Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Approaches for selectivity improvement of conductometric gas sensors: an overview
Jing
Li
,
Hongchao
Zhao
,
Yanjie
Wang
and
Yong
Zhou
 *
*
Key Laboratory of Optoelectronic Technology and System of Ministry of Education, College of Optoelectronic Engineering, Chongqing University, Chongqing 400044, People's Republic of China. E-mail: zhyf@cqu.edu.cn
First published on 10th January 2024
Abstract
Conductometric gas sensors (CGS) have been extensively explored in recent decades owing to easy fabrication and miniaturization, low cost and distributable detectability. Among numerous performance parameters, selectivity is a critical one to evaluate the operation quality of CGS in diverse application scenarios such as environment monitoring, food quality assessment, individual healthcare, etc. Nevertheless, in most preceding work either the underlying mechanism for the shown selectivity is not explained clearly or the strategies to improve the selectivity are not detailed, which necessitates an urgent need to address these. Also, there is still a lack of comprehensive summaries reported in this aspect thus far. A favorable selectivity always means a stronger sensitivity toward a specific gas than that toward other interference gases. Thus, it is very essential to conduct a comprehensive overview to understand the sensitivity, selectivity and their relationships from both quantitative and qualitative perspectives. In this review, the sensing mechanism of CGS is first studied according to the selectivity coefficient simultaneously concluding the factors that influence the detection selectivity. Subsequently, chemical and physical modification strategies to improve the selectivity are discussed. Finally, challenges and perspectives of the selectivity optimization methods are proposed for future research.
1. Introduction
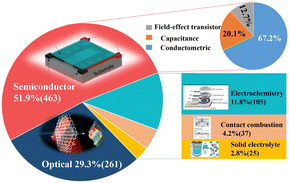
The global sensor market has witnessed an ever-increasing expansion. Among diverse sensor species, gas sensors are extensively applied in numerous scenarios such as explosive or toxic environments, Internet of Things (IOT), indoor air quality monitoring,1 medical diagnostics,2 food quality assessment,3etc. Its market is expected to reach 2.28 billion $ dollars by 2025. When surface-active nanomaterials come into contact with gas, the gas–solid interactions will change their inherent physicochemical parameters such as conductivity, capacitance, work function, optical properties, and reaction energy due to the large surface area, abundant functional groups, flexible adjustability of the structure–property relation, and environmental susceptibility.4–8 We roughly summarize approximately 900 research papers over the past decade with the search keywords “nanomaterials” and “gas sensing” via Web of Science as shown in Fig. 1. The results indicate that conductive gas sensors (CGS) with the merits of low cost, easy manufacturing and signal processing, and high sensitivity have gained the most popularity. However, in order to improve the cost-effectiveness of CGS in practical applications, further optimization and improvement are still needed in terms of sensitivity, selectivity, response/recovery time, operating temperature, etc.Of different performance parameters, selectivity is one of the most crucial indicators for evaluating the quality of CGS. Gas sensors with ideal selectivity can readily ascertain the detailed species and concentration of a specific gaseous component without any interference from other coexisting ones, which ensures an accurate and reliable gas detection. However, selectivity is often overlooked in most preceding efforts. Therefore, it is urgently essential to offer potential methods as theoretical basis for instructing the improvement process. Recently, the methods that leverage special catalytic components such as precious metals or rare earth metal oxides, filters, and sensor arrays, and the latest research using binary metal oxides9 and hierarchical metal oxides10 have emerged to boost the sensitivity and selectivity. Meanwhile, a few review articles in recent years had reported the modification of the selectivity of gas sensors.9–17 For example, G. Korotcenkov et al.9 discussed the engineering methods for improving the selectivity of CGS. Alternatively, M. Wusiman et al.14 provided chemical modification methods, but did not systematically elaborate the selectivity enhancement from the basic properties of gas molecules such as mass, charge, etc. In another report, H. Hashtroudi et al.13 studied the hybridization effect of two-dimensional (2D) materials on sensor selectivity and sensitivity as well as the sensing mechanism. As claimed by the authors, the hybridization offered more surface sites and then enhanced the collective performance. However, this research was only limited to 2D hybrid materials operated at room temperature. Another group18 detailed the factors that affected the selectivity of gas sensors as well as the techniques to improve the selectivity but from both spectral and nonspectral aspects.
According to the current endeavors mentioned above, this review comprehensively discusses the latest progress in improving the selectivity of CGS through both chemical and physical methods. The basic principles for each method are introduced from the perspectives of electron sensitization, chemical sensitization, and affinity modulation between target molecules and sensing materials. Also, the problems encountered are emphasized. Finally, an overall perspective on the issue of selectivity, as well as the challenges and prospects, is presented, with the aim of paving the way for optimizing future gas sensors with excellent selectivity.
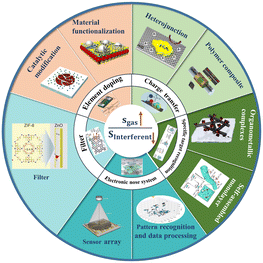
2. Strategies to improve the detection selectivity
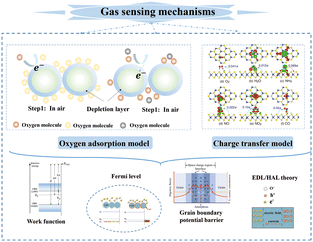
The fluctuation of the gaseous composition and concentration within the test environment would change the electronic properties of the sensing material for CGS. Recently, researchers have explained these phenomena from many perspectives, such as the electronic core–shell structure including the electron depletion layer (EDL) and hole accumulation layer (HAL),19,20 bulk resistance-control model,21 and gas-diffusion mechanism.22 However, the sensing mechanism of CGS is generally explained by gas–solid interactions such as ion oxygen adsorption and direct charge transfer, which is also well known as the receptor function of sensitive materials (as shown in Fig. 2). CGS mainly employ metal oxide semiconductors (MOS) and 2D layered nanomaterials including carbon nanomaterials (e.g., graphene and its derivatives), transition metal dichalcogenides (TMDs), MXene, black phosphorus (BP), etc., as the sensing materials. Although electron exchange occurs between gas molecules and sensitive materials in both cases, the sensing pathways are absolutely different. MOS predominantly follow the surface-oxygen adsorption model,23 wherein gas molecules directly react with these active oxygen species. However, 2D nanomaterials are more suitable for the charge transfer model with less dependence on surface oxygen species,24 which involves direct electron exchange between sensitive materials and gas molecules.However, the vast majority of CGS undergo electron exchange between their surfaces and gas molecules regardless of the gas species, which leads to the inherent weak selectivity. It is well known that selectivity indicates the ability to distinguish a specific gas from others. It could be quantitatively expressed by the ratio of the sensor signal toward the target gas (Sgas) to that toward the interfering one (Sinterferent), which was also termed as the selectivity coefficient. So the sensor selectivity could be improved by augmenting the target response and/or suppressing the interfering signal (Fig. 3). According to the sensing mechanism, the receptor function of CGS involves the ability of the material surface to interact with the target gas, which is determined by the adsorption and reaction processes. Therefore, the receptor function is closely related to the adsorption affinity, catalytic ability, and surface acidity/alkalinity of the sensing material as well as the reactivity of gas molecules. The enhancement of receptor function depends on the type and microstructure of the sensing material, which can be achieved through chemical methods such as doping, composite construction, and surface modification. In addition, the same target could be reached by reducing interference signals through filter membranes as well as utilizing gas sensor arrays and temperature/humidity regulation.
2.1 Element doping
As a common means of catalysis sensitization, element doping could optimize the sensing performance. According to the types and working principles of dopants, they can be divided into common metal doping (no noble metal), precious metal doping, and bimetallic doping. Common metal doping mainly improves the electron exchange rate by changing the band structure of the parent material, and focuses on chemical sensitization. Noble metal doping (Pd, Pt, Au, etc.) could lead to not only electron sensitization by changing the crystal structure, the content of oxygen vacancies and adsorption sites, and the reaction rate, but also chemical sensitization by dissociating the adsorbed gas molecules more easily. In addition, bimetallic doping was verified feasible to deliver better selectivity. Table 1 summarizes some related cases through element doping.25–36| Material type | Material | Dopant | Gas/Conc. (ppm) | Responsea | Selectivity coefficient | Ref. |
|---|---|---|---|---|---|---|
Conc.: concentration.a 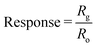 . . |
||||||
| Metal doping | SnO2 | Pd | o-Xylene/5 | 17 | HCHO, benzene, H2 (<7) | 25 |
| SnO2 | Pd–Au | Acetone/20 | 50 | CH3COCH3, C2H5OH, HCHO (<4) | 26 | |
| SnO2 | Pd | H2/1000 | 45.7 | — | 27 | |
| ZnO | Pt–Au | H2/1% | 1.58 | O2, NO2, CO, CO2, N2 (<1.04) | 170 | |
| ZnO–rGO | Ag | C2H2/100 | 21 | H2, O2, NO2, CO, CO2 (<3) | 29 | |
| NiO | Cr | o-Xylene/5 | 11 | Toluene, benzene, ethanol et al. (<3) | 30 | |
| Noble metal doping | rGO | Pd | H2/1000 | 1.07 | O2, NO2, CO, CO2, N2 (<1.01) | 31 |
| rGO | Ag | NH3/10 | 10 | NH3 (>15) | 32 | |
| rGO | Ag | NO2/50 | 1.74 | CH3OH, C2H5OH, C7H3, RH (<1.3) | 33 | |
| MoS2 | Au | Acetone/10 | 10 | Acetaldehyde, ethanol, toluene (<15) | 34 | |
| MoS2 | Pd | NH3/100 | 10 | NH3 (<4), NO2 (<1) | 35 | |
| WS2 | Ag | NO2/500 | 7.67 | — | 171 | |
| WS2 | Pt | NH3/250 | 10 | — | 172 | |
| BP | Pt | H2/500 | 500 | Acetone, ethanol, toluene (<15) | 36 | |
 | ||
| Fig. 4 (a) Cr-doped NiO hierarchical nanostructure based methyl benzene sensors. (b) The ratio of the response toward 5 ppm o-xylene to that toward 5 ppm ethanol (Sxylene/Sethanol) of NiO based sensors with temperature as well as the sensor response toward 5 ppm o-xylene (X), toluene (T), benzene (B), formaldehyde (F), ethanol (E), hydrogen (H) and carbon monoxide (C) at 400 °C of pure NiO and 1.15Cr-NiO sensors. Reprinted with permission from ref. 30 copyright 2013, The Royal Society of Chemistry. | ||
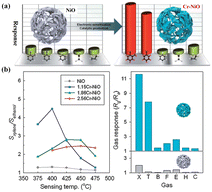
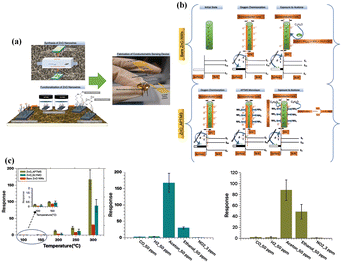
The catalyst could also dissociate the target gas molecules into gaseous intermediates with higher polarity and reactivity and thus favor a faster reaction. For instance, Y. J. Hong et al.25 demonstrated a continuous, single-step and large-scale preparation of Pd-supported SnO2 yolk–shell balls. These nanostructures exhibited high response to methyl groups (e.g., toluene) and a very low one to various interfering gases such as xylene and benzene, rendering them suitable for accurate indoor air-quality monitoring (Fig. 5). Within Pd-loaded SnO2 hollow microreactors, toluene was dissociated into smaller species with different chemical properties, resulting in more complex gas–solid reactions. As benzene was more stable than xylene and toluene, its difficult dissociation led to a low response. The weak reaction to ethanol, formaldehyde, and H2 could be explained by the complete oxidation of these gases to less reactive ones.
 | ||
| Fig. 5 Gas response of (a) dense SnO2 spheres, (b) SnO2 yolk–shell spheres and (c) Pd-loaded SnO2 yolk–shell spheres toward various gases with temperature (B: benzene, H: H2, E: C2H5OH, F: HCHO, X: o-xylene, T: toluene). Reprinted with permission from ref. 25 copyright 2014, The Royal Society of Chemistry. | ||
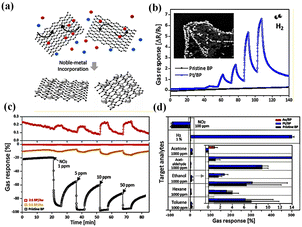 | ||
| Fig. 6 (a) Schematic illustration of Au- or Pt-modified few-layer BP, (b) gas response of pristine and modified BP sensors as function of H2 concentration, (c) tunable response behavior of Au/BP sensors by controlling the Au content, and (d) response of all sensors to various analytes. Reprinted with permission from ref. 36 copyright 2017, American Chemical Society. | ||
Different noble metals possess inconsistent oxidation activities towards a specific gas. Based on this, the selectivity to different target gases could be improved by doping different noble metals. C. R. Jung et al.41 prepared a CuO–CeO2 composite doped with precious metals (Pt, Pd, and Ru) and evaluated its selective oxidation of CO in hydrogen-rich gas streams. The results indicated that Pt doping shows the highest oxidation activity for CO while Pd or Ru doping for H2. This is because the doping behavior upshifts the binding energy of Cu within CuO–CeO2 and suppresses the degree of copper phase separation. In addition, the type and loading capacity of precious metals will determine the degree of movement and separation, which leads to varying oxidation activities towards different gases. By adopting a suitable precious metal, the selectivity could be improved by increasing the target response.
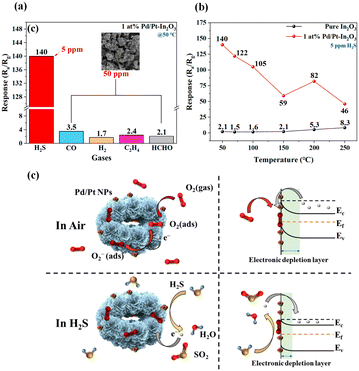 | ||
| Fig. 7 (a) Response of 1 at% Pd/Pt-In2O3 to various gases (CO, H2, C2H4, and HCHO). (b) Response of pure In2O3 and 1 at% Pd/Pt-In2O3 to 5 ppm H2S at different temperatures. (c) Demonstration of the gas-sensing mechanism of 1 at% Pd/Pt-In2O3, the left side is a simulation diagram of the microsphere, and the right side is an energy-band diagram. Reprinted with permission from ref. 42 copyright 2023, MOPI. | ||
Generally, element doping can introduce impurity levels within the band gap of the parent material. These impurity levels could act as recombination centres for electrons and holes. Also, doping semiconductors with a wide band gap may induce some undesirable properties or behaviors. This is detrimental to the sensor performance. In addition, the control of the doping ratio needs to be studied as more doping will cause severe agglomeration while less doping cannot achieve the desired effect.
2.2 Composite construction
To achieve a better selectivity, composite construction is another feasible method as it can suppress the problems of unwanted impurity levels and agglomeration encountered through element doping while achieving an improved performance compared with a single material. An interfacial depletion layer is always produced, accompanied with specific electron transfer, conformational changes, etc., which eventually promotes the selectivity. Thus, the resistance change during the sensing process is amplified for a stronger selectivity, as summarized in Table 2.46–62 In addition, the unique electron transfer, conformational changes, and target molecule-matched cavities probably enable a highly selective response for polymer-based composites, as concluded in Table 3.63–70| Material type | Materials | Gas/Conc. (ppm) | Response | Selectivity coefficient | Ref. |
|---|---|---|---|---|---|
| CED: cyclic electrochemical deposition; CVD: chemical vapor deposition. | |||||
| Metal oxide | ZnO–CuO | H2S /100 | 25 | NH3, EtOH, butanol, acetone (<2) | 46 |
| Cu2O–SnO2 | H2S /50 | 1.45 | NH3, NO, H2, toluene (<1.2) | 47 | |
| In2O3–SnO2–Al2O3 | NO2/450 | 632 | CO2 (<10) | 48 | |
| Fe2O3–In2O3 | NO2/5 | 5.4 | CO (<1.25) | 49 | |
| SnO2–NiO | Ethanol/1000 | 576 | Methanol, ammonia, acetone (<100) | 50 | |
| Al2O3–TiO2 | Ethanol/1000 | 1108 | H2S, CH3OH (>350) | 51 | |
| SnO2–TiO2 | Acetone/100 | 14 | HCHO, CH3OH,C2H5OH,NH3 (<7) | 52 | |
| ZnO–TiO2 | Acetone/100 | 23 | CO, C2H5OH (<7) | 53 | |
| SnO2–ZnO | Methanol/50 | 18 | Ammonia, benzene, acetone (<8) | 54 | |
| Low-dimensional materials | rGO–carbon nanodot | NO2/25 | 2.2 | DMMP, ethanol, methanol (<1.02) | 55 |
| g-C3N4–rGO | NO2/1 | 1.8 | NH3, SO2, toluene, hexane (<1.1) | 56 | |
| WS2–CuO | H2S /0.5 | 37 | SO2, NO2, NH3, CO, CO2 (<2) | 57 | |
| BPa–SnO2 | H2S /5 | 8.1 | CO2,HCHO, H2S, C3H8O (<3) | 58 | |
| BPa–ZnFe2O4 | Acetone /0.1 | 4.9 | SO2, NH3, NO, CO, CO2, H2S (<3) | 59 | |
| ZnFe2O4ZnSnO3 | Acetone /30 | 63.3 | Ammonia, benzene, toluene (<20) | 60 | |
| rGO/WS2 | NH3/10 | 2.78 | Acetone, ethanol, methanol (<1.2) | 61 | |
| MXene/SnO2 | NH3/50 | 1.4 | Formaldehyde, ethanol (<1.04) | 62 | |
| Polymer type | Materials | Gas/Conc. (ppm) | Response | Selectivity coefficient | Ref. |
|---|---|---|---|---|---|
| CPs | PANI/MWCNTs | NO2/100 | 1.28 | NH3 (<1.03) | 63 |
| PEDOT:PSS/cellulose nanofibers | NH3/1 | 1.05 | SO2, CO, H2S, acetone (<1.02) | 64 | |
| P3HT | NO2/1 | 50 | SO2, CO, H2S, NH3 (<3) | 65 | |
| n-Eicosane and carbon powder | Toluene/25 | 1.18 | Benzene, chloroform, hexane (<1.1) | 66 | |
| PMMA/graphene | NO2/10 | 1.06 | Hexane, ethanol, xylene (<1.02) | 67 | |
| Others | PDQT | NH3/10 | 30 | H2S, NO2, SO2 (<10) | 68 |
| DPPBu-BT | NH3/10 | 1.9 | Ethanol, ethylene (<1.009) | 69 | |
| PEI/carbon black | Nonanal/1 | 0.01 | Dodecane, ethanol, (<1.017) | 70 |
Heterojunctions could introduce greater interfacial adsorption especially for mixed-dimensional composites with difficult conformal combination. For instance, Y. Qin et al.72 prepared the core–shell stannous sulfide (SnS)/zeolitic imidazolate framework-8 (ZIF-8) heterostructure as a room-temperature methanol sensor. Tiny ZIF-8 nanocrystals were uniformly attached onto the surface of SnS nanosheets. During the synthesis process, S atoms on the SnS surface tightly attracted Zn ions, which not only induced the in situ growth of ZIF-8, but also promoted the formation of tight heterojunctions. Due to the difference in work function between SnS and ZIF-8, the hole accumulation layer (HAL) and electron accumulation layer (EAL) were generated at the interface, thus forming a heterogeneous junction. These heterojunctions amplified the conductivity variation after methanol exposure, and thus improved the selectivity. The selectivity factor for other interfering VOCs was k = Sgas/Smethanol. The k value of the interfering gases was much less than 1, indicating that the modification of ZIF-8 greatly improved the methane selectivity.
As a typical representative of heterojunctions composed of binary metal oxides, U. T. Nakate et al.73 fabricated a novel gas sensor based on the composites of n-type ZnO nanorods and p-type NiO nanoplates. In order to further enhance the sensor performance, Pd NPs were introduced. The morphology of the ternary composites is shown in Fig. 8a. With respect to NiO/ZnO counterparts, the Pd NP-decorated NiO/ZnO sensors exhibited higher response and selectivity toward H2 (Fig. 8b). The ternary sensor showed a gradient response with H2 concentration at 225 °C (Fig. 8d). The relevant H2-sensing mechanism is schematically illustrated in Fig. 8c. Upon close contact, an interfacial depletion region between NiO and ZnO was formed. When exposed to H2, the H2 molecules reacted with chemisorbed oxygen on the material surface and released electrons. These electrons consumed the holes which led to the increase of the sensor resistance. Meanwhile, with the increase of the potential barrier, the electrons migrated and the depletion region was extended, resulting in a further increase in resistance.
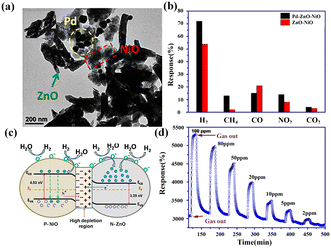 | ||
| Fig. 8 (a) TEM image of the Pd/ZnO/NiO composites, (b) the selectivity of ZnO/NiO and Pd/ZnO/NiO sensors separately at 225 and 237 °C, (c) schematic H2-sensing mechanism, and (d) dynamic resistance of the Pd/ZnO/NiO sensor as a function of H2 concentration at 225 °C. Reprinted with permission from ref. 73 copyright 2019, Elsevier. | ||
2D layered nanomaterials can also be functionalized by metal oxides such as SnO2, ZnO and TiO2 to construct heterojunctions. For example, the incorporation of TiO2 quantum dots could convert p-type WS2 nanosheets to an n-type one, simultaneously realizing a 17-fold larger response toward NH3 with high selectivity.74 It was reported that the rGO/ZnO sensor showed higher conductivity and better detection selectivity to H2 than the pure ZnO one.75 H. Yan et al.76 successfully synthesized the composites of SnO2 nanoparticles and MoS2 nanosheets by a two-step hydrothermal method. The SnO2@MoS2 sensor showed a high response to ethanol (Fig. 9c and d) and good selectivity (Fig. 9b). As shown in Fig. 9a, MoS2 nanosheets with a high surface area served as a platform to attach SnO2 nanoparticles which prevented their aggregation. This porous nanostructure provided numerous sorption sites and promoted the diffusion of ethanol molecules within the sensing layer. In addition, the activation energy was decreased.
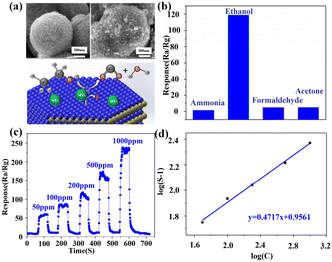 | ||
| Fig. 9 (a) SEM image of pure MoS2 and SnO2@MoS2 composites as well as the sensing mechanism for the composites, (b) selectivity, (c) dynamic response of the SnO2@MoS2 sensor as a function of ethanol concentration at 280 °C, and (d) dilogarithm fitting curve of the response–concentration relationship. Reprinted with permission from ref. 76 copyright 2015, The Royal Society of Chemistry. | ||
Also, the heterojunction of 2D/2D layered materials is a popular alternative. N. M. Tran et al.77 prepared a novel rGO/Ti3C2Tx heterostructure to detect NO2 gas (Fig. 10a). The morphological and structural analysis showed that MXene and rGO were hybridized well with sharp interfaces, accompanied with the increased specific surface area with regard to either single components (Fig. 10b and d). The obtained rGO/Ti3C2Tx sensor showed higher response to NO2 and better detection selectivity (Fig. 10c). The highly active surface terminal groups (–F, –OH, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of Ti3C2Tx MXene nanosheets tended to capture O2 molecules in synthetic air. Then partially adsorbed O2 molecules were dissociated into O atoms. Both O2 molecules and O atoms probably extracted electrons from MXene via these terminal groups, resulting in the formation of oxygen ions (O2− or O−).78,79 Due to the metallic nature of the Ti3C2Tx material, the electron loss on its surface was not significant during this process. These active surface sites also promoted the adsorption of NO2 molecules. Due to the higher electrophilicity, the physically-adsorbed NO2 molecules were likely to acquire electrons from vicinal sites supplied by the rGO component, resulting in a remarkable increase in the density of majority carriers (holes). Moreover, the close contact between rGO and MXene allowed more electrons to be transferred to NO2 molecules at a faster rate. Therefore, the heterojunctions increased the response and improved the selectivity.
O) of Ti3C2Tx MXene nanosheets tended to capture O2 molecules in synthetic air. Then partially adsorbed O2 molecules were dissociated into O atoms. Both O2 molecules and O atoms probably extracted electrons from MXene via these terminal groups, resulting in the formation of oxygen ions (O2− or O−).78,79 Due to the metallic nature of the Ti3C2Tx material, the electron loss on its surface was not significant during this process. These active surface sites also promoted the adsorption of NO2 molecules. Due to the higher electrophilicity, the physically-adsorbed NO2 molecules were likely to acquire electrons from vicinal sites supplied by the rGO component, resulting in a remarkable increase in the density of majority carriers (holes). Moreover, the close contact between rGO and MXene allowed more electrons to be transferred to NO2 molecules at a faster rate. Therefore, the heterojunctions increased the response and improved the selectivity.
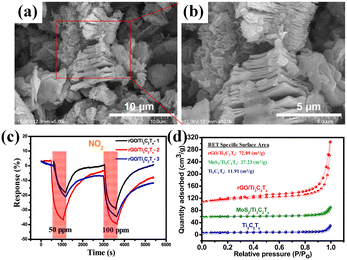 | ||
| Fig. 10 (a) Schematic illustration of the NO2-sensing mechanism of the rGO/Ti3C2Tx heterostructure. (b) The nitrogen adsorption and desorption isotherms of Ti3C2Tx MXene, rGO/Ti3C2Tx, and MoS2/Ti3C2Tx with BET surface areas. (c and d) rGO/Ti3C2Tx heterostructure. Reprinted with permission from ref. 77 copyright 2019, The Royal Society of Chemistry. | ||
In recent decades, conducting polymers such as polyaniline (PANI), poly(3,4-ethylenedioxythiophene):polystyrenesulfonate (PEDOT:PSS), and poly(3-hexylthiophene) (P3HT) have been extensively applied in this field.64 The most well-studied example is PANI and its composite with a secondary material such as MOS, carbon nanotubes (CNT), and graphene-based derivatives. During the synthesis process of PANI, hydrochloric acid (HCl) could protonate PANI with N+–H chemical bonds formed on the surface. The positive charge over nitrogen in PANI allowed a reversible interaction with NH3 together with the products of neutral PANI and positive ammonium ion (NH4+). Therefore, PANI is often combined with other sensing materials to improve the NH3 selectivity.80 Y. Guo et al.81 formed reduced graphene oxide (PPANI/rGO) by polymerizing aniline in a rGO solution. Next, the PANI nanoparticles (PPANI) and PANI nanofibers (FPANI) were successfully interlinked to produce the layered nanocomposite film (PPANI/rGO-FPANI) (Fig. 11c and d). The increased response of the composite to NH3 (Fig. 11a and b) was mainly attributed to the synergistic effect and the high specific surface area. After adding PANI, NH3 molecules could easily be adsorbed on PANI to provide electrons, thus increasing the resistance of PANI and further increasing the response (Fig. 11e). The lower electrical conductivity of PANI than that of rGO reduced the original resistance of the PPANI sensor. Moreover, PPANI strongly interacted with rGO through π–π interactions as an efficient sensing channel to enhance the charge transfer. Thus, the selectivity of the composite to NH3 gas was improved.
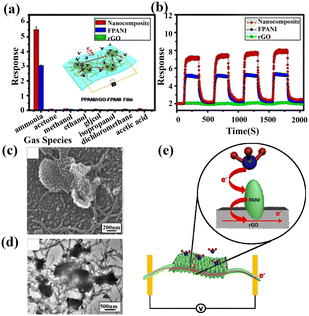 | ||
| Fig. 11 (a) Gas sensing selectivity (averaged results with error bar) of the rGO film, FPANI and PPANI/rGO-FPANI nanocomposite film devices to various volatile gases. (b) The repeatability of the rGO, FPANI, and PPANI/rGO-FPANI nanocomposite film sensors (6 h sample) to 10 ppm NH3. (c and d) SEM and TEM images of hierarchical PPANI/rGO-FPANI networks after polymerization. (e) Illustration of the NH3-sensing mechanism in the PPANI/rGO-FPANI network. Reprinted with permission from ref. 81 copyright 2016, The Royal Society of Chemistry. | ||
Molecular imprinting polymers (MIPs) are a class of cross-linked polymers that can bind target compounds with high specificity. Unlike ordinary polymers, the selectivity of MIPs depended not only on the solvation parameters of the gas molecules, but also on the shape and size of the template molecules. This allowed MIPs to distinguish target molecules from those with similar structures. Molecular imprinting techniques have been used to fabricate sensors with predetermined selectivity for gaseous molecules.67 For example, T. Alizadeh et al.66 successfully prepared a composite material of a nanoporous toluene-imprinted polymer, carbon powder, and n-eicosane. The MIP-based sensor exhibited a better toluene recognition than the non-imprinted polymer (NIP)-based counterpart (Fig. 12b). The interaction between the MIP and the analyte could be described by the following equation (eqn (1)):82
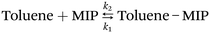 | (1) |
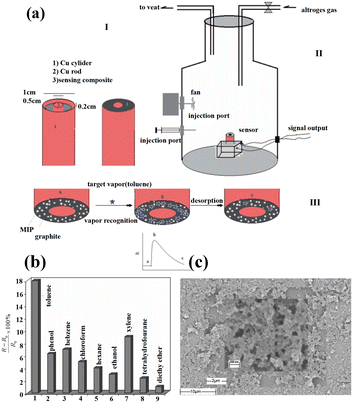 | ||
| Fig. 12 (a) Schematic representation of the sensor device (I), vapor sampling system (II) and the relationship between the electrical signal and recognition mechanism (III), (b) response for different vapors (25 ppm), and (c) SEM image of the MIP. Reprinted with permission from ref. 66 copyright 2013, Taylor & Francis. | ||
Herein, k1 and k2 represent the adsorption and desorption rate constants, respectively. According to eqn (1) and the diagram in Fig. 12a, when exposed to toluene vapor, the gas molecules diffused into the recognition cavity of the MIP. This caused the MIP between the adjacent conductive carbon black particles to swell, thereby distancing the conduction pathways and then increasing the sensor resistance. Since MIPs could be specifically designed, their selectivity could also be significantly enhanced compared to NIPs. Fig. 12c shows the SEM image of the as-prepared MIP. Obviously, the MIP was a kind of highly porous material full of nanoscale pores, which provided a high surface area for molecular adsorption. Thus, the response to the target gas was enhanced and then the selectivity was optimized.
Recent developments enabled a surface modification of polymer film via different strategies to ameliorate the selectivity and sensing performance of gas sensors.83 For instance, M. Castro et al.84 developed a series of poly(1-caprolactone)-grafted carbon nanotubes (PCL-g-CNT) via the spaying-layer-by-layer strategy. According to the Henry Clustering (HC) model of the PCL-g-CNT conductive polymer composite (CPC) sensor,85 at low concentrations (0 < f < 0.5) when only Henry diffusion occurred, solvent molecules could quickly enter the free volume of the amorphous phase at the scale of nanometer size, and finally changed the environment of CNT junctions through adsorption. For higher solvent concentrations (f > 0.5), the clustering of analytes led to a larger electrical signal due to swelling and important macromolecular conformational changes.86 In this diffusion mode, the selectivity of CPC was not only driven by the matrix diffusion (analyte/polymer interaction) but also by the direct analyte adsorption on CNT (analyte/CNT interactions) and volume expansion. This leads to additional clustering reactions. Fitting parameters for chloroform using the HC model as depicted in eqn (2) are summarized in this study.84 Among them, the fitting parameters for chloroform gas are: f′ = 0.001, n′ = 3.75, kH = 1.8, B = 4.26. Compared to other cases, the HC model for chloroform required additional clustering factor B = 4.25 (for others gas, B = 1), so AR for chloroform was 4 times larger than that for other gases, thus improving the selectivity.
| AR = kH × f + (f − f′) × (B × f)n′ | (2) |
Combining different materials to form heterojunctions is simple and widely studied, however, the selectivity is only improved to a limited extent. Due to the lack of accurate mechanisms to interpret the selectivity in polymer modification, the relevant discussion is usually treated with phenomenology. In addition, some limitations still exist such as high cost, high temperature sensitivity, low aging resistance, and poor storage stability.87
2.3 Specific target recognition
With respect to the previous two methods to improve the selectivity, specific target recognition does not alter the basic properties of sensitive materials such as energy levels, and is also relatively controllable. Moreover, this strategy introduces specific functional groups for target gases for selectivity optimization. Since some gases exhibit unique reactions with specific functional groups, we can incorporate these groups to match the key-to-lock interaction, and improve the chemical affinity between the material surface and the target molecules. In this regard, organometallic receptors and self-assembled monolayers (SAM) were usually employed. Table 4 summarizes some representative cases.88–95| Method | Sensor | Gas/Conc. (ppm) | Response | Selectivity coefficient | Ref. |
|---|---|---|---|---|---|
| NDMA: N-nitrosodimethylamine. | |||||
| Organometallic receptors | Carbon nanotube/polythiophene | DMMP/1% | 1.15 | Hexane, toluene (<1.03) | 88 |
| Functionalized single walled carbon nanotubes | CO/200 | 1.01 | CO2,O2 (<1.005) | 89 | |
| Carbon nanotube-based devices | Ethylene/50 | 1.8 | Benzene, acetone, methanol (<1.05) | 90 | |
| Chemiresistive carbon nanotube sensors | NDMA/200 | 17 | Hexane, water, acetone (<3) | 91 | |
| Self-assembled monolayer | Organosilane SAMs at the surface of organic | Acetone/1% | 2 | — | 92 |
| SAM-modified semiconductor nanowires | NO2/0.4 | 2100 | SO2, NO, CO2, NH3, CO (<20) | 93 | |
| SAM of alkanedithiol | NO2/100 | 16.02 | CO, CH3OH, H2, NH3 (<7) | 94 | |
| Densely packed SAM flexible sensor | Nitrobenzene/249 | 80 | Methanol, n-hexane, toluene (<40) | 95 | |
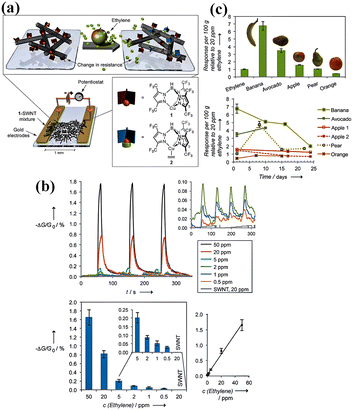 | ||
| Fig. 13 (a) Ethylene detection by a chemiresistive sensor: the sensing mechanism of a mixture of SWNTs and copper complex 1, (b) sensor performance in terms of (1) response of SWNT devices to 0.5, 1, 2, 5, 20, and 50 ppm ethylene and of pristine SWNTs to 20 ppm ethylene, (2) average response and (3) the plot of average response vs. ethylene concentration. (c) Top: response of the 1-SWNT device to 100 g of different fruits and 20 ppm ethylene; bottom: response to fruit over several weeks. Reprinted with permission from ref. 90 copyright 2012, The Royal Society of Chemistry. | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in acetone molecules that acted as electrophilic centres, thus resulting in the formation of imine ((H3C)2–C
O) in acetone molecules that acted as electrophilic centres, thus resulting in the formation of imine ((H3C)2–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and water molecule (Fig. 15b). Therefore, the gas–solid interactions enhanced the sensor response.
N) and water molecule (Fig. 15b). Therefore, the gas–solid interactions enhanced the sensor response.
 | ||
| Fig. 14 (a) Synthesis of ZnO nanowires using the vapor–liquid–solid mechanism and the surface functionalization of ZnO nanowires with APTMS and GLYMO self-assembled monolayers, and conductometric device, (b) the gas sensing mechanism of bare and SAM-functionalized ZnO NWs for acetone detection, (c) response versus temperature plot, and (d) the response of APTMS and GLYMO functionalized-ZnO nanowires toward 50 ppm acetone and other interfering gases at 300 °C. Reprinted with permission from ref. 99 copyright 2020, The Royal Society of Chemistry. | ||
 | ||
| Fig. 15 (a) Schematic illustration of the sensor fabrication, (b) structural characterization of the Co3O4/Pd-SnO2 composites, (c) the sensing performance and sensing mechanism of pristine and Co3O4-modified Pd-SnO2 sensors, and (d) cross-sectional SEM images and gas-sensing characteristics of both sensors as a function of temperature. Reprinted with permission from ref. 132 copyright 2020, The Royal Society of Chemistry. | ||
S. T. Marshall et al.100 modified conventional Pd/Al2O3 catalysts with an n-alkanethiol SAM coating. The catalysts showed a selectivity to epoxybutane at 313 K of 11%, while the coated counterparts greatly improved the selectivity to up to 94%. Since the effect of surface sulfur simultaneously changes the geometric and electronic structures of platinum group metals, the varied crystallinity of the underlying surface reduces the activity of reactions that require multiple metal atoms and tightly-bound intermediates such as epoxide hydrogen. This is conducive to the bonding reaction with epoxybutane and thus the selectivity. In addition, the n-alkanethiol SAM layer consisted of a sulfur atom “head” and a hydrocarbon “tail”. And the precise control of the surface structure could be utilized to produce a clear near-surface environment, which boosted the adsorption energy of the epoxy.
At present, the method of specific target recognition still has the following problems. Firstly, specific functional groups can only distinguish between different classes of gases rather than the same class. For example, coporphyrin receptors possess a good affinity for amines and can be used to distinguish amines from other volatile organic compounds, but not various amines.101 Secondly, not all gases can find unique functional groups to match with suitable sensitive materials.
2.4 Filters
Compared with specific target recognition, filters utilize other properties of the target analyte such as molecular size and surface affinity, which to some extent solves the problem of difficult matching between the target gas and functional groups.Filters are one of the best ways to improve the selectivity of gas sensors, which was first demonstrated in the 1990s.102 The filters were placed in front of the target sensor to control the composition of the gas mixture. There are two types of filter materials, one was prepared into a porous film that physically adsorbed gas molecules103,104 and the other into a catalytic film that decompose gases. For example, CeO2 could be utilized to separate oxygen at high temperatures,105 while Pt and Pd films could permeate hydrogen. There are four basic configurations including a packed bed, a covering layer, a membrane and a separation column.12 And Table 5 summarizes the work about filter-assisted gas sensors.106–115
| Filter Configuration | Filter material | Sensor | Gas/Conc. (ppm) | Response | Selectivity coefficient | Ref. |
|---|---|---|---|---|---|---|
| Packed bed | Carbon cloth | SnO2 | CO/15 | 1.25 | Ethanol, ethyl, acetate, heptane (∼0) | 115 |
| Charcoal | Pd/In2O3 | CH4/500 | 11 | Ethanol, methanol, acetone (<5) | 107 | |
| Zeolite 4A | Pd/SiO2 | CH4/1000 | 60 | CO, CO2,VOCs (<15) | 108 | |
| GC column | Porapak Q | SiO2 | Benzene/5 | 5 | SO2, CO (<2) | 109 |
| Not specified | ZnO | Acetone/50 | 1.05 | Acetone (<1.008) | 110 | |
| Separation column | Activated alumina | Pt/SnO2 | Isoprene/0.5 | 7 | Acetone, methanol, NH3 (<5) | 111 |
| Tenax TA | Pd/SnO2 | Methanol/5 | 20 | Acetone, ethanol, H2 (<10) | 112 | |
| Tenax TA | Pd/SnO2 | Formaldehyde/1 | 10 | Acetaldehyde, acetone, CH4 (<5) | 113 | |
| Others | Indigo | Organic semiconductor | NO2/0.1 | 4 | Ozone (>0.5) | 114 |
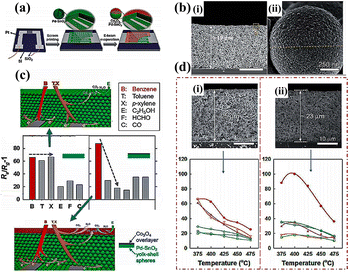
Adsorption filters harnessed the differences in polarity, hydrophilicity, boiling point, molecular weight, or size among multiple gas species to separate them. That is to say, the interference gases were adsorbed onto the filter while only allowing the target gas to pass through so as to enhance the selectivity of the downstream sensor. Recently, carbon-based filters,116 mesoporous (2–50 nm) adsorbents such as silica gel,117 porous polymers,118 and activated alumina, as well as micropores (<2 nm) including membranes,119 zeolites,120,121 perovskites,122,123 alumina,124 and copper oxide125 had been used for gas filtration. Recently, metal organic framework (MOF) materials, due to the high specific surface area, diverse structure and controllable porosity, have been developed to improve the selectivity of gas sensors.126,127 For instance, T. Zhou et al.128 synthesized two kinds of ZnO@zeolitic imidazolate framework (ZIF) core–shell structures with different pore sizes (∼3.4 Å for ZIF-8, ∼4.8 Å for ZIF-71) to detect H2, NH3, ethanol, acetone, benzene and other gases. The ZnO@ZIF-8 structure showed a much higher selectivity for H2 than that for ethanol and acetone, while the ZnO@ZIF-71 one exhibited an enhanced response to ethanol and acetone. The kinetic diameters of NH3 (2.90 Å) and H2 (2.89 Å) were respectively smaller than the pore sizes of ZIF-8 and ZIF-71, and could pass through the membranes easily. However, benzene molecules possessed a larger diameter (5.85 Å) than the pore size, thus leading to a negligible reaction. It could be concluded that the aperture of ZIFs determined the selectivity of ZnO@ZIF gas sensors. In addition, the BET data clearly unveiled that both ZnO@ZIF-8 and ZnO@ZIF-71 samples possessed a higher specific surface area and gas adsorption capacity than pure ZnO, which was also favorable for the improvement of selectivity.
As the gas-sensing measurement continued, the adsorption filter would be saturated with a deteriorated function. To overcome this issue, a catalytic filter was an effective alternative to enable a continuous measurement.129 Catalytic filters could eliminate the effect of interfering gases by harnessing their differences in chemical reactivity. In this case, the interference gas was completely converted into an inert species to improve the sensor selectivity. Common catalytic filters included ceramic carriers (alumina, silica and ferric oxide) on precious metals (such as Pt, Pd and Au),30 metal oxides,130 mixed metal catalysts,131etc. Their surface composition, structure and operating temperature jointly determined the overall reactivity and then the selectivity. For example, S.-Y. Jeong et al.132 prepared a Pd-loaded micro-reactor sensing layer with a catalytic Co3O4 overlayer to improve the selectivity for benzene (Fig. 15a). Fig. 15b shows the SEM image of the cross section and the material surface. It could be found that the surface of Pd-loaded SnO2 was covered by Co3O4. Then the performance of pristine and Co3O4-coated Pd-SnO2 sensors was measured (Fig. 15d). Obviously, the Co3O4 coating significantly improved the selectivity because the interference gases including ethanol and formaldehyde were oxidized by the filter into inactive or less active species (CO2 and H2O). Benzene was efficiently transferred to areas close to the sensor electrode and converted into smaller and more active species, thus enhancing the sensing response.
In fact, these filters also exhibit adsorption saturation. In addition, most work focused on the modification considerations of individual sensors, which resulted in a relatively high regulation difficulty.
2.5 Electronic nose systems
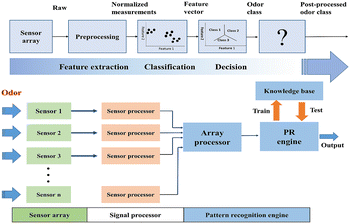
Due to the limitations of single sensor regulation when improving the selectivity, electronic nose systems are currently widely used to perfect this situation. The electronic nose is a kind of olfactory bionic technology to simulate the working principle of animal nose. In general, the signal fingerprint of the gas sensor arrays within the electronic nose system was harnessed to classify different odors. For each gaseous sample, a set of signals were generated, which were then analyzed via pattern recognition strategies. An intact electronic nose system mainly included three parts: sensor array, information pre-processing and pattern recognition (Fig. 16). As the first successful attempt,133 in 1982 three- and four-sensor arrays were used to simulate human olfaction.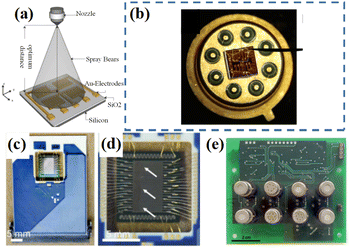 | ||
| Fig. 17 (a) A schematic for the sensor array layout as well as the spray setup. Reprinted with permission from ref. 134 copyright 2015, Elsevier. (b) Simulated thermal plot of the designed 2 × 2 microheater array. Reprinted with permission from ref. 135 copyright 2006, American Chemical Society. (c and d) rGO-based multi-sensor array. Reprinted with permission from ref. 136 copyright 2013, The Royal Society of Chemistry. (e) Real image of a sensor array with different selective sensing layers. Reprinted with permission from ref. 137 copyright 2017, Elsevier. | ||
For example, A. Star et al.135 modified SWNTs by site-selective electroplating of several catalytic metals (Pd, Pt, Rh, and Au) to prepare multiple sensors with different responses to analyte gases (H2, CH4, CO, and H2S). An integrated sensor array based on rGO was also used to improve the selectivity,136 with each sensor in the array possessing a unique response due to the irregular structure of the rGO film at the scale of nanosize. The resulting sensor system could reliably identify gases of nearly identical chemical properties. Also, the technology was equally applied for MOS gas sensors.138 The diversity of MOS and their ability to measure at different temperatures were critical for predicting the concentration of individual gases in a mixture, overcoming the selectivity limitation on individual MOS sensors.
With the increasing requirements of algorithm accuracy, it was necessary to carry out the compensation treatment on the effect of temperature, baseline drift, humidity and so on for the data set. So many methods were used to pre-process the test data. For example, the machine learning algorithm (ML) was more suitable to eliminate the influence of the response fluctuation. Advanced methods for drift component correction (CC) contained PCA and partial least squares algorithm (PLS) from the first principal component of the data set.147 This correction allowed the removal of highly correlated changes from one example to another. A generalization of this method was called component correction for common principal component analysis (CC-CPCA).148 The drift of a sensor response could also be compensated for by periodic functions such as Fourier and wavelet transform.148 Furthermore, V. V. Krivetskiy et al.149 employed statistical signal shape analysis (SSA) to implement the response preprocessing of temperature-modulated MOS gas sensors, which enabled a lower effect of the response fluctuation and baseline drift and then an improved recognition ability of PCA, DWT, PCF and ML algorithms for closely related gases in realistic atmospheric conditions.
2.6 Others strategies
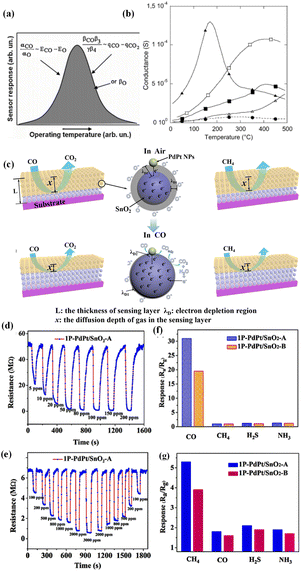 | ||
| Fig. 18 (a) Temperature-dependent response of MOS sensors toward CO, (b) temperature-dependent response of the SnO2 sensor toward C2H5OH (50 ppm), H2 (500 ppm), CO (300 ppm), and CH4 (1000 ppm). Reprinted with permission from ref. 151 copyright 2005, Elsevier. (c) Schematic illustration of the CO-sensing mechanism of PdPt/SnO2 at different working temperatures. The response of the obtained samples toward (d) 50 ppm CO and (e) 500 ppm CH4 at different working temperatures, the response of the PdPt/SnO2 sensors on successive exposure to 1000 ppm CH4 and 50 ppm of other hazardous gases in the coal mine at (f) 100 °C and (g) 320 °C, respectively. Reprinted with permission from ref. 156 copyright 2019, American Chemical Society. | ||
There were numerous reports utilizing this strategy to promote the selectivity. G. Li et al.156 realized the dual selectivity of CO and CH4 detection by using PdPt NPs with significant differences in the activation capacity of CO and CH4 at different temperatures of 100 and 320 °C. As shown in Fig. 18c–e, CO possessed a higher oxidation barrier at lower temperature to be activated by PdPt NPs, and then reacted with oxygen adsorbed on the MOS surface. However, the catalytic combustion of CH4 usually required high temperature (over 350 °C) due to its non-polarity and strong stability (Fig. 18f and j). F. Xu et al.157 investigated the response of ZnO toward four volatile organic compounds (ethanol, methanol, acetone and formaldehyde) at different temperatures from 20 to 400 °C, and analyzed the selectivity parameters. It was found that the stronger the broken bond, the higher the optimal temperature. In order to maximize the response, gas species with a larger molecular structure required higher temperature. Therefore, low temperature benefited the selectivity for small molecules with fewer and weaker bonds.
However, the approach discussed in the present section could not completely resolve the problem of low selectivity for MOS gas sensors because the temperature profiles of the sensing signals were too broad and there were too many gas species with similar temperature dependences.
In fact, humidity-activated sensing is a complex process that includes acid–base adsorption, ion-conduction formation, and reactions among various functional groups. In addition, the humidity activation mechanism can only be applied to specific conditions and sensitive materials, which needs more in-depth investigation.
However, this technique was very limited. For example, in order to distort the potential distribution, the applied bias voltage must be high enough,163 which was difficult to achieve. In addition, high bias voltage would induce the heating of the sensing film, causing the transient response to be remarkably different from the ideal situation.164
3. Summary and outlook
At present, how to improve the selectivity of gas sensors is a key challenge. In this review, the methods to achieve this goal are summarized. Firstly, we discussed two popular sensing mechanisms: the oxygen adsorption model and the charge transfer model. Subsequently, the methods to improve the selectivity were categorized including catalysis sensitization, composite construction, specific target recognition, filters, electronic nose systems, temperature/bias voltage control, and humidity modulation. For each method, examples were given to illustrate its concept and reveal its mechanism. Although each strategy showcases some success, the existing limitations could not be ignored.(I) For the single-element doping mentioned earlier, there may be drawbacks such as introducing unwanted impurity levels, agglomeration, and uncontrollability. In addition to bimetallic doping to overcome the limitations, researchers can further employ theoretical calculation to more accurately control the amount of doping.
(II) To address the issue of limited selectivity for heterojunctions, an emerging approach is to use hollow heterojunctions that combine hollow structures of two materials. The unique porous structure not only provides a large specific surface area and rich activity, but also enables short-distance charge carrier transport. For the drawbacks of polymer modifications, further research on their mechanisms is needed. For example, G. Becskereki et al.165 had thoroughly analyzed the selectivity mechanism of imprinted polymers in batch adsorption, binding analysis, chromatography, solid phase extraction, sensors, membranes, and catalysts. In addition, the polymer modification also demonstrates some limitations such as high cost and poor storage stability.87 Researchers can try various methods to eliminate these shortcomings such as adding hydrophobic clays, developing new active polymers, etc.
(III) As stressed above, organometallic receptors can only distinguish between different classes of gases rather than the same class. For example, ethylene receptors show high selectivity to ethylene under the interference of toluene, ethyl acetate, ethanol, chloroform and n-hexane gases, but similar reactions to acetonitrile, tetrahydrofuran and acetaldehyde. If the sensor is disturbed by these vapors of high concentration, a false positive reaction would occur.166 To address this limitation, organic ligands could be incorporated into composites. For example, various metals (Co, Ni, Cu, Zn, Cr, and Mn) were incorporated with N-nitrosodimethylamine (NDMA).91
(IV) To improve the selectivity via SAMs, electron transfer must be realized between the target gas and the sensing layer through SAMs so as to obtain a detectable response. Therefore, apart from determining the optimal SAMs, we also need to compare the Fermi level of the core sensing material with the energy level of the SAM–target complex.
(V) Currently, the most used filters are passive membranes with different diffusion parameters, which depended on the adsorption affinity of gas molecules on the screen and the pore–molecule size relationship. If there is no expected gas reaction or desorption, such adsorbent filters may become saturated when exposed to the interfering gas of large concentration. The selective catalytic reaction in the filter membrane is expected to overcome these limitations by catalyzing the conversion of interfering substances into harmless molecules.
(VI) The electronic nose system is not only more accurate and objective than the human nose in some cases, but also can be used in many harsh environments. It also expresses many limitations. First, the electronic nose tends to lose sensitivity in the presence of water vapor, which complicates the signal at high humidity.167 We can solve the problem by drying the target gas or using the algorithm to correct the humidity. Second, reproducibility is another common and important issue associated with electronic noses.168 To compensate for the temperature effect, some electronic noses employ an automatic sampler system to control the temperature. In addition, absolute calibration is impossible for a single target gas with high concentration. Besides, it is unable to obtain quantitative data on differences in gas types (fragrances, spoilage levels). All these problems need to be improved in the future.
To control the working temperature of CGS, light irradiation and doping with conducting nanofillers can be leveraged. In particular, introducing impurity energy levels to reduce the band gap width, and reducing interface density of states and grain boundaries will improve the material conductivity and lower the working temperature.169 In addition, the existing strategies for selectivity optimization mostly focus on the single-dimensional information of the unit sensor (i.e., the sensitivity toward target gas), which challenged the modulation flexibility. In the future, more parameters can be collectively considered, such as response polarity (i.e., resistance increase or decrease upon exposure to target gas), response speed, recovery degree, and temperature/humidity effects, so as to evaluate the selectivity from multiple-dimensional perspectives, which enables more accurate and stable data.
We reviewed a number of methods to greatly improve the performance parameters of gas sensors in this work. By expanding the application fields, the future CGS can certainly play a key role in the development of some burgeoning scenarios (e.g., Internet of Things) with unprecedented selectivity.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by the Fundamental and Frontier Research Project of Chongqing (Grant Nos. CSTB2023NSCQ-MSX0231 and cstc2019jcyj-msxmX0037) and National Natural Science Foundation of China (Grant No. 61704014).Notes and references
- A. Kumar, H. Kim and G. P. Hancke, IEEE Sens. J., 2012, 13, 1329–1339 Search PubMed.
- C. Di Natale, R. Paolesse, E. Martinelli and R. Capuano, Anal. Chim. Acta, 2014, 824, 1–17 CrossRef PubMed.
- A. Ponzoni, E. Comini, I. Concina, M. Ferroni, M. Falasconi, E. Gobbi, V. Sberveglieri and G. Sberveglieri, Sensors, 2012, 12, 17023–17045 CrossRef.
- T. Xu, Y. Wang, Z. Xiong, Y. Wang, Y. Zhou and X. Li, Nano-Micro Lett., 2023, 15, 6 CrossRef PubMed.
- Y. Wang and Y. Wang, SmartMat, 2023, 4, e1130 CrossRef.
- J. Li, Y. Wang, H. Song, Y. Guo, S. Hu, H. Zheng, S. Zhang, X. Li, Q. Gao and C. Li, Adv. Compos. Hybrid Mater., 2023, 6, 83 CrossRef.
- J. Li, Y. Wang, X. Li, Q. Gao and S. Zhang, J. Alloys Compd., 2021, 881, 160551 CrossRef.
- Y.-X. Zhang and Y.-H. Wang, RSC Adv., 2017, 7, 45129–45144 RSC.
- G. Korotcenkov and B. Cho, Sens. Actuators, B, 2013, 188, 709–728 CrossRef.
- J. Walker, P. Karnati, S. A. Akbar and P. A. Morris, Sens. Actuators, B, 2022, 355, 131242 CrossRef.
- J. M. Smulko, M. Trawka, C. G. Granqvist, R. Ionescu, F. Annanouch, E. Llobet and L. B. Kish, Sens. Rev., 2015, 35, 340–347 CrossRef.
- J. van den Broek, I. C. Weber, A. T. Güntner and S. E. Pratsinis, Mater. Horiz., 2021, 8, 661–684 RSC.
- H. Hashtroudi, I. D. Mackinnon and M. Shafiei, J. Mater. Chem. C, 2020, 8, 13108–13126 RSC.
- M. Wusiman and F. Taghipour, Crit. Rev. Solid State Mater. Sci., 2022, 47, 416–435 CrossRef.
- N. Joshi, T. Hayasaka, Y. Liu, H. Liu, O. N. Oliveira and L. Lin, Microchim. Acta, 2018, 185, 1–16 CrossRef PubMed.
- X. Liu, T. Ma, N. Pinna and J. Zhang, Adv. Funct. Mater., 2017, 27, 1702168 CrossRef.
- J. Zhang, X. Liu, G. Neri and N. Pinna, Adv. Mater., 2016, 28, 795–831 CrossRef.
- P. Barik and M. Pradhan, Analyst, 2022, 147, 1024–1054 RSC.
- P. T. Moseley, Meas. Sci. Technol., 2017, 28, 082001 CrossRef.
- H. Ji, W. Zeng and Y. Li, Nanoscale, 2019, 11, 22664–22684 RSC.
- M. Wang, T. Hou, Z. Shen, X. Zhao and H. Ji, Sens. Actuators, B, 2019, 292, 171–179 CrossRef.
- W. He, W. Lv and J. H. Dickerson, Gas Transport in Solid Oxide Fuel Cells, 2014, pp. 9–17 Search PubMed.
- P. Shankar and J. B. B. Rayappan, Sci. Lett. J., 2015, 4, 126 Search PubMed.
- Y. Li, Z. Li, C. Chi, H. Shan, L. Zheng and Z. Fang, Adv. Sci., 2017, 4, 1600430 CrossRef PubMed.
- Y. J. Hong, J. W. Yoon, J. H. Lee and Y. C. Kang, Chem. – Eur. J., 2014, 20, 2737–2741 CrossRef.
- A. Katoch, S.-W. Choi, H. W. Kim and S. S. Kim, J. Hazard. Mater., 2015, 286, 229–235 CrossRef PubMed.
- J. Zhao, W. Wang, Y. Liu, J. Ma, X. Li, Y. Du and G. Lu, Sens. Actuators, B, 2011, 160, 604–608 CrossRef.
- S.-J. Ha, J.-H. Kang, D. H. Choi, S. K. Nam, E. Reichmanis and J. H. Moon, ACS Photonics, 2018, 5, 3621–3627 CrossRef.
- A. I. Uddin, D.-T. Phan and G.-S. Chung, Sens. Actuators, B, 2015, 207, 362–369 CrossRef.
- H.-J. Kim, J.-W. Yoon, K.-I. Choi, H. W. Jang, A. Umar and J.-H. Lee, Nanoscale, 2013, 5, 7066–7073 RSC.
- D.-T. Phan and G.-S. Chung, Int. J. Hydrogen Energy, 2014, 39, 620–629 CrossRef.
- S. Cui, S. Mao, Z. Wen, J. Chang, Y. Zhang and J. Chen, Analyst, 2013, 138, 2877–2882 RSC.
- L. Huang, Z. Wang, J. Zhang, J. Pu, Y. Lin, S. Xu, L. Shen, Q. Chen and W. Shi, ACS Appl. Mater. Interfaces, 2014, 6, 7426–7433 CrossRef.
- S.-Y. Cho, H.-J. Koh, H.-W. Yoo, J.-S. Kim and H.-T. Jung, ACS Sens., 2017, 2, 183–189 CrossRef.
- B. Cho, J. Yoon, S. K. Lim, A. R. Kim, S.-Y. Choi, D.-H. Kim, K. H. Lee, B. H. Lee, H. C. Ko and M. G. Hahm, Sensors, 2015, 15, 24903–24913 CrossRef.
- S.-Y. Cho, H.-J. Koh, H.-W. Yoo and H.-T. Jung, Chem. Mater., 2017, 29, 7197–7205 CrossRef.
- T. Lan, Y. Zhao, J. Deng, J. Zhang, L. Shi and D. Zhang, Catal. Sci. Technol., 2020, 10, 5792–5810 RSC.
- H. Yan, P. Song, S. Zhang, J. Zhang, Z. Yang and Q. Wang, Ceram. Int., 2016, 42, 9327–9331 CrossRef.
- D. Sarkar, X. Xie, J. Kang, H. Zhang, W. Liu, J. Navarrete, M. Moskovits and K. Banerjee, Nano Lett., 2015, 15, 2852–2862 CrossRef.
- C. Kuru, C. Choi, A. Kargar, D. Choi, Y. J. Kim, C. H. Liu, S. Yavuz and S. Jin, Adv. Sci., 2015, 2, 1500004 CrossRef.
- C. R. Jung, A. Kundu, S. W. Nam and H.-I. Lee, Appl. Catal., A, 2007, 331, 112–120 CrossRef.
- K. Jiang, T. Chen, J. Sun, H. Quan and T. Zhou, Nanomaterials, 2023, 13, 668 CrossRef.
- J. Huang, J. Li, Z. Zhang, J. Li, X. Cao, J. Tang, X. Li, Y. Geng, J. Wang and Y. Du, Sens. Actuators, B, 2022, 373, 132664 CrossRef.
- G. Li, Z. Cheng, Q. Xiang, L. Yan, X. Wang and J. Xu, Sens. Actuators, B, 2019, 283, 590–601 CrossRef.
- C. Cullis, T. Nevell and D. Trimm, J. Chem. Soc., Faraday Trans. 1, 1972, 68, 1406–1412 RSC.
- L. Wang, Y. Kang, Y. Wang, B. Zhu, S. Zhang, W. Huang and S. Wang, Mater. Sci. Eng., C, 2012, 32, 2079–2085 CrossRef PubMed.
- G. Cui, M. Zhang and G. Zou, Sci. Rep., 2013, 3, 1–8 Search PubMed.
- A. Chen, X. Huang, Z. Tong, S. Bai, R. Luo and C. C. Liu, Sens. Actuators, B, 2006, 115, 316–321 CrossRef.
- M. Ivanovskaya, D. Kotsikau, G. Faglia, P. Nelli and S. Irkaev, Sens. Actuators, B, 2003, 93, 422–430 CrossRef.
- G. Sun, H. Chen, Y. Li, Z. Chen, S. Zhang, G. Ma, T. Jia, J. Cao, H. Bala and X. Wang, Sens. Actuators, B, 2016, 233, 180–192 CrossRef.
- M. Arafat, A. Haseeb, S. Akbar and M. Z. Quadir, Sens. Actuators, B, 2017, 238, 972–984 CrossRef.
- F. Li, X. Gao, R. Wang, T. Zhang and G. Lu, Sens. Actuators, B, 2017, 248, 812–819 CrossRef.
- D. Barreca, E. Comini, A. P. Ferrucci, A. Gasparotto, C. Maccato, C. Maragno, G. Sberveglieri and E. Tondello, Chem. Mater., 2007, 19, 5642–5649 CrossRef.
- W. Tang, J. Wang, P. Yao and X. Li, Sens. Actuators, B, 2014, 192, 543–549 CrossRef.
- J. Hu, C. Zou, Y. Su, M. Li, N. Hu, H. Ni, Z. Yang and Y. Zhang, J. Mater. Chem. C, 2017, 5, 6862–6871 RSC.
- A. Chen, R. Liu, X. Peng, Q. Chen and J. Wu, ACS Appl. Mater. Interfaces, 2017, 9, 37191–37200 CrossRef.
- J. Li, H. Zhao, Y. Wang, R. Zhang, C. Zou and Y. Zhou, Anal. Chem., 2022, 94, 16160–16170 CrossRef PubMed.
- Y. Zhou, Z. Hu, H. Zhao, Y. Wang, J. Li and C. Zou, Anal. Chim. Acta, 2023, 340825 CrossRef.
- H. Zhao, J. Li, Y. Wang, R. Zhang, C. Zou and Y. Zhou, IEEE Sens. J., 2022, 23, 1908–1916 Search PubMed.
- W. Guo, L. Huang, B. Zhao, X. Gao, Z. Fan, X. Liu, Y. He and J. Zhang, Sens. Actuators, B, 2021, 346, 130524 CrossRef.
- X. Wang, D. Gu, X. Li, S. Lin, S. Zhao, M. N. Rumyantseva and A. M. Gaskov, Sens. Actuators, B, 2019, 282, 290–299 CrossRef.
- T. He, W. Liu, T. Lv, M. Ma, Z. Liu, A. Vasiliev and X. Li, Sens. Actuators, B, 2021, 329, 129275 CrossRef.
- R. Mangu, S. Rajaputra and V. P. Singh, Nanotechnology, 2011, 22, 215502 CrossRef.
- R. Zhang, Y. Wang, J. Li, H. Zhao, Y. Wang and Y. Zhou, Microchim. Acta, 2022, 189, 308 CrossRef PubMed.
- S. Hou, J. Yu, X. Zhuang, D. Li, Y. Liu, Z. Gao, T. Sun, F. Wang and X. Yu, ACS Appl. Mater. Interfaces, 2019, 11, 44521–44527 CrossRef.
- T. Alizadeh and F. Rezaloo, Int. J. Environ. Anal. Chem., 2013, 93, 919–934 CrossRef.
- T. Alizadeh and L. Hamedsoltani, J. Environ. Chem. Eng., 2014, 2, 1514–1526 CrossRef.
- R. Song, X. Zhou, Z. Wang, L. Zhu, J. Lu, D. Xue, Z. Wang, L. Huang and L. Chi, Org. Electron., 2021, 91, 106083 CrossRef.
- D. Khim, G. S. Ryu, W. T. Park, H. Kim, M. Lee and Y. Y. Noh, Adv. Mater., 2016, 28, 2752–2759 CrossRef.
- A. Daneshkhah, S. Vij, A. P. Siegel and M. Agarwal, Chem. Eng. J., 2020, 383, 123104 CrossRef.
- W. Tang and J. Wang, Acta Phys.-Chim. Sin., 2016, 32, 1087–1104 Search PubMed.
- Y. Qin, J. Xie, S. Liu and Y. Bai, Sens. Actuators, B, 2022, 368, 132230 CrossRef.
- U. T. Nakate, R. Ahmad, P. Patil, Y. Wang, K. S. Bhat, T. Mahmoudi, Y. Yu, E.-K. Suh and Y.-B. Hahn, J. Alloys Compd., 2019, 797, 456–464 CrossRef.
- N. Huo, S. Yang, Z. Wei, S.-S. Li, J.-B. Xia and J. Li, Sci. Rep., 2014, 4, 5209 CrossRef PubMed.
- V. Galstyan, E. Comini, I. Kholmanov, G. Faglia and G. Sberveglieri, RSC Adv., 2016, 6, 34225–34232 RSC.
- H. Yan, P. Song, S. Zhang, Z. Yang and Q. Wang, RSC Adv., 2015, 5, 79593–79599 RSC.
- N. M. Tran, Q. T. H. Ta and J.-S. Noh, Mater. Chem. Phys., 2021, 273, 125087 CrossRef.
- Q. T. H. Ta, G. Namgung and J.-S. Noh, Electron. Mater. Lett., 2019, 15, 750–759 CrossRef.
- X. Yang, H. Li, T. Li, Z. Li, W. Wu, C. Zhou, P. Sun, F. Liu, X. Yan and Y. Gao, Sens. Actuators, B, 2019, 282, 339–346 CrossRef.
- N. R. Tanguy, M. Thompson and N. Yan, Sens. Actuators, B, 2018, 257, 1044–1064 CrossRef.
- Y. Guo, T. Wang, F. Chen, X. Sun, X. Li, Z. Yu, P. Wan and X. Chen, Nanoscale, 2016, 8, 12073–12080 RSC.
- L. Feng, Y. Liu, X. Zhou and J. Hu, J. Colloid Interface Sci., 2005, 284, 378–382 CrossRef PubMed.
- M. R. DashtArzhandi, A. Ismail, T. Matsuura, B. Ng and M. Abdullah, Chem. Eng. J., 2015, 269, 51–59 CrossRef.
- M. Castro, J. Lu, S. Bruzaud, B. Kumar and J.-F. Feller, Carbon, 2009, 47, 1930–1942 CrossRef.
- A. Bouvree, J.-F. Feller, M. Castro, Y. Grohens and M. Rinaudo, Sens. Actuators, B, 2009, 138, 138–147 CrossRef.
- J. F. Feller, D. Langevin and S. Marais, Synth. Met., 2004, 144, 81–88 CrossRef.
- C. Ouyang, S. Wang, Y. Zhang and Y. Zhang, Polym. Degrad. Stab., 2006, 91, 795–804 CrossRef.
- F. Wang, H. Gu and T. M. Swager, J. Am. Chem. Soc., 2008, 130, 5392–5393 CrossRef PubMed.
- S. Savagatrup, V. Schroeder, X. He, S. Lin, M. He, O. Yassine, K. N. Salama, X. X. Zhang and T. M. Swager, Angew. Chem., 2017, 129, 14254–14258 CrossRef.
- B. Esser, J. M. Schnorr and T. M. Swager, Angew. Chem., Int. Ed., 2012, 51, 5752–5756 CrossRef.
- M. He, R. G. Croy, J. M. Essigmann and T. M. Swager, ACS Sens., 2019, 4, 2819–2824 CrossRef PubMed.
- M. Calhoun, J. Sanchez, D. Olaya, M. Gershenson and V. Podzorov, Nat. Mater., 2008, 7, 84–89 CrossRef.
- M. W. Hoffmann, J. D. Prades, L. Mayrhofer, F. Hernandez-Ramirez, T. T. Järvi, M. Moseler, A. Waag and H. Shen, Adv. Funct. Mater., 2014, 24, 595–602 CrossRef.
- S.-H. Hsiao, J.-X. Wu and H.-I. Chen, Sens. Actuators, B, 2020, 305, 127269 CrossRef.
- M. Li, H. Chen, S. Li, G. Wang, F. Wei, X. Guo and H. Tu, Langmuir, 2020, 36, 1462–1466 CrossRef.
- B. M. Binder, Plant Sci., 2008, 175, 8–17 CrossRef.
- C. Paoletti, M. He, P. Salvo, B. Melai, N. Calisi, M. Mannini, B. Cortigiani, F. G. Bellagambi, T. M. Swager and F. Di Francesco, RSC Adv., 2018, 8, 5578–5585 RSC.
- C. A. Schoenbaum, D. K. Schwartz and J. W. Medlin, Acc. Chem. Res., 2014, 47, 1438–1445 CrossRef.
- M. Singh, N. Kaur, G. Drera, A. Casotto, L. Sangaletti and E. Comini, Adv. Funct. Mater., 2020, 30, 2003217 CrossRef.
- S. T. Marshall, M. O'Brien, B. Oetter, A. Corpuz, R. M. Richards, D. K. Schwartz and J. W. Medlin, Nat. Mater., 2010, 9, 853–858 CrossRef.
- S. F. Liu, A. R. Petty, G. T. Sazama and T. M. Swager, Angew. Chem., Int. Ed., 2015, 54, 6554–6557 CrossRef PubMed.
- M. Fleischer, S. Kornely, T. Weh, J. Frank and H. Meixner, Sens. Actuators, B, 2000, 69, 205–210 CrossRef.
- D. J. Wales, J. Grand, V. P. Ting, R. D. Burke, K. J. Edler, C. R. Bowen, S. Mintova and A. D. Burrows, Chem. Soc. Rev., 2015, 44, 4290–4321 RSC.
- M. Drobek, J.-H. Kim, M. Bechelany, C. Vallicari, A. Julbe and S. S. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 8323–8328 CrossRef PubMed.
- H. Li, R. Wu, X. Tian, L. Han, T. Chen, B. Yang, Z. Zhi, Z. Hua and S. Fan, Sens. Actuators, B, 2023, 389, 133872 CrossRef.
- K. Nagashima and S. Suzuki, Anal. Chim. Acta, 1984, 162, 153–159 CrossRef CAS.
- N. Lu, S. Fan, Y. Zhao, B. Yang, Z. Hua and Y. Wu, Sens. Actuators, B, 2021, 347, 130603 CrossRef CAS.
- B. Yang, Z. Zhang, C. Tian, W. Yuan, Z. Hua, S. Fan, Y. Wu and X. Tian, Sens. Actuators, B, 2020, 321, 128567 CrossRef CAS.
- J. Sun, Z. Geng, N. Xue, C. Liu and T. Ma, Micromachines, 2018, 9, 408 CrossRef.
- H. Jung, W. Cho, R. Yoo, H.-S. Lee, Y.-S. Choe, J. Y. Jeon and W. Lee, Sens. Actuators, B, 2018, 274, 527–532 CrossRef CAS.
- J. van den Broek, A. T. Güntner and S. E. Pratsinis, ACS Sens., 2018, 3, 677–683 CrossRef CAS PubMed.
- J. van den Broek, S. Abegg, S. E. Pratsinis and A. T. Güntner, Nat. Commun., 2019, 10, 4220 CrossRef CAS PubMed.
- J. van den Broek, D. K. Cerrejon, S. E. Pratsinis and A. T. Güntner, J. Hazard. Mater., 2020, 399, 123052 CrossRef CAS PubMed.
- J. Brunet, L. Spinelle, A. Pauly, M. Dubois, K. Guerin, M. Bouvet, C. Varenne, B. Lauron and A. Hamwi, Org. Electron., 2010, 11, 1223–1229 CrossRef CAS.
- M. Schweizer-Berberich, S. Strathmann, W. Göpel, R. Sharma and A. Peyre-Lavigne, Sens. Actuators, B, 2000, 66, 34–36 CrossRef CAS.
- X. Zhang, B. Gao, A. E. Creamer, C. Cao and Y. Li, J. Hazard. Mater., 2017, 338, 102–123 CrossRef CAS PubMed.
- A. A. Pesaran and A. F. Mills, Int. J. Heat Mass Transfer, 1987, 30, 1037–1049 CrossRef CAS.
- K. Sakodynskii, L. Panina and N. Klinskaya, Chromatographia, 1974, 7, 339–344 CrossRef CAS.
- P. Althainz, A. Dahlke, M. Frietsch-Klarhof, J. Goschnick and H. Ache, Sens. Actuators, B, 1995, 25, 366–369 CrossRef CAS.
- M. Vilaseca, J. Coronas, A. Cirera, A. Cornet, J. Morante and J. Santamarıa, Catal. Today, 2003, 82, 179–185 CrossRef CAS.
- G. Hagen, A. Dubbe, F. Rettig, A. Jerger, T. Birkhofer, R. Müller, C. Plog and R. Moos, Sens. Actuators, B, 2006, 119, 441–448 CrossRef CAS.
- K.-Y. Lee, J.-C. Hsieh, C.-A. Chen, W.-L. Chen, H.-F. Meng, C.-J. Lu, S.-F. Horng and H.-W. Zan, Sens. Actuators, B, 2021, 326, 128988 CrossRef CAS.
- Y. Zhuang, W. Yuan, L. Qian, S. Chen and G. Shi, Phys. Chem. Chem. Phys., 2017, 19, 12876–12881 RSC.
- A. Ryzhikov, M. Labeau and A. Gaskov, Sens. Actuators, B, 2005, 109, 91–96 CrossRef CAS.
- M. Frietsch, F. Zudock, J. Goschnick and M. Bruns, Sens. Actuators, B, 2000, 65, 379–381 CrossRef CAS.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- M. I. Nandasiri, S. R. Jambovane, B. P. McGrail, H. T. Schaef and S. K. Nune, Coord. Chem. Rev., 2016, 311, 38–52 CrossRef CAS.
- T. Zhou, Y. Sang, X. Wang, C. Wu, D. Zeng and C. Xie, Sens. Actuators, B, 2018, 258, 1099–1106 CrossRef CAS.
- I. C. Weber and A. T. Güntner, Sens. Actuators, B, 2022, 356, 131346 CrossRef CAS.
- M. Stoian, V. Rogé, L. Lazar, T. Maurer, J. C. Védrine, I.-C. Marcu and I. Fechete, Catalysts, 2021, 11, 427 CrossRef CAS.
- D. Tayde and M. Lande, Chem. Rev. Lett., 2021, 4, 30–36 CAS.
- S.-Y. Jeong, J.-W. Yoon, T.-H. Kim, H.-M. Jeong, C.-S. Lee, Y. Chan Kang and J.-H. Lee, J. Mater. Chem. A, 2017, 5, 1446–1454 RSC.
- K. Persaud and G. Dodd, Nature, 1982, 299, 352–355 CrossRef CAS PubMed.
- A. Abdelhalim, M. Winkler, F. Loghin, C. Zeiser, P. Lugli and A. Abdellah, Sens. Actuators, B, 2015, 220, 1288–1296 CrossRef CAS.
- A. Star, V. Joshi, S. Skarupo, D. Thomas and J.-C. P. Gabriel, J. Phys. Chem. B, 2006, 110, 21014–21020 CrossRef CAS PubMed.
- A. Lipatov, A. Varezhnikov, P. Wilson, V. Sysoev, A. Kolmakov and A. Sinitskii, Nanoscale, 2013, 5, 5426–5434 RSC.
- A. Vergara, J. Fonollosa, J. Mahiques, M. Trincavelli, N. Rulkov and R. Huerta, Sens. Actuators, B, 2013, 185, 462–477 CrossRef CAS.
- M. Leidinger, T. Sauerwald, W. Reimringer, G. Ventura and A. Schütze, J. Sens. Sens. Syst., 2014, 3, 253–263 CrossRef.
- L. Trizio, M. Brattoli, G. De Gennaro, D. Suriano, R. Rossi, M. Alvisi, G. Cassano, V. Pfister and M. Penza, in Sensors and Microsystems: AISEM 2011 Proceedings, Springer, 2011, pp. 139–144 Search PubMed.
- M. Kermit and O. Tomic, IEEE Sens. J., 2003, 3, 218–228 CrossRef.
- T. Itoh, Y. Koyama, Y. Sakumura, T. Akamatsu, A. Tsuruta, Y. Masuda and W. Shin, Sens. Actuators, B, 2023, 387, 133803 CrossRef CAS.
- W. Sears, K. Colbow, R. Slamka and F. Consadori, Sens. Actuators, B, 1990, 2, 283–289 CrossRef CAS.
- C. Distante, M. Leo and K. C. Persaud, Discrete Wavelet Transforms, Biomed. Appl., 2011, p. 177 Search PubMed.
- J. Yan, F. Tian, Q. He, Y. Shen, S. Xu, J. Feng and K. Chaibou, Sens. Mater., 2012, 24, 57–73 Search PubMed.
- H. Liu, R. Wu, Q. Guo, Z. Hua and Y. Wu, ACS Omega, 2021, 6, 30598–30606 CrossRef CAS.
- A. D. Wilson, Procedia Technol., 2012, 1, 453–463 CrossRef.
- O. Tomic, T. Eklöv, K. Kvaal and J.-E. Haugen, Anal. Chim. Acta, 2004, 512, 199–206 CrossRef CAS.
- N. J. Vickers, Curr. Biol., 2017, 27, R713–R715 CrossRef CAS PubMed.
- V. V. Krivetskiy, M. D. Andreev, A. O. Efitorov and A. M. Gaskov, Sens. Actuators, B, 2021, 329, 129187 CrossRef CAS.
- N. Yamazoe and K. Shimanoe, Sens. Actuators, B, 2011, 154, 277–282 CrossRef CAS.
- G. Tournier and C. Pijolat, Sens. Actuators, B, 2005, 106, 553–562 CrossRef CAS.
- H.-B. Na, X.-F. Zhang, M. Zhang, Z.-P. Deng, X.-L. Cheng, L.-H. Huo and S. Gao, Sens. Actuators, B, 2019, 297, 126816 CrossRef CAS.
- G. Sakai, N. Matsunaga, K. Shimanoe and N. Yamazoe, Sens. Actuators, B, 2001, 80, 125–131 CrossRef CAS.
- T.-H. Kim, S.-Y. Jeong, Y. K. Moon and J.-H. Lee, Sens. Actuators, B, 2019, 301, 127140 CrossRef CAS.
- S. Acharyya, S. Nag, S. Kimbahune, A. Ghose, A. Pal and P. K. Guha, ACS Sens., 2021, 6, 2218–2224 CrossRef CAS PubMed.
- G. Li, X. Wang, L. Yan, Y. Wang, Z. Zhang and J. Xu, ACS Appl. Mater. Interfaces, 2019, 11, 26116–26126 CrossRef CAS PubMed.
- F. Xu, C. Zhou and H.-P. Ho, J. Alloys Compd., 2021, 858, 158294 CrossRef CAS.
- L. Liu, T. Fei, X. Guan, H. Zhao and T. Zhang, Sens. Actuators, B, 2021, 334, 129625 CrossRef CAS.
- H. Zhao, L. Liu, X. Lin, J. Dai, S. Liu, T. Fei and T. Zhang, ACS Sens., 2019, 5, 346–352 CrossRef PubMed.
- F. Pourfayaz, Y. Mortazavi, A. Khodadadi and S. Ajami, Sens. Actuators, B, 2008, 130, 625–629 CrossRef CAS.
- A. Varpula, S. Novikov, A. Haarahiltunen and P. Kuivalainen, Sens. Actuators, B, 2011, 159, 12–26 CrossRef CAS.
- M. Liess, Sens. Actuators, B, 2003, 95, 46–50 CrossRef CAS.
- H. Tabata, Y. Sato, K. Oi, O. Kubo and M. Katayama, ACS Appl. Mater. Interfaces, 2018, 10, 38387–38393 CrossRef CAS.
- T. Sauerwald, D. Skiera and C. D. Kohl, Appl. Phys. A: Mater. Sci. Process., 2007, 87, 525–529 CrossRef CAS.
- G. Becskereki, G. Horvai and B. Tóth, Polymer, 2021, 13, 1781 CAS.
- B. Esser, J. M. Schnorr and T. M. Swager, Angew. Chem., Int. Ed., 2012, 51, 5752–5756 CrossRef CAS.
- D. R. Wijaya, R. Sarno and E. Zulaika, Data Brief, 2018, 21, 2414–2420 CrossRef.
- W. Jia, G. Liang, Y. Wang and J. Wang, Food Anal. Methods, 2018, 11, 2916–2924 CrossRef.
- D.-H. Guan, X.-X. Wang, F. Li, L.-J. Zheng, M.-L. Li, H.-F. Wang and J.-J. Xu, ACS Nano, 2022, 16, 12364–12376 CrossRef CAS.
- K. Hassan, A. I. Uddin and G.-S. Chung, Sens. Actuators, B, 2016, 234, 435–445 CrossRef CAS.
- K. Y. Ko, J.-G. Song, Y. Kim, T. Choi, S. Shin, C. W. Lee, K. Lee, J. Koo, H. Lee and J. Kim, ACS Nano, 2016, 10, 9287–9296 CrossRef CAS.
- C. Ouyang, Y. Chen, Z. Qin, D. Zeng, J. Zhang, H. Wang and C. Xie, Appl. Surf. Sci., 2018, 455, 45–52 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |