Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Convergent synthesis of thiodiazole dioxides from simple ketones and amines through an unusual nitrogen-migration mechanism
Kunlayanee
Punjajom
a,
Paul P.
Sinclair
a,
Ishika
Saha
b,
Mark
Seierstad
b,
Michael K.
Ameriks
b,
Pablo
García-Reynaga
*b,
Terry P.
Lebold
*b and
Richmond
Sarpong
*a
aDepartment of Chemistry, University of California, Berkeley, CA 94720, USA. E-mail: rsarpong@berkeley.edu
bJanssen Research and Development, San Diego, California 92121, USA. E-mail: pgarciar@its.jnj.com; terry.lebold@gmail.com
First published on 24th June 2024
Abstract
Correction for ‘Convergent synthesis of thiodiazole dioxides from simple ketones and amines through an unusual nitrogen-migration mechanism’ by Kunlayanee Punjajom et al., Chem. Sci., 2024, 15, 328–335, https://doi.org/10.1039/D3SC04478E.
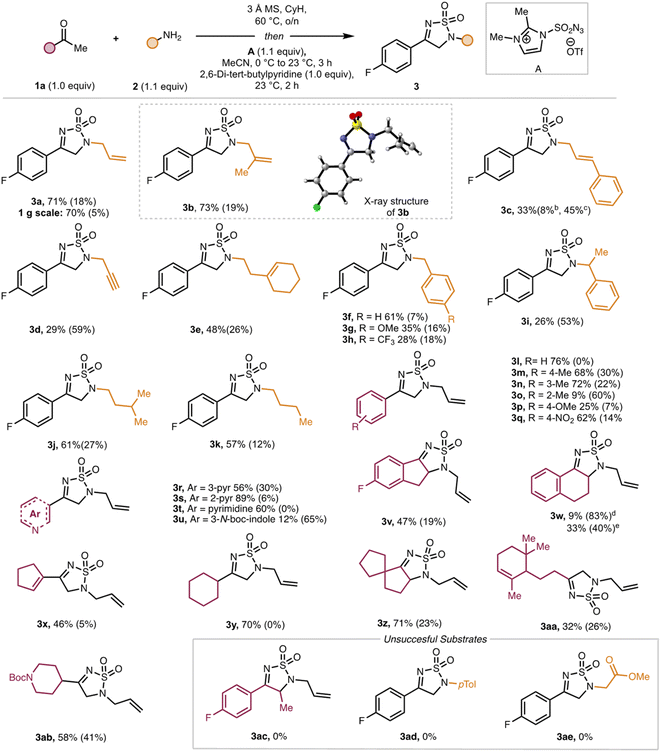
The original article contains errors in Scheme 1 which include: (1) the structure of 3w in which the compound is depicted as the 5,6,7,8-tetrahydroquinoline rather than the intended tetrahydronaphthalene; (2) the superscripts for the yields of 3w, in which the superscripts for compound 3c were repeated. The yields should read:
9% (83%)d
33% (40%)e
These changes do not affect the conclusions of the manuscript. An updated figure and caption are included here.
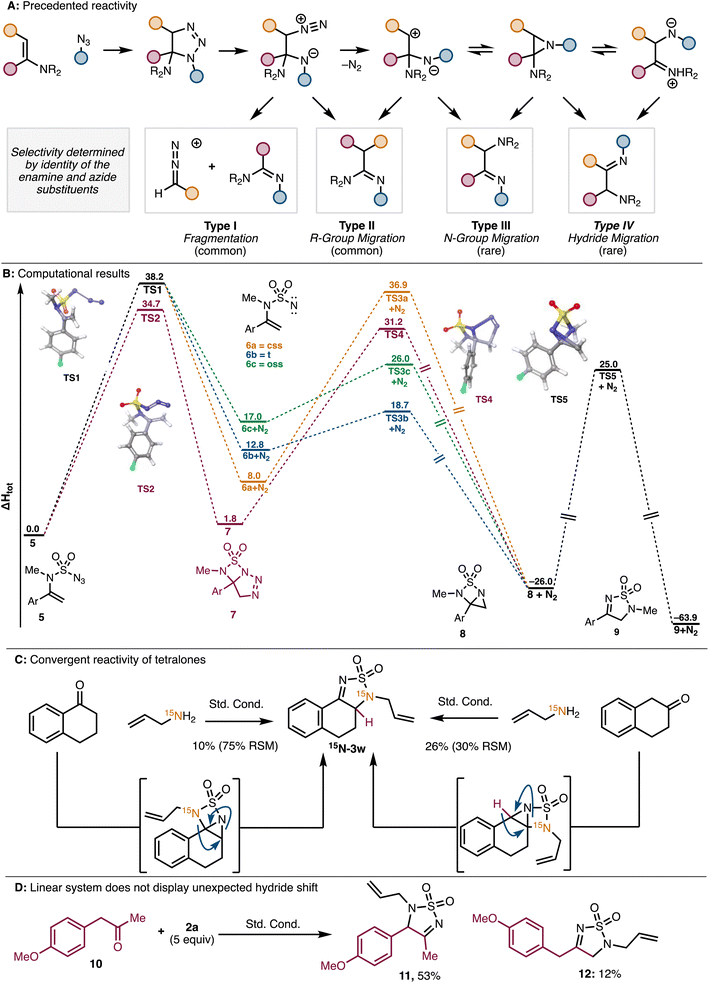
In Fig. 2C, the 15N product should be labeled 15N-3w and not 3x. In addition, the caption for Fig. 2 part D was missing in the original article. An updated figure and caption are provided here.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2024 |