Open Access Article
Open Access ArticleGram-scale enzymatic synthesis of 2′-deoxyribonucleoside analogues using nucleoside transglycosylase-2†
Admir
Salihovic
 ab,
Alex
Ascham
ab,
Alex
Ascham
 c,
Andrea
Taladriz-Sender
c,
Andrea
Taladriz-Sender
 ab,
Samantha
Bryson
ab,
Jamie M.
Withers
ab,
Iain J. W.
McKean
ab,
Paul A.
Hoskisson
ab,
Samantha
Bryson
ab,
Jamie M.
Withers
ab,
Iain J. W.
McKean
ab,
Paul A.
Hoskisson
 d,
Gideon
Grogan
d,
Gideon
Grogan
 *c and
Glenn A.
Burley
*c and
Glenn A.
Burley
 *ab
*ab
aDepartment of Pure & Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, UK G1 1XL. E-mail: glenn.burley@strath.ac.uk
bStrathclyde Centre for Molecular Bioscience, University of Strathclyde, UK
cDepartment of Chemistry, University of York, Heslington, York, YO10 5DD, UK. E-mail: gideon.grogan@york.ac.uk
dStrathclyde Institute of Pharmacy & Biomedical Sciences, University of Strathclyde, 161 Cathedral Street, Glasgow, G4 0RE, UK
First published on 27th August 2024
Abstract
Nucleosides are pervasive building blocks that are found throughout nature and used extensively in medicinal chemistry and biotechnology. However, the preparation of base-modified analogues using conventional synthetic methodology poses challenges in scale-up and purification. In this work, an integrated approach involving structural analysis, screening and reaction optimization, is established to prepare 2′-deoxyribonucleoside analogues catalysed by the type II nucleoside 2′-deoxyribosyltransferase from Lactobacillus leichmannii (LlNDT-2). Structural analysis in combination with substrate profiling, identified the constraints on pyrimidine and purine acceptor bases by LlNDT2. A solvent screen identifies pure water as a suitable solvent for the preparation of high value purine and pyrimidine 2′-deoxyribonucleoside analogues on a gram scale under optimized reaction conditions. This approach provides the basis to establish a convergent, step-efficient chemoenzymatic platform for the preparation of high value 2′-deoxyribonucleosides.
Introduction
Nucleosides are essential building blocks used throughout all forms of life.1 In addition, these analogues are used extensively in biotechnology and the pharmaceutical industry, serving as chemical probes when incorporated into oligodeoxyribonucleotides (ODNs) and genomic DNA,2–7 as well as anticancer/antiviral therapeutics.8–11 Underpinning many aspects of their utility is the need to modify the nucleobase of the nucleoside scaffold (1–4, Fig. 1A).12–14 Modifications range from the incorporation of bio-orthogonal reactive handles, modifications to enhance stability of the N-glycosidic linkage,15,16 and the incorporation of isotopes to aid structural characterization.17–20The preparation of nucleobase-modified nucleosides typically requires a multi-step synthetic sequence ranging from the elaboration of the existing purine/pyrimidine nucleobase through to more extensive preparation of analogues via N-glycosylation.21,22 Many of these synthetic steps to prepare 2′-deoxyribonucleosides by chemical synthesis require a series of protection/deprotection sequences, and in the context of N-glycosylation, produce regioisomeric and anomeric mixtures that can be challenging to purify.
Enzymatic synthesis of nucleoside analogues potentially offers a sustainable, cost and step-efficient alternative to purely chemical synthetic approaches. Of the various enzymes used in their biocatalytic synthesis,23 nucleoside phosphorylases (NPs) have been the most prominent (Fig. 1B).24,25 NPs catalyze a ‘nucleobase swap’ where, typically, the nucleobase of a natural nucleoside feedstock is exchanged for a non-natural nucleobase.26,27 Purine NPs show substrate tolerance for a range of purine nucleobases as well as a range of modifications to the ribosugar,25,28 whereas pyrimidine NPs can accept modifications to the pyrimidine nucleobase.29–31
A mechanistic hallmark of NPs is that nucleobase exchange proceeds via a two-step process involving N-glycosidic cleavage of a requisite nucleoside 5 by phosphate, producing pentose-1-phosphate 6 and a nucleobase.32 The formation of 7 then involves nucleophilic displacement of the C1′ phosphate in 6 with a nucleobase analogue, exclusively forming the desired β-anomer (7).
One major challenge of NP-catalysed nucleobase swapping is that the synthesis of the desired nucleoside product (e.g., 7) is in equilibrium with the competitive formation of 6. As such, efforts have focused on developing strategies to mitigate the undesirable reverse reactions by biasing the equilibrium towards formation of the desired nucleoside analogue,28,33 building a feedstock of 6,34 or through the application of flow-based synthetic routes.35–37
An alternative to the use of NPs to prepare nucleoside analogues is to use nucleoside 2′-deoxyribosyltransferases (NDTs).38,39 Whilst these enzymes catalyse the formation of nucleoside analogues via an overall nucleobase exchange akin to NPs, NDTs form a covalent adduct within the active site, thus controlling stereoselective nucleophilic attack of the incoming nucleobase on the β face (Fig. 1C). The mechanistic divergence of NDTs offers potential advantages over NPs as they do not form a phosphate intermediate such as 6.40,41
At present, in-depth knowledge of the substrate promiscuity of NDTs has yet to be established,40,42–46 particularly with respect to the ability of these enzymes to be used for scale-up. Herein, we describe a workflow for the gram-scale preparation of nucleoside analogues catalyzed by a type-2 NDT derived from Lactobacillus leichmannii (LlNDT-2).47–49 Structural studies highlight potential sites where modification of the nucleobase are tolerated. We use this knowledge to map the substrate promiscuity of both purine and pyrimidine substrates. Finally, we use this knowledge to establish sustainable conditions for the cost-effective preparation of prominent nucleoside analogues.
Results and discussion
Solvent tolerance of transglycosylation catalyzed by LlNDT-2
The first phase of establishing an optimization workflow was to survey reaction conditions for the synthesis of non-natural nucleosides (i.e., 7) where the higher value non-natural nucleobase is the limiting reagent. An excess (5 equiv.) of 2′-deoxycytidine (dC, 8, ∼£50 for 250 mg, Merck) was used as the corresponding 2′-deoxyribosyl donor relative to 1 equiv. of a non-natural purine 9 or pyrimidine 10 (Fig. 2A). Transglycosylation catalysed by LlNDT-2 showed remarkable tolerance to a range of organic solvents (20% v/v) and even pure water, producing the desired purine 11 or pyrimidine 12 nucleosides in conversions of up to 98% (11) and 85% (12, Fig. 2B).Based on the solvent screening we explored the further optimization of the synthesis of a high value pyrimidine nucleoside, 3. Nucleoside 3 (EdU, ∼£160 for 50 mg, Merck) is used extensively in cellular proliferation assays,4 as a Raman active reporter,50,51 and as a bio-orthogonal reactive group for the synthesis of bioconjugates.52 When 5 equiv. of 8 were used as the 2′-deoxyribosyl donor, the transformation resulted in 53% conversion to 3 using 13 as the nucleobase (entry 1, Fig. 2C). Increasing the enzyme loading from 2 to 4 μg mL−1 (from a 1 mg mL−1 stock solution), increased the conversion to a maximum of 66% (entries 2–4). Critical to increasing the conversion of 3 was increasing the equivalents of 13 from 1 to 10 whilst maintaining the catalytic loading of LlNDT-2 at 4 μg mL−1 (entries 5 and 6). A further increase to 20 equiv. of 13 resulted in 90% conversion to 3 (entry 8). The scope of these optimized conditions was used to scale up the synthesis of pyrimidine nucleoside analogues modified at the 5-position (Fig. 2D). 5-Modified nucleosides were then prepared on millimole scale to demonstrate the scalability of this biocatalytic reaction. In this way, 3 and 12 were prepared on 1 mmol scale in 52% (132 mg) and 55% (137 mg) yields, respectively. Preparation of 14 and 15 was also demonstrated affording the desired products in 56% (840 mg) and 31% (53 mg), respectively.
Structural basis for superior acceptance of purine nucleobases
A consistent observation that emerged from the solvent screen was that higher conversions resulted when purine 9 was used as the nucleobase ‘acceptor’ relative to pyrimidine 10. Although previous studies have reported lower transglycosylation conversions when pyrimidine nucleobases were used as acceptors,42,53,54 a structural basis for this has not been explored. We surmised that the generally lower conversions to non-natural pyrimidine nucleoside products is due to the reduced residence time of the smaller pyrimidine nucleobase relative to a larger purine analogue in the active site of LlNDT-2, which in turn could lead to competing hydrolysis.38,54–57 Indeed, a recent study of the reaction kinetics of a related NDT derived from Chroococcidiopsis thermalis (CtNDT) revealed preference for purine nucleobase donors, which was largely driven by the KM.58To shed further light on the substrate scope of LlNDT-2, we sought to obtain structures of the enzyme in complex with 2′-deoxyribonucleosides. The structure of WT-LlNDT-2 (EC 2.4.2.6) in complex with the C-nucleoside 5-methyl-2′-deoxypseudouridine (PDB: 1F8Y), which cannot undergo transglycosylation, was previously determined by Ealick and co-workers.39 Although that report also featured details of a complex of LlNDT with 2′-deoxyadenosine, no coordinate file for this complex is available in the PDB, so a reconsideration of complex interactions is timely.
Three datasets were obtained from crystals prepared under different substrate soak conditions. Each structure was obtained in the I213 space group and featured two monomers of LlNDT-2 in the asymmetric unit. We first obtained an apo structure of LlNDT-2, which had not been soaked with substrate ligands, and featured no ligand density in the active sites. The second structure was obtained from crystals soaked with 3 (EdU). Both active sites featured density between the side chains of E98 and D92 in a ‘closed’ conformation of the enzyme corresponding to that observed previously (Fig. 3A).39 This density was modelled as the covalent 2′-deoxyribosyl enzyme complex 16, evidenced by continuous density from the side chain of E98 to the C1′ atom of the deoxyribose sugar. Although crystallographic evidence for this intermediate in DRTase-I Class enzymes has previously been reported,59 the structure of a covalent 2′-deoxyribosyl adduct (Fig. 3B) has not been observed in a Class II enzyme. Additional density adjacent to the C-terminus of Y157 was not sufficiently large to model as a base.
A third structure was obtained by soaking the enzyme with nucleoside 50 (Fig. 3C and 5A). This structure contained one active site in the closed conformation containing 8 (dC). The non-covalent interactions with active site residues in this complex largely align with the previously reported 5-methyl-2′-deoxyuridine complex (PDB: 1F8Y).39 The exocyclic amine of 8 forms a non-covalent interaction with the C-terminus of Y157, whereas the endocyclic N3 forms a hydrogen bond with the side chain of Q46. Finally, hydrogen bond interactions were observed between O2 of 8 and both Q46 and D72 side chains.
The other enzyme monomer was observed in an ‘open’ conformation with the side chain of Q46 displaced because of a movement of the protein backbone between residues L43 and Y58. This active site featured electron density consistent with the covalent 2′-deoxyribosyl intermediate attached via an ester linkage from E98, and also two molecules of the nucleobase 17 in overlapping locations in a purine binding site (Fig. 3D). The purine interactions within the active site side chains are similar to those previously reported in the paper by Ealick et al., however no coordinates are available in the PDB for a thorough comparison.39 For the molecule of 17 situated closest to the 2′-deoxyribosyl adduct, N9 is 3.9 Å from the C1′ of the sugar, to which it would be bonded in an intact nucleoside; the N7 atom is 3.0 Å from the C-terminus of Y157, and N3 is 3.0 Å from the side-chain of D72. Another molecule of 17 is also present in the active site and is accommodated by movement of the Q46 side chain, forming a non-covalent interaction with the bromine atom at C6.
Taken collectively, our structural analyses suggest that the residence time of purine nucleobases within the active site of LlNDT-2 is greater than that observed for pyrimidine nucleobases. This is consistent with previous reaction kinetics analysis of LlNDT-2 where the equilibrium is biased towards complexation of a purine nucleobase within the active site.38,54
Substrate mapping defines the scope of the transglycosylation catalysed by LlNDT-2
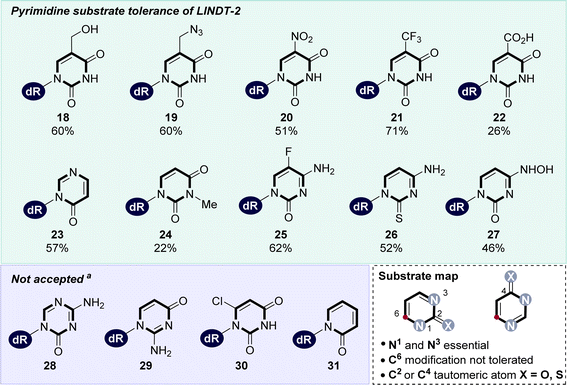
With the structural information in hand, we then sought to construct a detailed molecular map defining the substrate scope of non-natural nucleoside synthesis using the optimised biotransformation conditions as a basis (Fig. 4). Using either 8 (dC) or thymidine as the 2′-deoxyribosyl donor, the scope of nucleobase acceptance was explored using a range of pyrimidine analogues modified at the 5-position (18–22, Fig. 4). Our structures of LlNDT-2 suggest that the active site can accommodate substituents at this position as they project into a space between the side chains of Y157, F13 and N123. Unexpectedly, the lack of substituents in the 4-position (23) and nucleobase modifications to the 2- (26–27), 3- (24) and 4-positions (25–27) were also tolerated. However, an acceptor nucleobase with a substituent at position 6 (30) was not tolerated as this may clash with the side chain of N123. Nucleobases such as triazine (28),60 hydrogen bond donor groups in the 2-position (29) or pyridine (31) were not substrates, confirming that the N3 is essential.61 This is presumably due to the need for the nucleophilic nitrogen to be adjacent to a tautomeric oxygen.61The acceptance of purine nucleobase analogues by LlNDT-2 was far more extensive. Purines with modifications at all carbon positions were tolerated, producing non-natural nucleosides 32–51 with moderate to high conversions (Fig. 5A). Purine acceptors with a range of modifications at both C2 and C6 positions (32–40) were accepted, including precursors for the preparation of 6-thioguanosine (36), a known DNA damage adduct O6-methyl guanosine 37, and those including protecting groups used for solid phase DNA synthesis (e.g., 38).
The active site of LlNDT-2 in complex with the nucleobase 17 revealed that the mobility of the loop bearing Q46 permits the accommodation of a range of modifications at the C2 and C6 positions (e.g., 41–44).
Substrate mapping also identified tolerance for modifications at the N1-position. For example, transglycosylation using 1-deaza acceptors were tolerated (45–47) as well as modifications at the C8 (48–50) and C6 positions (51). Surprisingly, a trifluoromethyl substituent in the C8 position forms nucleoside 49, where N3 is the glycosylated position instead of N9.62 Another unexpected observation was the low conversion in the synthesis of 8-aza nucleoside 50, which was formed with 11% conversion. Nitrogen atoms at both N3 and N7 positions were essential for acceptor recognition, as the corresponding deaza-purine analogues were not transformed to the desired nucleoside products (52–56). This is presumably due to the removal of specific interactions with the C-terminal carboxylate of Y157 and the side chain of D72 respectively.
Having established the broader substrate specificity of LlNDT, we were able to scale up several syntheses of purine nucleoside analogues using our optimised reactions conditions (Fig. 5C). In this way, nucleosides 32, 36, 42, 45 and 51 were prepared on 0.6–6.5 mmol scale, affording products ranging from 90 mg to 1.37 g. Finally, to demonstrate the wider applicability of this enzymatic transglycosylation approach for the preparation of high value nucleoside building blocks, we sought to prepare the 15N-labelled nucleoside 58 starting from 51 (Fig. 5C). 15N-labelled nucleosides are used extensively in structural biology and as chemical probes to explore their enzymatic incorporation into DNA.19,63–66 The incorporation of 15N building blocks bearing non-natural nucleobases typically involve the incorporation of the 15N label, followed by a glycosylation step. Glycosylation is achieved enzymatically using a NP,67 or via a synthetic glycosylation step (e.g., Vorbrüggen)68,69 to prepare the final nucleoside.65 Accordingly, we prepared the novel 15N-labelled nucleoside 58 in two steps starting from 51. Acetyl protection of the hydroxyl groups afforded 57 in 90% yield, which was necessary to solubilise the nucleoside for the Pd-catalysed cross coupling with 15N-labelled benzoyl amide. The cross coupling and acetyl deprotection were performed in one-pot on a 0.3 mmol scale, affording 58 in 45% yield (48 mg).
Conclusions
The promiscuity of LlNDT-2-catalysed transglycosylations using non-natural nucleobases has been demonstrated using either 2′-deoxycytidine (8) or thymidine as the corresponding 2′-deoxyribosyl donor. Structural analysis of LlNDT-2 provided insight into the determinants of acceptor specificity by LlNDT, and thus the generally lower conversion to pyrimidine 2′-deoxyribonucleosides compared with purine analogues using equivalent stoichiometry. These transglycosylation reactions are inherently scalable, thus providing an alternative to the use of NPs, which can suffer from the need to prepare a pentose-1-phosphate (6) to drive the equilibrium to completion.70,71 This work highlights a workflow to prepare a wider range of nucleoside analogues with sugar modifications typically found in, for example, therapeutic oligonucleotides and nucleoside drugs.72–74 Future work will explore the potential to use engineered LlNDT-2 enzymes to enhance substrate scope for acceptors with modifications at the 2′-position44,75 and the subsequent potential to prepare nucleosides on scale.35,53Abbreviations
| LlNDT-2 | Type II nucleoside 2′-deoxyribosyltransferase from Lactobacillus leichmannii |
| NP | Nucleoside phosphorylase |
Data availability
The preparation of compounds and associated raw spectroscopic and HPLC data to confirm identity and purity will be made available through the University of Strathclyde's Pure repository (https://pure.strath.ac.uk). Protein crystallographic data has been uploaded to the PDB.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. Conceptualization was done by A. S., A. T. S., P. A. H., and G. A. B.; methodology was performed by A. S., A. A., S. B., J. M. W., I. J. W. M., G. G.; formal analysis was done by A. S., A. A. and G. G. Manuscript preparation was done by A. S., G. G. and G. A. B.Conflicts of interest
The authors declare no competing financial conflict of interest.Acknowledgements
A. S. and G. A. B. thank the University of Strathclyde and the EPSRC for a PhD studentship. A. A. was funded by a studentship from the BBSRC White Rose DTP in Mechanistic Biology (ref. 2737877). A. T. S. and G. A. B. thank the Biotechnology and Biological Research Council (BBSRC) for support (BB/V017586/1; BB/T000627/1). J. W. and G. A. B. thank the Leverhulme Trust for funding support (RPG-2018-149). P. A. H. acknowledges funding from the Royal Academy of Engineering Research Chair Scheme (RCSRF2021\11\15). A. S. and G. A. B. thank the MVLS Integrated Protein Analysis Facility at the University of Glasgow, for their guidance and advice with protein purification. We also thank Mr Sam Hart and Dr Johan P. Turkenburg for assistance with X-ray data collection, and the Diamond Light Source Didcot UK for access to beamline I03 under proposal mx32736.References
- Y. Wang, S. Zhang, W. Jia, P. Fan, L. Wang, X. Li, J. Chen, Z. Cao, X. Du, Y. Liu, K. Wang, C. Hu, J. Zhang, J. Hu, P. Zhang, H.-Y. Chen and S. Huang, Nat. Nanotechnol., 2022, 17, 976–983 CrossRef CAS PubMed
.
- X. Tang, J. Zhang, J. Sun, Y. Wang, J. Wu and L. Zhang, Org. Biomol. Chem., 2013, 11, 7814–7824 RSC
.
- J. Kuchlyan, L. Martinez-Fernandez, M. Mori, K. Gavvala, S. Ciaco, C. Boudier, L. Richert, P. Didier, Y. Tor, R. Improta and Y. Mély, J. Am. Chem. Soc., 2020, 142, 16999–17014 CrossRef CAS PubMed
.
- A. Salic and T. J. Mitchison, Proc. Natl. Acad. Sci. U.S.A., 2008, 105, 2415–2420 CrossRef CAS PubMed
.
- G. A. Burley, J. Gierlich, M. R. Mofid, H. Nir, S. Tal, Y. Eichen and T. Carell, J. Am. Chem. Soc., 2006, 128, 1398–1399 CrossRef CAS PubMed
.
- V. N. Schreier, M. O. Loehr, E. Lattmann and N. W. Luedtke, ACS Chem. Biol., 2022, 17, 1799–1810 CrossRef CAS PubMed
.
- N. Z. Fantoni, A. H. El-Sagheer and T. Brown, Chem. Rev., 2021, 121, 7122–7154 CrossRef CAS PubMed
.
- S. Westarp, F. Kaspar, P. Neubauer and A. Kurreck, Curr. Opin. Biotechnol., 2022, 78, 102829 CrossRef CAS PubMed
.
- G. Li, T. Yue, P. Zhang, W. Gu, L.-J. Gao and L. Tan, Molecules, 2021, 26, 923 CrossRef CAS PubMed
.
- D. C. Schultz, R. M. Johnson, K. Ayyanathan, J. Miller, K. Whig, B. Kamalia, M. Dittmar, S. Weston, H. L. Hammond, C. Dillen, J. Ardanuy, L. Taylor, J. S. Lee, M. Li, E. Lee, C. Shoffler, C. Petucci, S. Constant, M. Ferrer, C. A. Thaiss, M. B. Frieman and S. Cherry, Nature, 2022, 604, 134–140 CrossRef CAS PubMed
.
- C. M. Galmarini, J. R. Mackey and C. Dumontet, Lancet Oncol., 2002, 3, 415–424 CrossRef CAS PubMed
.
- A. Hottin and A. Marx, Acc. Chem. Res., 2016, 49, 418–427 CrossRef CAS PubMed
.
- A. Omumi, D. G. Beach, M. Baker, W. Gabryelski and R. A. Manderville, J. Am. Chem. Soc., 2011, 133, 42–50 CrossRef CAS PubMed
.
- M. Hocek and M. Fojta, Chem. Soc. Rev., 2011, 40, 5802–5814 RSC
.
- J. Štambaský, M. Hocek and P. Kočovský, Chem. Rev., 2009, 109, 6729–6764 CrossRef PubMed
.
- D. F. Vargas, E. L. Larghi and T. S. Kaufman, ACS Omega, 2021, 6, 19356–19363 CrossRef CAS PubMed
.
- L. T. Olenginski, K. M. Taiwo, R. M. LeBlanc and T. K. Dayie, Molecules, 2021, 26, 5581 CrossRef CAS PubMed
.
- K. Lu, Y.-C. Hsiao, C.-W. Liu, R. Schoeny, R. Gentry and T. B. Starr, Chem. Res. Toxicol., 2022, 35, 7–29 Search PubMed
.
- F. H. T. Nelissen, M. Tessari, S. S. Wijmenga and H. A. Heus, Prog. Nucl. Magn. Reson. Spectrosc., 2016, 96, 89–108 CrossRef CAS PubMed
.
- F. X. Theillet, Chem. Rev., 2022, 122, 9497–9570 CrossRef CAS PubMed
.
- E. K. Davison, D. A. Petrone, M. Meanwell, M. B. Nodwell, S. M. Silverman, L.-C. Campeau and R. Britton, Nat. Protoc., 2022, 17, 2008–2024 CrossRef CAS PubMed
.
- C. Maria and A. P. Rauter, Carbohydr. Res., 2023, 532, 108889 CrossRef CAS PubMed
.
- S. C. Cosgrove and G. J. Miller, Expert Opin. Drug Discovery, 2022, 17, 355–364 CrossRef CAS PubMed
.
- S. Kamel, I. Thiele, P. Neubauer and A. Wagner, Biochim. Biophys. Acta, Proteins Proteomics, 2020, 1868, 140304 CrossRef CAS PubMed
.
- A. Hatano, H. Wakana, N. Terado, A. Kojima, C. Nishioka, Y. Iizuka, T. Imaizumi and S. Uehara, Catal. Sci. Technol., 2019, 9, 5122–5129 RSC
.
- M. D. Erion, J. D. Stoeckler, W. C. Guida and R. L. Walter, Biochemistry, 1997, 36, 11735–11748 CrossRef CAS PubMed
.
- M. J. Pugmire and S. E. Ealick, Structure, 1998, 6, 1467–1479 CrossRef CAS PubMed
.
- F. Kaspar, M. Seeger, S. Westarp, C. Köllmann, A. P. Lehmann, P. Pausch, S. Kemper, P. Neubauer, G. Bange, A. Schallmey, D. B. Werz and A. Kurreck, ACS Catal., 2021, 11, 10830–10835 CrossRef CAS
.
- K. Szeker, X. Zhou, T. Schwab, A. Casanueva, D. Cowan, I. A. Mikhailopulo and P. Neubauer, J. Mol. Catal. B: Enzym., 2012, 84, 27–34 CrossRef CAS
.
- X. Zhou, K. Szeker, B. Janocha, T. Boehme, D. Albrecht, I. A. Mikhailopulo and P. Neubauer, FEBS J., 2013, 280, 1475–1490 CrossRef CAS PubMed
.
- F. Kaspar, P. Neubauer and A. Kurreck, ChemBioChem, 2021, 22, 1385–1390 CrossRef CAS PubMed
.
- T. H. Tahirov, E. Inagaki, N. Ohshima, T. Kitao, C. Kuroishi, Y. Ukita, K. Takio, M. Kobayashi, S. Kuramitsu, S. Yokoyama and M. Miyano, J. Mol. Biol., 2004, 337, 1149–1160 CrossRef CAS PubMed
.
- F. Kaspar, R. T. Giessmann, K. F. Hellendahl, P. Neubauer, A. Wagner and M. Gimpel, ChemBioChem, 2020, 21, 1428–1432 CrossRef CAS PubMed
.
- M. A. Huffman, A. Fryszkowska, O. Alvizo, M. Borra-Garske, K. R. Campos, K. A. Canada, P. N. Devine, D. Duan, J. H. Forstater, S. T. Grosser, H. M. Halsey, G. J. Hughes, J. Jo, L. A. Joyce, J. N. Kolev, J. Liang, K. M. Maloney, B. F. Mann, N. M. Marshall, M. McLaughlin, J. C. Moore, G. S. Murphy, C. C. Nawrat, J. Nazor, S. Novick, N. R. Patel, A. Rodriguez-Granillo, S. A. Robaire, E. C. Sherer, M. D. Truppo, A. M. Whittaker, D. Verma, L. Xiao, Y. Xu and H. Yang, Science, 2019, 366, 1255–1259 CrossRef CAS PubMed
.
- A. I. Benítez-Mateos and F. Paradisi, ChemSusChem, 2022, 15, e202102030 CrossRef PubMed
.
- A. I. Benítez-Mateos, C. Klein, D. Roura Padrosa and F. Paradisi, Catal. Sci. Technol., 2022, 12, 6231–6238 RSC
.
- X. Zhou, K. Szeker, L.-Y. Jiao, M. Oestreich, I. A. Mikhailopulo and P. Neubauer, Adv. Synth. Catal., 2015, 357, 1237–1244 CrossRef CAS
.
- S. A. Short, S. R. Armstrong, S. E. Ealick and D. J. T. Porter, J. Biol. Chem., 1996, 271, 4978–4987 CrossRef CAS PubMed
.
- S. Armstrong, W. J. Cook, S. A. Short and S. E. Ealick, Structure, 1996, 4, 97–107 CrossRef CAS PubMed
.
- J. Del Arco, J. Acosta and J. Fernández-Lucas, Biotechnol. Adv., 2021, 51, 107701 CrossRef CAS PubMed
.
- J. del Arco, A. Perona, L. González, J. Fernández-Lucas, F. Gago and P. A. Sánchez-Murcia, Org. Biomol. Chem., 2019, 17, 7891–7899 RSC
.
- E. Pérez, P. A. Sánchez-Murcia, J. Jordaan, M. D. Blanco, J. M. Mancheño, F. Gago and J. Fernández-Lucas, ChemCatChem, 2018, 10, 4406–4416 CrossRef
.
- J. Li, L. Yu, J. Li, L. Xie, R. Zhang and H. Wang, J. Biosci. Bioeng., 2019, 128, 22–27 CrossRef CAS PubMed
.
- Y.-J. Yoo, K.-H. Choi, B.-K. Kim, S.-S. Choi and E.-S. Kim, J. Microbiol. Biotechnol., 2022, 32, 1041–1046 CrossRef CAS PubMed
.
- J. Acosta, J. Del Arco, V. Pisabarro, F. Gago and J. Fernández-Lucas, Front. Bioeng. Biotechnol., 2020, 8, 593 CrossRef PubMed
.
- A. Fresco-Taboada, I. de la Mata, M. Arroyo and J. Fernández-Lucas, Appl. Microbiol. Biotechnol., 2013, 97, 3773–3785 CrossRef CAS PubMed
.
- S. Vichier-Guerre, T. C. Ku, S. Pochet and K. L. Seley-Radtke, ChemBioChem, 2020, 21, 1412–1417 CrossRef CAS PubMed
.
- S. Vichier-Guerre, L. Dugue, F. Bonhomme and S. Pochet, Org. Biomol. Chem., 2016, 14, 3638–3653 RSC
.
- S. Pochet, L. Dugué, A. Meier and P. Marlière, Bioorg. Med. Chem. Lett., 1995, 5, 1679–1684 CrossRef CAS
.
- S. Hong, T. Chen, Y. Zhu, A. Li, Y. Huang and X. Chen, Angew. Chem., Int. Ed., 2014, 53, 5827–5831 CrossRef CAS PubMed
.
- H. Yamakoshi, K. Dodo, A. Palonpon, J. Ando, K. Fujita, S. Kawata and M. Sodeoka, J. Am. Chem. Soc., 2012, 134, 20681–20689 CrossRef CAS PubMed
.
- J. Gierlich, K. Gutsmiedl, P. M. E. Gramlich, A. Schmidt, G. A. Burley and T. Carell, Chem.–Eur. J., 2007, 13, 9486–9494 CrossRef CAS PubMed
.
- F. Rinaldi, J. Fernández-Lucas, D. de la Fuente, C. Zheng, T. Bavaro, B. Peters, G. Massolini, F. Annunziata, P. Conti, I. de la Mata, M. Terreni and E. Calleri, Bioresour. Technol., 2020, 307, 123258 CrossRef CAS PubMed
.
- D. J. T. Porter and S. A. Short, J. Biol. Chem., 1995, 270, 15557–15562 CrossRef CAS PubMed
.
- J. Fernández-Lucas, L. A. Condezo, F. Martinez-Lagos and J. V. Sinisterra, Enzyme Microb. Technol., 2007, 40, 1147–1155 CrossRef
.
- M. Smar, S. A. Short and R. Wolfenden, Biochemistry, 1991, 30, 7908–7912 CrossRef CAS PubMed
.
- J. Acosta, E. Pérez, P. A. Sánchez-Murcia, C. Fillat and J. Fernández-Lucas, Biomolecules, 2021, 11, 120 CrossRef CAS PubMed
.
- P. Tang, C. J. Harding, A. L. Dickson, R. G. da Silva, D. J. Harrison and C. M. Czekster, ACS Catal., 2024, 14, 3090–3102 CrossRef CAS PubMed
.
- R. Anand, P. A. Kaminski and S. E. Ealick, Biochemistry, 2004, 43, 2384–2393 CrossRef CAS PubMed
.
- M. A. Konkina, M. S. Drenichev, D. I. Nasyrova, Y. B. Porozov and C. S. Alexeev, Sustainable Chem. Pharm., 2023, 32, 101011 CrossRef CAS
.
- R. Cardinaud and J. Holguin, Biochim. Biophys. Acta, 1979, 568, 339–347 CrossRef CAS PubMed
.
- M.-C. Huang, J. A. Montgomery, M. C. Thorpe, E. L. Stewart, J. A. Secrist and R. L. Blakley, Arch. Biochem. Biophys., 1983, 222, 133–144 CrossRef CAS PubMed
.
- G. Kupferschmitt, J. Schmidt, T. Schmidt, B. Fera, F. Buck and H. Riiterjans, Nucleic Acids Res., 1987, 15, 6225–6241 CrossRef CAS PubMed
.
- M. Terrazas, X. Ariza, J. Farràs, J. M. Guisado-Yang and J. Vilarrasa, J. Org. Chem., 2004, 69, 5473–5475 CrossRef CAS PubMed
.
- I. M. Lagoja and P. Herdewijn, Synthesis, 2002, 301–314 CrossRef CAS
.
- F. R. Traube, S. Schiffers, K. Iwan, S. Kellner, F. Spada, M. Müller and T. Carell, Nat. Protoc., 2019, 14, 283–312 CrossRef CAS PubMed
.
- J.-L. Abad, B. L. Gaffney and R. A. Jones, J. Org. Chem., 1999, 64, 6575–6582 CrossRef CAS PubMed
.
- T. K. Dayie, L. T. Olenginski and K. M. Taiwo, Chem. Rev., 2022, 122, 9357–9394 CrossRef CAS PubMed
.
- J. P. Henschke, X. Zhang, X. Huang, L. Mei, G. Chu, K. Hu, Q. Wang, G. Zhu, M. Wu, C. Kuo and Y. Chen, Org. Process Res. Dev., 2013, 17, 1419–1429 CrossRef CAS
.
- F. Kaspar, F. Brandt, S. Westarp, L. Eilert, S. Kemper, A. Kurreck, P. Neubauer, C. R. Jacob and A. Schallmey, Angew. Chem., Int. Ed., 2023, 62, e202218492 CrossRef CAS PubMed
.
- F. Kaspar, R. T. Giessmann, P. Neubauer, A. Wagner and M. Gimpel, Adv. Synth. Catal., 2020, 362, 867–876 CrossRef CAS
.
- K. J. D. Van Giesen, M. J. Thompson, Q. Meng and S. L. Lovelock, JACS Au, 2023, 3, 13–24 CrossRef CAS PubMed
.
- A. J. Burke, W. R. Birmingham, Y. Zhuo, T. W. Thorpe, B. Z. da Costa, R. Crawshaw, I. Rowles, J. D. Finnigan, C. Young, G. M. Holgate, M. P. Muldowney, S. J. Charnock, S. L. Lovelock, N. J. Turner and A. P. Green, J. Am. Chem. Soc., 2022, 144, 3761–3765 CrossRef CAS PubMed
.
- E. R. Moody, R. Obexer, F. Nickl, R. Spiess and S. L. Lovelock, Science, 2023, 380, 1150–1154 CrossRef CAS PubMed
.
- M. Willmott, W. Finnigan, W. R. Birmingham, R. Heath, S. Derrington, C. Schnepel, M. A. Hayes, P. D. Smith, F. Falcioni and N. J. Turner, ChemRxiv, 2023, preprint, DOI:10.26434/chemrxiv-2023-26t6z.
- T. Wenzel and F. Seela, Helv. Chim. Acta, 1996, 79, 169–178 CrossRef CAS
.
- S. Dey, C. L. Rühl and A. Jäschke, Chem.–Eur. J., 2017, 23, 12162–12170 CrossRef CAS PubMed
.
- F. Yang, Y. Zhu and B. Yu, Chem. Commun., 2012, 48, 7097–7099 RSC
.
- T. S. Rao, R. F. Rando, J. H. Huffman and G. R. Revankar, Nucleosides Nucleotides, 1995, 14, 1997–2008 CrossRef CAS
.
- W. Kabsch, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2010, 66, 133–144 CrossRef CAS PubMed
.
- P. Evans, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2006, 62, 72–82 CrossRef PubMed
.
- G. Winter, J. Appl. Crystallogr., 2010, 43, 186–190 CrossRef CAS
.
- P. Emsley and K. Cowtan, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2004, 60, 2126–2132 CrossRef PubMed
.
- G. N. Murshudov, A. A. Vagin and E. J. Dodson, Acta Crystallogr., Sect. D: Biol. Crystallogr., 1997, 53, 240–255 CrossRef CAS PubMed
.
- X. Xu, S. Yan, J. Hu, P. Guo, L. Wei, X. Weng and X. Zhou, Tetrahedron, 2013, 69, 9870–9874 CrossRef CAS
.
- S. Fernández-García, V. O. Chantzakou and F. Juliá-Hernández, Angew. Chem., Int. Ed., 2024, 63, e202311984 CrossRef PubMed
.
- D. Tauraitė, J. Jakubovska, J. Dabužinskaitė, M. Bratchikov and R. Meškys, Molecules, 2017, 22, 672 CrossRef PubMed
.
- K. Kraszewska, I. Kaczyńska, S. Jankowski, J. Karolak-Wojciechowska and E. Sochacka, Bioorg. Med. Chem., 2011, 19, 2443–2449 CrossRef CAS PubMed
.
- X.-Y. Jin, Y.-M. He, T.-H. Hui, L. Liu and L. Cheng, J. Org. Chem., 2024, 89, 3597–3604 CrossRef CAS PubMed
.
- M. J. Robins, M. MacCoss, S. R. Naik and G. Ramani, J. Am. Chem. Soc., 1976, 98, 7381–7389 CrossRef CAS PubMed
.
- T. Suzuki, A. Ozawa-Tamura, M. Takeuchi and Y. Sasabe, Chem. Pharm. Bull., 2021, 69, 1067–1074 CrossRef CAS PubMed
.
- C. H. Hwang, J. S. Park, J. H. Won, J. N. Kim and E. K. Ryu, Arch. Pharmacal Res., 1992, 15, 69–72 CrossRef CAS
.
- J. von Watzdorf and A. Marx, ChemBioChem, 2016, 17, 1532–1540 CrossRef CAS PubMed
.
- Z. Janeba, X. Lin and M. J. Robins, Nucleosides, Nucleotides Nucleic Acids, 2004, 23, 137–147 CrossRef CAS PubMed
.
- R. Xia, L.-S. Chen, S.-H. Xu, C. Xia and L.-P. Sun, Nucleosides, Nucleotides Nucleic Acids, 2022, 41, 593–604 CrossRef CAS PubMed
.
- P. Leonczak, P. Srivastava, O. Bande, G. Schepers, E. Lescrinier and P. Herdewijn, J. Org. Chem., 2019, 84, 13394–13409 CrossRef CAS PubMed
.
- M. J. Uddin, M. I. Schulte, L. Maddukuri, J. Harp and L. J. Marnett, Nucleosides, Nucleotides Nucleic Acids, 2010, 29, 831–840 CrossRef CAS PubMed
.
- H. Challa and T. C. Bruice, Bioorg. Med. Chem., 2004, 12, 1475–1481 CrossRef CAS PubMed
.
- H. Yehia, S. Westarp, V. Röhrs, F. Kaspar, R. T. Giessmann, H. F. T. Klare, K. Paulick, P. Neubauer, J. Kurreck and A. Wagner, Molecules, 2020, 25, 934 CrossRef CAS PubMed
.
- F. Seela, Y. Chen, U. Bindig and Z. Kazimierczuk, Helv. Chim. Acta, 1994, 77, 194–202 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: The authors have cited additional references within the ESI.39,76–100 Metadata of this work is deposited on https://pure.strath.ac.uk. See DOI: https://doi.org/10.1039/d4sc04938a |
| This journal is © The Royal Society of Chemistry 2024 |



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: