Open Access Article
Open Access ArticleAggregation-induced emission-active azines for chemosensing applications: a five-year update
Akhil A.
Bhosle
,
Mainak
Banerjee
 * and
Amrita
Chatterjee
* and
Amrita
Chatterjee
 *
*
Department of Chemistry, BITS-Pilani, K K Birla Goa Campus, NH 17B Bypass Road, Zuarinagar 403726, Goa, India. E-mail: p20190018@goa.bits-pilani.ac.in; mainak@goa.bits-pilani.ac.in; amrita@goa.bits-pilani.ac.in; Tel: +91 832 2580 347 Tel: +91 832 2580 320
First published on 11th April 2024
Abstract
Azines are an important class of compounds that display solvent-dependent fluorescence emission depending upon the substituents in the aromatic scaffolds. They are affordable, easy to synthesize, stable, and well-suited for numerous applications. Unlike most other AIE fluorophores, aromatic units of AIE-active azine derivatives are bridged by rotatable N–N bonds rather than C–C bonds. Their derivatives have been widely used in pharmaceuticals, drug delivery, organometallics, optoelectronics, dyes, etc., and most importantly in several sensing applications. This comprehensive review encapsulates the recent developments in the field of AIE-active azine molecules and their applications as chemosensors in the detection of various analytes. The review discusses the different chemosensing strategies involved in the detection of metal ions (Cu2+, Zn2+, Al3+, Fe3+, Cr3+, Hg2+, UO22+, etc.), anions (F−, CN−, ClO−, ONOO−, HSO3−), small molecules (thiols, hydrazine, hydrogen peroxide), and bio-analytes (protamine/heparin, HSA/BSA, neuraminidase, β-lactamase, β-galactosidase, etc.) with a focus on the development in the last five years. The review also highlights the advancements in azine-based systems for their use in imaging, supramolecular host–guest recognitions, AIE polymers, COFs/MOFs, etc.
1. Introduction
Azines, also known as 2,3-diazabutadienes, are formed by the condensation of carbonyl compounds with hydrazine molecules. They are either symmetrical or unsymmetrical depending upon the carbonyl compounds that combine.1 These compounds are highly stable, involve easy synthetic protocols, are inexpensive and suitable for diverse applications.2 Azines with aromatic units bridged by rotatable N–N bonds are one of the few classes of compounds with high fluorescence efficacy. These compounds have been widely used in molecular sensing, particularly for ion sensing, bio-analyte and enzyme detection, pharmaceuticals, dyes, drug delivery, bioimaging, organometallics, optoelectronics, solar cells, light-harvesting systems, etc.3 Polyazines displaying excellent mechanical, thermal, and semi-conductor properties have been developed from azines as monomers and utilized for electronic and photonic applications.4Conventional fluorophores exhibit strong fluorescence emissions in diluted organic solvents. However, a partial or complete quenching is displayed upon aggregation or in concentrated solutions, reducing their overall efficiency in sensing and bioimaging of the analytes.5 This effect is called the concentration quenching or the aggregation-caused quenching (ACQ) effect. One of the primary examples of the ACQ effect was studied by Förster and Kasper in 1955, wherein they observed a decrease in the fluorescence emission of pyrene upon increasing the concentration due to the formation of sandwich-shaped excimers and exciplexes (disc-like structures) at the excited state.6 However, Prof. B. Z. Tang introduced the exact reverse phenomenon in 2001 for a new set of luminophores that fluoresce strongly upon aggregation and could overcome the disadvantages of the ACQ molecules. They coined the term “aggregation-induced emission” to explain the strong emission of propeller-shaped non-planar molecule 1,1′,2,3,4,5-hexaphenyl-1H-silole (HPS) by increasing the water fractions in a THF solution.7 Subsequently, several molecules exhibiting AIE phenomena have been reported in the last two decades and are termed “AIEgens”.8 AIEgens display multiple advantages compared to traditional ACQ molecules in their high fluorescence quantum yield in aggregated states and at higher concentrations, significant Stokes shift, and low cytotoxicity.
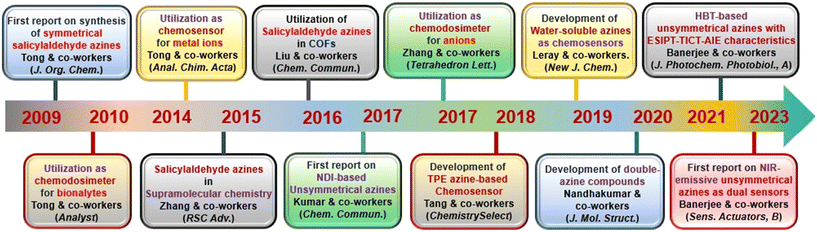
AIE-active azine molecules have paved the way for innovative technologies in diverse areas including chemosensing,3 organic electronic devices,9 nanomaterials,10 electro-optical devices,11 organic functional materials,12 battery materials,13 COFs,14 imaging studies,15,16 drug delivery,17 supramolecular chemistry,18 light-harvesting systems,19etc. Over the years, there have been many milestone developments in the field of AIE-active azine-based chemosensors (Scheme 1). As a first report, Tong and co-workers reported the synthesis of a range of salicylaldehyde azines which exhibited noteworthy AIE properties depending on the substitutions on the aromatic scaffold.20 In 2010, Chen et al. developed a carboxylic acid functionalized azine derivative that carries a negative charge in basic pH and demonstrated it for the detection of positively charged protamine owing to their electrostatic interactions.21 In 2014, an azine-derived flavone-based ratiometric probe was reported by Peng et al. for the detection of Al3+ ions in the solution phase which was one of the early developments in azines as chemosensors for metal ions.22 Subsequently, Xu et al. reported an amphiphilic AIE-active azine, a cationic bola-amphiphile with a salicylaldehyde azine moiety that undergoes a 30-fold emission enhancement upon the addition of γ-cyclodextrin due to the formation of a [2]pseudorotaxane inclusion complex.18 The azine probe can specifically localize in mitochondria of living cells for fluorescent imaging. In 2016, Liu and co-workers demonstrated an azine-linked luminescent 2D-covalent organic framework incorporating salicylaldehyde with bulky groups as building blocks, which was utilized for Cu2+ ion sensing in a selective and sensitive manner.23 The development of an unsymmetrical azine-derived probe with a naphthalimide moiety was reported by Kumar and co-workers which was utilized for the ratiometric detection of Al3+ ions in the solution phase.24 At a similar time, Zhang & co-workers developed an AIE-active azine-based chemodosimeter by tert-butyl dimethylsilyl protection for the sensing of fluoride ions.25 In 2018, Tang & co-workers reported a tetraphenylethylene-conjugated AIE-active azine by condensation of two TPE units that could recognize Cu2+ ions.26 A year later, Nguyen et al. synthesized a water-soluble azine probe to detect Al3+ in aqueous solutions.27 In 2020, Manigandan et al. reported a salicylaldehyde-based double azine probe as a highly reliable Fe3+ sensor.28 In the next year, an azine with ESIPT-TICT-AIE triple photophysical characteristics, which is fluorescent both in solution and solid phase featuring orange emission, was reported by our group for the first time and demonstrated in the turn-off sensing of Cu2+ ions both in solution and solid phase.29 Our group further envisaged an AIE active NIR-emissive unsymmetrical azine as a dual-mode-dual-chemodosimeter for the selective and sensitive detection of toxic analytes, N2H4 and HSO3−.30 The upcoming section will reflect the advancements in the realm of AIE-active luminogens based on the azine moiety, shedding light on their design, synthesis, optical properties and diverse chemosensing applications. Furthermore, the use of azines in supramolecular sensing, and various azine-derived materials like polymers, MOFs, and COFs in chemosensing applications has been captured in this review. Azines as dual sensors in multi-targeted sensing and imaging are also covered.
2. Design and exploration of photophysical properties of azines
Azines, owing to their structural skeleton, display excellent AIE characteristics depending upon the nature of substituents present on the aromatic scaffold. In addition, the presence of different substituents on the aromatic scaffold along the azine linkage induces excellent twisted-intramolecular charge transfer (TICT) characteristics in the scaffold. Similarly, the presence of a hydrogen bond donor functionality such as –OH or –NH2 induces stronger intramolecular hydrogen bonding between the donor functionality and the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N
N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– linkage resulting in the phenomenon known as excited state intramolecular proton transfer (ESIPT). This section will highlight the design of AIE-active azines for their tunable photophysical properties.
C– linkage resulting in the phenomenon known as excited state intramolecular proton transfer (ESIPT). This section will highlight the design of AIE-active azines for their tunable photophysical properties.
In one of the early attempts, Tong and co-workers synthesized a series of salicylaldehyde-based azine compounds (1) that showed appreciable aggregation-induced emission properties [Fig. 1A].20 These compounds fluoresced weakly in good organic solvents but strongly in poor solvents. Depending on the substituents in the aromatic scaffold, these azines displayed green to orange emission (λmax = 513 nm to 570 nm). The unsubstituted azine, 1b, displayed yellow emission at λmax 542 nm, whereas when the electron-donating OH group is at the 4-position, azine 1a showed intense emission at λmax 513 nm. Notably, with the presence of a –Cl substituent at the 5-position, the emission of 1c showed a red shift to λmax 570 nm. These azines were further explored in hydrazine sensing, which will be discussed later. The same group further envisaged that a planar conjugated core attached to a donor–acceptor (D–A) pair might be a design principle for developing new organic materials with solid luminescence-switching properties. Accordingly, they investigated a symmetrical azine, 2, for its solid-state fluorescence properties by incorporating a strong electron-donating diethylamino group at the 4-position of the salicylaldehyde azine [Fig. 1B].31 The azine was responsive to external stimuli like mechanical grinding and temperature. The yellow fluorescence of the compound with emission at 550 nm underwent a significant hypsochromic shift upon grinding or annealing to generate another crystalline form of 2 which emits at 529 nm. The solid-phase transformation with reduced π–π interactions also afforded an amorphous solid that emits at 529 nm with a shoulder peak at 550 nm with intense green fluorescence. The presence of the donor diethylamino group and the acceptor azine moiety functions as a push–pull system and is crucial for solid-state fluorescence-switching. The azines with such push–pull groups could potentially be used in new pressure/thermo-sensing devices. In their continuous study, the authors further synthesized a conformationally flexible symmetrical azine, 3, by condensing hydrazine with (2-hydroxy-4-methoxyphenyl)(phenyl)-methanone [Fig. 1C].32 They studied its ESIPT fluorescence changes with respect to the two different polymorphs, green and yellowish green fluorescence, respectively. It was observed that the green emitting polymorph, when annealed at 231 °C for 1 h, induced the phenyl units to stray from their energetically preferred vertical conformation by stronger interactions between them. In contrast, annealing the yellowish-green polymorph eliminated intermolecular connection, restoring the energetically favored conformation. Thus, by alternating annealing/melting procedures, the azine ESIPT fluorescence could be reversibly modified with different emission wavelengths having substantial Stokes shift, high stability and quantum yield.
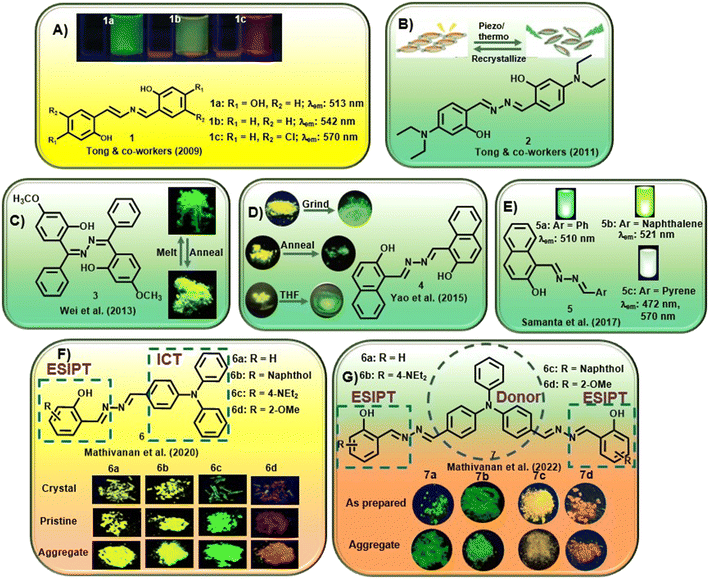 | ||
| Fig. 1 Selected examples of AIE-active azine compounds studied for their photophysical properties (adapted with permission from: (A) ref. 20. Copyright 2009, American Chemical Society; (B) ref. 31. Copyright 2011, American Chemical Society; (C) ref. 32. Copyright 2013, American Chemical Society; (D) ref. 33. Copyright 2015, Wiley-VCH; (E) ref. 34. Copyright 2017, Royal Society of Chemistry; (F) ref. 35. Copyright 2020, Royal Society of Chemistry; (G) ref. 36. Copyright 2022, Elsevier). | ||
Yao et al. could inculcate mechanochromism in a commercial dye, Pigment Yellow 101. The dye, 4, was susceptible to mechanical grinding or heat and switched between two structurally distinct polymorphs [Fig. 1D].33 It was observed that the yellow fluorescence at 543 nm from one polymorph gets converted to the second polymorph with green emission at 530 nm upon grinding in a mortar pestle. In contrast, adding THF or 1,4-dioxane and further grinding after removal of the solvent results in a piezofluorochromic behavior to afford the third polymorph with emission at 566 nm. When heated to 285 °C and annealed, the polymorph converts from yellow to green fluorescence. A reversible transition was obtained after melting and cooling quickly to afford the two original polymorphs.
Samanta et al. demonstrated unsymmetrical azines, 5, to emit white light from a single-component system [Fig. 1E].34 The naphthol moiety and the azine linkage induced AIE behavior in these compounds and the emission of the azine could be tuned with different water percentages in methanol–water and acetonitrile–water combinations. The introduction of a phenyl (5a), naphthyl (5b) and pyrene (5c) moiety was investigated for linking the AIE scaffold of the naphthyl moiety with dual emission behavior (comprising complementary emission colors) to obtain white light. It was observed that compounds 5a and 5b fluoresced strongly at 510 nm and 521 nm, respectively. However, the presence of the pyrene moiety in 5c afforded a distinct extra emission peak owing to its tendency to form an excimer and resulted in two peaks at 472 and 560 nm. 5c exhibited variable emission based on the change in the percentage of water and emitted white fluorescence at 70%, 80% and 90% water fractions.
Mathivanan et al. synthesized a short series of donor–acceptor unsymmetrical azines, 6, by condensation of triphenylamine hydrazone and various salicylaldehyde derivatives [Fig. 1F].35 The azines were designed by utilizing electron-rich triphenylamine as the donor and AIE-active core that also introduces ICT behavior in the scaffold. The imine nitrogen of the azine linkage acts as an electron acceptor and the incorporation of salicylaldehyde units further induces ESIPT behavior with tunable characteristics in the system. The crystal structures confirmed the formation of a supramolecular network from the weak solid-state interactions in addition to the solvent polarity-dependent positive solvatochromic behavior. Electron-donating triphenylamine and diethylamino groups on the scaffold induced pH-dependent emissive properties in the solution. In a continuous effort, the same group further reported a short series of triphenylamine-based symmetrical azines (7) involving an acceptor–donor–acceptor type moiety [Fig. 1G].36 In an attempt to incorporate the ESIPT property in the acceptor–donor–acceptor symmetrical systems, similar to the previous report, an electron-rich triphenylamine unit was utilized as the AIE core as well as the strong donor unit, whereas substituted salicylaldimine units were utilized as electron acceptor groups along with the ESIPT unit. The azines showed weak fluorescence in solution and significant multi-color emission in the aggregated/solid state, depending on the peripheral substituent owing to the ESIPT and AIE properties. The presence of the methoxy group on the aromatic scaffold was found to exhibit a bathochromic shift and ultimately afford red emission.
Recently, our group discussed the design principle for unsymmetrical azines displaying ESIPT-TICT-AIE triple photophysical properties by combining hydroxybenzothiazole and benzophenone hydrazones [Fig. 2A].37 The incorporation of a hydroxybenzothiazole (HBT) unit rendered the ESIPT property in the system by keto–enol tautomerism, whereas the azine linkage along with the two phenyl units of hydrazone induces TICT property in the scaffold. The greener and cost-effective mechanochemical method afforded the azine compounds within a short time, avoiding harmful solvents and tedious workup procedures. The orange-to-red emissive azines are highly fluorescent and displayed ESIPT characteristics in organic solvents and AIE characteristics with increased water fractions [Fig. 2A]. The azines revealed solvent-polarity-dependent emission intensities and wavelength shifts based on luminophore aggregation with the maximum emission at 90–93% water fractions in most cases. Incorporating an electron-donating group at the diphenyl methylene unit facilitates electron donation from the donor to the acceptor unit, and a noticeable red shift is observed with the highest red-emission up to 680 nm (8a–d). Similarly, the presence of an EWG at the donor moiety lowered the emission wavelength (8b). The azines exhibited high quantum yields in different solvents and showed high fluorescence emission in the solid state as well. The molecules displayed a D1–A–D2 (or D–π–A) character due to the presence of an EDG (or EWG) at the HBT unit and electron shift from the electron-rich donor diphenyl methylene unit to the acceptor benzothiazole moiety along the azine linkage.
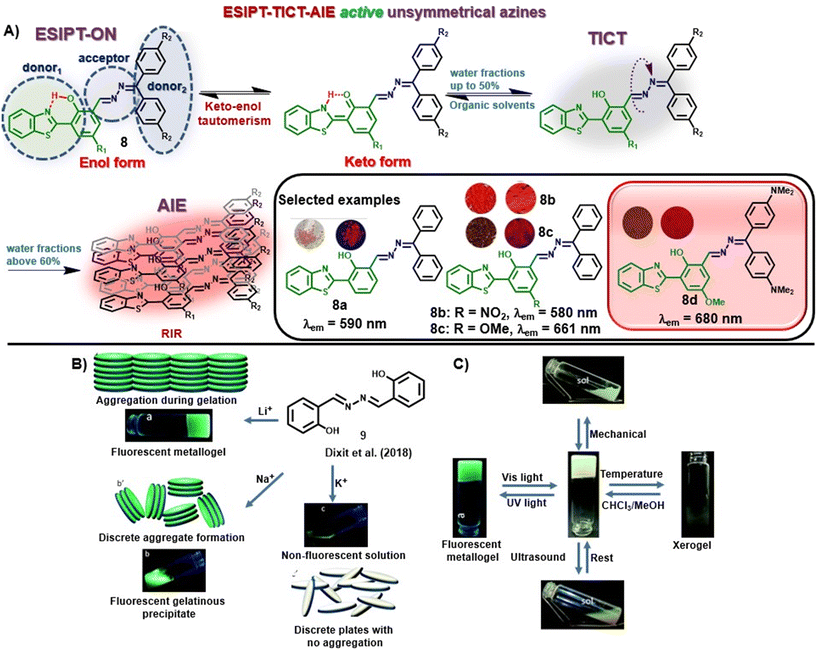 | ||
| Fig. 2 (A) HBT-based unsymmetrical azine dyes (8) having ESIPT-TICT-AIE triple photophysical characteristics (adapted with permission from: ref. 37. Copyright 2023, Wiley-VCH); (B) change in the response of 9 towards LiOH, NaOH, and KOH and their possible molecular arrangement; (C) multi-stimuli responses shown by the metallogel under UV irradiation, ultrasound, mechanical stress and temperature change (images B and C are adapted with permission from ref. 38. Copyright 2018, Royal Society of Chemistry). | ||
In another study, Dixit et al. synthesized a fluorescent metallogel (1% w/v) from a symmetrical azine (9) with LiOH in a CHCl3–MeOH mixture [Fig. 2B].38 The chelation of Li+ with 9 leads to the inhibition of excited state intramolecular proton transfer (ESIPT) or the origin of fluorescence through chelation-enhanced fluorescence (CHEF), and gelation from aggregation. The metallogel exhibited multi-stimuli responses towards thermal and mechanical stress as well as reswelling properties [Fig. 2C].
3. Azines as chemosensors
Chemosensors for tracing toxic analytes are preferable owing to their convenience, low cost, photostability, sensitivity, and selectivity to analytes. Chemosensors are chemical systems that respond to stimuli via fluorescence or color changes. Generally, a recognition site is coupled to a fluorophore, which induces a color or fluorescence change upon interaction with the site-specific analyte and generally involves processes such as ESIPT, PET, ICT, Förster resonance energy transfer (FRET), etc. Several chemosensors based on azines have been developed for the selective and sensitive detection of different analytes. The upcoming section will reflect the development of various azine-based chemosensors for the detection of different analytes in the last five years. The section is further classified into: a) metal ion sensors, b) anion sensors, c) small molecules and bioanalyte sensors.3.1. Metal ion sensors
Metal ions play crucial roles in various essential life processes including body metabolism and regulation of physiological mechanisms.39 They are categorized as biologically essential metal ions (Ca2+, Fe3+, Zn2+ and Cu2+), which must be maintained within an optimal range to ensure their normal biochemical functions, and biologically toxic metal ions (Hg2+ and Pb2+) necessitating timely detection. The selective and sensitive detection of these metal ions has become an enduring research objective as any alterations in their concentrations directly impact normal body and physiological functions.40 In previous years, several AIE-active azines have been designed for the selective detection of metal ions, especially Cu2+, Zn2+ and Al3+, mostly by complexation through the azine –N atoms.22,24,41–45 In general, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or a 1
1 or a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding between the azine and the metal ion is preferred wherein the analyte binds through the oxygen atom of the attached phenol moiety and an azine nitrogen atom. A snap-shot of the azine-based probes, the sensing mechanism, the limit of detection and applications are presented in Table 1.
1 binding between the azine and the metal ion is preferred wherein the analyte binds through the oxygen atom of the attached phenol moiety and an azine nitrogen atom. A snap-shot of the azine-based probes, the sensing mechanism, the limit of detection and applications are presented in Table 1.
| Sr. no. | Probe | Analyte | Mechanism/strategy | λ ab | Solvent system | LOD | Remark | Ref. |
|---|---|---|---|---|---|---|---|---|
| λ em | ||||||||
| (Δλ) | ||||||||
| 1 |
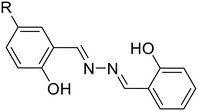 10a, R = H
10a, R = H |
Cu2+ | Turn-off/complexation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu2+ = 1 Cu2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
10a: 400 nm | Phosphate buffer–ACN (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 10 mM, pH 7.4) 1 v/v, 10 mM, pH 7.4) |
10a: 0.15 μM | Fluorescence retrieval with S2− | 46 |
| 552 nm | ||||||||
| (152 nm) | ||||||||
| 10b, R = Cl | 10b: 400 nm | 10b: 0.1 μM | ||||||
| Zhou et al. (2018) | 536 nm | |||||||
| (136 nm) | ||||||||
| 2 |
 8a, Bhosle et al. (2021)
8a, Bhosle et al. (2021) |
Cu2+ | Turn-off/complexation (8a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu2+ = 2 Cu2+ = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
373 nm | H2O–DMF (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
0.005 μM | Fluorescence retrieval with AA | 29 |
| 590 nm | ||||||||
| (217 nm) | ||||||||
| 3 |
 11, Tharmalingam et al. (2019)
11, Tharmalingam et al. (2019) |
Cu2+ | Turn-off/complexation (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu2+ = 1 Cu2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
430 nm | ACN–Tris·HCl buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 10 mM, pH 7.2) 1 v/v, 10 mM, pH 7.2) |
0.23 μM | Fluorescence retrieval with S2− | 47 |
| 470 nm | ||||||||
| (40 nm) | Application in solid-phase detection | |||||||
| 4 |
 12, Zhao et al. (2018)
12, Zhao et al. (2018) |
Cu2+ | Turn-off/complexation (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu2+ = 2 Cu2+ = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
380 nm | H2O–THF (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
0.03 μM | Fluorescence retrieval with cysteine | 26 |
| 581 nm | ||||||||
| (201 nm) | ||||||||
| 5 |
 13, Tiwari et al. (2018)
13, Tiwari et al. (2018) |
Cu2+ | Turn-off/complexation (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu2+ = 1 Cu2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
406 nm | EtOH–HEPES buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 10 mM, pH = 7.4) 1 v/v, 10 mM, pH = 7.4) |
0.34 μM | Fluorescence retrieval with PO43−, HPO42−, H2PO4− | 48 |
| 537 nm | ||||||||
| (131 nm) | ||||||||
| 6 |
 14, Ye et al. (2021)
14, Ye et al. (2021) |
Cu2+ | Turn-off/complexation (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu2+ = 1 Cu2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
400 nm | THF–HEPES buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v, 20 mM, pH 7.4) 19 v/v, 20 mM, pH 7.4) |
0.16 μM | Fluorescence retrieval with S2− | 49 |
| 515 nm | ||||||||
| (115 nm) | ||||||||
| 7 |
 10a, Liu et al. (2022)
10a, Liu et al. (2022) |
Cu2+ | Turn-off/complexation (10a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu2+ = 1 Cu2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
350 nm | H2O–ACN (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
0.57 μM | — | 50 |
| 544 nm | ||||||||
| (194 nm) | ||||||||
| 8 |
 15a, R = H
15a, R = H |
Cu2+ | Turn-on/complexation (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu2+ = 1 Cu2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
350 nm | CAN | 15a: 0.0008 μM | Fluorescence turn-on response toward CN− | 51 |
| 15b, R = Me | 510 nm | 15b: 0.0013 μM | ||||||
| 15c, R = F | (160 nm) | 15c: 0.0013 μM | ||||||
| Kumarasamy et al. (2022) | ||||||||
| 9 |
 16, Sharifi et al. (2023)
16, Sharifi et al. (2023) |
Cu2+ | Turn-on/complexation (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu2+ = 1 Cu2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
335 nm | H2O–ACN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) 4 v/v) |
4.2 μM | — | 52 |
| 671 nm | ||||||||
| (336 nm) | ||||||||
| 10 |
 17, Kumar et al. (2018)
17, Kumar et al. (2018) |
Al3+ | Turn-on/complexation (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ = 1 Al3+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
335 nm | MeOH | 0.27 μM | Application in solid-phase detection | 53 |
| 490 nm | ||||||||
| (155 nm) | ||||||||
| 11 |
 18, Nguyen et al. (2019)
18, Nguyen et al. (2019) |
Al3+ | Turn-on/complexation (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ = 1 Al3+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
390 nm | HEPES buffer (10 mM, pH 7) | 0.153 μM | Application in microfluidic detection | 27 |
| 511 nm | ||||||||
| (121 nm) | ||||||||
| 12 |
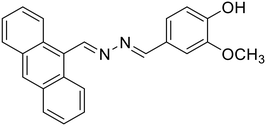 19, Khanra et al. (2020)
19, Khanra et al. (2020) |
Al3+ | Turn-on/complexation (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ = 1 Al3+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
326 nm | SDS media (0.1 M HEPES, pH 7.4) | 0.0628 μM | — | 54 |
| 400 nm | ||||||||
| (74 nm) | ||||||||
| 13 |
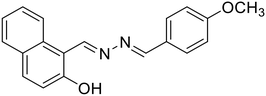 20, Khanra et al. (2019)
20, Khanra et al. (2019) |
Zn2+ | Turn-on/complexation (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn2+ = 1 Zn2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
400 nm | DMSO–bis-Tris buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH 7.4) 1 v/v, pH 7.4) |
0.0335 μM | — | 55 |
| 495 nm | ||||||||
| (95 nm) | ||||||||
| 14 |
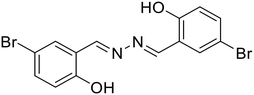 21, Das et al. (2021)
21, Das et al. (2021) |
Zn2+ | Turn-on/complexation (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn2+ = 1 Zn2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
410 nm | DMSO–HEPES buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH 7.2) 1 v/v, pH 7.2) |
2.18 μM | Fluorescence quenching with picric acid | 56 |
| 532 nm | ||||||||
| (122 nm) | Application in logic gates | |||||||
| 15 |
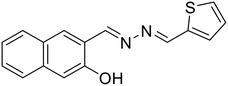 22, Musikavanhu et al. (2022)
22, Musikavanhu et al. (2022) |
Cr3+ | Turn-off/complexation (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cr3+ = 1 Cr3+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
408 nm | 0.2 M HEPES buffer, pH 7.2 | 0.041 μM | Application in solid-phase detection | 57 |
| 526 nm | ||||||||
| (118 nm) | ||||||||
| 16 |
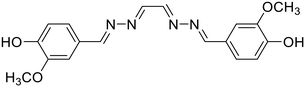 23, Manigandan et al. (2020)
23, Manigandan et al. (2020) |
Fe3+ | Turn-off/complexation (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe3+ = 1 Fe3+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
377 nm | H2O–DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
0.077 μM | Fluorescence retrieval with EDTA | 28 |
| 423 nm | ||||||||
| (46 nm) | ||||||||
| 17 |
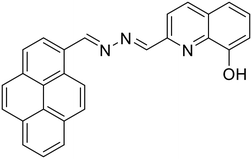 24, Mondal et al. (2020)
24, Mondal et al. (2020) |
Hg2+ | Turn-on/complexation (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hg2+ = 1 Hg2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
360 nm | H2O–EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) 9 v/v) |
0.22 μM | Fluorescence turn-off response towards picric acid | 58 |
| 447 nm | ||||||||
| (87 nm) | ||||||||
| 18 |
 25, Tong et al. (2020)
25, Tong et al. (2020) |
Hg2+ | Turn-on/complexation | 417 nm | H2O–THF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
7.07 μM | Fluorescence turn-off response towards Cu2+ | 59 |
| 558 nm | ||||||||
| (141 nm) | ||||||||
| 19 |
 26, Ghosh et al. (2018)
26, Ghosh et al. (2018) |
Mo6+ | Ratiometric/complexation (26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo6+ = 1 Mo6+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
342 nm | EtOAc–HEPES buffer (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 10 mM, pH 7.4) 1 v/v, 10 mM, pH 7.4) |
0.002 μM | Fluorescence retrieval with S2− | 60 |
| 501 nm ↑ and 415 nm ↓ | ||||||||
| (73 nm) | ||||||||
| 20 |
 27, Pham et al. (2019)
27, Pham et al. (2019) |
UO22+ | Turn-off/complexation (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) UO22+ = 1 UO22+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
365 nm | H2O–ACN (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) 2 v/v) |
0.023 μM | — | 61 |
| 550 nm | ||||||||
| (185 nm) | ||||||||
| 21 |
 28, Yadav et al. (2019)
28, Yadav et al. (2019) |
Al3+ and Cu2+ | Al3+: turn-on, Cu2+: turn-off/complexation (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+/Cu2+ = 1 Al3+/Cu2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
434 nm | DMSO–HEPES–MeOH (0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.0 v/v, pH 7.4) 8.0 v/v, pH 7.4) |
Al3+: 0.16 μM | Fluorescence quenching with F− and retrieval with EDTA | 62 |
| 534 nm | ||||||||
| (100 nm) | ||||||||
| Cu2+: 0.15 μM | Application in solid-phase detection | |||||||
| 22 |
 29, Sun et al. (2023)
29, Sun et al. (2023) |
Al3+ and Cu2+ | Turn-off/complexation (29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+/Cu2+ = 1 Al3+/Cu2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
365 nm | Al3+: H2O–DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) 4 v/v) |
Al3+: 8.47 μM | Application in solid-phase detection | 63 |
| 530 nm | ||||||||
| (165 nm) | ||||||||
Cu2+: H2O–DMSO (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) 3 v/v) |
Cu2+: 0.17 μM | |||||||
| 23 |
 30, Das et al. (2021)
30, Das et al. (2021) |
Al3+ and Zn2+ | Turn-on/complexation (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+/Zn2+ = 1 Al3+/Zn2+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
405 nm | MeOH–HEPES buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH 7.2) 1 v/v, pH 7.2) |
Al3+: 9.78 μM | Application in logic gates | 64 |
| 532 nm | ||||||||
| (127 nm) | Zn2+: 3.65 μM | |||||||
| 24 |
 31, Dolai et al. (2018)
31, Dolai et al. (2018) |
Al3+ and Cr3+ | Turn-on/complexation (31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+/Cr3+ = 1 Al3+/Cr3+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
360 nm | MeOH–HEPES buffer (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH 7.4) 1 v/v, pH 7.4) |
Al3+: 4.3 μM | — | 65 |
| Al3+: 490 nm | ||||||||
| (130 nm) | ||||||||
| Cr3+: 427 nm | Cr3+: 3.4 μM | |||||||
| (67 nm) | ||||||||
| 25 |
 32, Sawminathan et al. (2021)
32, Sawminathan et al. (2021) |
Th4+ and Fe3+ | Turn-on/complexation (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Th4+/Fe3+ = 1 Th4+/Fe3+ = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
442 nm | H2O–ACN (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
Th4+: 0.27 μM | Application in solid-phase detection | 66 |
| 532 nm | ||||||||
| (90 nm) | ||||||||
| Fe3+: 5.4 μM |
To further elaborate, in a recent study, Zhou et al. utilized salicylaldehyde-derived AIE-active azine, 10, for the detection of Cu2+ ions in a turn-off manner via a metal-induced self-assembly mechanism [Fig. 3A and Table 1, entry 1].46 Upon the addition of 2 equiv. of copper ions, its fluorescence emission at 552 nm was vastly quenched (146-fold). The 10–Cu2+ complex was further employed as an S2− sensor owing to the S2−-induced disassembly of AIE-active azine (10). Other metal ions did not complex with 10 and thus they are indifferent to azine, imparting a high selectivity towards Cu2+ ions [Fig. 3B]. The images of turn-off response upon the addition of Cu2+ ions and subsequent fluorescence revival with S2− were captured under 365 nm UV light and also utilized for acquiring the fluorescence microscopy images of KYSE510 cells [Fig. 3C and D].
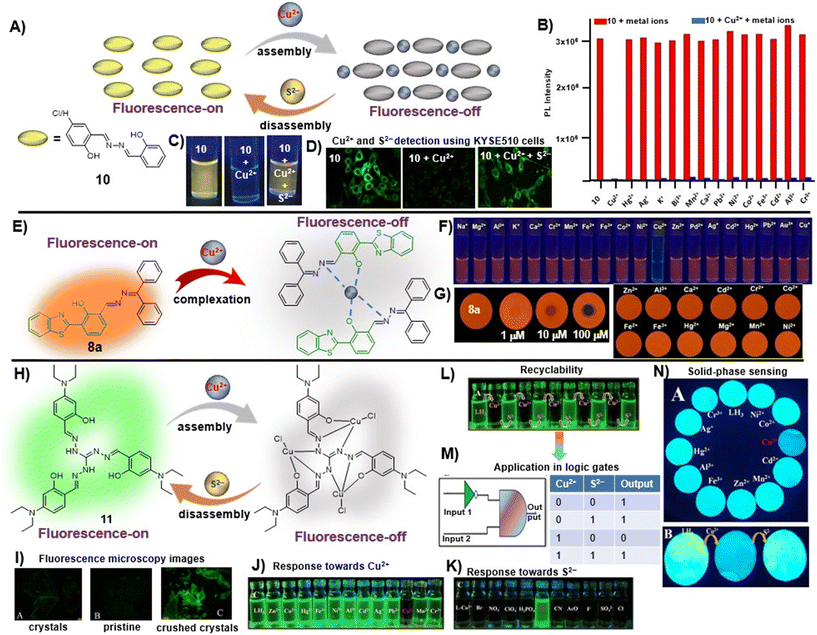 | ||
Fig. 3 (A) Salicylaldehyde-based azine, 10, as a turn-off chemosensor for Cu2+ ions showing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry and fluorescence retrieval upon the addition of sulfide ions; (B) competitive selectivity of 10 towards different metal ions; (C) images of 10 captured upon the addition of Cu2+ ions and S2− under 365 nm UV light depicting visual fluorescence changes; (D) the change in fluorescence microscopy images of KYSE510 cells consisting of 10 after incubation with Cu2+ and Cu2+ + S2− (images B–D are adapted with permission from ref. 46. Copyright 2018, Elsevier); (E) HBT-based unsymmetrical azine (8a) as a turn-off chemosensor for Cu2+ ions showing 2 1 binding stoichiometry and fluorescence retrieval upon the addition of sulfide ions; (B) competitive selectivity of 10 towards different metal ions; (C) images of 10 captured upon the addition of Cu2+ ions and S2− under 365 nm UV light depicting visual fluorescence changes; (D) the change in fluorescence microscopy images of KYSE510 cells consisting of 10 after incubation with Cu2+ and Cu2+ + S2− (images B–D are adapted with permission from ref. 46. Copyright 2018, Elsevier); (E) HBT-based unsymmetrical azine (8a) as a turn-off chemosensor for Cu2+ ions showing 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry; (F) images of 8a captured upon the addition of Cu2+ ions and other metal ions under 365 nm UV light depicting visual fluorescence changes; (G) on-site detection tool for the TLC-strip-based sensing of Cu2+ and selectivity of 8a towards different metal ions (images F and G are adapted with permission from ref. 29. Copyright 2021, Elsevier); (H) triaminoguanidine-based azine, 11, as a turn-off chemosensor for Cu2+ ions showing 3 1 binding stoichiometry; (F) images of 8a captured upon the addition of Cu2+ ions and other metal ions under 365 nm UV light depicting visual fluorescence changes; (G) on-site detection tool for the TLC-strip-based sensing of Cu2+ and selectivity of 8a towards different metal ions (images F and G are adapted with permission from ref. 29. Copyright 2021, Elsevier); (H) triaminoguanidine-based azine, 11, as a turn-off chemosensor for Cu2+ ions showing 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry and fluorescence retrieval upon the addition of S2− ions; (I) mechanoresponsive behavior of 11 showing weak emission in pristine form and intense fluorescence in ground form; selectivity of 11 towards (J) Cu2+ ions and (K) the 11–Cu2+ complex towards S2−; (L) the reversible turn-off–on response of 11 towards Cu2+ and S2− and (M) its application in the construction of logic gates; (N) on-site detection tool for the paper-strip-based sensing of Cu2+ and S2− (images I–N are adapted with permission from ref. 47. Copyright 2019, American Chemical Society). 1 binding stoichiometry and fluorescence retrieval upon the addition of S2− ions; (I) mechanoresponsive behavior of 11 showing weak emission in pristine form and intense fluorescence in ground form; selectivity of 11 towards (J) Cu2+ ions and (K) the 11–Cu2+ complex towards S2−; (L) the reversible turn-off–on response of 11 towards Cu2+ and S2− and (M) its application in the construction of logic gates; (N) on-site detection tool for the paper-strip-based sensing of Cu2+ and S2− (images I–N are adapted with permission from ref. 47. Copyright 2019, American Chemical Society). | ||
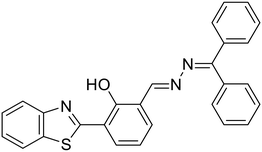
We recently demonstrated the use of the benzothiazole-based unsymmetrical azine (8a) as a fluorimetric sensor for the turn-off detection of Cu2+ ions in solution and solid phase utilizing its ESIPT property [Fig. 3E and Table 1, entry 2].29 The saturation point was reached at 0.5 equiv. of Cu2+ and the stoichiometry of 8a with Cu2+ was established as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 from Job's plot, EDX, time-resolved fluorescence measurements, and DFT studies. The sensing was highly selective towards Cu2+ ions and no other metal ions displayed any turn-off response [Fig. 3F]. The fluorescence of 8a can be retrieved by the addition of ascorbic acid (AA) by in situ generation of Cu+ and release of the probe from the complex. Notably, the LOD for 8a was found to be 0.005 μM, which is about 20 fold lower than Zhou et al.'s probe (10). The solid-phase sensing ability of the azine was explored using TLC plates which displayed a significant turn-off response at lower concentrations of the Cu2+ ions in a selective manner [Fig. 3G]. We demonstrated its practical applicability in real water samples with a high % recovery.
1 from Job's plot, EDX, time-resolved fluorescence measurements, and DFT studies. The sensing was highly selective towards Cu2+ ions and no other metal ions displayed any turn-off response [Fig. 3F]. The fluorescence of 8a can be retrieved by the addition of ascorbic acid (AA) by in situ generation of Cu+ and release of the probe from the complex. Notably, the LOD for 8a was found to be 0.005 μM, which is about 20 fold lower than Zhou et al.'s probe (10). The solid-phase sensing ability of the azine was explored using TLC plates which displayed a significant turn-off response at lower concentrations of the Cu2+ ions in a selective manner [Fig. 3G]. We demonstrated its practical applicability in real water samples with a high % recovery.
In another work, Tharmalingam et al. developed an AIE–ESIPT active star-shaped azine-based Cu2+ sensor (11) from a triaminoguanidine precursor [Fig. 3H and Table 1, entry 3].47 Its aggregation-enhanced emissive feature was exhibited in the water–ACN mixture showing an emission maximum at 470 nm. The probe, 11, displayed solvent-dependent dual emission in nonpolar and polar protic solvents, suggesting excited state ICT-coupled ESIPT characteristics. In this case, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 binding stoichiometry was observed between 11 and Cu2+ owing to the presence of three binding sites available in the structural scatffold of the chemosensor [Fig. 3H]. The azine derivative, 11, exhibited a mechanoresponsive behavior wherein the pristine sample was found to weakly fluoresce greenish yellow and the ground sample showed a bathochromic shift with fluorescence shift to intense green when observed under 365 nm UV light as well as with fluorescence microscopy [Fig. 3I]. The addition of Cu2+ resulted in a turn-off response which was found to be reversible towards S2−via a metal ion displacement method [Fig. 3J and K]. The reversible and selective on–off–on sensing characteristics of 11 toward Cu2+ and S2− were effective in the construction of an IMPLICATION logic gate [Fig. 3L and M]. The probe could also be utilized as an on-site detection tool for the paper-strip-based reversible sensing of Cu2+ and S2− ions [Fig. 3N]. Among other notable contributions on azine-based sensors for copper ions, Zhao et al. reported a tetraphenylethylene-based AIE-active azine-based compound (12) by condensation of two TPE units that could recognize Cu2+ ions in a turn-off manner [Table 1, entry 4].26 The introduction of the azine unit as an electron acceptor could inculcate a bathochromic shift from blue (about 465 nm for typical TPE emission) to orange emission (λmax 581 nm). The probe functioned well as a selective copper ion sensor by 1
3 binding stoichiometry was observed between 11 and Cu2+ owing to the presence of three binding sites available in the structural scatffold of the chemosensor [Fig. 3H]. The azine derivative, 11, exhibited a mechanoresponsive behavior wherein the pristine sample was found to weakly fluoresce greenish yellow and the ground sample showed a bathochromic shift with fluorescence shift to intense green when observed under 365 nm UV light as well as with fluorescence microscopy [Fig. 3I]. The addition of Cu2+ resulted in a turn-off response which was found to be reversible towards S2−via a metal ion displacement method [Fig. 3J and K]. The reversible and selective on–off–on sensing characteristics of 11 toward Cu2+ and S2− were effective in the construction of an IMPLICATION logic gate [Fig. 3L and M]. The probe could also be utilized as an on-site detection tool for the paper-strip-based reversible sensing of Cu2+ and S2− ions [Fig. 3N]. Among other notable contributions on azine-based sensors for copper ions, Zhao et al. reported a tetraphenylethylene-based AIE-active azine-based compound (12) by condensation of two TPE units that could recognize Cu2+ ions in a turn-off manner [Table 1, entry 4].26 The introduction of the azine unit as an electron acceptor could inculcate a bathochromic shift from blue (about 465 nm for typical TPE emission) to orange emission (λmax 581 nm). The probe functioned well as a selective copper ion sensor by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation and offered an LOD of 0.03 μM. Similarly, Tiwari et al.,48 Ye et al.,49 Liu et al.,50 Kumarasamy et al.,51 and Sharifi et al.52 demonstrated different symmetrical and unsymmetrical azine-based probes (10a, 13–16) with emission in the blue to green region for the detection of Cu2+ ions [Table 1, entries 5–9].
1 complexation and offered an LOD of 0.03 μM. Similarly, Tiwari et al.,48 Ye et al.,49 Liu et al.,50 Kumarasamy et al.,51 and Sharifi et al.52 demonstrated different symmetrical and unsymmetrical azine-based probes (10a, 13–16) with emission in the blue to green region for the detection of Cu2+ ions [Table 1, entries 5–9].
Kumar et al. synthesized a symmetrical azine (17) based on benzophenone and studied its application in the detection of Al3+ ions [Table 1, entry 10].53 The probe, 17, exhibited weak emission in MeOH due to intramolecular rotations and a typical AIE behavior in >70% H2O–CH3CN resulting in intense emission. The azine could detect Al3+ in a turn-on manner in MeOH with a limit of detection of 0.27 μM, along with a notable colorimetric response. The binding of Al3+ with 17 restricted the intramolecular rotation and caused enhancement in emission. The stoichiometry was studied by mass spectrometry, NMR titration and Job's plot, which suggested a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 17
1 ratio of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ in the complex. In another study, Nguyen et al. synthesized a salicylaldehyde-derived water-soluble azine (18) having terminal sulphonate functionalities to detect Al3+ in aqueous solutions [Table 1, entry 11].27 The sulfonate functional groups in the scaffold provide an enhanced water-solubility to the probe. The complexation of Al3+ with 18via the azine –N atom and phenolic –OH causes an aggregation-induced emission enhancement (AIEE) at λmax 511 nm leading to the formation of well-defined dendritic structures. 18 displayed high selectivity towards Al3+ ions with a moderate detection limit of 0.153 μM. Only a 15% response towards other metal cations such as Zn2+ and Pb2+ was observed at high concentrations of these analytes indicating selectivity towards Al3+ ions. Further, 18 was incorporated into a digital microfluidic sensor chip, resulting in a sub-micromolar portable detection system for water samples contaminated with Al3+. In another study in the similar direction, Khanra et al. synthesized 4-(anthracen-9-ylmethylene-hydrazonomethyl)-2-methoxy-phenol (19) and utilized it as a turn-on fluorimetric sensor for Al3+ detection in sodium dodecyl sulphate (SDS) medium [Table 1, entry 12].54
Al3+ in the complex. In another study, Nguyen et al. synthesized a salicylaldehyde-derived water-soluble azine (18) having terminal sulphonate functionalities to detect Al3+ in aqueous solutions [Table 1, entry 11].27 The sulfonate functional groups in the scaffold provide an enhanced water-solubility to the probe. The complexation of Al3+ with 18via the azine –N atom and phenolic –OH causes an aggregation-induced emission enhancement (AIEE) at λmax 511 nm leading to the formation of well-defined dendritic structures. 18 displayed high selectivity towards Al3+ ions with a moderate detection limit of 0.153 μM. Only a 15% response towards other metal cations such as Zn2+ and Pb2+ was observed at high concentrations of these analytes indicating selectivity towards Al3+ ions. Further, 18 was incorporated into a digital microfluidic sensor chip, resulting in a sub-micromolar portable detection system for water samples contaminated with Al3+. In another study in the similar direction, Khanra et al. synthesized 4-(anthracen-9-ylmethylene-hydrazonomethyl)-2-methoxy-phenol (19) and utilized it as a turn-on fluorimetric sensor for Al3+ detection in sodium dodecyl sulphate (SDS) medium [Table 1, entry 12].54
Khanra et al. further reported a β-naphthol derived azine-based chemosensor (20) for the detection of Zn2+ ions at the nano-molar level [Table 1, entry 13].55 The chelation of Zn2+ ions with 20 results in chelation-enhanced fluorescence (CHEF) through inhibition of ESIPT and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– isomerization and an enhanced emission was observed at 495 nm. The probe displayed negligible CHEF in the presence of other metal ions and showed a detection limit as low as 36.16 nM. The Job's plot and mass analysis confirmed a 1
N– isomerization and an enhanced emission was observed at 495 nm. The probe displayed negligible CHEF in the presence of other metal ions and showed a detection limit as low as 36.16 nM. The Job's plot and mass analysis confirmed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between 20 and Zn2+ ions. In contrast, Das et al. reported a turn-on fluorescence sensor using a salicylaldehyde derived azine (21) for the selective determination of Zn2+ in the presence of acetate ions (AcO−). The probe exhibited very weak fluorescence. Its emission intensity increases at λmax 532 nm in the presence of Zn2+ over several other metal ions, and the emission profile is augmented only when AcO− is present as the counter anion. They proposed that the addition of Zn2+ triggers a synergistic effect and results in pronounced fluorescence enhancement attributed to the Zn2+ assisted CHEF process, and inhibition of the PET and ESIPT process [Table 1, entry 14].56 The binding of Zn2+ associated with AcO− ions locks the free rotation around the –C
1 binding stoichiometry between 20 and Zn2+ ions. In contrast, Das et al. reported a turn-on fluorescence sensor using a salicylaldehyde derived azine (21) for the selective determination of Zn2+ in the presence of acetate ions (AcO−). The probe exhibited very weak fluorescence. Its emission intensity increases at λmax 532 nm in the presence of Zn2+ over several other metal ions, and the emission profile is augmented only when AcO− is present as the counter anion. They proposed that the addition of Zn2+ triggers a synergistic effect and results in pronounced fluorescence enhancement attributed to the Zn2+ assisted CHEF process, and inhibition of the PET and ESIPT process [Table 1, entry 14].56 The binding of Zn2+ associated with AcO− ions locks the free rotation around the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– resulting in the suppression of the non-radiative decay process in the excited state giving strong emission.
N– resulting in the suppression of the non-radiative decay process in the excited state giving strong emission.
Musikavanhu et al. derived a naphthol-based azine attached with thiophene (22) for the purpose of detecting trivalent chromium (Cr3+) [Table 1, entry 15].57 The turn-off response at λmax 526 nm with a low detection limit of 0.041 μM can be attributed to the fact that Cr3+ is a paramagnetic fluorescence quencher having empty d-shells. Also, the ligand-to-metal charge transfer (LMCT) results in chelation-enhanced quenching (CHEQ). Azine 22 was highly selective towards Cr3+ as compared to other competing metal ions owing to Pearson's hard and soft acids and bases (HSAB) theory that defines the greater affinity of hard acid, Cr3+, towards the electron donating imine nitrogen than others.
Manigandan et al. reported a symmetrical 4-hydroxy-3-methoxybenzaldehyde-derived azine probe (23) as a highly reliable Fe3+ sensor [Table 1, entry 16].28 In 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMF–H2O, the blue emissive azine (λem 423 nm) exhibits a sensitive fluorescence “turn-off” response towards Fe3+ ions with a limit of detection of 0.077 μM. The azine, 23, displayed high selectivity towards Fe3+ ions over other metal ions except for La3+ and Fe2+ ions which also showed an appreciable decrease in fluorescence intensities. The energy or electron transfer processes leading to the reverse photo-induced electron transfer process and the paramagnetic property of Fe3+ ions along with the unfilled d subshell contributed to the fluorescence quenching of 23.
1 DMF–H2O, the blue emissive azine (λem 423 nm) exhibits a sensitive fluorescence “turn-off” response towards Fe3+ ions with a limit of detection of 0.077 μM. The azine, 23, displayed high selectivity towards Fe3+ ions over other metal ions except for La3+ and Fe2+ ions which also showed an appreciable decrease in fluorescence intensities. The energy or electron transfer processes leading to the reverse photo-induced electron transfer process and the paramagnetic property of Fe3+ ions along with the unfilled d subshell contributed to the fluorescence quenching of 23.
Recently, Mondal et al. developed a pyrene-hydroxyquinoline-based azine (24) for selective turn-on detection of toxic Hg2+ ions [Table 1, entry 17].58 In EtOH–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) medium, 24 showed a turn-on response towards Hg2+ ions with a detection limit of 0.22 μM. The probe, 24, is weakly emissive in 9
1) medium, 24 showed a turn-on response towards Hg2+ ions with a detection limit of 0.22 μM. The probe, 24, is weakly emissive in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
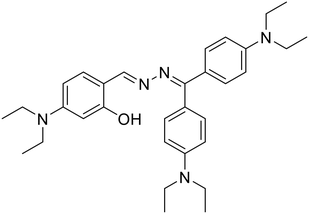
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOH–H2O medium and upon the addition of Hg2+ ions, it undergoes enhancement that is attributed to the CHEF effect owing to metal–ligand complexation which further triggers the aggregation of the complex and the AIEE effect dominates at higher Hg2+ concentrations. The DFT studies indicated that probe 24 acts as a tridentate ligand engaging one of the azine –N atoms, the quinoline –N atom and the –OH group to specifically bind to Hg2+. In another study, Tong et al. synthesized a series of azine compounds (25) and utilized them for the turn-on detection of Hg2+ ions [Table 1, entry 18].59
1 EtOH–H2O medium and upon the addition of Hg2+ ions, it undergoes enhancement that is attributed to the CHEF effect owing to metal–ligand complexation which further triggers the aggregation of the complex and the AIEE effect dominates at higher Hg2+ concentrations. The DFT studies indicated that probe 24 acts as a tridentate ligand engaging one of the azine –N atoms, the quinoline –N atom and the –OH group to specifically bind to Hg2+. In another study, Tong et al. synthesized a series of azine compounds (25) and utilized them for the turn-on detection of Hg2+ ions [Table 1, entry 18].59
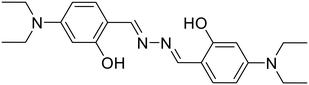
Ghosh et al. utilized a salicylaldehyde-based weakly fluorescent azine (26) for the detection of Mo6+ ions in an EtOH–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) medium [Table 1, entry 19].60 The planar geometry of 26 favors the PET process involving electron transition from imine –N to the conjugated aldehyde moiety and fluoresces weakly at 415 nm. A ratiometric response is observed with a decrease in peak at 415 nm and a simultaneous increase at 501 nm upon the addition of Mo6+ ions. The 26
1) medium [Table 1, entry 19].60 The planar geometry of 26 favors the PET process involving electron transition from imine –N to the conjugated aldehyde moiety and fluoresces weakly at 415 nm. A ratiometric response is observed with a decrease in peak at 415 nm and a simultaneous increase at 501 nm upon the addition of Mo6+ ions. The 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo6+ complex restricts the PET process, leading to CHEF. The formation of a 1
Mo6+ complex restricts the PET process, leading to CHEF. The formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex between 26 and Mo6+ was observed with a detection limit as low as 0.002 μM. The sensor was applied for the measurement of Mo6+ in certified steel samples. The selective extraction of Mo6+ from a mixture with other common metal ions using 26 was particularly effective.
2 complex between 26 and Mo6+ was observed with a detection limit as low as 0.002 μM. The sensor was applied for the measurement of Mo6+ in certified steel samples. The selective extraction of Mo6+ from a mixture with other common metal ions using 26 was particularly effective.
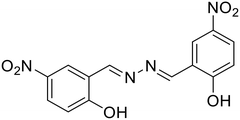
Similarly, Pham et al. developed a highly selective and sensitive azine-based probe (27) for the turn-off detection of uranyl ions in an organoaqueous media [Table 1, entry 20].61 The addition of UO22+ ions to 27 in 80% water–ACN fractions resulted in a small decrease in fluorescence intensities, whereas at 60% water fractions, a significant fluorescence quenching was noted. Similarly, the addition of UO22+ ions to the unsubstituted azine scaffold did not alter the emission to the extent that was possible with the presence of the –NO2 group in 27. This was attributed to the electron-withdrawing effect of –NO2 groups which stabilize the phenolate form of 27 in solution and the complexation with UO22+ ions was more efficient. The complexation of 27 with UO22+ ions destroyed the aggregation of the probe to solubilize the molecules in the solution resulting in a turn-off response with a 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) UO22+ complexation stoichiometry of 1
UO22+ complexation stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
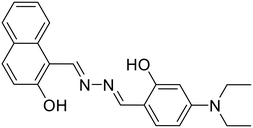
Some azine-based probes with dual metal ion detection ability have also been reported. Yadav et al. demonstrated an unsymmetrical azine (28) derived from 2-hydroxynaphthalene that exhibits viscochromic and mechanochromic properties and detects Al3+ and Cu2+ ions via two different sensing pathways in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HEPES
HEPES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (0.1
MeOH (0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9
1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.0, v/v, HEPES buffer, pH 7.4) [Table 1, entry 21].62 At the outset, a fluorescence turn-on and turn-off mechanism having 1
8.0, v/v, HEPES buffer, pH 7.4) [Table 1, entry 21].62 At the outset, a fluorescence turn-on and turn-off mechanism having 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometries was displayed upon the addition of Al3+ and Cu2+ with LODs of 0.165 μM and 0.152 μM, respectively. The stable 28
1 binding stoichiometries was displayed upon the addition of Al3+ and Cu2+ with LODs of 0.165 μM and 0.152 μM, respectively. The stable 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
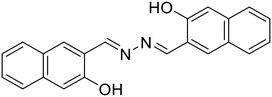
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al3+ complex formation imparts rigidity resulting in the CHEF effect, which subdues the PET process from the –N atom of azine to the large π-conjugation of the naphthalene moiety, thereby affording an increase in fluorescence intensity. Similarly, the turn-off response with Cu2+ is owing to the strong paramagnetic property of Cu2+ with a partially filled d-shell showing a high affinity for 28 with N or O as coordinating atoms. The binding of Cu2+ leads to CHEQ as the excited state of the fluorophore is suppressed by ligand-to-metal charge transfer (LMCT). F− and EDTA2− ions made 28 reversible for Al3+ and Cu2+ ions. Similarly, Sun et al. synthesized a series of azine derivatives (29) which displayed a turn-on response towards Al3+ in DMSO–H2O (4
Al3+ complex formation imparts rigidity resulting in the CHEF effect, which subdues the PET process from the –N atom of azine to the large π-conjugation of the naphthalene moiety, thereby affording an increase in fluorescence intensity. Similarly, the turn-off response with Cu2+ is owing to the strong paramagnetic property of Cu2+ with a partially filled d-shell showing a high affinity for 28 with N or O as coordinating atoms. The binding of Cu2+ leads to CHEQ as the excited state of the fluorophore is suppressed by ligand-to-metal charge transfer (LMCT). F− and EDTA2− ions made 28 reversible for Al3+ and Cu2+ ions. Similarly, Sun et al. synthesized a series of azine derivatives (29) which displayed a turn-on response towards Al3+ in DMSO–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and a turn-off response towards Cu2+ in DMSO–H2O (3
1) and a turn-off response towards Cu2+ in DMSO–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) [Table 1, entry 22].63
7) [Table 1, entry 22].63
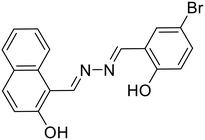
A naphthol-based fluorescent probe, 30, was demonstrated by Das et al. for the distinguishable turn-on detection of Al3+ and Zn2+ in different solvent systems (Al3+ in MeOH–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and Zn2+ in DMSO–H2O (9
1) and Zn2+ in DMSO–H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
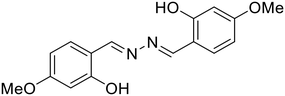
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) [Table 1, entry 23].64 In the less polar MeOH–H2O solvent system, Al3+ forms a strong complex with the hard oxophilic donor owing to its small size and high charge. At the same time, 30 is more nucleophilic by capturing the acidic O–H proton in the strong polar solvent DMSO–H2O. Al3+ is strongly passivated by DMSO–H2O compared with Zn2+, thereby making Zn2+ readily available for metal complex formation in the highly-polar solvent. In a similar manner, Dolai et al. utilized a weakly emissive salicylaldehyde-based azine probe (31) for the turn-on detection of Cr3+ and Al3+ by two distinct outputs [Table 1, entry 24].65 The addition of Cr3+ and Al3+ to 31 resulted in fluorescence enhancement at two different wavelengths (427 nm for Cr3+ and 490 nm for Al3+) which is attributed to the CHEF effect and the ICT (intermolecular charge transfer) between the metal ions (Al3+ and Cr3+) and 31. This in turn is due to the different radii of the two metal ions as well as the different ionic potentials (electronic charge/radius of ions).
1)) [Table 1, entry 23].64 In the less polar MeOH–H2O solvent system, Al3+ forms a strong complex with the hard oxophilic donor owing to its small size and high charge. At the same time, 30 is more nucleophilic by capturing the acidic O–H proton in the strong polar solvent DMSO–H2O. Al3+ is strongly passivated by DMSO–H2O compared with Zn2+, thereby making Zn2+ readily available for metal complex formation in the highly-polar solvent. In a similar manner, Dolai et al. utilized a weakly emissive salicylaldehyde-based azine probe (31) for the turn-on detection of Cr3+ and Al3+ by two distinct outputs [Table 1, entry 24].65 The addition of Cr3+ and Al3+ to 31 resulted in fluorescence enhancement at two different wavelengths (427 nm for Cr3+ and 490 nm for Al3+) which is attributed to the CHEF effect and the ICT (intermolecular charge transfer) between the metal ions (Al3+ and Cr3+) and 31. This in turn is due to the different radii of the two metal ions as well as the different ionic potentials (electronic charge/radius of ions).
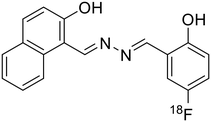
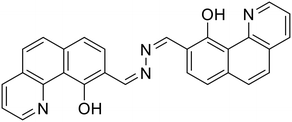
Sawminathan et al. presented a dual-emission responsive azine-based chemosensor (32) that uses a colorimetric and fluorescence turn-on technique to quickly, sensitively and selectively detect Th4+ and Fe3+ [Table 1, entry 25].66 When exposed to Th4+/Fe3+, the non-emissive 32 emits yellow fluorescence and it offers low detection limits of 2.1 nM and 3.3 nM for Th4+ and Fe3+, respectively. Initially, 32 is non-emissive due to the PET phenomenon that occurs from the electronegative –N atom to the highest occupied molecular orbital (HOMO) of the excited fluorophore. However, the addition of Th4+/Fe3+ results in turn-on response by blocking the PET process. Paper strips were made using 32 and their ability to sense Th4+ and Fe3+ in the solid phase was demonstrated. As real samples, several water bodies and human serum albumin were used to successfully assess the performance of 32 in detecting Th4+/Fe3+ ions.
3.2. Anion sensors
Anions play a vital role in numerous biological functions and are commercially important for industrial processes.67 For instance, an optimum concentration of fluoride (F−) ions is important for dental care and bone structure in the human body, whereas its excess intake results in skeletal fluorosis, leading to stiffness and calcification of bones in the body.68a An excess of the strong oxidant peroxynitrite (ONOO−) ions in the system damages proteins and DNA which may result in Alzheimer's disease, cancer, neurodegenerative disorders, inflammation, and diabetes.68b Cyanide (CN−) ions are one of the well-known environmental pollutants owing to their high physiological toxicity.68c Hypochlorite (ClO−) accumulation generates oxidative stress and can lead to various neurodegenerative and cardiovascular diseases and cancer.68d Bisulfite (HSO3−) is a common antioxidant and food preservative, however, excessive exposure can result in tissue damage and allergic reactions.68e Hence their selective and sensitive detection has gained considerable attention. In previous years, several azine-based probes with appropriate functionalities have been utilized for the selective detection of a number of anions such as F−,25 CN−,69–71 and tBuO−.72Among recent developments, Shen et al. synthesized a diphenylphosphinate-protected salicylaldehyde-based azine chemodosimeter (33) and utilized it for the detection of peroxynitrite (ONOO−) ions [Fig. 4A and Table 2, entry 1].73 The ONOO− ions could cleave the diphenylphosphinate group to afford the green fluorescent salicylaldehyde azine (λmax 520 nm) back in the solution to offer turn-on sensing for ONOO− ions [Fig. 4A]. The probe, 33, could detect endogenous ONOO− ions with high selectivity and excellent sensitivity and demonstrated a low detection limit of 0.08 μM [Fig. 4B]. The probe was further investigated for its ability to image ONOO− in living cells and it was found that the probe could specifically detect endogenous ONOO− in HeLa cells [Fig. 4C]. Yuan et al. synthesized another azine-based probe (34) for the detection of F− ions by incorporating 2-hydroxy-1-naphthaldehyde and 1,8-naphthalimide [Fig. 4D and Table 2, entry 2].74 In the presence of F− ions, 36 results in a color shift from yellow to light purple along with a fluorescence change in a turn-off manner [Fig. 4E]. The 1H NMR titration revealed that 36 undergoes deprotonation of phenolic –OH via a hydrogen-bond interaction between phenolic OH− and F− ions. The deprotonation blocks the ESIPT process and tautomerization from the enol form to keto form occurs which induces the PET effect in the system.
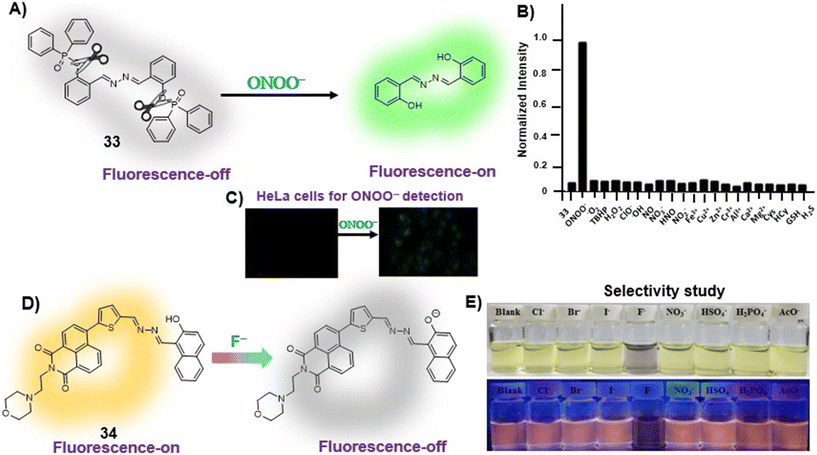 | ||
| Fig. 4 (A) Diphenylphosphinate modified salicylaldehyde-based azine (33) as a turn-on chemodosimeter for ONOO− ions by cleavage of the diphenylphosphinate group; (B) selectivity of 33 against different analytes depicting high selectivity towards ONOO− ions; (C) fluorescence microscopy images of HeLa cells in the presence of 33 and ONOO− ions (images B and C are adapted with permission from ref. 73. Copyright 2019, Elsevier); (D) naphthalimide-based azine (34) as a turn-off chemodosimeter for F− ions by the deprotonation strategy; (E) selectivity studies depicting a change in color from yellow to light purple and fluorescence quenching in the presence of F− ions (adapted with permission from ref. 74. Copyright 2018, Elsevier). | ||
| Sr. no. | Probe | Analyte | Mechanism/strategy | λ ab | Solvent system | LOD | Remark | Ref. |
|---|---|---|---|---|---|---|---|---|
| λ em | ||||||||
| (Δλ nm) | ||||||||
| 1 |
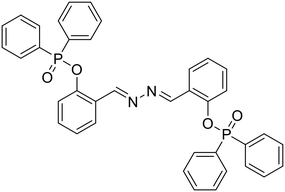 33, Shen et al. (2019)
33, Shen et al. (2019) |
ONOO− | Turn-on/diphenyl phosphinate cleavage | 403 nm | DMSO–PBS buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 v/v, 50 mM, pH 7.4) 99 v/v, 50 mM, pH 7.4) |
0.08 μM | — | 73 |
| 520 nm | ||||||||
| (117 nm) | ||||||||
| 2 |
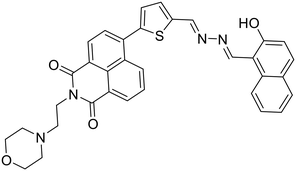 34, Yuan et al. (2018)
34, Yuan et al. (2018) |
F− | Turn-off/deprotonation | 420 nm | THF | 0.015 μM | — | 74 |
| 593 nm | ||||||||
| (173 nm) | ||||||||
| 3 |
 35a, R = H
35a, R = H |
CN− | Turn-on/nucleophilic addition | 320 nm | ACN | 0.058 μM | — | 75 |
| 35b, R = Me | 440 nm | |||||||
| 35c, R = F | (120 nm) | |||||||
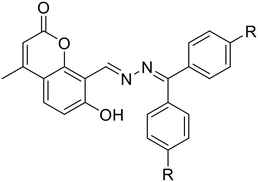
| 35, Devendhiran et al. (2021) | ||||||||
| 4 |
 36, Yao et al. (2022)
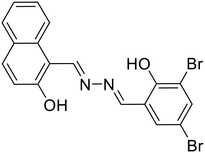
36, Yao et al. (2022) |
CN− | Turn-off/deprotonation | 413 nm | H2O–DMSO (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) 2 v/v) |
0.01 μM | Fluorescence retrieval with H+ | 76 |
| 562 nm | ||||||||
| (149 nm) | Application in solid-phase detection | |||||||
| 5 |
 37, Paul et al. (2023)
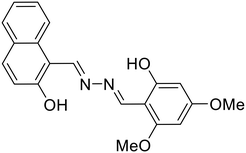
37, Paul et al. (2023) |
CN− | Turn-on/deprotonation | 365 nm | ACN–HEPES buffer (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, pH 7.3) 1 v/v, pH 7.3) |
0.045 μM | Solid state fluorescence quenching with TFA vapors and reversible with TEA vapors | 77 |
| 565 nm | ||||||||
| (200 nm) | ||||||||
| 6 |
 38a, R1 = R2 = H
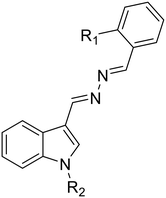
38a, R1 = R2 = H |
ClO− | Turn-on/deprotonation | 350 nm | DMSO–PBS buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v, pH 7.4) 4 v/v, pH 7.4) |
0.05 μM | Application in solid-phase detection | 78 |
| 38b, R1 = OH, R2 = H | 530 nm | |||||||
| 38c, R1 = OH, R2 = Me | (180 nm) | |||||||
| Singh et al. (2020) |
Devendhiran et al. reported a series of coumarin-based azines (35) and utilized them for the selective and sensitive turn-on detection of CN− ions [Table 2, entry 3].75 The probe works as a turn-off sensor by nucleophilic addition of the ions across the azine double bond with a detection limit of 0.058 μM. Similarly, Yao et al. developed a naphthalene-based unsymmetrical azine (36) to detect CN− in aqueous media via a turn-off fluorescence response resulting from the deprotonation of phenolic –OH [Table 2, entry 4].76 The probe, 36, recognizes CN− with good selectivity and sensitivity displaying an LOD of 0.01 μM. Paul et al. demonstrated a naphthalene hydrazone-based unsymmetrical azine (37) as a turn-on sensor for the detection of CN− ions [Fig. 5A and Table 2, entry 5].77 Azine 37 displayed typical AIE characteristics, having weak emission at 503 nm in ACN and the highest emission intensity at 80% water fractions with a bathochromic shift from 503 nm to 537 nm [Fig. 5B]. The probe was utilized for the turn-on detection of CN− ions in ACN–HEPES buffer. The sensing mechanism is based on the deprotonation by the mild base CN− to facilitate an extensive delocalization of charge between phenolate –O− and the aromatic rings in the planar form leading to fluorescence enhancement. They also proposed that the cleavage of the intramolecular hydrogen bonding might stop the ESIPT. The probe showed high sensitivity towards CN− ions with a limit of detection of 45.4 nM [Fig. 5C] with a high selectivity against most other interfering anions [Fig. 5D]. Azine 37 showed intense emission in the solid state and was therefore utilized for the sensitive detection of trifluoroacetic acid (TFA) vapors via fluorescence quenching with an LOD of 1.41 ppm [Fig. 5E]. The protonation-driven destruction of compacted arrangement in the solid state was assumed to be the quenching mechanism of 37. The turn-off system was further utilized for the reversible acidochromic behavior upon the sequential addition of triethylamine vapors [Fig. 5F]. The probe, 37, was reversible with TFA and triethylamine (TEA) and the system could easily be recycled several times, demonstrating its potent reusability [Fig. 5G].
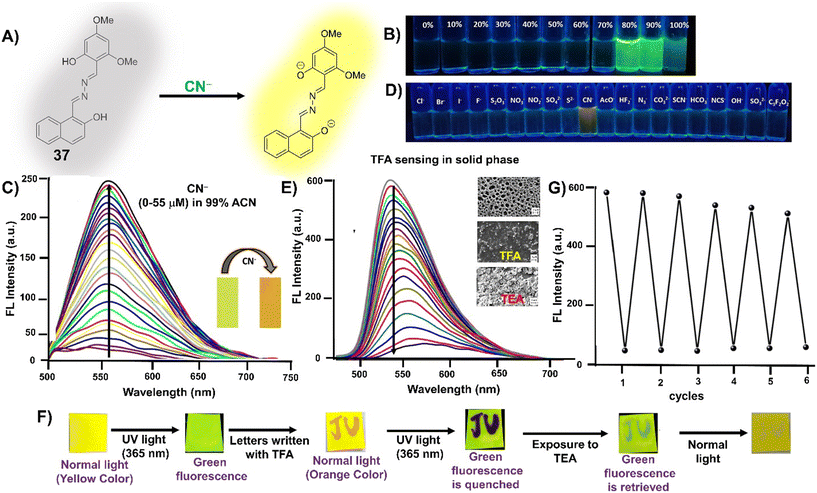 | ||
| Fig. 5 (A) Naphthalene-based azine (37) as a turn-on chemodosimeter for CN− ions by the deprotonation strategy; (B) AIE behavior exhibited by 37 with the highest intensity at 80% water fractions; (C) turn-on response of 37 upon the addition of CN− ions in 99% ACN; (D) selectivity of 37 against different analytes; (E) turn-off response of 37 upon the addition of TFA vapors in the solid state; (F) reversibility of the turn-off–on mechanism against addition of TFA followed by TEA; (G) reusability study of 37 against TFA vapors and TEA vapors showing high sensitivity and accuracy even after 6 cycles (adapted with permission from ref. 77. Copyright 2023, Royal Society of Chemistry). | ||
Singh et al. demonstrated a few azine-based sensors (38) for the reversible detection of hypochlorite in aqueous media employing the protonation–deprotonation strategy [Table 2, entry 6].78 The AIE–ESIPT-active assemblies of salicylaldehyde/indolium-based probes (38a) displayed sensitive detection of hypochlorite with a detection limit of 0.052 μM. The highly sensitive response to ClO− ions was owing to the elevated acidity and the formation of more organized structures following deprotonation. Additionally, the ‘dip strip’ of 38a has been used to show the real-time use for ‘on-site’ solid-phase detection of hypochlorite. Moreover, these assemblies were effectively employed for visualizing hypochlorite within cells and acted as antioxidants to avert cell death induced by hypochlorite.
3.3. Small molecule and bioanalyte sensors
Some small molecules such as hydrogen peroxide (H2O2) and hydrazine (N2H4) are crucial for human health and they can contaminate water and soil in higher concentrations. Elevated levels of H2O2 in the body are linked to various diseases, including malignant tumors, Parkinson's syndrome, Alzheimer's disease, etc.79a Meanwhile, excessive utilization of hydrazine results in elevated concentrations in water bodies and soil that readily move into humans through oral, dermal or inhalation routes owing to its volatile nature, leading to significant harm to the lungs, liver, kidneys, central nervous system and respiratory system.79b On the other hand, bioanalytes regulate a number of biological functions in the body and are important for the healthy functioning of physiological processes. Among a few important species of interest, protamine is used for the treatment of thrombotic diseases as an anticoagulant and in case of heparin overdosage. Higher concentrations of heparin induce thrombocytopenia, hemorrhages, and hyperkalemia.79c β-Lactamase acts as a key biomarker for pathogenic bacteria that show resistance to β-lactam antibiotics by effectively catalyzing the cleavage of the amide group in the antibiotics.79d Influenza virus proteins such as neuraminidase (NA) play a vital role in influenza virus infection and replication in host cells.79e Accurate quantification of biological thiols, including cysteine (Cys) and glutathione (GSH), is crucial as their aberrant levels are associated with various conditions such as cancer, liver damage, slow growth and skin lesions.79f In the past, several azine-based probes with appropriate functionalities have been utilized for the selective detection of bioanalytes and neutral molecules such as Cys,80 protamine,21 heparin,81 hydrophobic proteins (casein) or proteins composed of hydrophobic pockets (bovine serum albumin (BSA) and human serum albumin (HSA)),82,83 egg albumin,84 brown adipose cells,85 β-galactosidase,86 pyrophosphate,87 pH indicators,88etc. which is comprehensively reviewed by Kagatikar et al.3In recent times, Sathiyaraj et al. synthesized a short series of N,N-dimethylaminobenzaldehyde-based symmetrical azines (39) having electron-donating amino substituents as the recognition sites for the detection of nitro explosive, picric acid [Table 3, entry 1].89 The donor–π–donor probe displayed high selectivity in an aggregated form which arises from the substituents at the amine group. Notably, picric acid enhanced the fluorescence of the azine monomers in pure THF but quenched the same in the THF–water mixture. In THF, picric acid forms a hydrogen bonding network with one of the amine nitrogen atoms, resulting in an electron-accepting group instead of an electron donor. Further, the fluorescence quenching with PA was attributed to the inner filter effect and the disturbance of aggregates by the hydrogen bonding interaction between the nitrogen of PA and N,N-dialkylamino group. Similarly, our group utilized the red-emissive HBT-based unsymmetrical dyes, 8d, for the detection of nitroaromatic compounds (NACs) [Table 3, entry 2].37 The electron-deficient NACs in particular, picric acid, undergo noncovalent interactions with electron-rich azines resulting in the turn-off detection of NACs [Fig. 6A]. The disruption in the aggregation is due to the intercalation of nitroaromatics in an ordered array of 8 by more favorable π–π interactions. The red-emissive azine with the highest emission maxima, 8d, exhibited a turn-off response towards PA in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 v/v DMF–H2O with a limit of detection of 0.09 μM. The probe, 8d, displayed variable responses to other NACs such as 2,4-dinitro-chlorobenzene and 4-nitrotoluene and was non-responsive against other aromatic compounds with no or insignificant turn-off response [Fig. 6B].
19 v/v DMF–H2O with a limit of detection of 0.09 μM. The probe, 8d, displayed variable responses to other NACs such as 2,4-dinitro-chlorobenzene and 4-nitrotoluene and was non-responsive against other aromatic compounds with no or insignificant turn-off response [Fig. 6B].
| Sr. no. | Probe | Analyte | Mechanism/strategy | λ ab | Solvent system | LOD | Remark | Ref. |
|---|---|---|---|---|---|---|---|---|
| λ em | ||||||||
| (Δλ nm) | ||||||||
| 1 |
 39a, R = Me
39a, R = Me |
Picric acid | THF: turn-on, hydrogen bonding | THF: 415 nm | THF or H2O–THF (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) 3 v/v) |
THF: 26 μM | — | 89 |
| 570 nm | ||||||||
| (155 nm) | ||||||||
| 39b, R = Et | H2O–THF: turn-off, protonation at azine N | H2O–THF: 443 nm | H2O–THF: 38 μM | |||||
| 39c, R = Pr | 490 nm | |||||||
| 39d, R = Ph | (47 nm) | |||||||
| Sathiyaraj et al. (2020) | ||||||||
| 2 |
 8d, Bhosle et al. (2023)
8d, Bhosle et al. (2023) |
NACs | Turn-off/disruption of AIE property by intercalation of NACs | 398 nm | H2O–DMF (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
0.09 μM | Application in solid-phase detection | 37 |
| 675 nm | ||||||||
| (277 nm) | ||||||||
| 3 |
 40, Bhosle et al. (2022)
40, Bhosle et al. (2022) |
H2O2 | Turn-on/benzyl boronic acid cleavage | 384 nm | DMSO–PBS buffer (0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.9 v/v, 10 mM, pH 7.4) 9.9 v/v, 10 mM, pH 7.4) |
0.039 μM | Application in solid-phase detection | 90 |
| 617 nm | ||||||||
| (233 nm) | ||||||||
| 4 |
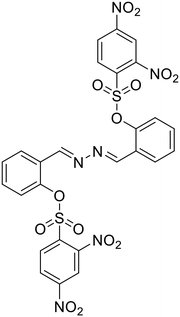 41, Song et al. (2018)
41, Song et al. (2018) |
Cys | Turn-on/dinitro benzene sulphonyl cleavage | 405 nm | DMSO–PBS buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v, 10 mM, pH 7.4) 9 v/v, 10 mM, pH 7.4) |
2.84 μM | Application in solid-phase detection | 91 |
| 547 nm | ||||||||
| (142 nm) | ||||||||
| 5 |
 42, Bhattu et al. (2023)
42, Bhattu et al. (2023) |
Azinphos-methyl | Turn-on/H-bonding interactions | 275 nm | H2O | 7.4 μM | — | 92 |
| 355 nm | ||||||||
| (80 nm) | ||||||||
| 6 |
 43, Thiagarajan and co-workers (2021)
43, Thiagarajan and co-workers (2021) |
Diethylchlorophosphate (DCP) | Turn-on/ACN: nucleophilic substitution at azine N | ACN: 373 nm | ACN or 80% H2O–ACN (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
ACN: 0.0099 μM | — | 93 |
| 495 nm | ||||||||
| (122 nm) | ||||||||
| ACN–H2O: protonation at azine N | ACN–H2O: 269 nm | ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O: 0.068 μM H2O: 0.068 μM |
||||||
| 432 nm | ||||||||
| (163 nm) | ||||||||
| 7 |
 44, Sathiyaraj et al. (2020)
44, Sathiyaraj et al. (2020) |
Diethylchlorophosphate (DCP) | Turn-on/THF: nucleophilic substitution at azine N | THF: 440 nm | THF or H2O: THF (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) 3 v/v) |
THF: 0.2 μM | — | 94 |
| 513 nm | ||||||||
| (73 nm) | ||||||||
THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O: protonation at azine N H2O: protonation at azine N |
THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O: 368 nm H2O: 368 nm |
THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O: 106 μM H2O: 106 μM |
||||||
| 570 nm | ||||||||
| (202 nm) | ||||||||
| 8 |
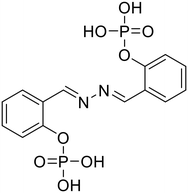 45, He et al. (2020)
45, He et al. (2020) |
Alkaline phosphatase (ALP) | Turn-on/phosphate cleavage | 356 nm | 50 mM Tris buffer, pH 9 | 0.012 U L−1 | — | 95 |
| 536 nm | ||||||||
| (180 nm) | ||||||||
| 9 |
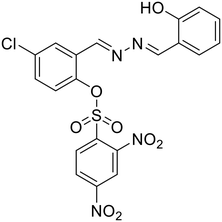 46, Tong and co-workers (2018)
46, Tong and co-workers (2018) |
β-Lactamase | Turn-on/dinitro benzene sulphonyl cleavage | 400 nm | PBS buffer (10 mM, pH 7.4) | 0.5 mU mL−1 | NA | 96 |
| 558 nm | ||||||||
| (158 nm) | ||||||||
| 10 |
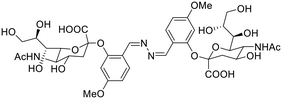 47, Chang et al. (2022)
47, Chang et al. (2022) |
Neuraminidase (NA) | Turn-on/sialic acid cleavage | 387 nm | 10 mM PBS buffer (pH 7.4) | 0.024 U mL−1 | — | 97 |
| 524 nm | ||||||||
| (137 nm) |
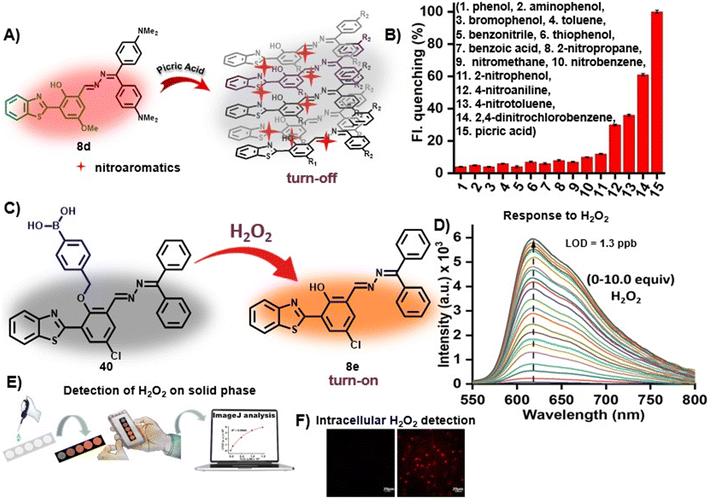 | ||
| Fig. 6 (A) HBT-based unsymmetrical azine dye (8d) as a turn-off chemosensor for the sensitive detection of nitroaromatic compounds showing a turn-off response by disruption in AIE owing to the intercalation of NACs; (B) the fluorimetric responses of 8d against different analytes depicting the high selectivity of 8d towards NACs (images A and B are adapted with permission from ref. 37. Copyright 2023, Wiley-VCH); (C) HBT-based chemodosimeter (40) for the detection of H2O2 ions by cleavage of the benzylboronic acid group; (D) turn-on detection of H2O2 ions by 40 with a low detection limit; (E) demonstration of solid-phase detection of H2O2 using TLC plates and ImageJ analysis for on-site quantitation of H2O2; (F) fluorescence microscopy images of HeLa cells in the presence of 40 and H2O2 ions (images C–F are adapted with permission from ref. 90. Copyright 2022, Elsevier). | ||
Our group further utilized one of the benzothiazole-derived unsymmetrical azines with a p-Cl substituent (8) and protected it with a benzyl boronic acid group as the recognition unit for the selective detection of H2O2 ions to afford a turn-on chemodosimeter [Fig. 6C and Table 3, entry 3].90 H2O2 spontaneously cleaves the benzyl boronic acid group of the chemodosimeter (40) in 1% DMSO in PBS (10 mM, pH 7.4) medium to produce its precursor 8, which emits intense orange AIE [Fig. 6D]. The probe was non-responsive to other ROS, cations, anions, oxidizing, and reducing agents. The high sensitivity of 40 towards H2O2 is attributed to a low limit of detection (LOD) of 3.9 × 10−8 M (1.3 ppb). The practical utility of 40 in H2O2 detection was demonstrated by spiking H2O2 in water samples collected from local water bodies and in blood serum. Azine 40 was successfully demonstrated in solid-phase detection of H2O2 using TLC plates as the platform, a smartphone for image-capturing, and ImageJ analysis for a practical demonstration of the on-site quantitation of H2O2 [Fig. 6E]. The probe, 40, could efficiently detect intracellular H2O2, as shown by imaging in live HeLa cells [Fig. 6F].
Song et al. developed a salicylaldehyde-based azine chemodosimeter, 41, protected with a dinitrobenzene sulphonyl group for the detection of Cys/Hcy in a turn-on manner [Table 3, entry 4].91 The addition of Cys/Hcy to the probe solution resulted in the cleavage of the 2,4-dinitro benzenesulfonate group of 41 to afford the turn-on response. The probe, 41, displayed a significant Stokes shift (148 nm), low cytotoxicity, and good photostability. It was further utilized as a portable kit for the on-site inspection of more than ten micro samples simultaneously which might successfully reduce the development of false positives and visual errors. In addition, it was also demonstrated for cell imaging using PC12 cells, establishing its potential in the detection of Cys/Hcy in live cells.
Azinphos-methyl (Guthion) is a broad spectrum organophosphate (OPP) insecticide and an acetylcholinesterase inhibitor. It is classified as an extremely hazardous substance by US-EPA making its sensitive detection as an important task. Bhattu et al. synthesized azine nanoparticles (E)-(4-chlorophenyl)-1,1-diamino-2,3-diazabutadiene (42) and utilized them for the detection of azinphos-methyl under aqueous conditions [Fig. 7A and Table 3, entry 5].92 H-bonding interactions between the analyte and guanidine-like unit of the probe, 42, were responsible for the selective turn-on detection of azinphos-methyl [Fig. 7B]. It showed a limit of detection of 7.4 μM. Its application in real samples like orange juice, water samples, etc. showed good recovery and selective fluorescence response. Thiagarajan's group synthesized a new unsymmetrical azine, 43, and utilized it for the selective and sensitive turn-on detection of the nerve agent mimic diethylchlorophosphate (DCP) by two different mechanisms in different solvent systems [Table 3, entry 6].93 The photoinduced electron transfer results in a turn-on emission at 495 nm in ACN due to nucleophilic substitution of DCP at the azine nitrogen close to the –OMe group giving rise to a new ICT state [Fig. 7C]. A 1763-fold enhancement in fluorescence was observed with a low detection limit of 9.9 nM. Contrastingly, the protonation at the azine nitrogen in an ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) mixture increased the fluorescence at 422 nm by 1188-fold with a limit of detection of 68 nM [Fig. 7D]. DCP sensing was also demonstrated on TLC strips and polymethylmethacrylate (PMMA) polymer film by coating the probe solution on the solid platforms and then recording the images in the presence and absence of DCP vapors under 365 nm UV light [Fig. 7E]. The absence of DCP vapors showed no fluorescence emission, however, the addition of DCP vapors afforded a green fluorescence indicating the potential of 43 to detect DCP vapors qualitatively from the environmental samples in solid platforms. The same group developed an unsymmetrical D–π–A type azine, 44, for the detection of DCP by two different channels [Table 3, entry 7].94 The probe, 44, forms a new ICT state with DCP in pure THF by phosphorylation at the imine nitrogen close to the donor moiety of 44. This results in color change to orange with a 203-fold fluorescence enhancement at 513 nm and this moiety acts as a strong withdrawing group. Azine 44 displayed intense fluorescence emission at 570 nm in THF–H2O fractions which undergoes protonation at the amine nitrogen upon the addition of DCP and causes a blue-shifted emission to 406 nm and fluorescence quenching at 570 nm. The probe also functions as a test-strip based detection assay to detect DCP vapors using Whatman filter paper which displays a visual color change of the test strip from yellow to orange immediately after exposure to DCP vapors.
4) mixture increased the fluorescence at 422 nm by 1188-fold with a limit of detection of 68 nM [Fig. 7D]. DCP sensing was also demonstrated on TLC strips and polymethylmethacrylate (PMMA) polymer film by coating the probe solution on the solid platforms and then recording the images in the presence and absence of DCP vapors under 365 nm UV light [Fig. 7E]. The absence of DCP vapors showed no fluorescence emission, however, the addition of DCP vapors afforded a green fluorescence indicating the potential of 43 to detect DCP vapors qualitatively from the environmental samples in solid platforms. The same group developed an unsymmetrical D–π–A type azine, 44, for the detection of DCP by two different channels [Table 3, entry 7].94 The probe, 44, forms a new ICT state with DCP in pure THF by phosphorylation at the imine nitrogen close to the donor moiety of 44. This results in color change to orange with a 203-fold fluorescence enhancement at 513 nm and this moiety acts as a strong withdrawing group. Azine 44 displayed intense fluorescence emission at 570 nm in THF–H2O fractions which undergoes protonation at the amine nitrogen upon the addition of DCP and causes a blue-shifted emission to 406 nm and fluorescence quenching at 570 nm. The probe also functions as a test-strip based detection assay to detect DCP vapors using Whatman filter paper which displays a visual color change of the test strip from yellow to orange immediately after exposure to DCP vapors.
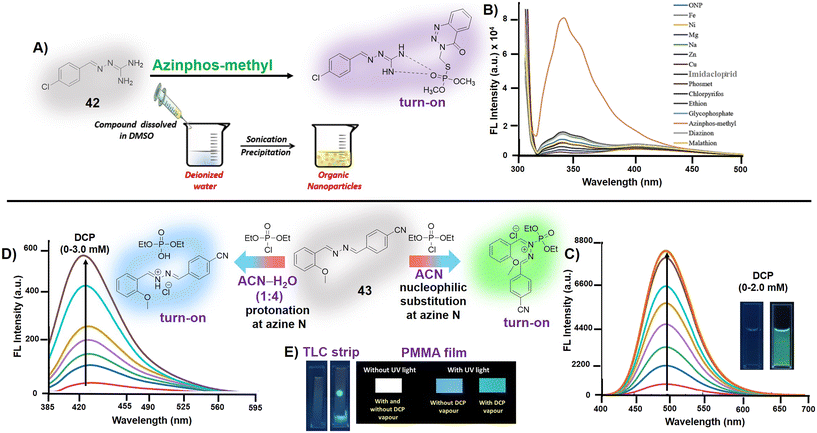 | ||
Fig. 7 (A) 1,1-Diaminoazine (42) as a chemosensor for organophosphorus pesticide azinphos-methyl; (B) selectivity study of 42 against various OPPs and metal ions (images A and B are adapted with permission from ref. 92. Copyright 2023, Elsevier); salicylaldehyde-based azine (43) as a dual-mode chemodosimeter for the detection of nerve agent mimic diethylchlorophosphate (DCP) by (C) turn-on emission at 495 nm in ACN due to nucleophilic substitution of the DCP at the azine nitrogen close to the –OMe group giving rise to a new ICT state and (D) turn-on emission at 422 nm due to protonation at the azine nitrogen in an ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture; (E) DCP sensing demonstrated on TLC strips and PMMA polymer film showing green fluorescence emission in the presence of DCP vapors under 365 nm light (images C–E are adapted with permission from ref. 93. Copyright 2021, Elsevier). H2O mixture; (E) DCP sensing demonstrated on TLC strips and PMMA polymer film showing green fluorescence emission in the presence of DCP vapors under 365 nm light (images C–E are adapted with permission from ref. 93. Copyright 2021, Elsevier). | ||
He et al. reported a phosphate-protected salicylaldehyde-based azine (45) and utilized it for the detection of alkaline phosphatase in aqueous medium [Fig. 8A and Table 3, entry 8].95 Additionally, 45 demonstrated strong water solubility and rapid response with a large Stokes shift. The addition of ALP resulted in more than 240-fold turn-on emission intensities [Fig. 8B]. The selectivity study confirmed the sensitive response of 45 towards ALP and no significant change was observed when it is exposed to metal ions, reactive oxygen species, reactive sulfur species (Cys, Hcy, and GSH) and enzymes (esterase, trypsin and lysozyme) [Fig. 8C]. ALP eliminates the phosphate groups of 45 by dephosphorylation affording an intermediate compound that contains one phosphate group and shows very weak fluorescence. The intermediate then undergoes the second dephosphorylation step and releases the unprotected salicylaldehyde-based azine which forms aggregates owing to the intramolecular hydrogen bond and increased hydrophobicity leading to a strong fluorescence signal due to the combined AIE and ESIPT mechanism. Azine-based probe 45 was further explored for differentiating the intracellular ALP activity in different cell lines such as MG-63, WI-38, B6F10, RAW264.7 and HEK293. It was found that MG-63 cells displayed the strongest fluorescence, indicating the highest expression level of ALP in MG-63 cells. WI-38, B16F10 and RAW 264.7 cell lines showed a moderate response, whereas a negligible response was displayed with HEK 293 cells [Fig. 8D]. In addition, MG-63 cells were investigated for the inhibition effect of the ALP inhibitor. The use of 1 mM Na3VO4 as an inhibitor showed negligible fluorescence intensity indicating that dephosphorylation of the ALP activity was inhibited by Na3VO4 [Fig. 8E].
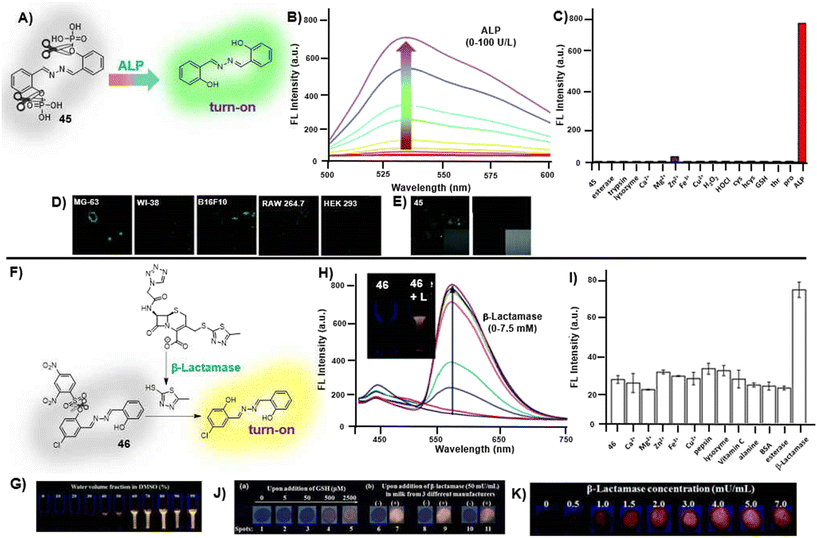 | ||
| Fig. 8 (A) Salicylaldehyde-based azine (45) as a chemodosimeter for alkaline phosphatase by cleavage of the phosphate group; (B) turn-on detection by fluorescence enhancement at 536 nm upon incremental addition of ALP; (C) change in fluorescence intensities towards ALP and various interfering analytes; confocal images of (D) MG-63, WI-38, B16F10, RAW 264.7 and HEK 293 cells after incubation with 45 showing intense fluorescence emission with MG-63 cells indicating the highest ALP expression and (E) MG-63 cells incubated with 45 in the absence and presence of 1 mM Na3VO4 wherein the inhibitor results in negligible turn-on response (images A–E are adapted with permission from ref. 95. Copyright 2020, Royal Society of Chemistry); (F) 2,4-dinitrobenzenesulfonyl protected salicylaldehyde-based azine (46) as a chemodosimeter for β-lactamase; (G) AIE–ESIPT behavior exhibited by the azine precursor; (H) turn-on detection of β-lactamase by fluorescence enhancement at λmax 558 nm; (I) fluorescence response of 46 towards different metal ions, proteins, enzymes, etc. confirming the high selectivity of 46 towards β-lactamase; (J) demonstration of solid-phase detection of β-lactamase on paper-strips; (K) demonstration of solid-phase detection of GSH and β-lactamase in milk samples on paper-strips (images F–K are adapted with permission from ref. 96. Copyright 2018, Royal Society of Chemistry). | ||
Tong's group utilized another non-fluorescent azine probe, 46, protected with a dinitrobenzene sulphonyl group for β-lactamase detection wherein the analyte first reacts with the substrate's lactam (cefazolin sodium) to form a secondary amine, commencing a spontaneous elimination event and yielding a thiol molecule [Fig. 8F and Table 3, entry 9].96 The thiol reacted with the sulfonate group of 46 to release the salicylaldehyde azine derivative which exhibited AIE–ESIPT properties [Fig. 8G]. β-Lactamase fluorescence measurement afforded a turn-on response at 558 nm with a detection limit of 0.5 mU mL−1 [Fig. 8H]. The probe, 46, was highly selective towards β-lactamase and no other analyte resulted in significant fluorescence emission [Fig. 8I]. Further, 46 was utilized as a portable test paper sensor for the detection of β-lactamase by dipping the filter paper strips coated with 46 into the sample solution containing cefazolin (4.8 mM) and different concentrations of β-lactamase (0–7.0 mU mL−1). A good linearity was obtained in the β-lactamase concentration range of 0–2.0 mU mL−1 [Fig. 8J]. 46 was also investigated for β-lactamase content in milk samples and the results showed good recoveries, suggesting the potential use for β-lactamase detection in milk samples [Fig. 8K].
Chang et al. demonstrated a dual sialic acid-protected salicylaldehyde-derived azine, 47, for the detection of neuraminidase (NA), a crucial enzyme for the replication of the influenza virus [Fig. 9A and Table 3, entry 10].97 The initially non-fluorescent probe 47 displayed a turn-on emission at 524 nm with a 30-fold fluorescence enhancement upon the addition of NA having a limit of detection of 0.024 U mL−1 due to the cleavage of the sialic acid group [Fig. 9B]. Azine 47 was further explored to detect the influenza virion using hemagglutination (HA) titer for the relative concentration of the virus. H3N2 viruses with different HA titers displayed enhanced fluorescence emission with the increment of H3N2 virions with an LOD of 2−1 HAU per 50 μL [Fig. 9C]. The probe 47 in combination with oseltamivir carboxylic acid (OC) was explored to distinguish oseltamivir-resistant mutant type NA (drug-resistant influenza virus strains) from wild types. It was observed that wild-type NA (WT) inhibited the hydrolysis of 47 in the presence of OC, whereas H274Y mutant NA (MT) hydrolyzed 47 both in the presence and absence of OC [Fig. 9D]. The probe, 47, was employed for NA detection in MDCK cells infected by the influenza virus. MDCK cells infected with H3N2 displayed high fluorescence under confocal imaging whereas the presence of OC showed extremely low background fluorescence [Fig. 9E].
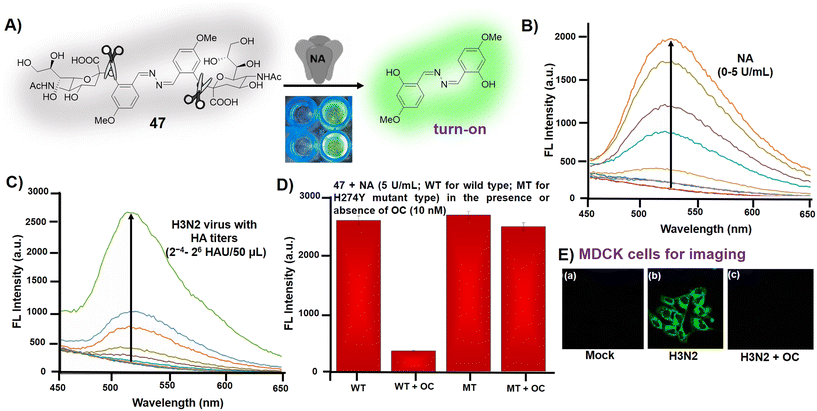 | ||
| Fig. 9 (A) Salicylaldehyde-based azine, 47, as a chemodosimeter for the detection of neuraminidase (NA) by cleavage of the sialic acid group; (B) turn-on emission at 524 nm upon addition of NA; (C) turn-on emission at 524 nm upon the addition of different concentrations of H3N2 viruses; (D) fluorescence intensities of 47 after addition of NA showing fluorescence quenching of wild type (WT) in the presence of OC; (E) fluorescence imaging in mock-infected (a), H3N2 infected (b) and OC pretreated infected MDCK cells (c) (adapted with permission from ref. 97. Copyright 2022, Elsevier). | ||
3.4. Azine as dual sensor
AIE-active probes for the simultaneous detection of two or more analytes with distinct emission outputs are rarely cited. Our group envisaged an AIE active NIR-emissive unsymmetrical azine, benzophenone-azine-phenyl-cyanovinyl-pyridinium chloride (48), as a dual-mode-dual-chemodosimeter for the selective and sensitive detection of toxic analytes, N2H4 and HSO3− [Fig. 10A].30 The D–π–A azine was designed to emit in the NIR region by keeping the electron-donating 4,4′-(hydrazineylidenemethylene)bis(N,N-dimethylaniline) and the electron-withdrawing cyano-pyridinium ethylene moiety at either ends of the azine linkage. The azine, 48, was synthesized by adopting one-pot tandem mechanochemistry. It displayed emission at 718 nm upon excitation at 385 nm with a large Stokes shift of 333 nm in 1% DMSO/H2O. The presence of two strong electron-withdrawing groups viz. –CN and –Py+ in the probe ensured a quick turn-off response against HSO3− by addition across the double bond and a ratiometric response at 525 nm against N2H4 by cleaving the cyano pyridyl group [Fig. 10B and C]. The excellent selectivity of 48 in the presence of other competing amines, cations, and anions resulted in a limit of detection (LOD) of 4 × 10−8 M (1.2 ppb) and 2.5 × 10−8 M (2 ppb) for N2H4 and HSO3−, respectively. The practical utility of the probe has been established for solid-phase and vapor-phase detection of the analytes on silica-coated TLC plates, followed by ImageJ analysis for on-site quantitation, and the real sample analysis was validated by spiking the analytes in various water, soil, and food samples [Fig. 10D–F]. The probe, 48, has also been utilized to detect intracellular N2H4 and HSO3− in living cells [Fig. 10G and H]. The sustainable tandem mechanochemical synthesis, NIR emission with large Stokes shift, ratiometric response, high selectivity and sensitivity, low response time, LOD in the ppb range, on-site solid-phase and vapor-phase detection, and potential in detection of the analytes in live cells are some of the merits of the sensing system.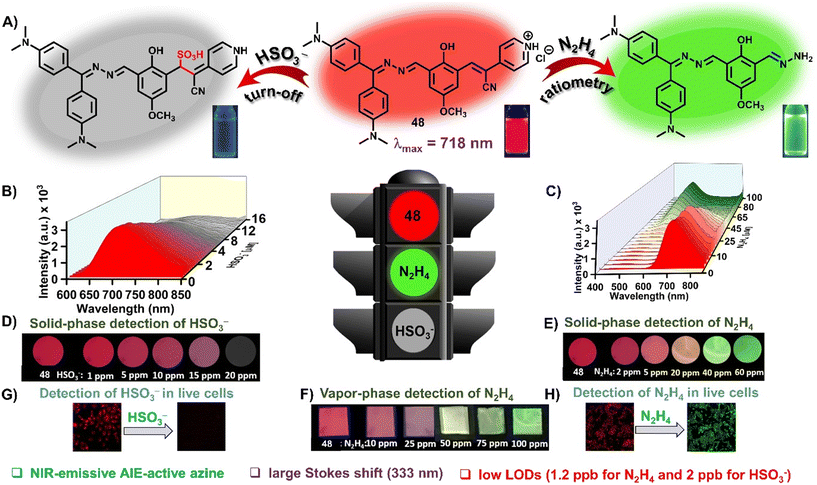 | ||
| Fig. 10 (A) NIR-emissive cationic azine (48) as a dual-chemodosimeter for N2H4 and HSO3− ions; (B) turn-off detection of HSO3− ions by addition across the double bond of the azine; (C) ratiometric response in the presence of N2H4 by cleavage of the cyano pyridyl group; demonstration of solid-phase detection of (D) N2H4 and (E) HSO3− ions; (F) demonstration of vapor-phase detection of N2H4; (E) fluorescence microscopy images of A549 cells in the presence of 48 and (G) N2H4 and (H) HSO3− ions (adapted with permission from ref. 30. Copyright 2023, Elsevier). | ||
3.5. Azines in multi-targeted sensing
Chemosensors capable of sensitively detecting multiple analytes with different fluorescence outputs are handy as they offer advantage of cost-efficient analysis when compared with single analyte-specific sensors.98 Azines have been occasionally used for multi-analyte detection. In this direction, Yadav et al. demonstrated an azine (49) for the multi-targeted sensing of metal ions, viscosity and pH sensing [Fig. 11].99 The azine-based probe, 49, displayed a distinctive color change to brown, dark yellow and greenish yellow and acts as a selective colorimetric sensor for Co2+, Cu2+ and Zn2+ ions, respectively, in DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (9
water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, HEPES 10 mM). However, the turn-on response in fluorimetric studies was found to be highly specific towards Zn2+ ions [Fig. 11A]. The probe exhibits a fluorescence turn-on response towards Zn2+ ions with an increase in emission intensities at 500 nm and displayed a lower LOD of 37 nM [Fig. 11B]. The azine 49 demonstrates remarkable viscochromism in DMF
1 v/v, HEPES 10 mM). However, the turn-on response in fluorimetric studies was found to be highly specific towards Zn2+ ions [Fig. 11A]. The probe exhibits a fluorescence turn-on response towards Zn2+ ions with an increase in emission intensities at 500 nm and displayed a lower LOD of 37 nM [Fig. 11B]. The azine 49 demonstrates remarkable viscochromism in DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol medium wherein with increasing viscosity a noteworthy 6-fold increase in the fluorescence intensity was observed [Fig. 11C]. At lower glycerol fractions, the skeletal framework of 49 rotates freely which relaxes the excited state through a non-radiative decay process. However, at higher glycerol fractions, intramolecular rotation around N–N bonds is restricted leading to strong green fluorescence. Similarly, 49 displayed a turn-on response against increasing pH and acts as a turn-on sensor for basic pH indicators [Fig. 11D]. The negligible fluorescence of 49 in the pH range of 2 to 9 enhanced rapidly in basic pH and a 5-fold enhancement in fluorescence intensity was observed at pH 13 owing to the deprotonation of phenolic hydroxyl protons. The reversibility of 49 towards acidic and basic media by subsequent addition of NaOH and HCl showed that the probe could detect the pH changes significantly for four cycles without much change in its responses [Fig. 11E]. Further, live cell imaging on SiHa cells has been demonstrated for the intra-cellular detection of Zn2+, basic pH and in mitotracking and monitoring cytoplasmic viscosity changes in SiHa cells [Fig. 11F–H]. It efficiently identified the apoptosis process by exhibiting an increase in fluorescence intensity, transitioning from cancerous SiHa cells to apoptotic cells.
glycerol medium wherein with increasing viscosity a noteworthy 6-fold increase in the fluorescence intensity was observed [Fig. 11C]. At lower glycerol fractions, the skeletal framework of 49 rotates freely which relaxes the excited state through a non-radiative decay process. However, at higher glycerol fractions, intramolecular rotation around N–N bonds is restricted leading to strong green fluorescence. Similarly, 49 displayed a turn-on response against increasing pH and acts as a turn-on sensor for basic pH indicators [Fig. 11D]. The negligible fluorescence of 49 in the pH range of 2 to 9 enhanced rapidly in basic pH and a 5-fold enhancement in fluorescence intensity was observed at pH 13 owing to the deprotonation of phenolic hydroxyl protons. The reversibility of 49 towards acidic and basic media by subsequent addition of NaOH and HCl showed that the probe could detect the pH changes significantly for four cycles without much change in its responses [Fig. 11E]. Further, live cell imaging on SiHa cells has been demonstrated for the intra-cellular detection of Zn2+, basic pH and in mitotracking and monitoring cytoplasmic viscosity changes in SiHa cells [Fig. 11F–H]. It efficiently identified the apoptosis process by exhibiting an increase in fluorescence intensity, transitioning from cancerous SiHa cells to apoptotic cells.
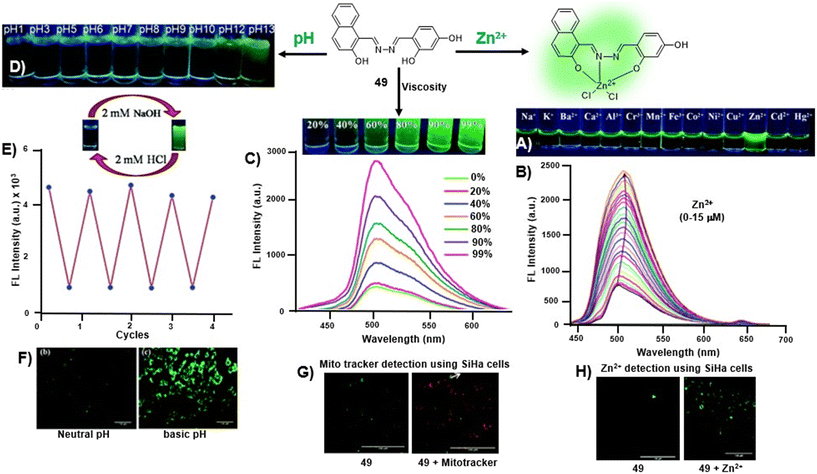 | ||
Fig. 11 (A) The fluorometric responses of 49 against various metal ions captured under 365 nm UV-light showing selective turn-on response towards Zn2+ ions; (B) turn-on fluorescence response of 49 at 500 nm upon incremental addition of Zn2+ ions; (C) the change in the fluorescence emission of 49 at various DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol mixtures and its corresponding images captured under 365 nm UV-light showing stronger emission at higher glycerol fractions; (D) the fluorimetric changes displayed by 49 as a function of pH captured under 365 nm UV-light; (E) the reversible behavior of 49 demonstrated against NaOH and HCl showing high reusability up to 4 cycles; fluorescence microscopy images of SiHa cells studied against (F) pH variation, (G) Mito tracker activity and (H) Zn2+ detection (adapted with permission from ref. 99. Copyright 2022, Royal Society of Chemistry). glycerol mixtures and its corresponding images captured under 365 nm UV-light showing stronger emission at higher glycerol fractions; (D) the fluorimetric changes displayed by 49 as a function of pH captured under 365 nm UV-light; (E) the reversible behavior of 49 demonstrated against NaOH and HCl showing high reusability up to 4 cycles; fluorescence microscopy images of SiHa cells studied against (F) pH variation, (G) Mito tracker activity and (H) Zn2+ detection (adapted with permission from ref. 99. Copyright 2022, Royal Society of Chemistry). | ||
4. Azines in supramolecular sensing
A supramolecular sensor generally helps in the detection of a particular analyte by the formation of a conjugate with a supramolecular host via host–guest interactions mediated by H-bonding, π–π interaction, etc. which upon the addition of the analyte displaces the probe from the complex and results in a fluorescence change. Similarly, amphiphilic AIE polymers can self-assemble in a selective medium resulting in various interesting nanostructures with turn-on fluorescence. The analyte can trigger a disassembly to generate a measurable optical change. The dynamic non-covalent interactions and adaptive nature of the supramolecular assemblies towards different sensing environments result in a selective and sensitive detection tool for a particular target analyte.100,1014.1. Host–guest based sensors
Macrocyclic structures are frequently involved in supramolecular host–guest interactions, which have been established as recognition units in the sensing assembly.102 The non-covalent trapping of analytes as guest molecules in the host cavity serves as the basis for the molecular recognition process for the majority of macrocyclic hosts. These macrocycles are chemically stable, simple to functionalize, and are excellent receptors for a variety of analytes as guest molecules. The cyclization of various aryl motifs through short linkers can produce macrocycles with various functionalities, often consisting of a hydrophobic inner half and a hydrophilic outer part.Ganesan et al. synthesized a symmetrical azine 4,4′-((1E,1′E)-hydrazine-1,2-diylidenebis(methanylylidene)) diphenol (50) from 4-hydroxybenzaldehyde and utilized it for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex formation with γ-cyclodextrin [Fig. 12A].103 The azine, 50, displayed poor water solubility, and thus resulted in weak fluorescence emission. The inclusion complexation of γ-CD/50 in an aqueous medium increased the water solubility and favored the metal ion sensing phenomenon. The fluorescent assembly was able to detect Al3+ selectively and sensitively in the presence of different analytes. The formation of a stable 1
1 inclusion complex formation with γ-cyclodextrin [Fig. 12A].103 The azine, 50, displayed poor water solubility, and thus resulted in weak fluorescence emission. The inclusion complexation of γ-CD/50 in an aqueous medium increased the water solubility and favored the metal ion sensing phenomenon. The fluorescent assembly was able to detect Al3+ selectively and sensitively in the presence of different analytes. The formation of a stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with Al3+ which predominantly uses their –OH and –N atoms afforded an LOD of 1.67 nM. The probe, 50, was further employed for fluorescence microscopy imaging which displayed a fluorescence quenching phenomenon upon the addition of Al3+ ions to the γ-CD–50 complex [Fig. 12B].
1 complex with Al3+ which predominantly uses their –OH and –N atoms afforded an LOD of 1.67 nM. The probe, 50, was further employed for fluorescence microscopy imaging which displayed a fluorescence quenching phenomenon upon the addition of Al3+ ions to the γ-CD–50 complex [Fig. 12B].
 | ||
Fig. 12 (A) Salicylaldehyde azine with terminal phenolic –OH (50) forming a strongly fluorescent 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complexation with γ-CD which acts as a turn-off chemosensor for supramolecular sensing of Al3+ ions; (B) fluorescence microscopy images of 50 in the presence of γ-CD and Al3+ ions displaying a turn-off sensing response (images A and B are adapted with permission from ref. 103. Copyright 2023, Elsevier); (C) syringaldehyde-based azine (51) as a chemosensor for Cu2+ and Pb2+ ions by CHEF and LMCT pathways, respectively; (D) the fluorescence enhancement of 51 with Cu2+ at 413 nm and Pb2+ ions at 473 nm; (E) the 51 1 inclusion complexation with γ-CD which acts as a turn-off chemosensor for supramolecular sensing of Al3+ ions; (B) fluorescence microscopy images of 50 in the presence of γ-CD and Al3+ ions displaying a turn-off sensing response (images A and B are adapted with permission from ref. 103. Copyright 2023, Elsevier); (C) syringaldehyde-based azine (51) as a chemosensor for Cu2+ and Pb2+ ions by CHEF and LMCT pathways, respectively; (D) the fluorescence enhancement of 51 with Cu2+ at 413 nm and Pb2+ ions at 473 nm; (E) the 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β-CD inclusion complex displaying high selectivity towards Al3+ without any interference from Cu2+ and Pb2+; (F) 51 β-CD inclusion complex displaying high selectivity towards Al3+ without any interference from Cu2+ and Pb2+; (F) 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β-CD complex displays a turn-on response against Al3+ addition with an increase in fluorescence intensity at 573 nm; (G) confocal fluorescence imaging indicating fluorescence enhancement with Cu2+ and Pb2+ in the green region and the red emission obtained after the 51 β-CD complex displays a turn-on response against Al3+ addition with an increase in fluorescence intensity at 573 nm; (G) confocal fluorescence imaging indicating fluorescence enhancement with Cu2+ and Pb2+ in the green region and the red emission obtained after the 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β-CD complex reacts with Al3+ ions (images C–G are adapted with permission from ref. 104. Copyright 2023, Elsevier). β-CD complex reacts with Al3+ ions (images C–G are adapted with permission from ref. 104. Copyright 2023, Elsevier). | ||
Narayanan et al. demonstrated a symmetrical azine (51) based on syringaldehyde for the dual detection of metal ions (Cu2+ and Pb2+) in different mechanistic pathways [Fig. 12C].104 The addition of Cu2+ to the azine solution resulted in an enhancement in the emission intensity at 413 nm via the chelation enhanced fluorescence mechanism (CHEF), whereas, in the case of Pb2+ ions, an enhancement in emission intensities with a red-shift to 473 nm was observed due to ligand to metal charge transfer (LMCT) [Fig. 12D]. Interestingly, the inclusion complex of 51 with β-cyclodextrin increases the water solubility and sensing probability and detects Al3+ without any interference from Cu2+ and Pb2+. The high selectivity towards Al3+ may be attributed to the interaction of the hydroxy group of β-CD with the metal ion [Fig. 12E]. The 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β-CD complex displays a turn-off response which gets drastically changed when Al3+ was added to the supramolecular conjugate and an increase in fluorescence intensity at 573 nm was obtained with a detection limit of 3.2 μM [Fig. 12F]. The fluorescence imaging studies were conducted using confocal microscopy which indicated that the presence of Cu2+ and Pb2+ enhances the green fluorescence and in the case of 51
β-CD complex displays a turn-off response which gets drastically changed when Al3+ was added to the supramolecular conjugate and an increase in fluorescence intensity at 573 nm was obtained with a detection limit of 3.2 μM [Fig. 12F]. The fluorescence imaging studies were conducted using confocal microscopy which indicated that the presence of Cu2+ and Pb2+ enhances the green fluorescence and in the case of 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β-CD + Al3+ a red emission is obtained [Fig. 12G].
β-CD + Al3+ a red emission is obtained [Fig. 12G].
4.2. Azine-polymer sensors
In recent years, the utilization of AIE polymers as sensors for different analytes has received considerable interest.105 AIE polymers are associated with numerous advantages such as tunable solubility, high thermal stability, good processability, biocompatibility, structural and functional diversity in comparison to AIE-active small molecules.106 Polymers with AIEgens in the mainchain or sidechain are quite vital in films and gel materials. As of now, numerous AIE-active azine-based polymers have been demonstrated.Huang et al. demonstrated a salicylaldehyde-based azine polymer with PEG arms (52) [Fig. 13A].107 Due to the hydrophilic PEG segments and hydrophobic salicylaldehyde moieties in the core, the polymer self-assembles into stable micelles in water at ambient temperature and fluoresces orange. Polymer micelles, produced at ambient temperature, can be used as a turn-off fluorescent probe to selectively detect Cu2+ in aqueous environments with a low detection limit of 53 nM. The 52–Cu2+ complex can be employed as a turn-on fluorescent probe for the selective detection of S2− displaying an LOD of 0.24 μM.
 | ||
| Fig. 13 (A) Utilization of salicylaldehyde-based azine for preparation of AIE-polymer 52 and its response towards Cu2+ and S2− ions (image A is adapted with permission from ref. 107. Copyright 2020, Elsevier); (B) and (F) AIE-active polymers 53 and 54 with salicylaldehyde backbones in the main chain via thiol–ene click copolymerization, respectively; (C) fluorescence response of 53 as a function of water content (0 to 8%) showing a decrease in intensity upon addition of water fractions to the amide solvents; (D) decrease in the fluorescence intensity of the supernatant from RBC solutions as a function of hemolysis ratio (0 to 4%); (E) turn-off response displayed by 53 upon addition of Cu2+ ions (images C–E are adapted with permission from ref. 108. Copyright 2021, American Chemical Society). | ||
Lu and co-workers used thiol–ene click copolymerization of the SA derivative diacrylate monomer, poly(ethylene glycol) diacrylate and 3,6-dioxa-1,8-octanedithiol for the preparation of a multi-functional polymer (53) [Fig. 13B].10853 unexpectedly exhibited intense emission in amide solvents, which gets significantly reduced by the addition of a small amount of water, serving as a water trace indicator in amide solvents [Fig. 13C]. Secondly, the presence of PEG segments facilitated its dispersion in water and the ROS-responsive thioether groups made it a promising scavenger for reactive oxygen species (ROS) [Fig. 13D]. Additionally, the azine moieties served as a fluorescent indicator for hemolysis determination and selective Cu2+ detection owing to the binding capacity of the azine [Fig. 13E]. The same group further demonstrated another amphiphilic AIE-active polymer with dithiothreitol, poly(ethylene glycol) diacrylate, and a salicylaldehyde-based azine (54) with increased water solubility [Fig. 13F].109 PEG segments and DTT units formed stable micelles to help the polymer disseminate in water and preferentially complex with Cu2+, making it a fluorescent sensor in aqueous media.
Wang et al. reported a linear AIE supramolecular polymer (57) from a salicylaldehyde azine-containing pillar[5]arene dimer (55) and an azine-based homoditopic guest (56) and utilized it as a turn-off Cu2+ sensor [Fig. 14].110 The polymer, 57, was strongly fluorescent at high concentration and the addition of Cu2+ ions results in quenching of the fluorescence as the linear supramolecular polymer changes into a cross-linked supramolecular polymer. The fluorescence of the sensor was re-established upon the addition of CN− ions. The cross-linkable AIE supramolecular polymer fabricated by the salicylaldehyde azine-containing pillar[5]arene dimer serves as a promising candidate for advanced materials such as metallogels, sensors, and adaptive coatings.
 | ||
| Fig. 14 Salicylaldehyde azine-containing pillar[5]arene dimer as a supramolecular assembly for detection of Cu2+ and CN− ions (adapted with permission from ref. 110. Copyright 2019, American Chemical Society). | ||
4.3. Azine-MOF as sensors
Metal–organic frameworks (MOFs) have been of great research interest for the detection of metal ions, anions, etc.111 They are formed by the coordination bonding of metal centers and organic linkers and usually display a high surface area, porosity, rich structural diversity, availability of in-pore functionality and scope for outer-surface modification. In particular, luminescent MOFs exhibit easily induced luminescence, low cost, high selectivity and sensitivity, diverse component scope and various detecting mechanisms.112 MOF fluorescent sensors typically respond by fluorescence enhancement or quenching depending upon the incorporation of guest molecules and ions into their pores.Farahani et al. utilized a pyridyl-based azine (58) to prepare a Zn-based metal–organic framework that could selectively detect Fe3+ ions in DMF [Fig. 15A].113 The photoinduced electron transfer between the Fe3+ ions and the ligand with the amino-functionalized structure in this MOF is responsible for the effective fluorescence quenching action. In addition, the azine –N donors also contribute their lone-pair electrons to the Fe3+ ions resulting in a notable improvement in the detection capability. With a response time of less than one minute and a detection limit of 0.7 μM, the detection of Fe3+ was highly selective, and there were no interferences from As3+, Cd2+, Zn2+, Co2+, Ni2+, Cu2+, Pb2+, Mn2+, and Al3+ [Fig. 15B].
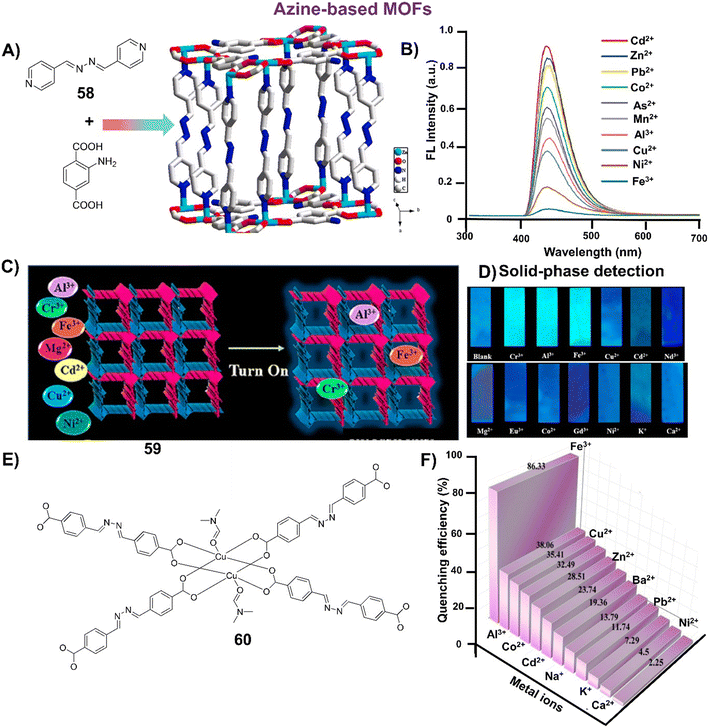 | ||
| Fig. 15 (A) Demonstration of a pyridyl-azine (58) for the formation of the cuboidal block Zn-based MOF showing Zn units linked by eight units of 58 and eight units of the amino-1,4-benzenedicarboxylic acid; (B) emission spectra of the Zn-based MOF against various metal ions showing the highest response towards Fe3+ (images A and B are adapted with permission from ref. 113. Copyright 2019, Elsevier); (C) pictorial description of a 3D porous Zn-based luminescent MOF (59); (D) test papers strips coated with 59 showing selective turn-on response towards metal ions such as Cr3+, Al3+ and Fe3+ under 365 nm UV-light (images C and D are adapted with permission from ref. 114. Copyright 2022, American Chemical Society); (E) core structure of the luminescent Cu2+-based MOF (60) with Lewis basic Schiff base sites; (F) % quenching depicted by the Cu-based MOF 60 against different metal ions (image F is adapted with permission from ref. 115. Copyright 2023, Springer). | ||
Mukherjee et al. developed another substituted pyridyl-based azine probe and used it in the preparation of a highly scalable 3D porous Zn-based MOF 59 by following a mixed ligand synthesis approach [Fig. 15C].114 The aromatic π-conjugated organic linker and N-rich spacer incorporating azine functionality as binding sites for metal ions immobilized within the pore spaces have rendered this MOF an excellent turn-on sensor. A turn-on phenomenon of this nature is uncommon, and a thorough examination indicated an enhancement mechanism attributed to absorbance, known as the absorbance-caused enhancement (ACE) mechanism. It exhibits exceptional sensitivity, selectivity and recyclability for Al3+, Cr3+ and Fe3+ ions. The azine MOF, 59, was further utilized as a simpler, convenient and portable solid-phase detection tool for the sensing of metal ions by turn-on response [Fig. 15D]. The visualization of the MOF 59 loaded silica gel test strips under 365 nm UV-light displayed an enhancement in the fluorescence emission against Cr3+, Al3+ and Fe3+, whereas no change was noted for the other metal ions. In a similar approach, Kaur et al. synthesized a Cu2+-based MOF 60 through the solvothermal method and demonstrated it as a highly selective sensor towards Fe3+ with a low LOD of 47 ppb [Fig. 15E].115 The sensing ability of 60 towards Fe3+ was evaluated against different metal ions and it was noted that no significant quenching was displayed by Ca2+, Ni2+, K+, Pb2+ and Na+ [Fig. 15F]. Ba2+, Cd2+, Zn2+, Co2+, Cu2+ and Al3+ showed a reasonable quenching effect, but the % quenching of 86% shown by Fe3+ ions was the best among the selected metal ions.
4.4. Azine-COF as sensors
Covalent organic frameworks (COFs) stand out as crucial and dynamic constituents within the category of porous organic materials.116 They are assembled through reticular chemistry, where building blocks are linked by covalent bonds. Their intriguing characteristics, including the substantial surface area, structural adaptability, ease of surface modification, and strong chemical stability, contribute to their significance. Numerous materials belonging to this class have been created and applied across various disciplines.117Xia et al. synthesized a salicylaldehyde azine-based 2D-COF (61) through the condensation of two non-AIE monomers, 2-hydroxy-1,3,5-triformylbenzene and hydrazine [Fig. 16A].118 The dispersibility and accessibility of the COF were improved by growing the COF in situ on ZIF-90 followed by digestion under mild acidic conditions to form a hollow COF (HOCOF) with a well-designed AIE structure, excellent dispersibility, small size, and low density. The strong red emission of the HOCOF at 635 nm was completely quenched by ammonia (NH3) owing to the break in the hydrogen bonding and unlocking of the rotors of azine segments thereby disabling the AIE modules [Fig. 16B]. The HOCOF displayed a low limit of detection of 157 nM and a broad response range of 0.1–300 μM for the detection of NH3. Further paper-based sensors were demonstrated by soaking disposable filter paper in HOCOF solution and exposing them to NH3. A visual color change from yellow to brownish red was observed in visible light along with a decrease in fluorescence emission under 365 nm UV light [Fig. 16C], indicating the potential of the sensor as an effective and economical strategy for the monitoring of ammonia in industrial production.
 | ||
| Fig. 16 (A) Azine-based emissive COF (61) showing turn off/on reversible response against NH3/HCl. Further utilization of 61 for the hollow COF by the sacrificial template strategy; (B) turn-off fluorescence response displayed by HOCOF with different concentrations of NH3; (C) paper-based HOCOF sensor upon exposure to NH3 for different times (adapted with permission from ref. 118. Copyright 2023, American Chemical Society). | ||
5. Azines as imaging agents
Since the discovery of the AIE phenomenon, the use of AIEgens for wash-free fluorescence detection and bioimaging has been attracting a lot of research attention.119 However, AIE-active azines are occasionally used as imaging agents. Among one such study, Tharmalingam et al. reported an AIE-active unsymmetrical azine, 62, by simple condensation of ethyl-α-cyano-5-formyl-2-pyrroleacrylate and 1-(hydrazonomethyl)naphthalene-2-ol and used it for the detection of Cu2+ ions.120 The red-emissive azine was further utilized for practical application in imaging latent fingerprints (LFPs). LFPs were demonstrated by accumulating the thumb impressions on the TLC plate surface and coated with the aggregated mixture of 62 in DMSO containing 60% water. The removal of the excess solution of 62 and washing with water developed the latent fingerprints when exposed to 365 nm UV light [Fig. 17A]. Next, the imaging was demonstrated over different surfaces such as a glass TLC slide, aluminium foil, cello tape, currency paper, stainless slide and marble slate which showed that regardless of the surface material, the solid-phase fluorescence is achieved with significant intensity outputs [Fig. 17B]. | ||
| Fig. 17 (A) and (B) Latent fingerprints of different individuals developed on glass TLC plates and various other surfaces such as aluminium foil, cello tape, currency paper, stainless slide, and marble slate showing the variable scope of latent fingerprinting regardless of the surface under investigation (images A and B are adapted with permission from ref. 120. Copyright 2023, Elsevier); (C) fusion images (transverse, coronal, and sagittal) of dynamic micro-PET/CT study of 63 in APP/PS1 mice and WT mice at different time intervals with the maximum intensity projection (MIP) image at 55–60 min after intravenous administration of 63; (D) fusion images (axial, sagittal, and coronal) of PET/CT study of 63 in the rhesus monkey's head at 0–10 min and 50–60 min post-injection along with the whole-body image at 60–70 min post-injection; (E) PET/CT images after injection in an AD patient and a HC subject showing accumulation of 63 in the cortex while the retention of 63 was mainly in the white matter in the HC subject (images C–E are adapted with permission from ref. 122. Copyright 2023, American Chemical Society). | ||
The accumulation of β-amyloid (Aβ) in the brain is a pathological biomarker of Alzheimer's disease (AD), manifesting years prior to the appearance of symptoms, and its identification is an integral part of clinical diagnosis.121 Li et al. demonstrated a class of diaryl-azines for detecting Aβ plaques in the AD brain using PET imaging.122 They developed an Aβ-PET tracer, 63, with a high binding affinity to the Aβ aggregates, significant binding ability with the AD brain sections, and optimal brain pharmacokinetic properties in rodents and non-human primates [Fig. 17C and D]. Dynamic PET studies of 63 demonstrated high initial brain uptake in APP/PS1 and WT mice, and subsequent clearance of the radiotracers from the brain was rapid [Fig. 17C]. An in vivo PET study in a rhesus monkey, devoid of Aβ pathology, suggested that 63 efficiently crossed the blood–brain barrier and accumulated in the cortex within 10 min, while rapidly decreasing the radioactivity to the baseline within 50 min [Fig. 17D]. Furthermore, the investigation of the overall body confirmed that no radio-defluorination takes place and 63 was highly stable in primates. The first-in-human PET study declared that 63 displayed low white matter uptake and could bind to Aβ pathology for distinguishing AD from healthy control subjects. The specific signal intensifications of 63 in the AD patient's cortex, especially in the frontal lobe and temporal lobe (common Aβ-burden regions), were confirmed [Fig. 17E]. Interestingly, the cortex of the HC subject did not show any presence of the relevant radioactivity retention and only a few signals were predominantly concentrated in the sub-cortical white matter.
6. Conclusion
Ever since the inception of the concept of aggregation-induced emission (AIE) in 2001, a new realm of research has unfolded, focusing on luminogenic molecules and materials based on their aggregation properties. Among various AIE-active systems, azines because of their easy synthesis and the ability to tune their luminescence emission through structural modifications are stand out performers with a range of practical applications. The introduction of bulky aromatic substituents and hindering intramolecular rotation through methods like metal chelation and hydrogen bonding can endow azines with AIE features. This review highlights the rationale behind the molecular design of azines and their tunable photophysical properties depending upon the substituents present on the aromatic scaffolds across the azine linkage. To date, many of these azines are derived from salicylaldehyde derivatives with fluorescence in the green to yellow region and are symmetrical across the azine bond. However, the condensation of two different aromatic aldehydes leads to unsymmetrical azines and although rarely documented, red to NIR emission can be achieved by proper tuning of the structure. The review primarily provides a comprehensive overview of the advancements in the realm of AIE-active azine molecules as chemosensors in detecting a diverse range of analytes emphasizing the developments in the last five years. It meticulously discusses the progress in various strategies employed for the detection of metal ions (Cu2+, Zn2+, Al3+, Fe3+, Cr3+, Th4+, Hg2+, Mo6+, UO22+), anions (F−, CN−, ClO−, ONOO−, HSO3−), small molecules (thiols, picric acid, hydrogen peroxide), and bio-analytes (diethylchlorophosphate, alkaline phosphatase, β-lactamase, neuraminidase) focusing on the sensing mechanism, analytical prowess, real sample analysis, etc. It also covers the utilization of azines as dual sensors and multi-analyte sensors. Furthermore, the review describes the developments of azines in supramolecular sensors and sensing materials such as polymers and COFs/MOFs with selected examples to give a wholesome view of AIE-active azines in chemo-/bio-sensing applications. In the end, a section is focused on the azines as imaging agents. From the above discussion, it is apparent that AIE-active azines have immense potential as efficient probes for diverse chemosensing applications as well as to serve as imaging agents. Till date, AIE-active azines for functional bioimaging and tracing are rarely cited. However, the presence of two aromatic scaffolds allows incorporation of multiple functionalities as per the desire to design various organelle targeted AIE-azines. As a future perspective, water-soluble NIR or red-emissive azines with low cytotoxicity and better cell-permeability may be devised with the scope for biomedical and theragnostic applications with a focus on organelle targeting and brain and neuron imaging.Author contributions
Writing of review manuscript, drawing of ChemDraw structures, all figures, A. A. B.; the inception of the concept, planning the template, writing, and reviewing, M. B.; the inception of the concept and reviewing, A. C.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
A. A. B. is indebted to BITS Pilani University for institute-fellowship for SRF.References
- (a) J. Safari and S. Gandomi-Ravandi, RSC Adv., 2014, 4, 46224–46249 RSC; (b) T. Curtius and K. Thun, J. Prakt. Chem., 1891, 44, 161–186 CrossRef; (c) J.-P. Schirmann and P. Bourdauducq, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2001 Search PubMed; (d) J. O. Bauer, G. Leitus, Y. Ben-David and D. Milstein, ACS Catal., 2016, 6, 8415–8419 CrossRef CAS PubMed; (e) G. Moss, P. Smith and D. Tavernier, Pure Appl. Chem., 1995, 67, 1307–1375 Search PubMed; (f) A. Ramakrishnan, S. S. Chourasiya and P. V. Bharatam, RSC Adv., 2015, 5, 55938–55947 RSC.
- S. S. Chourasiya, D. Kathuria, A. A. Wani and P. V. Bharatam, Org. Biomol. Chem., 2019, 17, 8486–8521 RSC.
- S. Kagatikar and D. Sunil, J. Mol. Liq., 2019, 292, 111371 CrossRef CAS.
- (a) M. Godara, R. Maheshwari, S. Varshney and A. K. Varshney, J. Serb. Chem. Soc., 2007, 72, 368–374 CrossRef; (b) A. I. Khodair and P. Bertrand, Tetrahedron, 1998, 54, 4859–4872 CrossRef CAS; (c) M. Chandra, A. N. Sahay, D. S. Pandey, R. P. Tripathi, J. K. Saxena, V. J. M. Reddy, M. C. Puerta and P. Valerga, J. Organomet. Chem., 2004, 689, 2256–2267 CrossRef CAS; (d) J. Granifo, M. E. Vargas, E. S. Dodsworth, D. H. Farrar, S. S. Fielder and A. B. P. Lever, J. Chem. Soc., Dalton Trans., 1996, 4369–4378 RSC; (e) A. Facchetti, A. Abbotto, L. Beverina, M. E. van der Boom, P. Dutta, G. Evmenenko, T. J. Marks and G. A. Pagani, Chem. Mater., 2002, 14, 4996–5005 CrossRef CAS; (f) G. H. Brown and W. G. Shaw, Chem. Rev., 1957, 57, 1049–1157 CrossRef; (g) N. Mukherjee, C. Sun, B. Marie, S. Jin and R. M. Peetz, Tetrahedron Lett., 2008, 49, 1037–1040 CrossRef CAS; (h) C. Amari, C. Pelizzi, G. Predieri, S. Destri and W. Porzio, Synth. Met., 1995, 72, 7–12 CrossRef CAS; (i) H. S. Nalwa, T. Hamada and A. Kakuta, Synth. Met., 1993, 57, 3901–3906 CrossRef CAS; (j) W. Hong, B. Sun, H. Aziz, W. T. Park, Y. Y. Noh and Y. Li, Chem. Commun., 2012, 48, 8413–8415 RSC; (k) J. J. Tindale, H. Holm, M. S. Workentin and O. A. Semenikhin, J. Electroanal. Chem., 2008, 612, 219–230 CrossRef CAS.
- Photophysics of Aromatic Molecules, ed. J. B. Birks, Wiley InterScience, London, 1970 Search PubMed.
- T. Förster and K. Kasper, Z. Elektrochem., 1955, 59, 976–980 CrossRef.
- (a) J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhuc and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC; (b) B. Z. Tang, X. Zhan, G. Yu, P. P. S. Lee, Y. Liu and D. Zhu, J. Mater. Chem., 2001, 11, 2974–2978 RSC.
- (a) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC; (b) J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed; (c) Q. Xia, Y. Zhang, Y. Li, Y. Li, Y. Li, Z. Feng, X. Fan, J. Qian and H. Lin, Aggregate, 2022, 3, e152 CrossRef CAS; (d) J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed; (e) H. Wang, E. Zhao, J. W. Y. Lam and B. Z. Tang, Mater. Today, 2015, 18, 365–377 CrossRef CAS; (f) G. R. Suman, M. Pandey and A. S. J. Chakravarthy, Mater. Chem. Front., 2021, 5, 1541–1584 RSC; (g) Z. He, C. Ke and B. Z. Tang, ACS Omega, 2018, 3, 3267–3277 CrossRef CAS PubMed; (h) R. T. K. Kwok, C. W. T. Leung, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2015, 44, 4228–4238 RSC; (i) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332–4353 RSC; (j) D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441–2453 CrossRef CAS PubMed.
- S. Poojary, D. Sunil, D. Kekuda and S. Sreenivasa, Opt. Mater., 2018, 85, 1–7 CrossRef CAS.
- T. Zheng, J.-L. Xu, X.-J. Wang, J. Zhang, X. Jiao, T. Wang and D. Chen, Chem. Commun., 2016, 52, 6922–6925 RSC.
- S. Kagatikar, D. Sunil, D. Kekuda, S. D. Kulkarni and A. A. A. Salam, J. Mol. Liq., 2020, 318, 114029 CrossRef CAS.
- T. Mondal, S. Roy, I. Mondal, M. V. Mane and S. S. Panja, J. Photochem. Photobiol., A, 2021, 406, 112998 CrossRef CAS.
- P. Acker, M. E. Speer, J. S. Wössner and B. Esser, J. Mater. Chem. A, 2020, 8, 11195–11201 RSC.
- D. Liu, K. Li, M. Li, Z. Wang, M. Shan and Y. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 37775–37784 CrossRef CAS PubMed.
- S. Bondock, T. Albarqi, I. A. Shaaban and M. M. Abdou, RSC Adv., 2023, 13, 10353–10366 RSC.
- S. Kaide, H. Watanabe, Y. Shimizu, S. Iikuni, Y. Nakamoto, M. Hasegawa, K. Itoh and M. Ono, ACS Chem. Neurosci., 2020, 11, 4254–4261 CrossRef CAS PubMed.
- S. Biswas, R. Mengji, S. Barman, V. Venugopal, A. Jana and N. D. P. Singh, Chem. Commun., 2018, 54, 168–171 RSC.
- J.-L. Xu, Y. Quan, Q.-Y. Li, H. Lu, H. Wu, J. Yin, X.-J. Wang and Q. Zhang, RSC Adv., 2015, 5, 88176–88180 RSC.
- S. Guo, Y. Song, Y. He, X.-Y. Hu and L. Wang, Angew. Chem., Int. Ed., 2018, 57, 3163–3167 CrossRef CAS PubMed.
- W. Tang, Y. Xiang and A. Tong, J. Org. Chem., 2009, 74, 2163–2166 CrossRef CAS PubMed.
- X. Chen, Y. Xiang, P. Song and A. Tong, Analyst, 2010, 135, 1098–1105 RSC.
- L. Peng, Z. Zhou, X. Wang, R. Wei, K. Li, Y. Xiang and A. Tong, Anal. Chim. Acta, 2014, 829, 54–59 CrossRef CAS PubMed.
- Z. Li, Y. Zhang, H. Xia, Y. Mua and X. Liu, Chem. Commun., 2016, 52, 6613–6616 RSC.
- N. Gupta, T. Kaur, V. Bhalla, R. D. Parihar, P. Ohri, G. Kaur and M. Kumar, Chem. Commun., 2017, 53, 12646–12649 RSC.
- Y. Zhao, Y. Li, Y. Long, Z. Zhou, Z. Tang, K. Deng and S. Zhang, Tetrahedron Lett., 2017, 58, 1351–1355 CrossRef CAS.
- Y.-H. Zhao, Y. Luo, H. Wang, T. Guo, H. Zhou, H. Tan, Z. Zhou, Y. Long and Z. Tang, ChemistrySelect, 2018, 3, 1521–1526 CrossRef CAS.
- H. L. Nguyen, N. Kumar, J.-F. Audibert, R. Ghasemi, J.-P. Lefevre, M.-H. Ha-Thi, C. Mongin and I. Leray, New J. Chem., 2019, 43, 15302–15310 RSC.
- S. Manigandan, A. Muthusamy, R. Nandhakumar and C. I. David, J. Mol. Struct., 2020, 1208, 127834 CrossRef CAS.
- A. A. Bhosle, S. D. Hiremath, A. C. Bhasikuttan, M. Banerjee and A. Chatterjee, J. Photochem. Photobiol., A, 2021, 413, 113265 CrossRef CAS.
- A. A. Bhosle, M. Banerjee, S. Saha, S. Garg, S. Ghosh and A. Chatterjee, Sens. Actuators, B, 2023, 397, 134661 CrossRef CAS.
- X. Chen, R. Wei, Y. Xiang, Z. Zhou, K. Li, P. Song and A. Tong, J. Phys. Chem. C, 2011, 115, 14353–14359 CrossRef CAS.
- R. Wei, P. Song and A. Tong, J. Phys. Chem. C, 2013, 117, 3467–3474 CrossRef CAS.
- X. Yao, J.-X. Ru, C. Xu, Y.-M. Liu, W. Dou, X.-L. Tang, G.-L. Zhang and W.-S. Liu, ChemistryOpen, 2015, 4, 478–482 CrossRef CAS PubMed.
- S. Samanta, U. Manna and G. Das, New J. Chem., 2017, 41, 1064–1072 RSC.
- M. Mathivanan, B. Tharmalingam, C.-H. Lin, B. V. Pandiyan, V. Thiagarajan and B. Murugesapandian, CrystEngComm, 2020, 22, 213–228 RSC.
- M. Mathivanan and B. Murugesapandian, Dyes Pigm., 2022, 203, 110367 CrossRef CAS.
- A. A. Bhosle, M. Banerjee, S. D. Hiremath, A. C. Bhasikuttan and A. Chatterjee, Chem. – Asian J., 2023, 18, e202300048 CrossRef CAS PubMed.
- M. K. Dixit and M. Dubey, Phys. Chem. Chem. Phys., 2018, 20, 23762–23772 RSC.
- D. H. Brown and W. E. Smith, Metal ions in biological systems, in Enzyme Chemistry, ed. C. J. Suckling, Springer, Dordrecht, 1984, DOI:10.1007/978-94-009-5574-5_6.
- (a) X. Zheng, W. Cheng, C. Ji, J. Zhang and M. Yin, Rev. Anal. Chem., 2020, 39, 231–246 CrossRef CAS; (b) Y. Shi, W. Zhang, Y. Xue and J. Zhang, Chemosensors, 2023, 11, 226 CrossRef CAS.
- A. K. Saini, K. Natarajan and S. M. Mobin, Chem. Commun., 2017, 53, 9870–9873 RSC.
- J.-H. Hu, Y. Sun, J. Qi, Q. Li and T.-B. Wei, Spectrochim. Acta, Part A, 2017, 175, 125–133 CrossRef CAS PubMed.
- A. Ghosh, S. Adhikari, S. Ta, A. Banik, T. K. Dangar, S. K. Mukhopadhyay, J. S. Matalobos, P. Brandão, V. Félix and D. Das, Dalton Trans., 2016, 45, 19491–19499 RSC.
- X. Chen, L. He, Y. Wang, B. Liu and Y. Tang, Anal. Chim. Acta, 2014, 847, 55–60 CrossRef CAS PubMed.
- X. Chen, L. Peng, M. Feng, Y. Xiang, A. Tong, L. He, B. Liu and Y. Tang, J. Lumin., 2017, 186, 301–306 CrossRef CAS.
- H. Zhou, B. Yang, G. Wen, X. Hu and B. Liu, Talanta, 2018, 184, 394–403 CrossRef CAS PubMed.
- B. Tharmalingam, M. Mathivanan, G. Dhamodiran, K. S. Mani, M. Paranjothy and B. Murugesapandian, ACS Omega, 2019, 4, 12459–12469 CrossRef CAS PubMed.
- K. Tiwari, S. Kumar, V. Kumar, J. Kaur, S. Arora and R. K. Mahajan, Spectrochim. Acta, Part A, 2018, 191, 16–26 CrossRef CAS PubMed.
- W. Ye, M. Xie, J. Wei, G. Li, Y. Tang, L. Hou, L. Wang, H. Yu, C.-S. Lee and H. Xu, Sens. Actuators, B, 2021, 343, 130106 CrossRef CAS.
- J. Liu, C. Leng, Q. Chen, Q. Liang, C. Li, S. Li, Z. Zhang and L. Xiao, ChemistrySelect, 2022, 7, e202203313 CrossRef CAS.
- K. Kumarasamy, T. Devendhiran, M.-C. Lin, W.-J. Chien, S. K. Ramasamy, S. Manickam and J.-C. Yang, Inorg. Chem. Commun., 2022, 145, 110020 CrossRef CAS.
- S. Sharifi, A. W. Mesbah and E. Iravani, Results Chem., 2023, 5, 100747 CrossRef CAS.
- G. Kumar, K. Paul and V. Luxami, Sens. Actuators, B, 2018, 263, 585–593 CrossRef CAS.
- S. Khanra, S. Ta, M. Ghosh, S. Chatterjee, P. Mukherjee and D. Das, New J. Chem., 2020, 44, 8477–8485 RSC.
- S. Khanra, S. Ta, M. Ghosh, S. Chatterjee and D. Das, RSC Adv., 2019, 9, 21302–21310 RSC.
- B. Das, M. Dolai, A. Dhara, S. Mabhai, A. Jana, S. Dey and A. Misra, Anal. Sci. Adv., 2021, 2, 447–463 CrossRef CAS.
- B. Musikavanhu, Y. Zhang, D. Zhu, Z. Xue, R. Yuan, S. Wang and L. Zhao, Spectrochim. Acta, Part A, 2022, 281, 121599 CrossRef CAS PubMed.
- T. Mondal, I. Mondal, S. Biswas, M. V. Mane and S. S. Panja, ChemistrySelect, 2020, 5, 9336–9349 CrossRef CAS.
- J. Tong, K. Zhang, J. Wang, H. Li, F. Zhou, Z. Wang, X. Zhang and B. Z. Tang, J. Mater. Chem. C, 2020, 8, 996–1001 RSC.
- M. Ghosh, S. Ta, J. S. Matalobos and D. Das, Dalton Trans., 2018, 47, 11084–11090 RSC.
- X.-Q. Pham, N. Kumar, M.-H. Ha-Thi and I. Leray, J. Photochem. Photobiol., A, 2019, 373, 139–145 CrossRef CAS.
- R. Yadav, A. Rai, A. K. Sonkar, V. Rai, S. C. Gupta and L. Mishra, New J. Chem., 2019, 43, 7109–7119 RSC.
- X.-M. Sun, J. Liu, Z.-H. Li, Y.-P. Fu, T.-T. Huang, Z.-D. Tang, B. Shi, H. Yao, T.-B. Wei and Q. Lin, Chin. Chem. Lett., 2023, 34, 107792 CrossRef CAS.
- B. Das, M. Dolai, A. Dhara, A. Ghosh, S. Mabhai, A. Misra, S. Dey and A. Jana, J. Phys. Chem. A, 2021, 125, 1490–1504 CrossRef CAS PubMed.
- M. Dolai, U. Saha, A. K. Das and G. S. Kumar, Anal. Methods, 2018, 10, 4063–4072 RSC.
- S. Sawminathan, S. Munusamy, S. Manickam, D. Jothi and S. KulathuIyer, Dyes Pigm., 2021, 196, 109755 CrossRef CAS.
- N. Kaur, G. Kaur, U. A. Fegade, A. Singh, S. K. Sahoo, A. S. Kuwar and N. Singh, TrAC, Trends Anal. Chem., 2017, 95, 86–109 CrossRef CAS.
- (a) J. F. Zhang, C. S. Lim, S. Bhuniya, B. R. Cho and J. S. Kim, Org. Lett., 2011, 13, 1190–1193 CrossRef CAS PubMed; (b) P. Pacher, J. S. Beckman and L. Liaudet, Physiol. Rev., 2007, 87, 315–424 CrossRef CAS PubMed; (c) F. Wang, L. Wang, X. Chen and J. Yoon, Chem. Soc. Rev., 2014, 43, 4312–4324 RSC; (d) B. Pan, H. Ren, X. Lv, Y. Zhao, B. Yu, Y. He, Y. Ma, C. Niu, J. Kong, F. Yu, W. Sun, Y. Zhang, B. Willard and L. Zheng, J. Transl. Med., 2012, 10, 65 CrossRef CAS PubMed; (e) A. F. Gunnison, D. W. Jacobsen and H. J. Schwartz, Crit. Rev. Toxicol., 1987, 17, 185–214 CrossRef CAS PubMed.
- B. Yu, C.-Y. Li, Y.-X. Sun, H.-R. Jia, J.-Q. Guo and J. Li, Spectrochim. Acta, Part A, 2017, 184, 249–254 CrossRef CAS PubMed.
- P.-X. Pei, J.-H. Hu, Y. Chen, Y. Sun and J. Qi, Spectrochim. Acta, Part A, 2017, 181, 131–136 CrossRef CAS PubMed.
- Y. Sun, J.-H. Hu, J. Qi and J.-B. Li, Spectrochim. Acta, Part A, 2016, 167, 101–105 CrossRef CAS PubMed.
- J. Yang, J. Rui, X. Xu, Y. Yang, J. Su, H. Xu, Y. Wang, N. Sun and S. Wang, RSC Adv., 2016, 6, 30636–30641 RSC.
- Y. Shen, M. Li, M. Yang, Y. Zhang, H. Li and X. Zhang, Spectrochim. Acta, Part A, 2019, 222, 117230 CrossRef CAS PubMed.
- X. Yuan, C.-X. Zhao, Y.-X. Lu, Y.-J. Shen and C.-Y. Wang, J. Photochem. Photobiol., A, 2018, 361, 41–47 CrossRef CAS.
- T. Devendhiran, K. Kumarasamy, M.-C. Lin and Y. X. Yang, Inorg. Chem. Commun., 2021, 134, 108951 CrossRef CAS.
- Y. Yao, X.-M. Fu and J.-H. Hu, Inorg. Chem. Commun., 2022, 141, 109557 CrossRef CAS.
- S. Paul, K. Debsharma, S. Dey, S. Halder, K. Jana and C. Sinha, Mater. Adv., 2023, 4, 3874–3891 RSC.
- G. Singh, A. Kaur, M. Sharma, V. Bhalla, D. Singh, S. Arora and M. Kumar, Mater. Adv., 2020, 1, 1347–1353 RSC.
- (a) J. R. Burgoyne, S. I. Oka, N. Ale-Agha and P. Eaton, Antioxid. Redox Signaling, 2013, 18, 1042–1052 CrossRef CAS PubMed; (b) S. Garrod, M. E. Bollard, A. W. Nicholls, S. C. Connor, J. Connelly, J. K. Nicholson and E. Holmes, Chem. Res. Toxicol., 2005, 18, 115–122 Search PubMed; (c) G. J. Despotis, G. Gravlee, K. Filos, J. Levy and D. M. Fisher, Anesthesiology, 1999, 91, 1122–1151 CrossRef CAS PubMed; (d) Q. Shao, Y. Zheng, X. Dong, K. Tang, X. Yan and B. Xing, Chem. – Eur. J., 2013, 19, 10903–10910 CrossRef CAS PubMed; (e) G. M. Air and W. G. Laver, Proteins: Struct., Funct., Bioinf., 1989, 6, 341–356 CrossRef CAS PubMed; (f) Y. Zhou and J. Yoon, Chem. Soc. Rev., 2012, 41, 52–67 RSC.
- L. Peng, Z. Zhou, R. Wei, K. Li, P. Song and A. Tong, Dyes Pigm., 2014, 108, 24–31 CrossRef CAS.
- H. Liu, P. Song, R. Wei, K. Li and A. Tong, Talanta, 2014, 118, 348–352 CrossRef CAS PubMed.
- L. Peng, R. Wei, K. Li, Z. Zhou, P. Song and A. Tong, Analyst, 2013, 138, 2068–2072 RSC.
- X.-T. Chen, Y. Xiang and A. J. Tong, Talanta, 2010, 80, 1952–1958 CrossRef CAS PubMed.
- X.-T. Chen and A. J. Tong, J. Lumin., 2014, 145, 737–740 CrossRef CAS.
- M. Gao, C. K. Sim, C. W. T. Leung, Q. Hu, G. Feng, F. Xu, B. Z. Tang and B. Liu, Chem. Commun., 2014, 50, 8312–8315 RSC.
- L. Peng, M. Gao, X. Cai, R. Zhang, K. Li, G. Feng, A. Tong and B. Liu, J. Mater. Chem. B, 2015, 3, 9168–9172 RSC.
- H. Liu, R. Wei, Y. Xiang and A. Tong, Anal. Methods, 2015, 7, 753–758 RSC.
- X. Ma, J. Cheng, J. Liu, X. Zhou and H. Xiang, New J. Chem., 2015, 39, 492–500 RSC.
- M. Sathiyaraj, K. Pavithra and V. Thiagarajan, New J. Chem., 2020, 44, 8402–8411 RSC.
- A. A. Bhosle, M. Banerjee, V. Gupta, S. Ghosh, A. C. Bhasikuttan and A. Chatterjee, New J. Chem., 2022, 46, 18961–18972 RSC.
- H. Song, Y. Zhou, H. Qu, C. Xu, X. Wang, X. Liu, Q. Zhang and X. Peng, Ind. Eng. Chem. Res., 2018, 57, 15216–15223 CrossRef CAS.
- M. Bhattu, A. A. Wani, M. Verma, P. V. Bharatam, D. Kathuria and J. Simal-Gandara, J. Photochem. Photobiol., A, 2023, 437, 114476 CrossRef CAS.
- R. Suhasini, R. Karpagam, K. Thirumoorthy and V. Thiagarajan, Spectrochim. Acta, Part A, 2021, 263, 120206 CrossRef CAS PubMed.
- M. Sathiyaraj and V. Thiagarajan, RSC Adv., 2020, 10, 25848–25855 RSC.
- Y. He, J. Yu, X. Hu, S. Huang, L. Cai, L. Yang, H. Zhang, Y. Jiang, Y. Jia and H. Sun, Chem. Commun., 2020, 56, 13323–13326 RSC.
- L. Peng, L. Xiao, Y. Ding, Y. Xiang and A. Tong, J. Mater. Chem. B, 2018, 6, 3922–3926 RSC.
- H. Chang, Y. Mei, Y. Li and L. Shang, Talanta, 2022, 247, 123583 CrossRef CAS PubMed.
- (a) S. Mukhopadhyay, R. K. Gupta, A. Biswas, A. Kumar, M. Dubey, M. S. Hundal and D. S. Pandey, Dalton Trans., 2015, 44, 7118–7122 RSC; (b) Y. Liang, R. Wang, G. Liu and S. Pu, ACS Omega, 2019, 4, 6597–6606 CrossRef CAS PubMed; (c) X. He, J. Zhang, X. Liu, L. Dong, D. Li, H. Qiu and S. Yin, Sens. Actuators, B, 2014, 192, 29–35 CrossRef CAS; (d) L. Wang, X. Gong, Q. Bing and G. Wang, Microchem. J., 2018, 142, 279–287 CrossRef CAS.
- P. Yadav, S. Gond, A. Shekher, S. C. Gupta, U. P. Singh and V. P. Singh, Dalton Trans., 2022, 51, 6927–6935 RSC.
- (a) T. L. Mako, J. M. Racicot and M. Levine, Chem. Rev., 2019, 119, 322–477 CrossRef CAS PubMed; (b) S. Lim, Y. Kuang and H. A. M. Ardoña, Front. Chem., 2021, 9, 723111 CrossRef CAS PubMed.
- (a) M. Huo, Q. Ye, H. Che, X. Wang, Y. Wei and J. Yuan, Macromolecules, 2017, 50, 1126–1133 CrossRef CAS; (b) Z. Zhang, Y. Lou, C. Guo, Q. Jia, Y. Song, J.-Y. Tian, S. Zhang, M. Wang, L. He and M. Du, Trends Food Sci. Technol., 2021, 118, 569–588 CrossRef CAS; (c) Y. Yue, D. Ji, Y. Liu and D. Wei, Chem. – Eur. J., 2023, e202302474 Search PubMed.
- (a) A. A. Bhosle, M. Banerjee, N. Barooah, A. C. Bhasikuttan, K. Kadu, S. R. Ramanan and A. Chatterjee, J. Photochem. Photobiol., A, 2022, 426, 113770 CrossRef CAS; (b) A. A. Bhosle, M. Banerjee, S. D. Hiremath, D. S. Sisodiya, V. G. Naik, N. Barooah, A. C. Bhasikuttan, A. Chattopadhyay and A. Chatterjee, J. Mater. Chem. B, 2022, 10, 8258–8273 RSC.
- V. Ganesan, M. K. Mani, V. Narayanan, E. Shanmugasundram, K. Vellaisamy, V. Baskaralingam, J. Jeyaraj, G. B. Veerakanellore, R. Rajamohan and S. Thambusamy, J. Photochem. Photobiol., A, 2023, 442, 114814 CrossRef CAS.
- V. Narayanan, V. Ganesan, E. Shanmugasundram, S. Durganandini, K. Vellaisamy, H. Amirthalingam, R. Rajamohan and S. Thambusamy, J. Photochem. Photobiol., A, 2023, 445, 115069 CrossRef CAS.
- (a) Z. Qiu, X. Liu, J. W. Y. Lam and B. Z. Tang, Macromol. Rapid Commun., 2019, 40, 1800568 CrossRef PubMed; (b) R. Hu, D. Xin, A. Qin and B. Z. Tang, Acta Polym. Sin., 2018, 2, 132–144 Search PubMed; (c) H. Chen, E. Zhang, G. Yang, L. Li, L. Wu, Y. Zhang, Y. Liu, G. Chen and M. Jiang, ACS Macro Lett., 2019, 8, 893–898 CrossRef CAS PubMed; (d) K. Morishima, F. Ishiwari, S. Matsumura, T. Fukushima and M. Shibayama, Macromolecules, 2017, 50, 5940–5945 CrossRef CAS.
- (a) M. Huo, Q. Ye, H. Che, X. Wang, Y. Wei and J. Yuan, Macromolecules, 2017, 50, 1126–1133 CrossRef CAS; (b) Y. Wu, L. Qu, J. Li, L. Huang and Z. Liu, Polymer, 2018, 158, 297–307 CrossRef CAS; (c) Y. Li, F. Gao, J. Guo, P. Ren, Z. Tian, J. Bai and J. Hua, Eur. Polym. J., 2020, 122, 109355 CrossRef CAS.
- J. Huang, H. Qin, H. Liang and J. Lu, Polymers, 2020, 202, 122663 CrossRef CAS.
- H. Qin, J. Huang, H. Liang and J. Lu, ACS Appl. Mater. Interfaces, 2021, 13, 5668–5677 CrossRef CAS PubMed.
- W. Liang, D. Liu, J. Huang, H. Liang and J. Lu, Dyes Pigm., 2022, 200, 110115 CrossRef CAS.
- P. Wang, B. Liang and D. Xi, Inorg. Chem., 2019, 58, 2252–2256 CrossRef CAS PubMed.
- (a) R. W. Flaig, T. M. Osborn Popp, A. M. Fracaroli, E. A. Kapustin, M. J. Kalmutzki, R. M. Altamimi, F. Fathieh, J. A. Reimer and O. M. Yaghi, J. Am. Chem. Soc., 2017, 139, 12125–12128 CrossRef CAS PubMed; (b) M. Tanhaei, A. R. Mahjoub and V. Safarifard, Mater. Lett., 2018, 227, 318–321 CrossRef CAS.
- (a) Y. Zhang, S. Yuan, G. Day, X. Wang, X. Yan and H.-C. Zhou, Coord. Chem. Rev., 2017, 354, 28–45 CrossRef; (b) Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC.
- Y. D. Farahani and V. Safarifard, J. Solid State Chem., 2019, 270, 428–435 CrossRef CAS.
- D. Mukherjee, A. Pal, S. C. Pal, A. Saha and M. C. Das, Inorg. Chem., 2022, 61, 16952–16962 CrossRef CAS PubMed.
- M. Kaur, M. Yusuf and A. K. Malik, J. Fluoresc., 2023, 33, 339–357 CrossRef CAS PubMed.
- X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC.
- R. K. Sharma, P. Yadav, M. Yadav, R. Gupta, P. Rana, A. Srivastava, R. Zbořil, R. S. Varma, M. Antonietti and M. B. Gawande, Mater. Horiz., 2020, 7, 411–454 RSC.
- Y.-D. Xia, Y. Cheng, Y.-J. Wu, Y. Xia and X.-B. Yin, Chem. Mater., 2023, 35, 2579–2587 CrossRef CAS.
- (a) J. Qian and B. Z. Tang, Chem, 2017, 3, 56–91 CrossRef CAS; (b) X. Huang, R. Zhang, C. Chen, R. T. K. Kwok and B. Z. Tang, Mater. Chem. Front., 2021, 5, 723–743 RSC; (c) Y. Chen, Mater. Today Chem., 2022, 25, 100975 CrossRef CAS; (d) S. Wang, K. Zhou, X. Lyu, H. Li, Z. Qiu, Z. Zhao and B. Z. Tang, Chem. Biomed. Imaging, 2023, 1, 509–521 CrossRef CAS.
- B. Tharmalingam, O. Anitha, J. Aiswarya, T. Thiruppathiraja, S. Lakshmipathi and B. Murugesapandian, J. Photochem. Photobiol., A, 2023, 442, 114757 CrossRef CAS.
- V. L. Villemagne, S. Burnham, P. Bourgeat, B. Brown, K. A. Ellis, O. Salvado, C. Szoeke, S. L. Macaulay, R. Martins, P. Maruff, D. Ames, C. C. Rowe and C. L. Masters, Lancet Neurol., 2013, 12, 357–367 CrossRef CAS PubMed.
- Y. Li, K. Zhou, X. Zhang, H. Zhao, X. Wang, R. Dong, Y. Wang, B. Chen, X.-x. Yan, J. Dai, Y. Sui, J. Zhang and M. Cui, J. Med. Chem., 2023, 66, 4603–4616 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |