Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
π-Extended ligands with dual-binding behavior: hindered rotation unlocks unexpected reactivity in cyclometalated Pt complexes†
Seiya
Ota
 a,
Miguel A.
Soto
a,
Miguel A.
Soto
 *a,
Brian O.
Patrick
*a,
Brian O.
Patrick
 a,
Saeid
Kamal
a,
Saeid
Kamal
 a,
Francesco
Lelj
a,
Francesco
Lelj
 b and
Mark J.
MacLachlan
b and
Mark J.
MacLachlan
 *acd
*acd
aDepartment of Chemistry University of British Columbia, 2036 Main Mall, Vancouver, V6T 1Z1, Canada. E-mail: msoto@chem.ubc.ca; mmaclach@chem.ubc.ca
bLa.M.I.and LaSCAMM INSTM Sezione Basilicata, Dipartiento di Scienze, Università della Basilicata, via dell'Ateneo Lucano 10, Potenza, 85100, Italy
cStewart Blusson Quantum Matter Institute University of British Columbia, 2355 East Mall, Vancouver, BC V6T 1Z4, Canada
dWPI Nano Life Science Institute Kanazawa University, Kanazawa, 920-1192, Japan
First published on 11th September 2024
Abstract
Cyclometalated platinum complexes play a crucial role in catalysis, bioimaging, and optoelectronics. Phenylpyridines are widespread cyclometalating ligands that generate stable and highly emissive Pt complexes. While it is common practice to modify these ligands to fine-tune their photophysical properties, the incorporation of polycyclic aromatic hydrocarbons into the ligand's structure has been largely overlooked. This report describes the cyclometalation of naphthalenyl- and anthracenylpyridine ligands, which has resulted in ten new luminescent PtII and PtIV complexes. These species are enabled by a dual-binding behavior discovered in our polyaromatic-containing ligands. The introduction of naphthalenyl and anthracenyl groups unlocks dual binding modes, with the Pt center bonding to either of two distant carbon atoms within the ligand. These complexes exhibit both symmetric structures with two 5-membered metallacycles and asymmetric structures with 5- and 6-membered metallacycles. This work presents a strategy for the regioselective synthesis of Pt complexes with bespoke structures and photophysical properties. Our findings offer new opportunities in platinum chemistry and beyond, with potential implications for materials and technologies.
Introduction
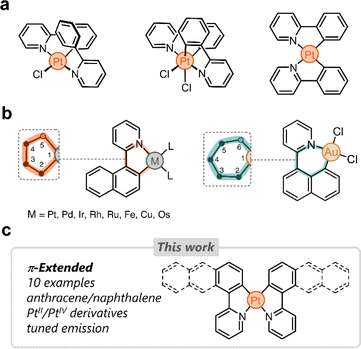
Cyclometalated platinum complexes have been the subject of extensive research in the last few decades due to their unique photophysical properties, reactivity, and stability.1–4 Many cyclometalating ligands have been reported in the literature, with arylpyridines5–7 and bis(aryl)pyridines7,8 being among the most studied. These structurally tailorable ligands are easy to access and once bound to Pt impart unique properties for various applications, including bioimaging,9–11 optoelectronics,12,13 sensing,14,15 and catalysis.16,17A number of cyclometalated Pt species reported to date rely on phenylpyridine-based ligands.8,18–30 The simplest examples of this family consist of 2-phenylpyridine and PtII or PtIV centers (Fig. 1a). Several research groups have tailored these systems in different directions to fine-tune the photophysical properties of the resulting complexes.31–33 Our group has been particularly inspired by these and other examples to incorporate phenylpyridine-based complexes in supramolecular assemblies,34 focusing primarily on (i) bridging phenylpyridines with a single oxygen atom,35 (ii) appending ring molecules to phenylpyridines,36,37 and (iii) incorporating flexible linkers to shape Pt-bridged macrocycles.38
Despite the growing interest in phenylpyridine ligands, studies on derivatives containing π-extended polyaromatics are scarce. Specifically, only 2-(naphthalen-1-yl)pyridine has been investigated in the cyclometalation of Pt,39 Pd,40 Ir,41 Rh,42 Ru,43 Fe,44 Cu,45 and Os.46 In these examples, the ligand binds to the metal via the pyridine's nitrogen donor and through a carbon atom from the naphthalenyl group (2-position, C2). This results in the formation of 5-membered metallacycles (Fig. 1b).47 When the same ligand forms a complex with Au, the naphthalenyl unit binds to the metal through the carbon at position 8 (C8), yielding a 6-membered metallacycle (Fig. 1b). This regioselective behavior has been observed only in heteroleptic Au complexes to date.48 We hypothesized that the dual binding behavior that the naphthalenyl motif displays could be exploited in other polyaromatics appended to a pyridine donor. This could uncover new directions in chemical reactivity as well as enable tailoring of the emission in the resulting Pt complexes.
Here we investigate the cyclometalation of phenyl-, naphthalenyl-, and anthracenylpyridine ligands (Fig. 1c). By exploiting the dual binding behavior of naphthalenyl and anthracenyl groups, we have isolated ten new cyclometalated complexes, with PtII and PtIV centers. These display tunable photoluminescence from blue to infrared light. All complexes have been characterized and analyzed through 1-D and 2-D nuclear magnetic resonance (NMR) spectroscopy, UV-vis spectroscopy, fluorescence spectroscopy, high-resolution mass spectrometry (HRMS), density-functional theory (DFT), and single-crystal X-ray diffraction (SCXRD).
Results and discussion
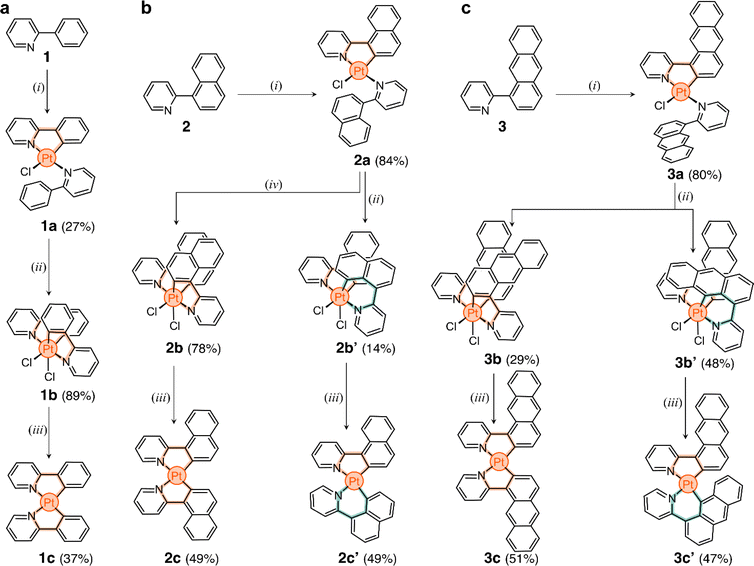
The three target ligands are shown in Scheme 1a–c. Compound 1 (2-phenylpyridine) was purchased and used without purification. Ligand 2-(naphthalen-1-yl)pyridine (2) was synthesized through the Suzuki cross-coupling of naphthalen-1-ylboronic acid and 2-bromopyridine (91% yield).49 2-(Anthracen-1-yl)pyridine (3) was obtained in three steps. First, 1-aminoanthracene-9,10-dione was converted to 1-bromoanthracene via a Sandmeyer reaction, followed by reduction.50 The product was borylated via a Miyaura borylation,51 and then coupled with 2-bromopyridine to obtain ligand 3 in 35% overall yield (full details in ESI†).In the direct metalation of 2-phenylpyridine, the ligand (1) is typically reacted with K2[PtCl4] in polar media, leading to the formation of the PtII square planar complex 1a (Scheme 1a).20 This compound has one cyclometalated phenylpyridine (forming a 5-membered metallacycle) and another one only N-coordinated, with a free phenyl ring. The coordination sphere of the metal center is completed by a Cl− ion. With this in mind, we tested the direct metalation of ligands 2 and 3, anticipating that their reactivity would be similar to 1.
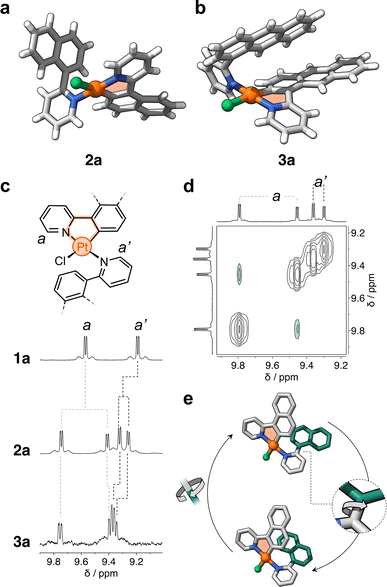
After metalation, complexes 2a and 3a (Scheme 1b and c) were isolated and their structures were unequivocally assigned by SCXRD. Both molecules have one cyclometalated ligand and another one coordinated only through the pyridine unit (Fig. 2a). Crystals of 2a have two molecules in the asymmetric unit, both with the cyclometalated and free naphthalenyl groups facing opposite directions. Similarly, 3a has two molecules in the asymmetric unit, one that has a similar geometry to 2a and a second one with the free and cyclometalated anthracenyl groups facing in the same direction, with an interplanar distance averaging 5.75 Å and a 48° deviation from coplanarity (see Fig. 2b).
Complexes 2a and 3a were characterized in solution using 1H NMR spectroscopy; the recorded spectra were more intriguing than expected (full data in Fig. S1†). For comparison, complex 1a displays distinct signals for each of its phenylpyridine units,20 with resonances attributed to protons a and a′ at 9.6 and 9.2 ppm (Fig. 2c), respectively. However, for 2a and 3a, each of these resonances split into two signals (e.g., d = 9.6, 9.3, 9.23 and 9.17 ppm for 2a, Fig. 2c). Exchange spectroscopy (EXSY) NMR of 2a and 3a confirmed that the two sets of protons undergo exchange at room temperature (Fig. 2d and S2†). This suggested that in solution two atropisomers may interconvert through slow rotation of the unbound aryl units (naphthalenyl and anthracenyl) in 2a and 3a.
The initial support for this hypothesis comes from the solid-state structure of 3a, which revealed two distinct atropisomers in the crystal lattice (Fig. S3†). Subsequent investigation using variable-temperature 1H NMR spectroscopy showed that the exchanging resonances for both 2a and 3a broaden as the temperature exceeds 90 °C (see Fig. S4 and S5†). DFT analysis showed that the interconversion is likely due to slow rotation about the C–C bond that connects the unbound aryl groups with the pyridine unit (Fig. 2e and ESI†). The computed activation barriers are 19.2 and 20.4 kcal mol−1 for 2a and 3a, respectively. These values are in good agreement with those obtained experimentally by 1H–1H EXSY NMR spectroscopy, which correspond to 18.4 (2a) and 19.1 kcal mol−1 (3a) in DCM-d2 at 25 °C (Fig. S6 and Table S1†). In contrast, the rotation along the same C–C bond in 1a (containing a phenyl ring) occurs with a much lower energy barrier (7.7 kcal mol−1, see ESI†) – this explains the observation of only one species by 1H NMR spectroscopy. It is noteworthy that the rotation about the N–Pt bond may have a negligible effect on the exchange process due to its higher activation barrier, estimated to range from 26 to 33 kcal mol−1 in 1a, 2a and 3a (see ESI, Fig. S7, S8 and Table S2†).
The unexpected slow interconversion of rotamers raised the question of whether this behavior would influence the reactivity of 2a and 3a towards oxidation (Scheme 1b and c). Typically, treating compound 1a with PhICl2 leads to oxidation of the metal center (PtII to PtIV) and cyclometalation of the free phenyl ring, generating compound 1b (Scheme 1a).22 A similar outcome was expected from 2a and 3a, and thus their oxidations were tested.
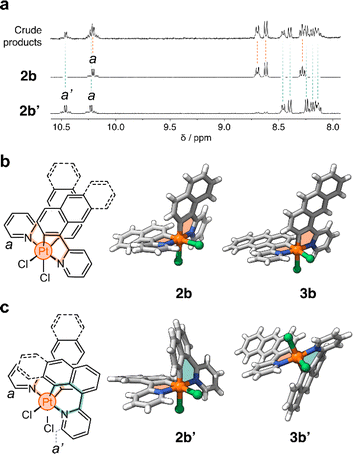
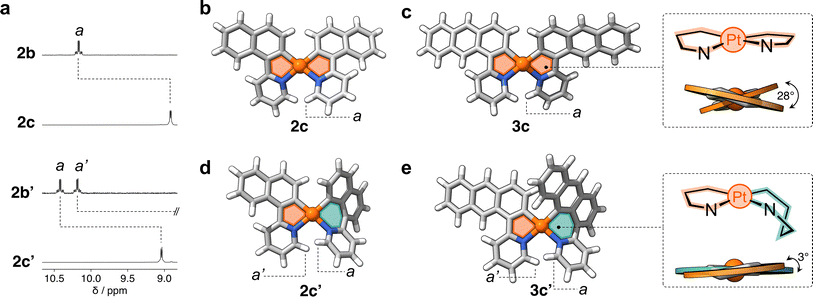
In a representative experiment, a solution of 2a in DCM-d2 was treated with 1 equiv of PhICl2 at room temperature, and the resulting mixture was analyzed by 1H NMR spectroscopy (Fig. S9†). Upon addition of the oxidant, all resonances corresponding to 2a disappeared, confirming its conversion into a new species. Based on the precedent of converting 1a to 1b, it was anticipated that the product would also have C2 symmetry. However, the recorded 1H NMR spectrum showed many resonances (Fig. 3a), suggesting the formation of more than one product. Taking advantage of subtle solubility differences between the products, a new compound, 2b′ (Scheme 1b), was first isolated in 14% yield. HRMS confirmed that 2b′ contains a Pt center and two deprotonated cyclometalating ligands ([2b′ − Cl]+, m/z (exp.) = 638.0953 Da, m/z (calc.) = 638.0963 Da). The 1H NMR spectrum of 2b′ showed individual sets of resonances for each naphthalenylpyridine unit, with the α-pyridine protons a and a′ appearing at 10.2 and 10.0 ppm (Fig. 3a). The solid-state structure (SCXRD) of 2b′ proved that each ligand binds to Pt with a distinct coordination mode. As shown in Fig. 3c, one of the ligands coordinates to the Pt center via the C2 naphthalenyl carbon atom, forming a 5-membered metallacycle. The second ligand cyclometalates to Pt generating a 6-membered ring via C8-ligation to the naphthalenyl group.
We were unable to isolate the anticipated product 2b (Scheme 1b) in sufficient purity. DFT calculations performed on both 2b and 2b′ revealed that 2b is thermodynamically more stable than 2b′ by 3–4 kcal mol−1 (see ESI, Table S3 and S4†). Considering this, 2a was treated with PhICl2 in 1,1,2,2-tetrachloroethane at 130 °C. A 1H NMR spectrum of the isolated product showed only one set of signals (see, e.g., proton a at 10.1 ppm, Fig. 3a), indicating the selective formation of 2b. This compound (78% yield) was analyzed by SCXRD (Fig. 3b), confirming that it contains a PtIV center coordinated to two Cl− ions and two cyclometalating ligands. Similar to 1b,222b also contains two ligands forming 5-membered metallacycles (C2-ligated).
Oxidation of the anthracene-based compound 3a was also tested, resulting in the detection of two products in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio in the crude reaction product (Fig. S10†). Compounds 3b and 3b′ (Scheme 1c) were obtained in 29% and 48% yield, respectively, after purification. Notably, we were able to purify 3b and 3b′ by silica gel column chromatography, in contrast to the more challenging purification of 2b and 2b′. SCXRD analysis confirmed that the two complexes have analogous structures to 2b and 2b′ (see Fig. 3b and c). Compound 3b′ has 5- and 6-membered metallacycles, with the PtIV center bound to the C2 and C9 carbon atoms of the anthracenyl units, respectively, while 3b has two 5-membered metallacycles (C2-bound).
2 ratio in the crude reaction product (Fig. S10†). Compounds 3b and 3b′ (Scheme 1c) were obtained in 29% and 48% yield, respectively, after purification. Notably, we were able to purify 3b and 3b′ by silica gel column chromatography, in contrast to the more challenging purification of 2b and 2b′. SCXRD analysis confirmed that the two complexes have analogous structures to 2b and 2b′ (see Fig. 3b and c). Compound 3b′ has 5- and 6-membered metallacycles, with the PtIV center bound to the C2 and C9 carbon atoms of the anthracenyl units, respectively, while 3b has two 5-membered metallacycles (C2-bound).
We envisioned that the unexpected reactivity of 2a and 3a, enabled by the dual-binding behavior of anthracenyl and naphthalenyl groups, would serve as a unique handle to trap other unpredicted cyclometalated species, especially in the form of PtII complexes. For this, reduction of the four PtIV species 2b, 2b′, 3b, and 3b′ was tested (Scheme 1b and c). All compounds were heated at 60 °C with 20 equiv of t-BuOK in THF under a N2 atmosphere.52 The reaction mixtures were purified using column chromatography and the expected products were isolated in yields of 47% to 51% (details in ESI†).
Fig. 4a shows the partial 1H NMR spectra of compounds 2c and 2c′ (full data in ESI†). In comparison to their precursors, 2b and 2b′, some of the resonances (e.g., a) show an upfield shift (Δδa = −1.3 ppm and −1.4 ppm, respectively), indicating the successful reduction of PtIV to the less electrophilic PtII center. The 1H NMR spectrum of 2c′ contains twice the number of resonances as its analogue 2c. This decreased symmetry is also observed in the 13C{1H} NMR spectrum, with thirty 13C signals detected for 2c′ and only fifteen for 2c (Fig. S11†). Similarly, the 1H NMR spectrum of 3c′ showed twice as many resonances as that of its analogue 3c, clearly indicating a significant reduction in symmetry. This observation suggests a more complex and less symmetrical environment in 3c′, which is also confirmed by the 38 resonances observed in the 13C{1H} NMR spectrum of this complex compared to the 19 signals observed for compound 3c (see Fig. S12†).
The molecular structures of complexes 2c and 3c were determined by SCXRD (Fig. 4b and c). Each molecule has two flat 5-membered metallacycles, although with noticeable deviation from the ideal square planar geometry of the Pt center. Specifically, the dihedral angles (θ) are 26° and 28° for 2c and 3c, respectively. This deviation, which has been observed in previous studies,19 originates from steric repulsion between hydrogen atoms on the ligand (e.g. a in Fig. 4b and c). Unfortunately, attempts to obtain single crystals of 2c′ and 3c′ failed, so insights into their structures were gained through DFT computations (see ESI, Table S5†). Complexes 2c′ and 3c′ show minimal deviation from first coordination square planar geometry, with θ of 5° and 3°, respectively (Fig. 4d and e). This distortion is attributed to the boat-like conformation adopted by the six-membered metallacycles, where the aryl and pyridine rings deviate from the plane of the coordination sphere. This deviation effectively mitigates the steric repulsion between adjacent hydrogen atoms in complexes 2c and 3c.
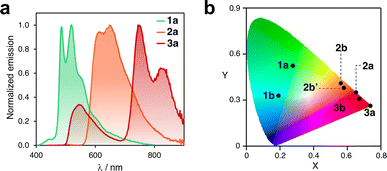
After isolating ten different complexes from ligands 2 and 3, we investigated their emission properties and compared them with those of 1a, 1b, and 1c,8,19,20 (Fig. S13 to S16†). All complexes except for 1c exhibited long emission lifetimes (up to 15 μs) in solution and relatively low photoluminescent quantum yields (Φ). Compound 2a had the highest Φ (2.3%, Table S6†).
In addition, the collected data shed light on several important aspects. First, the compounds exhibit a concomitant redshift of their absorption and emission bands as the ligand's π-conjugation extends from phenyl (1) to naphthalenyl (2) to anthracenyl (3). For instance, in solution, compounds 1a, 2a, and 3a emit green, orange, and red-to-infrared light, respectively (see Fig. 5a). Related complexes have been previously reported in the literature to undergo photoinduced isomerization.30 However, the consistency of the UV-vis absorption and excitation spectra indicated that this is not the case for the compounds investigated here. It should be noted that the excitation spectra for some complexes were not recorded owing to their very weak emission (see Fig. S15†). The CIE plot in Fig. 5b illustrates the significant differences in emission that can be achieved within this family of compact emitters. Additionally, the sets of isomers, such as 2b/2b′ and 3b/3b′, show similar photophysical properties. The emission bands of 2c and 2c′ differ in a range of 10 to 42 nm in solution, in a glassy matrix, and in the solid state (Fig. S17†), indicating that subtle structural differences can fine-tune emission properties. Finally, for some complexes, luminescence is activated by switching from phenyl to naphthalenyl or anthracenyl. Although compound 1c is not luminescent in solution, the analogous complexes formed with ligands 2 and 3 are emissive. This may be due to the significant deviation from planarity that 1c (θ > 80°) shows in the excited state, which promotes non-radiative decay.53,54 However, this phenomenon may be reduced in complexes 2c, 2c′, 3c, and 3c′ with bulkier ligands. Altogether, these observations indicate potential areas for further exploration, such as customizing the emission properties of cyclometalated Pt complexes by expanding to other polyaromatics. In addition, it may be beneficial to explore the circularly polarized emission of the complexes 2c′ and 3c′, and other parent compounds, following chiral resolution.
Conclusions
In summary, our exploration of cyclometalated platinum complexes bearing compact, π-extended ligands has led to significant findings. The hindered rotation around the aryl–pyridine bond led to the discovery of unique rotamers, which had a pivotal impact on both structural and reactivity aspects. In particular, selective oxidation yielded PtIV complexes with symmetric and asymmetric structures, providing valuable insights into their reactivity and isolation. Our synthesized complexes show promising photophysical properties, particularly in the near-infrared region, which makes them suitable for use in bioimaging and optical sensors. This study advances the understanding of Pt complexes with extended π-conjugation, providing opportunities for future research and applications.Data availability
All experimental procedures, mechanistic investigations, characterisation data, NMR spectra and computational data can be found in the article or in the ESI.† Crystallographic data for compounds analyzed by SCXRD have been deposited at the CCDC under deposition numbers CCDC Deposition Numbers: 2357023 (2b′), 2357024 (2b), 2357025 (3b), 2357026 (2c), 2357027 (3c), 2357028 (3a), 2357029 (2a), and 2357030 (3b′), and can be obtained from https://www.ccdc.cam.ac.uk/Author contributions
M. J. MacLachlan, M. A. Soto and S. Ota designed the research. S. Ota conducted the experiments. S. Ota, M. A. Soto, B. O. Patrick and S. Kamal analyzed the data. F. Lelj performed DFT calculations. M. A. Soto and S. Ota co-write the manuscript. M. J. MacLachlan and M. A. Soto conducted general guidance, project directing, and manuscript revision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. O. thanks Dr Maria Ezhova and Dr Zhicheng (Paul) Xia for help with NMR characterization. M. J. M thanks the Canada Research Chairs program and NSERC (Discovery Grant) for funding. We also acknowledge WPI Nano Life Science Institute (Kanazawa) for support.Notes and references
- G. van Koten, Pure Appl. Chem., 1989, 61, 1681–1694 CrossRef CAS.
- W. Paw and R. Eisenberg, Inorg. Chem., 1997, 36, 2287–2293 CrossRef CAS.
- P.-K. Chow, G. Cheng, G. S. M. Tong, W.-P. To, W.-L. Kwong, K.-H. Low, C.-C. Kwok, C. Ma and C.-M. Che, Angew. Chem., Int. Ed., 2015, 54, 2084–2089 CrossRef CAS.
- M. A. Soto, R. Kandel and M. J. MacLachlan, Eur. J. Inorg. Chem., 2021, 2021, 894–906 CrossRef CAS.
- A. Zucca, G. L. Petretto, S. Stoccoro, M. A. Cinellu, M. Manassero, C. Manassero and G. Minghetti, Organometallics, 2009, 28, 2150–2159 CrossRef CAS.
- M. V. Nikolaeva and M. V. Puzyk, Opt. Spectrosc., 2013, 114, 247–250 CrossRef CAS.
- C. A. Craig, F. O. Garces, R. J. Watts, R. Palmans and A. J. Frank, Coord. Chem. Rev., 1990, 97, 193–208 CrossRef CAS.
- J.-Y. Cho, K. Y. Suponitsky, J. Li, T. V. Timofeeva, S. Barlow and S. R. Marder, J. Organomet. Chem., 2005, 690, 4090–4093 CrossRef CAS.
- D. Septiadi, A. Aliprandi, M. Mauro and L. De Cola, RSC Adv., 2014, 4, 25709–25718 RSC.
- A. Colombo, F. Fiorini, D. Septiadi, C. Dragonetti, F. Nisic, A. Valore, D. Roberto, M. Mauro and L. De Cola, Dalton Trans., 2015, 44, 8478–8487 RSC.
- X. Wang, X. Wang and Z. Guo, Acc. Chem. Res., 2015, 48, 2622–2631 CrossRef CAS PubMed.
- H. Xu, R. Chen, Q. Sun, W. Lai, Q. Su, W. Huang and X. Liu, Chem. Soc. Rev., 2014, 43, 3259–3302 RSC.
- T. Fleetham, G. Li and J. Li, Adv. Mater., 2017, 29, 1601861 CrossRef.
- M. Kato, Bull. Chem. Soc. Jpn., 2007, 80, 287–294 CrossRef CAS.
- A. Aliprandi, D. Genovese, M. Mauro and L. De Cola, Chem. Lett., 2015, 44, 1152–1169 CrossRef CAS.
- G. Parrinello and J. K. Stille, J. Am. Chem. Soc., 1987, 109, 7122–7127 CrossRef CAS.
- I. E. Markó, S. Stérin, O. Buisine, G. Mignani, P. Branlard, B. Tinant and J.-P. Declercq, Science, 2002, 298, 204–206 CrossRef.
- D. S. Black, G. B. Deacon and G. L. Edwards, Aust. J. Chem., 1994, 47, 217–227 CrossRef CAS.
- L. Chassot, E. Mueller and A. Von Zelewsky, Inorg. Chem., 1984, 23, 4249–4253 CrossRef CAS.
- M. M. Mdleleni, J. S. Bridgewater, R. J. Watts and P. C. Ford, Inorg. Chem., 1995, 34, 2334–2342 CrossRef CAS.
- T. Yamaguchi, F. Yamazaki and T. Ito, J. Am. Chem. Soc., 2001, 123, 743–744 CrossRef CAS PubMed.
- C. P. Newman, K. Casey-Green, G. J. Clarkson, G. W. V. Cave, W. Errington and J. P. Rourke, Dalton Trans., 2007, 3170–3182 RSC.
- S. R. Whitfield and M. S. Sanford, Organometallics, 2008, 27, 1683–1689 CrossRef CAS.
- J. Mamtora, S. H. Crosby, C. P. Newman, G. J. Clarkson and J. P. Rourke, Organometallics, 2008, 27, 5559–5565 CrossRef CAS.
- D. M. Jenkins and S. Bernhard, Inorg. Chem., 2010, 49, 11297–11308 CrossRef CAS.
- F. Juliá, G. Aullón, D. Bautista and P. González-Herrero, Chem.–Eur. J., 2014, 20, 17346–17359 CrossRef PubMed.
- F. Juliá, D. Bautista, J. M. Fernández-Hernández and P. González-Herrero, Chem. Sci., 2014, 5, 1875–1880 RSC.
- F. Juliá, D. Bautista and P. González-Herrero, Chem. Commun., 2016, 52, 1657–1660 RSC.
- F. Juliá and P. González-Herrero, Dalton Trans., 2016, 45, 10599–10608 RSC.
- F. Juliá, M.-D. García-Legaz, D. Bautista and P. González-Herrero, Inorg. Chem., 2016, 55, 7647–7660 CrossRef.
- D. A. K. Vezzu, J. C. Deaton, J. S. Jones, L. Bartolotti, C. F. Harris, A. P. Marchetti, M. Kondakova, R. D. Pike and S. Huo, Inorg. Chem., 2010, 49, 5107–5119 CrossRef CAS PubMed.
- H. Fukagawa, T. Shimizu, H. Hanashima, Y. Osada, M. Suzuki and H. Fujikake, Adv. Mater., 2012, 24, 5099–5103 CrossRef CAS.
- J. C. López-López, D. Bautista and P. González-Herrero, Inorg. Chem., 2023, 62, 14411–14421 CrossRef.
- M. A. Soto and M. J. MacLachlan, Chem. Sci., 2024, 15, 431–441 RSC.
- M. A. Soto, V. Carta, R. J. Andrews, M. T. Chaudhry and M. J. MacLachlan, Angew. Chem., Int. Ed., 2020, 59, 10348–10352 CrossRef CAS.
- M. A. Soto, V. Carta, M. T. Cano, R. J. Andrews, B. O. Patrick and M. J. MacLachlan, Inorg. Chem., 2022, 61, 2999–3006 CrossRef CAS.
- M. A. Soto, M. T. Chaudhry, G. K. Matharu, F. Lelj and M. J. MacLachlan, Angew. Chem., Int. Ed., 2023, 62, e202305525 CrossRef CAS.
- M. A. Soto, V. Carta, I. Suzana, B. O. Patrick, F. Lelj and M. J. MacLachlan, Angew. Chem., Int. Ed., 2023, 62, e202216029 CrossRef CAS.
- H. Yang, H. Li, L. Yue, X. Chen, D. Song, X. Yang, Y. Sun, G. Zhou and Z. Wu, J. Mater. Chem. C, 2021, 9, 2334–2349 RSC.
- M. Kondrashov, D. Provost and O. F. Wendt, Dalton Trans., 2016, 45, 525–531 RSC.
- S. A. Denisov, Y. Cudré, P. Verwilst, G. Jonusauskas, M. Marín-Suárez, J. F. Fernández-Sánchez, E. Baranoff and N. D. McClenaghan, Inorg. Chem., 2014, 53, 2677–2682 CrossRef CAS PubMed.
- C.-Z. Luo, P. Gandeepan, J. Jayakumar, K. Parthasarathy, Y.-W. Chang and C.-H. Cheng, Chem.–Eur. J., 2013, 19, 14181–14186 CrossRef CAS PubMed.
- J. A. Fernández-Salas, S. Manzini, L. Piola, A. M. Z. Slawin and S. P. Nolan, Chem. Commun., 2014, 50, 6782–6784 RSC.
- G. Zhang, G. Lv, C. Pan, J. Cheng and F. Chen, Synlett, 2011, 2011, 2991–2994 CrossRef.
- J. Jin, Q. Wen, P. Lu and Y. Wang, Chem. Commun., 2012, 48, 9933–9935 RSC.
- R. Cerón-Camacho, S. Hernández, R. Le Lagadec and A. D. Ryabov, Chem. Commun., 2011, 47, 2823–2825 RSC.
- I. Omae, J. Organomet. Chem., 2017, 848, 184–195 CrossRef CAS.
- M. Kondrashov, S. Raman and O. F. Wendt, Chem. Commun., 2014, 51, 911–913 RSC.
- R. E. N. Njogu, P. Fodran, Y. Tian, L. W. Njenga, D. K. Kariuki, A. O. Yusuf, I. Scheblykin, O. F. Wendt and C.-J. Wallentin, Synlett, 2019, 30, 792–798 CrossRef CAS.
- N. P. Tsvetkov, E. Gonzalez-Rodriguez, A. Hughes, G. dos Passos Gomes, F. D. White, F. Kuriakose and I. V. Alabugin, Angew. Chem., Int. Ed., 2018, 57, 3651–3655 CrossRef CAS.
- X. Wang, W.-G. Liu, L.-T. Liu, X.-D. Yang, S. Niu, C.-H. Tung, L.-Z. Wu and H. Cong, Org. Lett., 2021, 23, 5485–5490 CrossRef CAS PubMed.
- I. Allison, H. Lim, A. Shukla, V. Ahmad, M. Hasan, K. Deshmukh, R. Wawrzinek, S. K. M. McGregor, J. K. Clegg, V. V. Divya, C. Govind, C. H. Suresh, V. Karunakaran, K. N. N. Unni, A. Ajayaghosh, E. B. Namdas and S.-C. Lo, ACS Appl. Electron. Mater., 2019, 1, 1304–1313 CrossRef CAS.
- A. F. Rausch, L. Murphy, J. A. G. Williams and H. Yersin, Inorg. Chem., 2012, 51, 312–319 CrossRef CAS.
- J. Wu, B. Xu, Y. Xu, L. Yue, J. Chen, G. Xie and J. Zhao, Inorg. Chem., 2023, 62, 19142–19152 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2357023–2357030. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc04799k |
| This journal is © The Royal Society of Chemistry 2024 |