Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Precision-engineered metal–organic frameworks: fine-tuning reverse topological structure prediction and design†
Xiaoyu
Wu
and
Jianwen
Jiang
 *
*
Department of Chemical and Biomolecular Engineering, National University of Singapore, 117576, Singapore. E-mail: chejj@nus.edu.sg
First published on 25th September 2024
Abstract
Digital discoveries of metal–organic frameworks (MOFs) have been significantly advanced by the reverse topological approach (RTA). The node-and-linker assembly strategy allows predictable reticulations predefined by in silico coordination templates; however, reticular equivalents lead to substantial combinatorial explosion due to the infinite design space of building units (BUs). Here, we develop a fine-tuned RTA for the structure prediction of MOFs by integrating precise topological constraints and leveraging reticular chemistry, thus transcending traditional exhaustive trial-and-error assembly. From an extensive array of chemically realistic BUs, we subsequently design a database of 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 precision-engineered MOFs (PE-MOFs) and further optimize their structures. The PE-MOFs are assessed for post-combustion CO2 capture in the presence of H2O and top-performing candidates are identified by integrating three stability criteria (activation, water and thermal stabilities). This study highlights the potential of synergizing PE with the RTA to enhance efficiency and precision for computational design of MOFs and beyond.
823 precision-engineered MOFs (PE-MOFs) and further optimize their structures. The PE-MOFs are assessed for post-combustion CO2 capture in the presence of H2O and top-performing candidates are identified by integrating three stability criteria (activation, water and thermal stabilities). This study highlights the potential of synergizing PE with the RTA to enhance efficiency and precision for computational design of MOFs and beyond.
1. Introduction
Porous crystals such as metal–organic frameworks (MOFs) represent a significant advancement in the field of functional materials, distinguished by their modular architectures and diverse chemical functionalities.1 With these salient features, MOFs are considered versatile materials for many potential applications including gas storage, separation, catalysis, etc.2 The evolution of MOF landscape has been remarkably enriched by the fusion of experimental synthesis and theoretical analysis, particularly through digital reticular chemistry.3Experimentally synthesized MOFs, through systematically orchestrated assembly of metal nodes and organic linkers, have provided tangible crystals for characterization and validation. Meanwhile, theoretical methods, facilitated by automated assembly,4ab initio crystal structure prediction,5,6 building unit (BU) replacement,7 and predominantly, the reverse topological approach (RTA),8 have exponentially expanded the conceivable landscape of MOFs and generated extensive hypothetical MOFs (hMOFs) without physical synthesis.9–11 The RTA operates by starting with a desired topology template and then mapping it back to specific BUs based on connectivity patterns and topological optimization. Consequently, high-throughput computational screenings have been conducted on experimental MOFs or customized hMOFs and shortlisted potential candidates for many applications, particularly CO2 capture.12–15 Yet, as the field progresses, a major challenge persists in combinatorial explosion due to a wide variety of BUs and their enormous arrangements. This explosion in structural possibilities, while broadening the horizon of MOFs, imposes a substantial challenge in computation.
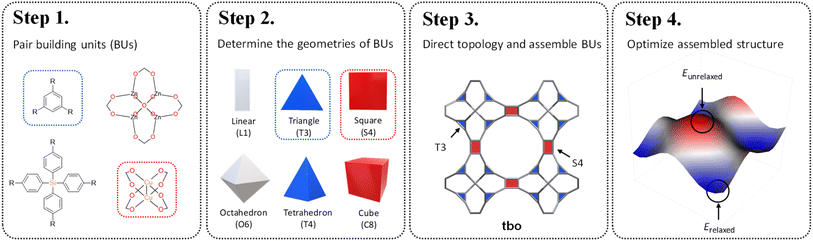
Reticular chemistry, a discipline that artfully weaves the threads of molecular architectures, offers an optimized palette of targeted nets controlled by the geometric and topological preference of BUs.16 By integrating topological constraints,17 one can specifically generate certain crystal configurations based on the geometric compatibility of BU combinations. Inspired by these benefits, in this work, we develop a fine-tuned RTA by introducing an additional layer of precision prerequisite. This refinement involves determining the geometries of BUs and selecting appropriate coordination templates guided by the reticular nature of MOFs. These criteria are governed by the local symmetry of BUs reticulated within a designated net. As illustrated in Scheme 1, our method starts with the one-on-one pairing of organic (red) and inorganic (blue) BUs, where their geometric signatures are subsequently matched and assigned. This approach strategically focuses on identifying a ‘reticular ideal’ topology, thereby alleviating the need for an exhaustive search across the entire topology space. The added dimension of precision engineering (PE) effectively mitigates the combinatorial explosion by filtering out infeasible topologies at an early stage, thereby catalyzing a substantially more focused and accurate search for synthesizable MOFs given a vast array of BUs.
Prompted by the combinatorial guarantee demonstrated above, we present how large-scale RTA-based structure prediction of MOFs can be fine-tuned by PE for vast recursive inorganic–organic combinations of BUs, sourced from the well-established HEALED library.18 To ensure a consistent basis for geometry determination, the connection points and the planarity of each candidate BU were standardized, followed by a similarity comparison against established geometric prototypes. Subsequently, we consulted a specialized table of topological constraints to specifically select topologies suited to the selected BUs before in silico synthesis and curation. Finally, a total of 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 PE-MOFs were distilled and carefully optimized by integrating the universal force field (UFF) and machine-learned atomic charges. The applicability of the PE-MOFs was demonstrated as adsorbents for post-combustion CO2 capture. Top-performing candidates were identified and quantitative structure–performance relationships were established. These endeavors not only enrich the existing MOF bank, but also set the stage for future advancement in digital discovery of MOFs. The systematic approach outlined here showcases the potential for computational construction and high-throughput discovery of superior MOFs, ensuring that the next generation of porous crystals will be precisely tailored for specific industrial applications such as CO2 capture and beyond.
823 PE-MOFs were distilled and carefully optimized by integrating the universal force field (UFF) and machine-learned atomic charges. The applicability of the PE-MOFs was demonstrated as adsorbents for post-combustion CO2 capture. Top-performing candidates were identified and quantitative structure–performance relationships were established. These endeavors not only enrich the existing MOF bank, but also set the stage for future advancement in digital discovery of MOFs. The systematic approach outlined here showcases the potential for computational construction and high-throughput discovery of superior MOFs, ensuring that the next generation of porous crystals will be precisely tailored for specific industrial applications such as CO2 capture and beyond.
2. Methodology
2.1. Geometric signatures
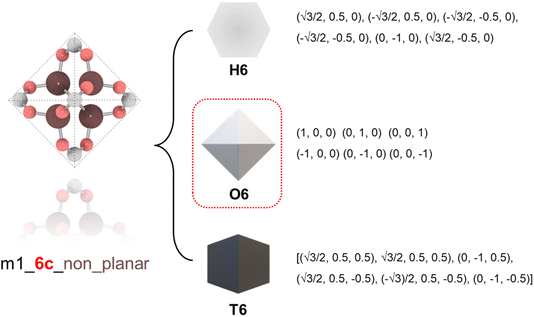
To ensure precise classification and compatibility check, we defined a comprehensive set of 13 geometric prototypes including 2-connected linear (L2), 3-connected triangle (T3), 4-connected square (S4), 4-connected tetrahedral (T4), 6-connected hexagonal (H6), 6-connected octahedral (O6), 6-connected trigonal prism (T6), 8-connected cubic (C8), 12-connected cuboctahedra (C12), 12-connected icosahedral (I12), 12-connected hexagonal prism (H12), 12-connected truncated tetrahedral (T12), and 24-connected truncated octahedron (T24). Classification of BUs began by determining the number of connection points, followed by evaluating the planarity where applicable, specifically for L2, T3, T4, S4, H6, C8, and T24 geometries. For instance, a BU with 3 connection points was directly classified as T3; a BU with 4 connection points was designated as tetrahedral if it failed the conservative planarity check. Should a BU not conform to these preliminary classifications, further steps would compare it with the detailed geometric prototypes by examining the root-mean squared deviations (RMSDs).19 An example is illustrated in Fig. 1 where a BU namely m1 from the HEALED library is assigned with an O6 geometric signature. However, it is worthwhile to note that there are challenges in classifying non-perfectly shaped BUs and highly connected BUs, particularly those with 12 or more connections. These BUs may not always align precisely with desired geometric signatures, even with thorough visual inspections. For instance, Fig. S1† demonstrates how a highly connected vertice BU, namely m345 (sourced from a MOF with fcu topology20), combined with an edge BU o13, leads to a structure that, while identical in coordination, diverges in linker shape due to the steric effects of the naphthalene linker. In cases where a BU exhibits an imperfect geometric signature, it can still be reticulated into a topology analogous to a perfect BU. As depicted in Fig. S2,† despite imperfect geometry, o165 (o255) can still assemble into edc (ftw) topology with m345, similar to o189 (o47) with an ideal BU. This adaptability enables the navigation of a broader topological diversity, more aligned with real-world scenarios where perfectly shaped BUs are less common, compared to methods that rely strictly on idealized BUs (Fig. S3†). The full list of the geometric prototypes mathematically constructed in this work is provided in the ESI.†2.2. Topological constraints
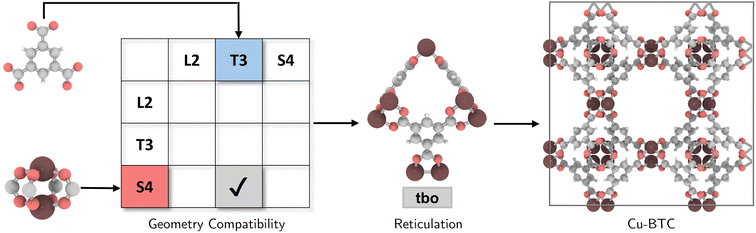
The coordination complexity of MOF structures arises from the wide variety of possible geometric signatures.17 By carefully selecting BUs with appropriate shapes, topological constraints can be targeted. We introduced a reticular reference table for binary combinations of geometric signatures (ESI†), providing information on the most ‘reticular ideal’ topology to form. For instance, Cu-paddlewheel (Cu2O8) and 1,3,5-benzene tricarboxylate (BTC) can be assigned geometric signatures as T3 and S4, respectively. While conventional connectivity-based methods10,21,22 would allow these BUs to assemble into several topologies such as ctn, bor, pto, and tbo (Fig. S4†), not all these combinations result in practical or favorable structures due to potential geometric distortions in structure prediction (Fig. S5†). Considering the shape compatibility of BUs, we further refined the selection of topologies, only targeting geometrically coherent structures, and constructed precise and ideal structures such as Cu-BTC23 or HKUST-1 (Fig. 2). It is important to note that certain topologies are absent from the publicly available topology database under the RCSR project24 and, as such, were not included in our table.2.3. Topological assembly
With topological constraints, the molecular LEGO-like PORMAKE algorithm10 was implemented for in silico assembly utilizing BUs from the HEALED library.18 A total of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 875 MOFs were initially generated with a RMSD cut-off at 0.3 Å. These structures were distilled to 94
875 MOFs were initially generated with a RMSD cut-off at 0.3 Å. These structures were distilled to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 PE-MOFs by employing a timeout-based strategy as previously used for computationally curating hMOFs11 and covalent–organic frameworks (COFs).25 The 94
823 PE-MOFs by employing a timeout-based strategy as previously used for computationally curating hMOFs11 and covalent–organic frameworks (COFs).25 The 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 PE-MOFs were structurally optimized by using the SMART algorithm of Forcite module in Materials Studio. The UFF26 and machine-learned PACMOF atomic charges27 were adopted to describe bonded and non-bonded interactions. In the calculations of atomic charges, a five-minute timeout was implemented to exclude structures being difficult to featurize for PACMOF. The bond information of both inter- and intra-BUs predefined using PORMAKE was preserved during optimizations to avoid unreasonable distance-based bond calculations, which might be caused by improperly configured BUs while fitting into selected topologies. During optimization, a twenty-minute wall-time limit was set to filter out structures that failed to converge. For each structure, bond information was retained and charge information was recalculated. The charge-recalculation and geometric optimization loop were repeated three times to ensure the accuracy of the final set of PE-MOF structures, with a wall-time limit consistently applied throughout these stages.
823 PE-MOFs were structurally optimized by using the SMART algorithm of Forcite module in Materials Studio. The UFF26 and machine-learned PACMOF atomic charges27 were adopted to describe bonded and non-bonded interactions. In the calculations of atomic charges, a five-minute timeout was implemented to exclude structures being difficult to featurize for PACMOF. The bond information of both inter- and intra-BUs predefined using PORMAKE was preserved during optimizations to avoid unreasonable distance-based bond calculations, which might be caused by improperly configured BUs while fitting into selected topologies. During optimization, a twenty-minute wall-time limit was set to filter out structures that failed to converge. For each structure, bond information was retained and charge information was recalculated. The charge-recalculation and geometric optimization loop were repeated three times to ensure the accuracy of the final set of PE-MOF structures, with a wall-time limit consistently applied throughout these stages.
2.4. Diversity analysis
Geometric and chemical features were used for diversity analysis of the designed PE-MOFs. The geometric features including pore limiting diameter (PLD), largest cavity diameter (LCD), channel dimensionality (Dimen), pore volume (PV), void fraction (VF), gravimetric surface area (GSA) and global cavity diameter (GCD) were computed via Zeo++![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 utilizing a probe radius of 1.86 Å. The diverse chemical features were encoded using revised autocorrelations (RACs),29 which were initially developed to fingerprint open-shell transition metal complexes30,31 and have been adapted to encode MOFs.32–35 The RACs were computed via MolSimplify,36 providing the product or difference of atomic heuristics based on non-weighted crystal graphs. A wide variety of properties such as Pauling electronegativity (χ), nuclear charge (Z) connectivity (T), atom identity (I), covalent radii (S) and polarizability (α) were included in the RACs. Disparity (D), variety (V) and balance (B) were computed as diversity metrics32 based on unsupervised machine-learned 2D embeddings of the RACs mapped by t-distributed Stochastic Neighbor Embedding (t-SNE).37
28 utilizing a probe radius of 1.86 Å. The diverse chemical features were encoded using revised autocorrelations (RACs),29 which were initially developed to fingerprint open-shell transition metal complexes30,31 and have been adapted to encode MOFs.32–35 The RACs were computed via MolSimplify,36 providing the product or difference of atomic heuristics based on non-weighted crystal graphs. A wide variety of properties such as Pauling electronegativity (χ), nuclear charge (Z) connectivity (T), atom identity (I), covalent radii (S) and polarizability (α) were included in the RACs. Disparity (D), variety (V) and balance (B) were computed as diversity metrics32 based on unsupervised machine-learned 2D embeddings of the RACs mapped by t-distributed Stochastic Neighbor Embedding (t-SNE).37
2.5. Molecular simulation for CO2 capture
The PE-MOFs were assessed as adsorbents for CO2 capture from a flue gas by pressure-swing adsorption. The flue gas was mimicked using a CO2/N2 mixture with a composition of 0.15/0.85. Considering the kinetic diameters of CO2 (3.30 Å) and N2 (3.64 Å), a subset of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 173 PE-MOFs with PLDs ranging from 3 to 7 Å was chosen. Grand-canonical Monte Carlo (GCMC) simulations were conducted to compute the adsorption of the flue gas at 298 K and 1 bar and the desorption of pure CO2 at 298 K and 0.01 bar. The PE-MOFs with performance superior to zeolite-13X38 were shortlisted and their water, thermal and activation stabilities were evaluated using MOFSimplify.39,40 For the stable ones, their CO2 capture performance was further evaluated in the presence of H2O. GCMC simulations were extended for the adsorption of a ternary mixture of CO2, N2 and H2O at 298 K and 1 bar, with 60% relative humidity. CO2 and N2 were represented using the transferable potentials for phase equilibria (TraPPE) force field,41 and H2O was mimicked by the four point TIP4P model.42 The Lennard-Jones interactions of framework atoms were modeled using the UFF26 and DREIDING force field.43 Table S1† lists the force field parameters. For cross LJ interactions, the potential parameters were estimated using the Lorentz–Berthelot mixing rules. The atomic charges of PE-MOFs were assigned using PACMOF,27 which efficiently estimates charges with comparable accuracy to the density derived electrostatic and chemical (DDEC) method.44 As exemplified in Fig. S6,† a determination coefficient (R2) of 0.933 was achieved by comparing PACMOF charges and DDEC charges in four representative PE-MOFs. Following the DDEC6 protocol,45 the DDEC charges here were computed using the CHARGEMOL package (https://github.com/berquist/chargemol) based on the calculations from the Vienna Ab initio Simulation Package (VASP)46,47 at the PBE-D3(BJ) level.48–50 Fig. S7† also shows CO2, N2 and H2O uptakes (at 60% relative humidity) simulated using PACMOF charges and DDEC charges, respectively, in the four PE-MOFs. The uptakes based on both charges are close, suggesting an insignificant effect of the charge assignment method. During GCMC simulations, the frameworks were treated as rigid with periodic boundary conditions applied to all three dimensions. The LJ interactions were calculated with a cutoff of 12 Å, while the electrostatic interactions were estimated using Ewald summation. The GCMC simulations were carried out using the RASPA package.51 Each simulation included 10
173 PE-MOFs with PLDs ranging from 3 to 7 Å was chosen. Grand-canonical Monte Carlo (GCMC) simulations were conducted to compute the adsorption of the flue gas at 298 K and 1 bar and the desorption of pure CO2 at 298 K and 0.01 bar. The PE-MOFs with performance superior to zeolite-13X38 were shortlisted and their water, thermal and activation stabilities were evaluated using MOFSimplify.39,40 For the stable ones, their CO2 capture performance was further evaluated in the presence of H2O. GCMC simulations were extended for the adsorption of a ternary mixture of CO2, N2 and H2O at 298 K and 1 bar, with 60% relative humidity. CO2 and N2 were represented using the transferable potentials for phase equilibria (TraPPE) force field,41 and H2O was mimicked by the four point TIP4P model.42 The Lennard-Jones interactions of framework atoms were modeled using the UFF26 and DREIDING force field.43 Table S1† lists the force field parameters. For cross LJ interactions, the potential parameters were estimated using the Lorentz–Berthelot mixing rules. The atomic charges of PE-MOFs were assigned using PACMOF,27 which efficiently estimates charges with comparable accuracy to the density derived electrostatic and chemical (DDEC) method.44 As exemplified in Fig. S6,† a determination coefficient (R2) of 0.933 was achieved by comparing PACMOF charges and DDEC charges in four representative PE-MOFs. Following the DDEC6 protocol,45 the DDEC charges here were computed using the CHARGEMOL package (https://github.com/berquist/chargemol) based on the calculations from the Vienna Ab initio Simulation Package (VASP)46,47 at the PBE-D3(BJ) level.48–50 Fig. S7† also shows CO2, N2 and H2O uptakes (at 60% relative humidity) simulated using PACMOF charges and DDEC charges, respectively, in the four PE-MOFs. The uptakes based on both charges are close, suggesting an insignificant effect of the charge assignment method. During GCMC simulations, the frameworks were treated as rigid with periodic boundary conditions applied to all three dimensions. The LJ interactions were calculated with a cutoff of 12 Å, while the electrostatic interactions were estimated using Ewald summation. The GCMC simulations were carried out using the RASPA package.51 Each simulation included 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-cycle equilibration and 10
000-cycle equilibration and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-cycle production. Five different trial moves including insertion, deletion, rotation, translation and identity change were attempted randomly with equal probability.
000-cycle production. Five different trial moves including insertion, deletion, rotation, translation and identity change were attempted randomly with equal probability.
3. Results and discussion
3.1. Analysis of PE-MOFs
We start with the geometric analysis of the PE-MOF database. Fig. 3 shows the distributions of three key geometric features (GSA, VF and LCD) in 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 PE-MOFs and also in 279
823 PE-MOFs and also in 279![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010 ARC-MOFs for comparison. The ARC-MOF database is a benchmark collection of both experimentally synthesized and computationally predicted MOF deposits.14 For each geometric feature, a similar ‘volcano’ pattern is observed in both databases; however, PE-MOFs exhibit a wider distribution. Specifically, GSA ranges from zero to 10
010 ARC-MOFs for comparison. The ARC-MOF database is a benchmark collection of both experimentally synthesized and computationally predicted MOF deposits.14 For each geometric feature, a similar ‘volcano’ pattern is observed in both databases; however, PE-MOFs exhibit a wider distribution. Specifically, GSA ranges from zero to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m2 g−1 in PE-MOFs and from zero to 8000 m2 g−1 in ARC-MOFs. The VF is between 0 and 1 in PE-MOFs and between 0 and 0.9 in ARC-MOFs. The LCD is from 2 Å to 80 Å in PE-MOFs and from 2 Å to 35 Å in ARC-MOFs. These results suggest that our fine-tuned RTA is capable of generating MOFs with a more variety of geometries. Fig. S8 and S9† provide a comparative analysis of topology distribution and its correlation with pore features between PE-MOFs and ARC-MOFs. Despite PE-MOFs being designed with a limited set of BUs, the enriched diversity in topologies significantly influences their porosity profiles, enhancing the variety of geometric features. Fig. S10† illustrates the t-SNE map with embeddings of geometric features for PE-MOFs and ARC-MOFs. Visually, PE-MOFs and ARC-MOFs are segregated into distinct regions. PE-MOFs primarily occupy the top-left quadrant of the map, suggesting that they are not extensively covered by ARC-MOFs but complement the unexplored geometric characteristics of ARC-MOFs.
000 m2 g−1 in PE-MOFs and from zero to 8000 m2 g−1 in ARC-MOFs. The VF is between 0 and 1 in PE-MOFs and between 0 and 0.9 in ARC-MOFs. The LCD is from 2 Å to 80 Å in PE-MOFs and from 2 Å to 35 Å in ARC-MOFs. These results suggest that our fine-tuned RTA is capable of generating MOFs with a more variety of geometries. Fig. S8 and S9† provide a comparative analysis of topology distribution and its correlation with pore features between PE-MOFs and ARC-MOFs. Despite PE-MOFs being designed with a limited set of BUs, the enriched diversity in topologies significantly influences their porosity profiles, enhancing the variety of geometric features. Fig. S10† illustrates the t-SNE map with embeddings of geometric features for PE-MOFs and ARC-MOFs. Visually, PE-MOFs and ARC-MOFs are segregated into distinct regions. PE-MOFs primarily occupy the top-left quadrant of the map, suggesting that they are not extensively covered by ARC-MOFs but complement the unexplored geometric characteristics of ARC-MOFs.
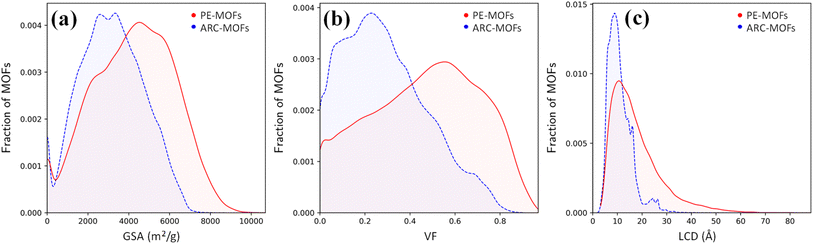 | ||
Fig. 3 Distributions of (a) GSA, (b) VF and (c) LCD in 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 PE-MOFs (red) and 279 823 PE-MOFs (red) and 279![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010 ARC-MOFs (blue).14 010 ARC-MOFs (blue).14 | ||
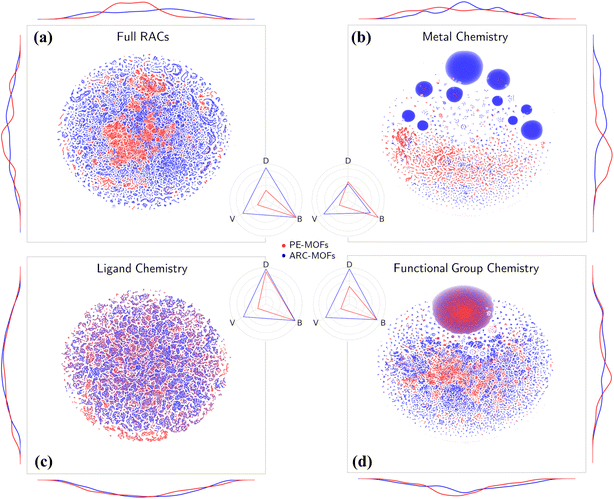
Chemical analysis of PE-MOFs is based on the RACs29 by characterizing three crucial aspects of MOF chemistry: metal, ligand, and functional group. As depicted in Fig. 4a, unlike the material space learned from geometric features (Fig. S10†), both PE-MOFs and ARC-MOFs are spread over the entire t-SNE map, with ARC-MOFs exhibiting a wider space. The difference is attributed to a greater structural freedom in ARC-MOFs compared with PE-MOFs, as the latter are constructed with precise or strict topological criteria. Table 1 lists the diversity metrics including variety, disparity and balance in PE-MOFs and ARC-MOFs. The variety of a database indicates the number of distinct types of structures present; the balance reflects how evenly distributed the structures are; the disparity measures how dissimilar the structures are. Apparently, PE-MOFs possess a smaller chemical space than ARC-MOFs as evidenced by a lower variety value. This likely arises from the sparse diversity of the BU reservoir14 exploited to construct PE-MOFs and a considerably more extensive ARC-MOF database, which is nearly three times the size of the PE-MOF database. Intriguingly, despite a limited population, a high balance value is observed in PE-MOFs (Fig. 4a–d), suggesting a well-distributed representation across different structural types. For metal chemistry and linker chemistry (Fig. 4b and c), both balance and disparity values of PE-MOFs are comparable with those of ARC-MOFs, indicating a dissimilarity-rich BU space of PE-MOFs. Although a greater diversity value in MOF banks is beneficial for computational screening and machine learning (ML),32,52 our approach intentionally fine-tunes exploration in the design space of MOFs. Such PE is particularly important for future RTA-based structure design employing a much wider array of BUs and becomes crucial as diffusion models have received increasingly more attention in the inverse design of MOFs.53–55
| Feature | Database | Variety | Balance | Disparity |
|---|---|---|---|---|
| Full RACs | PE-MOFs | 0.261 | 0.902 | 0.261 |
| ARC-MOFs | 0.720 | 0.941 | 0.871 | |
| Metal | PE-MOFs | 0.261 | 0.895 | 0.470 |
| ARC-MOFs | 0.720 | 0.661 | 0.416 | |
| Ligand | PE-MOFs | 0.261 | 0.920 | 0.885 |
| ARC-MOFs | 0.720 | 0.931 | 0.966 | |
| Functional group | PE-MOFs | 0.261 | 0.845 | 0.459 |
| ARC-MOFs | 0.720 | 0.837 | 0.918 |
3.2. CO2 capture performance
For CO2 capture, the performance of an adsorbent is usually quantified using three metrics: CO2 working capacity (ΔNCO2), CO2/N2 selectivity SCO2/N2, and a trade-off (TSN) between capacity and selectivity, TSN = log(SCO2/N2) × ΔNCO2. As illustrated in Fig. 5a–c, wide distributions are observed for ΔNCO2, log(SCO2/N2) and TSN in 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 173 PE-MOFs, specifically, in the range of 0–7 mol kg−1, 0.5–3.0, and 0–16, respectively. For comparison, the performance of zeolite-13X is included, which is benchmarked as a high-performing adsorbent for CO2 capture under dry conditions.38 A large number of PE-MOFs exhibit performance superior to 13X. Fig. 5d further shows log(SCO2/N2) versus ΔNCO2 with different TSN values. At a low ΔNCO2, a wide range of SCO2/N2 is observed approximately from 3 to 3000. With increasing ΔNCO2, SCO2/N2 generally drops and tends to approach a constant. PE-MOFs with greater TSN or superior performance are primarily clustered in the middle-right region. A total of 1207 PE-MOFs are found to surpass the TSN of 3.2 in 13X.
173 PE-MOFs, specifically, in the range of 0–7 mol kg−1, 0.5–3.0, and 0–16, respectively. For comparison, the performance of zeolite-13X is included, which is benchmarked as a high-performing adsorbent for CO2 capture under dry conditions.38 A large number of PE-MOFs exhibit performance superior to 13X. Fig. 5d further shows log(SCO2/N2) versus ΔNCO2 with different TSN values. At a low ΔNCO2, a wide range of SCO2/N2 is observed approximately from 3 to 3000. With increasing ΔNCO2, SCO2/N2 generally drops and tends to approach a constant. PE-MOFs with greater TSN or superior performance are primarily clustered in the middle-right region. A total of 1207 PE-MOFs are found to surpass the TSN of 3.2 in 13X.
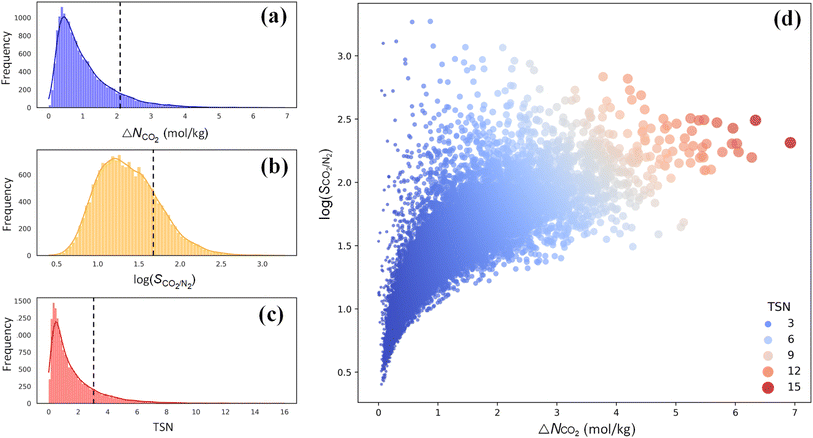 | ||
Fig. 5 Performance of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 173 PE-MOFs for CO2 capture. (a–c) Distributions of ΔNCO2, log(SCO2/N2) and TSN in 17 173 PE-MOFs for CO2 capture. (a–c) Distributions of ΔNCO2, log(SCO2/N2) and TSN in 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 173 PE-MOFs. The dashed line denotes the performance of zeolite-13X as a benchmark.38 (d) log(SCO2/N2) versus ΔNCO2, with the color-coding based on TSN. 173 PE-MOFs. The dashed line denotes the performance of zeolite-13X as a benchmark.38 (d) log(SCO2/N2) versus ΔNCO2, with the color-coding based on TSN. | ||
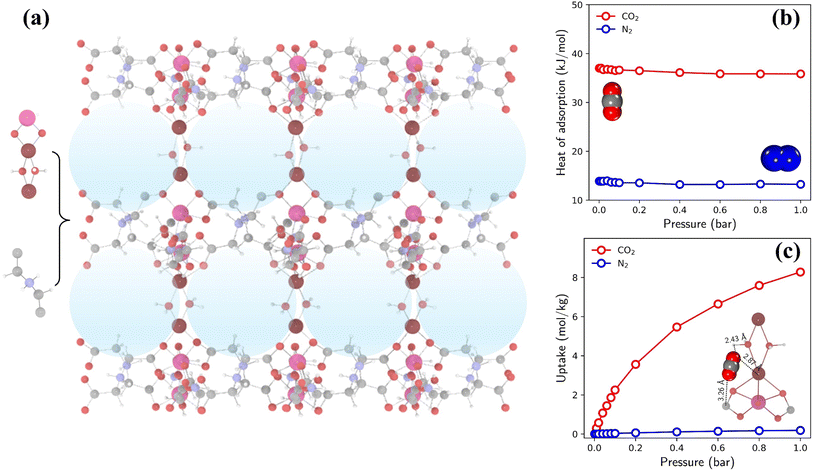
The highest TSN of 16.0 is observed in a PE-MOF assembled from metal vertice m160 and organic edge o323. With bcu topology and 8-connected metal vertices, as illustrated in Fig. 6a, this PE-MOF comprises three-dimensional pores with a diameter of 8.18 Å. Fig. 6b and c show the heats and isotherms of CO2 and N2 adsorption in this PE-MOF, calculated from GCMC simulations for a CO2/N2 mixture at 298 K and composition of 0.15/0.85. It is evident that CO2 adsorption significantly dominates over N2 across the entire pressure range, resulting in a CO2 adsorption capacity of 8.28 mol kg−1 and CO2/N2 selectivity of 346 at 1 bar. Such high CO2 capture performance is superior to or comparable with that of many experimentally synthesized and computationally designed MOFs reported in the literature, as compared in Table S2 and Fig. S11.†9,15,52,56
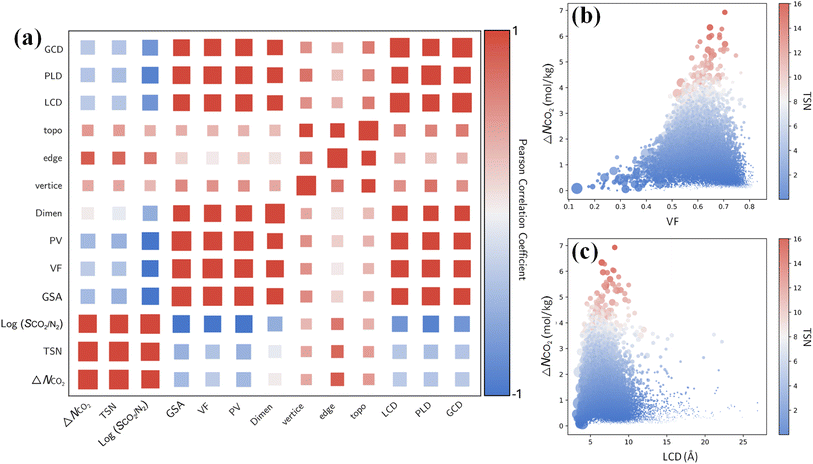
Furthermore, it is instructive to explore the interplay between CO2 capture performance and structure characteristics through multivariate analysis. From the Pearson correlation matrix in Fig. 7a, capture performance metrics ΔNCO2, TSN and log(SCO2/N2) exhibit negative correlations with GSA, VF, PV, LCD, PLD and GCD. These correlations are corroborated by the relationships of ΔNCO2 with the VF and LCD in 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 173 MOFs. As shown in Fig. 7b and c, with an increasing VF or LCD, ΔNCO2 and TSN first increase, reach maximum, and then drop; this suggests while a small pore in a MOF leads to high SCO2/N2, it restricts ΔNCO2 and TSN. The top-performing MOFs with great TSN possess VF from 0.6 to 0.7 and LCD around 7 Å. These dimensions are about one to two times the kinetic diameter of CO2 molecule, but less than twice that of N2 molecule, as also previously reported.57
173 MOFs. As shown in Fig. 7b and c, with an increasing VF or LCD, ΔNCO2 and TSN first increase, reach maximum, and then drop; this suggests while a small pore in a MOF leads to high SCO2/N2, it restricts ΔNCO2 and TSN. The top-performing MOFs with great TSN possess VF from 0.6 to 0.7 and LCD around 7 Å. These dimensions are about one to two times the kinetic diameter of CO2 molecule, but less than twice that of N2 molecule, as also previously reported.57
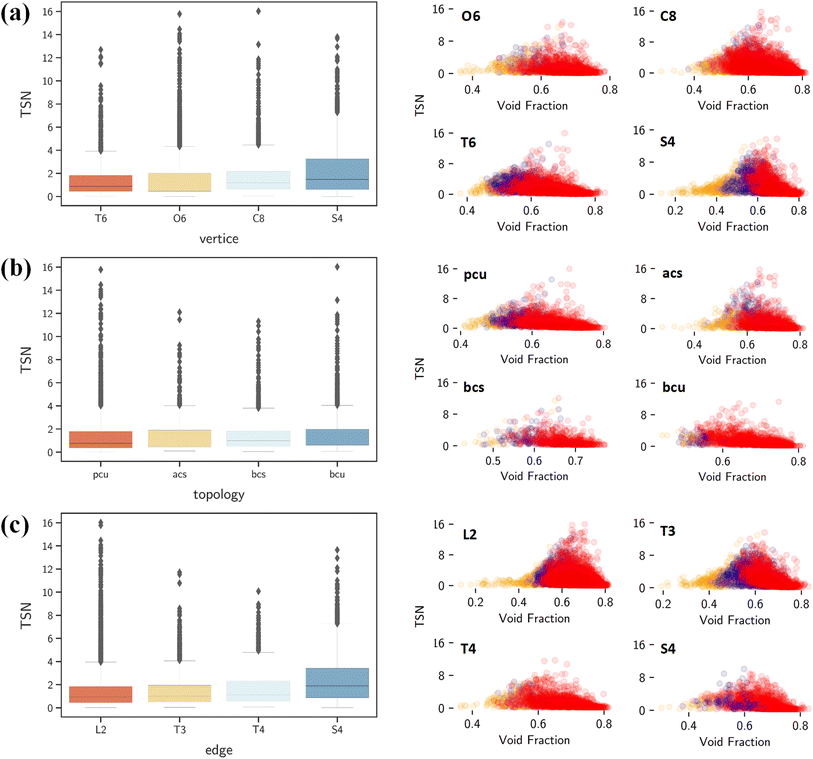
By contrast, ΔNCO2, TSN and log(SCO2/N2) show positive correlations with vertices, edges and topology as shown in Fig. 7a. This underscores the significant role of structural configuration in determining MOF performance. However, the impact may be overshadowed by the transformation of categorical variables into numerical values, which warrants deeper examination. As illustrated in Fig. 8a, MOFs with O6 and C8 vertices, fostering 3D geometries, exhibit a broad distribution of great TSN values (>8). This correlation aligns well with the finding in Fig. 8b, where 3D-MOFs with pcu and bcu topologies, characterized by larger void fractions, are densely represented among great TSN values. Regarding edges, as shown in Fig. 8c, our analysis reveals that high-performing MOFs incorporate L2 edge, which likely contributes to the formation of uniform pores; consequently, the pore space is optimized to accommodate CO2 molecules and enables them to align and stack efficiently.
To quantify the impact of chemical descriptors on CO2 capture, we utilized two separate Random Forest (RF) models58 and combined with their SHapley Additive exPlanations59 (SHAP) feature importance. The first model incorporates pore features along with bond types of reticulated BUs, and the second model focuses exclusively on bond types. The details of ML are provided in the ESI.† As depicted in Fig. S12,† the first model reveals that PV holds the greatest importance for CO2 capture. The second model shows that the presence and arrangement of carbon bonds within BUs—specifically C–H and C–C bonds—significantly affect the TSN value (Fig. 9a). These bonds typically lead to longer linkers, thereby enhancing porosity of MOFs.60,61 It is essential to note that the outcome of the feature importance analysis might differ depending on the dataset utilized for model training;32 thus, our conclusion is specific to our dataset.
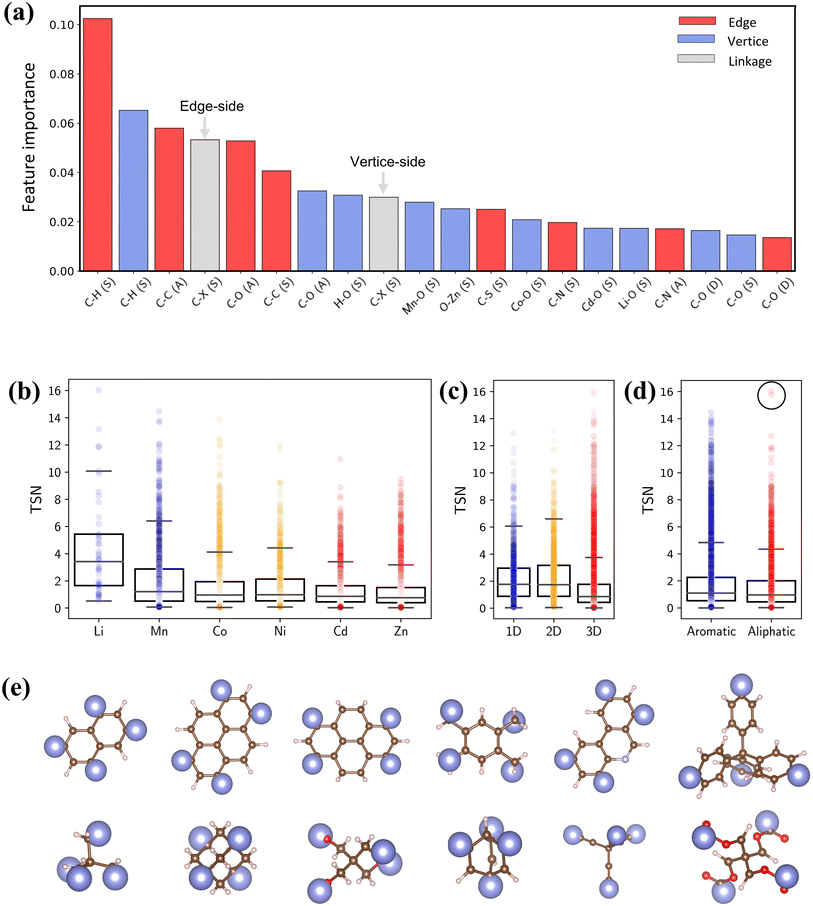 | ||
| Fig. 9 (a) SHAP interpreted feature importance of bond features. (b–d) Box plots of metal, dimensionality and linker type versus TSN. (e) Representative aromatic and aliphatic linkers in PE-MOFs. | ||
Fig. 9b–d show the relationships between TSN and three key MOF characteristics (metal, dimensionality and linker type). MOFs with metals such as Li and Mn typically possess a broader range of higher TSN values compared with others. However, metals in MOFs can form as clusters or complexes and associate with various BUs.17 The complex configurations of these metal sites are crucial in mediating interactions (e.g., dipole–dipole interactions). Regarding dimensionality, 1D and 2D MOFs, with constricted porosity as discussed previously, typically exhibit moderate TSN values due to their limited cavity space, which constrains deliverable capacity due to high surface affinity at a low pressure. In contrast, 3D MOFs, characterized by larger porosity, show significantly higher TSN values. Additionally, the edge BUs in our dataset are categorized into either aromatic or aliphatic. Aromatic linkers, with their uniform and planar geometry compared with aliphatic BUs (Fig. 9e), generally tend to exhibit higher TSN values, reflecting their capability to form MOFs with greater porosity and additional functional features. Notably, aliphatic linkers also achieve high performance in some cases (circled data points), highlighting that optimal CO2 capture cannot be attributed to a single chemical descriptor alone.
3.3. Stability and applicability of PE-MOFs
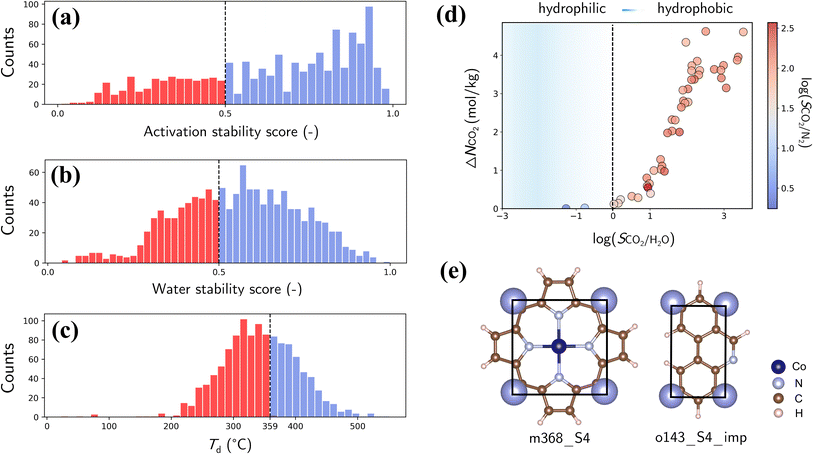
Finally, we assess the stability and applicability of the designed PE-MOFs in real-world applications, particularly for CO2 capture. Three critical stability metrics (activation, water and thermal)39,40 were used to evaluate the stability of 1207 PE-MOFs that outperform zeolite-13X for CO2 capture from a dry flue gas. As presented in Fig. 10a and b, the activation and water stability scores range from 0 to 1.0, categorizing PE-MOFs from unstable to stable. A threshold score of 0.5 is set to identify potentially activatable and water-stable structures. PE-MOFs with decomposition temperatures (Td) exceeding 359 °C, which is the average Td observed in the CoRE-MOF 2019 database,11,62 are considered thermally stable (Fig. 10c). Among the 1207 PE-MOFs, 224 satisfy the three stability criteria. It is worthwhile to note that the ML models39,40 facilitate rapid stability evaluation, to a certain extent, but they have limitations. For example, the models do not consider actual activation methods such as supercritical CO2 drying,63 which can stabilize MOFs prone to collapse by conventional activation techniques. For water stability, the models do not account for interactions under varied humidity conditions.64 Although post-combustion CO2 capture typically occurs near ambient temperature, the need for thermal activation of MOFs to expel trapped solvents necessitates higher thermal stability.65The 224 stable PE-MOFs were further evaluated for their CO2 capture from a wet flue gas (CO2/N2/H2O mixture at 298 K and 1 bar, with 60% relative humidity). As depicted in Fig. 10d, 34 PE-MOFs can be identified as hydrophobic (i.e., SCO2/H2O > 1) with SCO2/N2 not significantly compromised. A top-performing candidate, assembled from metal vertice m368 and organic edge o143, has exceptional performance with ΔNCO2 of 4.62 mol kg−1 and SCO2/N2 of 121.7, even in the presence of H2O. It also stands out in terms of stability metrics—an activation stability score of 0.95, a water stability score of 0.59, and a Td of 427 °C. As illustrated in Fig. 10e, this MOF possesses a planar geometry of BUs (common among other top-performers) and constricted pore apertures (4.13 × 4.83 Å) introduced by cdm topology, thus enhancing chemical robustness while minimizing H2O interference for CO2 capture.
4. Conclusions
We have developed a fine-tuned RTA for structure prediction toward the digital discovery of MOFs. By addressing combinatorial explosion and effectively navigating a large array of BUs, our approach enhances the efficiency and precision to assemble BUs. A total of 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 PE-MOFs are designed with a more variety of geometries compared with ARC-MOFs despite a relatively smaller chemical space. For CO2 capture, the performance of PE-MOFs exhibits negative correlations with several geometric features (i.e., GSA, VF, PV, LCD, PLD and GCD), but positive correlations with structural configuration (i.e., vertices, edges and topology). Further quantitative insights are probed with machine learning models, highlighting the indispensable role of pore volume and the configuration of carbon bonds in determining CO2 capture performance. A larger number of PE-MOFs are found to be superior to benchmarked zeolite-13X. By integrating three stability metrics (activation, water and thermal), stable PE-MOFs are identified with high-performance for CO2 capture from a wet flue gas.
823 PE-MOFs are designed with a more variety of geometries compared with ARC-MOFs despite a relatively smaller chemical space. For CO2 capture, the performance of PE-MOFs exhibits negative correlations with several geometric features (i.e., GSA, VF, PV, LCD, PLD and GCD), but positive correlations with structural configuration (i.e., vertices, edges and topology). Further quantitative insights are probed with machine learning models, highlighting the indispensable role of pore volume and the configuration of carbon bonds in determining CO2 capture performance. A larger number of PE-MOFs are found to be superior to benchmarked zeolite-13X. By integrating three stability metrics (activation, water and thermal), stable PE-MOFs are identified with high-performance for CO2 capture from a wet flue gas.
The strategic assembly of BUs based on geometric signatures and topological compatibility not only mitigates combinatorial explosion by filtering out infeasible topologies, but also demonstrates the potential of digital chemistry in discovering high-performing materials. Our approach highlights the transformative impact of synergizing precision engineering with digital reticular chemistry, and it would advance further innovation in the intelligent design of porous crystals beyond MOFs. In future research, we will focus on expanding the diversity of BUs, particularly by customizing property-triggered BUs and integrating mechanical-, water- and thermal-stability, and other important stability measures. Advanced machine learning techniques will be incorporated to streamline the design process and enhance practical applicability.
Data availability
Data and codes related to this study are available on GitHub https://github.com/xiaoyu961031/Fine-tuned-RTA. All computationally predicted structures are available on Zenodo https://zenodo.org/records/11480898.Author contributions
XW conceptualized the project, developed the approach and wrote the manuscript. All authors reviewed and edited the manuscript. JJ acquired the funding and supervised the project.Conflicts of interest
There is no conflict of interests to declare.Acknowledgements
We gratefully acknowledge the A*STAR LCER-FI projects (LCERFI01-0015 U2102d2004 and LCERFI01-0033 U2102d2006) and the National Research Foundation Singapore (NRF-CRP26-2021RS-0002) for financial support and the National University of Singapore and the National Supercomputing Centre (NSCC) Singapore for computational resources.References
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Design and synthesis of an exceptionally stable and highly porous metal-organic framework, Nature, 1999, 402, 276–279 CrossRef CAS.
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, The chemistry and applications of metal-organic frameworks, Science, 2013, 341, 1230444 CrossRef.
- H. Lyu, Z. Ji, S. Wuttke and O. M. Yaghi, Digital Reticular Chemistry, Chem, 2020, 6, 2219–2241 CAS.
- C. M. Draznieks, J. M. Newsam, A. M. Gorman, C. M. Freeman and G. Férey, De Novo Prediction of Inorganic Structures Developed through Automated Assembly of Secondary Building Units (AASBU Method), Angew. Chem., Int. Ed., 2000, 39, 2270–2275 CrossRef CAS.
- J. P. Darby, M. Arhangelskis, A. D. Katsenis, J. M. Marrett, T. Friščić and A. J. Morris, Ab Initio Prediction of Metal-Organic Framework Structures, Chem. Mater., 2020, 32, 5835–5844 CrossRef CAS.
- Y. Xu, J. M. Marrett, H. M. Titi, J. P. Darby, A. J. Morris, T. Friščić and M. Arhangelskis, Experimentally Validated Ab Initio Crystal Structure Prediction of Novel Metal–Organic Framework Materials, J. Am. Chem. Soc., 2023, 3515–3525 CrossRef CAS.
- C. E. Wilmer, M. Leaf, C. Y. Lee, O. K. Farha, B. G. Hauser, J. T. Hupp, R. Q. Snurr, C. E. Wilmer, M. Leaf and C. Y. Lee, et al., Large-scale screening of hypothetical metal–organic frameworks, Nat. Chem., 2011, 4, 83–89 CrossRef.
- J. Keupp, J. Keupp, R. Schmid and R. Schmid, TopoFF: MOF structure prediction using specifically optimized blueprints, Faraday Discuss., 2018, 211, 79–101 RSC.
- P. G. Boyd, A. Chidambaram, E. García-Díez, C. P. Ireland, T. D. Daff, R. Bounds, A. Gładysiak, P. Schouwink, S. M. Moosavi and M. M. Maroto-Valer, et al., Data-driven design of metal–organic frameworks for wet flue gas CO2 capture, Nature, 2019, 576, 253–256 CrossRef CAS.
- S. Lee, B. Kim, H. Cho, H. Lee, S. Y. Lee, E. S. Cho and J. Kim, Computational Screening of Trillions of Metal-Organic Frameworks for High-Performance Methane Storage, ACS Appl. Mater. Interfaces, 2021, 13, 23647–23654 CrossRef CAS.
- A. Nandy, S. Yue, C. Oh, C. Duan, G. G. Terrones, Y. G. Chung and H. J. Kulik, A database of ultrastable MOFs reassembled from stable fragments with machine learning models, Matter, 2023, 6, 1585–1603 CrossRef CAS.
- J. Jiang, Computational screening of metal–organic frameworks for CO2 separation, Curr. Opin. Green Sustainable Chem., 2019, 16, 57–64 CrossRef.
- A. S. Rosen, S. M. Iyer, D. Ray, Z. Yao, A. Aspuru-Guzik, L. Gagliardi, J. M. Notestein and R. Q. Snurr, Machine learning the quantum-chemical properties of metal–organic frameworks for accelerated materials discovery, Matter, 2021, 4, 1578–1597 CrossRef CAS.
- J. Burner, J. Luo, A. White, A. Mirmiran, O. Kwon, P. G. Boyd, S. Maley, M. Gibaldi, S. Simrod, V. Ogden and T. K. Woo, ARC-MOF: A Diverse Database of Metal-Organic Frameworks with DFT-Derived Partial Atomic Charges and Descriptors for Machine Learning, Chem. Mater., 2023, 35, 900–916 CrossRef CAS.
- S. A. Mohamed, D. Zhao and J. Jiang, Integrating stability metrics with high-throughput computational screening of metal–organic frameworks for CO2 capture, Commun. Mater., 2023, 4, 1–10 CrossRef.
- O. M. Yaghi, Reticular Chemistry in All Dimensions, ACS Cent. Sci., 2019, 5, 1295–1300 CrossRef CAS.
- M. J. Kalmutzki, N. Hanikel and O. M. Yaghi, Secondary building units as the turning point in the development of the reticular chemistry of MOFs, Sci. Adv., 2018, 4, eaat9180 CrossRef CAS.
- M. Gibaldi, O. Kwon, A. White, J. Burner and T. K. Woo, The HEALED SBU Library of Chemically Realistic Building Blocks for Construction of Hypothetical Metal-Organic Frameworks, ACS Appl. Mater. Interfaces, 2022, 14, 43372–43386 CrossRef CAS.
- K. M. Jablonka, A. S. Rosen, A. S. Krishnapriyan and B. Smit, An Ecosystem for Digital Reticular Chemistry, ACS Cent. Sci., 2023, 9, 563–581 CrossRef CAS.
- N. M. Padial, E. Q. Procopio, C. Montoro, E. López, J. E. Oltra, V. Colombo, A. Maspero, N. Masciocchi, S. Galli and I. Senkovska, et al., Highly Hydrophobic Isoreticular Porous Metal–Organic Frameworks for the Capture of Harmful Volatile Organic Compounds, Angew. Chem., 2013, 125, 8448–8452 CrossRef.
- D. A. Gómez-Gualdrón, Y. J. Colón, X. Zhang, T. C. Wang, Y.-S. Chen, J. T. Hupp, T. Yildirim, O. K. Farha, J. Zhang and R. Q. Snurr, Evaluating topologically diverse metal–organic frameworks for cryo-adsorbed hydrogen storage, Energy Environ. Sci., 2016, 9, 3279–3289 RSC.
- P. G. Boyd and T. K. Woo, A generalized method for constructing hypothetical nanoporous materials of any net topology from graph theory, CrystEngComm, 2016, 18, 3777–3792 RSC.
- S. S. Y. Chui, S. M. F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3](n), Science, 1999, 283, 1148–1150 CrossRef CAS.
- M. O'Keeffe, M. A. Peskov, S. J. Ramsden and O. M. Yaghi, The Reticular Chemistry Structure Resource (RCSR) database of, and symbols for, crystal nets, Acc. Chem. Res., 2008, 41, 1782–1789 CrossRef.
- R. Mercado, R. S. Fu, A. V. Yakutovich, L. Talirz, M. Haranczyk and B. Smit, In Silico Design of 2D and 3D Covalent Organic Frameworks for Methane Storage Applications, Chem. Mater., 2018, 30, 5069–5086 CrossRef CAS.
- A. K. Rappé, C. J. Casewit, K. S. Colwell, W. A. Goddard and W. M. Skiff, UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations, J. Am. Chem. Soc., 1992, 114, 10024–10035 CrossRef.
- S. Kancharlapalli, A. Gopalan, M. Haranczyk and R. Q. Snurr, Fast and Accurate Machine Learning Strategy for Calculating Partial Atomic Charges in Metal-Organic Frameworks, J. Chem. Theory Comput., 2021, 17, 3052–3064 CrossRef CAS.
- T. F. Willems, C. H. Rycroft, M. Kazi, J. C. Meza and M. Haranczyk, Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials, Microporous Mesoporous Mater., 2012, 149, 134–141 CrossRef CAS.
- J. P. Janet and H. J. Kulik, Resolving Transition Metal Chemical Space: Feature Selection for Machine Learning and Structure-Property Relationships, J. Phys. Chem. A, 2017, 121, 8939–8954 CrossRef CAS.
- A. Nandy, C. Duan, J. P. Janet, S. Gugler and H. J. Kulik, Strategies and Software for Machine Learning Accelerated Discovery in Transition Metal Chemistry, Ind. Eng. Chem. Res., 2018, 57, 13973–13986 CrossRef CAS.
- A. Nandy, C. Duan, M. G. Taylor, F. Liu, A. H. Steeves and H. J. Kulik, Computational Discovery of Transition-metal Complexes: From High-throughput Screening to Machine Learning, Chem. Rev., 2021, 121, 9927–10000 CrossRef CAS.
- S. M. Moosavi, A. Nandy, K. M. Jablonka, D. Ongari, J. P. Janet, P. G. Boyd, Y. Lee, B. Smit and H. J. Kulik, Understanding the diversity of the metal-organic framework ecosystem, Nat. Commun., 2020, 11, 1–10 CrossRef.
- J. Lee, I. Lee, J. Park, H. Kim, M. Kim, K. Min and S. Lee, Optimal Surrogate Models for Predicting the Elastic Moduli of Metal–Organic Frameworks via Multiscale Features, Chem. Mater., 2023, 35, 10457–10475 CrossRef CAS.
- H. Tang, Q. Xu, M. Wang and J. Jiang, Rapid screening of metal–organic frameworks for propane/propylene separation by synergizing molecular simulation and machine learning, ACS Appl. Mater. Interfaces, 2021, 13, 53454–53467 CrossRef CAS.
- Z. Zhang, H. Tang, M. Wang, B. Lyu, Z. Jiang and J. Jiang, Metal–Organic Frameworks for Water Harvesting: Machine Learning-Based Prediction and Rapid Screening, ACS Sustain. Chem. Eng., 2023, 11, 8148–8160 CrossRef CAS.
- E. I. Ioannidis, T. Z. H. Gani and H. J. Kulik, molSimplify: a toolkit for automating discovery in inorganic chemistry, J. Comput. Chem., 2016, 37, 2106–2117 CrossRef CAS.
- L. Van Der Maaten and G. Hinton, Visualizing Data using t-SNE, J. Mach. Learn. Res., 2008, 9, 2579–2605 Search PubMed.
- M. T. Ho, G. W. Allinson and D. E. Wiley, Reducing the Cost of CO2 Capture from Flue Gases Using Pressure Swing Adsorption, Ind. Eng. Chem. Res., 2008, 47, 4883–4890 CrossRef CAS.
- A. Nandy, G. Terrones, N. Arunachalam, C. Duan, D. W. Kastner and H. J. Kulik, MOFSimplify, machine learning models with extracted stability data of three thousand metal–organic frameworks, Sci. Data, 2022, 9, 74 CrossRef CAS.
- G. G. Terrones, S.-P. Huang, M. P. Rivera, S. Yue, A. Hernandez and H. J. Kulik, Metal–Organic Framework Stability in Water and Harsh Environments from Data-Driven Models Trained on the Diverse WS24 Data Set, J. Am. Chem. Soc., 2024, 146, 20333–20348 CrossRef CAS.
- J. J. Potoff and J. I. Siepmann, Vapor–liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen, AIChE J., 2001, 47, 1676–1682 CrossRef CAS.
- J. Vorholz, V. I. Harismiadis, B. Rumpf, A. Z. Panagiotopoulos and G. Maurer, Vapor+liquid equilibrium of water, carbon dioxide, and the binary system, water+carbon dioxide, from molecular simulation, Fluid Phase Equilib., 2000, 170, 203–234 CrossRef CAS.
- S. L. Mayo, B. D. Olafson and W. A. Goddard, DREIDING: a generic force field for molecular simulations, J. Phys. Chem., 1990, 94, 8897–8909 CrossRef CAS.
- T. A. Manz and D. S. Sholl, Chemically Meaningful Atomic Charges That Reproduce the Electrostatic Potential in Periodic and Nonperiodic Materials, J. Chem. Theory Comput., 2010, 6, 2455–2468 CrossRef CAS.
- N. G. Limas and T. A. Manz, Introducing DDEC6 atomic population analysis: part 4. Efficient parallel computation of net atomic charges, atomic spin moments, bond orders, and more, RSC Adv., 2018, 8, 2678–2707 RSC.
- G. Kresse, Ab initio molecular dynamics for liquid metals, J. Non-Cryst. Solids, 1995, 192–193, 222–229 CrossRef.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the damping function in dispersion corrected density functional theory, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS.
- D. Dubbeldam, S. Calero, D. E. Ellis and R. Q. Snurr, RASPA: molecular simulation software for adsorption and diffusion in flexible nanoporous materials, Mol. Simul., 2016, 42, 81–101 CrossRef CAS.
- S. Majumdar, S. M. Moosavi, K. M. Jablonka, D. Ongari and B. Smit, Diversifying Databases of Metal Organic Frameworks for High-Throughput Computational Screening, ACS Appl. Mater. Interfaces, 2021, 13, 61004–61014 CrossRef CAS.
- X. Fu, T. Xie, A. S. Rosen, T. Jaakkola and J. Smith, MOFDiff: Coarse-grained Diffusion for Metal-Organic Framework Design, arXiv, 2023, preprint, arXiv:2310.10732, DOI:10.48550/arXiv.2310.10732.
- H. Xiao, R. Li, X. Shi, Y. Chen, L. Zhu, X. Chen and L. Wang, An invertible, invariant crystal representation for inverse design of solid-state materials using generative deep learning, Nat. Commun., 2023, 14, 1–12 Search PubMed.
- H. Park, X. Yan, R. Zhu, E. A. Huerta, S. Chaudhuri, D. Cooper, I. Foster and E. Tajkhorshid, A generative artificial intelligence framework based on a molecular diffusion model for the design of metal-organic frameworks for carbon capture, Commun. Chem., 2024, 7, 1–18 CrossRef.
- J. B. Lin, T. T. T. Nguyen, R. Vaidhyanathan, J. Burner, J. M. Taylor, H. Durekova, F. Akhtar, R. K. Mah, O. Ghaffari-Nik and S. Marx, et al., A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture, Science, 2021, 374, 1464–1469 CrossRef CAS.
- X. Cao, Z. Wang, Z. Qiao, S. Zhao and J. Wang, Penetrated COF Channels: Amino Environment and Suitable Size for CO2 Preferential Adsorption and Transport in Mixed Matrix Membranes, ACS Appl. Mater. Interfaces, 2019, 11, 5306–5315 CrossRef CAS.
- T. K. Ho, Random decision forests, IEEE, Montréal, Canada, 1995 Search PubMed.
- S. M. Lundberg and S.-I. Lee, A unified approach to interpreting model predictions, in NIPS'17, Long Beach, California, USA, 2017 Search PubMed.
- T. Bailey, A. Jackson, R.-A. Berbece, K. Wu, N. Hondow and E. Martin, Gradient Boosted Machine Learning Model to Predict H2, CH4, and CO2 Uptake in Metal–Organic Frameworks Using Experimental Data, J. Chem. Inf. Model., 2023, 63, 4545–4551 CrossRef CAS.
- R. Wang, Q. Meng, L. Zhang, H. Wang, F. Dai, W. Guo, L. Zhao and D. Sun, Investigation of the effect of pore size on gas uptake in two fsc metal–organic frameworks, Chem. Commun., 2014, 50, 4911–4914 RSC.
- Y. G. Chung, E. Haldoupis, B. J. Bucior, M. Haranczyk, S. Lee, H. Zhang, K. D. Vogiatzis, M. Milisavljevic, S. Ling and J. S. Camp, et al., Advances, Updates, and Analytics for the Computation-Ready, Experimental Metal–Organic Framework Database: CoRE MOF 2019, J. Chem. Eng. Data, 2019, 64, 5985–5998 CrossRef CAS.
- D. M. Frimpong and L. Baojian, Recent Advances of Supercritical CO2 in Green Synthesis and Activation of Metal–Organic Frameworks, J. Inorg. Organomet. Polym. Mater., 2019, 30, 581–595 Search PubMed.
- N. C. Burtch, H. Jasuja and K. S. Walton, Water Stability and Adsorption in Metal–Organic Frameworks, Chem. Rev., 2014, 114, 10575–10612 CrossRef CAS.
- J. L. Woodliffe, R. S. Ferrari, I. Ahmed and A. Laybourn, Evaluating the purification and activation of metal-organic frameworks from a technical and circular economy perspective, Coord. Chem. Rev., 2021, 428, 213578 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc05616g |
| This journal is © The Royal Society of Chemistry 2024 |