Open Access Article
Open Access ArticleDearomative spirocyclization of ynamides†
Mohamed
Agbaria
,
Nwar
Egbaria
and
Zackaria
Nairoukh
 *
*
Institute of Chemistry, Casali Center of Applied Chemistry, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel. E-mail: z.nairoukh@mail.huji.ac.il
First published on 22nd October 2024
Abstract
Spiro N-heterocycles, particularly aza-spiro piperidines, have shown significant promise in pharmaceutical applications due to their ability to enhance physicochemical properties. Despite their potential, the preparation of these complex structures poses significant challenges. To address this, we propose a one-pot dearomative spirocyclization reaction of ynamides. This method involves a copper-catalyzed carbomagnesiation reaction, achieving chemo-, regio-, and stereoselective formation of vinyl metal intermediates. Upon the addition of a Lewis acid, these intermediates undergo a regioselective nucleophilic dearomatization event, facilitating the synthesis of diverse aza-spiro dihydropyridine scaffolds with multiple functional handles. Various Grignard reagents, diverse ynamides, and acylating reagents have been explored. A subsequent hydrogenation reaction provides access to both partially and fully reduced spirocyclic frameworks, broadening the scope of spirocyclic structures with potential medicinal applications.
Introduction
The majority of pharmaceuticals and natural products contain saturated and unsaturated five- and six-membered N-heterocycles,1 with a tendency towards increased saturation for improved physicochemical properties.2 However, during lead optimization, unconventional structures have been discovered to finely tune physicochemical properties such as basicity, lipophilicity, and solubility.1b–d Among these structures are spiro N-heterocycles, particularly spiro piperidines stand out for their ability to introduce conformational restrictions, potentially modulating binding potency and specificity (Fig. 1A).3,4 Consequently, their integration into lead structures has proven rewarding, particularly in antidiabetic,5 anticancer,6 and anti-Alzheimer pharmacological applications,7 with some successfully developed as commercial drugs (Fig. 1A).3 Nonetheless, the primary challenge lies in their preparation,8,9 which often entails a higher number of synthetic steps and/or unconventional reaction procedures,10 thus hindering further advancement of pharmacological applications. This challenge becomes even more evident in the preparation of diaza-spiro heterocyclic scaffolds.To address this issue, several protocols involving dearomatization reactions11 of pyridines towards the formation of aza-spiro piperidines have been developed. For instance, the addition of trifluoromethanesulfonic anhydride to N-arylisonicotinamides triggers an intramolecular nucleophilic attack of the aryl ring on the pyridinium salt, delivering diaza-spiro heterocycles in moderate to good yields, albeit with a poor regioisomeric ratio (Fig. 1B).12 Moreover, the semi-pinacol rearrangement of 4-(hydroxycyclobutyl)-pyridines elegantly facilitates the synthesis of spirocyclic piperidine derivatives (Figure 1B),13 although this approach has been less explored for the preparation of aza-spiro piperidines.
Additional elegant approaches towards the formation of aza-spiro piperidines involve the dearomatization of indole derivatives, delivering diverse six membered spiroindolenines with excellent stereoselectivity.14,15 Notably, the enantioselective formation of diaza-spirocycles has been achieved,16 albeit limited to spirocyclic 2H-pyrroles.
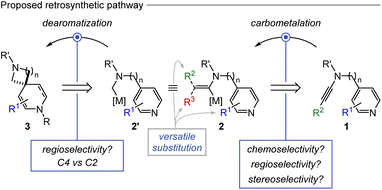
In light of the above, and considering our continuous efforts in constructing complex N-heterocycles through dearomative functionalization reactions,17 we sought to explore systematically the chemical space of aza-spiropiperidyl compounds. Envisioning the formation of diaza-spirocyclic 3 through a regioselective intramolecular nucleophilic dearomatization reaction of alkylmetal species 2′ seemed logical. However, fine-tuning the nature of the metalated nucleophile is essential to control the regioselective outcome (Fig. 2), especially in the presence of longer linkers.18 The synthesis of the latter species could be achieved via a carbometalation reaction of a pyridine derivative bearing a C–C triple bond 1. However, challenges related to the chemoselectivity of the carbo-addition reaction, as well as regio- and stereoselectivity of the carbometalation, need to be addressed. Note that this envisioned strategy allows the easy exploration of versatile substitution on both rings. Furthermore, it allows the preparation of different ring sizes of aza-spiropiperidyl scaffolds (Fig. 2).
Herein, we present a protocol for the carbometalation–dearomatization reaction of ynamides, offering a convenient, chemo-, regio- and stereoselective route to diverse aza-spiropiperidine scaffolds (Fig. 3). The resulting architectures could offer versatility for diverse modifications, allowing for the synthesis of medicinally relevant spiro heterocyclic scaffolds through multidirectional elaboration.
 | ||
| Fig. 3 This work: the dearomative spirocyclization of easily accessible ynamides delivers highly decorated spiro dihydropyridine derivatives. | ||
Results and discussion
Our journey began with the synthesis of ynamides 1,19 easily obtained by a C–N coupling reaction between bromoalkynes and aminopyridine derivatives.16,20 Subsequently we subjected the latter to a carbometalation reaction. Inspired by literature reports on carbocupration of ynamides21,22 and following extensive optimization studies,23 we identified the optimal reaction conditions as summarized in Table 1. Notably, the copper-catalyzed carbomagnesiation of ynamide 1a (R1 = H, R2 = Hex, R′ = COOMe) with EtMgBr in dichloromethane (DCM) enabled the clean formation of vinyl metal pyridin-4-ylmethanamine as a single regio- and stereoisomer. The stereochemistry of the intermediate was confirmed by NMR spectroscopy of the corresponding protonated product 2a (entry 1). Excellent selectivity values are attributed to the chelation of the metaled species with the electron-withdrawing group (R′ = COOMe).21| Entry | Deviation from standard conditions | Yieldb |
|---|---|---|
| a Reactions were performed under N2 with 0.2 mmol of 1a. b Yields were determined using 1H NMR spectroscopy with CH2Br2 as the internal standard. c Isolated yield. d The additive was added in the second step prior to the addition of the Lewis acid. See ESI, for more details on optimization studies. Me, methyl; Et, ethyl; Hex, hexyl; TMEDA, tetramethylethylenediamine; DMPU, N,N′-dimethylpropyleneurea. | ||
| 1 | None | 79% (75%)c (2a) |
| 2 | None | 72% (68%)c (3a) |
| 3 | Toluene instead of DCM | 48% (2a) |
| 4 | THF instead of DCM | 43% (2a) |
| 5 | CuI instead of CuBr·Me2S | 30% (2a) |
| 6 | CuBr instead of CuBr·Me2S | 33% (2a) |
| 7 | CuBr·Me2S (2.0 equiv.) | 60% (2a) |
| 8d | TMEDA as additive | 48% (3a) |
| 9d | DMPU as additive | 60% (3a) |
| 10 | 2nd step at −78 °C | 26% (3a) |
| 11 | 2nd step at 0 °C | 65% (3a) |
Additional control experiments provided insights into critical factors impacting the efficiency of this transformation. Interestingly, the choice of reaction solvent turned to be a crucial factor. Replacing DCM with standard carbomagnesiation solvents such as diethyl ether, THF and toluene resulted in decreased yields of the intermediate (entries 3 and 4). Furthermore, the presence of a catalytic amount of copper bromide dimethyl sulfide complex proved essential (entries 5–7).
Having achieved a highly regio- and stereoselective carbometalation reaction, the spirocyclization reaction was triggered through the addition of methyl chloroformate at room temperature (Table 1). The regioselectivity of the nucleophilic attack was ensured by the soft nature of the vinyl metal species.18 Subsequent efforts to enhance the efficiency of the second step led to the isolation of the final product 3a in a good overall yield (entry 2). The addition of metal scavengers such as TMEDA or DMPU to the reaction mixture decreased the overall yield (entries 8 and 9). Notably, conducting the second step under lower temperatures influenced the performance of this transformation (entries 10 and 11).
After identifying the optimal reaction conditions, we initiated scoping studies for the dearomative spirocyclization reaction (Scheme 1). Generally, the products were obtained as single isomers with moderate to high overall yields. Additionally, purification through standard column chromatography proved sufficient for obtaining pure compounds.
 | ||
| Scheme 1 Scope of the dearomative spirocyclization reaction. Reactions were carried out on a 0.5 mmol scale. Yields were determined after column chromatography. For detailed experimental procedures, see ESI.†aReaction was performed on a 0.2 mmol scale. Me, methyl; Et, ethyl; iPr, iso-propyl; Bu, butyl; Hex, hexyl; Hep, heptyl; Ph, phenyl; TIPS, triisopropylsilyl; Piv, pivaloyl. | ||
We began our investigation with ynamide 1a (R2 = Hex). Initially, we examined the addition of primary alkyl Grignard reagents, including methyl (Me), ethyl (Et), butyl (Bu), and hexyl (Hex) magnesium bromides. Pleasingly, the corresponding spiro heterocycles 3a–3d were obtained in moderate to good overall yields. This reaction was also successfully carried out on a gram scale, delivering 0.94 g of 3a as a single isomer with a good overall yield. The addition of secondary Grignard reagent, such iPrMgBr, resulted in the final product 3e with a reduced yield, primarily due to less efficient carbometalation reaction caused by steric hindrance. Similar behaviour was observed when phenethylmagnesium bromide was used, resulting in 3f being obtained in low yield. In contrast, the addition of aryl Grignard reagents delivered only the carbometalated products as single isomers.24 Further optimization studies aimed at facilitating the dearomatization reaction were ultimately unsuccessful.
Next, we sought to explore the influence of diverse ynamides on the reaction outcome (Scheme 1). Replacing the alkyl group R2 present on the alkyne with Me, Bu, heptyl (Hep) and cyclohexyl (Cy) provided the corresponding adducts 3g–3l in satisfactory yields. Interestingly, by exchanging R2 and R3 present on the alkyne and the Grignard reagent, respectively, and performing the carbometalation-spirocyclization reaction, the opposite isomer 3i was obtained in 46% yield, still as a single isomer. This demonstrates that both isomers of diazaspiro[3.5]nonadiene 3 can be readily accessed through this one-pot operation. Ynamides bearing triisopropylsilyl (TIPS)- and pivaloyl (Piv)-protected alcohols were well-tolerated under the reaction conditions, furnishing functionalized spiro heterocycles 3m–3n in moderate yields. Further investigation showed that replacing the carbamate with methanesulfonamide afforded the final product 3o with a good overall yield. To further demonstrate the utility of this methodology in the preparation of high complex spiropiperidines, substituted pyridines were incorporated, allowing for the formation of the desired products 3p–3q in good overall yields and thus providing new molecular structures that cannot be accessed using standard methods.
To provide a comprehensive scope, we also tested the effect of various Lewis acids on the dearomatization reaction (Scheme 2). Generally, alkyl chloroformates delivered the final products (3r) with higher efficiency compared to aryl chloroformates (3s), acyl chlorides (3t) or trifluoroacetic anhydride (3u and 3v). However, leveraging this approach allows the formation of diazaspiro[3.5]nonadiene 3 with orthogonally N-protected groups. Regrettably, attempts to trigger the dearomatization reaction with other Lewis acids, such as fluorenylmethoxycarbonyl chloride, were unsuccessful.
 | ||
| Scheme 2 Scope of the Lewis acids. Reactions were carried out on a 0.5 mmol scale. Yields were determined after column chromatography. For detailed experimental procedures, see ESI.† | ||
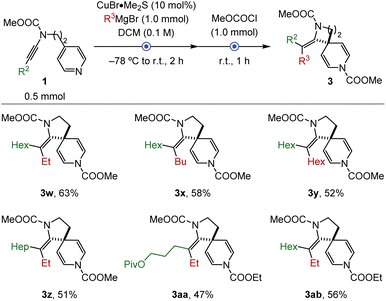
Motivated by these results, we then wondered whether the formation of diazaspiro[4.5]decadiene is feasible following the developed transformation. Suitable starting materials were obtained by adopting the previously mentioned protocol for the C–N coupling reaction.16,20 The resulting ynamides were then subjected to the carbometalation-spirocyclization sequence. To our delight, the addition of EtMgBr to ynamide 1a (R2 = Hex) furnished the desired compound 3w in a good overall yield and as a single isomer (Scheme 3). Several products were also obtained independent of the nature of the Grignard reagents (3x and 3y), the employed ynamides (3z and 3aa), or the acylating reagents (3ab).
 | ||
| Scheme 3 Scope of 2,8-diazaspiro[4.5]decadiene derivatives. Reactions were carried out on a 0.5 mmol scale. Yields were determined after column chromatography. For detailed experimental procedures, see ESI.† | ||
Finally, we demonstrated that the reduction of olefin moieties in dihydropyridine spirocycles 3 can be precisely controlled. Specifically, under partial reduction conditions, piperidine spirocycle 4a was selectively obtained, with remaining alkene functionality suitable for further functionalization. In contrast, extended reaction times facilitated complete hydrogenation, delivering fully saturated piperidine spirocyclic scaffolds 5a and 5b in high yields (Scheme 4). This controllable reduction pathway provides access to both partially and fully reduced spirocyclic frameworks, broadening the utility of the proposed dearomative spirocyclization reaction (Scheme 4).
 | ||
| Scheme 4 Partial and full hydrogenation of spiro dihydropyridine derivatives. Yields were determined after column chromatography. For detailed experimental procedures, see ESI.† | ||
Conclusions
In conclusion, the stereoselective formation of spiropiperidine derivatives can be efficiently achieved through a dearomative spirocyclization reaction. This approach utilizes a chemo-, regio-, and stereoselective carbometalation event followed by a regioselective dearomatization reaction. Notably, this methodology enables the synthesis of diverse diazaspiro heterocycles with varying ring sizes and functional groups, making it a valuable tool in synthetic and medicinal chemistry.Author contributions
Z. N. conceived the project. M. A. designed and conducted the experiments, with assistance from N. E. The manuscript was written with contributions from all authors.Conflicts of interest
There are no conflicts to declare.Data availability
Data for this article, including experimental procedures, and characterization data of all the substrates and products, are available in the ESI of the manuscript.Acknowledgements
We thank the Hebrew University of Jerusalem (Start-up grant), the Neubauer Foundation, and Israel Science Foundation (Grant no. 436/24) for the generous financial support.Notes and references
- (a) D. O'Hagan, Nat. Prod. Rep., 2000, 17, 435 RSC; (b) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257 CrossRef CAS PubMed; (c) R. D. Taylor, M. MacCoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845 CrossRef CAS PubMed; (d) P. Bhutani, G. Joshi, N. Raja, N. Bachhav, P. K. Rajanna, H. Bhutani, A. T. Paul and R. Kumar, J. Med. Chem., 2021, 64, 2339 CrossRef CAS PubMed.
- (a) D. C. Blakemore, L. Castro, I. Churcher, D. C. Rees, A. W. Thomas, D. M. Wilson and A. Wood, Nat. Chem., 2018, 10, 383 CrossRef CAS PubMed; (b) K. R. Campos, P. J. Coleman, J. C. Alvarez, S. D. Dreher, R. M. Garbaccio, N. K. Terrett, R. D. Tillyer, M. D. Truppo and E. R. Parmee, Science, 2019, 363, eaat0805 CrossRef CAS PubMed; (c) M. A. Hardy, B. A. Wright, J. Logan Bachman, T. B. Boit, H. M. S. Haley, R. R. Knapp, R. F. Lusi, T. Okada, V. Tona, N. K. Garg and R. Sarpong, ACS Cent. Sci., 2020, 6, 1017 CrossRef CAS PubMed.
- (a) Y. Zheng, C. M. Tice and S. B. Singh, Bioorg. Med. Chem. Lett., 2014, 24, 3673 CrossRef CAS PubMed; (b) K. Hiesinger, D. Dar’in, E. Proschak and M. Krasavin, J. Med. Chem., 2021, 64, 150 CrossRef CAS PubMed.
- (a) C. M. Marson, Chem. Soc. Rev., 2011, 40, 5514 RSC; (b) K. Acosta-Quiroga, C. Rojas-Peña, L. S. Nerio, M. Gutiérrez and E. Polo-Cuadrado, RSC Adv., 2021, 11, 21926 RSC.
- (a) C. M. Tice, W. Zhao, Z. Xu, S. T. Cacatian, R. D. Simpson, Y.-J. Ye, S. B. Singh, B. M. McKeever, P. Lindblom, J. Guo, P. M. Krosky, B. A. Kruk, J. Berbaum, R. K. Harrison, J. J. Johnson, Y. Bukhtiyarov, R. Panemangalore, B. B. Scott, Y. Zhao, J. G. Bruno, L. Zhuang, G. M. McGeehan, W. He and D. A. Claremon, Bioorg. Med. Chem. Lett., 2010, 20, 881 CrossRef CAS PubMed; (b) Y. Ohtake, T. Sato, T. Kobayashi, M. Nishimoto, N. Taka, K. Takano, K. Yamamoto, M. Ohmori, M. Yamaguchi, K. Takami, S.-Y. Yeu, K.-H. Ahn, H. Matsuoka, K. Morikawa, M. Suzuki, H. Hagita, K. Ozawa, K. Yamaguchi, M. Kato and S. Ikeda, J. Med. Chem., 2012, 55, 7828 CrossRef CAS PubMed; (c) Y. Wang, J. Liu, P. J. Dransfield, L. Zhu, Z. Wang, X. Du, X. Jiao, Y. Su, A.-R. Li, S. P. Brown, A. Kasparian, M. Vimolratana, M. Yu, V. Pattaropong, J. B. Houze, G. Swaminath, T. Tran, K. Nguyen, Q. Guo, J. Zhang, R. Zhuang, F. Li, L. Miao, M. D. Bartberger, T. L. Correll, D. Chow, S. Wong, J. Luo, D. C.-H. Lin and J. C. Medina, ACS Med. Chem. Lett., 2013, 4, 551 CrossRef CAS PubMed; (d) J. S. Scott, F. W. Goldberg and A. V. Turnbull, J. Med. Chem., 2014, 57, 4466 CrossRef CAS PubMed.
- (a) Y. Zhao, S. Yu, W. Sun, L. Liu, J. Lu, D. McEachern, S. Shargary, D. Bernard, X. Li, T. Zhao, P. Zou, D. Sun and S. Wang, J. Med. Chem., 2013, 56, 5553 CrossRef CAS PubMed; (b) A. Bertamino, M. Soprano, S. Musella, M. R. Rusciano, M. Sala, E. Vernieri, V. Di Sarno, A. Limatola, A. Carotenuto, S. Cosconati, P. Grieco, E. Novellino, M. Illario, P. Campiglia and I. Gomez-Monterrey, J. Med. Chem., 2013, 56, 5407 CrossRef CAS PubMed.
- (a) T. A. Dineen, M. M. Weiss, T. Williamson, P. Acton, S. Babu-Khan, M. D. Bartberger, J. Brown, K. Chen, Y. Cheng, M. Citron, M. D. Croghan, R. T. Dunn, J. Esmay, R. F. Graceffa, S. S. Harried, D. Hickman, S. A. Hitchcock, D. B. Horne, H. Huang, R. Imbeah-Ampiah, T. Judd, M. R. Kaller, C. R. Kreiman, D. S. La, V. Li, P. Lopez, S. Louie, H. Monenschein, T. T. Nguyen, L. D. Pennington, T. S. Miguel, E. A. Sickmier, H. M. Vargas, R. C. Wahl, P. H. Wen, D. A. Whittington, S. Wood, Q. Xue, B. H. Yang, V. F. Patel and W. Zhong, J. Med. Chem., 2012, 55, 9025 CrossRef CAS PubMed; (b) M. M. Weiss, T. Williamson, S. Babu-Khan, M. D. Bartberger, J. Brown, K. Chen, Y. Cheng, M. Citron, M. D. Croghan, T. A. Dineen, J. Esmay, R. F. Graceffa, S. S. Harried, D. Hickman, S. A. Hitchcock, D. B. Horne, H. Huang, R. Imbeah-Ampiah, T. Judd, M. R. Kaller, C. R. Kreiman, D. S. La, V. Li, P. Lopez, S. Louie, H. Monenschein, T. T. Nguyen, L. D. Pennington, C. Rattan, T. S. Miguel, E. A. Sickmier, R. C. Wahl, P. H. Wen, S. Wood, Q. Xue, B. H. Yang, V. F. Patel and W. Zhong, J. Med. Chem., 2012, 55, 9009 CrossRef CAS PubMed; (c) J. Yuan, S. Venkatraman, Y. Zheng, B. M. McKeever, L. W. Dillard and S. B. Singh, J. Med. Chem., 2013, 56, 4156 CrossRef CAS PubMed.
- S. Kotha, N. R. Panguluri and R. Ali, Eur. J. Org Chem., 2017, 2017, 5316 CrossRef CAS.
- For selected examples on the preparation of piperidine spirocycles that includes cyclization reactions, see: (a) H. Habashita, M. Kokubo, S.-I. Hamano, N. Hamanaka, M. Toda, S. Shibayama, H. Tada, K. Sagawa, D. Fukushima, K. Maeda and H. Mitsuya, J. Med. Chem., 2006, 49, 4140 CrossRef CAS PubMed; (b) I. D. Jenkins, F. Lacrampe, J. Ripper, L. Alcaraz, P. Van Le, G. Nikolakopoulos, P. De Almeida Leone, R. H. White and R. J. Quinn, J. Org. Chem., 2009, 74, 1304 CrossRef CAS PubMed; (c) D. G. Brown, P. R. Bernstein, A. Griffin, S. Wesolowski, D. Labrecque, M. C. Tremblay, M. Sylvester, R. Mauger, P. D. Edwards, S. R. Throner, J. J. Folmer, J. Cacciola, C. Scott, L. A. Lazor, M. Pourashraf, V. Santhakumar, W. M. Potts, S. Sydserff, P. Giguère, C. Lévesque, M. Dasser and T. Groblewski, J. Med. Chem., 2014, 57, 733 CrossRef CAS PubMed; (d) T. A. King, H. L. Stewart, K. T. Mortensen, A. J. P. North, H. F. Sore and D. R. Spring, Eur. J. Org Chem., 2019, 2019, 5219 CrossRef CAS PubMed.
- (a) P. S. Baran, T. J. Maimone and J. M. Richter, Nature, 2007, 446, 404 CrossRef CAS PubMed; (b) I. S. Young and P. S. Baran, Nat. Chem., 2009, 1, 193 CrossRef CAS; (c) P. A. Wender and B. L. Miller, Nature, 2009, 460, 197 CrossRef CAS PubMed; (d) K. E. Kim, A. N. Kim, C. J. McCormick and B. M. Stoltz, J. Am. Chem. Soc., 2021, 143, 16890 CrossRef CAS PubMed.
- For comprehensive reviews, see: (a) S. P. Roche and J. A. Porco Jr, Angew. Chem., Int. Ed., 2011, 50, 4068 CrossRef CAS; (b) Q. Ding, X. Zhou and R. Fan, Org. Biomol. Chem., 2014, 12, 4807 RSC; (c) W.-T. Wu, L. Zhang and S.-L. You, Chem. Soc. Rev., 2016, 45, 1570 RSC; (d) W. C. Wertjes, E. H. Southgate and D. Sarlah, Chem. Soc. Rev., 2018, 47, 7996 RSC; (e) C. J. Huck and D. Sarlah, Chem, 2020, 6, 1589 CrossRef CAS PubMed.
- G. Arnott, H. Brice, J. Clayden and E. Blaney, Org. Lett., 2008, 10, 3089 CrossRef CAS PubMed.
- J. C. Abell, C. P. Bold, L. Vicens, T. Jentsch, N. Velasco, J. L. Tyler, R. N. Straker, A. Noble and V. K. Aggarwal, Org. Lett., 2023, 25, 400 CrossRef CAS PubMed.
- For selected studies: (a) Q.-F. Wu, H. He, W.-B. Liu and S.-L. You, J. Am. Chem. Soc., 2010, 132, 11418 CrossRef CAS PubMed; (b) X. Zhang, W.-B. Liu, Q.-F. Wu and S.-L. You, Org. Lett., 2013, 15, 3746 CrossRef CAS PubMed; (c) T. D. Montgomery, A. E. Nibbs, Y. Zhu and V. H. Rawal, Org. Lett., 2014, 16, 3480 CrossRef CAS PubMed; (d) Y. Wang, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2017, 56, 15093 CrossRef CAS PubMed; (e) W.-T. Wu, L. Ding, L. Zhang and S.-L. You, Org. Lett., 2020, 22, 1233 CrossRef CAS PubMed; (f) G. Liang, Y. Ji, H. Liu, Y. Pang, B. Zhou, M. Cheng, Y. Liu, B. Lin and Y. Liu, Adv. Synth. Catal., 2020, 362, 192 CrossRef CAS; (g) A. Becker, C. P. Grugel and B. Breit, Org. Lett., 2021, 23, 3788 CrossRef CAS PubMed; (h) S. Ta, S. Ding, D. Li, L. Li, H. Zhao, M. Chai and J. Wang, Chem. Commun., 2021, 57, 10576 RSC; (i) Y. Zhu, S. Yang, E. Pu, L. Li, S. Ye, L. Wei, G. Ma, Y. Zhang, H. Zhang and J. Chen, Org. Lett., 2023, 25, 3533 CrossRef CAS PubMed.
- For recent reviews on the dearomatization of indoles, see: (a) C. Zheng and S.-L. You, ACS Cent. Sci., 2021, 7, 432 CrossRef CAS PubMed; (b) F. Buttard and X. Guinchard, ACS Catal., 2023, 13, 9442 CrossRef CAS.
- Y.-Q. Zhang, Y.-B. Chen, J.-R. Liu, S.-Q. Wu, X.-Y. Fan, Z.-X. Zhang, X. Hong and L.-W. Ye, Nat. Chem., 2021, 13, 1093 CrossRef CAS PubMed.
- (a) M. Hu, D. Ding, W. Desnoo, D. J. Tantillo and Z. Nairoukh, Angew. Chem., Int. Ed., 2023, 62, e202315108 CrossRef CAS PubMed; (b) R. Mondal, M. Agbaria, O. Cohen and Z. Nairoukh, Chem. - Eur. J., 2024, 30, e202303588 CrossRef CAS PubMed.
- J. A. Bull, J. J. Mousseau, G. Pelletier and A. B. Charette, Chem. Rev., 2012, 112, 2642 CrossRef CAS PubMed.
- (a) K. A. DeKorver, H. Li, A. G. Lohse, R. Hayashi, Z. Lu, Y. Zhang and R. P. Hsung, Chem. Rev., 2010, 110, 5064 CrossRef CAS PubMed; (b) G. Evano, A. Coste and K. Jouvin, Angew. Chem., Int. Ed., 2010, 49, 2840 CrossRef CAS PubMed; (c) F.-L. Hong and L.-W. Ye, Acc. Chem. Res., 2020, 53, 2003 CrossRef CAS PubMed; (d) C. C. Lynch, A. Sripada and C. Wolf, Chem. Soc. Rev., 2020, 49, 8543 RSC; (e) Y. -Bo Chen, P.-C. Qian and L.-W. Ye, Chem. Soc. Rev., 2020, 49, 8897 RSC; (f) S. Dutta, R. K. Mallick and A. K. Sahoo, Angew. Chem., Int. Ed., 2023, 62, e202300816 CrossRef CAS PubMed.
- (a) Y. Zhang, R. P. Hsung, M. R. Tracey, K. C. M. Kurtz and E. L. Vera, Org. Lett., 2024, 6, 1151 CrossRef PubMed; (b) P.-Y. Yao, Y. Zhang, R. P. Hsung and K. Zhao, Org. Lett., 2008, 10, 4275 CrossRef CAS PubMed; (c) J. P. Das, H. Chechik and I. Marek, Nat. Chem., 2009, 1, 128 CrossRef CAS PubMed.
- For selected reviews, see: (a) A. Basheer and I. Marek, Beilstein J. Org. Chem., 2010, 6, 77 Search PubMed; (b) Y. Minko, M. Pasco, H. Chechik and I. Marek, Beilstein J. Org. Chem., 2013, 9, 526 CrossRef CAS PubMed; (c) I. Marek, Y. Minko, M. Pasco, T. Mejuch, N. Gilboa, H. Chechik and J. P. Das, J. Am. Chem. Soc., 2014, 136, 2682 CrossRef CAS PubMed; (d) D. S. Mueller and I. Marek, Chem. Soc. Rev., 2016, 45, 4552 RSC.
- For selected recent studies, see: (a) Y. Minko, M. Pasco, L. Lercher, M. Botoshansky and I. Marek, Nature, 2012, 490, 522 CrossRef CAS PubMed; (b) R. Vabre, B. Island, C. J. Diehl, P. R. Schreiner and I. Marek, Angew. Chem., Int. Ed., 2015, 54, 9996 CrossRef CAS PubMed; (c) Z. Nairoukh and I. Marek, Angew. Chem., Int. Ed., 2015, 54, 14393 CrossRef CAS PubMed; (d) P. Starkov, J. T. Moore, D. C. Duquette, B. M. Stoltz and I. Marek, J. Am. Chem. Soc., 2017, 139, 9615 CrossRef CAS PubMed.
- See the ESI† for full optimization results.
- See the ESI† for more details.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc05541a |
| This journal is © The Royal Society of Chemistry 2024 |