearly dependent on both strain and temperature. By measuring the scattered signal time, the external perturbation can be localized along the optical fiber length.108Optical-frequency domain reflectometry (OFDR) works by sending a probe signal into an optical fiber and measuring the backscattered light as a function of frequency. It is possible to obtain information about the optical fiber by analyzing the frequency-dependent backscattered light, such as the location and magnitude of any changes in the fiber's refractive index or scattering properties. In this manner, high spatial resolution and sensitivity can be achieved with distributed sensing along the length of the fiber. OFDR has many applications, including monitoring structural health, measuring temperature and strain, and detecting and locating fiber faults.109 The working principle of optical-time domain reflectometry (OTDR) for distributed optical fiber sensing is based on the observation of coherent Rayleigh backscattering from a sensing fiber in the time domain. The backscattering field from each scattering element interferes with the receiver when light from a coherent pulse reaches the scattering elements. This backscattering field results in a distributed coherent speckle pattern whose local phase and intensity depend on local disturbances when measured over the entire fiber length. As a result of analyzing backscattered light changes, it is possible to detect and locate disturbances along the length of the fiber.110 Transmission-reflection analysis (TRA) is another approach to distributed sensing with optical fibers. Using an unmodulated continuous wave light source, it measures the transmitted and backscattered optical powers directly. In distributed monitoring systems, this technique is used to track single perturbations. The TRA method is simpler and less expensive than other methods including OTDR and OFDR, which require modulated light sources and require fast electronics to achieve high resolution.111,112Fig. 5 shows a schematic representation of the working principle of distributed sensors in optical fibers. In these sensors, part of incident light is scattered and this reflected signal can be used to measure external perturbations. They can be employed in different healthcare applications, such as upper limb mechanical perturbation detection,18 liver tissue temperature distribution measurement,113 and shape-sensing guiding system.114Table 1 summarizes the key information about the optical fiber sensors employed in healthcare settings discussed in this chapter.
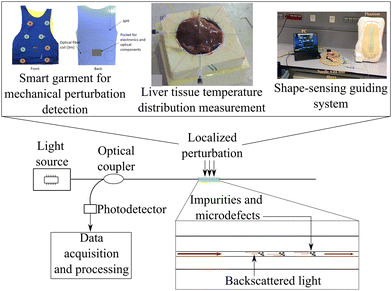 | ||
| Fig. 5 Optical fiber distributed sensor working principle schematic representation and examples of healthcare applications. Reproduced from ref. 18, 113 and 114 with permission from IEEE, copyright 2020, IOP Publishing Group, copyright 2018, and Elsevier, copyright 2024, respectively. | ||
| Application | Optical fiber material | Sensor sensitivity | Sensor technique | Ref. |
|---|---|---|---|---|
| Plantar pressure monitoring | Polymer | — | Intensity variation | 17 |
| Smart pants for pose classification | Polymer | Ranging from 1.43 mV mm−1 to 568.09 mV mm−1 | Intensity variation | 56 |
| Smart carpet | Polymer | — | Intensity variation | 19 |
| Joint angle measurement | Polymer | 0.0229 V Å−1 | Intensity variation | 115 |
| Joint angle measurement | Silica | 1.20 pm με−1 | Bragg gratings | 116 |
| Smart walker instrumentation | Polymer | 1.57 ± 0.15 pm με−1 for strain and 20.17 pm °C−1 for temperature | Bragg gratings | 88 |
| Instrumented insole | Polymer | From 7.71 pm kPa−1 to 8.51 pm kPa−1 for pressure and from 18.1 pm °C−1 to 18.9 pm °C−1 for temperature | Bragg gratings | 87 |
| Instrumented carpet for gait analysis | Polymer | — | Bragg gratings | 117 |
| Respiration monitoring | Silica | — | Mach–Zehnder interferometer | 102 |
| Arterial pulse wave measurement | Silica | — | Michelson interferometer | 103 |
| Rapid monitoring of human physiological activity | Polymer | — | Sagnac interferometer | 118 |
| Thrombus detection | Silica | 7 nm μL−1 for the micro-tip fiber type and 8.7 nm μL−1 for the single-mode fiber type | Fabry–Pérot interferometer | 105 |
| Respiratory rate monitoring | Polymer | 0.35 nm/% | Optical time domain reflectometry combined with Bragg gratings | 119 |
| Liver tissue temperature distribution measurement | Silica | 10 pm °C−1 | Optical backscatter reflectometry | 113 |
| Protein concentration quantifying | Silica | Ranging from 2.6 nm RIU−1 to 19.7 nm RIU−1 | Optical backscatter reflectometry | 120 |
4 Optical biosensor functionalization
Generally, as the next step after the definition of the transduction mechanism to be used (among the different ones in optical fiber sensors121), functionalization plays a key role on biosensor development.122 In this case, the functionalization methods and common approaches to achieve higher selectivity and sensitivity on the biosensors are discussed below.Surface chemical functionalization is one of the most common strategies to modify the surface of materials used as a sensor. It is a simple and effective approach to altering the surface properties of a material to achieve a specific goal.123 The success of a biosensor in detecting a specific analyte will depend on how effective its surface functionalization process is. This means that both the adhesion mechanism of a receptor on a substrate (transducer) and the selectivity provided by it are fundamental steps in the functionalization of a surface and in the construction of a sensory device.
Briefly, the functionalization of a surface to act as a biosensor has important aspects, such as the orientation of the receptor, the inert area, and the total area. When united, these aspects result in fundamental parameters in the detection of an analyte. The first one is the sensitivity, which is defined as the variation of the sensory signal as a function of the variation of the analyte; and the second is selectivity, which is defined as the difference in response under a variety of different analytes.124
Plasma-based biosensing can be performed either by means of a frequency associated with the surface plasmon resonance in the presence of an analyte (thin film-based biosensors), or by the intensity of the signal associated with the analyte (nanostructure-based biosensors).124,125 These plasmonic nanostructures can be obtained by different approaches, namely: top-down and bottom-up. Considered the most expensive and time-consuming, the top-down approach leads to the fabrication of periodic nanoarrays by more complex techniques, such as lithography, laser ablation, ion milling, and chemical etching. In other words, in the top-down approach, bulk materials are reduced to nanoscale materials by chemical, mechanical and physical methods. The bottom-up strategy is the most commonly used because it is a low-cost, large-scale approach. It results in the non-periodic or quasi-periodic arrangement of nanostructures, obtained from the synthesis of self-assembled plasmonic nanoparticles on transparent and reflective substrates. That is, the bottom-up approach atomic or molecular substances are assembled through physical processes or chemical reactions to produce nanoscale materials. Although this approach is less sensitive than biosensing, it can be applied in a scalable way to ensure greater use of the technique. Manufacturing techniques include physical or chemical vapor deposition and evaporation, molecular beam epitaxy, and bio/chemical processes (production of supramolecular complexes, self-assembled monolayers, and protein–polymer nanocomposites).126–128
Several materials with plasmonic properties can be used for biosensing, among which metals, oxides and nitrides can be mentioned. Noble metals such as palladium, gold and silver have excellent plasmonic properties that help to disperse resonance and absorb light in the infrared and visible regions, but have disadvantages such as high cost or ease of oxidation, like silver.124,129 Aluminum has low cost and process compatibility with silicon technologies and exhibits plasmonic resonance in the ultraviolet range. Despite being a promising metal compared to others, aluminum has high chemical instability and easily forms oxides, which decreases the effect of plasmonic resonance.130–132 Copper has near-infrared plasmonic resonance, low cost and high plasmonic conductivity, but like aluminum, its surface can be easily oxidized and the plasmonic effect decreases.130,133,134 Semiconductor materials, such as metallic oxides, metallic chalcogenides, metallic nitrides, and silicon, also exhibit plasmonic properties that result in absorption, scattering and near field enhancement in nanostructures. Unlike metals that have a high concentration of carriers and natural resonant frequencies, the concentration of carriers in semiconductors is tunable by the doping procedure, allowing control of the resonant frequencies from visible to far-infrared.130,135
SAMs can be obtained by various combinations of adsorbate–substrate pairs, such as normal alkanoic acids on aluminum oxide or silver, alkylsilanes on hydroxylated surfaces, disulfides on gold, alkanethiols on coinage metals such as platinum, gold, silver, and copper, and non-metals such as gallium arsenide (GaAs), indium phosphide (InP), and indium tin oxide.136 Among these, it is likely that SAMs based on alkanethiols on gold are one of the best options in biosensing because they show great advantages in device fabrication, such as flexibility and stability. This occurs because the bond between gold and sulfur (substrate–adsorbate) has a high affinity for each other and, therefore, it is difficult to desorb the organosulfur species (binding enthalpy is between 167 and 188 kJ mol−1).137
Detection based on bioreceptors has become an excellent detection alternative to more advanced analytical techniques, such as scanning electron microscopy,138 transmission electronic microscopy,139 Raman and infrared spectroscopy,140 among others. Bioreceptors are key components in the detection of an analyte of biological (e.g. cortisol141 and cholesterol142) or synthetic (e.g. micro- and nanoplastics143) origin and play a fundamental role in the development of biosensors. These components are constituted by molecules or biological elements that undergo a conformational change or a biochemical reaction when they recognize a certain analyte. This phenomenon generates a type of signal (optical, electrochemical, thermal, acoustic, etc.) that can be transduced into an electrical signal and processed by electronics.127,144
To achieve a highly effective biosensor that is able to accurately recognize its target, some requirements are necessary that are directly related to its bioreceptor. The selectivity of a biosensor depends specifically on the bioreceptor's compatibility with the analyte and ability to distinguish it from other contaminating species that are mixed in the sample. Stability also plays a key role in biosensing when it requires the analyte to be continuously monitored. In this case, the stability of the biosensor against environmental weather conditions requires that the bioreceptor has affinity with the analyte and that it does not suffer degradation over time.127
Since bioreceptors are primary components in the construction of biosensors (alongside transducers and electronics/display), they provide a broad category based on the principle of biorecognition.145 The first of these is called catalytic biosensors, in which their receptors interact with the analyte and result in a new product of a biochemical reaction.146 Receptors based on enzymes, cells, tissues and microorganisms are considered catalytic biosensors.147 On the other hand, non-catalytic biosensors (commonly called affinity biosensors) are those in which the analyte irreversibly binds to the receptors, preventing any biochemical reaction.146 Antibodies and aptamers (DNA and RNA) can be classified as non-catalytic biosensors.148 Next, the main types of bioreceptors found in the literature are summarized and how their mechanism of biorecognition with the analyte occurs.
1. Enzymes: enzymes are globular proteins composed of amino acids and whose function is to catalyze biochemical reactions. They are elements widely used as bioreceptors due to their high capacity for biorecognition and excellent catalytic properties when selectively reacting with the substrate. Enzyme-based biosensors have a recognition mechanism whose detection efficiency depends on the catalytic activity of the enzyme and its binding capacity. The product of the biochemical reaction with the analyte can be detected directly or in the presence of an indicator.149–151 Approaches to improve the use of enzymes in biosensors can be done by genetic and chemical modification.146 Genetic modification consists of facilitating the access of the substrate to the active site of the enzyme through fusion protein technology or site-directed mutagenesis.152 Chemical modification is used to change the properties of enzyme key residues or the overall surface153 and approaches such as site-specific chemical modification, nonspecific modification of the enzyme surface, chemical cross-linking, and use of polymers can be cited.146
A greater number of enzymes with specific biorecognition elements have been used for direct optical detection of disease biomarkers, such as glucose, cholesterol, urea, creatine, lactate (further information is described elsewhere154). However, a direct-assay-type SPR biosensor has been constructed in recent years to detect chiral amino acids.155 Albeit chiral recognition techniques are widely used, ultra-low concentration of amino acid isomers still remains a challenge for detection. The enzyme Rasamsonia emersoniiD-amino acid oxidase was used as the receptor for capture of the specific D-amino acid enantiomer. The detection of the linear range and the signal amplitude has been improved when gold nanorods and graphene oxide were combined on gold film and used to fabricate a sensor assembled with the enzyme. As a result, a limit of detection of 1.09 × 10−9 mM was achieved, providing a strong capability to determine the composition of the isomer mixture.
2. Cells: cell-based biosensors consist of genetically modified living cells and can be of both animal and microbial origin (bacteria, fungi, algae, protozoa and viruses). Due to their ability to survive in harsh environments, similar to bacteria and fungi, whole-cell biosensors are used to detect specific analytes under extreme conditions, such as low pH, high temperatures, presence of pollutants, etc. While many microbial cell-based biosensors utilize the natural ability of a microorganism to detect a specific analyte, animal cell-based biosensors are more complex due to their growth rate, morphological and structural characteristics, and nutritional requirements.127,156,157 Microorganism immobilization techniques can be described as physical methods, such as adsorption and entrapment, or chemical methods, like chemical binding and cross-linking.158 The immobilization matrix of microorganisms also plays an important role in their immobilization and can be reviewed elsewhere.159
Different microorganisms, such as bacteria, algae and yeasts, have been used in microbial biosensors in environmental monitoring applications and antimicrobial susceptibility testing. Tahirbegi et al. developed a fast pesticide detection method through a microfluidic device with integrated optical pH, oxygen and algal fluorescence for in situ analysis. The detection of pesticide concentration was made via metabolism/photosynthesis of Chlamydomonas reinhardtii algal cells in tap water. The device provides a fast quantification of pesticides in less than 10 min and detection in nanomolar concentrations.160 However, in the case of animal cells, the growth rate, nutritional requirement and structural characteristics are very different from those of microbial cells. Therefore, this specific kind of cell-based biosensor needs proper design and suitable fabrication.161 For example, Caluori et al. placed cardiomyocytes on a microelectrode array to record the beating-force of diseased cardiomyocytes through atomic force microscopy.162
3. Antibodies: antibody-based bioreceptors (or immunosensors) are extensively used as diagnostic tools due to their excellent specificity and affinity with their cognate antigen. These biosensors have antibodies as biorecognition components and use the antibody–antigen interaction as an analyte detection mechanism. Antibodies are proteins composed of two heavy and two light peptide chains, and have the shape of a Y, through which interactions occur. Typically, these interactions develop between antibody paratopes and antigen epitopes and allow multiple recognitions to take place according to the nature of the antibody.163–165 Technically, the immobilization of antibodies on substrates can occur using two different approaches, namely random and site-directed antibody immobilization. The first consists of a simpler method of random adsorption of antibodies onto a surface, while the second approach improves the availability of the antigen binding site.166
Recently, Ucci et al. designed a lab-on-fiber optrode assisted by an oriented antibody immobilization strategy.167 Lab-on-fiber technologies integrate functional materials onto optical fiber substrates at the micro and nanoscale to provide a multifunctional all-in-fiber device for many applications, ranging from optical processing, safety, and environmental monitoring.168 The authors investigated the immobilization strategies to improve them through the use of hinge carbohydrates by involving homemade antibodies. First, an optimized protocol using microfluid surface plasmon resonance was created and then transferred to the lab-on-fiber platform to improve the biofunctionalization protocols. The capability of antigen recognition in ultra-low detection limits was achieved and the proposed oriented antibody immobilization strategy was able to detect Cripto-1 at a concentration of 0.05 nM, i.e., a 10-fold enhancement compared to the random approach.
4. Aptamers: aptamers are synthetic nucleic acids (DNA and RNA sequences) formed by small single-stranded oligonucleotides that bind to their cognate target (proteins, peptides, amino acids, drugs, metal ions and whole cells) with high affinity and selectivity. The high affinity is due to its ability to be able to integrate into the structure of macromolecules or to incorporate small molecules into its own structure. The high stability of aptamers over a wide range of temperature and pH, the possibility of chemically synthesizing them and the ability of thermal refolding bring several advantages to biosensors based on aptamers.169–173 As approaches for the immobilization of aptamers on a gold surface, the techniques that have recently been used can be cited, such as immobilization via direct thiolation or thiolated short linkers, streptavidin/biotin interaction, as well as DNA nanostructures and reduced graphene oxide as immobilization platforms. More information can be found in more detail elsewhere.174
In this regard, Ning et al. developed a J-shaped optical fiber localized surface plasmon resonance (LSPR) aptasensor able to detect pathogenic bacteria from actual water samples in one step for only 30 min without any sample pre-treatment. For the first time an LSPR aptamer biosensor was designed for the rapid and ultrasensitive detection of Helicobacter pylori (H. pylori), reaching a detection limit as low as 45 CFU mL−1 and a wide linear range from 1.0 × 102 CFU mL−1 to 1.0 × 108 CFU mL−1. A further enhancement in LSPR signal response occurs when a spacer nucleic acid with a short stem-loop structure is adopted to control the aptamer density on gold nanoparticles on the surface of the J-shaped optical fiber probe.175
In addition to the preparation of the device using functionalization mechanisms, the detection of physical and chemical quantities is performed following a transduction mechanism, some of them were discussed in section 4 for optical fiber sensor applications. It is also worth noting that the evolution of artificial intelligence associated with the IoT indicates that the use of sensors tends to increase, increasingly integrating with human beings.176
Sensors can be classified into several categories.177 Depending on the type of measurement performed and the detection signal identified, it is possible to classify different types of sensors, as shown in Table 2. Those based on recognition of biological systems are called biosensors. Receptor-based biosensors provide more efficient binding in terms of sensitivity and selectivity. In general, biosensors can be classified according to the biomolecule which can successfully identify the biological analyte, i.e., anti-bodies, aptamers or enzymes (Fig. 6), main bioreceptors.178
| Signal detection | Signal conversion | Measurand | Ref. |
|---|---|---|---|
| Physical | PhotoElastic | Acoustic wave – sound pressure (dB) | 179 |
| Piezoelectric | Physiological signals – pressure (kPa) | 180 | |
| Thermoelectric | Heat flux of human body – calorific power (W) | 181 | |
| Thermomagnetic | Temperature – radiation (Gy h−1) | 182 | |
| Magnetoelectric | Magnetic microbeads – magnetic field (kA m−1) | 183 | |
| Chemical | Chemiluminescence (CL) | Propranolol – CL intensity (au) | 184 |
| Chemiacoustic | Ammonia – S11 (dB) | 185 | |
| Chemoresistive | Breath acetone – resistance (au) | 186 | |
| Chemocapacitive | CWA and VX gas – capacitance change (%) | 187 | |
| Electrochemical | Nitrate – current (μA) | 188 | |
| Biological | Photoelectric | Urea (enzyme) – transmission (%) | 189 |
| Chemoresistive | miRNA-21(DNA) – resistance (au) | 190 | |
| Chemiluminescence (CL) | Bacteria (DNA) – CL intensity (au) | 191 | |
| Electrochemical | TCA and nitrite (Hb) – current (μA) | 192 | |
| Electrochemical | Cortisol (antibody) – current (μA) | 193 |
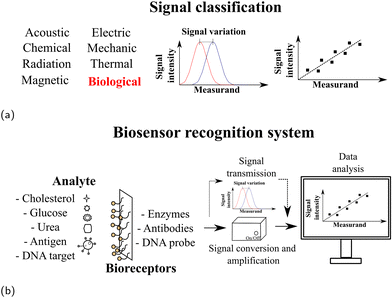 | ||
| Fig. 6 (a) Classification of sensors based on the detection signal and (b) biosensor biorecognition system. | ||
In summary, analytes contained in biological samples are bound to biological macromolecules and produce a biological signal that is passed to the transducer for further processing. Once the analytes are trapped on the surface of these bioreceptors, the interaction can cause changes in the form of light, heat, pH, mass change, charge change, etc.194
This leads to another common classification of sensors that occurs by the way the detected signals are converted for analysis, which can be optical, thermal, electrical, electrochemical or piezoelectrical mechanisms.183
In this context, the science of biosensors has become an area independent of modern analytical chemistry, due to the combination of a biological component with a physical–chemical detector.195 Optical biosensors combine the high specificity of the bioreceptor with the high sensitivity of optical transducers, i.e. the analyte/bioreceptor interaction causes changes in light parameters, specifically (Fig. 7).
Currently, the main research centers in the world's demand for biosensors with greater efficiency, precision and economy, seeking to meet the growing demand for clinical purposes related to the constant advancement of molecular biology and the need for diagnosis and disease prevention.
5 Energy harvesting for wearable sensor systems
In the development of actuators, sensors and active components, the power supply is always a significant element.196 Considering the additional demands of size and power density of wearable devices, the demands of such systems are even larger and significant research in this field has been conducted in the last few years.197 In this context, self-powered sensors and devices emerged in the last few years as a sustainable and affordable option for wearable device development.198 For this reason, different technologies for energy harvesting are proposed and we discuss some of them in this section. It is important to mention that this is not an exhaustive review in energy harvesting (interested readers are advised to check review papers focused on this topic199–202). However, piezoelectric, triboelectric, and solar concentrators, as well as thermoelectric approaches are discussed in this section.Certain crystals and ceramics have a unique way to generate energy. The phenomenon of the materials that are capable of generating an electrostatic charge when subjected to mechanical stress are known as piezoelectricity.203 The stress causes a polarization in the piezoelectric crystal, which leads to an opposite charge configuration, when the stress is released, it goes back to the initial state. The conservation of mechanical energy is satisfied generating electrical energy in an external circuit.204
The mechanical energy from movements and vibrations surrounds us in multiple ways and typically is wasted due to the lack of commercialization of the appropriate technology for its capture. This kind of technology has been studied since the end of the last century and brought recent progress that can be used into our daily life such as promising technology for medical applications and other areas where energy is limited or difficult to obtain.
An important piezoelectric energy harvesting method was proposed by ref. 205. The study focuses on the human gait as an energy harvesting model and how different loads impact the project. Also, the material selection for this kind of project is essential to obtain high quality results. Bimorphs and plectra were responsible for generating electrical energy from mechanical movements. The research concluded that the replacement of batteries with the renewable source of energy afforded by energy harvesters has reduced maintenance and consumption. Another application of piezoelectric technology is in the implantable field, such as an organ harvesting system.206 This research focuses on conformal piezoelectric energy harvesters (CPEHs) that utilize the natural contractile and relaxation movements of organs to generate electrical energy. The primary organs under analysis are the heart, lungs, and diaphragm, all of which exhibit a significant energy potential. This could enhance the applicability of such technology in biomedical monitors and wearable electronics.
The concept of tribology appears in many situations during our daily tasks. For example, wheels, trains and moving cars.207 Triboelectricity is based on the friction between different materials to generate electrostatic charges that can be harvested by an external circuit.204 Furthermore, it allows us to construct or integrate wearable power supply mechanisms. As another important approach for energy harvesting in wearable applications, the triboelectric nanogenerators (TENGs) represent an important recent technology that offers numerous advantages, including energy harvesting capabilities, biocompatibility and self-powering capabilities.208
Utilizing human biomechanical energy and TENG technology,207 a self-sustaining system that provides a continuous source of electricity for mobile electronics is proposed. The project involves a multilayered attached-electrode contact-mode TENG and a power management circuit. This specific type of TENG was designed with a Kapton film and electrodes to harness human biomechanical energy.209 The act of walking or running by humans creates contact between the Kapton film and the electrodes, generating energy. The advantage of this technology is the ability to stay connected without the need to pause and recharge devices, including wearable technology, sensors and more.
Alopecia is a common topic of debate within the scientific community. This is primarily due to the fact that 50% of all men suffer from alopecia by the age of fifty,210 prompting the exploration of new solutions for this issue. The study of triboelectric nanogenerators (TENGs) is proposed as a means of providing therapeutic electrical stimulation (ES). TENG-ES enhances hair growth cycles by promoting capillary blood flow, stimulating cellular proliferation and differentiation, and, more importantly, can serve as a self-sufficient treatment without the need for third-party equipment interventions.208
Moreover, a wearable electrical stimulation device (M-ESD) was designed by ref. 211. As is common in TENG projects, it harnesses body movements as a source of energy to enhance blood vessel growth factors and increase the number of hair follicles. The M-ESD consists of a TENG with two friction layers connected by a soft Ecoflex belt and a pair of interlaced electrodes. Such results indicate that the TENGs are not only used for energy harvesting proposes, but also can enhance the wearable and healthcare device capabilities by promoting therapeutic alternatives for different applications.
The solar energy harvesting technology involves using solar cells to convert sunlight energy into electrical energy via the photovoltaic effect.212 Solar cells are made of semiconductor junctions that have a characteristic energy level called a band gap. When incident photons have higher energy than this band gap, the solar cell generates an electrical current.213 The generated power is then optimized by a conditioning circuit and stored in an energy storage device (ESD) or used to power an electronic load.214 The input from the solar cell goes through a boost converter controlled by a Maximum Power Point Tracking (MPPT) algorithm. MPPT algorithms force the solar cells to work around their Maximum Power Point (MPP), which is normally at 80% of their open circuit voltage.213 The performance of solar energy harvesting systems depends on many factors, such as the operating conditions of the load (sensor measurements and connectivity), time, location and position of the solar cells. It is also difficult to theoretically estimate the generated power when solar cells are included in a wearable device. Therefore, testing the performance of the solar energy harvesting system under different daily life scenarios is necessary.214 Additionally, the cost of large-scale production and fabrication of energy harvesting wearable devices is a concern.
For example, they can be used to power physiological sensors that monitor vital signs such as the heart rate, blood pressure, and body temperature. This can be especially useful for patients who need continuous monitoring, such as those with chronic conditions or those recovering from surgery.215 Additionally, solar-powered wearables can be used to power wireless communication modules that transmit data from the sensors to healthcare providers, allowing for remote monitoring and telemedicine. This can improve patient outcomes and reduce healthcare costs.212
In addition, wearable luminescent solar concentrators (LSCs) can be integrated with fiber solar cells to harvest additional photons and fully utilize the advantage of harvesting light from all directions.143 LSCs are made from an amphiphilic polymer conetwork matrix and luminescent dyes, and they assist in enlarging the photon harvesting area, recycling lost photons, and utilizing the complete surface of the fiber dye-sensitized solar cells (FDSSCs). The results of the study showed a remarkable FDSSC power conversion efficiency enhancement of 84%, LSC concentration factor of 1.57, and device optical efficiency of 7.85%.143 Additionally, wearable LSCs demonstrated a sustainable power conversion efficiency even after 1000 bending cycles, which demonstrates their wearability.
A thermoelectric generator (TEG) is a device that converts heat energy into electrical energy using the Seebeck effect.200 The Seebeck effect is a phenomenon where a temperature difference between two dissimilar conductors or semiconductors produces a voltage difference between them.216 In a TEG, two different types of semiconductors (p-type and n-type) are connected in series, forming a thermocouple. When one side of the thermocouple is heated and the other side is cooled, a voltage difference is generated between the two sides, which can be used to power an electrical load. The efficiency of a TEG is determined by the thermoelectric properties of the semiconductors used, such as the Seebeck coefficient, electrical conductivity, and thermal conductivity.200 TEGs have a wide range of applications, including waste heat recovery, power generation in remote locations, and energy harvesting from body heat. Drawbacks of thermoelectric generators include their relatively low efficiency compared to other energy conversion technologies, their high cost, and their limited power output. Additionally, thermoelectric generators are often sensitive to temperature changes and may require complex thermal management systems to maintain optimal performance.217
Applications can be found in various wearable electronic devices, such as health monitoring systems, performance monitoring systems, and wireless communication devices. These TEGs can harvest energy from the human body heat and convert it into electrical energy to power these devices, eliminating the need for external power sources or batteries. The TEGs can be attached to the skin or clothing, making them convenient and unobtrusive for the user. The high power density of these TEGs also makes them suitable for applications that require continuous and long-term monitoring. Additionally, they can be used for personalized thermoregulation.217Fig. 8 shows an illustration of harvesting energy applications: energy harvesting and storage from motions of the heart,218 smart insole for biomechanical energy harvesting,219 and knee-joint piezoelectric energy harvester.220 These devices are capable of harvesting energy to develop self-powered sensor systems in healthcare settings. Table 3 summarizes some key features of the aforementioned energy harvesting systems.
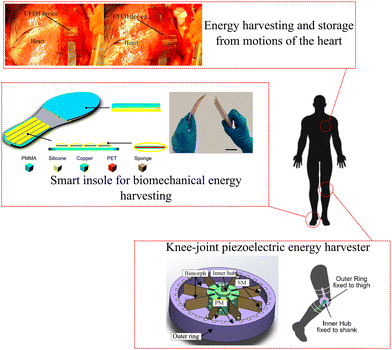 | ||
| Fig. 8 Applications of energy harvesting using human physiological systems. Reproduced from ref. 218–220 with permission from IOP Publishing Group, copyright 2016, Springer Science and Business Media LLC, copyright 2015, and American Chemical Society (ACS), copyright 2020, respectively. | ||
| Application | Energy type | System voltage | Output power | Ref. |
|---|---|---|---|---|
| Human gait as an energy harvesting mode | Piezoelectricity | 20 V (peaks observed during the gait cycle) | 2.06 ± 0.3 mW | 205 |
| Organ harvesting system | Piezoelectricity | 3.7 V | Time-averaged power density of 1.2 μW cm−2 | 206 |
| Energy harvesting from wheels, trains, and cars | Triboelectricity | — | 1.044 mW (7.34 W m−3) | 207 |
| Energy harvesting in wearable applications using triboelectric nanogenerators | Triboelectricity | 10–100 V | 1–100 mW | 208 |
| Energy harvesting from human activities (e.g. running and walking) | Triboelectricity | — | — | 209 |
| Harnessing of body movements as a source of energy to enhance blood vessel growth factors and increase the number of hair follicles | Triboelectricity | 6.2 V | 5.488 to 70 μW | 211 |
| Feeding physiological sensors for vital sign monitoring | Solar energy | Ranging from 90 V to 2 kV | Ranging from 90 μW to 0.2 mW | 215 |
| Feeding wireless communication modules used in healthcare devices | Solar energy | 1.7996 V | 159.1 mW | 212 |
| Skin-attached device for energy harvesting | Thermoelectricity | Ranging from 1.27 to 3 V | — | 217 |
6 Sensor integration into textiles and components
A number of challenges are involved in developing and implementing smart textiles for biomedical applications, including their dependability and washability, their high manufacturing costs, and their effectiveness after washing. Researchers around the globe are continuously working to advance smart textile-based biomedical devices, but the development of wearable smart textile standards and testing procedures is now the focus of intense activity. Additionally, smart textiles have the potential to provide doctors with a complete picture of physiological responses, motivate people to take more active roles in their healthcare, assist in exercise, and record progress.221–226Structures that incorporate sensors, actuators, and other components have the capability of sensing, responding, and adapting to changes in their environment. A variety of materials can be incorporated to construct these structures, called smart structures. These materials include metals, polymers, and composites, and they can be rigid or flexible. In smart structures, sensors and other components can be used to monitor their conditions, detect changes in their environment, and respond to those changes. A smart structure can, for instance, be used to detect and diagnose equipment failures, monitor production lines, and track the movement of goods and materials within a manufacturing facility.227 Besides their use in aerospace and civil engineering,228,229 they can also be applied to biomedical engineering.230,231
Due to their high flexibility and impact resistance, optical fiber sensors, specifically POFs, are well-suited to smart textiles and intrusive applications. Optical fibers based on silica can be more prone to breakage than polymer fibers, which may result in injury to users when there is a breakage due to glass punctures. Moreover, POFs can be embedded in soft structures, making them a suitable instrumentation option for wearable robots and biomedical applications.51 Compared to electronic sensors, they include compactness, lightweightness, flexibility, immunity to electromagnetic interference, chemical stability, and multiplexing capabilities.56,232 These features have pushed POF sensor technology into a wide range of smart textile applications.56,232,233
Optical fibers can be embedded into textile fabrics using various textile fabrication techniques. Weaving, knitting, and non-weaving techniques are among them. In weaving, the most commonly used technique, warp and weft yarns are interlaced in a basic woven structure. In order to ensure effective transmission and sensing, optical fibers are commonly woven into fabrics in an unbent condition or with a limited bending angle through the weaving technique.234 External perturbations can modulate the optical output signal in sensing applications, as reported in ref. 235, where a POF is embedded into a textile for respiration frequency monitoring using a smartphone-based interrogation technique and remote access to information through a Web server. Leal-Junior, Arnaldo, et al. have proposed a smart textile in a similar approach, where POFs embedded into a textile were used to monitor the displacement caused by respiration, vibration due to heartbeat, and bending induced in the gait while a user is operating a smart walker236 and to assess the heart rate under dynamic movements.237 Najafi, Bijan, et al. have demonstrated smart socks embedded with highly flexible optical fibers where temperature and pressure were assessed for the management of biomechanical risk factors associated with diabetic foot amputation.238 Li, Tianliang, et al. have proposed a smart-textile-based gesture recognition application with additional monitoring of respiratory signals under different postures using fiber Bragg gratings embedded into textiles.239 Avellar, Leticia, et al. have proposed POF-based pants for a fully optical fiber-integrated smart textile for remotely monitoring the biomechanics of lower limbs.56 These reported applications highlight the suitability of optical fiber sensors for the development of wearable devices and smart textiles due to the aforementioned advantages compared to electronic sensors.
Besides the aforementioned biomedical applications, optical fiber sensors can be embedded into structures for structural health monitoring, as reported in a review study conducted by Sasy Chan, Y. W., et al. where they provided state-of-the-art optical fiber sensing technologies and their practical application in railway infrastructures240 and for real-time damage detection in smart composites proposed in ref. 241. Moreover, the recent advances in aerospace composite structure monitoring through optical fiber-based sensors are reported in ref. 242 and 243. Finally, it is worth mentioning that these applications demonstrate that optical fiber sensors are a flexible technology that has been explored in sensor integration for a wide variety of applications, from biomedical to industrial applications, enhancing the efficiency and safety of these applications by providing measurements that can be utilized for data-driven decisions. Fig. 9 shows some examples of textiles integrated with sensors for plantar pressure assessment,244 impact monitoring,245 and gesture recognition (Table 4).246
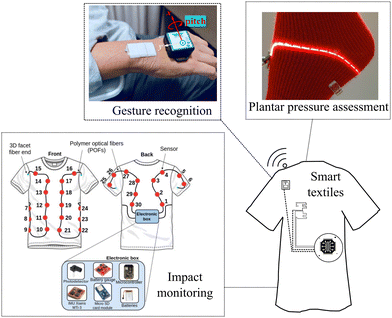 | ||
| Fig. 9 Applications of textile integrated with sensors as examples. Reproduced from ref. 244–246 with permission from MDPI AG, copyright 2019, IEEE, copyright 2021, and Optica Publishing Group, copyright 2024, respectively. | ||
| Application | Structure | Sensor technique | Ref. |
|---|---|---|---|
| Respiration frequency monitoring using a smartphone-based interrogation technique | Smart garment | Intensity variation-based using POF | 235 |
| Cardiorespiratory frequency monitoring during smartwalker operating | Smart garment | Intensity variation-based using POF | 236 |
| Plantar pressure and temperature monitoring | Smart socks | Intensity variation-based using POF | 239 |
| Remote monitoring of the lower limb biomechanics for pose classification and motion quantifying | Smart pants | Intensity variation-based using POF | 56 |
| Real-time damage detection in smart composite | Composites | Fiber Bragg gratings and Fabry–Pérot interferometer | 241 |
| Aerospace composite structure health monitoring | Aerospace composites | Brillouin-based distributed sensing | 243 |
7 Sensors for vital sign monitoring
In healthcare settings, monitoring vital signs is crucial as it enables early detection of deterioration. This can be achieved by, for example, analyzing blood pressure, respiratory rate, temperature, and oxygen saturation. The use of sensors in vital sign monitoring enables healthcare providers to obtain accurate and real-time data regarding a patient's physiological parameters. When analyzing the data obtained by the sensors, clinical decisions can be made with respect to treatment plans, medication doses, and the need for a further evaluation.247 Furthermore, the interest in development of sensors to monitor vital signs is growing, with the World Health Organization predicting that 1 in 6 people will be over the age of 60 by 2030. The number of people aged 60 years and older is expected to increase from 1 billion in 2020 to 1.4 billion by 2050.248 Additionally, aging is accompanied by a natural decline in physical capabilities, including muscle strength, power, and endurance. Several factors contribute to this decline, including changes in muscle mass, neuromuscular function, and cardiovascular capacity.249 As a result of these factors, there is an increasing demand for sensor development for healthcare applications.In addition to optical fiber sensors, various sensor modalities such as electromechanical sensors, thermistors, accelerometers, electrodermal activity sensors, acoustic sensors, and RFID tags find utility within healthcare contexts.250 Optical fiber sensors offer different advantages for healthcare applications, including high sensitivity allowing precise measurement of vital signs, miniaturization facilitating integration into wearable or implantable devices for continuous monitoring, immunity to electromagnetic interference ensuring compatibility with medical equipment, and multiplexing capability enabling simultaneous monitoring of diverse vital signs at different anatomical locations.251 The employment of optical fiber sensors for monitoring vital signs is already well-consolidated.
A POF-based sensor was proposed by Leal-Junior et al. for simultaneous measurement of breath and heart rate under dynamic movement.237 As part of the employed setup, the sensor is positioned on the subject's chest in various regions to record both breathing and heartbeat-induced body vibrations. The sensor is lightweight and flexible, allowing for comfortable wear during a variety of activities. Furthermore, signal processing techniques were applied to the sensor data to eliminate the influence of body movement on the sensor response. To further reduce the effects of movement, the analysis is conducted in the frequency domain. As a result of this approach, the sensor was able to accurately measure both the breath rate and heart rate even during dynamic movements such as walking or other activities.
Haseda Y. et al. have proposed a polymer optical fiber Bragg grating sensor for blood pressure measurement.252 The objective of this study was to measure pulse wave signals and calculate blood pressure using a setup comprising polymer optical fiber Bragg grating (POFBG) sensors. A POFBG sensor was placed on the brachial artery of the left elbow, while a silica-FBG sensor was located nearby for comparative purposes. Using reference blood pressure measurements obtained from an electrical sphygmomanometer, a calibration curve was constructed using pulse wave signals sampled at 1 kHz. In order to measure pressure changes, the FBG sensor system consists of a laser light source and an interrogator that detects shifts in the Bragg wavelength. To enhance the accuracy of blood pressure calculations, data processing techniques such as filtering and partial least squares regression (PLSR) were applied. As a whole, the setup and working principle of POFBG sensors demonstrated their potential for non-invasive and wearable vital sign monitoring with improved safety and reliability.
Leal-Junior et al. have proposed smart textiles for wearable sensing using optical fiber sensors.253 This study proposes a smart textile system that integrates multiplexed optical fiber sensors within a neoprene textile fabric. Between two layers of the fabric, sensors consisting of POFs and flexible light-emitting diodes (LEDs) are embedded into the textiles. In this setup, it is possible to simultaneously measure multiple parameters, such as temperature and interaction forces, with high sensitivity, linearity, repeatability, and resolution. Using controlled pressures and different temperatures to the sensors, the sensors are able to detect pressure interactions between the user and the environment, enabling the measurement of body temperature and the tracking of activity levels. With low crosstalk between sensors, the system ensures accurate and independent measurements, making it a promising technology for wearable health monitoring.
Avellar L. et al. have proposed a fully portable smart textile integrated with optical fiber sensors.56 This setup involves integrating POF sensors into a flexible textile for monitoring lower limb biomechanics and activity recognition. These sensors used multiplexed intensity variation to detect movements such as walking, squatting, and kicking. Each sensor was characterized by vertical displacements to enable machine learning algorithms to normalize the data. These data can be used for posture monitoring, which provides valuable insights into daily activities and biomechanical patterns. Patients with mobility impairments or chronic conditions may benefit from the ability to monitor their movements in real time, which can contribute to early detection of movement abnormalities and improve their quality of life. Finally, Fig. 10 summarizes the aforementioned applications for monitoring vital signs using optical fiber sensors. Table 5 summarizes the vital sign monitoring using optical fiber sensors discussed in this chapter concerning the employed sensing technique.
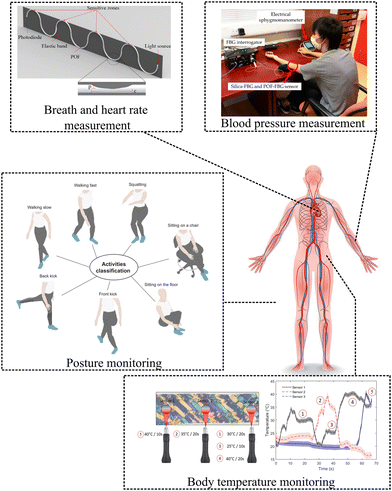 | ||
| Fig. 10 Examples of vital sign monitoring applications using optical fiber sensors. Reproduced from ref. 56, 237, 252 and 253 with permission from Elsevier, copyright 2019, MDPI AG, copyright 2019, Springer Science and Business Media LLC, copyright 2020, and Optica Publishing Group, copyright 2023, respectively. | ||
| Application | Sensor type | Ref. |
|---|---|---|
| Simultaneous measurement of breath and heart rate under dynamic movement | Intensity variation-based using POF | 237 |
| Blood pressure measurement | Polymer optical fiber Bragg gratings | 252 |
| Body temperature monitoring | Intensity variation-based using POF | 253 |
| Posture monitoring | Intensity variation-based using POF | 56 |
8 Biosensors and intrusive applications
The need to continuously monitor the metabolites of the human body can provide additional understanding about the patient's diagnosis and improve the treatment needed for his recovery. Alternative monitoring is often done by devices located in specialized laboratories and requires skilled labor to carry out the measurements. Typically, fluids are sampled from patients at regular intervals and taken for analysis. This means that monitoring performed in this way has a high susceptibility to human errors and can mask the diagnosis. As an alternative to these typical procedures, biosensors can be used as invasive monitoring devices and provide continuous detection of chemical and biological species in medical applications.254Briefly, there are three different types of biosensors that can invasively monitor patients: wearable (applied to the skin), ingestible (ingested into the gastrointestinal tract) and implantable (implanted into the body directly in contact with the skin).201 Despite the advantages that invasive biosensing can provide in clinical analysis, it still has some problems that need to be overcome, such as its stability, biocompatibility, and calibration. In addition, no approach has been able to couple all the analytical requirements for biosensing, often presenting different performance on the benchtop with in vivo monitoring.255 However, new materials and manufacturing processes have emerged to minimize these deficiencies associated with biosensors and overcome the inherent challenges of invasive monitoring.
Regarding the manufacturing processes of these implantable and wearable biosensors, it is essential to consider the mechanisms for converting biological recognition into a detectable signal. This requires that the manufacturing process be seen as an important step in the development of a biosensor, not only considering the production costs with respect to the technique used, but also the minimization of instrumental errors and the possibility of transitioning to non-invasive sensing.256 Invasive or minimally invasive biosensor fabrication techniques, such as lithography, screen printing technology and inkjet printing, can be cited as viable and low-cost approaches and will be described as follows.
1. Lithography is an excellent procedure that allows the components of a biosensor to be built systematically by superimposing layers. It is a technique with wide applications and that covers several possibilities of surface fabrication. Lithography is a technique that depends on the medium which is used to transfer the geometric pattern to the substrate and therefore is broadly classified in different ways, which can be cited as the most used photolithography, stereolithography, and electron beam lithography.256,257 Without a doubt, photolithography is one of its best-established and most widely used aspects in industry, mainly due to its large-scale production and low cost.258 In this process, UV light is used to transfer a geometric pattern established by a mask onto a substrate coated with a photoresist material. Despite its low resolution of approximately 1 μm, approximation and contact photolithography are still widely used throughout industry and modern research.259
2. The stereolithography process relies on photopolymerization or light-initiated polymerization and allows three-dimensional structures to be constructed. First, the digital models of the 3D figure are converted into a standard tessellation language file format so that they are virtually cut into sequential layers. This information is sent to the stereolithography apparatus which builds the 3D figure. The apparatus emits a high-energy laser that writes on the surface of the resin and traces the model of the 3D figure layer by layer by sequential polymerization. In other words, the construction of the 3D object is done by polymerizing two-dimensional cross sections.260 Its application in biosensing can be done by the manufacture of biosensors and their components or in the development of integrated devices. 3D printing technology in electrochemical biosensors, for example, has been widely used because it can provide customizable manufacturing of its electrodes, allowing full control over the shape and size of the desired electrode and printing on a specific matrix.261
3. Electron beam lithography (EBL) is a process used to create nanostructures with a high standard of resolution and alignment accuracy. These nanostructured arrangements are formed by sweeping a focused electron beam over a surface coated with an electron resist film. After immersing the device in an appropriate solution, exposed or unexposed resist will be completely removed from the surface.135,262 In the field of biosensors, silver nanodisks have already been designed by EBL on a sapphire substrate and used to detect heat-killed Escherichia coli.263 Nanoelectrode arrays were also obtained by EBL by creating nanoholes in thin polycarbonate films deposited on a macroelectrode.262 In another case, EBL was used to fabricate nanostructures resembling filamentous virions on Si/SiO2 substrate, as reported by Agrawal et al.135
4. Screen printing technology (SPT) is a manufacturing process for microelectronic devices or components. The method is highly competitive among other means of manufacturing microelectrodes due to its low cost, easy operation and scalability. It consists of painting different types of substrates, such as ceramics, plastics, and paper, using specific paints or pastes that allow adaptability, design and modifications according to manufacturing needs.264 This ink or paste is squeezed through a frame or woven mesh and printed onto a matrix.265 SPT is widely used in the fabrication of electrode systems used in electrochemical biosensors. The working electrode, reference electrode and counter electrode can be printed on inert substrates and enable an electrochemical system for the manufacture of the biosensor.266 In addition, its miniaturized dimension allows only one drop of solution, i.e., on a microliter scale, to be analyzed. The diffusion distance is also reduced (distance from the analyte to the surface of the receptor), decreasing the incubation time and providing a lighter measurement.267
5. Finally, inkjet printing technology consists of a printer containing an ink chamber and a channel that takes the ink to an injector nozzle, where the ink is digitally controlled. An inkjet printer is classified as a drop-on-demand (DOD) printer, which implies the ejection of ink only when necessary to avoid spending on necessary materials.268 The versatility of the technique, added to its low cost and manufacturing speed, allowed the technology to be used to print electronics269 and allowed manufacturing 2D270 and 3D structures.271 The development of biosensors manufactured using inkjet printing began in the mid-1990s and since then, it has been a field extensively investigated by academic research and highly viable for industry. They fall into virus sensors, enzymatic biosensors and non-enzymatic biosensors, which may be reviewed in greater detail elsewhere.268
In summary, biosensor devices of high quality and precision require techniques that allow the fabrication of complex arrangements and that use multiple components. Lithography, screen printing technology and inkjet printing technology start to play a role as more functional techniques in the manufacture of these devices by enabling the formation of sensory components by simplified methods and offering versatility of operation. In addition, variations in techniques allow the miniaturization of biosensor devices to be manufactured and used in an invasive way, in which the size of the device plays a fundamental role when implanted in the individual to monitor body fluids.
Ingestible biosensors are used as an alternative to implantable and wearable biosensors as they allow access to places within the human body that are impossible for the other two types. Thanks to advances in bioengineering, microelectronics and materials science, these biosensors could be manufactured in the form of capsules (and for this reason they are called ingestible biosensing capsules, IBCs) that are capable of monitoring multiple functions.272 Its small capsule size (in the order of a few centimeters) and its tablet format contribute to the IBC being swallowed easily and allowing the monitoring of biomarkers of interest in the gastrointestinal tract to be carried out.273
Lu et al. have classified IBCs into two categories according to their locomotion, with passive IBCs being those that follow the peristaltic motion of the gastrointestinal tract and active IBCs being those that can be magnetically guided.272 This was only possible thanks to the significant advances in the miniaturization of processors that made it possible to manufacture devices that are smaller, smarter, and more operationally efficient. Advances in battery technology also played an important role in the development of ingestible biosensors, allowing them to operate for long periods with a tiny device.274
The use of ingestible biosensors makes it possible to measure a wide range of biomarkers from the mucous membrane of the guts, including electrolytes, metabolites,273 hormones, enzymes, and gases,275 for example. The by-products of the microorganism ecosystem that inhabit the gastrointestinal tract, i.e., the human gut microbiota, can also be monitored by these devices.274,276 A list of possible markers can be monitored in this way, such as the gastric juice electrolytes that control pH (hydrochloric acid, potassium chloride and sodium chloride),277,278 digestive enzymes (amylase, pepsin, trypsin, etc.),274,279 O2-equivalent, H2 and CO2 gases, and microbial community,280,281 beyond imaging,282 temperature monitoring,283 pressure,284 and drug delivery units.285
Regarding materials for manufacturing this kind of device, some essential components can be highlighted, such as structural materials and packaging, materials for electronic components, logic devices, communications and power sources, as reported by Bettinger, C.286 For encapsulation, some substances are considered generally recognized as safe (GRAS), such as gelatin, cellulose, zinc oxide, and metallic iron, among others. Polymers, ceramics, pigments, metals, and minerals are also classified as GRAS and can be used in encapsulation as well as device electronics.286 Galvanic couples, such as Zn|Cu, Mg|Cu,287 and melanin|ceramics288 are very energy efficient and can supply large voltages to the system when needed. On the other hand, energy collected from bodies of living subjects in chemical, thermal and mechanical ways is under development and could be used for self-powered electronics.289
In summary, the amount of information that an ingestible biosensor can provide about the gastrointestinal tract is enormous. Knowledge about the microbiome and how it impacts the other organs of the human body, the behavior of metabolites and hormones in the face of changes in the gastrointestinal environment and understanding about electrolytes in the intestine in response to the ingestion of food, medication and supplements are some of the questions that can be answered with the use of ingestible devices.274Fig. 11 shows a schematic representation of an ingestible biosensor with its main parts highlighted. Table 6 summarizes some key information about the aforementioned biosensors used in intrusive applications.
| Application | Locomotion | Sensor description | Ref. |
|---|---|---|---|
| Biomarkers for metabolites | Passive | The proposed sensor composition includes a battery-free ingestible biosensing system that integrates a self-powered glucose biofuel cell biosensor | 273 |
| Biomarkers for gases | Passive | This biosensor is an ingestible electronic capsule that incorporates electronic components for measuring H2, CO2, and O2 concentrations in the gut. It uses a combination of thermal conductivity and semiconducting sensors, with high accuracy for measuring gas concentrations | 275 |
| Monitoring of by-products of the microorganism ecosystem that inhabit the gastrointestinal tract | Passive | The capsule utilizes a combination of thermal conductivity and semiconducting sensors, with the ability to adjust sensor heating elements for selectivity and sensitivity to different gases | 274, 276 |
| Monitoring of microbial community | Passive | The proposed ingestible sensor is a miniaturized detector and transmitter packed into a capsule for tracking and quantifying bioactive compounds in the gut to assess microbiome effectiveness | 280, 281 |
| Drug delivery unity | Passive | Ingestible device designed for highly predictable intraluminal drug delivery in the gut. It features a drug reservoir, pH and temperature sensors, a stepper motor, and a transceiver for real-time wireless communication. The device can relay data to a computer, allowing for precise drug delivery control based on pH and temperature changes in the gut | 285 |
9 Conclusions
This paper presented a review of wearable sensor applications for healthcare using optical fiber sensors. In this case, the overview of population aging and the demands that led to the development of new healthcare technologies are discussed. Then, the development of wearable and healthcare sensors is discussed considering all steps and components on the wearable optical fiber sensor fabrication, namely the optical fiber sensor approaches, fiber functionalization and novel technologies for power supply and self-powered sensor systems. In addition, the applications with textile integrated sensors as well as biosensors and intrusive applications are presented. Thus, this review presents an overview of wearable devices and indicates the possibility of combining the aforementioned technologies for the new generation of wearable devices, where the self-powered integrated optical systems for both unobtrusive and intrusive applications can be used for different healthcare devices and approaches.It is possible to envisage the popularization of the optical fiber biosensing techniques using low cost platforms due to their advantages related to miniaturization, flexibility and multiplexing techniques, where multipurpose structures can be developed for vital sign monitoring. In the future, it may be possible to integrate the optical fiber biosensing platforms with the energy harvesting systems discussed in this paper to achieve a self-powered optical fiber sensing platform for biomedical applications. Another advantage of such systems is the possibility of using both in continuous monitoring and as need-based devices, since the optical fiber sensors can provide continuous monitoring of different parameters with low energy consumption. It is also worth mentioning that commercially available optical fiber sensors are already available with some startup companies beginning the commercialization of smart textiles and glucose sensors.
Author contributions
A. L-J. conceptualization, funding acquisition, investigation, methodology, project administration, supervision, visualization, writing – original draft, writing – review & editing; J. S. conceptualization, investigation, methodology, visualization, writing – original draft; L. M. methodology, visualization, writing – original draft; A. M. methodology, visualization, writing – original draft; S. M. investigation, visualization, writing – original draft; J. V. conceptualization, investigation, methodology, visualization, writing – original draft; F. V. investigation, visualization, writing – original draft; M. C. conceptualization, investigation, methodology, visualization, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is financed by CNPq (440064/2022-8, 310709/2021-0 and 405336/2022-5), FAPES (1004/2022, 29/2022 and 316/2023) and MCTI/FNDCT/FINEP 2784/20 and 0036/21.Notes and references
- S. Li, L. D. Xu and S. Zhao, J. Ind. Inf. Integr., 2018, 10, 1–9 Search PubMed.
- S. Sicari, A. Rizzardi and A. Coen-Porisini, Comput. Netw., 2020, 179, 107345 CrossRef.
- A. Zanella, N. Bui, A. Castellani, L. Vangelista and M. Zorzi, IEEE Internet Things J., 2014, 1, 22–32 Search PubMed.
- G. A. Akpakwu, B. J. Silva, G. P. Hancke and A. M. Abu-Mahfouz, IEEE Access, 2017, 6, 3619–3647 Search PubMed.
- T. Islam, S. C. Mukhopadhyay and N. K. Suryadevara, IEEE Sens. J., 2017, 17, 577–584 Search PubMed.
- L. Romeo, A. Petitti, R. Marani and A. Milella, Sensors, 2020, 20, 1–23 CrossRef PubMed.
- A. Leal-Junior and C. Marques, IEEE Instrum. Meas. Mag., 2021, 24, 41–49 Search PubMed.
- P. P. Jayaraman, A. R. M. Forkan, A. Morshed, P. D. Haghighi and Y. B. Kang, Wiley Interdiscip. Rev., Data Min. Knowl. Discov., 2020, 10, 1–23 Search PubMed.
- A. Turner, Philos. Trans. R. Soc., B, 2009, 364, 3009–3021 CrossRef PubMed.
- E. Jaul and J. Barron, Front. Public Health, 2017, 5, 1–7 Search PubMed.
- S. Majumder, T. Mondal and M. Deen, Sensors, 2017, 17, 130 CrossRef PubMed.
- L. P. Malasinghe, N. Ramzan and K. Dahal, J. Ambient Intell. Humaniz. Comput., 2019, 10, 57–76 CrossRef.
- G. H. Lee, H. Moon, H. Kim, G. H. Lee, W. Kwon, S. Yoo, D. Myung, S. H. Yun, Z. Bao and S. K. Hahn, Nat. Rev. Mater., 2020, 5, 149–165 CrossRef PubMed.
- Kenry, J. C. Yeo and C. T. Lim, Microsyst. Nanoeng., 2016, 2, 16043 CrossRef CAS PubMed.
- A. Leal-Junior, L. Avellar, V. Biazi, M. S. Soares, A. Frizera and C. Marques, Opto-Electron. Adv., 2022, 210098 Search PubMed.
- S. Abdulmalek, A. Nasir, W. A. Jabbar, M. A. M. Almuhaya, A. K. Bairagi, M. A.-M. Khan and S.-h. Kee, Healthcare, 2022, 10, 1993 CrossRef PubMed.
- A. G. Leal-Junior, C. R. Díaz, C. Marques, M. J. Pontes and A. Frizera, Opt. Laser Technol., 2019, 116, 256–264 CrossRef CAS.
- A. G. Leal-Junior, D. Ribeiro, L. M. Avellar, M. Silveira, C. A. R. Diaz, A. Frizera-Neto, W. Blanc, E. Rocon and C. Marques, IEEE Sens. J., 2021, 21, 2995–3003 Search PubMed.
- L. Avellar, A. Leal-Junior, C. Diaz, C. Marques and A. Frizera, Sensors, 2019, 19(14), 3156 CrossRef PubMed.
- S. M. A. Iqbal, I. Mahgoub, E. Du, M. A. Leavitt and W. Asghar, npj Flexible Electron., 2021, 5, 9 CrossRef.
- W. G. Macena, L. O. Hermano and T. C. Costa, Revista Mosaicum, 2018, 27, 223–236 CrossRef.
- M. Latash, M. Levin, J. Scholz and G. Schöner, Medicina, 2010, 46, 382 CrossRef PubMed.
- W. J. Chodzko-Zajko, D. N. Proctor, M. A. F. Singh, C. T. Minson, C. R. Nigg, G. J. Salem and J. S. Skinner, Med. Sci. Sports Exercise, 2009, 41, 1510–1530 CrossRef PubMed.
- S. Usmani, A. Saboor, M. Haris, M. A. Khan and H. Park, Sensors, 2021, 21(15), 5134 CrossRef PubMed.
- L. A. de Melo and K. C. de Lima, Cien. Saude Colet., 2020, 25, 3869–3877 CrossRef PubMed.
- G. M. D. Miranda, A. da Cruz Gouveia Mendes and A. L. A. da Silva, Rev. Bras. Geriatr. Gerontol., 2016, 19, 507–519 CrossRef.
- S. de Liz Sofiatti, M. M. de Oliveira, L. M. Gomes and K. V. S. Vieira, Revista Brasileira Militar de Ciências, 2021, 7, 17 Search PubMed.
- R. A. Lourenço, V. G. Moreira, R. G. B. de Mello, I. de Souza Santos, S. M. Lin, A. L. F. Pinto, L. P. Lustosa, Y. A. de Oliveira Duarte, J. A. Ribeiro, C. C. Correia, H. N. Mansur, E. Ribeiro, R. R. D. Corte, E. Ferriolli, C. A. Uehara, A. Maeda, T. Petroni, T. S. Lima, S. F. Durão, I. Aprahamian, C. M. Avesani and W. J. Filho, Geriatr. Gerontol. Aging., 2018, 12, 121–135 CrossRef.
- W. H. Organization, WHO global report on falls, 2007, pp. 1–47 Search PubMed.
- J. E. Lewis and M. B. Neider, Ergon. Des., 2017, 25, 4–10 Search PubMed.
- M. Wu and J. Luo, Online Journal of Nursing Informatics Contributors, 2019, Available at https://www.himss.org/resources/wearable-technology-applications-healthcare-literature-review Search PubMed.
- N. Niknejad, W. B. Ismail, A. Mardani, H. Liao and I. Ghani, Eng. Appl. Artif. Intell., 2020, 90, 103529 CrossRef.
- J. Li, Q. Ma, A. H. Chan and S. S. Man, Appl. Ergon., 2019, 75, 162–169 CrossRef PubMed.
- T. G. Stavropoulos, A. Papastergiou, L. Mpaltadoros, S. Nikolopoulos and I. Kompatsiaris, Sensors, 2020, 20, 2826 CrossRef PubMed.
- A. C. Kogan, K. Wilber and L. Mosqueda, J. Am. Geriatr. Soc., 2016, 64, e1–e7 CrossRef PubMed.
- K. Kulinski, C. DiCocco, S. Skowronski and P. Sprowls, Front. Public Health, 2017, 5, 4 Search PubMed.
- D. Podsiadlo and S. Richardson, J. Am. Geriatr. Soc., 1991, 39, 142–148 CrossRef CAS PubMed.
- L. C. A. Andrade, G. L. dos Anjos Costa, L. G. B. Diogenes and P. H. R. Pimentel, Res., Soc. Dev., 2021, 10, e321101321615 CrossRef.
- M. R. Kaysir, A. Stefani, R. Lwin and S. Fleming, 2017 Conference on Lasers and Electro-Optics Pacific Rim (CLEO-PR), 2017, pp. 1–2 Search PubMed.
- H.-E. Joe, H. Yun, S.-H. Jo, M. B. Jun and B.-K. Min, Int. J. Precis. Eng. Manuf. - Green Technol., 2018, 5, 173–191 CrossRef.
- Z. Liu, Z. Zhang, H.-Y. Tam and X. Tao, Photonics, 2019, 6, 48 CrossRef CAS.
- Z. Liu, M. Zhang, Y. Zhang, Y. Xu, Y. Zhang, X. Yang, J. Zhang, J. Yang and L. Yuan, Sens. Actuators, A, 2020, 313, 112179 CrossRef CAS.
- Y. Koike and M. Asai, NPG Asia Mater., 2009, 1, 22–28 CrossRef.
- M. Elsherif, A. E. Salih, M. G. Muñoz, F. Alam, B. AlQattan, D. S. Antonysamy, M. F. Zaki, A. K. Yetisen, S. Park, T. D. Wilkinson and H. Butt, Adv. Photonics Res., 2022, 3, 2100371 CrossRef.
- K. Peters, Polymer optical fiber sensors - A review, 2011 Search PubMed.
- O. Strobel and J. Lubkoll, 2010 20th International Crimean Conference “Microwave Telecommunication Technology”, 2010, pp. 16–20 Search PubMed.
- J. Ballato and P. Dragic, Materials, 2014, 7, 4411–4430 CrossRef PubMed.
- A. Leal-Junior, L. Macedo, A. Frizera and M. J. Pontes, IEEE Photonics Technol. Lett., 2022, 34, 611–614 Search PubMed.
- S. Liehr, P. Lenke, M. Wendt, K. Krebber, M. Seeger, E. Thiele, H. Metschies, B. Gebreselassie and J. C. Munich, IEEE Sens. J., 2009, 9, 1330–1338 CAS.
- L. Macedo, R. W. M. Pires Junior, A. Frizera, M. J. Pontes and A. Leal-Junior, Opt. Fiber Technol., 2022, 72, 103001 CrossRef CAS.
- A. G. Leal-Junior, C. A. Diaz, L. M. Avellar, M. J. Pontes, C. Marques and A. Frizera, Sensors, 2019, 19, 3156 CrossRef PubMed.
- M. Rocha, P. Antunes, P. André, N. Alberto and M. F. Domingues, 2023 IEEE 7th Portuguese Meeting on Bioengineering (ENBENG), 2023, pp. 76–79 Search PubMed.
- A. G. Leal-Junior, A. Frizera, L. M. Avellar, C. Marques and M. J. Pontes, IEEE Sens. J., 2018, 18, 2362–2368 Search PubMed.
- A. D. Kersey, in Distributed and Multiplexed Fiber Optic Sensors, Wiley, 2011, pp. 277–314 Search PubMed.
- A. G. Leal-Junior, C. R. Díaz, C. Marques, M. J. Pontes and A. Frizera, Opt. Laser Technol., 2019, 111, 81–88 CrossRef CAS.
- L. Avellar, A. Frizera and A. Leal-Junior, Biomed. Opt. Express, 2023, 14, 3689–3704 CrossRef CAS PubMed.
- K. Hill and G. Meltz, J. Lightwave Technol., 1997, 15, 1263–1276 CrossRef CAS.
- C. Giles, J. Lightwave Technol., 1997, 15, 1391–1404 CrossRef.
- Y. Liu, A. Zhou, Q. Xia, Y. Zhao, H. Deng, S. Yang and L. Yuan, IEEE Sens. J., 2019, 19, 10728–10735 CAS.
- X. Xiaoqiang, S. Ziqi, M. Yan and D. Yang, 2021 33rd Chinese Control and Decision Conference (CCDC), 2021, pp. 3150–3157 Search PubMed.
- A. Theodosiou and K. Kalli, Opt. Fiber Technol., 2020, 54, 102079 CrossRef CAS.
- Y. Qiu, Q. B. Wang, H. T. Zhao, J. A. Chen and Y. Y. Wang, Shanghai Jiaotong Daxue Xuebao, 2013, 18, 129–139 CrossRef.
- H. Zhou, Y. Wang, Z. Zhou and C. Ma, Measurement, 2023, 222, 113565 CrossRef.
- G. Kashaganova, A. Kozbakova, T. Kartbayev, G. Balbayev, K. Togzhanova, Z. Alimseitova and S. Orazaliyeva, Electronics, 2023, 12(11), 2491 CrossRef.
- A. Kerrouche, T. Najeh and P. Jaen-Sola, Sensors, 2021, 21(11), 3639 CrossRef PubMed.
- T. Li, J. Guo, Y. Tan and Z. Zhou, IEEE Sens. J., 2020, 20, 12074–12087 Search PubMed.
- J. Huang, J. Zeng, Y. Bai, Y. Wang, K. Wang, X. Wu, Z. Cheng and D. Liang, Opt. Fiber Technol., 2020, 57, 102216 CrossRef CAS.
- M. Liao, S. Liang, R. Luo and Y. Xiao, Constr. Build. Mater., 2022, 342, 128029 CrossRef.
- H. Hisham, Fiber bragg grating sensors: development and applications, CRC Press, 2019 Search PubMed.
- J. Albert, L. Y. Shao and C. Caucheteur, Tilted fiber Bragg grating sensors, 2013 Search PubMed.
- X. Dong, H. Zhang, B. Liu and Y. Miao, Tilted fiber bragg gratings: Principle and sensing applications, 2011 Search PubMed.
- X. Chen, J. Xu, X. Zhang, T. Guo and B.-O. Guan, IEEE Photonics Technol. Lett., 2017, 29, 719–722 CAS.
- L.-Y. Shao, L. Xiong, C. Chen, A. Laronche and J. Albert, J. Lightwave Technol., 2010, 28, 2681–2687 Search PubMed.
- P. Kisała, D. Harasim and J. Mroczka, Opt. Express, 2016, 24, 29922–29929 CrossRef PubMed.
- D. Kinet, P. Mégret, K. W. Goossen, L. Qiu, D. Heider and C. Caucheteur, Sensors, 2014, 14, 7394–7419 CrossRef PubMed.
- S. Korganbayev, M. De Landro, A. Wolf, D. Tosi and P. Saccomandi, IEEE Sens. J., 2022, 22, 15999–16007 Search PubMed.
- Y. Singh, S. K. Raghuwanshi, O. Prakash and P. K. Saini, Opt. Quantum Electron., 2021, 53, 664 CrossRef CAS.
- G. Allwood, G. Wild and S. Hinckley, Electronics, 2017, 6(4), 92 CrossRef.
- W. Bao, F. Chen, H. Lai, S. Liu and Y. Wang, Sens. Actuators, B, 2021, 349, 130794 CrossRef CAS.
- A. Lopez Aldaba, A. González-Vila, M. Debliquy, M. Lopez-Amo, C. Caucheteur and D. Lahem, Sens. Actuators, B, 2018, 254, 1087–1093 CrossRef CAS.
- C. Massaroni, M. Zaltieri, D. Lo Presti, A. Nicolò, D. Tosi and E. Schena, IEEE Sens. J., 2021, 21, 14069–14080 Search PubMed.
- C. Zhang, X. Chen, X. Liu, C. Shen, Z. Huang, Z. Wang, T. Lang, C. Zhao, Y. Zhang and Z. Liu, Int. J. Hydrogen Energy, 2022, 47, 6415–6420 CrossRef CAS.
- B. Raju, R. Kumar, M. Senthilkumar, R. Sulaiman, N. Kama and S. Dhanalakshmi, Sustain. Energy Technol. Assessments, 2022, 52, 102306 CrossRef.
- W. Du, X. Tao, H. Tam and C. Choy, Compos. Struct., 1998, 42, 217–229 CrossRef.
- A. Leal-Junior, L. Avellar, W. Blanc, A. Frizera and C. Marques, IEEE Internet Things J., 2024, 11, 9587–9598 Search PubMed.
- C. Perez-Ramirez, D. Almanza-Ojeda, J. Guerrero-Tavares, F. Mendoza-Galindo, J. Estudillo-Ayala and M. Ibarra-Manzano, Sensors, 2014, 14, 24483–24501 CrossRef PubMed.
- D. Vilarinho, A. Theodosiou, C. Leitão, A. Leal-Junior, M. Domingues, K. Kalli, P. André, P. Antunes and C. Marques, Sensors, 2017, 17, 2924 CrossRef PubMed.
- A. G. Leal-Junior, A. Frizera, A. Theodosiou, C. Díaz, M. Jimenez, R. Min, M. J. Pontes, K. Kalli and C. Marques, IEEE Sens. J., 2019, 19, 9221–9228 CAS.
- W. Li, Y. Yuan, J. Yang and L. Yuan, Photonic Sens., 2021, 11, 31–44 CrossRef.
- X. Chen, S. He, D. Li, K. Wang, Y. Fan and S. Wu, IEEE Sens. J., 2016, 16, 349–354 Search PubMed.
- L. Liu, P. Lu, H. Liao, S. Wang, W. Yang, D. Liu and J. Zhang, IEEE Sens. J., 2016, 16, 3054–3058 CAS.
- Z. Tian and S. S.-H. Yam, IEEE Photonics Technol. Lett., 2009, 21, 161–163 CAS.
- X. Wang, D. Chen, H. Li, G. Feng and J. Yang, IEEE Sens. J., 2017, 17, 100–104 Search PubMed.
- D. Lyu, J. Peng, Q. Huang, W. Zheng, L. Xiong and M. Yang, IEEE Sens. J., 2021, 21, 57–61 CAS.
- Y. Chen, Y. Zheng, H. Xiao, D. Liang, Y. Zhang, Y. Yu, C. Du and S. Ruan, Sensors, 2022, 22(15), 5748 CrossRef CAS PubMed.
- B. Culshaw and A. Kersey, J. Lightwave Technol., 2008, 26(9), 1064–1078 Search PubMed.
- Y. Wang, H. Yuan, X. Liu, Q. Bai, H. Zhang, Y. Gao and B. Jin, IEEE Access, 2019, 7, 85821–85837 Search PubMed.
- J. Zhao, Y. Zhao, L. Bai and Y. Zhang, Sens. Actuators, A, 2020, 314, 112245 CrossRef CAS.
- M. Szczerska, Chemosensors, 2022, 10(6), 228 CrossRef CAS.
- S. Wu, L. Wang, X. Chen and B. Zhou, J. Lightwave Technol., 2018, 36, 2216–2221 CAS.
- X. Li, D. Liu, R. Kumar, W. P. Ng, Y. Q. Fu, J. Yuan, C. Yu, Y. Wu, G. Zhou, G. Farrell, Y. Semenova and Q. Wu, Meas. Sci. Technol., 2017, 28, 035105 CrossRef.
- R. Wang, J. Zhao, Y. Sun, H. Yu, N. Zhou, H. Zhang and D. Jia, Biomed. Opt. Express, 2019, 11, 316 CrossRef PubMed.
- S. Eom, J. Park and J. Lee, Microw. Opt. Technol. Lett., 2010, 52, 1318–1321 CrossRef.
- C. Ke, Y. Cai, T. Zhao and Z. Li, IOP Conf. Ser. Earth Environ. Sci., 2021, 769, 042039 CrossRef.
- M. Ghasemi, J. Oh, S. Jeong, M. Lee, S. Bohlooli Darian, K. Oh and J. K. Kim, Biosensors, 2023, 13, 817 CrossRef CAS PubMed.
- P. Lu, N. Lalam, M. Badar, B. Liu, B. T. Chorpening, M. P. Buric and P. R. Ohodnicki, Appl. Phys. Rev., 2019, 6, 041302 Search PubMed.
- X. Bao and Y. Wang, Advanced Devices Instrumentation, 2021, 2021 DOI:10.34133/2021/8696571.
- S. Adachi, 2008 SICE Annual Conference, 2008, pp. 329–333 Search PubMed.
- K. Yuksel, M. Wuilpart, V. Moeyaert and P. Megret, 2009 11th International Conference on Transparent Optical Networks, 2009, pp. 1–5 Search PubMed.
- Y. Muanenda, J. Sens., 2018, 2018, 1–16 CrossRef.
- M. Silveira, A. Frizera, A. Leal-Junior, D. Ribeiro, C. Marques, W. Blanc and C. A. Camilo, Opt. Fiber Technol., 2020, 58, 102303 CrossRef CAS.
- L. Avellar, A. Frizera, H. Rocha, M. Silveira, C. Díaz, W. Blanc, C. Marques and A. Leal-Junior, Photonics Res., 2023, 11, 364 CrossRef.
- R. Correia, S. James, S.-W. Lee, S. P. Morgan and S. Korposh, J. Opt., 2018, 20, 073003 CrossRef.
- Z. Katrenova, S. Alisherov, T. Abdol and C. Molardi, Sens. Bio-Sens. Res., 2024, 43, 100616 CrossRef.
- A. G. Leal-Junior, A. Frizera-Neto, M. J. Pontes and T. R. Botelho, Meas. Sci. Technol., 2017, 28, 125103 CrossRef.
- S. Umesh, S. Padma, T. Srinivas and S. Asokan, IEEE Sens. J., 2018, 18, 216–222 Search PubMed.
- A. Leal, L. Avellar, M. J. Pontes, C. A. Díaz, C. Marques and A. Frizera Neto, Seventh European Workshop on Optical Fibre Sensors, 2019 Search PubMed.
- X. Wang, H. Zhou, M. Chen, Y. He, Z. Zhang, J. Gan and Z. Yang, Sci. China Inf. Sci., 2024, 67, 1300–1310 Search PubMed.
- A. Grillet, D. Kinet, J. Witt, M. Schukar, K. Krebber, F. Pirotte and A. Depre, IEEE Sens. J., 2008, 8, 1215–1222 Search PubMed.
- D. Tosi, C. Molardi, M. Sypabekova and W. Blanc, IEEE Sens. J., 2021, 21, 12667–12678 CAS.
- C. Broadway, R. Min, A. G. Leal-Junior, C. Marques and C. Caucheteur, J. Lightwave Technol., 2019, 37, 2605–2615 CAS.
- M. S. F. Soares, PhD Thesis, University of Aveiro, 2021 Search PubMed.
- J. Wang, Coatings for Biomedical Applications, Woodhead Publishing, 2012, pp. 143–175 Search PubMed.
- M. Oliverio, S. Perotto, G. C. Messina, L. Lovato and F. D. Angelis, ACS Appl. Mater. Interfaces, 2017, 9, 29394–29411 CrossRef CAS PubMed.
- R. T. Hill, WIREs Nanomed. Nanobiotechnol., 2014, 7, 152–168 CrossRef PubMed.
- V. Nocerino, B. Miranda, C. Tramontano, G. Chianese, P. Dardano, I. Rea and L. D. Stefano, Chemosensors, 2022, 10, 150 CrossRef CAS.
- V. Naresh and N. Lee, Sensors, 2021, 21, 1109 CrossRef CAS PubMed.
- P. I. Dolez, Nanoengineering, Elsevier, 2015, pp. 3–40 Search PubMed.
- M. E. Hamza, M. A. Othman and M. A. Swillam, Biology, 2022, 11, 621 CrossRef CAS PubMed.
- A. Negm, M. M. R. Howlader, I. Belyakov, M. Bakr, S. Ali, M. Irannejad and M. Yavuz, Materials, 2022, 15, 7289 CrossRef CAS PubMed.
- M. J. McClain, A. E. Schlather, E. Ringe, N. S. King, L. Liu, A. Manjavacas, M. W. Knight, I. Kumar, K. H. Whitmire, H. O. Everitt, P. Nordlander and N. J. Halas, Nano Lett., 2015, 15, 2751–2755 CrossRef CAS PubMed.
- M. W. Knight, N. S. King, L. Liu, H. O. Everitt, P. Nordlander and N. J. Halas, ACS Nano, 2013, 8, 834–840 CrossRef PubMed.
- G. V. Naik, V. M. Shalaev and A. Boltasseva, Adv. Mater., 2013, 25, 3264–3294 CrossRef CAS PubMed.
- K. M. McPeak, S. V. Jayanti, S. J. P. Kress, S. Meyer, S. Iotti, A. Rossinelli and D. J. Norris, ACS Photonics, 2015, 2, 326–333 CrossRef CAS PubMed.
- A. Agrawal, S. H. Cho, O. Zandi, S. Ghosh, R. W. Johns and D. J. Milliron, Chem. Rev., 2018, 118, 3121–3207 CrossRef CAS PubMed.
- C. Park, R. Colorado, A. Jamison and T. Lee, Reference Module in Materials Science and Materials Engineering, Elsevier, 2016 Search PubMed.
- G. M. Whitesides and P. E. Laibinis, Langmuir, 1990, 6, 87–96 CrossRef CAS.
- T. Yu, F. Zhong, F. Zhang, C. Ying and G. Geng, J. Phys.: Conf. Ser., 2021, 2002, 012010 CrossRef CAS.
- P. Roingeard, P.-I. Raynal, S. Eymieux and E. Blanchard, Rev. Med. Virol., 2018, 29, e2019 CrossRef PubMed.
- L. Mandrile, A. M. Giovannozzi, F. Pennecchi, A. Saverino, C. Lobascio and A. M. Rossi, Anal. Methods, 2015, 7, 2813–2821 RSC.
- C. Leitão, A. Leal-Junior, A. R. Almeida, S. O. Pereira, F. M. Costa, J. L. Pinto and C. Marques, Biotechnol. Rep., 2021, 29, e00587 CrossRef PubMed.
- V. Narwal, R. Deswal, B. Batra, V. Kalra, R. Hooda, M. Sharma and J. Rana, Steroids, 2019, 143, 6–17 CrossRef CAS PubMed.
- C.-J. Huang, G. V. Narasimha, Y.-C. Chen, J.-K. Chen and G.-C. Dong, Biosensors, 2021, 11, 219 CrossRef CAS PubMed.
- Y. Tang, T. J. Hardy and J.-Y. Yoon, Biosens. Bioelectron., 2023, 234, 115361 CrossRef CAS PubMed.
- S. Shukla, P. Govender and A. Tiwari, Advances in Biomembranes and Lipid Self-Assembly, Elsevier, 2016, pp. 143–161 Search PubMed.
- H. H. Nguyen, S. H. Lee, U. J. Lee, C. D. Fermin and M. Kim, Materials, 2019, 12, 121 CrossRef CAS PubMed.
- A. Hasan, M. Nurunnabi, M. Morshed, A. Paul, A. Polini, T. Kuila, M. A. Hariri, Y. Kyu Lee and A. A. Jaffa, BioMed Res. Int., 2014, 2014, 1–18 Search PubMed.
- E. C. Wilkirson, K. L. Singampalli, J. Li, D. D. Dixit, X. Jiang, D. H. Gonzalez and P. B. Lillehoj, Anal. Bioanal. Chem., 2023, 415, 3983–4002 CrossRef CAS PubMed.
- D. W. G. Morrison, M. R. Dokmeci, U. Demirci and A. Khademhosseini, Clinical Applications of Micro- and Nanoscale Biosensors, in Biomedical Nanostructures, ed. K. E. Gonsalves, C. R. Halberstadt, C. T. Laurencin and L. S. Nair, 2007, DOI:10.1002/9780470185834.ch17.
- Y.-C. Zhu, L.-P. Mei, Y.-F. Ruan, N. Zhang, W.-W. Zhao, J.-J. Xu and H.-Y. Chen, Advances in Enzyme Technology, Elsevier, 2019, pp. 201–223 Search PubMed.
- D. J. Monk and D. R. Walt, Anal. Bioanal. Chem., 2004, 379, 931–945 CrossRef CAS PubMed.
- M. Campàs, B. Prieto-Simón and J.-L. Marty, Semin. Cell Dev. Biol., 2009, 20, 3–9 CrossRef PubMed.
- R. C. Rodrigues, Á. Berenguer-Murcia and R. Fernandez-Lafuente, Adv. Synth. Catal., 2011, 353, 2216–2238 CrossRef CAS.
- K. Sadani, P. Nag, X. Thian and S. Mukherji, Biosens. Bioelectron.: X, 2022, 12, 100278 CAS.
- Z. Zhou, Z. Yang, L. Xia and H. Zhang, Biosens. Bioelectron., 2022, 198, 113836 CrossRef CAS PubMed.
- N. Gupta, V. Renugopalakrishnan, D. Liepmann, R. Paulmurugan and B. D. Malhotra, Biosens. Bioelectron., 2019, 141, 111435 CrossRef CAS PubMed.
- M. E. Inda, M. Mimee and T. K. Lu, J. Allergy Clin. Immunol., 2019, 144, 645–647 CrossRef PubMed.
- L. Su, W. Jia, C. Hou and Y. Lei, Biosens. Bioelectron., 2011, 26, 1788–1799 CrossRef CAS PubMed.
- N. Lobsiger and W. J. Stark, Anal. Sci., 2019, 35, 839–847 CrossRef PubMed.
- I. B. Tahirbegi, J. Ehgartner, P. Sulzer, S. Zieger, A. Kasjanow, M. Paradiso, M. Strobl, D. Bouwes and T. Mayr, Biosens. Bioelectron., 2017, 88, 188–195 CrossRef CAS PubMed.
- N. Gupta, V. Renugopalakrishnan, D. Liepmann, R. Paulmurugan and B. D. Malhotra, Biosens. Bioelectron., 2019, 141, 111435 CrossRef CAS PubMed.
- G. Caluori, J. Pribyl, M. Pesl, S. Jelinkova, V. Rotrekl, P. Skladal and R. Raiteri, Biosens. Bioelectron., 2019, 124–125, 129–135 CrossRef CAS PubMed.
- B. Byrne, E. Stack, N. Gilmartin and R. O'Kennedy, Sensors, 2009, 9, 4407–4445 CrossRef CAS PubMed.
- H. W. Schroeder and L. Cavacini, J. Allergy Clin. Immunol., 2010, 125, S41–S52 CrossRef PubMed.
- S. Sharma, H. Byrne and R. J. O'Kennedy, Essays Biochem., 2016, 60, 9–18 CrossRef PubMed.
- A. Makaraviciute and A. Ramanaviciene, Biosens. Bioelectron., 2013, 50, 460–471 CrossRef CAS PubMed.
- S. Ucci, S. Spaziani, G. Quero, P. Vaiano, M. Principe, A. Micco, A. Sandomenico, M. Ruvo, M. Consales and A. Cusano, Biosensors, 2022, 12(11), 1040 CrossRef CAS PubMed.
- M. Pisco and A. Cusano, Sensors, 2020, 20(17), 4705 CrossRef PubMed.
- E. M. McConnell, J. Nguyen and Y. Li, Front. Chem., 2020, 8 DOI:10.3389/fchem.2020.00434.
- S. Song, L. Wang, J. Li, C. Fan and J. Zhao, TrAC, Trends Anal. Chem., 2008, 27, 108–117 CrossRef CAS.
- S. Tombelli, M. Minunni and M. Mascini, Biomol. Eng., 2007, 24, 191–200 CrossRef CAS PubMed.
- T. Hermann and D. J. Patel, Science, 2000, 287, 820–825 CrossRef CAS PubMed.
- J. Arlett, E. Myers and M. Roukes, Nat. Nanotechnol., 2011, 6, 203–215 CrossRef CAS PubMed.
- F. V. Oberhaus, D. Frense and D. Beckmann, Biosensors, 2020, 10, 45 CrossRef CAS PubMed.
- W. Ning, S. Hu, C. Zhou, J. Luo, Y. Li, C. Zhang, Z. Luo and Y. Li, Anal. Chim. Acta, 2023, 1278, 341733 CrossRef CAS PubMed.
- Y. Zheng, C. Liu, N. Y. G. Lai, Q. Wang, Q. Xia, X. Sun and S. Zhang, Front. Bioeng. Biotechnol., 2023, 11 DOI:10.3389/fbioe.2023.1190211.
- V. Naresh and N. Lee, Sensors, 2021, 21(4), 1109 CrossRef CAS PubMed.
- L. Farzin, M. Shamsipur, L. Samandari and S. Sheibani, Microchim. Acta, 2018, 185, 276 CrossRef PubMed.
- C. Chen, R. Wang, X. Chen and S. Liu, Optics Frontiers Online 2020: Distributed Optical Fiber Sensing Technology and Applications, 2021, p. 116070O Search PubMed.
- L. Xie, X. Zi, Q. Meng, Z. Liu and L. Xu, Sensors, 2019, 19(7), 1656 CrossRef CAS PubMed.
- C. Jesús, F. Socorro, H. J. R. D. Rivera and M. R. D. Rivera, J. Therm. Anal. Calorim., 2014, 116, 151–155 CrossRef.
- A. V. Tregubov, V. V. Prikhodko, A. S. Alekseyev, S. G. Novikov, G. V. Tertyshnikova and A. V. Zhukov, IEEE Sens. Lett., 2022, 6, 1–4 Search PubMed.
- J. Rife, M. Miller, P. Sheehan, C. Tamanaha, M. Tondra and L. Whitman, Sens. Actuators, A, 2003, 107, 209–218 CrossRef CAS.
- Y. Qi and F.-R. Xiu, J. Lumin., 2016, 171, 238–245 CrossRef CAS.
- N. Wang, N. Zhang, T. Wang, F. Liu, X. Wang, X. Yan, C. Wang, X. Liu, P. Sun and G. Lu, Sens. Actuators, B, 2022, 350, 130854 CrossRef CAS.
- A. T. Güntner, I. C. Weber, S. Schon, S. E. Pratsinis and P. A. Gerber, Sens. Actuators, B, 2022, 367, 132182 CrossRef.
- S. G. Song, S. Ha, H. J. Cho, M. Lee, D. Jung, J. H. Han and C. Song, ACS Appl. Nano Mater., 2019, 2, 109–117 CrossRef CAS.
- R. Amali, H. Lim, I. Ibrahim, Z. Zainal and S. Ahmad, J. Electroanal. Chem., 2022, 918, 116440 CrossRef CAS.
- L. Cheng, W. Zheng, Y.-N. Zhang, X. Li and Y. Zhao, IEEE Trans. Instrum. Meas., 2023, 72, 1–7 Search PubMed.
- M. Govindasamy, C. R. Jian, C. F. Kuo, A. H. Hsieh, J. L. Sie and C. H. Huang, Microchim. Acta, 2022, 189, 374 CrossRef CAS PubMed.
- Z. Gu, A. Fu, L. Ye, K. Kuerban, Y. Wang and Z. Cao, ACS Sens., 2019, 4, 2922–2929 CrossRef CAS PubMed.
- W. Weng, J. Liu, C. Yin, H. Xie, G. Luo, W. Sun and G. Li, Int. J. Electrochem. Sci., 2019, 14, 4309–4317 CrossRef CAS.
- O. Parlak, S. T. Keene, A. Marais, V. F. Curto and A. Salleo, Sci. Adv., 2018, 4, eaar2904 CrossRef CAS PubMed.
- S. Qazi and K. Raza, Smart Biosensors in Medical Care, Academic Press, 2020, pp. 65–85 Search PubMed.
- A. A. Karyakin, Sensors for Environment, Health and Security, Dordrecht, 2009, pp. 255–265 Search PubMed.
- C. Gonçalves, A. F. da Silva, J. Gomes and R. Simoes, Inventions, 2018, 3, 1–13 CrossRef.
- F. Narita and M. Fox, Adv. Eng. Mater., 2018, 20, 1–22 CrossRef.
- S. Khalid, I. Raouf, A. Khan, N. Kim and H. S. Kim, International Journal of Precision Engineering and Manufacturing-Green Technology, 2019, 6, 821–851 CrossRef.
- Y. Zhang, J. Zhou, Y. Zhang, D. Zhang, K. T. Yong and J. Xiong, Adv. Sci., 2022, 9, 1–28 Search PubMed.
- S. Zhu, Z. Fan, B. Feng, R. Shi, Z. Jiang, Y. Peng, J. Gao, L. Miao and K. Koumoto, Energies, 2022, 15, 1–27 Search PubMed.
- T. Lu, S. Ji, W. Jin, Q. Yang, Q. Luo and T.-L. Ren, Sensors, 2023, 23, 2991 CrossRef CAS PubMed.
- S. Naval, A. Jain and D. Mallick, Eng. Res. Express, 2022, 4, 045022 CrossRef.
- A. Toprak and O. Tigli, Appl. Phys. Rev., 2014, 1, 031104 Search PubMed.
- L. Wang, Z. Fei, Y. Qi, C. Zhang, L. Zhao, Z. Jiang and R. Maeda, ACS Appl. Energy Mater., 2022, 5, 7091–7114 CrossRef CAS.
- M. Pozzi, M. S. H. Aung, M. Zhu, R. K. Jones and J. Y. Goulermas, Smart Mater. Struct., 2012, 21, 075023 CrossRef.
- C. Dagdeviren, B. D. Yang, Y. Su, P. L. Tran, P. Joe, E. Anderson, J. Xia, V. Doraiswamy, B. Dehdashti, X. Feng, B. Lu, R. Poston, Z. Khalpey, R. Ghaffari, Y. Huang, M. J. Slepian and J. A. Rogers, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 1927–1932 CrossRef CAS PubMed.
- S. Niu, X. Wang, F. Yi, Y. S. Zhou and Z. L. Wang, Nat. Commun., 2015, 6, 8975 CrossRef CAS PubMed.
- G. Conta, A. Libanori, T. Tat, G. Chen and J. Chen, Adv. Mater., 2021, 33, 2007502 CrossRef CAS PubMed.
- S. Niu, S. Wang, L. Lin, Y. Liu, Y. S. Zhou, Y. Hu and Z. L. Wang, Energy Environ. Sci., 2013, 6, 3576–3583 RSC.
- J. Qi and L. A. Garza, Cold Spring Harb. Perspect. Med., 2014, 4, a013615 CrossRef PubMed.
- Z. Zhao, Y. Lu, Y. Mi, J. Meng, X. Wang, X. Cao and N. Wang, Biosensors, 2022, 12, 1127 CrossRef CAS PubMed.
- A. Páez-montoro, M. García-valderas, E. Olías-ruíz and C. López-ongil, Sensors, 2022, 22(10), 3950 CrossRef PubMed.
- T. Zhang, Z. Wen, Y. Liu, Z. Zhang, Y. Xie and X. Sun, iScience, 2020, 23, 101813 CrossRef CAS PubMed.
- C. Xu, Y. Song, M. Han and H. Zhang, Microsyst. Nanoeng., 2021, 7, 25 CrossRef PubMed.
- A. Ali, H. Shaukat, S. Bibi, W. A. Altabey, M. Noori and S. A. Kouritem, Energy Strat. Rev., 2023, 49, 101124 CrossRef.
- M. N. Hasan, M. Nafea, N. Nayan and M. S. Mohamed Ali, Adv. Mater. Technol., 2022, 7, 2101203 CrossRef.
- A. J. Bandodkar, S. P. Lee, I. Huang, W. Li, S. Wang, C. J. Su, W. J. Jeang, T. Hang, S. Mehta, N. Nyberg, P. Gutruf, J. Choi, J. Koo, J. T. Reeder, R. Tseng, R. Ghaffari and J. A. Rogers, Nat. Electron., 2020, 3, 554–562 CrossRef CAS.
- B. Lu, Y. Chen, D. Ou, H. Chen, L. Diao, W. Zhang, J. Zheng, W. Ma, L. Sun and X. Feng, Sci. Rep., 2015, 5, 16065 CrossRef CAS PubMed.
- Z. Zhou, L. Weng, T. Tat, A. Libanori, Z. Lin, L. Ge, J. Yang and J. Chen, ACS Nano, 2020, 14, 14126–14133 CrossRef CAS PubMed.
- Y. Kuang, Z. Yang and M. Zhu, Smart Mater. Struct., 2016, 25, 085029 CrossRef.
- M. P. Sikka, S. Kumar and S. Garg, 2023 Joint International Conference on Digital Arts, Media and Technology with ECTI Northern Section Conference on Electrical, Electronics, Computer and Telecommunications Engineering (ECTI DAMT NCON), 2023, pp. 511–516 Search PubMed.
- M. Popescu and C. Ungureanu, Materials, 2023, 16(11), 4075 CrossRef CAS PubMed.
- I. I. Shuvo and P. I. Dolez, Functional and Technical Textiles, Woodhead Publishing, 2023, pp. 333–372 Search PubMed.
- M. K. Kangazi and A. A. Merati, Advances in Healthcare and Protective Textiles, Woodhead Publishing, 2023, pp. 23–56 Search PubMed.
- J. Yin, S. Wang, A. Di Carlo, A. Chang, X. Wan, J. Xu, X. Xiao and J. Chen, Med-X, 2023, 1, 3 CrossRef.
- H. Li, R. Fan, B. Zou, J. Yan, Q. Shi and G. Guo, J. Nanobiotechnol., 2023, 21, 73 CrossRef PubMed.
- A. Leal-Junior and A. Frizera-Neto, Optical Fiber Sensors for the Next Generation of Rehabilitation Robotics, Elsevier, 2022 Search PubMed.
- S. G. Farag, 2019 4th MEC International Conference on Big Data and Smart City (ICBDSC), 2019, pp. 1–5 Search PubMed.
- S. Javadinasab Hormozabad, M. Gutierrez Soto and H. Adeli, Expert Syst., 2021, 38(8), e12845 CrossRef.
- E. Yarali, M. Baniasadi, A. Zolfagharian, M. Chavoshi, F. Arefi, M. Hossain, A. Bastola, M. Ansari, A. Foyouzat, A. Dabbagh, M. Ebrahimi, M. J. Mirzaali and M. Bodaghi, Appl. Mater. Today, 2022, 26, 101306 CrossRef.
- M. Ramezani and Z. Mohd Ripin, J. Funct. Biomater., 2023, 14(7), 347 CrossRef PubMed.
- A. Leal-Junior, L. Avellar, A. Frizera and C. Marques, Sci. Rep., 2020, 10, 13867 CrossRef CAS PubMed.
- A. Issatayeva, A. Beisenova, D. Tosi and C. Molardi, Sensors, 2020, 20, 1–16 CrossRef PubMed.
- Z. Gong, Z. Xiang, X. OuYang, J. Zhang, N. Lau, J. Zhou and C. C. Chan, Materials, 2019, 12(20), 3311 CrossRef CAS PubMed.
- L. G. Gomes, R. de Mello and A. Leal-Junior, Opt. Fiber Technol., 2023, 78, 103313 CrossRef.
- A. G. Leal-Junior, C. R. Díaz, M. F. Jiménez, C. Leitão, C. Marques, M. J. Pontes and A. Frizera, IEEE Sens. J., 2019, 19, 567–574 Search PubMed.
- A. G. Leal-Junior, C. R. Díaz, C. Leitão, M. J. Pontes, C. Marques and A. Frizera, Opt. Laser Technol., 2019, 109, 429–436 CrossRef CAS.
- B. Najafi, H. Mohseni, G. S. Grewal, T. K. Talal, R. A. Menzies and D. G. Armstrong, J. Diabetes Sci. Technol., 2017, 11, 668–677 CrossRef PubMed.
- T. Li, F. Qiao, P. Huang, Y. Su, L. Wang, X. Li, H. Li, Y. Tan and Z. Zhou, IEEE Sens. J., 2022, 22, 19336–19345 CAS.
- Y. W. Sasy Chan, H.-P. Wang and P. Xiang, Symmetry, 2021, 13(12), 2251 CrossRef.
- R. de Oliveira, O. Frazão, J. Santos and A. Marques, Comput. Struct., 2004, 82, 1315–1321 CrossRef.
- H. Rocha, C. Semprimoschnig and J. P. Nunes, Eng. Struct., 2021, 237, 112231 CrossRef.
- S. Minakuchi and N. Takeda, Photonic Sens., 2013, 3, 345–354 CrossRef.
- C. Guignier, B. Camillieri, M. Schmid, R. M. Rossi and M.-A. Bueno, Sensors, 2019, 19, 3011 CrossRef CAS PubMed.
- L. Avellar, G. Delgado, C. Marques, A. Frizera, A. Leal-Junior and E. Rocon, IEEE Sens. J., 2021, 21, 20078–20085 CAS.
- K. Xiao, Z. Wang, Y. Ye, C. Teng and R. Min, Biomed. Opt. Express, 2024, 15, 1892 CrossRef PubMed.
- W. Q. Mok, W. Wang and S. Y. Liaw, Int. J. Nurs. Pract., 2015, 21, 91–98 CrossRef PubMed.
- W. H. Organization, Ageing and health — who.int, https://www.who.int/news-room/fact-sheets/detail/ageing-and-health#:~:text=By%202030%2C%201%20in%206,will%20double%20(2.1%20billion), 2022, [Accessed 14 mar 2024].
- H. Suominen, Eur. Rev. Aging Phys. Act., 2010, 8, 37–42 CrossRef.
- E. Batista, M. A. Moncusi, P. López-Aguilar, A. Martínez-Ballesté and A. Solanas, Sensors, 2021, 21, 6886 CrossRef CAS PubMed.
- W. Lyu, S. Chen, F. Tan and C. Yu, Photonics, 2022, 9, 50 CrossRef CAS.
- Y. Haseda, J. Bonefacino, H.-Y. Tam, S. Chino, S. Koyama and H. Ishizawa, Sensors, 2019, 19, 5088 CrossRef PubMed.
- A. Leal-Junior, L. Avellar, A. Frizera and C. Marques, Sci. Rep., 2020, 10, 13867 CrossRef CAS PubMed.
- E. P. Córcoles and M. G. Boutelle, Biosensors and Invasive Monitoring in Clinical Applications, Springer International Publishing, 2013 Search PubMed.
- G. Rong, S. R. Corrie and H. A. Clark, ACS Sens., 2017, 2, 327–338 CrossRef CAS PubMed.
- P. C. Pandey, G. Pandey and R. J. Narayan, Front. Bioeng. Biotechnol., 2020, 8, 894 CrossRef PubMed.
- D. Ma, S. Chon, S. Cho, Y. Lee, M. Yoo, D. Kim, D. Y. Lee and J. K. Lim, J. Electroanal. Chem., 2020, 876, 114720 CrossRef CAS.
- J. Lietard, D. Ameur, M. J. Damha and M. M. Somoza, Angew. Chem., Int. Ed., 2018, 57, 15257–15261 CrossRef CAS PubMed.
- S. Fruncillo, X. Su, H. Liu and L. S. Wong, ACS Sens., 2021, 6, 2002–2024 CrossRef CAS PubMed.
- R. Raman and R. Bashir, Essentials of 3D Biofabrication and Translation, Elsevier, 2015, pp. 89–121 Search PubMed.
- G. Remaggi, A. Zaccarelli and L. Elviri, Chemosensors, 2022, 10, 65 CrossRef CAS.
- F. Virgilio, M. Prasciolu, P. Ugo and M. Tormen, Microelectron. Eng., 2013, 111, 320–324 CrossRef CAS.
- N. A. Cinel, S. Butun and E. Özbay, Opt. Express, 2012, 20, 2587 CrossRef CAS PubMed.
- S. Singh, J. Wang and S. Cinti, ECS Sens. Plus, 2022, 1, 023401 CrossRef CAS.
- M. U. Ahmed, M. M. Hossain, M. Safavieh, Y. L. Wong, I. A. Rahman, M. Zourob and E. Tamiya, Crit. Rev. Biotechnol., 2015, 1–11 CrossRef PubMed.
- D. Albanese, M. D. Matteo and C. Alessio, Computer Aided Chemical Engineering, Elsevier, 2010, pp. 283–288 Search PubMed.
- F. Arduini, L. Micheli, D. Moscone, G. Palleschi, S. Piermarini, F. Ricci and G. Volpe, TrAC, Trends Anal. Chem., 2016, 79, 114–126 CrossRef CAS.
- A. Hussain, N. Abbas and A. Ali, Chemosensors, 2022, 10, 103 CrossRef CAS.
- V. Beedasy and P. J. Smith, Materials, 2020, 13, 704 CrossRef CAS PubMed.
- J. Li, M. C. Lemme and M. Östling, ChemPhysChem, 2014, 15, 3427–3434 CrossRef CAS PubMed.
- K. Zub, S. Hoeppener and U. S. Schubert, Adv. Mater., 2022, 34, 2105015 CrossRef CAS.
- T. Lu, S. Ji, W. Jin, Q. Yang, Q. Luo and T.-L. Ren, Sensors, 2023, 23(6), 2991 CrossRef CAS PubMed.
- E. De la Paz, N. H. Maganti, A. Trifonov, I. Jeerapan, K. Mahato, L. Yin, T. Sonsa-ard, N. Ma, W. Jung, R. Burns, A. Zarrinpar, J. Wang and P. P. Mercier, Nat. Commun., 2022, 13, 7405 CrossRef CAS PubMed.
- K. Kalantar-zadeh, N. Ha, J. Z. Ou and K. J. Berean, ACS Sens., 2017, 2, 468–483 CrossRef CAS PubMed.
- K. Kalantar-Zadeh, K. J. Berean, N. Ha, A. F. Chrimes, K. Xu, D. Grando, J. Z. Ou, N. Pillai, J. L. Campbell, R. Brkljača, K. M. Taylor, R. E. Burgell, C. K. Yao, S. A. Ward, C. S. Mc-Sweeney, J. G. Muir and P. R. Gibson, Nat. Electron., 2018, 1, 79–87 CrossRef.
- K. Oliphant and E. Allen-Vercoe, Microbiome, 2019, 7(1), 91 CrossRef PubMed.
- J. P. Geibel and M. Physiology, World J. Gastroenterol., 2005, 11, 5259–5265 CrossRef CAS PubMed.
- C. Cheng, Y. Wu, X. Li, Z. An, Y. Lu, F. Zhang, B. Su and Q. Liu, Sens. Actuators, B, 2021, 349, 130781 CrossRef CAS.
- W. Xu, H. Yang, W. Zeng, T. Houghton, X. Wang, R. Murthy, H. Kim, Y. Lin, M. Mignolet, H. Duan, H. Yu, M. Slepian and H. Jiang, Adv. Mater. Technol., 2017, 2, 1700181 CrossRef.
- S. Mukherjee, S. Suleman, R. Pilloton, J. Narang and K. Rani, Sensors, 2022, 22(11), 4228 CrossRef CAS PubMed.
- D. Smith and S. Jheeta, Gastrointestinal Disorders, 2020, 2, 3–11 CrossRef.
- S. N. Adler, Y. Metzger, C. Misrachi and R. Klar, Gastrointest. Endosc., 2007, 65, AB165 Search PubMed.
- P. L. Rajbhandary and G. Nallathambi, 2020 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), 2020, pp. 4652–4655 Search PubMed.
- M. Brun, W. Michalek, B. Surjanhata and B. Kuo, Gastroenterology, 2011, 140, S-865 CrossRef.
- P. J. van der Schaar, F. Dijksman, J. Shimizu, C. Wanke and P. D. Siersema, Gastroenterology, 2011, 140, S-766 CrossRef.
- C. J. Bettinger, Angew. Chem., 2018, 130, 17190–17203 CrossRef.
- P. Nadeau, D. El-Damak, D. Glettig, Y. L. Kong, S. Mo, C. Cleveland, L. Booth, N. Roxhed, R. Langer, A. P. Chandrakasan and G. Traverso, Nat. Biomed. Eng., 2017, 1, 0022 CrossRef CAS PubMed.
- Y. J. Kim, W. Wu, S. E. Chun, J. F. Whitacre and C. J. Bettinger, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 20912–20917 CrossRef CAS PubMed.
- C. Dagdeviren, Z. Li and Z. L. Wang, Annu. Rev. Biomed. Eng., 2017, 19, 85–108 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |